Abstract
Antigen presentation by major histocompatibility complex class II molecules is essential for antibody production and T cell activation. For most class II alleles, peptide binding depends on the catalytic action of human histocompatibility leukocyte antigens (HLA)-DM. HLA-DO is selectively expressed in B cells and impedes the activity of DM, yet its physiological role remains unclear. Cell surface iodination assays and mass spectrometry of major histocompatibility complex class II–eluted peptides show that DO affects the antigenic peptide repertoire of class II. DO generates both quantitative and qualitative differences, and inhibits presentation of large-sized peptides. DO function was investigated under various pH conditions in in vitro peptide exchange assays and in antigen presentation assays using DO− and DO+ transfectant cell lines as antigen-presenting cells, in which effective acidification of the endocytic pathway was prevented with bafilomycin A1, an inhibitor of vacuolar ATPases. DO effectively inhibits antigen presentation of peptides that are loaded onto class II in endosomal compartments that are not very acidic. Thus, DO appears to be a unique, cell type–specific modulator mastering the class II–mediated immune response induced by B cells. DO may serve to increase the threshold for nonspecific B cell activation, restricting class II–peptide binding to late endosomal compartments, thereby affecting the peptide repertoire.
Keywords: antigen presentation, immune response, selection, HLA-DM, autoimmunity
Introduction
The immune response against exogenous antigens requires presentation of antigenic fragments by MHC class II molecules at the cell surface of APCs 1. As these peptides are predominantly generated in the endosomal and lysosomal pathway, MHC class II molecules are directed into this pathway after synthesis. For this, they associate in the endoplasmic reticulum to the invariant chain (Ii), containing the targeting signal for endosomal sorting 2. During transport to lysosomal-like compartments, where the majority of antigen loading occurs (termed MHC class II peptide-loading compartment [MIIC]), the Ii is proteolytically removed, leaving only a small fragment (class II–associated invariant chain peptides [CLIP]) in the class II peptide binding groove 3 4.
Release of CLIP is needed to allow antigen binding to class II 5. It requires the action of HLA-DM, another MHC-like molecule 6 7 that predominantly localizes to the MIICs 8. In mutant cells or mice lacking functional DM, CLIP remains associated to class II, which severely hampers antigen presentation 9 10 11 12. In addition, DM catalyzes dissociation of other nonstably bound peptides from class II, thus acting as a peptide editor that favors presentation of stably bound peptides 13 14 15 16 17 18. During peptide loading, some class II molecules escape the editing action of DM, as demonstrated by the presence of a low, but detectable amount of class II/CLIP at the cell surface 5.
Recently, another MHC-encoded heterodimer, HLA-DO 19 20, was found in tight association with DM in B cells 21. This association is a prerequisite for exit of DO from the endoplasmic reticulum and subsequent targeting into the endosomal pathway. Like DM, the predominant localization of the DM–DO complex is in the MIICs. The function of DO is still controversial. DO inhibits the catalytic action of DM, impeding class II–CLIP dissociation and reducing, but not abolishing, presentation of specific antigenic peptides 22 23. Moreover, B cells of H2-O–deficient mice (H2-O is the murine equivalent of DO) have a changed capacity to present particular antigens 24. Since DO appears to be best expressed in resting B cells (our unpublished results), these findings suggest that DO may be involved in controlling B cell–driven immune responses. Still, the physiological function and purpose of DO remain elusive.
We here report that DO forms a modulator of the antigenic peptide repertoire that is presented by MHC class II molecules. DO both limits antigen presentation as a whole, and simultaneously alters the actual composition of the set of class II–associated peptides. Mechanistically, DO is demonstrated to inhibit DM-mediated class II–peptide loading best at the pH of the early endocytic compartments. As a consequence, DO skews the peptide repertoire to those peptides that are loaded onto class II in acidic compartments, like the MIICs, while impeding peptide loading in earlier, less acidic compartments of the endosomal/lysosomal pathway.
Materials and Methods
Cell Lines, Transfectants, and Antibodies.
Stable transfectants of the melanoma cell line Mel JuSo (HLA-A1, HLA-B8, HLA-Cw7, HLA-DR3, and HLA-DQ2 by DNA typing) either transfected with HLA-DOα 19, HLA-DOβ–green fluorescent protein (GFP), or vector only 20 23, were grown in Iscove's medium with 10% FCS, 2,000 μg/ml G418, and/or 600 μg/ml hygromycin (GIBCO BRL). Continuous and homogenous expression of the GFP-tagged proteins was ensured by regular selection of the GFP+ cells by FACS®. B lymphocytes, derived from peripheral blood from healthy donors, were purified with anti-CD19 Dynabeads® and detached with the corresponding DETACHaBEAD®, according to the manufacturer's instructions (Dynal). The HLA-DR–specific mAbs L243 25 and 1B5 26, the CLIP-specific antibody CerCLIP.1 27, the DR3–antigenic peptide specific mAb 16.23 28 29, the DMα-specific mAb 5C1 30, the rabbit anti-DOβ serum 23, and the Ii-specific polyclonal sera ICN2 and ICC5 31 have been described 8. The antiactin mAb Ab-1 was obtained from Oncogene Research Products and the Texas red–conjugated secondary antibodies from Molecular Probes.
DNA Constructs.
The cDNAs encoding HLA-DOα 19 and HLA-DOβ 20 were respectively cloned into pcDNA3 (Invitrogen) and a variant of pCEP4 (Invitrogen) disabled for episomal replication. The generation of a fusion construct between GFP and the COOH terminus of DOβ (DOβGFP) has been described 23. Other DNA manipulations were carried out using standard procedures 32.
Biochemical and Western Blot Analyses.
Cell surface labeling of proteins was performed by lactoperoxidase-catalyzed iodination with 1 mCi Na125I on cells grown to subconfluency in a 75-cm2 tissue culture flask. Cells were washed four times with cold PBS, lysed in Tris lysis buffer (pH 7.4) containing 0.5% NP-40 and 2 μM PMSF (Sigma Chemical Co.), and used for immunoprecipitations with normal rabbit serum, mAb 1B5, and the polyclonal sera ICN2 and ICC5. Samples were loaded such that they were related to equal amounts of recovered radioactive class II molecules and separated by 12.5% SDS-PAGE. For pulse–chase analysis, cells (grown to subconfluency in 6 Ø cm dishes) were pulsed for 30 min with 0.1 mCi l-[35S]methionine and l-[35S]cysteine (Amersham Pharmacia Biotech) in methionine- and cysteine-free RPMI 1640 medium after deprivation of the cells of methionine and cysteine for 30 min and chased upon addition of cold methionine/cysteine to a final concentration of 1 μM. Cells were either lysed in NP-40–containing lysis buffer immediately or after chase periods for up to 8 h in complete medium at 37°C. Class II molecules were recovered from equal amounts of TCA-precipitable radioactivity using the 1B5 antibody. Immunoprecipitates were analyzed by 12.5% SDS-PAGE. To determine the relative expression levels of DM and DO, equal amounts of total protein were Western blotted using the anti-DM mAb 5C1 30, the antiactin mAb Ab-1 (Oncogene), or the anti-DOβ serum, and specific protein amounts were quantified using the FluorChem™ imaging system and analyzed with AlphaEase™ FluorChem™ software (Alpha Innotech Corp.).
FACS® Analysis.
106 cells were stained with saturating amounts of unlabeled primary antibody and PE-conjugated F(ab′)2 rabbit anti–mouse IgG (heavy and light chains; Zymed) and analyzed on a FACScan™ flow cytometer (Becton Dickinson).
MHC Class II Peptide Isolation, Reversed Phase HPLC, Mass Spectrometry, and Peptide Sequencing.
HLA-DR3–peptide complexes were purified from >1010 Mel JuSo cells transfected with DOαβGFP (FACS®-sorted for homogenous DOαβGFP expression) or with vector only. Peptides were eluted and separated by RP-HPLC on a SMART system equipped with a μRPC C2/C18 SC2.1/10 column (Amersham Pharmacia Biotech) using an acetonitrile gradient in 0.1% TFA as described 23.The RP-HPLC profiles obtained from independent separations were reproducible and equivalent for the respective class II eluates of DO− or DO+ cells. 0.5 μl aliquots were sampled from each HPLC fraction and analyzed by matrix-assisted laser-desorption time-of-flight mass spectrometry using a TofSpec 2E mass spectrometer (Micromass) fitted with a time-lag focusing source 33 34.
Peptide Association Assay.
HLA-DR3–CLIP complexes were affinity purified from T2.DR3 cells using L243-coupled CNBr-activated Sepharose as described previously 17. Mel JuSo cells either transfected with DOαβGFP or with vector only were lysed in 50 μM Tris-HCl, pH 8.0, containing 0.5% NP-40, 5 μM EDTA, and protease inhibitors, followed by removal of nuclei and debris by centrifugation. Protein concentrations of the different lysates were determined by BCA (Pierce Chemical Co.) measurements using BSA (Sigma Chemical Co.) as standard and adjusted to equal values. Exchange of CLIP from DR3–CLIP complexes for biotinylated apolipoprotein B (ApoB[2877–2894]) was determined by adding 2 μM of biotinylated peptide to 30 nM DR3–CLIP in buffer containing 50 μM Tris-HCl, 25 μM Na2CO3, 2 μM EDTA, 0.1% NP-40, and 0.1 μM PMSF titrated to the desired pH with 1 M citrate 35. Lysates from ∼5 × 105 cells or lysis buffer only were added when appropriate. Western blot analysis, using the DMα-specific mAb 5C1 30 or the antiactin mAb Ab-1 (Oncogene) followed by quantification using the FluorChem™ imaging system and AlphaEase™ FC software (Alpha Innotech Corp.), confirmed that the lysates contained identical amounts of DM and DM/DO. The time course of ApoB(2877–2894) association at 37°C was followed until saturation of peptide binding (24 h for pH 4.5–6.0 and 50 h for pH 7.0). MHC–peptide complexes were immunoprecipitated with immobilized L243 antibody and peptides were detected via peroxidase-conjugated streptavidin (Amersham Pharmacia Biotech). The absorbance at 405 nm was measured by an ELISA reader (Multiskan Plus; Titertek) and nonspecific signals (quadruplicates, typically 15% of maximal absorbance) were subtracted from the data 17 35.
T Cell Proliferation Assay.
Antigen presentation experiments were performed essentially as described 23. In brief, Mel JuSo cells were incubated for 4 h with 100 nM bafilomycin A1 (Biomol) when appropriate, and subsequently pulsed with varying amounts of sonicate of Mycobacterium tuberculosis either in the presence or not of 40 nM bafilomycin A1. Subsequently, the cells were irradiated at 8,000 rads and seeded in 96-well flat-bottomed microtiter plates at cell concentrations triggering optimal T cell proliferation 36, together with 104 T cells from the HLA-DR3–restricted, p3-13–specific T cell clone Rp15 1-1. As a control, the HLA-DR3–restricted epitope, the p56-65 peptide of the 30–31-kD protein of M. tuberculosis, was added to nontreated, irradiated cells. To check for toxicity due to the Bafilomycin A1 treatment, T cell proliferation was measured in the presence of APCs and 10% IL-2 (Lymphocult-T; Biotest). After 66 h in culture, 1 μCi [3H]thymidine was added, cells were collected 18 h later on glass fiber filter strips, and the radioactivity incorporated in the DNA was assessed by liquid scintillation counting 36.
Results and Discussion
DO Interferes with CLIP Release from Class II Molecules and Reduces Antigenic Peptide Presentation.
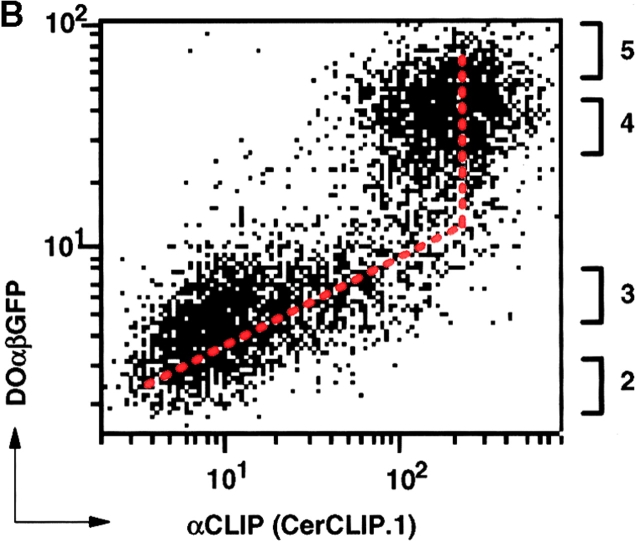
The function of DO was studied using the human cell line Mel JuSo (DO−, DM+, and HLA–DR3+; 23) and stable transfectants expressing DOαβGFP with GFP 37 linked to the cytoplasmic tail of DOβ without affecting DO function 23. Although DO hampers the release of CLIP from MHC class II molecules, it affects presentation of some particular antigenic peptides, but not of others 22 23 24. To get insight in the effect of DO on the overall efficiency of antigenic peptide presentation by class II, FACS® analysis was performed on both DO− and DO+ cells. The amount of DOαβGFP in the DO+ cells was such that the whole DM pool was quantitatively associated to DO 23. DOαβGFP expression caused an ∼50% reduction in staining with the antibody 16.23 that recognizes HLA-DR3–antigenic peptide complexes 28 29, concomitant with an increase in the relative amount of class II/CLIP ( Fig. 1 A). The total level of cell-surface expressed class II remained unchanged ( Fig. 1 A), nor did the level of DM vary significantly 23. Thus, in cells with DM quantitatively associated to DO, the pool of class II presenting antigenic peptides is reduced, but not eliminated. Since it was recently suggested 38 that the inhibitory effect of DO on CLIP release only occurred at high DO expression levels, FACS® analysis on a population of transfectants with various DOαβGFP levels was performed. This demonstrated an almost linear correlation between DOαβGFP expression and cell surface–expressed class II/CLIP, ranging from a low, but detectable, amount of class II/CLIP in DO− cells to an almost two-log increase in cells with relatively high DOαβGFP levels ( Fig. 1 B). Additional augmentation of DOαβGFP expression did not increase the relative CLIP expression any further, suggesting that from this point on DO expression was saturating for quantitative association of DM to DO ( Fig. 1 B). Identical results were obtained when GFP and DOβ were expressed as separate proteins from one bicistronic transcript (data not shown). FACS® sorting of different DOαβGFP transfectant populations (populations 2–5, Fig. 1 B) was performed to quantify DM and DO expression (Table ). Comparison of the relative DO/DM levels with that observed in primary B cells showed that the relative DO/DM level in primary B cells falls within the range of values obtained from the transfectants studied (Table ). Thus, DO invariably and quantitatively impedes CLIP removal from newly synthesised class II molecules, also when expressed at levels that are comparable to the physiological B cell levels.
Figure 1.
DOαβGFP affects peptide presentation by HLA-DR3. (A) FACS® analysis of 5,000 Mel JuSo cells transfected either with DOαβ2 GFP (GFP+ population) or vector only (GFP− population) showing staining with secondary antibody only (−), or staining that is specific for class II molecules (L243), CLIP-bound HLA-DR3 (CerCLIP.1), or HLA-DR3 complexed to stably bound peptides (16.23). (B) FACS® analysis of 5,000 Mel JuSo cells expressing various levels of DOαβGFP, showing specific staining of HLA-DR3–CLIP complexes by CerCLIP.1 antibody. The vertical axis represents GFP and the horizontal axis PE-fluorescence derived from the secondary antibody, each in arbitrary units on a logarithmic scale. Values next to the right axis indicate which DOαβGFP populations were FACS® sorted for analysis of relative DO/DM expression levels.
Table 1.
Relative DO/DM Expression Levels in Mel JuSO/DOαβGFP Transfectants and Primary B Cells
| Actin | DMα | DOβ | DOβ/DMα | |
|---|---|---|---|---|
| aU | aU | aU | aU | |
| Mel JuSo/DOαβGFP | ||||
| Population 2 | 1 | 1.2 | 0.04 | 0.03 |
| Population 3 | 1 | 1.1 | 0.07 | 0.06 |
| Population 4 | 1 | 1.1 | 0.9 | 0.82 |
| Population 5 | 1 | 1 | 1 | 1 |
| Primary B Cells | 1 | 1.0–1.4 | 0.45 | 0.3–0.5 |
Quantification of DMα and DOβ expression levels in transfectant Mel JuSo cells sorted for different expression levels of DOαβGFP and primary B cells was performed using the FluorChem™ imaging system and AlphaEase™ FC software. Relative DMα and DOβ expression levels (in arbitrary units, aU) are correlated to cellular actin levels (in arbitrary units).
DO Abolishes Presentation of a Set of Long Peptides Associated to Class II.
The effect of DO on the composition of surface class II–peptide complexes was investigated more closely using surface iodinated class II complexes from DO+ and DO− cells. Strikingly, MHC class II immunoprecipitation using the HLA-DRα–specific mAb 1B5 led to the coprecipitation of a set of iodinated peptides with apparent molecular sizes <18 kD in the DO− cells, but not in the DO+ cells ( Fig. 2 A). These fragments were not recovered upon immunoprecipitation using mAbs specific for the N or COOH terminus of the Ii, indicating that they were not derived from the class II–associated Ii fragments ( Fig. 2 A). Moreover, when class II–Ii maturation was compared between both DO+ and DO− in pulse–chase analysis, DO did not affect the intracellular transport of class II ( Fig. 2 B, top), nor the rate of breakdown of the associated Ii. Ii breakdown intermediates 39 were recovered at the same time points in both DO+ and DO− cells, apart from the expected recovery of CLIP at late time points in DO+ cells only ( Fig. 2 B, bottom). Notably, the set of large peptides associated with class II at the cell surface in DO− cells did not correspond to the Ii fragments observed in pulse–chase analysis (compare Fig. 2a and Fig. b). Together, these data show that DO generates both quantitative differences in the pool of class II–associated peptides at the cell surface of APCs (as demonstrated by a 50% reduction of 16.23 staining; Fig. 1 A), as well as qualitative differences in that class II–mediated presentation of long, iodinable fragments is abrogated.
Figure 2.
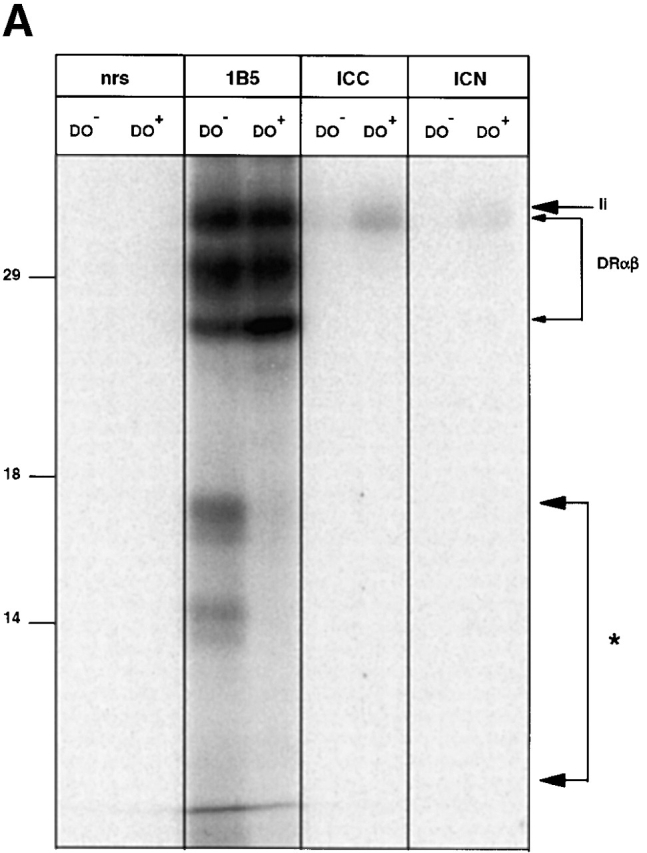
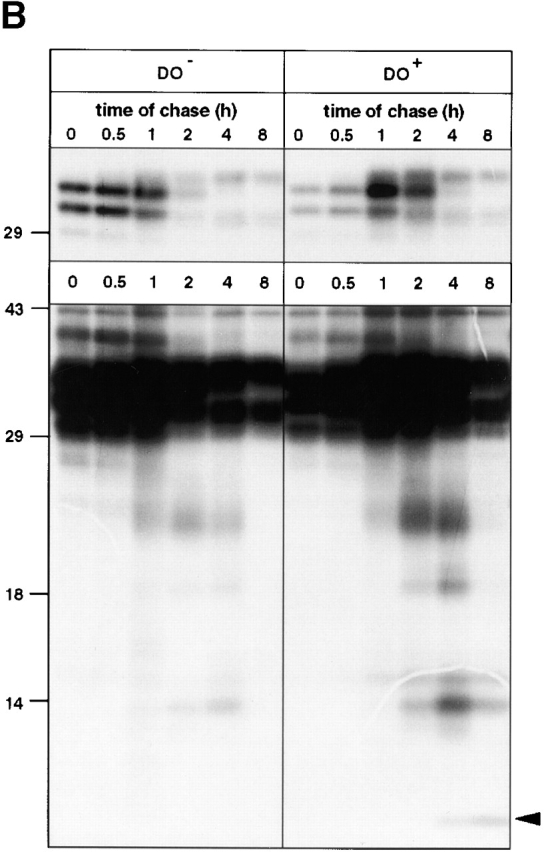
DO alters the class II–associated peptides at the cell surface. (A) SDS-PAGE of immunoprecipitated material from surface iodinated Mel JuSo cells either transfected (DO+) or not (DO−) with DOαβGFP. Proteins were precipitated using normal rabbit serum (nrs), the anti–HLA-DRα mAb 1B5, or the anti-Ii mAbs ICC5 (anti–COOH terminus) and ICN2 (anti–NH2 terminus). The positions of the DR chains and Ii are marked by arrows. The area containing iodinated fragments associated with class II only in DO− cells (even upon prolonged exposure of the autoradiogram) is indicated with an asterisk. Molecular marker sizes are shown on the left (in kD). (B) Pulse–chase analysis of [35S]Met-Cys–labeled MHC class II complexes of control Mel JuSo cells (DO−) or transfected with DOαβ− GFP (DO+). Cells were labeled for 30 min and lysed after chase in nonradioactive medium for the indicated times, followed by isolation using the anti-DRα antibody 1B5. The top and bottom show a short and long exposure, respectively, of the same autoradiogram. Molecular marker sizes are indicated on the left (in kD).
DO Generates Qualitative Differences in the Class II–associated Antigenic Peptide Repertoire.
To identify the nature of the differences in the class II peptide repertoire generated by DO, HLA-DR3 molecules were purified from both DO+ and DO− cells. The associated peptides were eluted, separated into >100 different fractions by RP-HPLC and analyzed by matrix-assisted laser-desorption time-of-flight mass spectrometry 33 34. Analysis of the fractions showed that the DO+ cells were highly enriched in peptides with masses corresponding to various CLIP variants in the late eluting fractions of the RP-HPLC, as observed presiously 23. Both for DO− and for DO+, >500 individual mass species in antigenic peptide size (Mr range of 13–21 amino acid residues) were identified in the RP-HPLC fractions eluting before CLIP. Analysis of all of these masses upon calibration showed that ∼90% of the peptides were identical for both eluates ( Fig. 3), with masses being regarded as identical when 1 differing <0.5 daltons and 2 eluting in equivalent fractions from the RP-HPLC. Strikingly, ∼10% of the peptides were unique either for the DO+ or DO− cells ( Fig. 3). These unique peptides were found in several subsequent fractions of either DO− or DO+ eluate, but not in any of the fractions of the other eluate. The average size of the unique peptides did not vary significantly between both eluates. Thus, apart from abrogating association of long peptide fragments to class II, DO also alters the set of class II–associated antigenic peptides by suppressing presentation of certain peptides, while inducing presentation of others. Therefore, DO has the unique property of modulating the class II–mediated peptide repertoire, both by positive and negative discrimination. This level of fastidiousness in selecting peptides for loading onto presenting class II molecules points to an unprecedented function for the DO molecule in the immune system.
Figure 3.

DO modulates the antigenic peptide repertoire of HLA-DR3. Mass spectrometry profiles of four sets of equivalent RP-HPLC fractions containing peptides eluted from HLA-DR3 molecules from DO− and DO+ cells. Peptides that are found in both depicted DO− and DO+ fractions are denoted with a number. Nonannotated peptide peaks are found in both DO− and DO+ eluates, but not in the corresponding fractions depicted. Peptides denoted with a specific mass are found in several subsequent fractions of either DO− or DO+ eluate, but not in any of the fractions of the other eluate.
DO Modulates DM-mediated Class II–peptide Loading in a pH-dependent Manner.
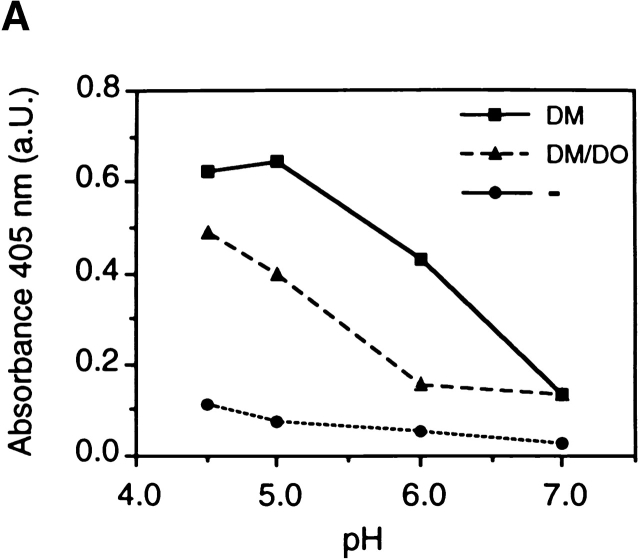
By which mechanism does DO generate qualitative differences in the class II–associated peptide repertoire? Different class II–peptide complexes can be generated in distinct compartments of the endosomal/lysosomal pathway in different pH environments 40 41 42 43 44 45. The DM–DO complex constitutively recycles between the MIICs and the plasma membrane (data not shown), implying that en route to the MIIC, both DM and DO traverse all endocytic compartments where they may support and modulate peptide loading. Recently, recombinant DO was reported to affect DM function in a pH-sensitive manner in vitro 24. If true, this may illustrate how DO modulates the peptide repertoire. To determine if DO modulates DM activity in a pH-dependent fashion in our transfectants, in vitro peptide loading experiments were performed using DM and DM–DO complexes obtained from either DO− or DO+ cells in which the whole DM pool was quantitatively associated to DO 23 30 35. Equal amounts of DM and DM–DO were applied in the experiments as quantified using the FluorChem™ imaging system and AlphaEase™ FC software (data not shown). Exchange of CLIP from purified HLA-DR3–CLIP for the antigenic peptide ApoB(2877–2894) was indeed increasingly catalyzed by DM on acidification with an optimum pH ∼5 ( Fig. 4 A). DO-mediated inhibition of DM was strongest at the pH normally observed in early lysosomes (pH ∼6), conditions in which the catalytic capacity of DM is almost abrogated. DO still inhibited DM at the pH described for the MIICs (pH 4.5–5), but only by half ( Fig. 4 A). Thus, these in vitro data suggests that DO inhibits DM preferentially at the pH of earlier endosomal compartments, while allowing substantial DM-mediated peptide loading at the pH present in MIICs.
Figure 4.
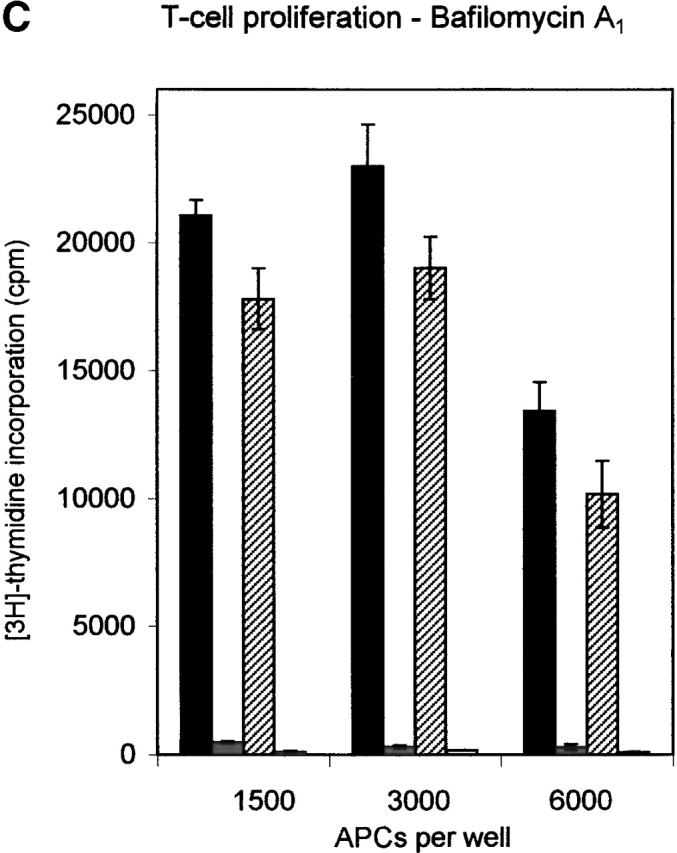
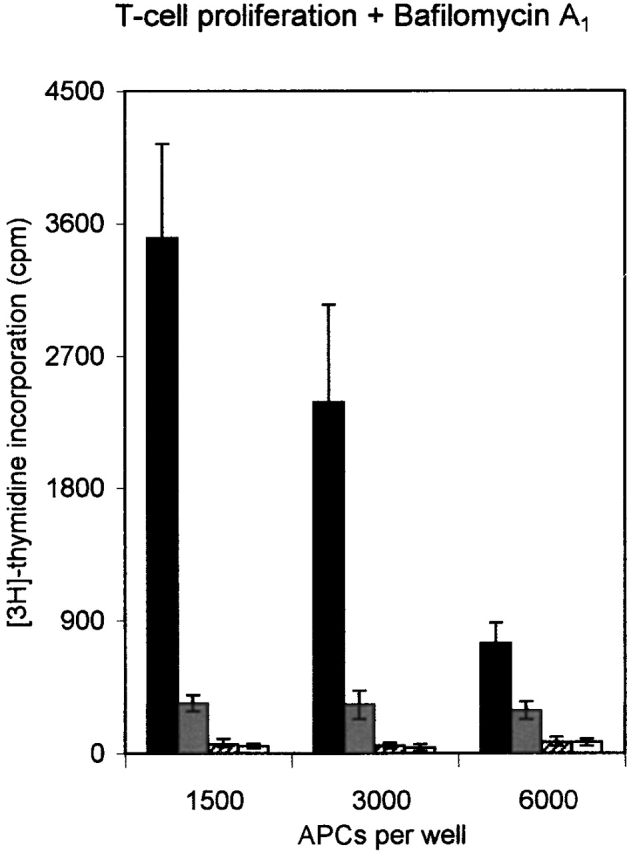
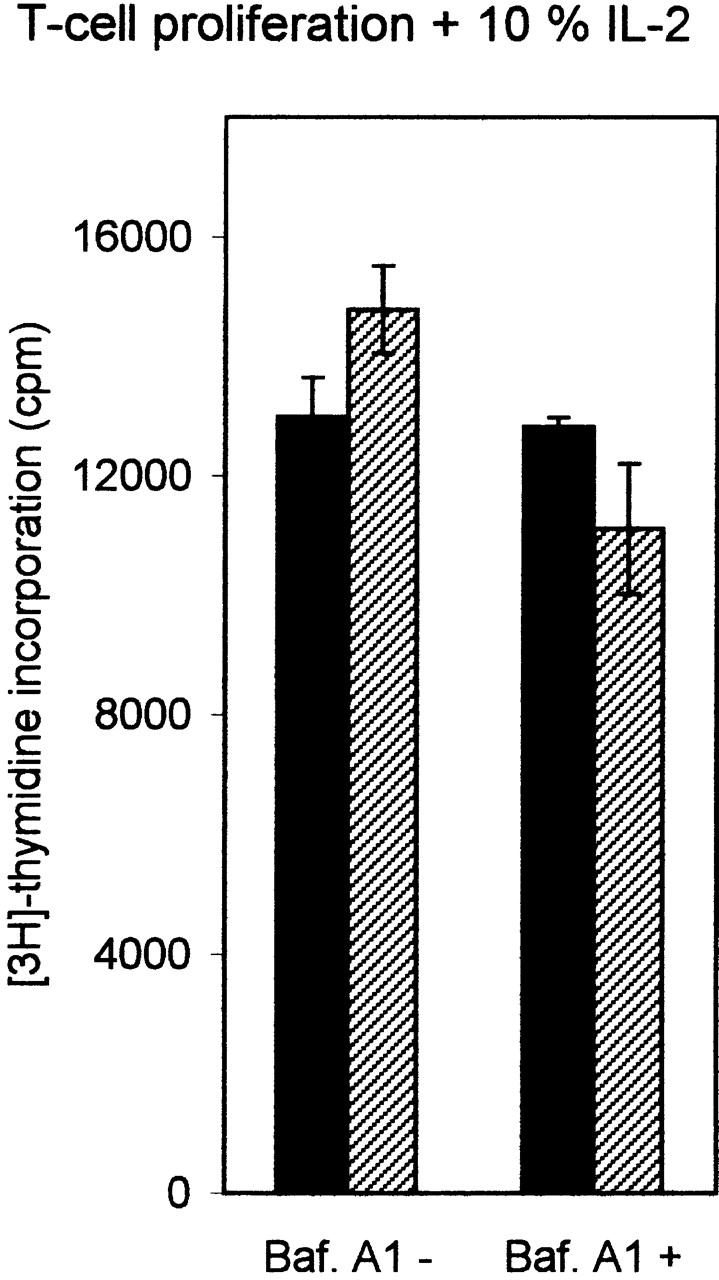
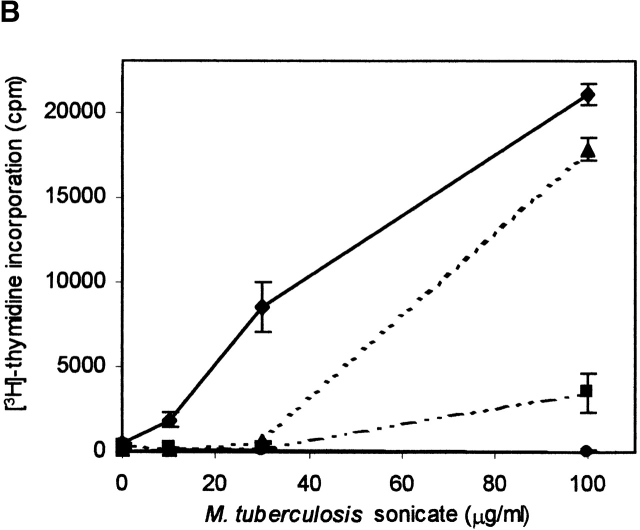
DO modulates DM function in a pH-dependent fashion. (A) In vitro association of biotinylated ApoB(2877–2894) to purified DR3–CLIP complexes upon CLIP dissociation was measured at different pH values in the presence of cell lysates containing equal amounts of DM or DM–DOαβ2 GFP complexes (DM/DO) or lysis buffer only (−). Association is depicted as absorbance at 405 nm in arbitrary units (a.U.) upon saturation of the peptide exchange reaction. The data shown were derived from a representative set of experiments (n = 4). (B) Antigen-presenting capacity of p3-13–reactive T cell clone Rp15 1-1 using control (DM) and DOαβGFP-expressing cells (DM/DO) at prevention of acidification of the endosomal/lysosomal pathway. T cell proliferation is expressed as the amount of [3H]thymidine incorporation in cpm and was determined for varying amounts of M. tuberculosis sonicate with 1,500 APCs/assay well. Data shown were derived from a representative experiment performed three times; SEM <10%. T cell proliferation in response to Mel JuSo cells pulsed with HLA-DR3–restricted peptide p56-65 was not significant, and application of Mel JuSo transfected with DO without GFP tag resulted in the same effects as the DOαβGFP transfectants (data not shown). (C) Antigen-presenting capacity of p3-13–reactive T cell clone Rp15 1-1 using varying amounts of control (DM) and DOαβGFP-expressing cells (DM/DO) at prevention of acidification of the endosomal/lysosomal pathway. T cell proliferation (measured by [3H]thymidine incorporation) was determined using 100 μg/ml M. tuberculosis sonicate. Data shown were derived from a representative experiment performed three times; SEM <10%. Application of Mel JuSo transfected with DO without GFP tag yielded the same results as the DOαβGFP transfectants (data not shown). Black bars, DM + M. tuberculosis; gray bars, DM + medium; lined bars, DM-DO + M. tuberculosis; empty bars, DM-DO + medium.
The pH-sensing activity of DO was investigated under more physiological conditions in antigen presentation assays 36 using bafilomycin A1, an inhibitor of vacuolar ATPases 44. Confocal analyses showed that this strongly reduced the acidification of the endosomal/lysosomal compartments, with the majority of DM–DO still locating to the MIICs (data not shown). Antigen presentation of the p3-13 peptide of the hsp65 protein from M. tuberculosis 36 was studied, as this peptide is loaded onto newly synthesized class II molecules in acidic MIICs (its presentation is abrogated upon brefeldin A incubation; data not shown). Incubation of both DO− and DO+ cells with varying amounts of M. tuberculosis sonicate showed that both transfectants presented the p3-13 peptide in a dose-dependent manner ( Fig. 4 B). DO expression reduced presentation ( Fig. 4 B), in line with the in vitro peptide loading abilities of DM–DO and DM at acidic pH ( Fig. 4 A) and our previous results 23. bafilomycin A1 strongly affected p3-13 presentation upon incubation of DO− cells with M. tuberculosis sonicate, but still allowed presentation at higher antigen concentrations ( Fig. 4 B). However, bafilomycin A1 completely inhibited p3-13 peptide presentation in DO+ cells at all concentrations of M. tuberculosis sonicate ( Fig. 4 B). To exclude the possibility that bafilomycin A1 inhibited antigen presentation in DO+ cells due to the initial lower presentation capacities of nontreated cells, antigen presentation was studied using a very high amount of M. tuberculosis sonicate (100 μg/ml) with varying amounts of APC. In this way, the T cell proliferation values induced by 1,500 and 3,000 nontreated DO+ cells were higher than the proliferation values obtained with 6,000 nontreated DO− cells ( Fig. 4 C). Still, bafilomycin A1 again completely abrogated p3-13 peptide presentation at all DO+ concentrations, while only partially inhibiting presentation by DO− cells (even upon application of 6,000 DO− cells/assay well; Fig. 4 C). Antigen presentation was not affected by bafilomycin A1 when IL-2 was added to the T cell–APC mixture, indicating that bafilomycin A1 was not toxic to the cells ( Fig. 4 C). Thus, DO completely inhibits DM-mediated class II–peptide loading in intracellular compartments that are not effectively acidified. These data demonstrate that DO indeed acts in a pH-dependent fashion to regulate DM function. Translated to the physiological situation, DO will inhibit DM-mediated peptide loading in early endosomal compartments, while allowing peptide binding to class II molecules in more acidic compartments, like the MIICs. In concordance with this are descriptions of DM mediating class II–peptide binding in MIICs, but not in early endosomes 42 43 45, considering that in those experiments DM was most likely complexed to DO. The skewing by DO to DM-mediated peptide loading in acidic compartments provides an explanation for how DO inhibits class II–mediated presentation of certain epitopes, especially large ones, while favoring presentation of others.
DO May Serve to Control B Cell-driven Immune Responses.
DO is the first example of a physiological relevant protein that both limits and skews the class II–presented antigenic peptide repertoire. Two distinct features of the action of DO on class II–mediated antigen presentation are apparent, one quantitative, the other qualitative. First, DO downmodulates the levels of class II–antigen loading as a whole by impeding CLIP release from class II, resulting in a reduced, but not completely inhibited, presentation of antigens and thus a diminished CD4+ response. The observed linearity between DO and DR–CLIP expression indicates that even relatively low DO expression modulates the class II response in this way. Second, DO results in the presentation of certain peptides while suppressing others, thus modulating the antigenic peptide repertoire. Presentation of long peptides is abrogated by DO, probably because these fragments have already been trimmed down in the later, acidic peptidase–containing 46 compartments where DO allows peptide loading of class II. The finding that DO is mainly expressed in B cells, and thus forms a cell type–specific modulator, points to a B cell–specific need for skewing peptide loading to acidic compartments. Since in a B cell, specific antigens are taken up via the B cell receptor and targeted to acidic MIICs 47 48, DO may favor presentation of these antigens, while repressing presentation of antigens continuously internalized via fluid phase endocytosis. These modulatory actions of DO may increase the threshold for nonspecific B cell activation, preventing nonspecific activation of the immune system and autoantibody production.
Acknowledgments
We gratefully acknowledge G. Malcherek for the biotinylated ApoB(2877–2894) peptide and P. Cresswell for the T2.DR3 cells and the CerCLIP.1 antibody. We thank A. Griekspoor, L. de Bruin, and W. Haagmans for helpful assistance.
This work was supported by a Pioneer Grant from The Netherlands Organisation for Scientific Research (NWO) (to M. van Ham and M. van Lith), by The Wellcome Trust (to J. Trowsdale), and by European Community grant CT960069 (to J. Neefjes and J. Trowsdale).
Footnotes
M. van Lith and B. Lillemeier contributed equally to this work.
Abbreviations used in this paper: ApoB, apolipoprotein B; CLIP, class II–associated invariant chain peptides; GFP, green fluorescent protein; Ii, invariant chain; MIIC, MHC class II compartment; RP-HPLC, reversed phase HPLC.
References
- Lanzavecchia A. Mechanisms of antigen uptake and presentation. Curr. Opin. Immunol. 1996;8:348–354 . doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716 . doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Roche P.A., Cresswell P. Invariant chain association with DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–618 . doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Amaya M., Mellins E., Wiley D.C. The structure of an intermediate in MHC maturationCLIP bound to HLA-DR3. Nature. 1995;378:457–462 . doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- Avva R.R., Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994;1:763–774 . doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- Fremont D.H., Crawford F., Marrack P., Hendrickson W.A., Kappler J. Crystal structure of mouse H2-M. Immunity. 1998;9:385–393 . doi: 10.1016/s1074-7613(00)80621-4. [DOI] [PubMed] [Google Scholar]
- Mosyak L., Zaller D.M., Wiley D.C. The structure of HLA-DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation. Immunity. 1998;9:377–383 . doi: 10.1016/s1074-7613(00)80620-2. [DOI] [PubMed] [Google Scholar]
- Sanderson F., Kleijmeer M.J., Kelly A.P., Verwoerd D., Tulp A., Neefjes J.J., Geuze H.J., Trowsdale J. Accumulation of HLA-DM, a regulator of antigen presentation, in MHC class II compartments. Science. 1994;266:1566–1569 . doi: 10.1126/science.7985027. [DOI] [PubMed] [Google Scholar]
- Fling S.P., Arp B., Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–558 . doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- Morris P., Shaman J., Attaya M., Amaya M., Goodman S., Bergman C., Monaco J.J., Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–554 . doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- Martin W.D., Hicks G.G., Mendiratta S.K., Leva H.I., Ruley H.E., Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550 . doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Wolf P., Tourne S., Waltzinger C., Dierich A., Barois N., Ploegh H., Benoist C., Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541 . doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Denzin L.K., Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165 . doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Sherman M.A., Weber D.A., Jensen P.E. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205 . doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Sloan V.S., Cameron P., Porter G., Gammon M., Amaya M., Mellins E., Zaller D.M. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806 . doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Vogt A.B., Moldenhauer G., Hammer J., Blum J.S., Hämmerling G.J. Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO (Eur. Mol. Biol. Org.) J. 1996;15:6144–6154 . [PMC free article] [PubMed] [Google Scholar]
- van Ham S.M., Grüneberg U., Malcherek G., Bröker I., Melms A., Trowsdale J. Human histocompatibility leukocyte antigen (HLA)-DM edits peptides presented by HLA-DR according to their ligand binding motifs. J. Exp. Med. 1996;184:2019–2024 . doi: 10.1084/jem.184.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D.A., Evavold B.D., Jensen P.E. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618–620 . doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]
- Young J.A., Trowsdale J. The HLA-DNA (DZA) gene is correctly expressed as a 1.1 kb mature mRNA transcript. Immunogenetics. 1990;31:386–388 . doi: 10.1007/BF02115015. [DOI] [PubMed] [Google Scholar]
- Tonnelle C., DeMars R., Long E.O. DO betaa new beta chain gene in HLA-D with a distinct regulation of expression. EMBO (Eur. Mol. Biol. Org.) J. 1985;4:2839–2847 . doi: 10.1002/j.1460-2075.1985.tb04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M., Kuwana T., Fung-Leung W.-P., Jackson M.R., Peterson P.A., Karlsson L. HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO (Eur. Mol. Biol. Org.) J. 1996;15:4817–4824 . [PMC free article] [PubMed] [Google Scholar]
- Denzin L.K., Sant'Angelo D.B., Hammond C., Surman M.J., Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–109 . doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- van Ham S.M., Tjin E.P.M., Lillemeier B.F., Grüneberg U., van Meijgaarden K.E., Pastoors L., Verwoerd D., Tulp A., Canas B., Rahman D. HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr. Biol. 1997;7:950–957 . doi: 10.1016/s0960-9822(06)00414-3. [DOI] [PubMed] [Google Scholar]
- Liljedahl M., Winqvist O., Surh C.D., Wong P., Ngo K., Teyton L., Peterson P.A., Brunmark A., Rudensky A.Y., Fung-Leung W.-P., Karlsson L. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–243 . doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- Lampson L.A., Levy R. Two populations of Ia-like molecules on a human cell line. J. Immunol. 1980;125:293–299 . [PubMed] [Google Scholar]
- Adams T.E., Bodmer J.G., Bodmer W.F. Production and characterization of monoclonal antibodies recognizing the α-chain subunits of human Ia alloantigens. Immunology. 1983;50:613–624 . [PMC free article] [PubMed] [Google Scholar]
- Denzin L.K., Robbins N.F., Carboy-Newcomb C., Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994;1:1–20 . doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Pious D., Dixon L., Levine F., Cotner T., Johnson R. HLA class II regulation and structureanalysis with HLA-DR3 and HLA-DP point mutants. J. Exp. Med. 1985;162:1193–1207 . doi: 10.1084/jem.162.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins E., Kempin S., Smith L., Monji T., Pious D. A gene required for class II–restricted antigen presentation maps to the major histocompatibility complex. J. Exp. Med. 1991;174:1607–1615 . doi: 10.1084/jem.174.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson F., Thomas C., Neefjes J., Trowsdale J. Association between HLA-DM and HLA-DR in vivo. Immunity. 1996;4:1–20 . doi: 10.1016/s1074-7613(00)80301-5. [DOI] [PubMed] [Google Scholar]
- Morton P.A., Zacheis M.L., Giacoletto K.S., Manning J.A., Schwartz B.D. Delivery of nascent MHC class II-invariant chain complexes to lysosomal compartments and proteolysis of invariant chain by cysteine proteases precedes peptide binding in B-lymphoblastoid cells. J. Immunol. 1995;154:137–150 . [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual 1989. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1–6.20, 16.30–16.40. pp. 5 [Google Scholar]
- Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988;60:2299–2301 . doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Vestal M.L., Juhasz P., Martin S.A. Delayed extraction matrix-assisted laser-desorption time-of-flight mass-spectrometry. Rapid Commun. Mass Spectrom. 1995;9:1044–1050 . [Google Scholar]
- Malcherek G., Gnau V., Jung G., Rammensee H.-G., Melms A. Supermotifs enable natural invariant chain–derived peptides to interact with many major histocompatibility complex-class II molecules. J. Exp. Med. 1995;181:527–536 . doi: 10.1084/jem.181.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geluk A., van Meijgaarden K.E., Janson A.A.M., Drijfhout J., Meloen R.H., De Vries R.R.P., Ottenhoff T.H. Functional analysis of DR17(DR3)-restricted mycobacterial T cell epitopes reveals DR17 binding motif and enables the design of allele-specific competitor peptides. J. Immunol. 1992;149:2864–2871 . [PubMed] [Google Scholar]
- Heim R., Cubbit A.B., Tsien R.Y. Improved green fluorescence. Nature. 1995;373:663–664 . doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Vogt A.B., Thery C., Armandola E.A., Li B.-C., Moldenhauer G., Amigorena S., Hämmerling G.J. A role for HLA-DO as a co-chaperone of HLA-DM in peptide loading of MHC class II molecules. EMBO (Eur. Mol. Biol. Org.) J. 1998;17:101–111 . doi: 10.1093/emboj/17.11.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters J., Horstmann H., Bakke O., Griffiths G., Lipp J. Intracellular transport and localization of major histocompatibility complex class II molecules and associated invariant chain. J. Cell Biol. 1991;115:1213–1223 . doi: 10.1083/jcb.115.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola J.-M., Grivel J.-C., Chavrier P., Gorvel J.-P. Different endocytic compartments are involved in the tight association of class II molecules with processed hen egg lysozyme and ribonuclease A in B cells. J. Cell Sci. 1995;108:2337–2345 . doi: 10.1242/jcs.108.6.2337. [DOI] [PubMed] [Google Scholar]
- Pinet V.M., Long E.O. Peptide loading onto recycling HLA-DR molecules occurs in early endosomes. Eur. J. Immunol. 1998;28:799–804 . doi: 10.1002/(SICI)1521-4141(199803)28:03<799::AID-IMMU799>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pinet V., Malnati M.S., Long E.O. Two processing pathways for the MHC class II-restricted presentation of exogenous influenza virus antigen. J. Immunol. 1994;152:4852–4860 . [PubMed] [Google Scholar]
- Lindner R., Unanue E.R. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO (Eur. Mol. Biol. Org.) J. 1996;15:6910–6920 . [PMC free article] [PubMed] [Google Scholar]
- Bowman E.J., Siebers A., Altendorf K. Bafilomycinsa class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA. 1988;85:7972–7976 . doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.P., Chu R., Harding C.V. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J. Immunol. 1997;158:1523–1532 . [PubMed] [Google Scholar]
- Fernandez-Borja M., Verwoerd D., Sanderson F., Aerts H., Trowsdale J., Tulp A., Neefjes J. HLA-DM and MHC class II molecules co-distribute with peptidase-containing lysosomal subcompartments. Int. Immunol. 1996;8:625–640 . doi: 10.1093/intimm/8.5.625. [DOI] [PubMed] [Google Scholar]
- Mitchell R.N., Barnes K.A., Grupp S.A., Sanchez E., Misulovin Z., Nussenzweig M.C., Abbas A.K. Intracellular targeting of antigens internalized by membrane immunoglobulin in B lymphocytes. J. Exp. Med. 1995;181:1705–1714 . doi: 10.1084/jem.181.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemasko K., Eisfelder B.J., Williamson E., Kabak S., Clark M.R. Signals from the B lymphocyte antigen receptor regulate MHC class II containing late endosomes. J. Immunol. 1998;160:5203–5208. [PubMed] [Google Scholar]