Abstract
Initial biologic events that underlie sexual transmission of HIV-1 are poorly understood. To model these events, we exposed human immature Langerhans cells (LCs) within epithelial tissue explants to two primary and two laboratory-adapted HIV-1 isolates. We detected HIV-1Ba-L infection in single LCs that spontaneously emigrated from explants by flow cytometry (median of infected LCs = 0.52%, range = 0.08–4.77%). HIV-1–infected LCs downregulated surface CD4 and CD83, whereas MHC class II, CD80, and CD86 were unchanged. For all HIV-1 strains tested, emigrated LCs were critical in establishing high levels of infection (0.1–1 μg HIV-1 p24 per milliliter) in cocultured autologous or allogeneic T cells. HIV-1Ba-L (an R5 HIV-1 strain) more efficiently infected LC–T cell cocultures when compared with HIV-1IIIB (an X4 HIV-1 strain). Interestingly, pretreatment of explants with either aminooxypentane-RANTES (regulated upon activation, normal T cell expressed and secreted) or cellulose acetate phthalate (potential microbicides) blocked HIV-1 infection of LCs and subsequent T cell infection in a dose-dependent manner. In summary, we document HIV-1 infection in single LCs after exposure to virus within epithelial tissue, demonstrate that relatively low numbers of these cells are capable of inducing high levels of infection in cocultured T cells, and provide a useful explant model for testing of agents designed to block sexual transmission of HIV-1.
Keywords: AIDS, dendritic cells, cellulose acetate phthalate, aminooxypentane-RANTES, transmission
Introduction
Unprotected sexual intercourse is the most common mode of HIV-1 transmission worldwide 1 2 3. Many biologic factors have been implicated in influencing rate of transmission from individual exposures, including virus strain and inoculum in semen or vaginal fluid, concomitant ulcerative anogenital infection, traumatic intercourse, menstrual cycle, and use of oral contraceptives 4 5 6 7. In addition, genetic studies have shown that individuals with homozygous deletions of their CCR5 alleles, the major HIV-1 coreceptor used by macrophage-tropic strains of virus (R5 viruses), are relatively protected from initial HIV-1 infection despite numerous exposures 8 9 10 11. This fact points to the importance of CCR5 as a critical cofactor involved in sexual transmission of HIV-1 and also correlates with the finding that >90% of HIV-1 strains isolated from patients soon after primary infection are R5 viruses 12 13. Given this finding, potent inhibitors of CCR5, such as aminooxypentane (AOP)1-RANTES (regulated upon activation, normal T cell expressed and secreted) 14, may eventually prove useful in blocking sexual transmission of HIV-1.
Condom use and behavioral interventions have only been partially successful in slowing the spread of HIV-1 infection. Thus, there is an urgent need for additional interventions to prevent new HIV-1 infections, including the development of female-controlled topical formulations of anti–HIV-1 compounds that could be used as a suppository, cream, or gel before sexual intercourse 15 16 17 18 19. Ideally, these topical microbicides should be inexpensive, easy to use, stable under low pH conditions, colorless, tasteless, and nonirritating to genital mucosal tissues and inactivate a variety of sexually transmitted microbes within genital secretions and/or block relevant HIV-1 receptors on initial target cells. To date, the spermicide nonoxynol-9 is the only compound that has been clinically tested for its ability to block sexual transmission of HIV-1, but results from this study were disappointing 20. One promising compound currently under development, cellulose acetate phthalate (CAP) (New York Blood Center, New York, NY), is a component of enteric film coating for tablets and capsules and has been shown to inactivate HIV-1 and other sexually transmitted agents in vitro 21 22, inhibit herpes simplex type 2 infection in vivo in mice 23, and block vaginal transmission of SIV in rhesus monkeys 24.
To further understand the pathophysiology of initial HIV-1 entry and to preclinically test potential topical microbicides, appropriate models of sexual HIV-1 transmission are needed. Our previous work in this area has focused on cell culture models that include Langerhans cells (LCs), members of the dendritic cell family, because they exhibit the following characteristics: (a) location within mucosal epithelium at sites of HIV-1 exposure; (b) expression of CD4, CCR5, and CXCR4 capable of supporting HIV-1 infection; (c) ability to emigrate to paracortical T cell–rich areas of regional lymph nodes after contact with virus or other antigens; and (d) ability to efficiently transmit HIV-1 to cocultured CD4+ T cells during the process of antigen-specific activation 25 26 27 28 29. No other potential initial target cell type for HIV-1, including T cells, macrophages, and epithelial cells, possesses all of these salient features. In this report, LCs within epithelial tissue explants were infected with R5, X4, and dual-tropic HIV-1 isolates, and we tested the ability of two potential microbicides, AOP-RANTES and CAP, for their ability to block infection. The strength of this system relies on the use of human epithelium–derived LCs in a relatively “native” state, in that trypsinization, exogenous cytokine stimulation, and culture (all capable of altering coreceptor expression and maturation state) were avoided before HIV-1 infection.
Materials and Methods
Preparation of Epithelial Tissue Explants and CD4+ T Cells.
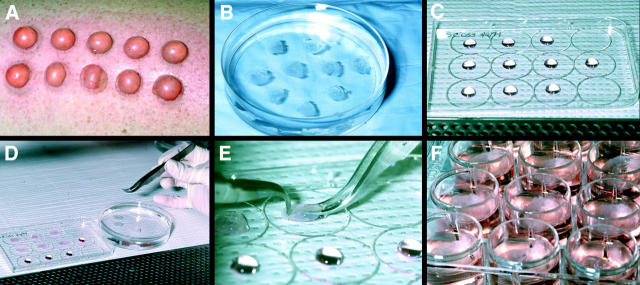
The Institutional Review Board of the National Cancer Institute approved the suction blistering procedure 30, and informed consent was obtained from all healthy volunteers. In brief, normally appearing skin of the anterior thighs was shaved and cleaned with a povidone-iodine solution followed by 70% ethanol. 20 blisters (1 cm in diameter) were then induced by vacuum suction and heat over a 2-h period (see Fig. 1 A). Blister roofs (i.e., epidermal sheets) were removed with scissors, washed in three consecutive plastic dishes containing sterile PBS (Biofluids, Inc.) (see Fig. 1 B), and then used for HIV-1 infection assays. To show the LC-specific nature of HIV-1 transmission to T cells, in some experiments single epidermal cell suspensions were made from explants by limited trypsinization immediately after HIV-1 exposure, and half of the suspensions were depleted of LCs by immunomagnetic bead selection just before CD4+ T cell coculture 31.
Figure 1.
Experimental set-up for infecting LCs within epithelial tissue explants and for evaluating potential microbicides. (A) Suction blisters. (B) Blister roofs, i.e., epithelial tissue explants, floating in PBS after surgical removal. (C) 50-μl droplets containing HIV-1 before LC infection (droplets containing drugs in the microbicide experiments appeared identical). (D and E) Epithelial tissue explants being draped over HIV-1 droplets. (F) Epithelial tissue explants floating in complete medium after HIV-1 infection. The pictures shown are representative of a typical experiment.
PBMCs were isolated by density gradient centrifugation from either heparinized blood of volunteers undergoing suction blistering or from buffy coats of HIV-1–uninfected blood donors. CD4+ T cell enrichment was performed by negative selection using a commercially prepared mAb cocktail/complement reagent (Lympho-Kwik™; One Lambda Inc.) according to manufacturer's guidelines.
Viral Strains and Infection Assays.
Purified, pelleted, and titered HIV-1Ba-L (R5) (stock at 1.8 × 1010 virus particles per milliliter) and HIV-1IIIB (X4) (stock at 6.7 × 1010 virus particles per milliliter) were purchased from Advanced Biotechnologies Inc. The HIV-1 primary isolates HIV-1CJI.3 (R5) and HIV-1CI-8 (R5X4) were gifts of David I. Cohen (Queens College, Flushing, NY). Predominant coreceptor usage for each strain was determined using a method as previously described by others 32. Low passage stocks of these HIV-1 primary isolates were generated from supernatants of phytohemagglutinin- (PHA; Difco Laboratories) and IL-2– (Boehringer Mannheim) stimulated PBMCs. In brief, PBMCs were cultured at 2 × 106 cells/ml for 3 d at 37°C in a humidified 5% CO2 environment in upright flasks containing RPMI 1640 medium GIBCO BRL), 20% heat-inactivated FBS (GIBCO BRL), 100 U/ml penicillin (GIBCO BRL), 100 μg/ml streptomycin (GIBCO BRL), and 2 mM l-glutamine (GIBCO BRL) (complete medium) supplemented with PHA (5 μg/ml). PHA-stimulated PBMCs were then centrifuged in 15- or 50-ml conical tubes, and cell pellets were resuspended with low passage cell-free virus (0.1–1 ml of stock per 107 cells) and incubated for 1 h at 37°C. After infection, cells were resuspended at 106 cells/ml in complete medium supplemented with recombinant human IL-2 (20 U/ml) and incubated as above for an additional 3–10 d (depending on the strain). At the appropriate time, cultures were centrifuged and supernatants were filtered, aliquoted, and frozen at −70°C. Before use, HIV-1 p24 content of supernatant stocks, as well as purified laboratory-adapted viral stocks, was assessed by ELISA (Coulter Corp.) according to the manufacturer's instructions.
For infection of epithelial tissue explants, 50-μl droplets containing HIV-1 at various dilutions were placed on the inside surfaces of sterile plastic culture dish covers (see Fig. 1 C). In all experiments with primary HIV-1 isolates, explants were exposed to stock supernatants containing 50–200 ng of HIV-1 p24 protein. Explants were either draped over droplets with the basal epithelial cell surface or the stratum corneum surface facing downward (see Fig. 1D and Fig. E). Virus and explants were incubated together in this manner at 37°C in a humidified 5% CO2 environment for 2 h. Explants were washed in three separate wells in 6-well plates containing sterile PBS and then floated with the basal epithelial cell sides down in 12-well plates containing 2 ml of complete medium (as above, except that FBS was replaced with 20% human AB serum (Sigma-Aldrich), without exogenous stimulants or cytokines (see Fig. 1 F). For some experiments, floating explants were cocultured with 2 × 106 resting autologous or allogeneic CD4+ T cells (106 cells/ml); for these experiments, explants were removed from cultures after 2 d and discarded. To show that HIV-1 p24+ LCs had true intracellular HIV-1 infection (as opposed to positive staining due to extracellular bound virus), in some experiments “crawl-out” LCs collected from media 2 d after HIV-1 exposure of explants were collected, and half of the cells were exposed to 0.1% trypsin for 10 min at 37°C to remove extracellular bound virus. Trypsin-treated and untreated LCs were then cocultured with CD4+ T cells.
Assessment of LC and LC–T Cell Infection by HIV-1.
HIV-1 infections in LC and LC–T cell populations were assessed by flow cytometry for intracellular HIV-1 p24 protein, ELISA for HIV-1 p24 protein levels in culture supernatants, and transmission electron microscopy for visualization of virions. To determine the sensitivity of the flow cytometric analyses, we mixed varying numbers of HIV-1–infected H9 cells (a gift from R. Yarchoan, NCI, Bethesda, MD) with uninfected H9 cells, stained these cell mixtures for intracellular HIV-1 p24 (see below), and determined the percentage of infected cells by flow cytometry. For the explant experiments, LCs that had emigrated from tissue into media 2–4 d after HIV-1 exposure of explants were collected, passed through a 70-μm mesh, washed three times in 2% human AB serum/PBS (staining buffer), preincubated in staining buffer for 10 min at room temperature to block nonspecific staining, and then incubated with 10 μg/ml PE-conjugated mouse anti–human mAbs for 30 min at 4°C. Specifically, we used anti-MHC class I and II, CD80, CD86, CD4, CD83, or isotype control mAbs to label surface antigens on LCs (all from PharMingen). Cells were then washed three times in staining buffer, incubated with Dead Red (Molecular Probes, Inc.) for 20 min at room temperature to label dead cells, washed three times in staining buffer, and fixed and permeabilized with Cytofix/Cytoperm reagents (PharMingen) for 20 min at 4°C. Cells were then washed three times in PermWash (PharMingen), incubated with 10 μg/ml unconjugated rat anti–HIV-1 p24 IgG (Biosource International) diluted in Perm/Wash for 30 min at 4°C, washed three times in PermWash, and finally incubated with FITC-conjugated goat anti–rat F(ab′)2 (Biosource International) for 30 min at 4°C. Cells were then washed and examined by flow cytometry using a FACScan® (Becton Dickinson) equipped with Lysis™ II software (Becton Dickinson). Dead cells, i.e., Dead Red–positive cells, were excluded from all analyses.
For detection of secreted HIV-1 p24 protein, supernatants were harvested every other day from cocultures of emigrated LCs and CD4+ T cells, inactivated with 2% (vol/vol) Triton X-100 (Sigma-Aldrich) in PBS and frozen at −20°C. After collecting supernatants for 2–3 wk, samples were thawed and examined for HIV-1 p24 protein content by ELISA.
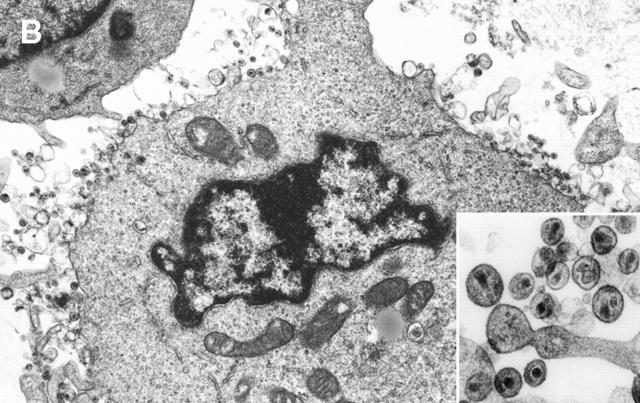
To specifically identify individually infected cells by transmission electron microscopy, LCs migrating from explants in the absence of cocultured T cells were collected 2–3 d after infection of explants. In addition, LCs and T cells were collected 4 and 7 d after infection of explants. Harvested cells were fixed overnight in neutral buffered 2.5% (vol/vol) glutaraldehyde, postfixed in 1% OsO4, dehydrated in graded ethanol and propylene oxide, and embedded in Spurr's epoxy. Thin sections were stained with uranyl acetate and lead citrate and examined for the presence and location of budding and mature HIV-1 virions using a Zeiss EM10 electron microscope at 60 kV.
Evaluation of Potential Microbicides.
AOP-RANTES and CAP were evaluated for their ability to block HIV-1 infection of LCs within explants at doses of 1, 10, and 100 nM (for AOP-RANTES) and 0.5, 1, 5, and 10 mg/ml (for CAP). AOP-RANTES was dissolved in PBS, whereas CAP was dissolved in 0.3 M sodium acetate, pH 5.5. Hydroxypropylmethylcellulose dissolved in 0.3 M sodium acetate and 0.3 M sodium acetate alone were used as controls for CAP because they demonstrated similar viscosity and pH to CAP/sodium acetate, respectively. Notably, a 140 mg/ml gel formulation of CAP has been tested as a microbicide in SIV-macaque studies monkeys 24. 50-μl droplets containing drug at various concentrations were placed on the inside surface of sterile plastic culture dish covers (see Fig. 1 C), and explants were draped over droplets with the basal epithelial cell layer down (see Fig. 1D and Fig. E) for 20 min at 37°C; 50 μl of virus at various dilutions was then added to drug under the explants and incubated for an additional 2 h at 37°C. Drug and virus were then washed from explants in three separate dishes containing sterile PBS and floated in complete medium with the basal epithelial cell layer down (see Fig. 1 F), with or without underlying CD4+ T cells. Notably, no exogenous drugs or virus were added during the subsequent coculture of HIV-1–exposed explants and CD4+ T cells.
Results
HIV-1 Infects LCs within Epithelial Tissue Explants.
It is known that LCs within peripheral epithelial tissues are in a relatively immature or resting state 25 26. To mimic conditions present after mucosal exposure to HIV-1, we obtained epithelial tissue containing immature LCs by suction blistering of healthy human skin. This procedure splits skin through the lamina lucida, leaving the blister roof (i.e., the epidermis) histologically intact and devoid of subepithelial cells, including T cells (data not shown). Epithelial tissue explants were cocultured with HIV-1, washed three times in PBS to remove excess virus, and floated in complete medium with the basal epithelial sides of the tissues facing downward (Fig. 1). LCs spontaneously emigrated from epithelial tissues into surrounding media over the following 1–4 d. From one epithelial tissue explant 1 cm in diameter, ∼2 × 104 LCs could be collected from media after floating for 3 or 4 d. There were more keratinocytes in cell populations collected at days 3 and 4 compared with cell populations collected on days 1 and 2.
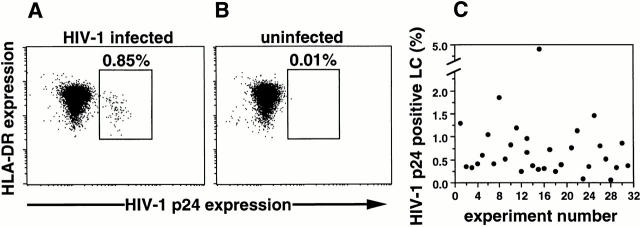
Using mixed populations containing known amounts of HIV-1–infected and uninfected H9 cells, we determined the sensitivity to detect HIV-1–infected cells by flow cytometry to be 0.01% (data not shown). Using this assay, we were able to readily and reproducibly document HIV-1 infection in single LCs (Fig. 2). Specifically, HIV-1Ba-L infection was detected in “crawl-out” LCs collected 3 d after HIV-1 exposure of explants in >30 experiments (median percentage HIV-1 p24+ LCs = 0.52%, 25% quartile = 0.33%, 75% quartile = 0.87, range = 0.08–4.77%, n = 31; Fig. 2). Isotype control Ab staining of HIV-1–infected LCs was always negative, whereas uninfected LCs stained with anti–HIV-1 p24 mAb showed occasional low positive staining (median = 0.01%, 25% quartile = 0%, 75% quartile = 0.13%, range = 0–0.23%, n = 31), confirming the specificity of the HIV-1 p24 staining (Fig. 2). For each FACS® experiment, this latter background staining was always subtracted from the specific staining values to generate the “true” numbers of HIV-1 p24+ LCs shown in Fig. 2 C. No other cell types present within crawl-out cell preparations (mostly keratinocytes) showed p24 positivity (data not shown). In addition, we routinely checked for p24+CD3+ T cells within crawl-out populations, but these were never detected (data not shown). Notably, we were unable to reproducibly detect HIV-1 p24+ LCs by FACS® analyses when using HIV-1 isolates other then HIV-1Ba-L, probably due to low levels of HIV-1 infection that approach the sensitivity of this assay (i.e., 0.01%). However, we have been able to detect proviral DNA in crawl-out LCs exposed to these isolates in preliminary PCR experiments, including in LCs exposed to the X4 HIV-1 isolate HIVIIIB (data not shown).
Figure 2.
HIV-1 infection of single crawl-out LC demonstrated by flow cytometry. Representative intracellular HIV-1 p24 and surface MHC class II mAb double-staining of LCs that have emigrated from HIV-1Ba-L–exposed (A and C) or uninfected (B) epithelial tissue explants, demonstrating 0.85% productively infected cells in A and low nonspecific staining in B. Dead cells were excluded from all analyses. HIV-1–infected cells stained with an isotype control Ab were always negative (data not shown). (C) Plot of percentage of HIV-1 p24+ LCs (with background staining subtracted) obtained in 31 separate experiments showing the range of intracellular HIV-1 p24 positivity depending on the skin donor.
HIV-1 virions could not be visualized in LCs using electron microscopy (data not shown), probably due to the low sensitivity of this method to detect relatively few productively infected cells.
Number and Phenotype of HIV-1-Infected LCs.
When purified laboratory-adapted HIV-1 was used to infect LCs, the number of crawl-out LCs per epithelial sheet exposed to HIV-1 was approximately equal to the number LCs per epithelial sheet not exposed to HIV-1 (2 × 104 LCs per epithelial sheet). However, when using primary isolates of HIV-1, there were approximately two- to threefold greater numbers of LCs that migrated from HIV-1–exposed epithelial sheets when compared with LCs collected from unexposed epithelial sheets. This, however, could have been due to LC exposure to cytokines present in the primary isolate viral preparations (that were not present in the laboratory-adapted viral preparations).
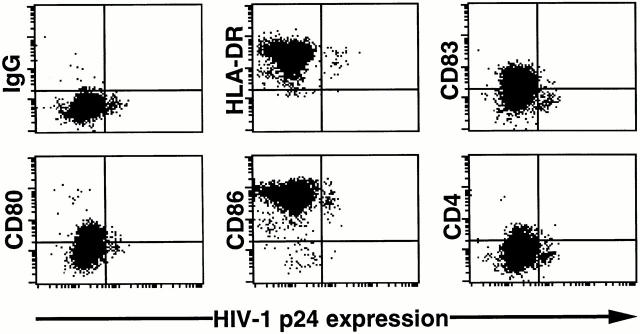
We next examined the phenotype of HIV-1 p24+ LCs by two-color flow cytometry. HIV-1 p24+ LCs expressed equal amounts of cell surface MHC class II, CD80, and CD86 when compared with HIV-1 p24− LCs (Fig. 3). However, cell surface CD4 and CD83 were clearly downregulated on HIV-1–infected LCs when compared with uninfected LCs (Fig. 3). This particular pattern of staining (no change in MHC class II, CD80, and CD86 with downregulation of CD4, and CD83) is similar to what we have recently observed on HIV-1 p24+ monocyte-derived dendritic cells (Kawamura, T., and A. Blauvelt, manuscript in preparation).
Figure 3.
Downregulation of CD4 and CD83 on HIV-1–infected LCs. LCs that had emigrated from HIV-1Ba-L–exposed skin were double stained for the surface antigens shown and intracellular HIV-1 p24. Dead cells were excluded from all analyses. Data shown are representative of at least three experiments.
HIV-1–infected LCs That Have Emigrated from Epithelial Tissue Explants Transmit Virus to Cocultured Allogeneic or Autologous CD4+ T Cells.
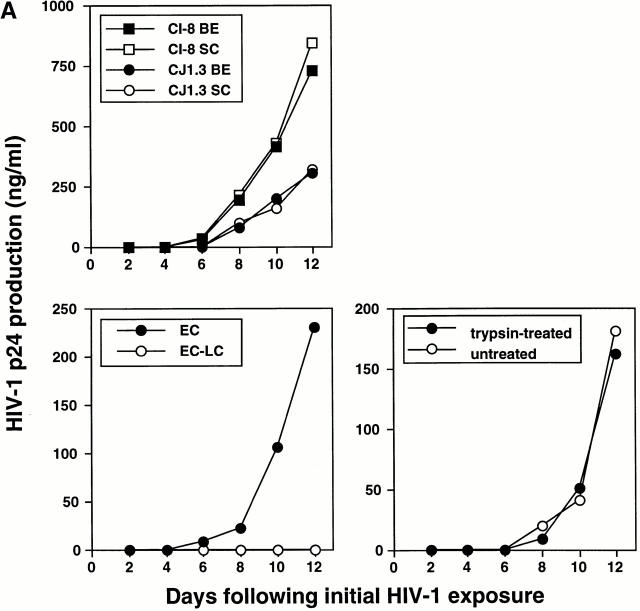
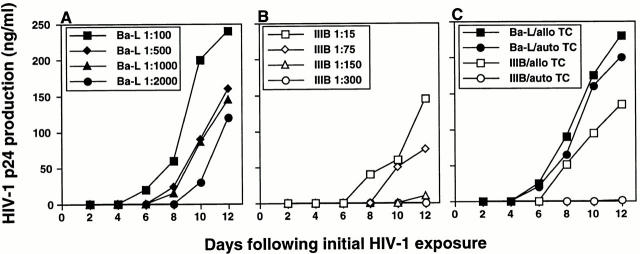
We next cocultured crawl-out LCs with T cells in an approximate LC/T cell ratio of 1:100 (2 × 104 LCs per 2 × 106 T cells). Although only relatively few productively infected LCs could be identified in crawl-out populations (Fig. 2), these cells could induce high levels of HIV-1 infection in cocultured T cells (Fig. 4 Fig. 5 Fig. 6 Fig. 7). LCs could transmit both primary and laboratory-adapted HIV-1 isolates to T cells (Fig. 4 Fig. 5 Fig. 6 Fig. 7). Notably, levels of HIV-1 infection in cocultured T cells were similar when either the stratum corneum surfaces or the basal epithelial surfaces were exposed to virus (Fig. 4). Interestingly, electron microscopy of LC–T cell cocultures 4 and 7 d after HIV-1 exposure of epithelial tissue explants revealed numerous virions budding from the surfaces of T cells, but not LCs (Fig. 4 B). T cell infection, as localized by electron microscopy and quantitated by p24 supernatant levels, was critically dependent on HIV-1–infected LCs, as evidenced by the following: (a) LC-depleted epidermal cell suspensions made from HIV-1–exposed epithelial tissue explants failed to transmit infection to cocultured T cells (Fig. 4) and (b) HIV-1–infected crawl-out LCs exposed briefly to trypsin (which effectively cleaved off any extracellular bound HIV-1 virions) 33 transmitted infection to cocultured T cells as well as infected LCs not exposed to trypsin (Fig. 4). Importantly, these two types of experiments rule out the possibility that free HIV-1 virions nonspecifically adhered to epithelial cells and were passively transferred to T cells.
Figure 4.
HIV-1–infected LCs are responsible for transmitting high levels of infection to cocultured CD4+ T cells. (A, top left) Tissue explants were exposed to two HIV-1 primary isolates, HIV-1CJI.3 (R5) or HIV-1CI-8 (R5X4), on the basal epithelial (BE) side or the stratum corneum (SC) side, washed, and then floated in media containing allogeneic CD4+ T cells. (B) electron micrograph taken from an LC–T cell coculture 7 d after HIV-1CI-8 exposure of explants showing numerous HIV-1 virions surrounding and budding from a CD4+ T cell (×64,000; inset ×440,000). (A, bottom left) Epidermal cell suspensions were made immediately after HIV-1 exposure of epithelial tissue explants to HIV-1CJI.3, and half of the suspensions were then depleted of LCs by immunomagnetic bead separation. Nondepleted epidermal cells (EC) and LC-depleted epidermal cells (EC-LC) were then cocultured with allogeneic CD4+ T cells. (A, bottom right) Emigrated LCs were collected from media 2 d after HIV-1CJI.3 exposure of epithelial tissue explants, and half of the cells were exposed to trypsin treatment and the other half left untreated. Trypsin-treated LCs and untreated LCs were then cocultured with allogeneic CD4+ T cells. HIV-1 infection levels were assessed by measuring HIV-1 p24 protein content by ELISA in coculture supernatants collected every other day (A, all panels). Data shown are representative of at least three separate experiments that showed similar results.
Figure 5.
Preferential infection of HIV-1Ba-L in LC–T cell cocultures when compared directly with HIV-1IIIB. Known amounts, as determined by HIV-1 p24 content, of HIV-1Ba-L (an R5 virus) and HIV-1IIIB (an X4 virus) were incubated with epithelial tissue explants, washed, and floated in media containing either allogeneic (A–C) or autologous (C) CD4+ T cells. For data shown in C, high titers (1:100 for HIV-1Ba-L and 1:15 for HIV-1IIIB) of each HIV-1 strain were used for infection. Infection levels were assessed by measuring HIV-1 p24 protein content by ELISA in coculture supernatants collected every other day. Data shown are representative of at least three separate experiments that showed similar results.
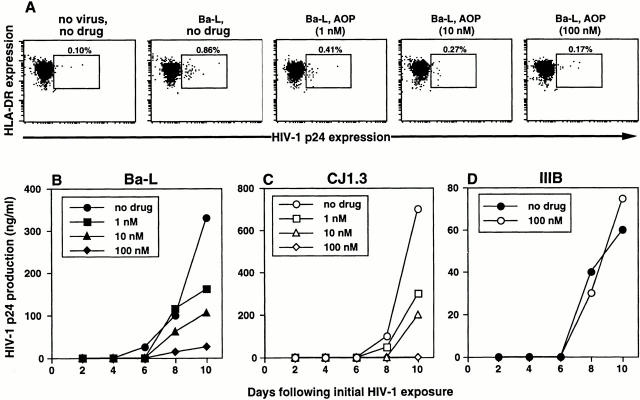
Figure 6.
Preincubation of epithelial tissue explants with AOP-RANTES specifically blocks subsequent HIV-1Ba-L and HIVCJ1.3, but not HIV-1IIIB, infection in LC and LC–T cell cocultures in a dose-dependent manner. Epithelial tissue explants were incubated with 1, 10, or 100 nM of AOP-RANTES, a CCR5 ligand, for 20 min before exposure to 1:100 HIV-1Ba-L (A and B) or HIVCJ1.3 (C), both R5 viruses, or 1:15 HIV-1IIIB (D), an X4 virus. After infection, explants were washed and floated in media alone (A) or in media containing allogeneic CD4+ T cells (B–D). HIV-1 infection levels were assessed by intracellular HIV-1 p24 staining (A) or by measuring HIV-1 p24 protein content by ELISA in coculture supernatants collected every other day (B–D). Data shown are representative of at least three separate experiments that showed similar results.
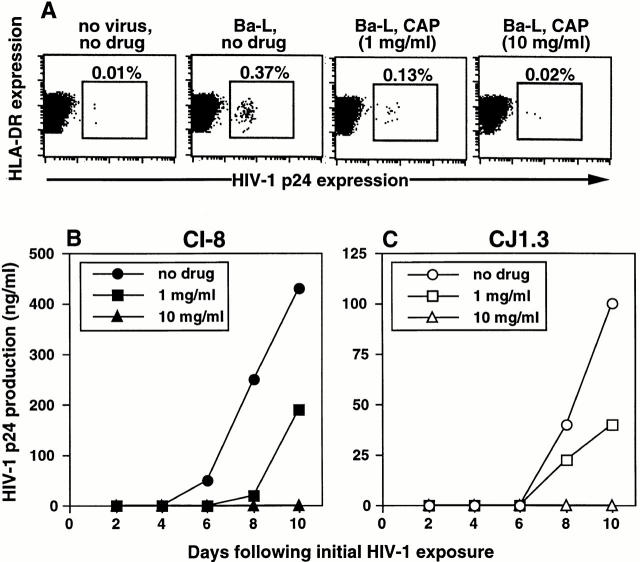
Figure 7.
Preincubation of epithelial tissue explants with CAP blocks subsequent HIV-1 infection in LC and LC–T cell cocultures in a dose-dependent manner. Epithelial tissue explants were incubated with 1 or 10 mg/ml of CAP, a candidate microbicide, for 20 min before exposure to HIV-1. After infection, explants were washed and floated in media alone (A) or in media containing allogeneic CD4+ T cells (B and C). HIV-1 infection levels were assessed by intracellular HIV-1 p24 staining (A) or by measuring HIV-1 p24 protein content by ELISA in coculture supernatants collected every other day (B and C). Data shown are representative of at least three separate experiments that showed similar results.
To compare efficiency of R5 virus infection to X4 virus infection, LCs within tissue explants were exposed to known amounts (as determined by HIV-1 p24 content) of either the laboratory-adapted R5 isolate HIV-1Ba-L or the laboratory-adapted X4 isolate HIV-1IIIB. Because these viruses were grown in different cell types, TCID50 values were not directly comparable. We chose what we believed to be the next best measure, HIV-1 p24 content, to normalize virus input. Using this method of normalization, the X4 virus HIV-1IIIB was clearly transmitted less well when compared with infection with the R5 virus HIV-1Ba-L (Fig. 5). This difference was even more dramatic when crawl-out LCs were cocultured with autologous T cells (Fig. 5 C) instead of allogeneic T cells (Fig. 5). These data are consistent with the HIV coreceptor phenotype that we have documented on immature and mature LCs 34 35.
AOP-RANTES and CAP Each Block HIV-1 Infection of LCs and Subsequent Infection of CD4+ T Cells.
To test whether our new explant model system could be useful as a preclinical method to screen potential microbicides, epithelial sheets were incubated with the compounds AOP-RANTES or CAP for 20 min prior to HIV-1 infection. Preincubation with AOP-RANTES, a synthetic chemo-kine analogue of RANTES that binds to CCR5 14, blocked R5 HIV-1 infection of LCs in a dose-dependent manner (Fig. 6 A). Subsequent R5 HIV-1 infection in LC–T cell cocultures was also blocked in a dose-dependent manner (Fig. 6B and Fig. C), but not subsequent infection with the X4 virus HIV-1IIIB (Fig. 6 D). AOP-RANTES blocked infection of both laboratory-adapted and primary isolate R5 viruses. This data demonstrates the specificity of the blocking effect of AOP-RANTES and shows that coreceptor usage is important for LC infection. Preincubation of epithelial sheets with AOP-RANTES did not cause cell toxicity or changes in the number of emigrated LCs at the doses used in this study (data not shown).
Preincubation with CAP, a compound used for enteric coating of tablets and capsules 21 22 23, also blocked infection of LCs (Fig. 7 A) as well as subsequent infection in LC–T cell cocultures (Fig. 7B and Fig. C). As with AOP-RANTES, CAP demonstrated clear dose-dependent blocking effects. Both laboratory-adapted and primary isolates, as well as both R5 and X4 viruses, were blocked by CAP. Preincubation of epithelial sheets with CAP did not lead to cell toxicity nor changes in the number of emigrated LCs at the doses used in this study (data not shown).
Discussion
We believe that tissue culture models for sexual transmission of HIV-1 should focus on LCs, members of the dendritic cell family, because LCs represent the most likely initial targets for HIV-1. LCs reside in mucosal surfaces at sites of HIV-1 exposure, express CD4 and HIV-1 coreceptors, have been shown to be infectable with HIV-1 in vivo and in vitro, are capable of emigrating to lymph nodes, and are efficient at transmitting virus to CD4+ T cells 25 26 27 28 29. Despite this, two recently published explant models have failed to sufficiently evaluate the role of LCs in genital tissue infection by HIV-1 36 37. Understandably, several problems are inherent in working with LCs under experimental conditions. Isolation of true tissue LCs is laborious because it is difficult to obtain large numbers of relatively pure cell populations. Most problematic, however, is that standard isolation procedures require trypsinization of epithelial tissues and/or a culture period to collect single-cell suspensions of LCs 31 34 35 38 39. Use of trypsin interferes with subsequent HIV-1 infection experiments because it cleaves CD4 that is required for virus entry into LCs 33. Culturing epithelial tissues to obtain crawl-out LCs avoids trypsin, although this procedure dramatically changes the phenotype and function of LCs 40. More specifically, freshly isolated LCs, which resemble LCs in situ, express surface CCR5 and no surface CXCR4, whereas crawl-out LCs and LCs cultured for as few as 16 h resemble mature dendritic cells; these cells express CXCR4 and comparatively low levels of surface CCR5 34 35 41. In this report, these problems are avoided by exposing LCs to HIV-1 as they reside within epithelial tissue (Fig. 1). Thus, this model is likely to mimic biologic conditions present during sexual transmission of HIV-1.
Like our model, Reece et al. recently reported on the use of skin to model early events of HIV-1 transmission 38. These investigators used abraded surgical skin specimens and were able to show selective infection by R5 viruses when compared with X4 viruses using a PCR-based assay. Unlike this previously published study, abrasion of epidermis was not necessary for LC infection to occur through stratum corneum surfaces in our model system (Fig. 4). We postulate that either the stratum corneum was not acting as an effective barrier to infection or that virus was gaining access to LCs by infecting these cells through small breaks in the stratum corneum or through the lateral aspects of the tissue. In addition, and something not demonstrated in the Reece study, we were able to document infection in single LCs by intracellular staining for HIV-1 p24 protein (Fig. 2), utilize this technique to phenotype HIV-1-infected LCs (Fig. 3), and demonstrate that potential microbicides could block LC infection (Fig. 6 and Fig. 7). Interestingly, CD4 and CD83 were downregulated on the surfaces of HIV-1 p24+ LCs, whereas MHC class II, CD80, and CD86 were unchanged. To our knowledge, this represents the first study to document the phenotype of HIV-1 infected LCs, although the functional significance of these phenotypic changes remains to be determined. A recent study has demonstrated downregulation of CD83 and functional impairment in dendritic cells infected with herpes simplex virus type 1 42.
Similar to the Reece study 38, R5 viral infection was much more efficient than X4 virus infection in the system described here, although this selection was not absolute (Fig. 5). Additionally, our model suggests that use of autologous instead of allogeneic T cells in LC–T cell cocultures contributes to relative selection of R5 virus infection. This finding is consistent with data from others suggesting that resting or nonproliferating T cells may preferentially replicate R5 viruses in the setting of dendritic cell–T cell clustering 43 44. Thus, we propose that both differential HIV-1 coreceptor expression on resident LCs 34 and the ability of autologous T cells to preferentially replicate R5 viruses that are transmitted by HIV-1–infected LCs contribute to preferential infection by R5 viruses during sexual transmission of HIV-1 12 13.
One difficulty in firmly establishing a role for LCs in initial HIV-1 infection has been the interpretation of results obtained in SIV-macaque studies. When mucosal tissues have been examined after atraumatic cell-free SIV inoculation, most studies have shown no evidence of intraepithelial LC infection 43 45 46. However, virus-infected LCs would be expected to emigrate from epithelium within a few hours after exposure, so that tissue from time points earlier than 24 h after inoculation would need to be examined to document infection within intraepithelial LCs. Second, assays that detect only productive HIV-1 infection within tissue, e.g., HIV-1 p24 staining, would be expected to show negative staining in HIV-1–exposed activated LCs. This assumption is based on the in vitro findings that immature dendritic cells can be productively infected with HIV-1 but that initiation of the activation process rapidly leads to nonproductive latent HIV-1 infection within mature dendritic cells 39 47 48. Interestingly, Hu et al. have recently documented SIV-infected LCs within mucosal epithelium 18 h after vaginal exposure to virus 49. Thus, proving a definitive role for LCs in initial HIV-1/SIV infection requires specialized technical expertise. Here, in a novel ex vivo model that is consistent with the results of Hu et al. 49, we show definitive evidence that HIV-1–infected LCs are critically involved in the subsequent transmission of virus to T cells (Fig. 4). Interestingly, relatively few HIV-1–infected LCs (usually <1%) were capable of inducing high levels of infection in cocultured T cells (0.1–1 μg HIV-1 p24 per milliliter).
Faced with the daunting task of controlling sexual spread of HIV-1 around the world, many investigators and drug companies have focused on developing topical barrier compounds to prevent new infections 15 16 17 18 19. Several of these compounds have been shown to be safe and are now being tested in clinical settings to determine efficacy in blocking HIV-1 transmission. These microbicides, as well as newer potential microbicides, are screened in the laboratory with a CD4- and HIV-1 coreceptor–independent assay that involves HIV-1 infection of a transformed cervical epithelial cell line 50; however, the relevance of this assay to sexual transmission of HIV-1 is unclear. In addition, more newly described explant models fail to elucidate a role for LCs in genital tissue HIV-1 infection 36 37. Thus, essentially no current tissue culture model appears to be adequate for preclinical testing of these agents. Importantly, the LC infection model described in this report provides a physiologic system to evaluate potential microbicides in the laboratory. We demonstrate the usefulness of this approach by showing that AOP-RANTES and CAP, two potential microbicides currently under investigation, can block both LC infection and LC-mediated infection of T cells (Fig. 6 and Fig. 7). Furthermore, it is possible that this model system could be adapted to study transmission characteristics of other sexually transmitted pathogens. In this regard, Wu et al. have recently reported on dengue virus infection of cutaneous dendritic cells that had emigrated from virus-exposed skin explants 51.
In summary, we describe a novel, physiologically relevant ex vivo model for sexual transmission of HIV-1 that takes advantage of the biologic properties of LCs, likely initial targets for HIV-1 after mucosal exposure to virus. We have used this model to document infection of LCs on a single-cell level and to show that two candidate microbicides are capable of blocking LC infection and subsequent transmission of virus to T cells. Our system should prove valuable in further understanding early cellular and molecular events involved in primary HIV-1 infection and in the preclinical testing of additional agents designed to block mucosal transmission of virus.
Acknowledgments
We thank David I. Cohen for primary HIV-1 isolates and coreceptor usage assay, Inga Tokar, Ana Hancox, and Laura Musse for assisting with healthy volunteers, Harry Schaefer for preparing figures, and Mark C. Udey, Stephen I. Katz, and Roberta J. Black for helpful discussions.
Footnotes
Abbreviations used in this paper: AOP, aminooxypentane; CAP, cellulose acetate phthalate; LCs, Langerhans cells; PHA, phytohemagglutinin; RANTES, regulated upon activation, normal T cell expressed and secreted.
T. Kawamura and S.S. Cohen contributed equally to this work.
References
- Royce R.A., Sena A., Cates W., Cohen M.S. Sexual transmission of HIV. N. Engl. J. Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- Kahn J.O., Walker B.D. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- Fauci A.S. The AIDS epidemic-considerations for the 21st century. N. Engl. J. Med. 1999;341:1046–1050. doi: 10.1056/NEJM199909303411406. [DOI] [PubMed] [Google Scholar]
- Cohen M.S. Sexually transmitted diseases enhance HIV transmissionno longer a hypothesis Lancet 351 1998. 5 7(Suppl [DOI] [PubMed] [Google Scholar]
- Vernazza P.L., Eron J.J., Fiscus S.A., Cohen M.S. Sexual transmission of HIVinfectiousness and prevention. AIDS. 1999;13:155–166. doi: 10.1097/00002030-199902040-00003. [DOI] [PubMed] [Google Scholar]
- Cohen O.J., Fauci A.S. Host factors that affect sexual transmission of HIV. Int. J. Infect. Dis. 1998;2:182–185. doi: 10.1016/s1201-9712(98)90049-2. [DOI] [PubMed] [Google Scholar]
- Miller C.J. Host and viral factors influencing heterosexual HIV transmission. Rev. Reprod. 1998;3:42–51. doi: 10.1530/ror.0.0030042. [DOI] [PubMed] [Google Scholar]
- Dean M., Carrington M., Winkler C., Huttley G.A., Smith M.W., Allikmets R., Goedert J.J., Buchbinder S.P., Vittinghoff E., Gomperts E. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Samson M., Libert F., Doranz B.J., Rucker J., Liesnard C., Farber C.M., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Huang Y., Paxton W.A., Wolinsky S.M., Neumann A.U., Zhang L., He T., Kang S., Ceradini D., Jin Z., Yazdanbakhsh K. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Zhang L.Q., MacKenzie P., Cleland A., Holmes E.C., Brown H.J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D.S., Cao Y., Koup R.A., Ho D.D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- Simmons G., Clapham P.R., Picard L., Offord R.E., Rosenkilde M.M., Schwartz T.W., Buser R., Wells T.N.C., Proudfoot A.E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- Elias C.J., Coggins C. Female-controlled methods to prevent sexual transmission of HIV AIDS 10 1996. S43 51(Suppl [PubMed] [Google Scholar]
- Pauwels R., De Clercq E. Development of vaginal microbicides for the prevention of heterosexual transmission of HIV. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;11:211–221. doi: 10.1097/00042560-199603010-00001. [DOI] [PubMed] [Google Scholar]
- Rosenthal S.L., Cohen S.S., Stanberry L.R. Topical microbicides. Current status and research considerations for adolescent girls. Sex. Transm. Dis. 1998;25:368–377. doi: 10.1097/00007435-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Gross, M., S.P. Buchbinder, C. Celum, P. Heagerty, and G.R. Seage, 3rd. 1998. Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET Vaccine Preparedness Study Protocol Team. Sex. Transm. Dis. 25:296–302. [DOI] [PubMed]
- Wainberg M.A. The need for microbicideswhy aren't women's groups more involved in the fight against AIDS? AIDS Res. Hum. Retroviruses. 1999;15:405–406. doi: 10.1089/088922299311141. [DOI] [PubMed] [Google Scholar]
- Roddy R.E., Zekeng L., Ryan K.A., Tamoufe U., Weir S.S., Wong E.L. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- Neurath A.R., Strick N., Li Y.Y., Lin K., Jiang S. Design of a “microbicide” for prevention of sexually transmitted diseases using “inactive” pharmaceutical excipients. Biologicals. 1999;27:11–21. doi: 10.1006/biol.1998.0169. [DOI] [PubMed] [Google Scholar]
- Neurath A.R., Li Y.Y., Mandeville R., Richard L. In vitro activity of a cellulose acetate phthalate topical cream against organisms associated with bacterial vaginosis. J. Antimicrob. Chemother. 2000;45:713–714. doi: 10.1093/jac/45.5.713. [DOI] [PubMed] [Google Scholar]
- Gyotoku T., Aurelian L., Neurath A.R. Cellulose acetate phthalate (CAP)an “inactive” pharmaceutical excipient with antiviral activity in the mouse model of genital herpesvirus infection. Antiviral Chem. Chemother. 1999;10:327–332. doi: 10.1177/095632029901000604. [DOI] [PubMed] [Google Scholar]
- Manson K.H., Wyand M.S., Miller C., Neurath A.R. Effect of a cellulose acetate phthalate topical cream on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 2000;44:3199–3202. doi: 10.1128/aac.44.11.3199-3202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Jakob T., Udey M.C. Epidermal Langerhans cellsfrom neurons to nature's adjuvants. Adv. Dermatol. 1999;14:209–259. [PubMed] [Google Scholar]
- Weissman D., Fauci A.S. Role of dendritic cells in immunopathogenesis of human immunodeficiency virus infection. Clin. Microbiol. Rev. 1997;10:358–367. doi: 10.1128/cmr.10.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeteweij J.P., Blauvelt A. HIV-dendritic cell interactions promote efficient viral infection of T cells. J. Biomed. Sci. 1998;5:253–259. doi: 10.1007/BF02255856. [DOI] [PubMed] [Google Scholar]
- Cameron P., Pope M., Granelli-Piperno A., Steinman R.M. Dendritic cells and the replication of HIV-1. J. Leukoc. Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- Cooper K.D., Neises G.R., Katz S.I. Antigen-presenting OKM5+ melanophages appear in human epidermis after ultraviolet radiation. J. Invest. Dermatol. 1986;86:363–370. doi: 10.1111/1523-1747.ep12285600. [DOI] [PubMed] [Google Scholar]
- Blauvelt A., Asada H., Klaus-Kovtun V., Altman D.J., Lucey D.R., Katz S.I. Interleukin-15 mRNA is expressed by human keratinocytes, Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J. Invest. Dermatol. 1996;106:1047–1052. doi: 10.1111/1523-1747.ep12338641. [DOI] [PubMed] [Google Scholar]
- Deng H.K., Unutmaz D., Kewal-Ramani V.N., Littman D.R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- Tang S., Levy J.A. Inactivation of HIV-1 by trypsin and its use in demonstrating specific virus infection of cells. J. Virol. Methods. 1991;33:39–46. doi: 10.1016/0166-0934(91)90005-k. [DOI] [PubMed] [Google Scholar]
- Zaitseva M., Blauvelt A., Lee S., Lapham C.K., Klaus-Kovtun V., Mostowski H., Manischewitz J., Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophagesimplications for HIV primary infection. Nat. Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- Zoeteweij J.P., Golding H., Mostowski H., Blauvelt A. Cutting edgecytokines regulate expression and function of the HIV coreceptor CXCR4 on human mature dendritic cells. J. Immunol. 1998;161:3219–3223. [PubMed] [Google Scholar]
- Collins K.B., Patterson B.K., Naus G.J., Landers D.V., Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- Greenhead P., Hayes P., Watts P.S., Laing K.G., Griffin G.E., Shattock R.J. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece J.C., Handley A.J., Anstee E.J., Morrison W.A., Crowe S.M., Cameron P.U. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M., Betjes M.G.H., Romani N., Cameron P.U., Hoffman L., Gezelter S., Schuler G., Steinman R.M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Steinman R., Hoffman L., Pope M. Maturation and migration of cutaneous dendritic cells. J. Invest. Dermatol. 1995;105:2S–7S. doi: 10.1111/1523-1747.ep12315162. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C.R., Qin S., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kruse M., Rosorius O., Kratzer F., Stelz G., Kuhnt C., Schuler G., Hauber J., Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Q., Schuler T., Zupancic M., Wietgrefe S., Staskus K.A., Reimann K.A., Reinhart T.A., Rogan M., Cavert W., Miller C.J. Sexual transmission and propagation of SIV and HIV in resting and activated CD4(+) T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Pope M., Inaba K., Steinman R.M. Coexpression of NF-kappa B/Rel and Sp1 transcription factors in human immunodeficiency virus 1-induced, dendritic cell-T-cell syncytia. Proc. Natl. Acad. Sci. USA. 1995;92:10944–10948. doi: 10.1073/pnas.92.24.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A.I., Marx P.A., Patterson B.K., Mahoney J., Koup R.A., Wolinsky S.M., Ho D.D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Hennig C., Steinman R.M., Tenner-Racz K., Pope M., Stolte N., Matz-Rensing K., Grobschupff G., Raschdorff B., Hunsmann G., Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Moser B., Pope M., Chen D., Wei Y., Isdell F., O'Doherty U., Paxton W., Koup R., Mojsov S. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J. Exp. Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A., Asada H., Saville M.W., Klaus-Kovtun V., Altman D.J., Yarchoan R., Katz S.I. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Invest. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Gardner M.B., Miller C.J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D.M., Tan X., Pearce-Pratt R., Zacharopoulos V.R. An assay for HIV infection of cultured human cervix-derived cells. J. Virol. Methods. 1995;52:1–13. doi: 10.1016/0166-0934(94)00053-j. [DOI] [PubMed] [Google Scholar]
- Wu S.J.L., Grouard-Vogel G., Sun W., Mascola J.R., Brachtel E., Putvatana R., Louder M.K., Filgueira L., Marovich M.A., Wong H.K. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]