Abstract
Hemofiltrate CC chemokine (HCC)-1 is a recently described human chemokine that is constitutively expressed in numerous tissues and is present at high concentrations in normal plasma. Using a cell line expressing CC chemokine receptor (CCR)5 as a bioassay, we isolated from human hemofiltrate an HCC-1 variant lacking the first eight amino acids. HCC-1[9–74] was a potent agonist of CCR1, CCR3, and CCR5 and promoted calcium flux and chemotaxis of T lymphoblasts, monocytes, and eosinophils. It also blocked entry of HIV-1 strains using CCR5 as coreceptor. Limited tryptic digestion of HCC-1 generated the active variant. Conditioned media from several tumor cell lines activated HCC-1 with a high efficiency, and this activity could be inhibited by serine protease inhibitors. Our results indicate that HCC-1 represents a nonfunctional precursor that can be rapidly converted to the active chemokine by proteolytic processing. This process represents an additional mechanism by which tumor cells might generate chemoattractant molecules and recruit inflammatory cells. It might also affect HIV-1 replication in infected individuals and play an important role in AIDS pathogenesis.
Keywords: receptors, CCR5; chemokines, CC; biological assay; endopeptidase; HIV
Introduction
Chemokines constitute a superfamily of small (8–10 kD) secreted cytokines that mediate chemotaxis, trafficking, and activation of leukocyte populations. From a structural viewpoint, they are classified into two major subgroups, CC and CXC chemokines, according to the relative position of the first two of four conserved cysteine residues 1. From a functional viewpoint, they may be classified into inflammatory chemokines, which are mostly regulated at the transcriptional level and involved in the recruitment of leukocytes to inflammatory sites, and constitutive chemokines, which are responsible for the trafficking and homing of leukocyte populations 1 2. Chemokines mediate their activity through G protein–coupled receptors and pertussis toxin–sensitive heterotrimeric G proteins of the Gi family 3. Most chemokine receptors are promiscuous, binding and responding functionally to a variety of chemokines, and many chemokines activate several receptors 4. The constitutive or regulated expression of chemokines by various cell types and the pattern of receptor distribution on immune cell populations determine the range of activities these mediators play in leukocyte trafficking 2. Besides their role in the recruitment of leukocytes, chemokines have been shown to be involved in an increasing range of other functions, including the control of hematopoiesis 5 and angiogenesis 6. Accordingly, chemokine receptors are expressed on a variety of cell types besides leukocyte populations, including epithelial 7, smooth muscle 8, and endothelial cells 6. Chemokines and their receptors play major roles in tumorigenesis. Monocyte chemotactic protein (MCP)1-1 and other chemokines are produced by various tumors and account for the infiltration of these tumors by host inflammatory cells 9. Chemokine receptors (mainly CCR5 and CXCR4) were also shown to constitute the coreceptors for HIV 10. CCR5 is the major coreceptor for the macrophage-tropic strains of HIV 11 12 13 14 that are responsible for disease transmission. Chemokines acting on CCR5, including RANTES (regulated upon activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1α, MIP-1β, and MCP-2 were shown to inhibit HIV-1 entry 15 16. Considerable efforts are currently underway to explore the therapeutic properties of chemokines, chemokine analogues, mAbs, or chemical ligands acting on CCR5 as antiviral agents 3 .
Hemofiltrate CC chemokine (HCC)-1 is a member of the CC chemokine family, originally isolated from hemofiltrate of patients with chronic renal failure 17. HCC-1 consists of 74 amino acids, after cleavage of a 19-residue leader sequence, and shares 46% sequence identity with MIP-1α and MIP-1β. Its gene (SCYA14) is colocalized with other CC chemokine genes in a cluster located in the 17q11.2 region of the human genome 18. Two unusual features of HCC-1 are its constitutive expression in a large variety of tissues and its high concentration range, which is 1.5–10 nM in normal human plasma and 2.5–80 nM in plasma from patients with chronic renal failure 17. However, the major source for circulating HCC-1 is unknown. HCC-1 was described as a weak activator of monocytes 17, and CCR1 was later identified as responding functionally to high (and supraphysiological) concentrations of HCC-1 19.
In this report, we describe the purification from hemofiltrate of a new high-affinity CCR5 ligand and its characterization as a truncated form of HCC-1. This variant, resulting from the proteolytic processing of the precursor, was also found to be highly active on CCR1 and CCR3 to activate and recruit monocytes, T lymphoblasts, and eosinophils and to block HIV entry.
Materials and Methods
Materials.
All chemicals, including protease inhibitors, were obtained from Sigma-Aldrich unless stated. Cell culture media were from GIBCO BRL, chemokines were from R & D Systems, and radioligands were from Amersham Pharmacia Biotech. Primary cell lines were obtained from the American Type Culture Collection.
Aequorin Assays.
CHO-K1 cell lines expressing chemokine receptors Gα16 and mitochondrial apoaequorin were established. A functional assay based on the luminescence of mitochondrial aequorin after intracellular Ca2+ release 20 was performed as described 21. In brief, cells were collected from plates with PBS containing 5 mM EDTA, pelleted, and resuspended at 107 cells/ml in DMEM-F12 medium and incubated with 5 μM coelenterazine H (Molecular Probes) for 4 h at room temperature. Cells were then washed in DMEM-F12 medium and resuspended at a concentration of 2 × 106 cells/ml. Cells were then mixed with the chemokines, and the light emission was recorded over 30 s using a Microlumat™ luminometer (PerkinElmer). Results are expressed as relative light units (RLU).
Purification of HCC-1[9–74].
Peptides were extracted from 10,000 liters of human hemofiltrate derived from 40 adult patients with chronic renal disease and fractionated by strong cation exchange chromatography as previously reported 22. The resulting fractions were subjected to reverse-phase (RP) chromatography (Source RPC column; 15 μm, 10 × 12.5 cm; Amersham Pharmacia Biotech) as a second separation step (0–50% acetonitrile, 10 mM HCl, 200 ml/min). Fractions of 200 ml were collected and tested for their ability to stimulate CCR5. Active fractions were subsequently separated by four further analytical RP-HPLC steps using techniques described elsewhere 23. Mass determination of the biologically active compounds was carried out on a Sciex API III quadrupole mass spectrometer (PerkinElmer) with an electrospray interface (ESI-MS) and with a LaserTec RBT II MALDI-MS (PerSeptive Biosystems). Sequence analysis was performed on a 473 A gas-phase sequencer (Applied Biosystems) by Edman degradation with on-line detection of phenylthiohydantoin amino acids.
Synthesis of HCC-1[9–74].
HCC-1[9–74] was prepared by Fmoc solid-phase peptide synthesis as described 24. The purified product was characterized by HPLC, capillary zone electrophoresis, electrospray mass spectrometry, and sequence analysis and was used for biological testing according to the net peptide content as determined by amino acid analysis.
Radioligand Binding Assays.
Competition binding assays were performed as described 21 25 on crude membrane fractions prepared from CHO-K1 cell lines expressing CCR5 or CCR1 and a K562 cell line expressing CCR3. In brief, 1–10 μg crude membrane extracts were incubated in binding buffer (50 mM HEPES, pH 7.4, 5 mM MgCl2, 1 mM CaCl2, and 0.5% [wt/vol] protease-free BSA) containing 0.1 nM radioligand 125I–MIP-1α, 125I–RANTES, or 125I–eotaxin and competitors over 90 min at 27°C. Bound tracer was separated by filtration through GF/B filters (Millipore) presoaked in 1% BSA or 0.3% polyethylenimine. Filters were then counted by gamma scintillation counting. Results were normalized for total binding in the absence of competitor (100%) and nonspecific binding (0%) in the presence of a 100-fold excess of the competitor and were analyzed by nonlinear regression using a single-site competition model (Graph-Pad Prism™ Software).
Calcium and Chemotactic Assays.
Donor blood buffy coats for the isolation of neutrophils, monocytes, and lymphocytes were supplied by the Central Laboratory of the Hôpital Erasme (Brussels, Belgium). Eosinophils were purified from the venous blood of healthy volunteers by using a negative selection based on CD16 microbeads (Miltenyi Biotec) and further isolated according to established methods 26. Lymphoblasts were cultured as described 27, and ∼60% of the cells expressed CCR5, as determined by flow cytometry using the 2D7 mAb (R & D Systems). For intracellular calcium measurements, the cells were loaded for 30 min at room temperature with Fura-2AM (Molecular Probes). Calcium transients were monitored by a LS 50B spectrofluorimeter (PerkinElmer) as described 28. Chemotaxis was assessed in 48-well chambers using polycarbonate filter membranes with 5-μM pores (Neuroprobe Inc.) as previously described 29. The results are represented as chemotactic index.
HIV Infection Assays.
P4-CCR5 cells (National Institutes of Health AIDS Research and Reference Reagent program; reference 30) were grown in DMEM supplemented with 10% FCS and 1 μg/ml puromycin (GIBCO BRL). Cells were seeded in flat-bottomed 96-well dishes, cultured overnight, and incubated with the chemokines for 2 h before infection with virus containing 20 ng of p24 antigen in a total volume of 50 μl of medium. After overnight incubation, cells were washed twice and cultivated in fresh DMEM without chemokines. 3 d after infection, the cells were lysed, and β-galactosidase activity (Tropix) was measured. PBMCs were isolated using lymphocyte separation medium (Organon Teknika Corporation). Cells were cultured in RPMI 1640 medium with 20% FCS (GIBCO BRL) and 50 U/ml IL-2 (Chiron Corp.), and virus production was measured by a reverse transcriptase assay as described 31.
Activation of HCC-1 by Conditioned Media.
Human tumoral cell lines DU-145 (HTB-81), 143B (CRL-8303), MCF-7 (HTB-22), and MeWo were maintained in MEM supplemented with 10% FCS. For the screening of these cell lines for proteolytic activities on HCC-1, 3 × 106 cells were plated in 9-cm dishes and grown for 48 h to 70–80% confluence, at which time 1 μM HCC-1[1–74] was added to the medium. After an incubation at 37°C for 48 h, the medium was collected, clarified by centrifugation at 13,000 g, and stored at −80°C until testing on the CCR5-expressing and control CHO cells using the aequorin-based assay. Conditioned medium was prepared from DU-145 cells for testing the activity of protease inhibitors. Serum-free medium was incubated for 5 d with confluent cultures and clarified by centrifugation and 0.22-μm filtration. This conditioned medium was incubated with HCC-1[1–74] alone or in the presence of protease inhibitors for 6 h at 37°C and tested immediately on CCR5-expressing cells. Potential interference of the protease inhibitors with the aequorin assay was excluded by testing their effect on the response to 2 nM HCC-1[9–74]. Non–CCR5-specific activities of conditioned media and protease inhibitors were assayed on CHO cell lines expressing no chemokine receptors.
Results
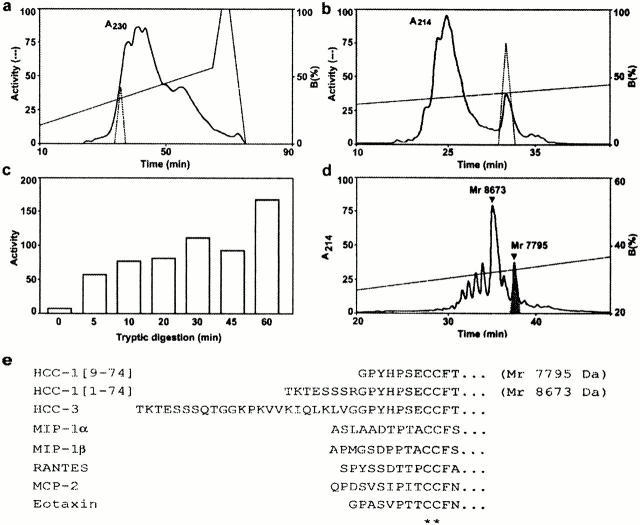
In the process of isolating natural ligands of various G protein–coupled receptors from human hemofiltrate fractions, we used, among others, a cell line expressing human CCR5. Hemofiltrate was fractionated by cation exchange and RP chromatography. A peak of biological activity specific for CCR5-expressing cells was obtained (Fig. 1 a) and was further purified by additional chromatographic steps. After the last step (Fig. 1 b), the active fraction was analyzed by mass spectrometry, revealing two major components with molecular masses of 8,673 and 7,795 daltons. Edman degradation identified the NH2-terminal sequences TKTESSSRGPYHP and GPYHPSEXXFTYT. These data corresponded respectively to full size human HCC-1 and to a variant of the same protein, HCC-1[9–74], truncated after an arginine residue (Fig. 1 e). As full size HCC-1 is inactive on CCR5 19 21, we focused on HCC-1[9–74]. We investigated whether trypsin could generate this variant by performing a limited tryptic digestion of full size HCC-1 highly purified from another hemofiltrate batch and testing the biological activity at various time points. The activity of the tryptic digest increased progressively (Fig. 1 c), and analysis of the products by RP chromatography, mass spectrometry, and sequencing identified HCC-1[9–74] (7,795 daltons) as the single active compound (Fig. 1 d). Chemical synthesis of HCC-1[9–74] further confirmed the biological activity of the truncated chemokine on a CCR5-expressing cell line. Both the purified chemokine variant and chemically synthesized HCC-1[9–74] were used for further characterization of the molecule.
Figure 1.
Purification of HCC-1[9–74] from human hemofiltrate. (a) A fraction resulting from cation exchange chromatography was applied to RP-HPLC, and the biological activity of the fractions on human CCR5 was assayed (dotted line). (b) Fifth and final purification step of the active material by RP-HPLC. The active peak (dotted line) was analyzed by mass spectrometry and Edman degradation. (c) Time course of the CCR5-stimulatory activity generated by tryptic digestion of HCC-1. (d) RP-HPLC chromatogram of trypsin-digested HCC-1 (1-h incubation), showing the generation of HCC-1[9–74] (shaded). (e) Amino acid sequence of the NH2-terminal part of HCC-1, HCC-1[9–74], HCC-3, and other chemokines active on CCR1, CCR3, or CCR5. Asterisks mark the first two conserved cysteines.
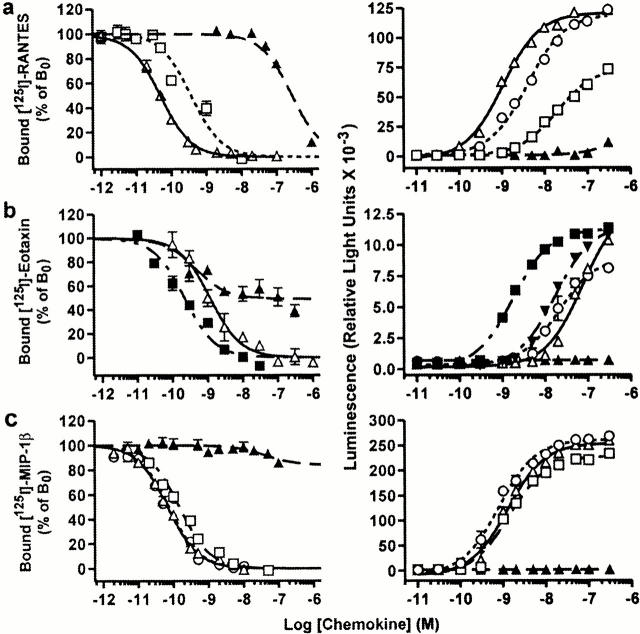
The activity of HCC-1[9–74] was tested on CCR5 and other chemokine receptors using an aequorin-based assay. The truncated chemokine appeared as a high-affinity agonist of CCR5 (EC50 4.8 ± 1.2 nM) with a potency similar to that of RANTES and MIP-1α (Fig. 2 and Table ). It was also more active than RANTES on CCR1 (EC50 2.8 ± 0.8 nM) and a reasonably good agonist for CCR3 (EC50 78 ± 14 nM), whereas it was inactive on CCR2, CCR4, CCR6, CCR7, CCR8, CCR9, CXCR1, and CX3CR1. The properties of HCC-1[9–74] were confirmed by competition radioligand binding assays on CCR1, CCR3, and CCR5. The binding parameters were consistent with the functional data (Fig. 2 and Table ). The affinity of HCC-1[9–74] for CCR5 (K i 0.04 ± 0.01 nM) was similar to that of RANTES and was, for CCR1, higher (K i 0.023 ± 0.007 nM) than that of MIP-1α. The affinity for CCR3 (K i 2.7 ± 0.8 nM) was ∼10-fold lower than that of eotaxin. Full size HCC-1 tested in parallel in functional and binding studies appeared as a fairly weak agonist of CCR1 (K i > 100 nM) and was totally inactive on CCR5, in agreement with previous reports 19 21. It was found to compete partially for eotaxin binding on CCR3, despite the absence of functional response of this receptor (Fig. 2 b).
Figure 2.
Binding and functional activity of HCC-1[9–74] on human recombinant receptors expressed in CHO-K1 cells. Competition binding assays (left panels) and functional aequorin-based assays (right panels) were performed with CCR1 (a), CCR3 (b), and CCR5 (c). HCC-1[9–74] (▵), HCC1[1–74] (▴), MIP-1α (□), RANTES (○), Eotaxin (▪), and MCP-4 (▾) were used as ligands in both assays. Binding and functional assay results are representative of at least three independent experiments. The data represent the mean and SEM for measurements performed in triplicate.
Table 1.
Binding and Functional Parameters of CCR1, CCR3, and CCR5 for Full Size HCC-1, HCC-1[9–74], and Reference Chemokines for Each Receptor
| CCR1 | CCR3 | CCR5 | ||||
|---|---|---|---|---|---|---|
| Ligand | K i ± SEM | EC50 ± SEM | K i ± SEM | EC50 ± SEM | K i ± SEM | EC50 ± SEM |
| HCC-1 | >100 | >1,000 | >1,000 | >1,000 | >1,000 | >1,000 |
| HCC-1[9–74] | 0.023 ± 0.007 | 2.8 ± 0.8 | 2.7 ± 0.8 | 78 ± 14 | 0.04 ± 0.01 | 4.8 ± 1.2 |
| RANTES | ND | 6.3 ± 1.1 | ND | 15 ± 4 | 0.05 ± 0.01 | 2.4 ± 0.5 |
| MIP-1α | 0.32 ± 0.06 | 15 ± 5 | ND | ND | 0.11 ± 0.06 | 3.3 ± 0.8 |
| MIP-1β | ND | ND | ND | ND | ND | 1.3 ± 0.5 |
| Eotaxin | ND | ND | 0.31 ± 0.03 | 2.4 ± 0.4 | ND | ND |
Values, expressed in nM, are the mean and SEM for at least three independent determinations.
The biological activity of HCC-1[9–74] was further tested on natural cell populations for its calcium mobilization and chemotactic properties. In monocytes, HCC-1[9–74] induced robust calcium fluxes, comparable to those evoked by RANTES (Fig. 3 a). 50 nM HCC-1[9–74] totally desensitized these cells to further stimulation by the same ligand (data not shown) and abrogated further response to 50 nM RANTES as well, whereas HCC-1[9–74] remained capable of mobilizing calcium at reduced levels after a first stimulation by up to 100 nM RANTES (Fig. 3 a). Prior stimulation by HCC-1[1–74] did not modify the response to HCC-1[9–74], whereas a first stimulation by MIP-1β (50 nM) resulted in a mild calcium mobilization response with no reduction of the subsequent response to HCC-1[9–74] (data not shown). Similar results were obtained with the monocytic cell line THP-1 (not shown). In chemotaxis assays with monocytes, HCC-1[9–74] was as potent (maximal migration observed at 10 nM) and efficient as RANTES. It was 100-fold more potent than full size HCC-1, which appeared as a weak but efficient chemoattractant 19. MIP-1β had weak or no chemotactic activity on monocytes, depending on the donors (Fig. 3 a). These results suggest that CCR1 mediates most of the functional response of monocytes to HCC-1[9–74] but that CCR5 and other as yet unidentified receptor(s) may also contribute to this activity.
Figure 3.
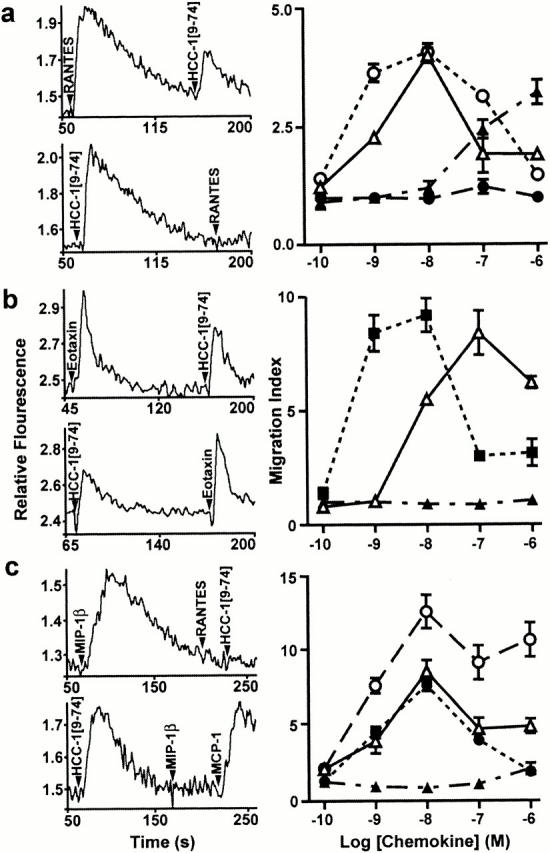
Calcium mobilization and chemotaxis induced by HCC-1[9–74] in primary cell populations. Monocytes (a), eosinophils (b), and IL-2–stimulated lymphoblasts (c) were tested for their functional response to HCC-1[9–74], full size HCC-1, and reference chemokines. Stimulation of calcium mobilization and cross-desensitization experiments (left panels) were performed with 50 nM chemokine concentrations. For chemotaxis assays (right panels), cell migration in response to HCC-1[9–74] (▵), HCC-1 (▴), RANTES (○), Eotaxin (▪), or MIP-1β (•) is reported as a migration index. Each panel is representative of at least three independent experiments. The chemotaxis data represent means and SEM for triplicate wells.
HCC-1[9–74] mobilized calcium less efficiently than eotaxin in eosinophils (Fig. 3 b). The eotaxin response was slightly reduced after prior exposure to HCC-1[9–74], whereas the activity of HCC-1[9–74] was unaltered after eotaxin stimulation (Fig. 3 b) and strongly inhibited after MIP-1α stimulation (data not shown). These results substantiate the role of HCC-1[9–74] as a weak agonist of CCR3 and support the involvement of CCR1 in the functional response of eosinophils to HCC-1[9–74]. In chemotaxis assays, HCC-1[9–74] was less potent but almost as efficacious as eotaxin on eosinophils from most donors. For one of the donors, however, HCC-1 was weakly chemotactic at high concentrations only (data not shown), which was attributed to the low CCR1 expression observed on eosinophils from some individuals 32. HCC-1[9–74] but not full size HCC-1 mobilized calcium in IL-2–conditioned lymphoblasts. Complete cross-desensitization was observed between the responses to HCC-1[9–74] and MIP-1β, indicating that CCR5 is the principal receptor used by HCC-1[9–74] in these cells. Migration of lymphoblasts was stimulated by HCC-1[9–74] and MIP-1α but not by full size HCC-1 (Fig. 3 c).
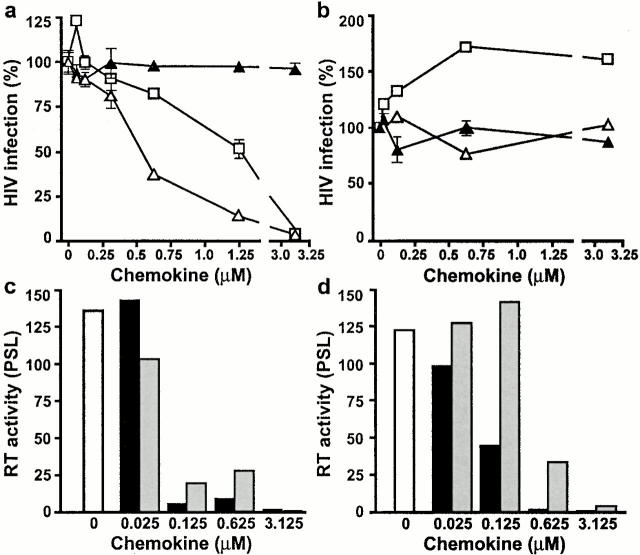
Given the role of CCR5 as the major coreceptor for macrophage-tropic strains of HIV 11 12 13 14 and the role ascribed to chemokines acting on CCR5 as entry inhibitors 15, we investigated the antiviral activity of HCC-1[9–74] in an infection inhibition assay. Both HCC-1[9–74] and RANTES inhibited infection by the macrophage-tropic strain YU2 (Fig. 4 a). RANTES reduced YU2 infection of P4-CCR5 cells by >95% at 3.2 μM and by 50% at 1.3 μM. HCC-1[9–74] was slightly more efficient, blocking YU2 infection by 50% at 0.5 μM. Similar results were obtained using the macrophage-tropic strain JR-CSF (not shown). No inhibitory effect on YU2 infection was observed with full-length HCC-1. None of the three chemokines inhibited the T-tropic strain NL4-3 (Fig. 4 b). In agreement with previously published results 33, RANTES enhanced infectivity of NL4-3 by ∼50% at concentrations over 0.6 μM. In contrast, no enhancing effect on infectivity was observed with HCC-1[9–74] (Fig. 4 b). We also tested whether HCC-1[9–74] could inhibit HIV-1 replication in human PBMCs. As shown in Fig. 4c and Fig. d, replication of the macrophage-tropic strain JR-CSF was blocked significantly by HCC-1[9–74] and RANTES at concentrations of 125 nM, whereas concentrations of 625 nM were necessary for the YU2 strain. No inhibition of NL4-3 replication was observed, and full size HCC-1 was ineffective for all strains (not shown).
Figure 4.
Inhibition of HIV-1 infection. Cells expressing both CCR5 and CXCR4 coreceptors were infected with the YU2 strain (a), which uses CCR5, and the NL4-3 strain (b), which uses CXCR4, in the presence of full size HCC-1 (▴), HCC-1[9–74] (▵), or RANTES (□). The data represent the mean and SEM for points performed in triplicate. Human PBMCs were infected with JR-CSF (c) and YU-2 (d) in the presence of RANTES (black bars) or HCC-1[9–74] (gray bars). The data represent the reverse transcriptase activity measured in culture cell supernatants harvested 10 d (JR-CSF) or 14 d (YU-2) after infection. Results are expressed as photostimulated luminescence (PSL) units.
Considering the ability of trypsin to generate HCC-1[9–74] in vitro, we hypothesized that other and potentially more specific proteases could be involved in the in vivo processing of HCC-1. As tumor cells are known to release a number of proteases active on extracellular matrix and mediators, we tested conditioned media from a set of human tumor cell lines for HCC-1 processing activities using the CCR5-expressing cell line as a bioassay. The prostate carcinoma cell line DU-145 34, the osteosarcoma cell line 143B, the mammary adenocarcinoma cell line MCF-7 35, and the melanoma cell line MeWo 36 were used as sources of proteases potentially involved in the activation of HCC-1. Incubation of 1 μM HCC-1 in the medium of monolayer cell cultures generated a clear CCR5-stimulatory activity for the cell lines DU-145 and 143B (Fig. 5 a). Weak activities were recovered from MCF-7 and MeWo supernatants. A CCR5-stimulatory activity was also obtained when HCC-1[1–74] was incubated with acellular conditioned medium from DU-145 cells (data not shown). Preliminary time course experiments indicated that maximal activity was reached after 10 h of incubation in these conditions, after which a plateau was maintained for over 24 h (data not shown). Based on this maximal activity, it was estimated that up to 10% of HCC-1 was converted into a CCR5-activating form by proteolysis during the incubation. We next tested whether inhibitors of various protease families could inhibit what was expected to represent the proteolytic processing of inactive HCC-1. HCC-1[1–74] (200 nM) was coincubated with protease inhibitors in serum-free conditioned medium from the DU-145 cell line (Fig. 5 b). AEBSF (4-[2-aminoethyl]-benzylsulfonyl fluoride), an inhibitor of serine proteases, and leupeptin, an inhibitor of serine and cysteine proteases, reduced the activation of HCC-1 by ∼75% to near control levels. Other serine protease inhibitors, such as aprotinin and α2-macroglobulin, the soybean trypsin inhibitor, an inhibitor of cysteine proteases (E-64), as well as inhibitors of metalloproteases (EDTA, 1,10-phenanthroline) and of aspartic proteases (pepstatin) had weak (<10% of inhibition) or no effects on the generation of CCR5-stimulatory activity.
Figure 5.
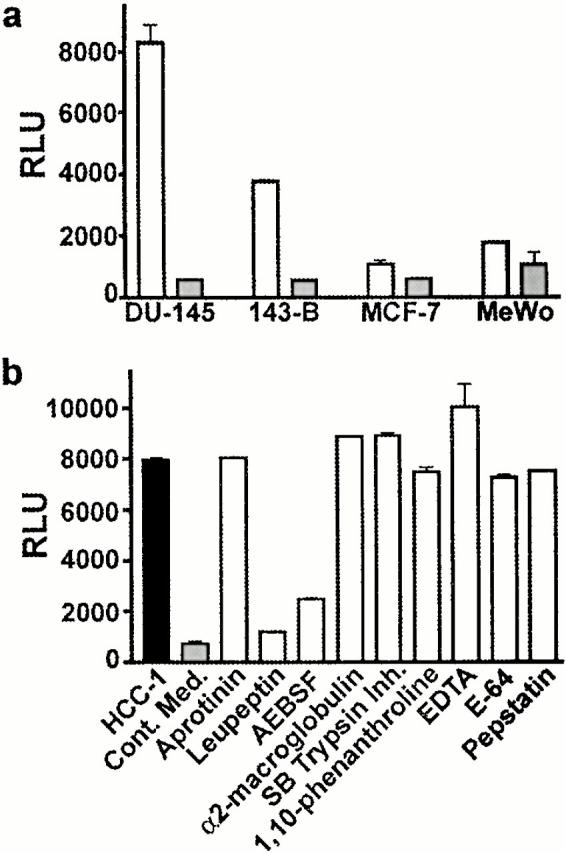
Generation of CCR5-stimulatory activities from HCC-1 [1–74] by conditioned media from human tumor cell lines. CHO-K1 cells coexpressing CCR5 and apoaequorin were used to test the biological activity generated from HCC-1[1–74] in conditioned media. (a) The medium from monolayer cultures of the cell lines DU-145, 143B, MCF-7, and MeWo, incubated without (gray bars) or after addition of 1 μM HCC-1[1–74] (white bars), was tested for CCR5-stimulatory activities. (b) Conditioned medium from the DU-145 cell line (gray bar) was incubated with 200 nM HCC-1[1–74] alone (black bar) or in the presence of various protease inhibitors: aprotinin (0.3 μM), leupeptin (1 μM), AEBSF (4 mM), soybean trypsin inhibitor (5 μM), α2-macroglobulin (20 nM), 1,10-phenanthroline (10 μM), EDTA (1 mM), pepstatin (1 μM), and E-64 (N-[N-[l-3-trans-carboxirane-2-carbonyl]-l-leucyl]-agmatine, 2.5 μM). The results in RLU represent the mean and SEM of triplicate data points and are representative of two independent experiments. Cont. Med., conditioned medium without added HCC-1[1–74].
Discussion
We have shown in this work that a truncated variant of HCC-1, HCC-1[9–74], acts as a high-affinity agonist on CCR1, CCR5, and, to a lesser extent, CCR3. Using calcium mobilization and chemotaxis assays, we have shown that HCC-1[9–74] is active on monocytes, and our data suggest that CCR1 is the main receptor involved in the functional response of these cells, in accordance with the weak expression of CCR5 found in freshly prepared monocytes 11. CCR1 was also identified as mediating the effects of HCC-1[9–74] on eosinophils, whereas the functional response of T lymphoblasts was attributed to CCR5, in agreement with the high expression of this receptor on these cells 37 38. As expected for a potent CCR5 agonist, HCC-1[9–74] acted as an efficient blocker of HIV entry, with a potency similar to that of RANTES, the most efficient natural chemokine so far. Full size HCC-1, produced constitutively by numerous tissues and present in normal human plasma, was inactive in all assays, with the exception of its modest activity on CCR1.
We therefore propose that HCC-1 represents an inactive precursor that can be processed by proteases to an active chemokine, able to recruit monocytes/macrophages, T lymphocytes, and eosinophils through CCR1, CCR3, and CCR5 and possibly other receptors that remain to be characterized. Limited proteolysis by trypsin in vitro was able to generate the truncated chemokine, which was also extracted from a natural source, human hemofiltrate. Furthermore, conditioned media from the prostatic adenocarcinoma cell line DU-145 and the osteosarcoma cell line 143-B were shown to contain an activity able to process HCC-1 into a form that could activate CCR5. The inhibition profile obtained with the DU-145 cell line suggests that this activity is mediated by a serine protease. We speculate that the result of this processing is the generation of HCC-1[9–74], which is consistent with the cleavage downstream of an arginine residue. Future work will aim at demonstrating this fact. We will also investigate whether the release of HCC-1–activating proteases is shared by a broader range of human tumoral cell lines. The precise nature of the protease(s) responsible for the cleavage in conditioned media and in vivo remains to be determined, but various trypsin-like serine proteases, many of which are known to be activated in the course of inflammatory or tumoral processes, could be involved.
Thus, HCC-1[9–74] represents the naturally processed, biologically active form of the human chemokine HCC-1. HCC-3, a splice variant of HCC-1 with a different NH2 terminus (Fig. 1 e; reference 17), might also generate HCC-1[9–74] by proteolytic cleavage, although the arginine present at the cleavage site of HCC-1 is not conserved in HCC-3. From the relative recovery of HCC-1[1–74] and HCC-1[9–74] during the purification procedure from human hemofiltrate, we estimate that the concentration of HCC-1[9–74] in hemofiltrate (and presumably in plasma) might be 100–1,000-fold lower than that of HCC-1[1–74]. Local concentrations of HCC-1[9–74] might, however, be significantly higher at specific sites or under specific pathophysiological situations, depending on the expression and/or activation of the protease(s) responsible for the processing of the abundant HCC-1[1–74].
Proteolytic processing has been described for other chemokines. Neutrophil-activating peptide 2, a neutrophil chemoattractant, is processed from two platelet-specific precursors by the action of cathepsin G 39 40. Dipeptidyl peptidase IV (CD26) removes the NH2-terminal dipeptide from various chemokines (RANTES, stromal cell–derived factor 1, macrophage-derived chemokine, eotaxin), usually resulting in a reduction of their biological activities 25 41 42. The processing of HCC-1 is unique, however, as HCC-1 is widely expressed in tissues and abundant in plasma, and its proteolytic processing transforms an almost inactive precursor to a wide-range chemoattractant. This mechanism therefore represents an alternative to transcriptional control for the generation of inflammatory chemokines. It also appears to couple leukocyte recruitment to the proteolytic pathways activated in tissue remodeling and tumors 6.
When tested in parallel under highly stringent conditions, using high doses of virus and fully activated PBMCs, HCC-1[9–74] and the most potent natural HIV-1 inhibitor known thus far, RANTES, showed comparable inhibitory activities. As the concentration of HCC-1 in normal human plasma is ∼10-fold higher than that of RANTES 43, the conversion of this precursor into the active chemokine HCC-1[9–74] might affect HIV-1 replication in infected individuals and play an important role in AIDS pathogenesis. Modifications of HCC-1, such as additional truncations or NH2-terminal modifications, similar to what has been performed for RANTES, might generate more active agonists or antagonists with enhanced interest as anti-HIV molecules 44 45. Delayed progression to AIDS has been correlated to high levels of chemokines acting at CCR5 46. Nevertheless, chemokines and analogues have strong limitations as therapeutic agents given the size of the molecules, their production costs, and the need for repeated parenteral delivery. However, given the abundance of full size HCC-1 in normal human plasma 17, it may be considered to induce the conversion of this precursor into the active chemokine HCC-1[9–74], thus generating in vivo an efficient blocker of HIV entry.
Acknowledgments
We thank U. Block, G. Cassano, F. Naveau, U. Schrameck, and I. Uhrlandt for expert technical assistance, Dr. B. Renneboog for providing lymphoblasts, and Dr. E. Klüver for providing synthetic peptides.
This work was supported by the Actions de Recherche Concertées of the Communauté Française de Belgique, the French Agence Nationale de Recherche sur le SIDA, the Belgian programme on Interuniversity Poles of attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, the BIOMED and BIOTECH programmes of the European Community, the Fonds de la Recherche Scientifique Médicale of Belgium, Télévie and the Fondation Médicale Reine Elisabeth (to M. Parmentier), and by the German government (to W.G. Forssmann). Scientific responsibility is assumed by the authors. J. Vakili is supported by a Télévie grant.
Footnotes
Abbreviations used in this paper: HCC, hemofiltrate CC chemokine; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T cell expressed and secreted.
M. Detheux, L. Ständker, and J. Vakili contributed equally to this work.
References
- Baggiolini M., Dewald B., Moser B. Human chemokinesan update. Annu. Rev. Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Ward S.G., Bacon K., Westwick J. Chemokines and T lymphocytesmore than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- Murphy P.M., Baggiolini M., Charo I.F., Hebert C.A., Horuk R., Matsushima K., Miller L.H., Oppenheim J.J., Power C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Mantovani A. The chemokine systemredundancy for robust outputs. Immunol. Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Graham G.J., Wright E.G., Hewick R., Wolpe S.D., Wilkie N.M., Donaldson D., Lorimore S., Pragnell I.B. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- Strieter R.M., Polverini P.J., Arenberg D.A., Walz A., Opdenakker G., Van Damme J., Kunkel S.L. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J. Leukoc. Biol. 1995;57:57752–57762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- Agace W.W., Roberts A.I., Wu L., Greineder C., Ebert E.C., Parker C.M. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur. J. Immunol. 2000;30:819–826. doi: 10.1002/1521-4141(200003)30:3<819::AID-IMMU819>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schecter A.D., Calderon T.M., Berman A.B., McManus C.M., Fallon J.T., Rossikhina M., Zhao W., Christ G., Berman J.W., Taubman M.B. Human vascular smooth muscle cells possess functional CCR5. J. Biol. Chem. 2000;275:5466–5471. doi: 10.1074/jbc.275.8.5466. [DOI] [PubMed] [Google Scholar]
- Wang J.M., Deng X., Gong W., Su S. Chemokines and their role in tumor growth and metastasis. J. Immunol. Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- Berger E.A., Murphy P.M., Farber J.M. Chemokine receptors as HIV-1 coreceptorsroles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Alkhatib G., Combadiere C., Broder C.C., Feng Y., Kennedy P.E., Murphy P.M., Berger E.A. CC CKR5a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Doranz B.J., Rucker J., Yi Y., Smyth R.J., Samson M., Peiper S.C., Parmentier M., Collman R.G., Doms R.W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- Dragic T., Litwin V., Allaway G.P., Martin S.R., Huang Y., Nagashima K.A., Cayanan C., Maddon P.J., Koup R.A., Moore J.P. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P.D., Wu L., Mackay C.R., LaRosa G., Newman W. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Grego G., Mackewics C., Levy J.A. Sensitivity of human immunodeficiency virus infection to various alpha, beta and gamma chemokines. J. Gen. Virol. 1999;80:2369–2373. doi: 10.1099/0022-1317-80-9-2369. [DOI] [PubMed] [Google Scholar]
- Schulz-Knappe P., Magert H.J., Dewald B., Meyer M., Cetin Y., Kubbies M., Tomeczkowski J., Kirchhoff K., Raida M., Adermann K. HCC-1, a novel chemokine from human plasma. J. Exp. Med. 1996;183:295–299. doi: 10.1084/jem.183.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maho A., Carter A., Bensimon A., Vassart G., Parmentier M. Physical mapping of the CC-chemokine gene cluster on the human 17q11. 2 region. Genomics. 1999;59:213–223. doi: 10.1006/geno.1999.5850. [DOI] [PubMed] [Google Scholar]
- Tsou C.L., Gladue R.P., Carroll L.A., Paradis T., Boyd J.G., Nelson R.T., Neote K., Charo I.F. Identification of C-C chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C chemokine (HCC)-1 receptor. J. Exp. Med. 1998;188:603–608. doi: 10.1084/jem.188.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables J., Green A., Marshall F., Fraser N., Knight E., Sautel M., Milligan G., Lee M., Rees S. A bioluminescent assay for agonist activity at potentially any G-protein-coupled receptor. Anal. Biochem. 1997;252:115–126. doi: 10.1006/abio.1997.2308. [DOI] [PubMed] [Google Scholar]
- Blanpain C., I. Migeotte B., Lee J., Vakili B.J., Doranz C., Govaerts G., Vassart R.W., Doms, Parmentier M. CCR5 binds multiple CC-chemokinesMCP-3 acts as a natural antagonist. Blood. 1999;94:1899–1905. [PubMed] [Google Scholar]
- Schulz-Knappe P., Schrader M., Standker L., Richter R., Hess R., Jurgens M., Forssmann W.G. Peptide bank generated by large-scale preparation of circulating human peptides. J. Chromatogr. A. 1997;776:125–132. doi: 10.1016/s0021-9673(97)00152-0. [DOI] [PubMed] [Google Scholar]
- Mark S., Forssmann W.G., Ständker L. Strategy for identifying circulating fragments of insulin-like growth factor binding proteins in a hemofiltrate peptide bank. J. Chromatogr. A. 1999;852:197–205. doi: 10.1016/s0021-9673(99)00356-8. [DOI] [PubMed] [Google Scholar]
- Escher S.E., Sticht H., Forssmann W.G., Rösch P., Adermann K. Synthesis and characterization of the human CC chemokine HCC-2. J. Peptide Res. 1999;54:505–513. doi: 10.1034/j.1399-3011.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- Struyf S., Proost P., Schols D., De Clercq E., Opdenakker G., Lenaerts J.P., Detheux M., Parmentier M., De Meester I., Scharpe S. CD26/dipeptidyl-peptidase IV down-regulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor 3. J. Immunol. 1999;162:4903–4909. [PubMed] [Google Scholar]
- Forssmann U., Uguccioni M., Loetscher P., Dahinden C.A., Langen H., Thelen M., Baggiolini M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 1997;185:2171–2176. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D., Rabin R.L., Arthos J., Rubbert A., Dybul M., Swofford R., Venkatesan S., Farber J.M., Fauci A.S. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Pardigol A., Forssmann U., Zucht H.D., Loetscher P., Schulz-Knappe P., Baggiolini M., Forssmann W.G., Magert H.J. HCC-2, a human chemokinegene structure, expression pattern, and biological activity. Proc. Natl. Acad. Sci. USA. 1998;95:6308–6313. doi: 10.1073/pnas.95.11.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charneau P., Mirambeau G., Roux P., Paulous S., Buc H., Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- Potts B.J. Mini reverse transcriptase RT assays. In: Aldovini A., Walker B.D., editors. Techniques in HIV Research. Stockton; New York: 1987. pp. 103–106. [Google Scholar]
- Sabroe I., Hartnell A., Jopling L.A., Bel S., Ponath P.D., Pease J.E., Collins P.D., Williams T.J. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 1999;162:2946–2955. [PubMed] [Google Scholar]
- Kinter A., Catanzaro A., Monaco J., Ruiz M., Justement J., Moir S., Arthos J., Oliva A., Ehler L., Mizell S. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4(+) T cellsrole of signal transduction. Proc. Natl. Acad. Sci. USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festuccia C., Dolo V., Guerra F., Violini S., Muzi P., Pavan A., Bologna M. Plasminogen activator system modulates invasive capacity and proliferation in prostatic tumor cells. Clin. Exp. Metastasis. 1998;16:513–528. doi: 10.1023/a:1006590217724. [DOI] [PubMed] [Google Scholar]
- Emonard H.P., Remacle A.G., Noel A.C., Grimaud J.A., Stetler-Stevenson W.G., Foidart J.M. Tumor cell surface-associated binding site for the M(r) 72,000 type IV collagenase. Cancer Res. 1992;52:5845–5848. [PubMed] [Google Scholar]
- Meissauer A., Kramer M.D., Hofmann M., Erkell L.J., Jacob E., Schirrmacher V., Brunner G. Urokinase-type and tissue-type plasminogen activators are essential for in vitro invasion of human melanoma cells. Exp. Cell. Res. 1991;192:453–459. doi: 10.1016/0014-4827(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Bleul C.C., Wu L., Hoxie J.A., Springer T.A., Mackay C.R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P., Uguccioni M., Bordoli L., Baggiolini M., Moser B., Chizzolini B., Dayer J.M. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- Car B.D., Baggiolini M., Walz A. Formation of neutrophil-activating peptide 2 from platelet-derived connective-tissue-activating peptide III by different tissue proteinases. Biochem. J. 1991;275:581–584. doi: 10.1042/bj2750581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.B., Stevens M.D., Miller E.J., Atkinson M.A., Mullenbach G. Generation of the neutrophil-activating peptide-2 by cathepsin G and cathepsin G-treated human platelets. Am. J. Physiol. 1992;263:L249–256. doi: 10.1152/ajplung.1992.263.2.L249. [DOI] [PubMed] [Google Scholar]
- Oravecz T., Pall M., Roderiquez G., Gorrell M.D., Ditto M., Nguyen N.Y., Boykins R., Unsworth E., Norcross M.A. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J. Exp. Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester I., Korom S., Van Damme J., Scharpe S. CD26, let it cut or cut it down. Immunol. Today. 1999;8:367–375. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- Malnati M.S., Tambussi G., Clerici E., Polo S., Algeri M., Nardese V., Furci L., Lazzarin A., Lusso P. Increased plasma levels of the C-C chemokine RANTES in patients with primary HIV-1 infection. J. Biol. Regul. Homeost. Agents. 1997;11:40–42. [PubMed] [Google Scholar]
- Mosier D.E., Picchio G.R., Gulizia R.J., Sabbe R., Poignard P., Picard L., Offord R.E., Thompson D.A., Wilken J. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 1999;73:3544–3550. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M., Luckow B., Nelson P.J., Cihak J., Simmons G., Clapham P.R., Signoret N., Marsh M., Stangassinger M., Borlat F. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recyclinga novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagury D., Lachgar A., Chams V., Fall L.S., Bernard J., Zagury J.F., Bizzini B., Gringeri A., Santagostino E., Rappaport J. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc. Natl. Acad. Sci. USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]