Abstract
CD8+ cytotoxic T lymphocytes (CTLs) recognize antigen in the context of major histocompatibility complex (MHC) class I molecules. Class I epitopes have been classified as dominant or subdominant depending on the magnitude of the CTL response to the epitope. In this report, we have examined the in vitro memory CTL response of H-2d haplotype murine CD8+ T lymphocytes specific for a dominant and subdominant epitope of influenza hemagglutinin using activation marker expression and staining with soluble tetrameric MHC–peptide complexes. Immune CD8+ T lymphocytes specific for the dominant HA204-210 epitope give rise to CTL effectors that display activation markers, stain with the HA204 tetramer, and exhibit effector functions (i.e., cytolytic activity and cytokine synthesis). In contrast, stimulation of memory CD8+ T lymphocytes directed to the subdominant HA210-219 epitope results in the generation of a large population of activated CD8+ T cells that exhibit weak cytolytic activity and fail to stain with the HA210 tetramer. After additional rounds of restimulation with antigen, the HA210-219–specific subdominant CD8+ T lymphocytes give rise to daughter cells that acquire antigen-specific CTL effector activity and transition from a HA210 tetramer–negative to a tetramer-positive phenotype. These results suggest a novel mechanism to account for weak CD8+ CTL responses to subdominant epitopes at the level of CD8+ T lymphocyte differentiation into effector CTL. The implications of these findings for CD8+ T lymphocyte activation are discussed.
Keywords: immunodominance, MHC class I tetramer, CD8+ T cell, anergy, self-peptide
Introduction
The cell-mediated immune response is critical for the clearance of viral pathogens 1. In particular, CD8+ CTLs have been demonstrated to be effective in the elimination of several different viruses, including influenza 2 3 4 and lymphocytic choriomeningitis virus (LCMV) 5 6 7. CTLs recognize peptide antigens, derived from intracellular processing of proteins, complexed with MHC class I molecules on the cell surface 8. Although most pathogens are composed of numerous foreign proteins, the T cell response is often directed at only a limited number of peptide epitopes 9 10. The magnitude of the response to these determinants is variable: some epitopes elicit a strong or immunodominant response, whereas others generate only a weak or subdominant response, and still others are described as cryptic, eliciting little if any detectable immune response 11. The factors regulating immunodominance have not been clearly defined, although the complex mechanisms of antigen processing and presentation, T cell priming and expansion, as well as limitations in the T cell repertoire are likely to play some role 12.
The CD8+ T cell response to influenza virus infection has been well characterized in several laboratories 13. Three epitopes recognized by murine CTLs in association with Kd molecules in H-2d mice have been identified in the influenza hemagglutinin (HA [14, 15]). Two of these epitopes are partially overlapping, spanning the residues HA204-212 and HA210-219, whereas the third, HA529-537, is derived from the transmembrane domain of the HA molecule.
The two overlapping epitopes, HA204-212 and HA210-219, are of particular interest because they differ markedly in immunogenicity. The HA204-212 epitope, like the HA529-537 epitope, is immunodominant and elicits a robust in vitro secondary CTL response in immune BALB/c mice in response to infectious A/Japan/57 influenza virus 14 16. In comparison, the in vitro secondary CTL response to the HA210-219 epitope is weak and variable 17. Previous studies suggested that the efficiency of antigen processing and peptide transport cannot account for the difference in immunogenicity of the two epitopes 18. It was also shown that the HA204-212 and HA210-219 peptides were equally efficient at stabilizing cell surface expression of Kd molecules on transfected RMA-S cells, indicating that inefficient binding of the HA210-219 peptide to Kd molecules was not the underlying cause for the weak response to this epitope 19. However, additional work using the limiting dilution assay (LDA) to quantitate the number of precursor CTLs (pCTLs) specific for each epitope demonstrated a 10–20-fold lower frequency of HA210-219–specific pCTLs compared with HA204-212–specific pCTLs, suggesting that the subdominant response to the HA210-219 epitope is a reflection of a limited number of HA210-219 pCTLs in the T cell pool 17.
Although the LDA has been widely used to quantitate relative numbers of effector T cells, recent technological advances have provided tools for direct staining of antigen-specific cells without relying on effector readouts 20. Virus-specific CTLs have been detected by staining with tetrameric MHC class I–peptide complexes in response to HIV 20, simian immunodeficiency virus 21, EBV 22, LCMV 23, and influenza 24. Reevaluation of the immune response to acute LCMV infection using tetramer staining has demonstrated that massive expansion of the CD8+ T cell population is almost entirely due to virus-specific CTL, and that previous reports had thus underestimated the antigen-specific response as measured by LDA 25.
In light of these observations, we have reexamined the magnitude of the memory CTL response to the HA204-212 and HA210-219 epitopes using activation marker expression and MHC class I–peptide tetramer staining. After in vitro stimulation of immune pCTLs with virus, we detected high levels of antigen-specific cytolytic activity and higher percentages of tetramer-positive, IFN-γ–producing CD8+ T cells responding to the dominant HA204-214 epitope than to the subdominant HA210-219 epitope. This result is consistent with our previous observations on the subdominance of the response to the HA210-219 epitope. Despite the low level of specific cytolytic activity and the low percentage of cells staining with HA210 tetramer, cultures responding to the subdominant epitope had a high percentage of CD8+ lymphoblasts displaying markers of activated T cells. To evaluate the antigen specificity of the activated CD8+ cells responding to the subdominant epitope, and to further characterize these cells, we subjected the cultures to two successive rounds of restimulation with antigen. We found that there was selective expansion and enrichment of both HA210 tetramer–positive and –negative CD8+ T lymphocytes in response to the subdominant HA210-219 epitope, with HA210 tetramer–positive cells representing an increasing percentage of the total CD8+ cells over successive stimulations. After restimulation, the subdominant CD8+ blast cells acquired antigen-specific cytolytic activity and the ability to secrete IFN-γ. This CTL effector activity was exhibited by both HA210 tetramer–positive and –negative CD8+ T lymphocyte populations. We further demonstrated that tetramer-negative HA210-219–specific CD8+ cells give rise to HA210 tetramer–positive cells in response to antigenic stimulation. These results suggest a novel mechanism for an apparently weak or subdominant CD8+ T lymphocyte response to viral antigen where specific CTL precursors are present at high frequency and activate in response to initial antigen stimulation, but do not fully differentiate into effector CTLs.
Materials and Methods
Mice.
6-wk-old female BALB/cAnNTac (H-2d) mice were purchased from Taconic Farms.
Viruses.
Influenza virus strains A/Ann Arbor/57 (AA), A/Guiyang/57-variant 17 (GV), and B/Lee were grown in the allantoic cavity of 10-d-old chicken embryos and stored as infectious allantoic fluid at −70°C 26. Recombinant vaccinia viruses expressing the A/Japan/305/57 HA protein lacking the transmembrane region (rVV HA A−) were prepared from monolayer cultures of BSC-40 cells as described 27.
Cell Lines.
The P815 (H-2d) mastocytoma cell line was maintained in DMEM (Life Technologies) supplemented with 10% FCS and 1% glutamine. CTL clones E2 and D4 were isolated pCTLs primed with influenza A/Japan/305/57 virus and established as described previously 27a. Cloned CTL lines were stimulated weekly with irradiated BALB/c splenocytes infected with influenza virus and human rIL-2 (10 U/ml).
Bulk CTL Populations.
Mice were primed intraperitoneally with 107 units of infectious rVV HA A− virus. 3 wk later, splenocytes were removed and restimulated in vitro with irradiated influenza virus–infected splenocytes. Subsequent in vitro stimulations were performed as above with the addition of rIL-2 (10 U/ml) to the medium.
Peptides.
Synthetic oligopeptides were produced on an automated Solid Phase Peptides Synthesizer (PE Biosystems). HPLC analysis showed that peptide preparations were >95% pure. Stock solutions of synthetic peptides were dissolved in 100% DMSO at concentrations of 5 mg/ml and diluted to the desired concentrations immediately before use in in vitro stimulations or cytotoxicity assays.
CTL-mediated Cytotoxicity Assays.
P815 cells were used as target cells in a sodium chromate (51Cr)-release assay as described previously(14). In brief, 51Cr-labeled target cells were washed twice and plated in 96-well microtiter plates at 104 per well in 0.1 ml assay medium. Peptide solutions or medium were added to each well in a 0.05-ml volume. Finally, T cells from day 5 bulk cultures were added in a volume of 0.05 ml per well. After incubation for 5 h at 37°C, 0.1 ml culture supernatant from each well was removed and counted for radioactivity. Values for percentage of specific release were calculated as described 14.
MHC Class I–Peptide Tetramers.
MHC class I–peptide tetramers were produced as described by Altman et al. 20. Plasmid DNA encoding the extracellular domain of the H2-Kd heavy chain and human β2-microglobulin were provided Eric Pamer (Yale University, New Haven, CT). Recombinant proteins were expressed in Escherichia coli, purified from inclusion bodies, solubilized, and refolded in the presence of A/Japan/305/57 influenza HA epitopes (204-212 or JHA210-219) or an H2-Kd–restricted Janus kinase (JAK)-1–derived self-peptide, as described 28. Folded complexes consisting of H2-Kd, β2-microglobulin, and antigenic peptide were purified by gel filtration over a Superdex 200 HR column (Amersham Pharmacia Biotech), then enzymatically biotinylated with the biotin–protein ligase BirA (Avidity) and tetramerized with PE-labeled streptavidin (Molecular Probes). Tetramers were stored at 5 mg/ml at 4°C in PBS (pH 8.0) containing 0.02% sodium azide, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 0.5 mM EDTA.
Cell Staining and Flow Cytometry.
T lymphocytes were isolated from a centrifuged Ficoll-Hypaque (Fico-Lite; Atlanta Biologicals) gradient of the bulk splenocyte cultures and stained at 4°C for 1 h. Tetrameric H2-Kd–peptide complexes or the following fluorochrome-conjugated antibodies (BD PharMingen) were used: anti-CD8α–CyChrome or –perCP (53-6.7), anti-TCR Vβ–FITC, anti-CD62L–FITC (MEL-14), and anti-CD44–FITC. After washing, the cells were fixed in 1% paraformaldehyde, then analyzed on a FACSCalibur™ (Becton Dickinson) using CELLQuest™ software. In some experiments, tetramer staining was carried out at 37°C with identical results.
Cell proliferation was assayed by labeling with carboxyfluorescein succinimidyl diacetate ester (CFSE; Molecular Probes) as described 29. Resting lymphocytes were incubated with 1.8 μM CFSE in PBS (pH 7.4) at room temperature for 7 min, washed twice, and then restimulated with virus-infected APCs as above. Cells were examined 4–5 d after stimulation by flow cytometry for evidence proliferation, as indicated by loss of CFSE intensity.
For cell sorting, cells were stained as above and a sterile sort was performed at the University of Virginia FACS® Core Facility using a FACS Vantage™ cell sorter (Becton Dickinson). Sorted populations were examined for purity and then either restimulated or used in cytolysis assays. Alternatively, cells were separated by MACS (Miltenyi Biotec) after staining with tetramer–PE and anti-PE microbeads. For both FACS® and MACS separations, cell populations were observed to be >99% pure.
Intracellular Cytokine Staining.
T lymphocytes from bulk cultures were incubated with peptide-pulsed P815 target cells (E/T = 1:2) for 5 h in the presence of 10 U/ml IL-2 and 2 μM monensin. After staining for extracellular markers, cells were permeabilized with 0.5% saponin, stained with antibodies to IFN-γ–FITC (BD PharMingen), and examined by flow cytometry as above.
Results
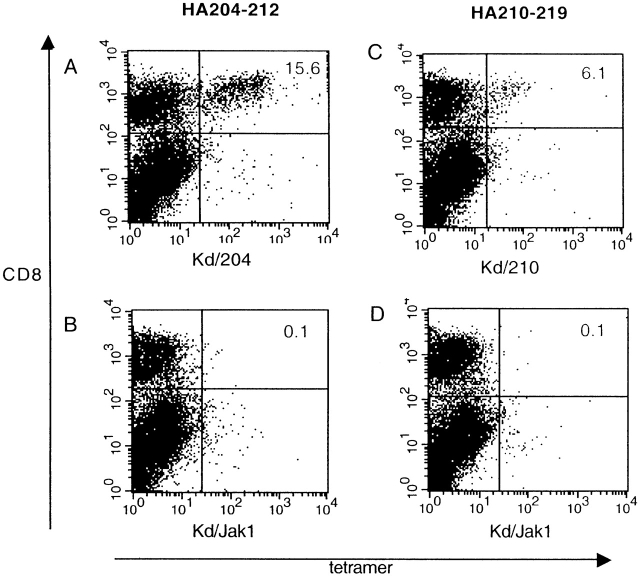
The CD8+ T cell response to the A/Japan/57 HA in H2d haplotype BALB/c mice is directed to three H2-Kd–restricted epitopes 15. Two of these epitopes, HA204-212 and HA529-537, elicit vigorous memory CD8+ CTL responses and are classified as dominant. The third epitope, HA210-219, is subdominant and elicits a weak, variable CTL response after priming and in vitro challenge with A/Japan/57 virus 17. The weak response to HA210-219 is not due to inefficient processing or presentation of the epitope 30, and was attributed to an apparent low frequency of CD8+ memory T cells directed to this site 17. As the designation of HA-specific CD8+ CTL epitopes as dominant or subdominant was based on the in vitro lytic activity of CTL effectors, we wanted to directly evaluate the number of memory CTL effectors directed to the subdominant HA210-219 epitope and a dominant epitope, HA 204-212, by MHC class I tetramer staining. Tetramers were prepared that consisted of the H2-Kd molecules loaded with the HA204-212 peptide (HA204 tetramer) or the HA210-219 peptide (HA210 tetramer), respectively. The specificity of binding of these HA tetramers was established by flow cytometry using clonal populations of HA204-212–specific and HA219-219–specific CTLs. As Fig. 1 demonstrates, HA204 tetramers selectively stained the HA204-212–specific CTL clone E2 (Fig. 1 A), whereas the HA210 tetramers selectively bound the HA210-219–specific CTL clone D4 (Fig. 1 B). Comparable specificity of tetramer staining was obtained with other HA-specific CD8+ CTL clones. Neither CTL clone bound a control tetramer containing a Kd-binding self-peptide derived from the murine JAK-1 kinase, further establishing the specificity of tetramer staining.
Figure 1.
Specificity of tetramer staining on cloned CTL lines. (A) Staining of HA204-specific cloned CTL line E2 with a panel of tetramer reagents. (B) Staining of HA210-specific cloned CTL cell line D4 with the same panel of reagents. Unstained cells (dashed line), Kd–HA204 tetramer (broken line), Kd–HA210 tetramer (solid line), Kd–JAK-1 tetramer (dotted line).
As noted above, infection with the A/Japan/57 virus stimulates Kd-restricted responses to three HA epitopes and to a dominant site on the influenza nucleoprotein (NP 147–155). Because antigenic competition has been suggested to influence the magnitude of CD8+ CTL responses to specific epitopes 31 32, we wanted to quantitate the memory CTL response to the dominant and subdominant HA CTL epitopes independently while still using a physiological stimulus (i.e., infectious virus) as the recall stimulus. To avoid the simultaneous induction of a primary CD8+ CTL response during infection in vivo, the memory CTL response was analyzed in vitro. To do so, we used the following priming and recall strategy. BALB/c mice were primed with a recombinant vaccinia virus expressing a truncated A/Japan/57 HA (HA A−) lacking the transmembrane domain and cytoplasmic tail of the HA gene product. This mutant HA lacks the dominant HA529-537 transmembrane epitope, but it does express the dominant HA204-212 and the subdominant HA210-219 epitopes. Therefore, this vaccination strategy should prime memory CD8+ T lymphocytes directed only to the HA204-212 and HA210-219 epitopes. Since in vitro restimulation of immune cells with infectious influenza virus preferentially activates memory CD8+ T lymphocytes, irradiated splenocyte APCs infected with either the AA or the GV influenza strain were used to reactivate A/Japan/57–specific memory CD8+ T lymphocytes directed to the HA204-212 or HA210-219 epitopes, respectively. The HA proteins of these two H2N2 influenza strains are serologically and structurally closely related to the A/Japan/57 HA, but they differ slightly in amino acid sequence from the A/Japan/57 HA. The AA HA is identical to the A/Japan/57 HA at 204–212, but the sequence differs at one residue in the HA210-219 epitope. This glycine to serine change at position 215 results in an epitope that is not recognized by A/Japan/57 HA210-219–specific CTLs 15. Likewise, the GV HA retains the HA210-219 epitope of the A/Japan/57 HA but has a single amino acid substitution at position 207 (N207K) in the HA204-212 epitope, which is not recognized by A/Japan/57 HA204-212–specific CTLs.
Immune splenocytes from two or three HA A−–primed donors were pooled and restimulated in vitro with irradiated splenocyte APCs infected with either the AA virus, the GV virus, or the unrelated type B influenza strain LEE. 5 d after in vitro restimulation, cultures were examined for HA tetramer staining and cell surface expression of the activation-associated markers CD62L and CD44, as well as for cytolytic activity and IFN-γ production. Representative results from these experiments are shown in Fig. 2 and Fig. 3 and in Table . CD8+ T cells that stained positive with the HA204 tetramer were readily demonstrated in cultures stimulated with the AA virus, and 15.6% of total CD8+ T cells were observed to bind tetramer in the representative experiment shown (Fig. 2 A). In addition to HA204 tetramer staining, stimulation of memory CD8+ T cells with the AA virus was associated with a vigorous cytolytic response directed toward HA204-212 peptide–pulsed targets (Fig. 3 A). These cytolytic effectors did not lyse target cells pulsed with the HA210-219 or HA529-537 peptide, suggesting that stimulation of immune cells with the AA virus had selectively activated HA204-212–specific memory CD8+ T lymphocytes. The strong cytolytic response to the dominant HA204-212 epitope is consistent with our earlier observations using A/Japan/57 virus for priming and in vitro reactivation of memory CTL effectors directed to this epitope 17. Analysis of the fraction of CD8+ T lymphocytes staining for IFN-γ production (12%; Fig. 3 C) and for the CD62LloCD44hi phenotype (18.3%; Table ) demonstrated a good correlation with the percentage of HA204-212–specific CTLs estimated by tetramer staining (15.6%; Fig. 2 A).
Figure 2.
Activation of HA-specific memory CTLs. BALB/c mice were immunized with a recombinant vaccinia virus expressing a truncated version of the HA protein. 3 wk later, immune splenocytes were stimulated in vitro with APCs infected with (A and B) AA virus (for HA204-212 responses) or (C and D) GV virus (for HA210-219 responses). 5 d after in vitro stimulation, cells were harvested and stained with anti-CD8–CyChrome and the Kd–JAK-1 or indicated HA tetramer, then analyzed by flow cytometry. Numbers in the corner of the upper right quadrant represent the percentage of total CD8+ T cells that stained positive with HA tetramer. These results are representative of five independent experiments.
Figure 3.
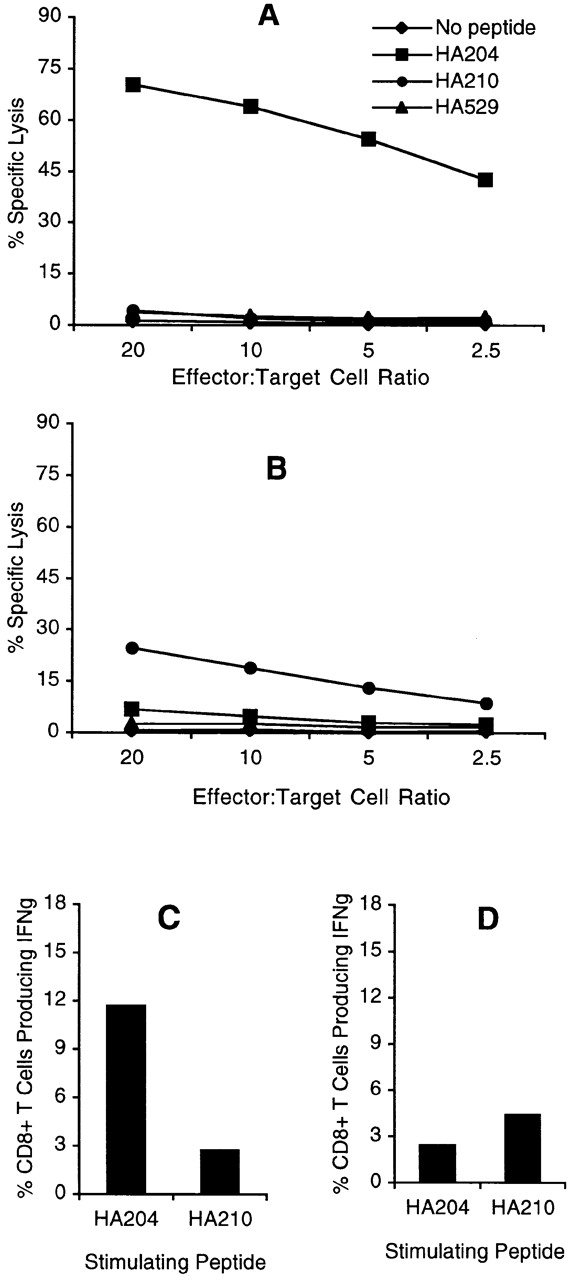
Effector activity of bulk cultures. Cytolytic activity and cytokine production by HA-specific CTLs was measured on day 5 after in vitro stimulation. For cytolysis assays, bulk immune cell populations were incubated with 51Cr-labeled P815 target cells pulsed with 10−9 M concentration of the indicated peptide over a range of E/T ratios for 5 h at 37°C. (A) AA-stimulated cultures (HA204 enriched). (B) GV-stimulated cultures (HA210 enriched). Intracellular cytokine staining was performed by incubating day 5 cells with 10−6 M HA204-212 or HA210-219 peptide–pulsed P815 target cells (E/T = 1:2) in the presence of IL-2 and monensin for 6 h at 37°C. Cells were stained with anti-CD8–CyChrome, then permeabilized and stained with anti–FITC-conjugated IFN-γ or isotype control antibody (not shown). Histogram values are the average percentage of IFN-γ–secreting CD8+ T cells in response to peptide from duplicate cultures. (C) AA-stimulated cultures (HA204 enriched). (D) GV-stimulated cultures (HA210 enriched).
Table 1.
Activated Lymphocytes in Bulk Cultures
| Stimulus | |||
|---|---|---|---|
| Percentage of CD8+ T cells | AA | GV | B/Lee |
| HA-specific (tetramer positive) | 15.6 | 6.1 | <1 |
| Activated phenotype (CD44hiCD62Llo) | 18.3 | 25.2 | 3.1 |
Analysis of the frequency of subdominant HA210-219–specific CTL effectors elicited by stimulation with the GV virus was carried out in parallel using the same pool of immune splenocytes. In the representative experiment shown (Fig. 2 C), only 6.1% of CD8+ T cells were HA210 tetramer positive in the GV-stimulated culture. The lower percentage of HA210-staining CD8+ T cells directed to the subdominant epitope was associated with a weak cytolytic response that was specific for the HA210-219 epitope (Fig. 3 B). Consistent with our earlier observations, the cytolytic response to the HA210-219 epitope was at least 10-fold lower in cytolytic activity than the response to the dominant HA204-212 epitope 17. In keeping with the low percentage of HA210 tetramer binding cells and the weak cytolytic response, GV virus–stimulated cultures had a low frequency of CD8+ T lymphocytes that produced IFN-γ in response to the HA210-219 peptide (4.3%; Fig. 3 D). However, cultures of immune cells stimulated with the GV virus appeared highly activated by visual inspection. When these cultures were examined for expression of CD62L and CD44, 25.2% of CD8+ T cells in the GV-stimulated cultures exhibited an activated phenotype (CD62LloCD44hi), as shown in Table . The percentage of CD8+ T cells with an activated phenotype was consistently higher in the subdominant cultures than in the dominant HA204-212 cultures (Table ).
This high percentage of activated CD8+ T cells in GV-stimulated cultures was not readily attributable to exposure of CD8+ T cells to virus-infected APCs, as companion cultures of HA A− immune splenocytes exposed to the unrelated influenza B/Lee virus exhibited only minimal CD8+ T cell activation (Table ). Identical results were obtained when cytolytic activity was examined at days 6–10 after stimulation (data not shown), which implies that the absence of effector activity in these highly activated HA210-219–specific cultures was not due to delayed kinetics of the expression of effector activity. To determine if the generation of activated noncytolytic CD8+ T lymphocytes in response to the subdominant HA210-219 epitope was due to an unrelated property of the GV virus, the responses of immune splenocytes to GV-infected APCs and APCs pulsed with the synthetic HA210-219 peptide were compared. As Table demonstrates, peptide-stimulated cultures contained an even higher percentage of activated CD8+ T cells than virus-stimulated cultures at day 5–6 after stimulation; however, like the GV virus–stimulated culture, these activated CD8+ T cells exhibited minimal cytolytic activity on HA210-219–expressing target cells. These results raised the possibility that HA210-219–specific memory CD8+ T cells are present at a high frequency in immune animals and are capable of responding to the subdominant HA210-219 epitope. However, the responding CD8+ T cell population fails to exhibit effector activity or efficiently bind tetramers incorporating the HA210-219 epitope.
Table 2.
Effector Activity of Virus- and Peptide-stimulated Cultures
| Cytolytic activity | ||||
|---|---|---|---|---|
| Stimulus | 1:1 | 5:1 | 10:1 | Percentage of activated CD8+ T cells (CD44hiCD62Llo) |
| GV virus | 9 [7] | 5 [0] | 9 [8] | 45 |
| HA210-219 peptide (1×) | 6 [0] | 1 [8] | 7 [7] | 61 |
| HA210-219 peptide (mx) | 49 [5] | 60 [1] | 75 [8] | >95 |
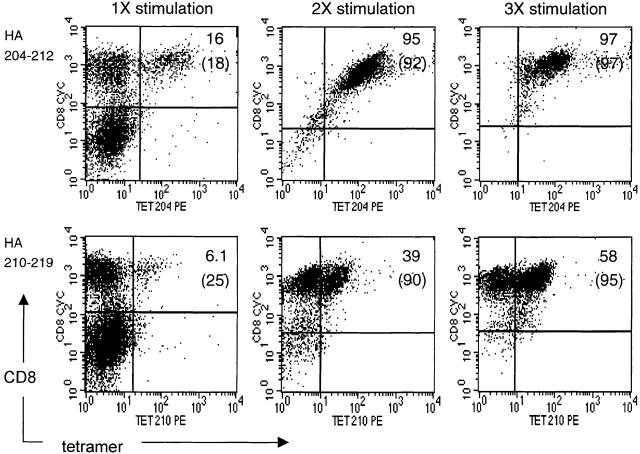
Cultures of antigen-specific CD8+ CTLs can be maintained and expanded by successive rounds of restimulation with antigen at periodic intervals. To further evaluate if the activated noncytolytic CD8+ T cells in cultures stimulated with the GV virus were specific for the subdominant HA210-219 epitope, these cultures were subjected to two further rounds of in vitro stimulation at 14-d intervals. At day 5 after the second and third round of stimulation, viable cells were examined for expression of CD8 and CD44 (or CD62L) and for staining with HA tetramer. Companion cultures of HA204-212–specific CD8+ T cells were expanded by restimulation with AA virus and were examined in parallel. As Fig. 4 (top) demonstrates, >90% of viable cells after the second stimulation with the AA virus were CD8+ and bound the HA204 tetramer. This phenotype was maintained after the third round of in vitro stimulation. Therefore, as expected, in vitro restimulation with specific antigen enriched for these dominant HA204-212–specific CD8+ T cells in culture.
Figure 4.
Effect of antigenic stimulation on tetramer staining by CD8+ T cells. Immune splenocytes were stimulated once (1×) in vitro with A/AA/57-infected or GV-infected APCs to selectively activate HA204-212–specific or HA210-219–specific CD8+ T cells, respectively. Immune cell cultures were successively restimulated twice (2×, 3×) at 14-d intervals with homologous virus. 5 d after each in vitro stimulation, cells were harvested and viable cells were stained with anti-CD8–CyChrome, HA tetramer (TET) PE and either CD44-FITC or CD62L-FITC. HA204-212 cultures were stained with HA204 tetramers, and HA210-219 cultures were stained with HA210 tetramers. The values within each panel are the percentage of total CD8+ T cells that are tetramer positive and, in parentheses, the percentage of total CD8+ T cells with an activated phenotype (CD44hiCD62Llo). These results are representative of five independent experiments.
When cultures containing the subdominant HA210-219–specific CTLs were restimulated with GV virus in vitro, a distinctly different pattern of tetramer staining was observed (Fig. 4, bottom). After the second round of in vitro stimulation with HA210-219–expressing GV-infected APCs, 90% of viable cells in the culture were CD8+, indicating the expected selective enrichment of antigen-specific CD8+ T cells after restimulation. Approximately 40% of the CD8+ T cells stained positive for the HA210 tetramer. However, after a second round of antigenic stimulation, 60% of the stimulated CD8+ T cells retained the tetramer-negative phenotype. After a third round of stimulation, >90% of viable cells in these cultures were CD8+, with HA210 tetramer–binding cells now representing ∼60% of the total cells. Despite the increased frequency of HA210 tetramer–positive cells in these cultures, activated CD8+ HA210 tetramer–negative cells still represented a substantial fraction (40%; Fig. 4) of the total CD8+ T cells in these cultures after three successive rounds of in vitro stimulation. Expansion of the small number (6.1%) of HA210 tetramer–positive cells in the initial cultures could account for the progressive increase in tetramer-staining cells after repeated exposure to HA210-219–expressing GV-infected APCs. However, the continued presence of activated CD8+ HA210 tetramer–negative T cells in these cultures most likely reflects specific stimulation and antigen-dependant proliferation of HA210-219–specific cells that fail to interact with HA210 tetramer.
As HA210 tetramer–positive CD8+ T cells represented ∼50–60% of activated cells in tertiary GV-stimulated cultures, it was of interest to determine if the cells in these cultures now exhibited CD8+ T cell effector activities. Tertiary cultures containing HA210-219 and HA204-212–specific CD8+ T cells, described above, were analyzed for cytolytic activity on peptide-pulsed target cells and for intracellular IFN-γ staining (Fig. 5). Tertiary cultures of AA-stimulated HA204-219–specific CD8+ T cells (Fig. 5 A) and GV-stimulated HA210-219–specific CD8+ T cells (Fig. 5 B) both exhibited high levels of epitope-specific cytolytic activity. Comparable results were obtained when HA210-specific immune splenocytes underwent multiple rounds of in vitro restimulation with the synthetic HA210-219 peptide (Table ). Thus, after two or more rounds of restimulation with antigen, the CD8+ CTLs in the subdominant cultures now displayed a level of specific cytolytic effector activity comparable to that of the HA204-212–specific CTLs present in the dominant cultures. Although the potent lytic activity of the subdominant CD8+ T cell population could be attributed to the 50–60% of CD8+ T cells that are HA210 tetramer positive, the analysis of intracellular IFN-γ staining did not support this view. As shown in Fig. 5C and Fig. D, ∼92% of the cells in AA-immune tertiary cultures and 86% of the cells in GV-immune tertiary cultures exhibit antigen-dependent IFN-γ production. However, the presence of a high percentage of IFN-γ–secreting cells with potent lytic activity in the HA210-219–specific culture suggested that both HA210 tetramer–positive and –negative CD8+ T cells contributed to the CTL effector activity observed.
Figure 5.
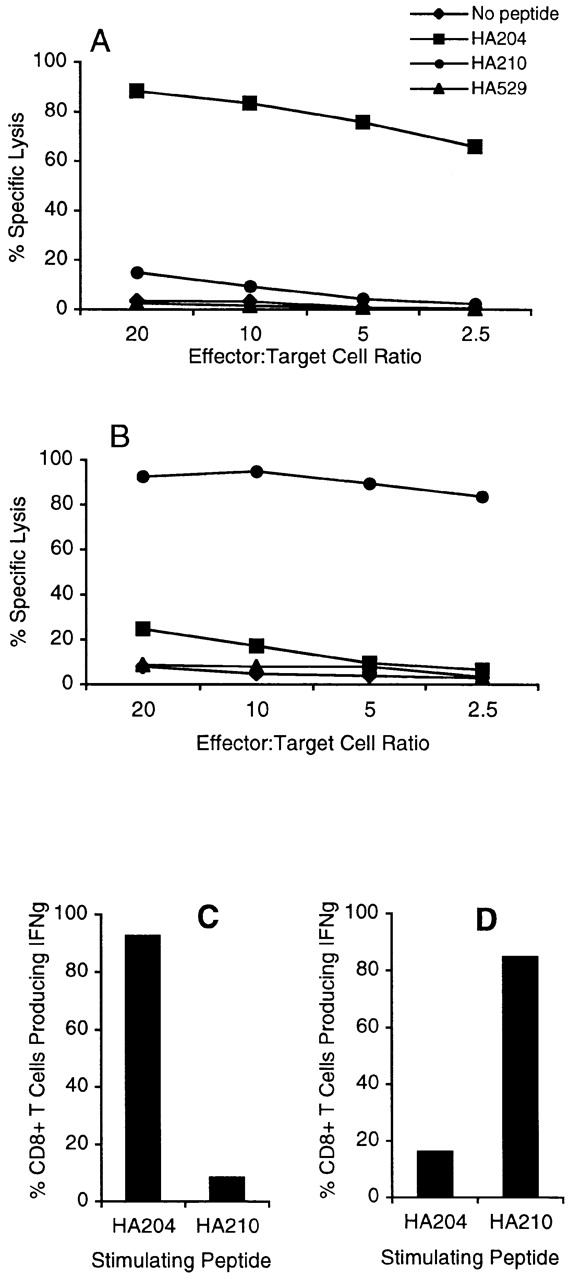
Effector activity of tertiary bulk cultures. 5 d after the third in vitro stimulation with homologous virus–infected APCs, bulk cultures were analyzed for cytolytic activity and IFN-γ production as described in the legend to Fig. 3. A and C represent activity of tertiary HA204-21–specific AA virus–stimulated cultures. B and D represent tertiary HA210-219–specific GV virus–stimulated cultures.
To directly evaluate this point, day 5 effectors from tertiary GV-stimulated cultures were sorted by flow cytometry into HA210 tetramer–positive and –negative fractions. The tetramer-positive and -negative fractions were tested along with unfractionated cells for in vitro cytolytic activity. For technical reasons, we were unable to take sorted HA210 tetramer–positive and –negative cells and simultaneously monitor tetramer binding and intracellular IFN-γ accumulations. So it was not possible to directly demonstrate IFN-γ production by the HA210-negative CD8+ T cells. As shown in Fig. 6, epitope-specific cytolytic activity was demonstrable in both the tetramer-positive and -negative CD8+ T cell populations. The diminished cytolytic activity exhibited by the HA210 tetramer–positive cell fraction may represent interference with T cell receptor recognition events as a result of tetramer binding to these cells or some loss of cell viability during the cell sorting procedure. Nevertheless, these results demonstrate that activated HA210 tetramer–negative CD8+ T cells not only can be expanded and propagated continuously in culture, but also acquire antigen-specific effector activity after repeated antigenic stimulation. This includes both the development of antigen-specific cytolytic activity and antigen-dependent cytokine production.
Figure 6.
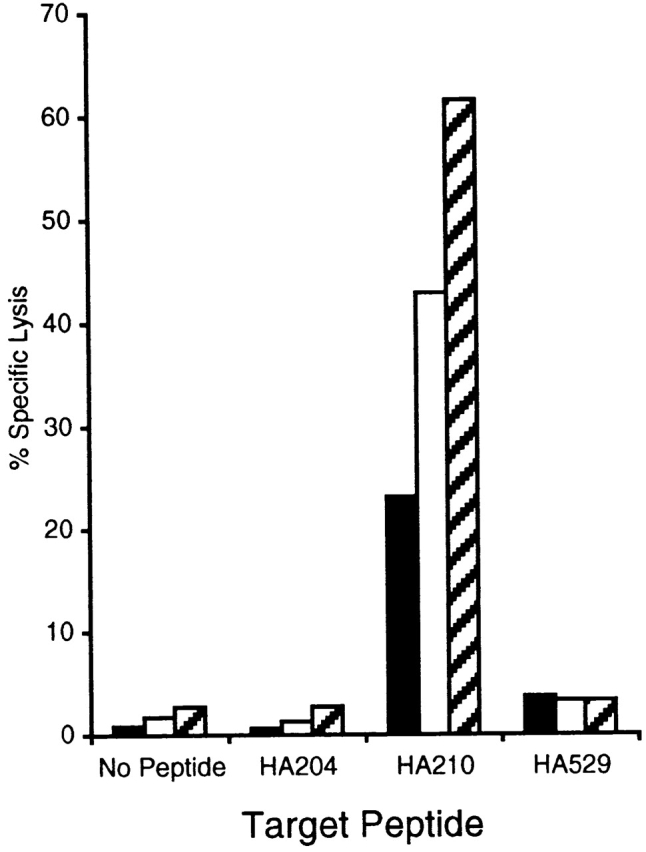
Cytolytic activity of sorted HA210 tetramer–positive and tetramer-negative CTLs. Bulk CTL populations, stimulated three times in vitro with GV virus–infected APCs, were stained with HA210 tetramer–PE and anti-CD8–FITC, then sorted via FACS® into >99% pure tetramer-positive and -negative populations. Sorted populations, as well as samples of the unsorted bulk culture, were assayed for cytolytic activity in a 51Cr-release assay using P815 target cells pulsed with 10−9 M of the indicated peptide (E/T = 5:1). These results are representative of three independent experiments. Black bars, HA210 tetramer–positive cells; white bars, HA210 tetramer–negative cells; hatched bars, unsorted tertiary GV bulk culture.
The presence of subdominant HA210-219–specific CTL effectors that failed to bind the HA210 tetramer was unexpected. These tetramer-negative CD8+ T cells were detected in multiple independent experiments using several different preparations of HA210 tetramers. The percentage of HA210 tetramer–positive and –negative cells in GV-stimulated cultures was identical whether the staining procedure was carried out at 4°C or 37°C, and remained unchanged over a 30-fold concentration of HA210 tetramer used in the staining procedure (data not shown). One explanation for the failure of activated antigen-specific CD8+ effector cells to bind the HA210 tetramer is that the cells express a lower level of the TCR complex than the tetramer-positive cells present in cultures of HA210-219– or HA204-212–specific cells. To examine this possibility, we analyzed the level of expression of the TCR β chain, CD3, and CD8 present on effector cells in cultures after tertiary stimulation with either AA virus (>90% HA204 tetramer–positive cells) or GV virus (50–60% HA210 tetramer–positive cells; Fig. 4). If subdominant HA210-219–specific CD8+ effectors contained a mixture of tetramer-positive TCRhi cells and tetramer-negative TCRlo cells, then these cultures would exhibit either a bimodal distribution of TCR complex staining or a lower overall level of TCR complex expression. As shown in Fig. 7, cultures of dominant HA204-212–specific CD8+ CTLs and cultures of subdominant HA210-219–specific CD8+ CTLs expressed comparable levels of TCR β chain, CD3, and CD8.
Figure 7.
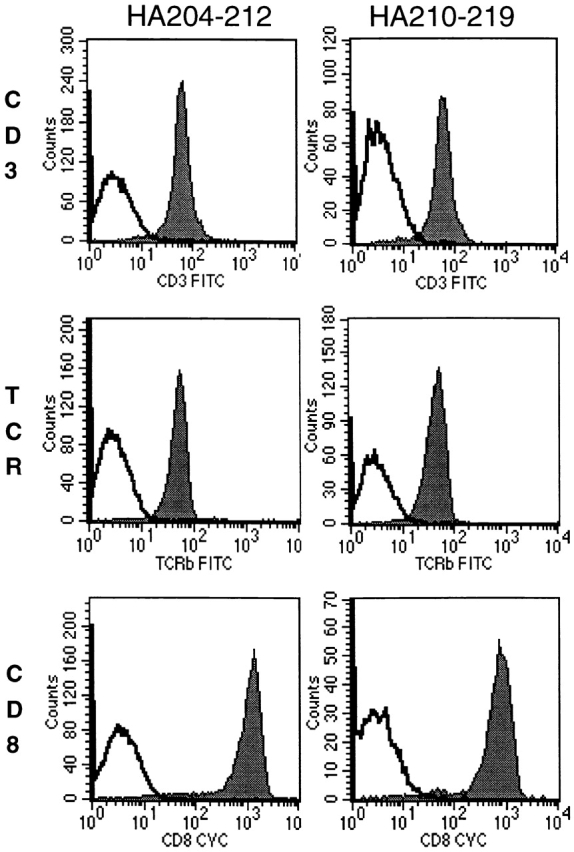
Surface expression of TCR complex on dominant and subdominant CTLs. 5 d after a third in vitro stimulation with virus-infected APCs, dominant (HA204) and subdominant (HA210) bulk cultures were stained with anti–TCR-β or anti-CD3 and anti-CD8 (solid histograms) or with corresponding isotype control antibodies (open histograms), as indicated.
TCR Vβ gene usage by dominant and subdominant CD8+ T cell populations was also determined by flow cytometry after one or three rounds of in vitro stimulation. Not unexpectedly, both the HA204-specific and the HA210-specific CD8+ T cells were heterogeneous in terms of Vβ gene usage with TCR Vβ2, 3, 6, 7, 8.1/8.2, 8.3, 10b, 13, and 14 positive cells representing 4% or more of the activated CD8+ cells. Representative percentages of Vβ cells for HA210 specific CD8+ T cells after three rounds of in vitro stimulation are: Vβ2 [5.4]; Vβ4 [6.2]; Vβ6 [16.4]; Vβ7 [5.0]; Vβ8.1/8.2 [25.5]; Vβ8.3 [12.4]; Vβ10b [10.0]; Vβ13 [4.2]; Vβ14 [14.3], with values in brackets indicating the percentage of activated CD8+ cells expressing the indicated Vβ gene. We noted no significant difference in the range of Vβ gene usage displayed by HA210-specific tetramer-positive and -negative cells. Much greater variability in the percentage of CD8+ cells expressing specific Vβ genes was evident between individual HA210-specific donors in different experiments.
An alternative explanation for the inability of these subdominant CTL effectors to bind the HA210 tetramer is that they represent a population of low-affinity CTLs with TCRs that are capable of binding to peptide–MHC ligand on the surface of infected APCs or peptide-pulsed target cells, but they are unable to bind soluble MHC tetramers with sufficient avidity to prevent tetramer dissociation. Therefore, cultures of subdominant HA210-219–specific CTLs may contain a mixture of high-affinity tetramer-positive CTLs and lower-affinity tetramer-negative CTLs, whereas the cultures of dominant HA204-212–specific CTLs consist exclusively of high-affinity tetramer-positive CD8+ effectors. To evaluate the relative affinity of dominant and subdominant CTL populations, we examined the peptide dose dependence of target cell lysis by HA204-212– and HA210-219–specific cytolytic effectors present at day 5 after tertiary stimulation with AA or GV virus. The latter cultures consisted of ∼40% HA210 tetramer–negative CD8+ T cells. As Fig. 8 demonstrates, both HA204-212–specific and HA210-219–specific CTL effectors had comparable peptide dose dependencies for target cell lysis, with optimal cytolysis observed at 10−9–10−10 M peptide concentrations. A comparable peptide dose dependence for target cell recognition and lysis was obtained using HA210 tetramer–negative HA210-219–specific CD8+ effectors selected by cell sorting before use in the in vitro cytotoxicity assay (Fig. 8 C), although both tetramer-positive and -negative CD8+ cells exhibited lower lytic activity on a per cell basis than the heterogeneous cell population before sorting.
Figure 8.
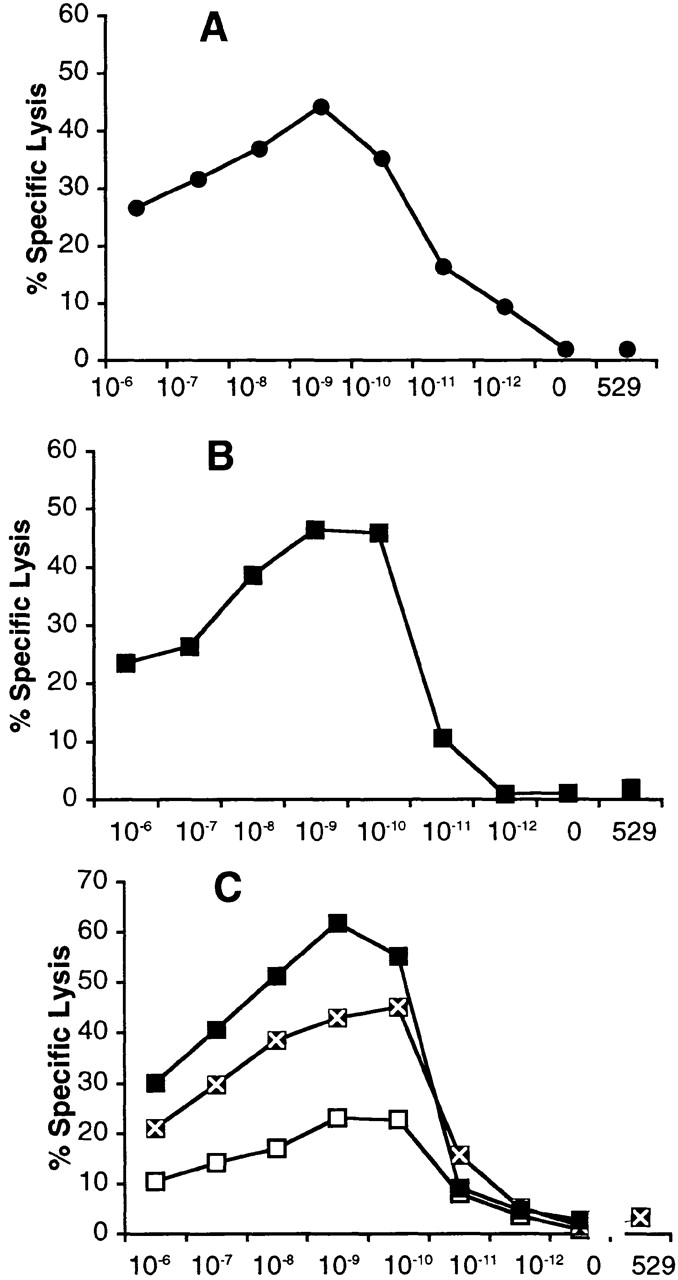
Peptide dose dependence of dominant and subdominant CTLs. On day five or six after tertiary in vitro stimulation with virus-infected APCs, cytolytic activity was examined on peptide-pulsed targets over the indicated peptide concentrations as described. (A) AA-stimulated cultures tested on HA204-212 peptide–pulsed P815 target cells. (B) GV-stimulated cultures tested on HA210-219 peptide–pulsed P815 target cells. (C) FACS®-sorted HA210 tetramer–positive (□), tetramer-negative (x), and unsorted (▪) CD8+ T cells from tertiary stimulated GV cultures tested on HA210-219–pulsed targets. Assay time was 5 h at an E/T ratio of 10:1. Values are the mean percentage of lysis of quadruplicate samples. SE values were ≤3% of mean values and are not shown. “0” indicates target cells not treated with peptide, and “529” indicates target cells treated with 10−8 M concentration of the irrelevant HA529 synthetic peptide.
The results in Fig. 8 indicated that the heterogeneous population of tetramer-positive and -negative CD8+ T cells comprising the cultures of HA210-219 CTL effectors had the same overall affinity as the uniform population of tetramer-positive CTLs present in the HA204-212–specific cultures. Because HA210 tetramer–positive cells progressively accumulated upon repeated stimulation with the GV virus (Fig. 4), we considered the possibility that, rather than representing a distinct population of low-affinity effector T cells, the HA210 tetramer–negative CTL effectors gave rise to HA210 tetramer–positive cells upon antigenic stimulation.
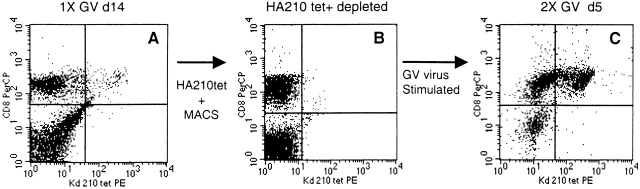
To assess the precursor–product relationship between tetramer-negative and tetramer-positive HA210-219–specific CD8+ T cells, cell depletion experiments were carried out. Immune cells consisting of a mixture of HA210 tetramer–positive and –negative cells were harvested 14 d after the first in vitro stimulation with GV virus. The HA210 tetramer staining profile of this resting cell population before depletion is shown in Fig. 9 A. HA210 tetramer–binding cells were depleted from these cultured cells by magnetic bead separation. The remaining viable cells, which consisted of >99% HA210 tetramer–negative T cells (Fig. 9 B), underwent a second round of in vitro stimulation with GV-infected APCs. 5 d after stimulation, the cells were evaluated for HA210 tetramer staining. As Fig. 9 C demonstrates, both HA210 tetramer–positive and –negative CD8+ T cells were detected in the culture. Tetramer-positive cells represented ∼35% of the viable CD8+ T cells in culture. These results support the view that subdominant tetramer-negative CD8+ T cells can give rise to cells that stain positive for HA210 tetramer after antigenic stimulation. However, it is formally possible that the tetramer-negative CD8+ T cells did not undergo extensive proliferation, and that a small number of HA210 tetramer-positive CD8+ T cells contaminating the cell culture after magnetic bead separation underwent preferential proliferative expansion in response to antigenic stimulation.
Figure 9.
Generation of HA210 tetramer–positive CD8+ cells from HA210 tetramer–negative cells in response to antigen. Freshly isolated immune splenocytes were stimulated one time in vitro with GV-infected APCs. At day 14 after stimulation, the residual viable cells were incubated with a 10-fold excess of PE-labeled HA210 tetramer, washed, and incubated with anti-PE antibody–coated magnetic microbeads. Tetramer-binding cells were depleted from the culture by magnetic cell separation. The column flow-through (CD8+, HA210 tetramer–negative cells) was washed and then restimulated by coculture with GV-infected APCs. 5 d after stimulation, viable cells were harvested and stained with CD8-perCP and HA210 tetramer–PE, then examined by flow cytometry. (A) Resting GV cultures on day 14 (1× GV d14). (B) Tetramer-negative column flow-through cells before stimulation (HA210 tet+ depleted). (C) Composition of cultures 5 d after stimulation of flow-through cells (2× GV d5).
To examine the proliferative potential of the HA210 tetramer–negative cells, these day 14 cells, after magnetic bead depletion, were labeled with the fluorescent dye CFSE before antigenic stimulation. This dye decreases proportionately in fluorescence intensity with each cell division, allowing for quantitation of cell divisions by flow cytometry 29. At the time of in vitro stimulation with GV-infected APCs, the HA210 tetramer–negative cells showed a uniform pattern of high-intensity CFSE staining (Fig. 10 A). 5 d after in vitro antigenic stimulation, viable cells were evaluated for CFSE staining intensity and HA210 tetramer binding. As Fig. 10 B illustrates, CFSE-stained cells fell into two distinct distributions. A large fraction of cells (R1) stained weakly for the dye, indicating a population of cells that had undergone five or more cell divisions in response to antigenic stimulation. When this population of recently dividing CD8+ T cells was examined for HA210 tetramer staining, tetramer-positive and tetramer-negative cells were equally represented (Fig. 10 C). These data indicate that HA210 tetramer–negative precursor cells proliferate in response to antigen and give rise to both tetramer-positive and tetramer-negative cells. By contrast, the second cell fraction (R2) stained strongly for CFSE, indicating a population of cells that had undergone only a limited number of cell divisions. Analysis of CD8 expression and HA210 tetramer staining by this population revealed that all cells in this fraction were tetramer negative and primarily CD8 negative (Fig. 10 D). These residual nondividing cells in the cultures are probably not antigen specific.
Figure 10.
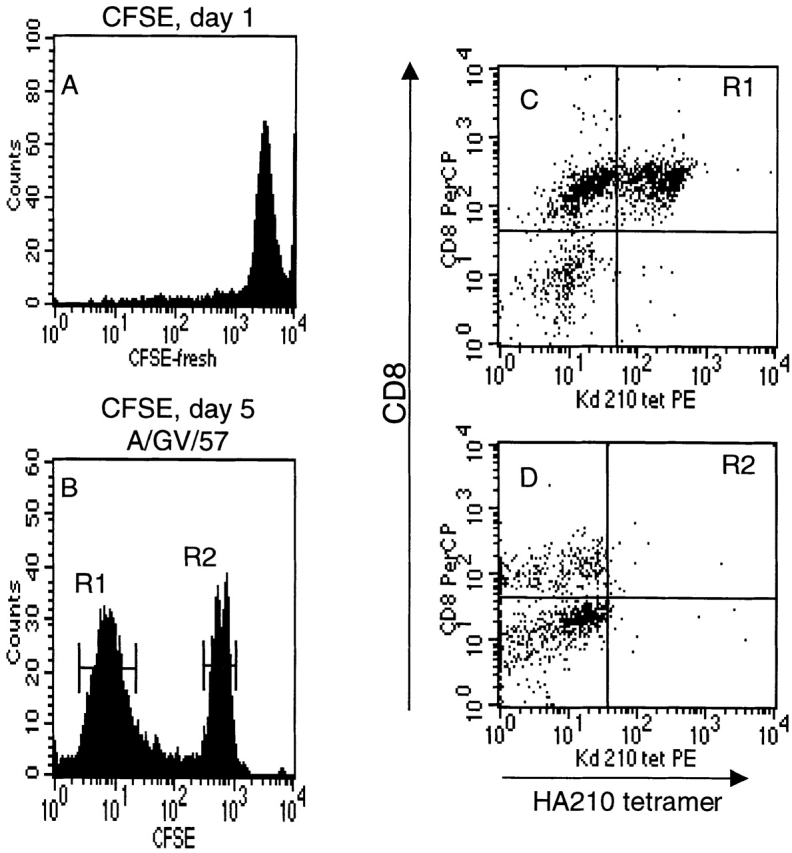
Proliferation of separated HA210 tetramer-negative cells after antigenic stimulation. Tetramer-negative cells were isolated as described in the legend to Fig. 9. Before antigenic stimulation, cells were labeled with the CFSE dye to monitor cell division. (A) CFSE fluorescence intensity of freshly labeled cells. (B) Dye intensity of CFSE-labeled cells at day 5 after stimulation with GV-infected APCs. (C and D) HA210 tetramer and CD8 staining of day 5 cells in dye peak R1 (C) and R2 (D).
Discussion
In this report, we have used MHC class I tetramer staining and activation marker expression to examine the memory murine CTL response in the spleen to a dominant (HA204-212) and a subdominant (HA210-219) epitope of the HA protein. We found that the frequency of subdominant HA210-specific cells responding to antigen was higher than predicted from the cytolytic activity, cytokine release, or MHC class I tetramer staining of the responding CD8+ T cells. A large number of immune CD8+ T cells proliferated in response to the HA210-219 epitope and displayed an activated phenotype, but the majority of these cells did not express effector activity as measured by in vitro cytolysis assay or intracellular IFN-γ staining. Unexpectedly, these antigen-reactive cells also failed to bind HA210 tetramer. However, upon repeated exposure to antigen, these subdominant CD8+ T cells gained effector activity and ultimately switched from a tetramer-negative to a tetramer-positive phenotype. These results suggest a novel mechanism for the occurrence of subdominant or weak CTL responses at the level of CD8+ T cell activation and differentiation.
The weak response to the subdominant HA210 epitope is not an exclusive property of memory CTL precursors in the spleen undergoing activation in vitro. The weak response of CD8+ T cells to the HA210 epitope is also observed in the lungs of mice undergoing primary pulmonary infection with A/Japan/57 virus (Lawrence, C.W., and T.J. Braciale, unpublished observations). This unusual property of HA210-specific CD8+ T cells is not readily attributable to the use of the GV influenza strain as the immunogen for in vitro stimulation, because immune splenocytes stimulated with a synthetic peptide corresponding to the HA210-219 epitope give rise to effector cells with a similar phenotype as immune cells stimulated with virus-infected APCs (Table ; Spencer, J.V. and T.J. Braciale, unpublished observations). Our results strongly suggest that the subdominant response to the HA210 epitope is not due to a low frequency of antigen-specific CD8+ T cells, but rather to the inability of the majority of HA210-specific memory CTL precursors to fully differentiate into activated CTL effectors after initial exposure to the viral antigen. Full expression of effector activity requires subsequent rounds of antigenic stimulation and further cell division to complete the differentiation process.
Selective induction of specific effector activities has been reported in the response of fully differentiated CD8+ CTL effectors to antigen. Valitutti et al. 33 demonstrated that the expression of cytolytic activity by human CD8+ CTL clones could be elicited at low peptide concentrations by engagement of a very small number of T cell receptors 33. Only at increasing levels of TCR occupancy were proliferation and production of IFN-γ observed. Selective expression of cytolytic activity to an antigenic stimulus by CD8+ CTLs in the absence of other effector responses has also been reported in other systems 34 35. Much less information is available concerning the antigen-driven transition of CD8+ T cells from the resting state into activated CTL effectors. Although CD8+ T cell responses ranging from anergy to exhaustion have been observed, in only one report dealing with the response of H-Y–specific TCR transgenic CD8+ T cells to the male antigen has antigen-specific proliferation of resting, naive CD8+ T cells without the expression of cytolytic activity been observed 36. The results reported here suggest that a heterogeneous population of memory CD8+ T cells directed to a viral epitope can activate and proliferate in response to antigen without fully differentiating into armed CD8+ T cell effectors.
Memory CD8+ T cells directed to the dominant HA204-212 epitope and the subdominant HA210-219 epitope, although apparently comparable in frequency, differ dramatically in their response to viral antigen. Dominant CD8+ T cells fully differentiate into effector CTLs in response to the viral agonist, whereas subdominant CD8+ T cells appear to activate, but not fully differentiate. One explanation for the incomplete differentiation of the T cells directed to the HA210 epitope is that these CD8+ T cells are in an altered activation state before initial contact with the agonist HA210 epitope. A mechanism that may account for this altered activation state of the subdominant CD8+ T cells is suggested by our earlier observation that CD8+ CTLs directed to the HA210 epitope exhibit cross-reactive recognition of a self-IgVH–derived peptide. This self-peptide acts as a partial agonist/antagonist ligand capable of stimulating a subset of CTL effector activities from mature CD8+ CTL effectors 19. It is possible that contact with this and/or other self-peptides in vivo before virus infection partially antagonizes the HA210-219–specific CD8+ T cells, rendering the antagonized cells unable to fully differentiate upon subsequent encounters with the HA210-219 agonist ligand. Interference with normal T cell activation/differentiation is a well-recognized consequence of T cell interaction with altered peptide ligands that exhibit partial agonist or antagonist properties 37 38. Preliminary studies in which HA210-specific memory CD8+ T cells have been stimulated in short-term culture in the absence of B lymphocytes have not demonstrated the rapid conversion of the majority of responding subdominant T cells from a tetramer-negative noncytolytic phenotype to the tetramer-positive cytolytic phenotype exhibited by the dominant HA204-specific CTLs. Whether this result is due to our inability to completely eliminate B lymphocytes during priming in vivo and restimulation in vitro, or whether this result indicates that other self-antigens not displayed on B lymphocytes alter the activation state of the HA210-specific CD8+ T cells is currently under investigation.
An unexpected finding in this analysis was the observation that the majority of CD8+ T cells responding to HA210-219 do not bind the HA210 tetramer after initial proliferation in response to antigen. HA210 tetramer–positive cells increasingly accumulate only after restimulation of activated CD8+ T cells by the subdominant HA210-219 epitope. This unusual phenotype was not exhibited by CD8+ T cells directed to the dominant HA204-212 epitope, where there was a direct correspondence among antigen-driven T cell activation, the expression of effector activity, and HA204 tetramer binding. The observation that tetramer-negative HA210-reactive T cells displayed cell surface TCR, CD3, and CD8 at levels comparable to that of the tetramer-positive HA204-reactive cells argues against antigen-induced TCR downmodulation 39 40 as a likely explanation for this phenotype. Although differences in TCR avidity have been detected based on the rate of tetramer dissociation 41, the tetramer-negative HA210-219–specific CD8+ T cells did not appear to represent a distinct subpopulation of T cells with low-avidity TCRs. We noted no difference in the peptide dose dependence of target cell recognition between tetramer-positive and -negative CTL effector cells. More importantly, when purified HA210 tetramer–negative CD8+ T cells were stimulated by antigen, they gave rise to daughter cells that exhibited a tetramer-positive phenotype. This finding strongly suggests that tetramer-negative T cells are the precursors of tetramer-positive T cells, and that this transition requires antigenic stimulation. In support of this idea, we have found in preliminary studies that exposure of tetramer-negative CD8+ T cells to a strong mitogenic stimulus rapidly converts the cells from a tetramer-negative to a tetramer-positive phenotype within 12–18 h. In addition, after multiple cycles of in vitro restimulation with antigen, all HA210-specific CD8+ T cells present in long-term cultures are tetramer positive.
The conversion of tetramer-negative CTLs to a tetramer-positive phenotype in response to antigen could reflect a change in TCR conformation, rendering the receptors displayed by CTLs capable of efficiently binding the soluble tetramer. Alternatively, the TCRs on tetramer-negative CD8+ T cells could be arrayed on the cell surface in a manner unfavorable for efficient tetramer binding. Upon subsequent exposure to antigen, the TCRs on these cells would then undergo activation-dependent redistribution to form a cell surface array more favorable to efficient tetramer binding. The recent reports of the asymmetric display of TCR-associated signaling molecules on cell surfaces in sequestered membrane domains 42 43 suggest possible mechanisms to explain the inability of the TCR to efficiently bind tetramers. Why this tetramer-negative phenotype is associated only with HA210-reactive CD8+ T cells is not clear. It is possible that prior exposure to partial agonist/antagonist self-peptides in vivo triggers the re-distribution of TCRs on these subdominant T cells into a cell surface array unfavorable to efficient tetramer binding. Nevertheless, the CD8+ cells must be still capable of engaging HA210-219 peptide–MHC complexes on infected cell surfaces. Only after repeated stimulation by the agonist HA210-219 epitope do the TCRs on these cells redistribute into a cell surface array allowing MHC tetramers to irreversibly bind the TCR.
The observations reported here indicate that the subdominant or weak CD8+ CTL response to the HA210-219 epitope is not due to a low frequency of memory CTL precursors, but is instead due to the inability of the majority of CTL precursors to efficiently differentiate into effector CTLs after antigenic stimulation. Memory CD8+ T cells to this epitope proliferate in response to antigen and acquire cell surface markers of activated T cells, but they do not exhibit effector activity (i.e., IFN-γ secretion or cell-mediated cytotoxicity) and fail to bind MHC tetramers. With repeat antigenic stimulation, these CD8+ T cells transition first to tetramer-negative cells with full effector function, and then to tetramer-positive CTL effector cells. These findings provide a potential explanation for the higher frequency of antigen-specific CD8+ effectors as defined by intracellular cytokine staining rather than by tetramer binding, as recently reported in experimental palmy virus infection 44. More importantly, these data suggest that repeated vaccination may not only expand the pool of memory CD8+ T cells directed to viral antigens, but may also drive memory CD8+ T cells into a state of differentiation where cells with full effector potential are rapidly generated in response to viral infection. At present, we do not know whether this altered activation and delayed differentiation is a feature of other subdominant CD8+ CTL responses. Our results do suggest that along with control at the level of antigen processing and presentation and antigenic competition 11 45, the ability of CD8+ T cells to differentiate into effector cells may be critical in regulating the CTL response to foreign antigen.
Acknowledgments
We thank Drs. Dirk Busch and Erik Pamer (Yale University School of Medicine) for providing several tetramer preparations used in this work and for their help and guidance in tetramer preparation. We would also like to thank Shawn Gill, Barbara Small, and Sarah Dressel for excellent technical assistance. We also thank William Ross for valuable assistance with flow cytometry and FACS® sorting, as well as Dr. Richard Enelow for many helpful discussions.
This work was supported by US Public Health Service grants AI15608 and HL33391 (to T.J. Braciale). J.V. Spencer is a Parker B. Francis Fellow in Pulmonary Research.
Footnotes
J.V. Spencer's present address is ChemoCentryx, 1539 Industrial Rd., San Carlos, CA 94070.
Abbreviations used in this paper: AA, A/Ann Arbor/57 influenza virus strain; GV, A/Guiyang/57-variant 17 influenza virus strain; HA, influenza hemagglutinin; HA A−, truncated A/Japan/57 HA; JAK, Janus kinase; LCMV, lymphocytic choriomeningitis virus; LDA, limiting dilution assay; pCTL, precursor CTL; rVV HA A−, recombinant vaccinia virus expressing the A/Japan/305/57 HA protein lacking the transmembrane region.
References
- Doherty P.C., Allan W., Eichelberger M., Carding S.R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu. Rev. Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Askonas B.A., Taylor P.M., Esquivel F. Cytotoxic T cells in influenza infection. Ann. NY Acad. Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- Bender B.S., Crighan T., Zhang L., Small P.A., Jr. Transgenic mice lacking class I major histocompatibility complex–restricted T cells have delayed clearance and increased mortality after influenza virus challenge. J. Exp. Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A., Braciale V.L., Braciale T.J. In vivo effector function of influenza virus–specific cytotoxic T lymphocyte clones is highly specific. J. Exp. Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson B.D., Butler L.D., Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone-marrow derived cells. J. Virol. 1987;61:3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J.A., Oldstone M.B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virusclearance of virus in vivo. J. Virol. 1984;51:682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Grube F., Moskophidis D., Lohler J. Recovery from acute virus infection. Role of cytotoxic T lymphocytes in the elimination of lymphocytic choriomeningitis virus from spleens of mice. Ann. NY Acad. Sci. 1988;532:238–256. doi: 10.1111/j.1749-6632.1988.tb36343.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R.M., Doherty P.C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or a semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- Townsend A.R.M., McMichael A.J., Carter N.P., Huddlestone J.A., Brownlee G.G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted cytotoxic T lymphocytes. Annu. Rev. Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Sercacz E.E., Lehman P.V., Ametani A., Benichou G., Miller A., Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- Yewdell J.W., Bennink J.R. Immunodominance in major histocompatibility complex class 1-resticted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- McMichael A. Cytotoxic T lymphocytes specific for influenza virus. Curr. Top. Microbiol. Immunol. 1994;189:75–91. doi: 10.1007/978-3-642-78530-6_5. [DOI] [PubMed] [Google Scholar]
- Braciale T.J., Sweetser M.T., Morrison L.A., Kittleson D.J., Braciale V.L. Class I major histocompatibility complex-restricted cytolytic T lymphocytes recognize a limited number of sites on the influenza hemagglutinin. Proc. Natl. Acad. Sci. USA. 1989;86:277–281. doi: 10.1073/pnas.86.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser M.T., Braciale V.L., Braciale T.J. Class I major histocompatibility complex–restricted T lymphocyte recognition of the influenza hemagglutinin. J. Exp. Med. 1989;179:1357–1368. doi: 10.1084/jem.170.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T.J., Braciale V.L., Henkel T.J., Sambrook J., Gething M.-J. Cytotoxic T lymphocyte recognition of the influenza hemagglutinin gene product expressed by DNA-mediated gene transfer. J. Exp. Med. 1984;159:341–354. doi: 10.1084/jem.159.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Myers-Powell B.A., Braciale T.J. The weak CD8+ T cell response to an influenza hemagglutinin epitope reflects limited T cell availability. J. Immunol. 1996;157:505–511. [PubMed] [Google Scholar]
- Yang B., Braciale T.J. Characteristics of ATP-dependent peptide transport in isolated microsomes. J. Immunol. 1995;155:3889–3896. [PubMed] [Google Scholar]
- Cao W., Tykodi S.S., Esser M.T., Braciale V.L., Braciale T.J. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Kuroda M.J., Schmitz J.E., Barouch D.H., Craiu A., Allen T.M., Sette A., Watkins D.I., Forman M.A., Letvin N.L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus–infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I–peptide complex. J. Exp. Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan M.F.C., Tan L., Annels N., Ogg G.S., Wilson J.D.K., O'Callaghan C.A., Steven N., McMichael A.J., Rickinson A.B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore A., Glithero A., Godkin A., Tissot A.C., Pluckthun A., Elliott T., Hengartner H., Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus–specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K.J., Belz G.T., Altman J.D., Ahmed R., Woodland D.L., Doherty P.C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J.D., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen specific CD8 T cellsa reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Braciale T.J. Immunological recognition of influenza virus–infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J. Exp. Med. 1977;146:673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G.L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J. Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L.A., Lukacher A.E., Braciale V.L., Fan D.P., Braciale T.J. Differences in antigen presentation to MHC class I– and class II–restricted influenza virus–specific cytolytic T lymphocyte clones. J. Exp. Med. 1986;163:903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch D.H., Pilip I.M., Vijh S., Pamer E.G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–862. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- Lyons A.B., Parish C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Hahn Y.S., Yang B., Braciale T.J. Regulation of antigen processing and presentation to class I MHC restricted CD8+ T lymphocytes. Immunol. Rev. 1996;151:31–49. doi: 10.1111/j.1600-065x.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Weidt G., Utermohlen O., Heukeshoven J., Lehmann-Grubbe F., Deppert W. Relationship among immunodominance of single CD8+ T cell epitopes, virus load, and kinetics of primary antiviral CTL response. J. Immunol. 1998;160:2923–2931. [PubMed] [Google Scholar]
- Wettstein P.J. Immunodominance in the T-cell response to multiple non-H-2 histocompatibility antigens. II. Observation of a hierarchy among dominant antigens. Immunogenetics. 1986;24:24–31. doi: 10.1007/BF00372294. [DOI] [PubMed] [Google Scholar]
- Valitutti S., Muller S., Dessing M., Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois N., Guilloux Y., Diez E., Jotereau F. Suboptimal activation of melanoma infiltrating lymphocytes (TIL) due to low avidity of TCR/MHC–tumor peptide interactions. J. Exp. Med. 1996;183:2403–2407. doi: 10.1084/jem.183.5.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollsberg P., Weber W.E.J., Dangond F., Batra V., Sette A., Hafler D. Differential activation of proliferation and cytotoxicity in human T-cell lymphotropic virus type I Tax specific-CD8 T cells by an altered peptide ligand. Proc. Natl. Acad. Sci. USA. 1995;92:4036–4040. doi: 10.1073/pnas.92.9.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsov I., Vukmanovic S. Altered effector responses of H-Y transgenic CD8+ cells. Int. Immunol. 1997;9:1423–1430. doi: 10.1093/intimm/9.10.1423. [DOI] [PubMed] [Google Scholar]
- Cao W., Braciale T.J. Partial activation of foreign antigen specific T lymphocytes by a self peptidepossible roles of altered peptide ligands in regulating T lymphocyte mediated immune responses. J. Mol. Med. 1996;74:573–582. doi: 10.1007/s001090050061. [DOI] [PubMed] [Google Scholar]
- Evavold B.D., Sloan L.J., Allen P.M. Tickling the TCRselective T cell functions stimulated by altered peptide ligands. Immunol. Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Cai Z., Kishimot H., Brunmark A., Jackson M.R., Peterson P.A., Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J. Exp. Med. 1997;185:641–652. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Hemmer B., Martin R., Germain R.N. Serial TCR engagement and down-modulation by peptide:MHC molecule ligandsrelationship to the quality of individual TCR signaling events. J. Immunol. 1999;162:2073–2080. [PubMed] [Google Scholar]
- Busch D.H., Pamer E.G. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. The immunological synapsea molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Lukacher A.E., Moser J.M., Hadley A., Altman J.D. Visualization of polyomavirus-specific CD8+ T cells in vivo during infection and tumor rejection. J. Immunol. 1999;163:3369–3378. [PubMed] [Google Scholar]
- Busch D.H., Pamer E.G. MHC class I/peptide stabilityimplications for immunodominance, in vitro proliferation, and diversity of responding CTL. J. Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]