Abstract
Previous pharmacologic and genetic studies have demonstrated a critical role for the low molecular weight GTP-binding protein RhoA in the regulation of cell-mediated killing by cytotoxic lymphocytes. However, a specific Rho family guanine nucleotide exchange factor (GEF) that activates this critical regulator of cellular cytotoxicity has not been identified. In this study, we provide evidence that the Rho family GEF, Vav-2, is present in cytotoxic lymphocytes, and becomes tyrosine phosphorylated after the cross-linking of activating receptors on cytotoxic lymphocytes and during the generation of cell-mediated killing. In addition, we show that overexpression of Vav-2 in cytotoxic lymphocytes enhances cellular cytotoxicity, and this enhancement requires a functional Dbl homology and Src homology 2 domain. Interestingly, the pleckstrin homology domain of Vav-2 was found to be required for enhancement of killing through some, but not all activating receptors on cytotoxic lymphocytes. Lastly, although Vav and Vav-2 share significant structural homology, only Vav is able to enhance nuclear factor of activated T cells–activator protein 1–mediated gene transcription downstream of the T cell receptor. These data demonstrate that Vav-2, a Rho family GEF, differs from Vav in the control of certain lymphocyte functions and participates in the control of cell-mediated killing by cytotoxic lymphocytes.
Keywords: natural killer cell, cytotoxic T cell, Vav-2, RhoA, signal transduction
Introduction
The low molecular weight GTP-binding proteins of the Rac and Rho family of GTPases have been identified as critical transducers of signals downstream of activating receptors on lymphocytes 1. The Dbl homology (DH) domain-containing guanine nucleotide exchange factors (GEFs) are a family of proteins that interact exclusively with members of the Rho/Rac family of GTPases and promote GTP for GDP exchange 2. Members of this family of proteins are widely expressed, and many of them can promote tumor formation when overexpressed in either their wild-type or oncogenic forms 2. In fact, Vav, which is a Dbl family member, was identified due to its ability to transform mouse fibroblasts 3 4. The Vav protooncogene is primarily expressed in hematopoietic cells and is among the most extensively studied member of this family of proteins 5 6. It has been shown that Vav undergoes tyrosine phosphorylation after the cross-linking of many activating receptors on hematopoietic cells, including the TCR, B cell receptor, FcR 7 8 9 10, and during the generation of natural cytotoxicity 11. Tyrosine phosphorylation of Vav is required for Vav GEF activity 12 13, and inactivation of the Vav Src homology 2 (SH2) domain inhibits receptor-mediated tyrosine phosphorylation 14 15. In addition to the SH2 domain, Vav contains numerous other protein subdomains including two SH3 domains, an NH2-terminal calponin homology (CH) domain, an acidic region, a DH domain followed by a pleckstrin homology (PH) domain, a cysteine-rich region, and a proline-rich region (PR) 6. The presence of so many protein subdomains in Vav suggests that it has the ability to interact with many signaling molecules.
Recently, Vav-2, a new member of this family of GEFs, has been identified 16 17 18. Overall, these two proteins share similar arrangements of their structural domains. In addition, like Vav, tyrosine phosphorylation of Vav-2 is required for intrinsic GEF activity, and it was recently shown that Vav-2 can be phosphorylated by Src family and Syk family protein tyrosine kinases (PTKs) in vitro 18. However, whereas Vav has been found to undergo tyrosine phosphorylation after stimulation through numerous lymphocyte activation receptors, receptor-mediated tyrosine phosphorylation of Vav-2 has not been reported. Also, in contrast to Vav, which is primarily expressed in hematopoietic cells, Vav-2 is more ubiquitously expressed 16 17. Moreover, whereas Vav acts as a GEF toward Rac-1, Rac-2, and RhoG 12 13 18, Vav-2 displays GEF activity toward RhoA subfamily members and RhoG 18. These data provide an excellent view into the biochemical properties of these two Vav family GEFs. However, the identification of cell surface receptors that are linked to Vav-2 activation and the role that Vav-2 plays in the development of receptor-initiated events remains unclear.
The generation of cell-mediated killing by NK cells requires activation of proximal PTKs 19 20 21. We and others have previously shown that Vav undergoes receptor-mediated tyrosine phosphorylation after cross-linking of FcγRIIIA on human NK cells and during the generation of natural cytotoxicity 10 11. In addition, it has been shown that Vav and one of its target molecules, Rac-1, regulate the generation of cell-mediated killing by NK cells and CD8+ CTLs 11 22. Furthermore, inactivation of the RhoA GTPase by pharmacologic or genetic approaches has been shown to block the development of cellular cytotoxicity by these two cell populations 11 23. Therefore, the regulation of cell-mediated killing by cytotoxic lymphocytes involves receptor-mediated regulation of both Rac and Rho GTPases. However, the identification of a Rho subfamily GEF that is regulated by activating receptors in these cells has not been identified.
Northern blot analysis has identified Vav-2 RNA in lymphoid organs including the thymus and spleen 16 17. We therefore sought to determine: (a) if Vav-2 is present in cytotoxic lymphocytes; (b) whether Vav-2 undergoes receptor-mediated tyrosine phosphorylation during the generation of cytotoxicity by these two cell types; (c) whether Vav-2 can regulate the generation of cell-mediated killing; and (d) what subdomains of Vav-2 are critical for its activity. To this end, we have found that Vav-2 is expressed in cytotoxic lymphocytes and undergoes receptor-mediated tyrosine phosphorylation after the cross-linking of activating receptors on cytotoxic lymphocytes and during the generation of natural cytotoxicity. Also, although overexpression of Vav-2 in cytotoxic lymphocytes results in enhanced cellular cytotoxicity, Vav-2 mutants containing inactivating mutations in the DH or SH2 domain cannot. Moreover, we have found that the PH domain of Vav-2 is required for its activation downstream of some but not all activating receptors on cytotoxic lymphocytes. Lastly, in contrast to Vav, Vav-2 is unable to regulate nuclear factor of activated T cells (NFAT)–activator protein (AP)-1–mediated gene transcription after TCR cross-linking. Together, our data highlight Vav-2 as a regulatory molecule for the development of cell-mediated killing by cytotoxic lymphocytes.
Materials and Methods
Reagents, Cells, and Antibodies.
Unless otherwise stated, all chemicals were from Sigma-Aldrich. The Jurkat T cell line, anti-CD3–producing hybridoma OKT3 (murine IgG2a), K562 erythroid leukemia cell line, HeLa epithelial cell line, and murine mastocytoma cell line P815 were obtained from American Type Culture Collection. Human NK cells and CD8+ T cells were cloned and passaged as previously described 24. The long-term melanoma-specific CTL line recognizing the G9209 peptide of the melanoma-associated antigen gp100 was provided by E. Celis (Mayo Clinic, Rochester, MN). Antibodies used in this study included the anti-FLAG murine mAb FLAG-M2 (Sigma-Aldrich), antiphosphotyrosine mAb 4G10 (Upstate Biotechnology), and goat anti–mouse IgG F(ab′)2 (ICN Biochemicals). Anti-FcγRIIIA mAb 3G8 and anti-CD3 mAb OKT3 were purified from ascites by affinity chromatography over protein A–agarose. Rabbit polyclonal antiserum to Vav-1 was obtained after immunization of rabbits with KLH-conjugated Vav peptide 566–593 (HGQDFAGTMKKDKLHRRAQDKKRNELGL, anti-Vav; Cocalico Biologicals, Inc.). Two separate rabbit polyclonal antisera to Vav-2 were obtained by immunization of KLH-conjugated peptides derived from Vav-2, amino acids 134–155 (TTENDDDVYRSLEELADEHDLG, anti–Vav-2.1), and amino acids 448–473 (EIIELLFHKMTDDPMNNKDVKKSHGK, anti–Vav-2.2). Both anti–Vav-2 polyclonal rabbit sera were affinity purified using the Sulfolink kit from Pierce Chemical Co. and the synthetic peptide as per manufacturer protocol.
DNA Constructs and Recombinant Vaccinia Generation.
The recombinant vaccinia expressing FLAG-tagged Vav (F.Vav) has been previously described 11. To obtain Vav-2 recombinant vaccinia, the Vav-2 coding sequence was isolated by double digestion with HindIII and NotI from a Vav-2 cDNA provided by D.J. Kwiatkowski (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) 16 and then subcloned into the pSHN11 vaccinia recombination substrate. The Vav-2 construct was FLAG tagged using the previously described FLAG adaptor 11. The fragment was then subcloned using standard molecular biology techniques into similarly digested pCDNA3. All recombinant vaccinia viruses were produced via homologous recombination with the WR strain of virus as previously described 11. Using the Site-Directed Mutagenesis kit (CLONTECH Laboratories Inc.) all of the mutations were obtained as previously described 11. The single amino acid point mutations were generated using the pSHN11 selection oligonucleotide along with the specific Vav-2 mutagenic oligonucleotides, Y213F/L217Q (5′-GAGACCGAGGCCAAGTTCTACCGCACCCAGGAGGACATTGAGAAG-3′), W503L (5′-GATATGAAGAGGAAGCTGATGGAGCAGTTTGAG-3′), and R698A (5′-GGGACCTACCTGATGGCGGAGCGGCCTGCCGAG-3′). The Vav-2 TRUNC mutant was generated by introduction of a HindIII site at amino acids 573/574 using the mutagenic oligonucleotide (5′-GTGATACCTCCCTGCAAGCTTACTTCTCCTGCAGAT-3′). The mutant was digested with HindIII to remove the 5′ coding sequence and religated, and a FLAG adaptor was added. The nucleotides in bold represent the mutational changes resulting in either amino acid changes or in the case of the TRUNC mutant, the addition of a HindIII site.
Electroporation and Luciferase Assays.
Jurkat T cells (107) were electroporated with 20 μg of pCDNA3 control vector, Vav-1, or Vav-2-expressing vectors along with 10 μg of the pNFAT3–luciferase construct as previously described 25. After 18–24 h of incubation the cells were stimulated as indicated in the figure legend and luciferase activity was measured as previously described 25. The percentage of maximal NFAT activity was determined by dividing the luciferase activity obtained in the absence or presence of stimulation, by that obtained by stimulation with PMA + ionomycin. The maximum NFAT–AP-1 responses did not differ significantly between transfection conditions. Protein expression of the electroporated constructs was determined by immunoprecipitating F.Vav-1 and F.Vav-2 proteins from 2 × 106 electroporated cells using the anti-FLAG mAb, followed by anti-FLAG immunoblotting.
Cytotoxicity Assays.
The 51Cr-release assays were performed as described previously 24. In all cases, spontaneous release did not exceed 10% of maximum release. In redirected cytotoxicity assays, NK clones and CD8+ T cell clones were only able to kill the P815 target cell in the presence of anti-FcR or anti-CD3 mAb, respectively. Lytic units were calculated based on 20% cytotoxicity 26.
Ca2+ Mobilization Assays.
Changes in levels of intracellular Ca2+ of vaccinia-infected, Indo-1–loaded cells was assessed by flow cytometer using a FACStar® (Becton Dickinson) as previously described 27. In brief, Jurkat T cells were infected with the indicated nonrecombinant (WR) or recombinant vaccinia virus for 2 h in a humidified 37°C incubator at a multiplicity of infection of 10:1. For the last 30 min of the infection, the cells were loaded with 5 μM Indo-1 (Calbiochem-Novabiochem). They were then washed in PBS containing 1% BSA and resuspended in RPMI-10 until analyzed. For analysis, the Indo-1–loaded Jurkat T cells were incubated with either goat anti–mouse IgG F(ab′)2 alone (as a baseline control) or a combination of anti-CD3 mAb OKT3 (2.5 μg/ml) and goat anti–mouse IgG F(ab′)2. The samples were immediately analyzed by flow cytometry using a UV laser for excitation with violet (390 nm) and blue (500 nm) fluorescence emissions recorded. The data plots were generated using the FlowJo software program (TreeStar Inc.).
Cell Stimulation and Immunoblot Analysis.
In experiments analyzing endogenous Vav and Vav-2 proteins, Vav and Vav-2 were specifically immunoprecipitated for 1–2 h from the cell lysates of the indicated cell lines using either normal rabbit antiserum as a control or the specific anti-Vav or anti–Vav-2.1 rabbit polyclonal antisera. In experiments involving viral infection, NK clones, CD8+ T clones, or Jurkat T cells were infected with the indicated FLAG-tagged recombinant vaccinia virus at an multiplicity of infection of 20:1 for 5 h (NK and T cell clones) or 10:1 for 2 h (Jurkat T cells). For experiments where NK cells were activated by target cells, 5 × 106 cloned NK cells were briefly pelleted with 2.5 × 106 target cells and then incubated at 37°C for the indicated period of time. In experiments involving specific cell surface receptor cross-linking, the indicated quantity of cells were incubated for 3 min on ice with anti-CD3 mAb OKT3 (1 μg/ml) or anti-FcγRIIIA mAb 3G8 (1 μg/ml). Washed cells were then incubated with goat anti–mouse IgG F(ab′)2 at 37°C for the indicated period of time. After stimulation, the cells were lysed on ice for 10 min in 1 ml of buffer containing 20 mM Tris-HCl, 40 mM NaCl, 5 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, 0.1% BSA, 1 mM Na3VO4, 1 mM PMSF, 5 μg/ml aprotinin, 10 μg/ml leupeptin, and 1% Triton X-100. Cellular debris was removed by centrifugation at 14,000 rpm for 5 min at 4°C. Endogenous Vav and Vav-2 were immunoprecipitated from the cell lysates for 1–2 h at 4°C using either anti-Vav or anti–Vav-2.1 polyclonal rabbit antisera bound to protein A–Sepharose beads. F.Vav-2 was immunoprecipitated from the lysate for 1–2 h at 4°C using 1 μg of anti–FLAG-M2 mAb bound to goat anti–mouse IgG–agarose beads. Protein complexes were washed four times in wash buffer (lysis buffer lacking BSA). Bound proteins were then eluted in 40 μl of SDS sample buffer, resolved by SDS-PAGE, and transferred to Immobilon-P membranes (Millipore). In some experiments, Vav and Vav-2 were detected using specific anti-Vav and anti–Vav-2.2 polyclonal rabbit antisera followed by protein A coupled to horseradish peroxidase (Amersham Pharmacia Biotech) and the ECL detection system from Amersham. Tyrosine-phosphorylated proteins were detected using the anti-pTYR mAb 4G10, and FLAG-tagged proteins were detected using anti-FLAG mAb, M2 followed by sheep anti–mouse IgG coupled to horseradish peroxidase and the ECL detection system.
Results
Vav-2 Is Expressed in Cytotoxic Lymphocytes.
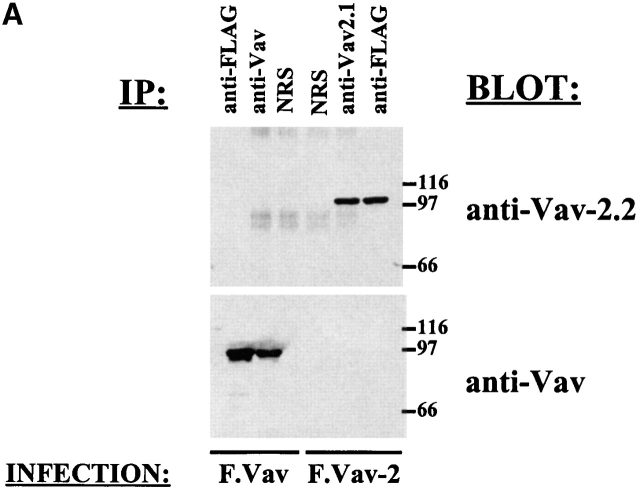
Previous Northern blot analysis has demonstrated that Vav-2 message is present in the spleen and the thymus 16 17, two lymphoid organs rich in NK and T cells, respectively. We therefore wanted to determine if Vav-2 protein is present in cytotoxic lymphocytes. Analysis of the Vav-2 protein sequence identified several potential antigenic sites, and two of them, amino acids 134–155 (TTENDDDVYRSLEELADEHDLG) and amino acids 448–473 (EIIELLFHKMTDDPMNNKDVKKSHGK), were significantly different in sequence from Vav. The synthesized peptides were conjugated to KLH and used to generate the rabbit polyclonal Vav-2–specific sera, anti–Vav-2.1 and anti–Vav-2.2. To demonstrate the specificity of the antisera, we infected 5 × 106 Jurkat T cells with recombinant vaccinia virus expressing FLAG-tagged versions of either Vav (F.Vav) or Vav-2 (F.Vav-2). After a 2-h infection, the recombinant proteins were immunoprecipitated with normal rabbit serum (NRS) or the antibodies indicated in Fig. 1 A, separated by SDS-PAGE, and transferred to a nylon membrane. As shown in Fig. 1 A, anti–Vav-2.1 sera immunoprecipitates as much F.Vav-2 as the anti-FLAG mAb (Fig. 1 A, top, compare anti–Vav-2.1 to anti-FLAG). In addition, the anti–Vav-2.2 blotting antibody specifically reacts with F.Vav-2 and not with F.Vav (Fig. 1 A, top). Lastly, the rabbit polyclonal anti-Vav sera is specific for Vav, as it did not immunoprecipitate or Western blot Vav-2 protein (Fig. 1 A, bottom). These data demonstrate that the Vav-2 and Vav polyclonal rabbit antisera are specific for their intended proteins.
Figure 1.
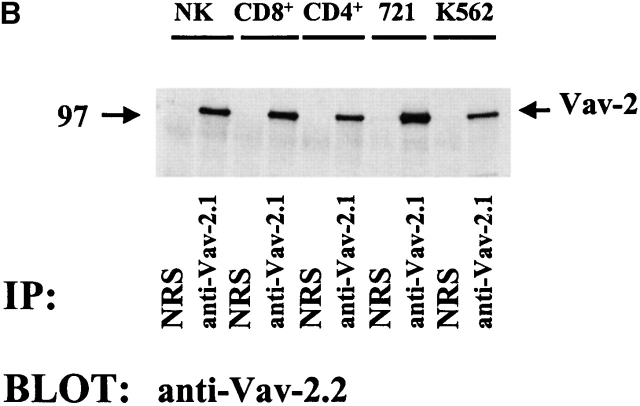
The Vav-2 protein is present in hematopoietic cells. (A) Jurkat T cells (5 × 106) were infected with F.Vav or F.Vav-2 recombinant vaccinia virus and then specifically immunoprecipitated with anti-FLAG mAb (1 μg/ml), anti-Vav (10 μg/ml), or anti–Vav-2.1 (10 μg/ml) specific polyclonal rabbit antisera or NRS as a control. The immunoprecipitates were resolved by SDS-PAGE, transferred to a nylon membrane, and probed with either anti–Vav-2.2 polyclonal rabbit antisera (top, anti–Vav-2.2) or anti-Vav polyclonal rabbit antisera (bottom, anti-Vav). This is a representative example of three separate experiments. (B) Endogenous Vav-2 was specifically immunoprecipitated from the indicated hematopoietic clones and cell lines (25 × 106) using the anti–Vav-2.1 polyclonal rabbit sera or NRS as a control. The immunoprecipitates were resolved by SDS-PAGE, transferred to a nylon membrane, and probed with anti–Vav-2.2 polyclonal rabbit sera. This is a representative example of two independent experiments.
To determine if Vav-2 protein is present in hematopoietic cells, we used the anti–Vav-2.1 sera to specifically immunoprecipitate endogenous Vav-2 from a series of lymphoid cells. We initially immunoprecipitated Vav-2 from a limited number of cells (5 × 106) but only observed a weak band after Western blotting with the anti–Vav-2.2 sera. This was in contrast to the amount of Vav protein that could be detected after immunoprecipitation from the same number of cells (data not shown). In fact, there is approximately three- to fourfold more Vav protein in NK cells, as determined by either flow cytometry analysis of intracellular staining or by immunoblotting (data not shown). The low level of protein expression is consistent with previous Northern blot analysis demonstrating lower levels of Vav-2 RNA in the thymus and spleen compared with Vav RNA 17. However, when we immunoprecipitated Vav-2 from 25 × 106 cells, Vav-2 protein was readily detectable in not only the NK and CD8+ and CD4+ T cell clones, but also in the 721 B lymphoblastoid cell line and the K562 erythroid cell line (Fig. 1 B). These data demonstrate that Vav-2 protein is present in the various hematopoietic cell types tested, albeit at a lower level than Vav.
Vav-2 Becomes Tyrosine Phosphorylated after FcR and TCR Cross-Linking.
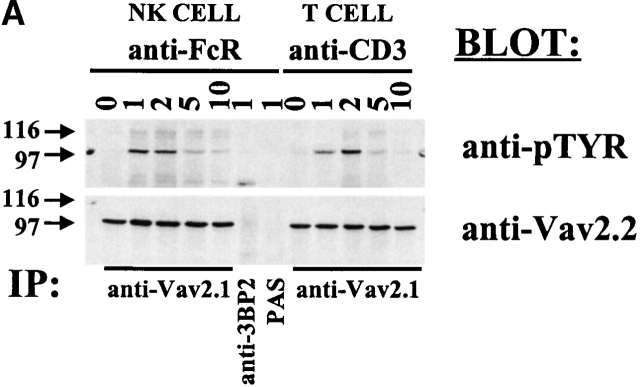
Tyrosine phosphorylation of Vav and Vav-2 is required for their functional activity 12 13 18. It has been shown that Vav will undergo tyrosine phosphorylation after cross-linking of activating receptors on hematopoietic cells. In addition, it has recently been shown that both the Src and the Syk family PTKs can phosphorylate Vav-2 in vitro 18. We therefore wanted to determine if Vav-2 would undergo tyrosine phosphorylation after the cross-linking of the FcγRIIIA on NK clones and the TCR on a CD8+ T cell clone. As indicated in Fig. 2 A, the NK clone or a CD8+ T cell clone were left unstimulated or stimulated through the FcR or TCR, respectively, over the indicated time course. Endogenous Vav-2 protein was then specifically immunoprecipitated using anti–Vav-2.1, separated by SDS-PAGE, transferred to a nylon membrane, and probed with anti-pTYR mAb 4G10. As can be seen in Fig. 2 A, cross-linking of either the FcR or the TCR results in Vav-2 tyrosine phosphorylation (top panel). The tyrosine phosphorylation of Vav-2 is rapid, peaking between 1 and 2 min of cross-linking and decreasing to background levels by 10 min (Fig. 2 A, top). Tyrosine-phosphorylated proteins migrating at the same size as Vav-2 were not evident from FcR-stimulated NK cells immunoprecipitated with protein A–Sepharose (Fig. 2 A, PAS, top), or with anti-3BP2 peptide-purified polyclonal rabbit sera (anti-3BP2, top). These results demonstrate that Vav-2 is coupled to specific activating receptors on these two distinct lymphocyte populations.
Figure 2.
Vav-2 is tyrosine phosphorylated after the cross-linking of activating receptors on cytotoxic lymphocytes. (A) NK clones (25 × 106) or a CD8+ T cell clone (25 × 106) was stimulated with anti-FcR mAb 3G8 (1 μg/ml) or anti-CD3 mAb OKT3 (1 μg/ml) over the indicated time course. After the stimulation, Vav-2 was specifically immunoprecipitated with anti–Vav-2.1 rabbit polyclonal sera. As negative controls, stimulated cell lysates were immunoprecipitated with an irrelevant peptide-purified polyclonal rabbit antibody (anti-3BP2) or protein A–Sepharose alone (PAS). The immunoprecipitates were resolved by SDS-PAGE, transferred to a nylon membrane, and probed with either anti-pTYR mAb 4G10 (top) or anti–Vav-2.2 polyclonal rabbit sera (bottom). (B) NK clones (5 × 106) were infected with F.Vav-2 recombinant vaccinia virus for 5 h. After the infection, the cells were incubated with 2.5 × 106 NK-sensitive K562 target cells for the indicated time, and then F.Vav-2 was specifically immunoprecipitated from the infected NK clone using anti-FLAG mAb. The immunoprecipitates were resolved by SDS-PAGE, transferred to a nylon membrane, and probed with either anti-pTYR mAb 4G10 (top) or anti-FLAG mAb (bottom).
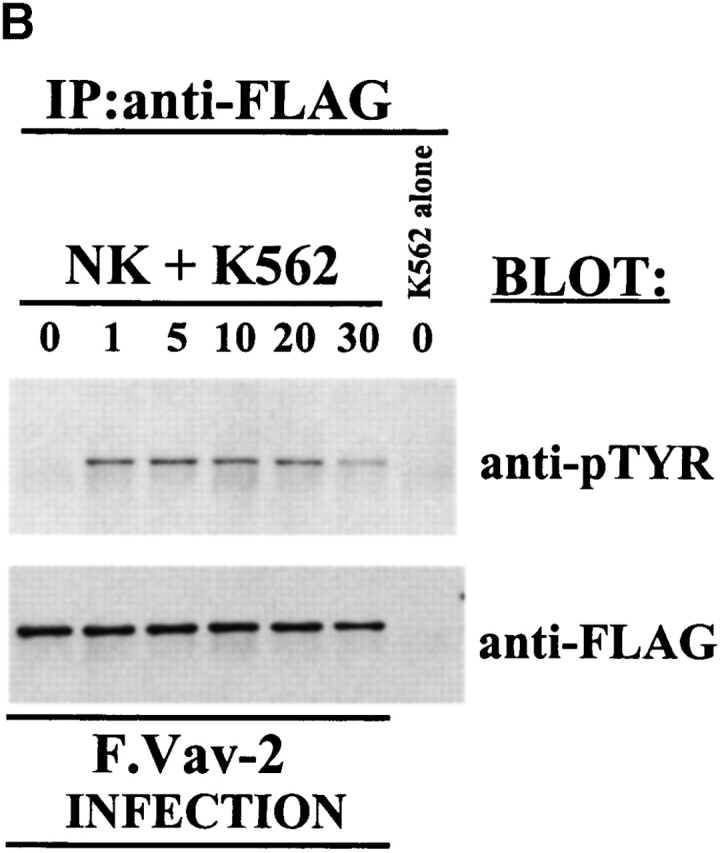
Since it has been shown that different intracellular signals can be required for the generation of antibody-dependent cellular cytotoxicity (ADCC) and natural killing 28, we wanted to determine if Vav-2 would become biochemically modified during the generation of natural cytotoxicity. To do this, we infected NK clones with the F.Vav-2 recombinant vaccinia virus. This allows us to distinguish Vav-2 protein derived from NK cells from that of the NK-sensitive target cells, since both cell types express Vav-2 (Fig. 1 B). The F.Vav-2–infected NK clones were then incubated with the erythroblastic leukemia cell line K562 for the indicated time (Fig. 2 B), and the FLAG-tagged recombinant protein was then specifically immunoprecipitated and analyzed for tyrosine phosphorylation. As shown in Fig. 2 B, F.Vav-2 underwent tyrosine phosphorylation during the generation of natural cytotoxicity against the K562 cell line with tyrosine phosphorylation peaking between 1 and 5 min and decreasing to basal levels by 10 min. Similar results were obtained when F.Vav-2 tyrosine phosphorylation was analyzed after the incubation of F.Vav-2 recombinant vaccinia–infected NK clones with the NK-sensitive C1R or 721 B lymphoblastoid target cell lines (data not shown). These data indicate that Vav-2 is linked to activating receptors on NK cells that initiate the development of natural cytotoxicity toward these susceptible targets.
Vav-2 Enhances Cell-mediated Killing by Cytotoxic Lymphocytes.
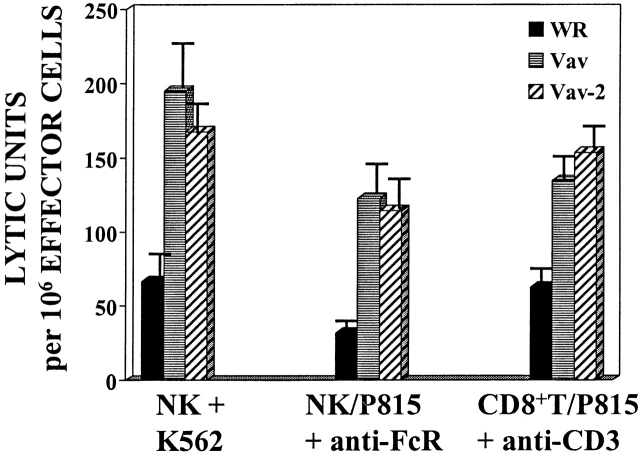
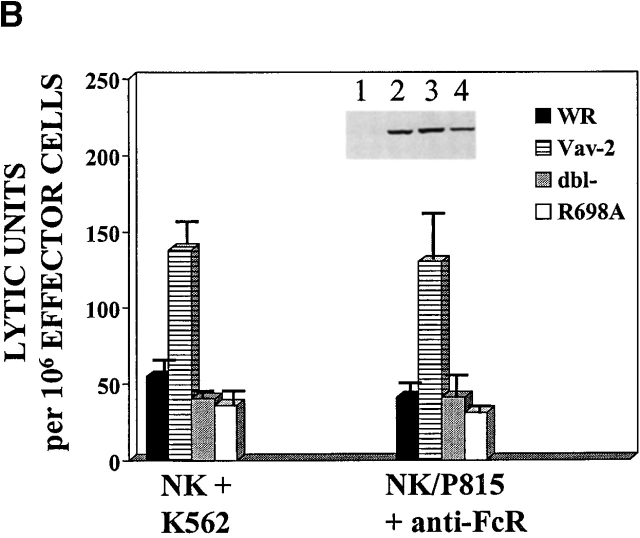
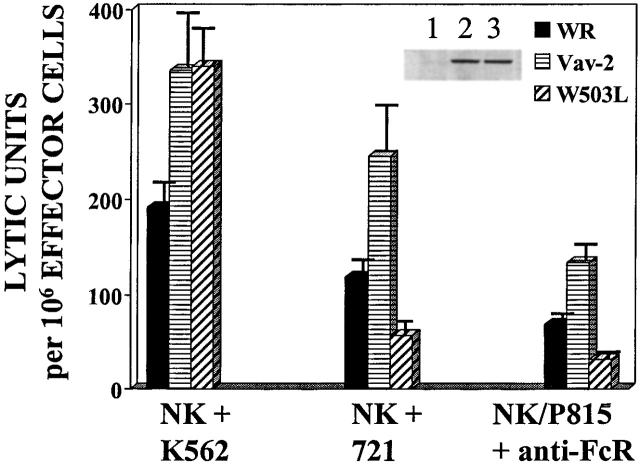
The above data identify Vav-2 as a signaling molecule that is biochemically modified as a result of cross-linking of cell surface activating receptors on cytotoxic lymphocytes. To determine if Vav-2 influences cell-mediated killing, we infected NK clones or a CD8+ T cell clone with recombinant vaccinia virus expressing FLAG-tagged versions of Vav-2, Vav as a positive control, or the parental virus WR as a control for viral effect on killing. As previously noted 11, NK cells overexpressing Vav have significantly enhanced killing of K562 target cells (Fig. 3, NK/K562 compare WR to Vav). In addition, NK cells overexpressing Vav-2 show comparable enhanced killing toward the K562 target cell (Fig. 3, compare Vav and Vav-2). The ability of Vav-2 to enhance natural cytotoxicity is not restricted to killing of K562 target cells, since Vav-2–overexpressing NK clones similarly enhance killing of both the C1R and 721 B lymphoblastoid target cell lines (see below and data not shown). Also, using reverse ADCC and the FcR-bearing P815 target cell, we found that similar to NK clones overexpressing Vav, Vav-2–overexpressing NK clones increase killing initiated through the FcR (Fig. 3, NK/P815 + α-FcR). Importantly, Vav-2–overexpressing NK cells and T cells did not kill P815 in the absence of the appropriate stimulatory antibody or upon incubation with an isotype-matched control antibody (data not shown). Lastly, in a reverse ADCC using α-CD3 mAb, we found that killing by a CD8+ T cell clone was enhanced by overexpression of Vav-2 to levels observed by Vav overexpression (Fig. 3, CD8+ T/P815 + α-CD3). In addition, this enhancement by Vav-2 was observed in other CTL lines stimulated with α-CD3 in a reverse ADCC assay, as well as tumor-initiated killing by a melanoma-specific CTL (data not shown). Taken together, these data suggest that Vav-2 is involved in the regulation of the cytolytic machinery during the generation of natural killing and FcR-mediated killing by NK clones and TCR-initiated killing by CD8+ T cells.
Figure 3.
Overexpression of Vav-2 enhances killing of cytotoxic lymphocytes. NK clones (2 × 106) and CD8+ T cell clones (106) were infected with the indicated recombinant vaccinia virus. The infected NK clones were then incubated with either 51Cr-labeled K562 cells (NK + K562) or 51Cr-labeled P815 cells coated with 0.15 μg/ml of the anti-FcR mAb 3G8 (NK/P815 + anti-FcR). The infected CD8+ T cell clones were incubated with 51Cr-labeled P815 cells coated with 0.15 μg/ml of anti-CD3 mAb OKT3 (CD8+ T/P815 + anti-CD3). The data are expressed as lytic units. Data shown is representative of four separate experiments.
The Vav-2 DH and SH2 Domains Are Required for Enhanced Cellular Cytotoxicity.
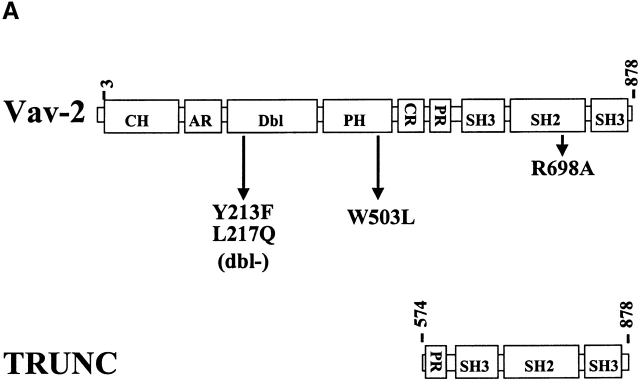
The above data indicate that Vav-2 is involved in the regulation of cell-mediated killing by cytotoxic lymphocytes. To determine what structural features of Vav-2 are required for its function, we generated a series of inactivating point mutations (Fig. 4 A) and a truncation mutant containing the Vav-2 PR–SH3–SH2–SH3 domains (TRUNC; amino acids 574–878). Mutation of highly conserved residues within the Vav DH domain produce a protein lacking GEF activity 12 29. To determine if Vav-2 GEF activity was required for enhanced cellular cytotoxicity, we mutated the analogous residues in the DH domain of Vav-2 (Fig. 4 A, Y213F/L217Q [Dbl−]). We then infected NK clones with recombinant vaccinia encoding wild-type F.Vav-2 or the Dbl− mutant and measured their cytolytic potential. As shown in Fig. 4 B, overexpression of Vav-2 in NK clones results in enhancement of both forms of cellular cytotoxicity, whereas overexpression of the Dbl− mutant does not. The inability of the Dbl− mutant to enhance killing is not due to the level of expression of the recombinant protein, as both Vav-2 and the Dbl− mutant are expressed to similar levels (Fig. 4 B, inset, compare lanes 2 and 4). These data suggest that a functional DH domain is required for Vav-2 enhancement of cellular cytotoxicity.
Figure 4.
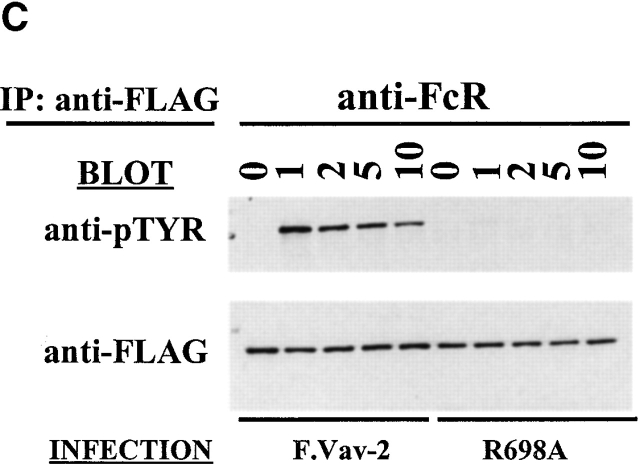
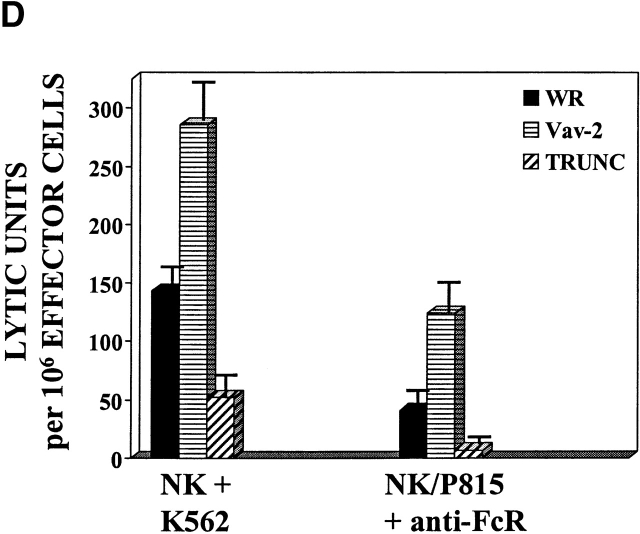
The Vav-2 Dbl homology and SH2 domains are required for enhanced cellular cytotoxicity. (A) The human Vav-2 protooncogene is an 878–amino acid protein that has a number of complex structural domains, including a calponin homology (CH), acidic (AR), Dbl homology (Dbl), pleckstrin homology (PH), cysteine-rich (CR), proline-rich (PR), SH3, and SH2 domains. Wild-type Vav-2 is schematically depicted in the top line of this figure (Vav-2). The mutants used in this study include: the inactivating Dbl homology domain mutant Y213F/L217Q (Dbl−), the inactivating W503L PH domain mutant, and an inactivating SH2 domain mutant R698A. In addition, a truncated version of Vav-2 was produced that contains the PR–SH3–SH2–SH3 domains (TRUNC). All mutants contain an NH2-terminal FLAG epitope. (B and D) NK clones (2 × 106) were infected with the indicated recombinant vaccinia virus. Cellular cytotoxicity of the infected NK clones was assayed as described in Fig. 3. The levels of recombinant protein were determined by Western blot of whole cell lysates prepared from infected NK clones (106). The data are expressed as lytic units. Data shown is representative of six separate experiments. (C) NK clones (5 × 106) were infected with the indicated recombinant vaccinia virus, stimulated with anti-FcR mAb 3G8 (1 μg/ml) at 37°C over the indicated time course, and then specifically immunoprecipitated with anti-FLAG mAb (1 μg/ml). The immunoprecipitates were resolved by SDS-PAGE, transferred to a nylon membrane, and probed with either antiphosphotyrosine mAb 4G10 (top, anti-pTYR) or anti-FLAG mAb (bottom, anti-FLAG). This is a representative example of three separate experiments.
Tyrosine phosphorylation of Vav family members is required for GEF activity. The ability of Vav to couple to activating receptors and undergo receptor-initiated tyrosine phosphorylation requires an intact SH2 domain 14 15. To determine if the SH2 domain of Vav-2 is required for its ability to couple to activating receptors in NK cells, we generated an inactivating mutation in the highly conserved FLVR sequence of the Vav-2 SH2 domain (Fig. 4 A, R698A). We subsequently infected NK clones with FLAG-tagged versions of wild-type Vav-2 or the R698A mutant and measured tyrosine phosphorylation after FcR cross-linking. As shown in Fig. 4 C, in contrast to wild-type Vav-2, the R698A mutant does not undergo tyrosine phosphorylation after cross-linking of the FcR on NK cells, indicating that a functional SH2 domain is required for its ability to couple to the FcR. The R698A mutant was also unable to couple to activating receptors during the generation of natural cytotoxicity toward the K562 or 721 target cell lines, or after TCR cross-linking on a CD8+ T cell clone (data not shown). As expected, overexpression of the R698A mutant in NK clones does not result in enhanced natural cytotoxicity or FcR-initiated killing (Fig. 4 B, compare Vav-2 to R698A). The R698A mutant was also unable to enhance TCR-initiated killing by a CD8+ T cell clone (data not shown). These data indicate that the SH2 domain of Vav-2 is required for its ability to link to activating receptors and undergo tyrosine phosphorylation.
It has been demonstrated that overexpression of a truncated version of Vav, Vav-C, that contains the COOH-terminal SH2 and SH3 domains could inhibit Vav-regulated NFAT–AP-1–mediated gene transcription 15. These data suggest that the SH2 domain of Vav plays a significant role in the regulation of Vav activation after receptor cross-linking. We therefore wanted to determine if a similar mutant of Vav-2 (Fig. 4 A, TRUNC) would antagonize cell-mediated killing. As shown in Fig. 4 D, NK cell–mediated cellular cytotoxicity can be inhibited by overexpression of the truncation mutant of Vav-2. The ability of the Vav-2 TRUNC mutant to inhibit cell-mediated killing requires a functional SH2 domain, as an inactivating mutation (R698A) in the TRUNC mutant reverses its inhibitory effect (data not shown). Interestingly, although the Vav-C truncation mutant undergoes TCR-mediated tyrosine phosphorylation, the Vav-2 TRUNC mutant does not become tyrosine phosphorylated after FcR or TCR cross-linking (data not shown). Therefore, in contrast to Vav, potential tyrosine phosphorylation sites are not present in the COOH terminus of Vav-2.
The PH Domain Is Important in the Activation of Vav-2 from Some but Not All NK Cell Activating Receptors.
It was previously suggested that the PH domain is required for Vav tyrosine phosphorylation and GEF activity 30. Interestingly, mutation of the Vav-3 PH domain had no effect on its GEF activity 31. We therefore mutated a highly conserved tryptophan in the Vav-2 PH domain in an attempt to determine the role of this domain in regulating Vav-2 tyrosine phosphorylation and activity during the generation of cell-mediated killing (Fig. 4 A, W503L). This mutation has been shown to inactivate the PH domain of other Rho and Rac GEFs 31 32. We initially analyzed the W503L mutants ability to couple to activating receptors in cytotoxic lymphocytes. Interestingly, we found no difference in the level or duration of receptor-mediated tyrosine phosphorylation of Vav-2 or the W503L mutant after FcR or target stimulation of NK clones or after TCR cross-linking in the CD8+ T cell clone (data not shown). However, it remained possible that qualitative differences in tyrosine phosphorylation or localization of the protein might impact on Vav-2 function. As shown in Fig. 5, overexpression of the W503L mutant in NK clones results in enhanced killing of the K562 target cell line, but natural cytotoxicity toward the 721 B lymphoblastoid cell line was not similarly enhanced (Fig. 5, compare NK + K562 to NK + 721). In addition, the W503L mutant was unable to enhance killing initiated through the NK FcR or TCR-initiated killing by a CD8+ T cell clone (Fig. 5, NK/P815 + α-FcR, and data not shown). In fact, overexpression of the W503L mutant consistently inhibited killing to below that observed by WR, indicating that this mutant functions as a dominant negative (Fig. 5, compare WR to W503L). These data suggest that the PH domain is required for Vav-2 enhancement of cell-mediated killing after engagement of certain activating receptors on cytotoxic lymphocytes but not others.
Figure 5.
The Vav-2 PH domain is required for enhanced cell-mediated killing after stimulation through some, but not all activating receptors. NK clones (2 × 106) were infected with the indicated recombinant vaccinia virus (inset). Cellular cytotoxicity of the infected NK clones was assayed as described in Fig. 3. In addition to K562, natural cytotoxicity toward the B lymphoblastoid cell line 721 was also assessed. The levels of recombinant protein were determined by Western blot of whole cell lysates prepared from infected NK clones (106). The data are expressed as lytic units. Data shown is representative of four separate experiments.
Vav-2 Does Not Activate NFAT–AP-1–mediated Gene Transcription after TCR Cross-Linking.
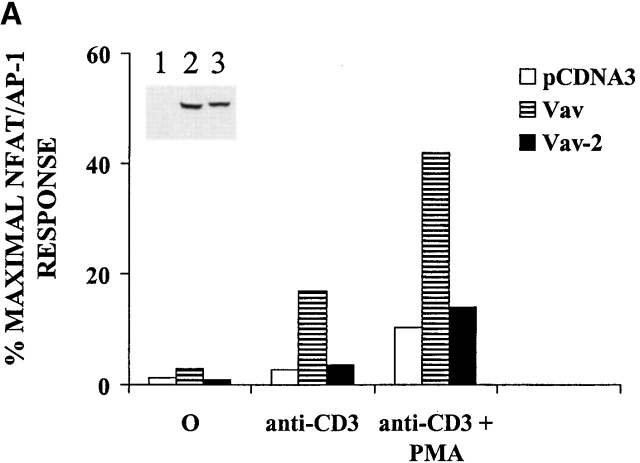
Previous studies have focused on a significant role for Vav in the regulation of the NFAT–AP-1 response element in the IL-2 promoter after TCR cross-linking 33 34. We therefore tested whether Vav-2 could regulate the IL-2 promoter after TCR cross-linking. Jurkat T cells were electroporated with a control vector, FLAG-tagged Vav expression vector, or Vav-2 expression vector along with an NFAT–AP-1 luciferase reporter construct. After an overnight incubation, the cells were stimulated as indicated in Fig. 6 A, and luciferase activity was measured and is expressed as the percent maximal NFAT–AP-1 response. As can be seen in Fig. 6 A, overexpression of Vav in Jurkat T cells results in increased levels of NFAT–AP-1 activity after anti-CD3 and anti-CD3 + PMA stimulation. However, overexpression of Vav-2 had no significant effect on the levels of NFAT–AP-1 activation under any stimulation conditions (Fig. 6 A, compare Vav to Vav-2). The inability of Vav-2 to regulate the NFAT–AP-1 response was not due to levels of expression of recombinant protein, as both Vav and Vav-2 were expressed at similar levels (Fig. 6 A, inset). In addition, compared with Vav, Vav-2 underwent similar kinetics of tyrosine phosphorylation in Jurkat T cells after TCR cross-linking (data not shown).
Figure 6.
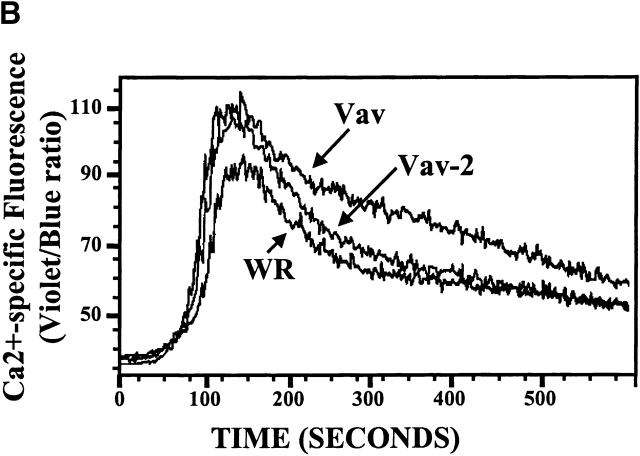
Vav-2 does not regulate the IL-2 promoter after TCR cross-linking. (A) Jurkat T cells (107) were electroporated with 10 μg of an NF-AT/AP-1.luciferase reporter construct and 20 μg of the indicated regulatory plasmids. Both Vav and Vav-2 constructs contain an NH2-terminal eight–amino acid FLAG epitope. After an overnight rest, the cells were either left unstimulated or stimulated for 6 h with anti-CD3 mAb OKT3 (1 μg/ml), a combination of anti-CD3 (1 μg/ml) and PMA (20 ng/ml), or a combination of PMA (20 ng/ml) and ionomycin (2 μM). Cells were then harvested and assayed for luciferase activity as described in Materials and Methods. The data are presented as the percentage of maximal NFAT–AP-1 activity obtained by stimulation with PMA + ionomycin. Maximum NFAT–AP-1 responses did not vary significantly between transfected samples. (Top left insets) Protein expression in the transfected cells was verified by immunoprecipitation of 2 × 106 electroporated cells with anti-FLAG mAb (1 μg/ml), followed by immunoblotting with the same antibody. The data shown is representative of three separate experiments. (B) Jurkat T cells (5 × 106) were infected with nonrecombinant vaccinia (WR) or recombinant vaccinia expressing Vav or Vav-2. After infection, the cells were loaded with Indo-1 (5 μM), divided in half, and treated with goat anti–mouse IgG F(ab′)2 alone or stimulated with a combination of anti-CD3 (2.5 μg/ml) and goat anti–mouse IgG F(ab′)2. The samples were immediately analyzed by flow cytometry over the indicated time course. The data from the anti-CD3– and goat anti–mouse IgG F(ab′)2–stimulated cells is shown. Goat anti–mouse IgG F(ab′)2 treated cells did not demonstrate Ca2+ fluxes in the absence of anti-CD3 mAb (data not shown). This is a representative example of four separate experiments.
Activation of NFAT Requires a Sustained Level of Intracellular Ca2+ after TCR Engagement.
It has recently been found that Vav is involved in the regulation of intracellular Ca2+ after TCR engagement 27 35. One possible reason for the inability of Vav-2 to regulate NFAT–AP-1–mediated gene transcription is that it cannot regulate intracellular Ca2+ levels after TCR engagement. Indeed, as shown in Fig. 6 B, TCR cross-linking of Indo-1–loaded Jurkat T cells overexpressing Vav leads to a sustained level of intracellular Ca2+ over the duration of the assay compared with the control-infected population (Fig. 6 B, compare Vav to WR). However, whereas Vav-2–overexpressing cells demonstrate an initial increase in intracellular Ca2+ after TCR engagement compared with control infected cells, the sustained response is diminished compared with Vav-expressing cells (Fig. 6 B, compare Vav to Vav-2). Although Vav-2 overexpression does not lead to sustained levels of intracellular Ca2+ after TCR engagement, its inability to regulate the NFAT–AP-1 response element of the IL-2 promoter may be due in part to its activation of different Rho family proteins compared with those activated by Vav. Taken together, these data suggest that Vav-2 does not impact NFAT–AP-1–mediated gene transcription after TCR cross-linking.
Discussion
The Rac and Rho families of GTPases are intimately involved in the regulation of various hematopoietic cell effector functions, including gene transcription, phagocytosis, and the development of cell-mediated killing 1 11 36 37. Previous genetic and pharmacologic approaches have demonstrated that RhoA is a critical regulator of cell-mediated killing by cytotoxic lymphocytes 11 23. It is clear that Vav plays a critical role in the regulation of Rac family effector functions downstream of activating receptors on lymphocytes 38 39, including NK cells 11 22. However, a specific Rho family GEF has not been identified that links activating receptors on cytotoxic lymphocytes to Rho-regulated effector functions. In this study, we have identified that Vav-2 is expressed in various hematopoietic cells, including NK cells and T cells, and have shown for the first time that Vav-2 is coupled to activating receptors on cytotoxic lymphocytes. Furthermore, we found that overexpression of Vav-2 in cytotoxic lymphocytes results in increased cell-mediated killing of susceptible targets. In addition, Vav-2 GEF activity toward Rho family GTPases is required for its ability to enhance cellular cytotoxicity, since an inactivating DH mutant was unable to enhance killing (Fig. 4 B, Dbl−). Taken together, these data highlight a role for the Vav-2 GEF in the generation of cell-mediated killing by cytotoxic lymphocytes.
Many proteins, in particular those with enzymatic function, when overexpressed in an inactive form function as dominant negatives by presumably linking to their appropriate upstream signaling cascades and inhibiting downstream signals. It is interesting that neither the Vav-2 GEF inactive mutant (Dbl−) nor a similar Dbl-inactivating Vav mutant function as dominant negatives even though the mutant protein is still capable of undergoing receptor-initiated tyrosine phosphorylation, implying that it is recruited to the activating receptor complex (data not shown). Although it has been shown that point mutations or small deletions within the Dbl domain result in the loss of GEF activity, it is not clear if this is due to a lack of interaction of the GEF with its respective GTP-binding protein or decreased GEF activity toward bound GTPases 12 29 32. Therefore, if the Vav-2 Dbl− protein was still recruited to the receptor complex but fails to interact with its target GTPases, then endogenously activated GEFs could still function to regulate their respective GTP-binding proteins. Further studies will be required to elucidate the mechanism by which mutation of the Vav family Dbl domain influences its GEF activity.
It was previously demonstrated in vitro that both Src and Syk family PTKs can phosphorylate Vav-2 and that this tyrosine phosphorylation was required for Vav-2 GEF functional activity 12 13. In this study, we demonstrate that Vav-2 becomes tyrosine phosphorylated after FcR and TCR cross-linking on NK cells and T cells, respectively, and during the development of natural killing. However, the specific Src or Syk family protein tyrosine kinase(s) that mediate Vav-2 tyrosine phosphorylation downstream of these activating receptors remains to be identified. The SH2 domain of Vav is required for its ability to couple to activating receptors and carry out Vav-regulated effector functions 14 15. Clearly, the ability of Vav-2 to regulate cell-mediated killing downstream of these activating receptors requires a functional SH2 domain, since inactivation of the SH2 domain uncouples Vav-2 from receptor-mediated tyrosine phosphorylation and the generation of cell-mediated killing (Fig. 4b and Fig. c). The inability of this mutant to function as a dominant negative is most likely due to lack of recruitment to the activating receptors, where it would putatively interact not only with its downstream GTP-binding proteins but potentially other cell signaling molecules. Indeed, Vav has been shown to interact with numerous growth factor receptors upon ligand engagement and also with numerous signaling molecules that undergo tyrosine phosphorylation after receptor cross-linking 5 6 38 39. SH2 domains contain highly conserved phosphotyrosine binding residues and variable residues that mediate binding to the side chains of amino acids that flank the phosphotyrosine 40. The SH2 domain–containing proteins can be grouped on the basis of these variable residues, and the presence of a threonine at the βD5 position of the Vav SH2 domain places it in group II, possessing specificity toward pYMEP-containing peptides 41 42. Interestingly, neither Vav-2 nor Vav-3 encode a threonine at this position. Therefore, determining whether the Vav, Vav-2, or Vav-3 SH2 domains interact with distinct or an overlapping set of tyrosine-phosphorylated proteins will provide insight into how this family of proteins is regulated.
The overexpression of a truncated version of Vav containing the COOH-terminal SH2 and SH3 domains, Vav-C, was found to block TCR-mediated, NFAT–AP-1–mediated gene transcription 15. However, a mutant Vav-C, Vav-CSH2RK, containing an inactivating mutation in the SH2 domain could not similarly suppress NFAT–AP-1 gene transcription, suggesting that the Vav SH2 domain must serve to couple Vav to upstream TCR-initiated signals. Similarly, we have found that a Vav-2 truncation mutant containing the COOH-terminal PR–SH3–SH2–SH3 domains (TRUNC; Fig. 4 D, compare Vav-2 to TRUNC), but not a TRUNC mutant carrying an SH2 inactivating mutation, inhibits cell-mediated killing when overexpressed in cytotoxic lymphocytes (our unpublished observation). Interestingly, although the Vav-C mutant underwent tyrosine phosphorylation after TCR cross-linking 15, we have not observed tyrosine phosphorylation of the Vav-2 TRUNC mutant after FcR or TCR cross-linking (data not shown). This suggests that in contrast to Vav, Vav-2 contains no tyrosine within this domain that become phosphorylated after cross-linking of these specific receptors. We are currently investigating whether the Vav-2 TRUNC mutant functions as a dominant negative by antagonizing Vav-2 tyrosine phosphorylation or by preventing recruitment to areas of activation during the generation of cell-mediated killing.
The PH domains of numerous proteins have been shown to interact with membrane phospholipids generated by phosphatidyl inositol 3-kinase (PI3-K; reference 43). Interestingly, all Dbl family proteins have the characteristic of a DH domain followed by a PH domain 2. This conserved structural architecture within the Dbl family of proteins suggests that these two domains have important roles in the regulation of their activity. However, the role that the PH domain plays in the regulation of GEF activity among the various Dbl family members appears to be protein specific. For instance, it has been shown that deletion or mutation of the PH domains of Dbl, Vav-3, or onco-Lbc does not impair their intrinsic GEF activity 31 32 44. However, it was recently shown in vitro that the Vav PH domain interacts with PI3-K–generated phospholipids, and that this interaction is required for not only protein tyrosine kinase–mediated tyrosine phosphorylation of Vav but also its GEF activity 30. Interestingly, the W503L PH domain mutant of Vav-2 behaves similarly to a deletion mutant of Vav 27 in that it enhances killing through some but not all activating receptors on NK cells and T cells. We have previously shown that treatment of NK cells with wortmannin, a drug that inhibits PI3-K activity, decreased FcR-mediated but not natural killing of the K562 target cell line 28. Moreover, initial experiments have suggested that NK cells treated with wortmannin have decreased cytotoxicity toward 721 target cells (data not shown). It is therefore possible that certain activating receptors (TCR, FcR, and those that mediate killing of 721 cells) rely on the generation of membrane phospholipids by PI3-K for the subsequent recruitment and compartmentalization of PH domain–containing molecules required for the generation of specific effector functions. We are currently investigating the role that the Vav and Vav-2 PH domains have in targeting these molecules to the correct compartment during lymphocyte activation.
It is well known that Vav plays a significant role in the regulation of T cells after TCR cross-linking 45. Most importantly, Vav has been shown to be a potent regulator of the NFAT–AP-1 transcription complex within the IL-2 promoter 33 34. In contrast to Vav, we found that overexpression of Vav-2 in the Jurkat T cell line did not result in enhanced NFAT–AP-1–mediated gene transcription after TCR cross-linking (Fig. 6 A). Recently, using thymocytes from Vav-deficient mice, it was found that Vav plays a significant role in the regulation of intracellular Ca2+ after TCR cross-linking 35. Sustained levels of intracellular Ca2+ are required for the activation of the phosphatase calcineurin. Upon activation, calcineurin dephosphorylates cytoplasmic NFAT, resulting in nuclear localization and accumulation of NFAT 46. We have recently shown that the CH domain of Vav is important in regulating the increase in intracellular Ca2+ after TCR cross-linking in the Jurkat T cell 27. As shown in Fig. 6 B, compared with Vav, Vav-2 does not lead to sustained levels of intracellular Ca2+ after TCR cross-linking of Indo-1–loaded Jurkat T cells. One possible reason for this is that the CH domains of Vav and Vav-2, although homologous, have different regulatory functions. Consistent with this is the observation that although partial deletion of the CH domain of Vav produces an oncogenic protein, truncation of both the CH and acidic regions of Vav-2 is required for production of an oncogenic protein 18. However, the fact that Vav and Vav-2 regulate distinct Rac and Rho family members may also be a reason why Vav, but not Vav-2, regulates NFAT–AP-1–mediated gene transcription.
It was recently found that a constitutively active mutant of Rho, V14Rho could synergize with PMA in the regulation of an isolated AP-1 response element from the IL-2 promoter 47. Interestingly, V14Rho did not regulate the transcriptional activity of luciferase reporter constructs containing isolated NFAT–AP-1, Oct-1, or nuclear factor κB response elements 47. Furthermore, inactivation of Rho by overexpression of C3 exoenzyme in the thymus results in a small thymus and decreased cellularity 48. It has also been shown that Rho is important for the survival of pre-T cells and regulates their cell cycle progression 49. Therefore, it is possible that the Rho family GEF Vav-2 plays a significant role downstream of activating receptors in maturing T cells, resulting in the activation of Rho family members and their downstream signaling cascades.
In this study, we have shown for the first time that Vav-2 protein is present in hematopoietic cells and that it undergoes receptor-mediated tyrosine phosphorylation after cross-linking of activating receptors on NK and T cells. In addition, we have shown that Vav-2 plays a significant role in the regulation of cell-mediated killing by cytotoxic lymphocytes. Clearly, identifying molecules that interact with Vav-2 and determining the role that Vav-2 has in the regulation of other hematopoietic cells will be an important step toward understanding this new member of the Vav family of GEFs.
Acknowledgments
We would like to thank Dr. David J. Kwiatkowski for kindly providing the human Vav-2 cDNA.
This research was supported by the Mayo Foundation and by National Institutes of Health grant CA-47752 to P.J. Leibson. D.D. Billadeau is a Special Fellow of the Leukemia and Lymphoma Society and is also supported by a grant from the Levy Foundation.
Footnotes
Abbreviations used in this paper: ADCC, antibody-dependent cellular cytotoxicity; AP, activator protein; CH, calponin homology; DH, Dbl homology; GEFs, guanine nucleotide exchange factors; NFAT, nuclear factor of activated T cells; NRS, normal rabbit serum; PH, pleckstrin homology; PI3-K, phosphatidyl inositol 3-kinase; PR, proline-rich region; PTKs, protein tyrosine kinases; SH, Src homology; WR, wild-type vaccinia virus.
References
- Henning S.W., Cantrell D.A. GTPases in antigen receptor signalling. Curr. Opin. Immunol. 1998;10:322–329. doi: 10.1016/s0952-7915(98)80171-4. [DOI] [PubMed] [Google Scholar]
- Cerione R.A., Zheng Y. The Dbl family of oncogenes. Curr. Opin. Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. Vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S., Cleveland J.L., Helsop H.E., Pulido D. Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Mol. Cell Biol. 1991;11:1912–1920. doi: 10.1128/mcb.11.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S. VavCaptain hook for signal transduction? Crit. Rev. Oncog. 1995;6:87–97. [PubMed] [Google Scholar]
- Bustelo X.R. Regulatory and signaling properties of the Vav family. Mol. Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo X.R., Ledbetter J.A., Barbacid M. Product of the vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992;356:68–71. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- Bustelo X.R., Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992;256:1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- Darby C., Geahlen R.L., Schreiber A.D. Stimulation of the macrophage FcγRIIIA activates the receptor-associated protein tyrosine kinase Syk and induces phosphorylation of multiple proteins including p95Vav and p62/GAP-associated protein. J. Immunol. 1994;152:5429–5437. [PubMed] [Google Scholar]
- Xu X., Chong A.S. Vav in natural killer cells is tyrosine phosphorylated upon cross-linking of FcγRIIIA and is constitutively associated with a serine/threonine kinase. Biochem. J. 1996;318:527–532. doi: 10.1042/bj3180527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billadeau D.D., Brumbaugh K.M., Dick C.J., Schoon R.A., Bustelo X.R., Leibson P.J. The Vav–Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J. Exp. Med. 1998;18:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P., Schuebel K.E., Ostrom A.A., Gutkind S.J., Bustelo X.R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;38:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Han J., Das B., Wei W., Van Aelst L., Mosteller R.D., Khosravi-Far R., Westwick J.K, Der C.J., Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S. Single point mutations in the SH2 domain impair the transforming potential of vav and fail to activate proto-vav. Oncogene. 1993;8:1757–1763. [PubMed] [Google Scholar]
- Wu J., Motto D.G., Koretzky G.A., Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- Henske E.P., Short M.P., Jozwiak S., Bovey C.M., Ramlakhan S., Haines J.L., Kwiatkowski D.J. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann. Hum. Genet. 1995;5:25–37. doi: 10.1111/j.1469-1809.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Schuebel K.E., Bustelo X.R., Nielsen D.A., Song B.-J., Barbacid M., Goldman D., Lee I.J. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- Schuebel K.E., Movilla N., Rosa J.L., Bustelo X.R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K.J., Abraham R.T., Binstadt B.A., Uehara Y., Leibson P.J. Tyrosine phosphorylation provides and early and requisite signal for the activation of natural killer cell cytolytic function. Proc. Natl. Acad. Sci. USA. 1991;88:6279–6283. doi: 10.1073/pnas.88.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J.J., McVicar D.W., Kuhns D.B., Ortaldo J.R. A role for protein tyrosine kinase activity in natural cytotoxicity as well as antibody-dependent cellular cytotoxicity. J. Immunol. 1992;148:2497–2502. [PubMed] [Google Scholar]
- Brumbaugh K.M., Binstadt B.A., Billadeau D.D., Schoon R.A., Dick C.J., Ten R.M., Leibson P.J. Functional role for the Syk tyrosine kinase in NK cell–mediated natural cytotoxicity. J. Exp. Med. 1997;186:1965–1974. doi: 10.1084/jem.186.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrini R., Palmieri G., Piccoli M., Frati L., Santoni A. Role for the Rac1 exchange factor Vav in the signaling pathways leading to NK cell cytotoxicity. J. Immunol. 1999;162:3148–3152. [PubMed] [Google Scholar]
- Lang P., Guizani L., Vitte-Mony I., Stancou R., Dorseuil O., Gacon G., Bertoglio J. ADP-ribosylation of the ras-related, GTP-binding protein RhoA inhibits lymphocyte-mediated cytotoxicity. J. Biol. Chem. 1992;267:11677–11680. [PubMed] [Google Scholar]
- Windebank K.P., Abraham R.T., Powis G., Olsen R.A., Barna T.J., Leibson P.J. Signal transduction during human natural killer cell activationinositol phosphate generation and regulation by cyclic AMP. J. Immunol. 1988;141:3951–3957. [PubMed] [Google Scholar]
- Hedin K.E., Bell M.P., Kalli K.R., Huntoon C.J., Sharp B.M., McKean D.J. δ-Opioid receptors expressed by Jurkat T cells enhance IL-2 secretion by increasing AP-1 complexes and activity of the NF-AT/AP-1-binding promoter element. J. Immunol. 1997;159:5431–5440. [PubMed] [Google Scholar]
- Pross H.F., Baines M.G., Rubin P., Shragge P., Patterson M.S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J. Clin. Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Billadeau D.D., Mackie S.M., Schoon R.A., Leibson P.J. Specific subdomains of Vav differentially affect T cell and NK cell activation. J. Immunol. 2000;164:3971–3981. doi: 10.4049/jimmunol.164.8.3971. [DOI] [PubMed] [Google Scholar]
- Bonnema J.D., Karnitz L.M., Schoon R.A., Abraham R.T., Leibson P.J. Fc receptor stimulation of phosphatidyl inositol 3-kinase in natural killer cells is associated with protein kinase C–independent granule release and cell-mediated cytotoxicity. J. Exp. Med. 1994;180:1427–1435. doi: 10.1084/jem.180.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P., Bustelo X.R., Aaronson D.S., Coso O.A., Lopez-Barahona M., Barbacid M., Gutkind S.J. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- Han J., Luby-Phelps K., Das B., Shu X., Xia Y., Mosteller R.D., Krishna U.M., Falck J.R., White M.A., Broek D. Role of substrates and products of PI3-kinase in regulating activation of rac-related guanosine triphosphates by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Movilla N., Bustelo X.R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M.P., Sterpetti P., Nagata K., Toksoz D., Hall A. Distinct roles for DH and PH domains in the lbc oncogene. Oncogene. 1996;15:2827–2832. doi: 10.1038/sj.onc.1201594. [DOI] [PubMed] [Google Scholar]
- Holsinger L.J., Spencer D.M., Austin D.J., Schreiber S.L., Crabtree G.R. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc. Natl. Acad. Sci. USA. 1995;92:9810–9814. doi: 10.1073/pnas.92.21.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Katzav S., Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol. Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello P.S., Walters A.E., Mee P.J., Turner M., Reynolds L.F., Prisco A., Sarner N., Zamoyska R., Tybulewicz V.L.J. The rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc. Natl. Acad. Sci. USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Chang P., Zhang Q., Reddy P.G., Bokoch G.M., Greenberg S. Requirements for both Rac1 and CDC42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam D.J., Rotstein O.D., Schreiber A., Zhang W.J., Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J. Exp. Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T.L., Deckert M., Altman A. Views on Vav. Immunol. Today. 1997;18:221–225. doi: 10.1016/s0167-5699(97)01037-2. [DOI] [PubMed] [Google Scholar]
- Cantrell D. Lymphocyte signallinga coordinating role for Vav? Curr Biol. 1998;8:R535–R538. doi: 10.1016/s0960-9822(07)00341-7. [DOI] [PubMed] [Google Scholar]
- Waksman G., Kominos D., Robertson S.C., Pant N., Baltimore D., Birge R.B., Cowburn D., Hanafusa H., Mayer B.J., Overduin M. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine phosphorylated peptides. Nature. 1992;358:646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S.E., Chaudhuri M., Gish G., Pawson T., Haser W.G., King F., Roberts T., Ratnofsky S., Lechleider R.J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S.E., McGlade J., Olivier P., Pawson T., Bustelo X.R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M.A., Ferguson K.M., Schlessinger J. PH domainsdiverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Zangrilli D., Cerione R.A., Eva A. The pleckstrin homology domain mediates transformation by oncogenic Dbl through specific intracellular targeting. J. Biol. Chem. 1996;271:19017–19020. doi: 10.1074/jbc.271.32.19017. [DOI] [PubMed] [Google Scholar]
- Fischer K.-D., Tedford K., Penninger J.M. Vav links antigen-receptor signaling to the actin cytoskeleton. Semin. Immunol. 1998;10:317–327. doi: 10.1006/smim.1998.0124. [DOI] [PubMed] [Google Scholar]
- Crabtree G.R. Generic signals and specific outcomesSignaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Chang J.-H., Pratt J.C., Sawasdikosol S., Kapeller R., Burakoff S.J. The small GTP-binding protein Rho potentiates AP-1 transcription in T cells. Mol. Cell Biol. 1998;18:4986–4993. doi: 10.1128/mcb.18.9.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrini R., Henning S.W., Cantrell D.A. Different functions of the GTPase Rho in prothymocytes and late pre-T cells. Immunity. 1997;7:163–174. doi: 10.1016/s1074-7613(00)80519-1. [DOI] [PubMed] [Google Scholar]
- Henning S.W., Galandrini R., Hall A., Cantrell D.A. The GTPase Rho has a critical regulatory role in thymus development. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]