Abstract
Little is known about innate immunity to bacteria after birth in the hitherto sterile fetal intestine. Breast-feeding has long been associated with a lower incidence of gastrointestinal infections and inflammatory and allergic diseases. We found in human breast milk a 48-kD polypeptide, which we confirmed by mass spectrometry and sequencing to be a soluble form of the bacterial pattern recognition receptor CD14 (sCD14). Milk sCD14 (m-sCD14) concentrations were up to 20-fold higher than serum sCD14 from nonpregnant, pregnant, or lactating women. In contrast, lipopolysaccharide (LPS)-binding protein was at very low levels. Mammary epithelial cells produced 48-kD sCD14. m-sCD14 mediated activation by LPS and whole bacteria of CD14 negative cells, including intestinal epithelial cells, resulting in release of innate immune response molecules. m-sCD14 was undetectable in the infant formulas and commercial (cows') milk tested, although it was present in bovine colostrum. These findings indicate a sentinel role for sCD14 in human milk during bacterial colonization of the gut, and suggest that m-sCD14 may be involved in modulating local innate and adaptive immune responses, thus controlling homeostasis in the neonatal intestine.
Keywords: innate immunity, neonatal immunity, mucosal immunity, intestinal immune response, breast-feeding
Introduction
After birth, the sterile fetal intestine is heavily colonized mainly by Escherichia coli and Streptococci 1. The predominance of endotoxin (LPS)-producing Gram-negative bacteria may contribute to the pathogenesis of a variety of immune and inflammatory neonatal conditions, such as necrotizing enterocolitis and gut-originated sepsis 2. However, the epithelial layer, together with intraepithelial and lamina propria immunocompetent cells, coordinates local innate and adaptive immune responses, thus maintaining gut homeostasis 3.
Importantly, it has been demonstrated that breast-fed newborns experience a lower incidence of gastrointestinal infections and inflammatory, respiratory, and allergic diseases 4 5 6. Protection by milk has been variously ascribed to maternal immunocompetent cells, immunoglobulins, immune reactive peptides, antiinfectious oligosaccharides, growth factors, cytokines, lysozyme, lactoferrin, and complement components 7. However, we speculated that more specific factors may be present in milk that would continuously sense and signal to the neonate the presence of microorganisms, thus contributing to maintain intestinal immune homeostasis.
The bacterial pattern recognition receptor (PRR) CD14 plays a pivotal role in the recognition of and cell activation induced by microbial cell wall components of Gram-negative and Gram-positive bacteria as well as mycobacteria 8 9. Two soluble forms of CD14, sCD14α and sCD14β, are found in normal human plasma at a concentration of 2–3 μg/ml 10 11. sCD14 binds to whole bacteria and bacterial cell wall components, and mediates bacterial-induced activation of cells that do not express membrane-bound CD14 as well as CD14-bearing cells 12 13 14.
In view of this PRR activity of CD14, we tested whether sCD14 is present in human milk and contributes to the innate immune mechanisms controlling gut homeostasis in newborns.
Materials and Methods
Reagents.
Abs: anti-human CD14 mAb MY4 (IgG2b; Beckman Coulter) and MEM-18 (IgG1κ; provided by Dr. V. Horejsi, Academy of Sciences, Czech Republic) and their isotype-matched controls MOPC 141 and MOPC 21, respectively (Sigma-Aldrich). The rabbit anti-CD14 Ab (Sanofi) used in Western blot analyses was raised in animals immunized with human recombinant sCD14 and cross-reacts with bovine and porcine CD14. LPS and nonpathogenic E. coli serotype O55:B5 were from Sigma-Aldrich.
Human Milk and Serum, Infant Milk Formulas, and Bovine Milk.
Human serum and breast milk samples were collected from healthy donors after written consent. Milk was processed within 2 h of collection. After centrifugation, the cellular pellet was used for analysis of macrophage-derived sCD14, and the milk samples were kept at −80°C until testing for sCD14 and LPS-binding protein (LBP). Commercial infant milk formulas were tested for sCD14 content and bioactivity after reconstitution. Commercial bovine milk (bottled, pasteurized cows' milk) was kept at 4°C before testing for sCD14. Bovine colostrum was obtained from animals kept in local barns. sCD14 (Immuno-Biological Laboratories) and LBP (Hycult Biotechnology) concentrations were determined by ELISA.
Western Blot Analysis.
Samples were separated by 12.5% SDS-PAGE under reducing conditions (PhastSystem®; Amersham Pharmacia Biotech, or Mini Protean II; Bio-Rad) and analyzed by Western blotting as described 11.
Isolation and Purification of m-sCD14, Mass Spectrometry, and Sequencing.
m-sCD14 was isolated from a pool of 3-mo-postpartum breast milk samples by affinity chromatography in a Sepharose 4B precolumn (Amersham Pharmacia Biotech) in tandem to a CN-Br Sepharose 4B–coupled MEM-18 mAb (20 mg) affinity column. The bound protein was eluted (PBS/50 mM diethylamine, pH 11.5), dialyzed against PBS, and concentrated. Some preparations were further purified by gel filtration on a Superose 12 column (fast protein liquid chromatography [FPLC]; Amersham Pharmacia Biotech). The purity of the isolated protein was confirmed by SDS-PAGE followed by silver staining, which showed a single 48-kD polypeptide. For mass spectrometric analyses, purified m-sCD14 was reduced, alkylated, and digested with porcine trypsin and lysyl endopeptidase C (Lys-C) according to standard protocols 15. Mass spectrometry (MS) and tandem MS (MS/MS) of peptides were recorded in a nanoelectrospray Q-TOF mass spectrometer (Micromass). Liquid chromatography/MS was performed with an UltiMate NanoHPLC pump equipped with a NanoColumn PepMap reverse phase (RP)HPLC (LC Packings) on line with the nanoelectrospray source. NH2-terminal sequencing was performed in an Applied Biosystems sequencer according to the Edman degradation protocol.
Cell Lines, Cell Activation, and Cytokine and Chemokine Determinations.
The human colon carcinoma epithelial cell lines HT29 and SW620, myeloid cell line U937 (American Type Culture Collection), astrocytoma line U373 MG, and breast adenocarcinoma cell line with characteristics of differentiated mammary epithelium, MCF-7 (European Collection of Animal Cell Cultures), were cultured as indicated by the purveyor. To test for CD14 expression, MCF-7 cells were cultured in AIM-V (GIBCO BRL) serum-free medium (5 × 105 cells/ml) for 72 h before analysis of cell lysates and filtered supernatants by Western blotting. For cell activation, 90% confluent cultures of HT29 (in 24-well plates), SW620, and U373 (in 96-well plates) cells and the U937 cell line (5 × 105 cells/ml) were washed and cultured for an additional 24 h in medium supplemented with normal human serum, FCS, or milk in the absence or presence of E. coli or E. coli LPS before culture supernatants were tested for TNF-α (Diaclone), epithelial neutrophil activator (ENA)-78 (R&D Systems), IL-6, or IL-8 by ELISA (IL-6– and IL-8–specific matched-pair Abs were from Immunokontact).
Results
Detection of sCD14 in Human Milk.
Western blot analysis using anti-CD14–specific Abs of breast milk samples (n = 10) taken after the first week postpartum showed a strong single 48-kD polypeptide band (Fig. 1 A). The parallel serum samples had the typical doublet of sCD14β (56-kD) and sCD14α (50-kD) polypeptides, as described 11. The sCD14 pattern in milk from the same subject at early (<6 d), and late (>8 d) times postpartum was different (Fig. 1 B). Most of the early samples had a complex sCD14 pattern with three polypeptide bands: a strong 48-kD sCD14 polypeptide and two slower migrating polypeptides of ∼50 and 56 kD, which resembled serum sCD14α and β, respectively. The later samples from the same subject had a single 48-kD sCD14 band.
Figure 1.
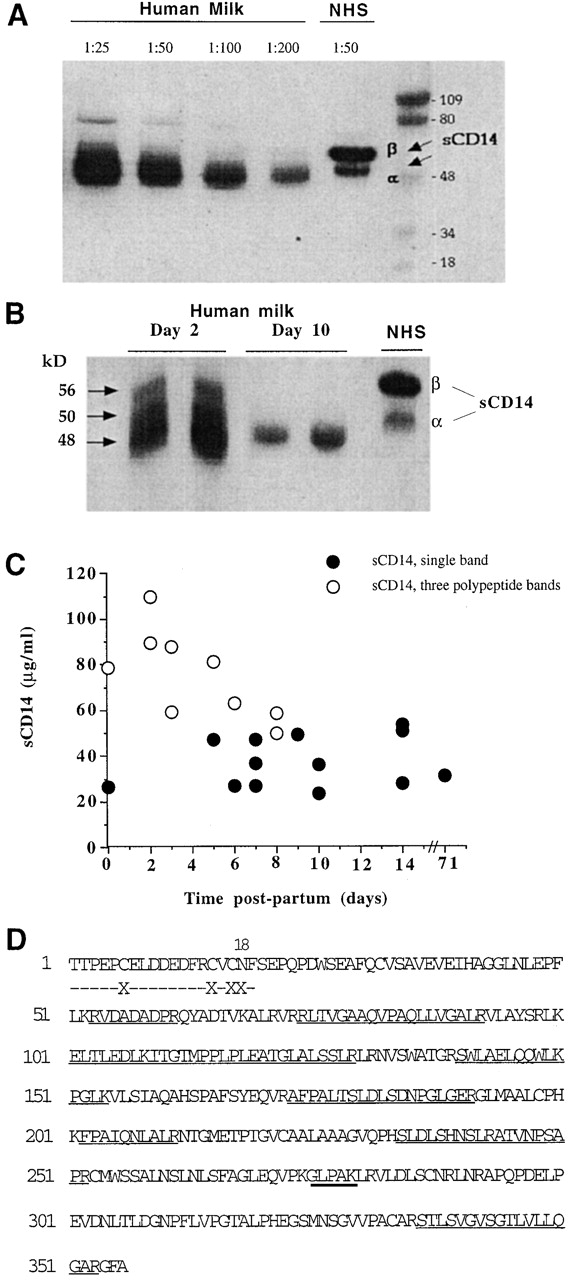
Detection and characterization of sCD14 in human milk. (A) Milk samples (1:25 to 1:200 dilutions) taken after the first week postpartum and normal human serum (NHS) were tested for sCD14 by Western blotting. Shown is the result of one sample representative of 10 donors. (B) Analysis of m-sCD14 in milk samples taken at day 2 or day 10 postpartum from the same mother. Samples from two donors are shown. NHS, normal human serum. (C) m-sCD14 levels determined by ELISA in multiple samples taken from 10 donors at different times postpartum. Values correspond to the mean of triplicate determinations (SD ≤ 6%). The m-sCD14 molecular pattern of each sample was determined by Western blotting and is indicated by the symbols. (D) NH2-terminal sequence (dashed line) and mass spectrometric analysis followed by amino acid sequencing (solid line) of 48-kD m-sCD14 tryptic peptides showing homology with the predicted sequence from monocyte CD14 cDNA. Thick solid line underlines a peptide analyzed only by mass spectrometry. X, not determined.
Levels of m-sCD14 in multiple milk samples from 10 donors, taken at different times postpartum (Fig. 1 C), were very high (52.9 ± 24.0 μg/ml; n = 22) compared with those reported for normal serum (2-3 μg/ml; reference 10). The highest m-sCD14 levels were detected in the relatively early samples (≤6 d, 67.09 ± 27.61 μg/ml; n = 10). The majority of these early samples showed the three-sCD14 polypeptide pattern (Fig. 1 B). m-sCD14 concentration declined over the time to values of 41.12 ± 11.91 μg/ml (n = 12; ≥7 d postpartum). The serum sCD14 concentrations in mothers after three and eight mo of pregnancy and after three and six mo postpartum during lactation (Table ) remained similar to those reported for normal donors (pregnancy, 3.71 ± 0.57 μg/ml; n = 20, and postpartum, 3.76 ± 0.56 μg/ml; n = 20). However, the milk samples from the same mothers showed significantly higher levels of m-sCD14 compared with serum sCD14 (14.84 ± 6.40 μg/ml vs. 3.76 ± 0.56 μg/ml; n = 20; P < 0.001; milk vs. serum, three and six months postpartum). Thus, the high levels of m-sCD14 did not reflect a systemic increase in sCD14 during pregnancy and postpartum.
Table 1.
Levels of sCD14 and LBP in Human Serum and Milk during Pregnancy and Postpartum
| sCD14 | LBP | ||||
|---|---|---|---|---|---|
| Serum | Milk | Serum | Milk | ||
| μg/ml | μg/ml | ||||
| Pregnancy | 3 mo | 3.50 ± 0.48 | – | 13.52 ± 4.58 | – |
| 8 mo | 3.92 ± 0.61 | – | 17.21 ± 3.01 | – | |
| Postpartum | 5 d | – | 20.10 ± 8.74 | – | 0.03 ± 0.01 |
| 1 mo | – | 12.06 ± 4.77 | – | 0.01 ± 0.02 | |
| 3 mo | 3.71 ± 0.59 | 12.16 ± 3.75 | 10.65 ± 2.39 | 0.01 ± 0.01 | |
| 6 mo | 3.82 ± 0.47 | 15.05 ± 4.08 | 8.68 ± 1.27 | 0.01 ± 0.01 | |
Data represent mean ± SD of 10 (sCD14) and 8 (LBP) samples tested by ELISA.
LBP, the protein that accelerates the binding of LPS to sCD14 16, was at a 1000-fold lower concentration in milk than in serum of the same donor (Table ), in marked contrast with the high levels of m-sCD14. Together, these results indicated that sCD14 but not LBP is specifically expressed at high concentration in human milk.
Biochemical Characterization of m-sCD14.
The NH2-terminal sequence of the 48-kD milk polypeptide (Fig. 1 D), present during most of the lactating period, was identical to that reported for sCD14 from urine of nephrotic patients and monocyte-derived CD14 10 17. No signal was detected at position 18, which was consistent with an N-glycosylation at asparagine, as predicted by the CD14 cDNA 17. Analysis of tryptic peptides from the purified material by Q-TOF mass spectrometry and sequencing indicated high homology with the amino acid sequence predicted by the CD14 cDNA (Fig. 1 D). These results confirmed the identity of m-sCD14 and indicated that differences in glycosylation may account for the difference in electrophoretic mobility and pattern between 48-kD m-sCD14 and serum sCD14. Furthermore, m-sCD14 did not incorporate into lipid micelles or elute with the void volume when subjected to size exclusion chromatography (not shown), indicating that it lacks the glycolipid tail 18.
Cellular Origin of m-sCD14.
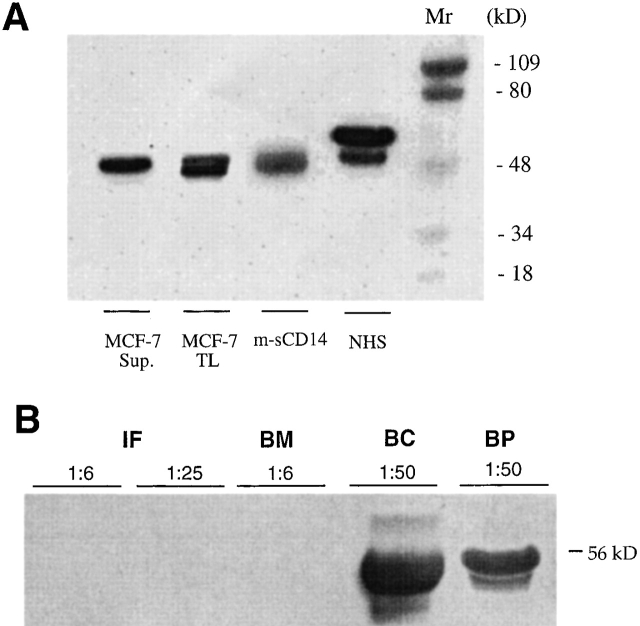
The extremely high concentration of sCD14 in milk and the presence, during most of the lactating period, of a single 48-kD sCD14 polypeptide contrasted with the level and pattern of monocyte-derived sCD14 present in serum (reference 11; Table and Fig. 1). We therefore asked whether mammary gland cells produced m-sCD14. Flow cytometric analysis of the mammary epithelial cell line MCF-7 did not show surface expression of CD14 (not shown). However, cell lysates and culture supernatants from serum-free cultured cells showed CD14 polypeptide bands (Fig. 2 A). The culture supernatant showed a 48-kD sCD14 polypeptide similar to that detected in relatively late milk samples. The cell lysate showed two closely migrating CD14 polypeptides of ∼48 kD, one of them of slightly faster mobility, most likely representing a precursor of the mature sCD14 glycoprotein. Supernatants of serum-free cultured milk–derived macrophages showed low levels of the typical serum sCD14α and sCD14β isoforms (not shown).
Figure 2.
48-kD sCD14 production by mammary epithelial cells and absence from commercial infant milk formulas and cows' milk. (A) Detection of sCD14 polypeptides by Western blotting in culture supernatants (Sup.) and total lysates (TL) from serum-free medium–cultured mammary epithelial cell line MCF-7. Parallel analysis of m-sCD14 in milk and normal human serum (NHS) is shown. Mr, relative molecular mass. (B) Infant milk formulas (IF, 1:6 and 1:25 dilutions), cows' milk (bottled milk [BM]), bovine colostrum (BC), and bovine plasma (BP) were tested for sCD14 by Western blotting. Shown is a representative result of 5 IF, 8 BM, 2 BC, and 10 BP.
m-sCD14 Is Absent from Infant Milk Formulas and Commercial (Cows') Milk.
We asked whether presence of m-sCD14 is limited to breast milk. sCD14 was not detected in the infant milk formulations (powdered milk) and commercial cows' milk (bottled, pasteurized milk) tested. However, it was detectable in bovine colostrum and plasma (Fig. 2 B).
Endotoxin and Whole Bacteria Induce Cell Activation by a Human Milk–mediated m-sCD14–dependent Mechanism.
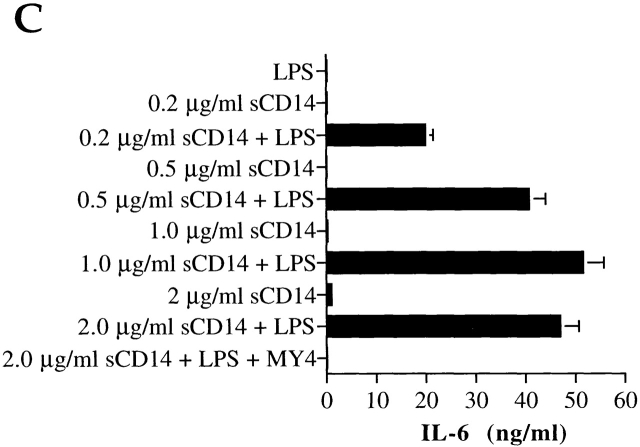
Next, we asked whether m-sCD14 could function as a mediator of endotoxin activation of cells that lack cell membrane CD14, as demonstrated for serum sCD14 13. Human milk mediated LPS-induced IL-6 production by the CD14-negative cell line U373 in a dose-dependent manner (Fig. 3 A). Two anti-CD14 mAbs (MY4, MEM-18) abrogated the IL-6 production, suggesting that m-sCD14 was critically involved in this activity. Importantly, bovine colostrum and plasma, but not the powdered milk tested, mediated IL-6 production by LPS-stimulated U373 cells (not shown). Human milk also mediated LPS-induced IL-8 production by the CD14-negative myeloid cell line U937, and this effect was blocked by MY4 (Fig. 3 B). We confirmed that m-sCD14 was responsible for the milk-mediated cell activation by LPS, as purified m-sCD14 was able to reproduce the effect of milk (Fig. 3 C).
Figure 3.
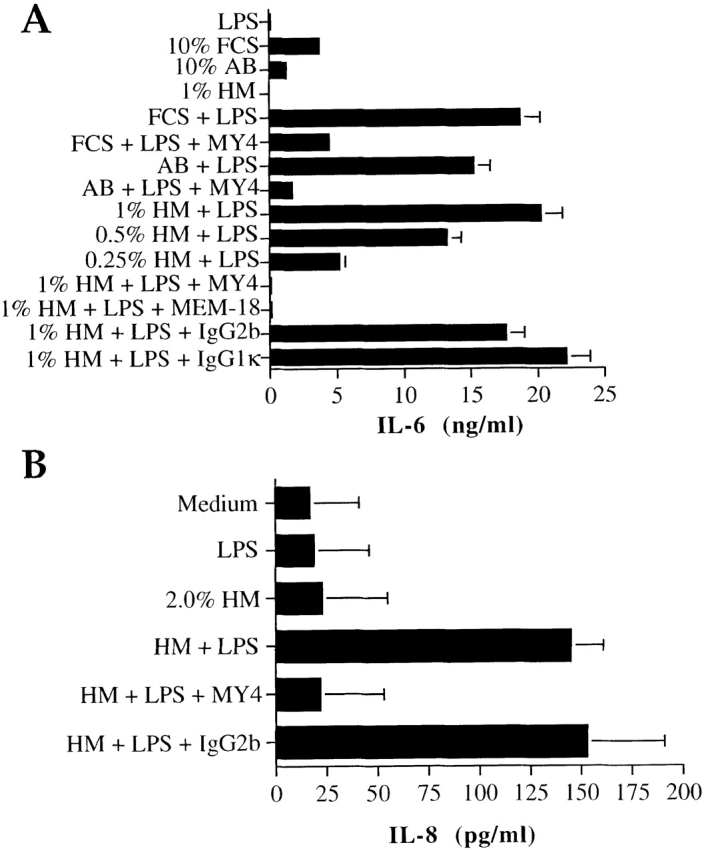
m-sCD14 mediates LPS activation of CD14-negative cells. (A and B) IL-6 and IL-8 production by the astrocytoma cell line U373 (A) and the myeloid cell line U937 (B), respectively, stimulated with E. coli LPS (100 ng/ml) in medium supplemented with FCS, human AB serum (AB), or human milk (HM). The anti-CD14 mAb MY4 and MEM-18 but not their isotype-matched controls (20 μg/ml) blocked the LPS-induced cell activation. (C) IL-6 production by LPS stimulated U373 cells in the presence of different amounts of purified milk–derived sCD14. All cytokines were tested by ELISA. Results in A and C are means ± SD of triplicate cultures of one experiment representative of four; results in B are the mean ± SD of three independent experiments.
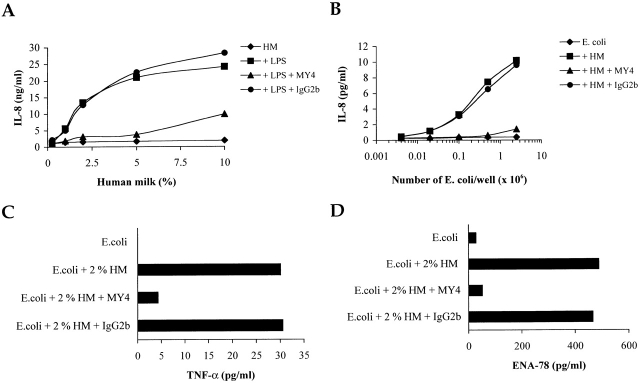
We also tested the capacity of intestinal epithelial cell (IEC) lines (HT-29 and SW620 lines) to produce immune and proinflammatory molecules when challenged by endotoxin or whole bacteria in the presence of human milk. IL-8 production by LPS-activated IEC was mediated by human milk, and this activity was inhibited by an anti-CD14 mAb (Fig. 4 A). Similarly, human milk mediated IL-8 production by IECs challenged with E. coli, and this effect was blocked by an anti-CD14 mAb (Fig. 4 B). IECs also responded to bacteria by producing TNF-α and the CXC chemokine, ENA-78; these responses were inhibited by anti-CD14 mAb (Fig. 4C and Fig. D). The m-sCD14–mediated IEC activation by bacteria appears to be selective, as expression of other immune response molecules (i.e., IL-7, IL-15, IL-18, MHC class I and class II, and CD80) was not affected (data not shown).
Figure 4.
IECs can be activated by LPS or whole bacteria by an m-sCD14–dependent mechanism. (A–D) IL-8, TNF-α, and ENA-78 production was tested by ELISA in supernatants of HT29 and SW620 cell lines cultured in medium supplemented with human milk (HM) and stimulated with E. coli LPS or varying numbers of whole E. coli (C and D; 2.5 × 106 E. coli). The anti-CD14 mAb MY4 (IgG2b) but not its isotype-matched control blocked LPS and bacterial stimulation. Representative results with HT29 cells are shown.
Discussion
Here, we demonstrate that the soluble form of the receptor critically involved in innate recognition of bacteria, CD14, is present and at high concentration in human milk. Furthermore, we show that m-sCD14 can mediate IEC cell activation induced by endotoxin and whole bacteria, resulting in the production of potent immune response and proinflammatory mediators. Importantly, we found that differentiated mammary epithelial cells are able to produce sCD14 whose molecular pattern is identical to that of sCD14 present in human milk during most of the lactating period. This, together with the lack of correlation between the high concentration of m-sCD14 and the corresponding serum sCD14 levels during pregnancy and lactation, point at the mammary gland epithelium as the main source of m-sCD14. It should be noted that milk-derived macrophages showed expression of the typical serum sCD14α and β isoforms. These sCD14 polypeptides were similar to those which, in addition to the strong 48-kD sCD14 polypeptide band, are part of the complex sCD14 pattern detectable in early milk samples (Fig. 1 B). Thus, it is possible that milk macrophage–derived sCD14 contributes to the total pool of m-sCD14 within the first week postpartum. Additionally, serum sCD14 may reach the milk by the paracellular pathway, which allows components of the interstitial space to pass between the alveolar epithelial cells 7. This process does not operate during full lactation, explaining the disappearance of sCD14α and β isoforms from late milk samples.
At first sight, it was surprising to find very low levels of LBP in milk, in view of the role that this serum protein plays in facilitating the interaction of LPS with CD14. However, the low concentration of LBP may be beneficial by leading to a more controlled immune response against bacteria, as it was demonstrated that sCD14 can mediate CD14-negative cell activation by LPS in the absence of LBP, albeit with slower kinetics 16.
We consider two possible roles for m-sCD14: (i) in protection of the mammary gland against bacterial infection during lactation, and (ii) in protection of the neonatal gut. We have so far focused on the latter role.
The data show that m-sCD14–mediated in vitro stimulation of IECs by LPS and Gram-negative bacteria results in the release of molecules involved in cellular recruitment and innate defense at the site of infection. However, a number of mediators of epithelial–T cell communication were not affected. These findings indicate that the m-sCD14–mediated gut response is selective and modulated. Indeed, our findings pose the question of why, given the heavy bacterial colonization of the newborn's intestine, the presence at high concentration of m-sCD14 does not result in an excessive and deleterious immune response? One possibility is that m-sCD14 loses bioactivity during transit through the newborn's stomach and proximal intestine. The high load of m-sCD14 in the mother's milk may be necessary to compensate for such losses. We speculate that high concentrations of m-sCD14 are provided for the newborn to cope with the vast microbial inoculum and signal its presence to the neonatal intestine, which in turn will initiate innate and adaptive immune responses. Modulation of these responses, thus avoiding excessive immune and inflammatory reactions, may be achieved by several contributing mechanisms in which m-sCD14 is involved, including the slow kinetics of cell activation by bacteria as a consequence of the very low levels of LBP, endotoxin tolerization 19 resulting from this regulated level of cell activation, the selective response of the IEC to bacterial challenge described here, and the production of the potent immunosuppressant prostaglandin E2 by the LPS-stimulated lamina propria mononuclear cells, as has been suggested 20. In addition, m-sCD14 may participate in modulating the gut immune response by interacting directly (without LPS) with local T and B cells, which will cause modulation of T and B cell activation and function, as we recently described 21 22.
In conclusion, the findings reported here indicate a sentinel role for m-sCD14 during the neonatal period, consistent with its PRR function in innate immunity, and suggest an immunomodulatory activity of m-sCD14. We propose that sCD14 in breast milk is responsible, at least in part, for the association of breast-feeding with a lower incidence of gastrointestinal infections and other inflammatory and allergic conditions, which depends on adequate stimulation of the gut immune system during microbial exposure early in life.
Acknowledgments
We thank Professor M. Rowe for helpful discussions and for critical reading of this manuscript.
M. Arias is supported by a fellowship from Colciencias, Colombia.
References
- Mackie R.I., Sghir A., Gaskins H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- Peter C.S., Feuerhahn M., Bhonhorst B., Schlaud M., Ziesing S., von der Hardt H., Poets C.F. Necrotising enterocolitisis there a relationship to specific pathogens? Eur. J. Pediatr. 1999;158:67–70. doi: 10.1007/s004310051012. [DOI] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel diseaseprotective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Howie P.W., Forsyth J.S., Ogston S.A., Clark A., Florey C.D. Protective effect of breast feeding against infection. BMJ. 1990;300:11–16. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- César J.A., Victora C.G., Barros F.C., Santos I.S., Flores J.A. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazilnested case-control study. BMJ. 1999;318:1316–1320. doi: 10.1136/bmj.318.7194.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddy W.H., Holt P.G., Sly P.D., Read W., Landau L.I., Stanley F.J., Kendall G.E., Burton P.R. Association between breast feeding and asthma in 6 year-old childrenfindings of a prospective birth cohort study. BMJ. 1999;319:815–819. doi: 10.1136/bmj.319.7213.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M.C. Lactogenesis in womena cascade of events revealed by milk composition. In: Jensen R.D., editor. The Composition of Milks. Academic Press; San Diego, CA: 1995. pp. 87–98. [Google Scholar]
- Pugin J., Heumann D., Tomasz A., Kravchenko V.V., Akamatsu Y., Nishijima M., Glauser M.P., Tobias P.S., Ulevitch R.J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Wright S.D. CD14 and innate recognition of bacteria. J. Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- Bazil V., Horejsi V., Baudys M., Kristofova H., Strominger J.L., Kostka W., Hilgert I. Biochemical characterization of a soluble form of the 54-kDa monocyte surface antigen. Eur. J. Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- Durieux J.J., Vita N., Popescu O., Guette F., Calzada-Wack J., Munker R., Schmidt R.E., Lupker J., Ferrara P., Ziegler-Heitbrock H.W., Labéta M.O. The two soluble forms of the lipopolysaccharide receptor, CD14characterization and release by normal human monocytes. Eur. J. Immunol. 1994;24:2006–2012. doi: 10.1002/eji.1830240911. [DOI] [PubMed] [Google Scholar]
- Jack R.S., Grunwald U., Stelter F., Workalemahu G., Schütt C. Both membrane bound and soluble forms of CD14 bind to Gram-negative bacteria. Eur. J. Immunol. 1995;25:1436–1441. doi: 10.1002/eji.1830250545. [DOI] [PubMed] [Google Scholar]
- Frey E.A., Miller D.S., Jahr T.G., Sundan A., Bazil V., Espevik T., Finlay B.B., Wright S.D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J. Exp. Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labéta M.O., Durieux J.J., Fernandez N., Herrmann R., Ferrara P. Release from a human monocyte-like cell line of two different soluble forms of the lipopolysaccharide receptor, CD14. Eur. J. Immunol. 1993;23:2144–2151. doi: 10.1002/eji.1830230915. [DOI] [PubMed] [Google Scholar]
- Smith B.J. Protein Sequencing Protocols 1997. Humana Press; Totowa, NJ: pp. 392 pp [Google Scholar]
- Hailman E., Lichenstein H.S., Wurfel M., Miller D.S., Johnson D.A., Kelley M., Busse L.A., Zukowski M.M., Wright S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to sCD14. J. Exp. Med. 1994;79:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E., Goyert S.M. Nucleotide sequence of the gene encoding the monocyte differentiation antigen, CD14. Nucleic Acids Res. 1988;16:4173. doi: 10.1093/nar/16.9.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney I.A., Atkinson J.P., Krul E.S., Sconfeld G., Polakoski K., Saffitz J.E., Morgan B.P. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasmaCD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement mediated lysis. J. Exp. Med. 1993;177:1409–1420. doi: 10.1084/jem.177.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H.W., Frankenberger M., Wedel A. Tolerance to lipopolysaccharide in human blood monocytes. Immunobiology. 1995;193:217–223. doi: 10.1016/s0171-2985(11)80546-2. [DOI] [PubMed] [Google Scholar]
- Newberry R.D., Stenson W.F., Lorenz R.G. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigens. Nat. Med. 1999;8:900–912. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- Rey Nores J.E., Bensussan A., Vita N., Stelter F., Arias M.A., Jones M., Lefort S., Borysiewicz L.K., Ferrara P., Labéta M.O. Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur. J. Immunol. 1999;29:265–276. doi: 10.1002/(SICI)1521-4141(199901)29:01<265::AID-IMMU265>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Arias M.A., Rey Nores J.E., Vita N., Stelter F., Borysiewicz L.K., Ferrara P., Labéta M.O. Cutting edgehuman B cell function is regulated by interaction with soluble CD14: opposite effects on IgG1 and IgE production. J. Immunol. 2000;164:3480–3486. doi: 10.4049/jimmunol.164.7.3480. [DOI] [PubMed] [Google Scholar]