Abstract
2B4 is a surface molecule involved in activation of the natural killer (NK) cell–mediated cytotoxicity. It binds a protein termed Src homology 2 domain–containing protein (SH2D1A) or signaling lymphocyte activation molecule (SLAM)-associated protein (SAP), which in turn has been proposed to function as a regulator of the 2B4-associated signal transduction pathway. In this study, we analyzed patients with X-linked lymphoproliferative disease (XLP), a severe inherited immunodeficiency characterized by critical mutations in the SH2D1A gene and by the inability to control Epstein-Barr virus (EBV) infections. We show that, in these patients, 2B4 not only fails to transduce triggering signals, but also mediates a sharp inhibition of the NK-mediated cytolysis. Other receptors involved in NK cell triggering, including CD16, NKp46, NKp44, and NKp30, displayed a normal functional capability. However, their activating function was inhibited upon engagement of 2B4 molecules. CD48, the natural ligand of 2B4, is highly expressed on the surface of EBV+ B cell lines. Remarkably, NK cells from XLP patients could not kill EBV+ B cell lines. This failure was found to be the consequence of inhibitory signals generated by the interaction between 2B4 and CD48, as the antibody-mediated disruption of the 2B4–CD48 interaction restored lysis of EBV+ target cells lacking human histocompatibility leukocyte antigen (HLA) class I molecules. In the case of autologous or allogeneic (HLA class I+) EBV+ lymphoblastoid cell lines, restoration of lysis was achieved only by the simultaneous disruption of 2B4–CD48 and NK receptor–HLA class I interactions. Molecular analysis revealed that 2B4 molecules isolated from either XLP or normal NK cells were identical. As expected, in XLP-NK cells, 2B4 did not associate with SH2D1A, whereas similar to 2B4 molecules isolated from normal NK cells, it did associate with Src homology 2 domain–containing phosphatase 1.
Keywords: X-linked lymphoproliferative disease, Epstein-Barr virus infection, natural killer cell, natural cytotoxicity receptor, 2B4 molecule
Introduction
X-linked lymphoproliferative disease (XLP; Mendelian inheritance in man [MIM] 308240) is a severe inherited immune deficiency characterized by abnormal immune responses to EBV 1 2. After EBV infection, most XLP males succumb to fulminant infectious mononucleosis, whereas those who survive develop lymphoma, hypo- or dysgammaglobulinemia, and/or histiocytic infiltration leading to marrow aplasia. The overall prognosis of XLP is very poor, with >70% of affected patients dying by 10 years of age 3. In most cases, before EBV infection, the immunological status of genotypically affected males is normal 4, whereas expansion of CD8+ T cells, reduced NK cytotoxic activity, and diminished response to delayed-type hypersensitivity tests are observed after exposure to EBV 5 6 7.
Recently, different groups have independently identified in XLP families mutations in a novel gene 8 9 10. This gene encodes for a short intracytoplasmic protein of 128 amino acids, termed Src homology 2 domain–containing protein (SH2D1A) or signaling lymphocyte activation molecule (SLAM)-associated protein (SAP). SH2D1A is predominantly expressed in lymphoid tissues (thymus, spleen), and particularly in T lymphocytes 8 9 10. Controversial data have been reported on the expression of SH2D1A in B cells and B cell lines 8 9 10. Although the SH2D1A transcript has also been detected in NK cells, no information is available on the actual presence of the encoded protein 8. The molecular and cellular mechanisms that account for XLP are still poorly defined. It has been proposed that the SH2D1A protein functions as a regulator of the signal transduction pathways initiated by at least two distinct human surface receptors belonging to the CD2 family: namely, signaling lymphocyte activation molecule, which is expressed on T and B cells 9 11, and 2B4, which is primarily expressed on NK cells and on a subset of CD8+ T lymphocytes 12 13 14 15 16. Tyrosine phosphorylation of 2B4 molecules and its association with SH2D1A or Src homology 2 domain–containing phosphatase (SHP)-2 have been detected in cell transfectants. In this context, it has been suggested that binding of SH2D1A to 2B4 may prevent its association with SHP-2. This molecular interaction may be crucial for transducing activating signals via 2B4 17. However, in normal NK cells, although tyrosine phosphorylation of 2B4 was observed upon treatment with sodium pervanadate, neither SH2D1A nor SHP-2 association to 2B4 has been described so far 14. Although the role of 2B4 in T cell functions is still unknown, in NK cells the engagement of 2B4 by specific mAbs 12 13 14 15 or by its natural ligand (i.e., CD48 [14, 18 19 20]) has been shown to trigger cytolytic activity. More recently, we showed that the NK cell triggering via 2B4 is strictly dependent upon the simultaneous cross-linking of other triggering NK receptors. These data suggested that 2B4 may function as a coreceptor in the process of NK cell activation and natural cytotoxicity 21.
In this study, we analyzed the NK cell function in XLP patients. Different triggering receptors including CD16 22 and the “natural cytotoxicity receptors” (NCRs [23, 24]) NKp46 25 26, NKp44 27 28, and NKp30 29 were found to display normal functional capabilities. In contrast, 2B4, upon interaction with its ligand (CD48) on target cells, failed to activate but rather inhibited the NK cell–mediated cytotoxicity against EBV-infected cells that is primarily mediated via NKp46 receptor. The abnormal function of 2B4 molecules appeared to be directly involved in the inability of XLP-NK cells to lyse EBV-infected cells, as mAb-mediated disruption of the 2B4–CD48 interaction resulted in killing of EBV-infected cells.
Materials and Methods
mAbs.
The following mAbs were used in this study: JT3A (IgG2a, anti-CD3); BAB281 and KL247 (IgG1 and IgM, respectively, anti-NKp46); Z231 and KS38 (IgG1 and IgM, respectively, anti-NKp44); KD1 and c127 (IgG2a and IgG1, respectively, anti-CD16); c218 and FS280 (IgG1 and IgG2a, respectively, anti-CD56); A6-136 (IgM, anti-HLA class I [30]); GL183 (IgG1, anti-p58.2); EB6 (IgG1, anti-p58.1); Z199 (IgG2b, anti-NKG2A); PP35 15 21 and S39 (IgG1 and IgG2a, respectively, anti-2B4); and P192 (IgG2a, anti-IRp60). Other anti-2B4 mAbs used in this study were BAB57 (IgG1), BAB102 (IgG1), and MA344 (IgM).
D1.12 (IgG2a, anti–HLA-DR) mAb was provided by Dr. R.S. Accolla (University of Pavia, Pavia, Italy). HP2.6 (IgG2a, anti-CD4) mAb was provided by Dr. P. Sanchez-Madrid (Hospital de la Princesa, Madrid, Spain). TU145 (IgM, anti-CD48) was purchased from BD PharMingen.
Purification of PBLs and Generation of Polyclonal or Clonal NK Cell Populations.
PBLs were derived from healthy or XLP donors by Ficoll-Hypaque gradients and depletion of plastic-adherent cells. To obtain enriched NK cells, PBLs were incubated with anti-CD3 (JT3A), anti-CD4 (HP2.6), and anti–HLA-DR (D1.12) mAbs (30 min at 4°C) followed by goat anti–mouse coated Dynabeads (Dynal; 30 min at 4°C) and immunomagnetic depletion 25 29. CD3−4−DR− cells were cultured on irradiated feeder cells in the presence of 100 U/ml rIL-2 (Proleukin; Chiron Corp.) and 1.5 ng/ml PHA (GIBCO BRL) to obtain polyclonal NK cell populations or, after limiting dilution 25, NK cell clones.
Flow Cytofluorimetric Analysis.
Cells were stained with the appropriate mAb followed by PE- or FITC-conjugated isotype-specific goat anti–mouse second reagent (Southern Biotechnology Associates, Inc.). Samples were analyzed by one- or two-color cytofluorimetric analysis (FACScan™; Becton Dickinson) as described previously 27.
Cell Lines and Cytolytic Assays.
The following target cells were used in this study: P815 (murine mastocytoma, FcγR+), EBV lymphoblastoid cell line (LCL) 721.221 target cell line (FcγR−HLA class I−CD48+), LCLs from patient A and from donor LM, Daudi Burkitt lymphoma, SMMC hepatocarcinoma, and MEL15 melanoma 29.
Cells were tested for cytolytic activity in a 4-h 51Cr-release assay as described previously 25 29, in the absence or presence of various mAbs. The concentrations of the various mAbs were 10 μg/ml for the masking experiments and 0.5 μg/ml for the redirected killing experiments unless otherwise specified. The E/T ratios are indicated in the text.
Biochemical Characterization of 2B4 Molecules.
20 × 106 cells were incubated (15 min at 20°C) in 1 ml PBS, pH 8 containing 250 μg of EZ-Link™ Sulfo-NHS-LC-LC-Biotin (Pierce Chemical Co.) and washed three times in washing buffer (10 mM Tris, pH 8, 0.14 M NaCl). Cells were then lysed in 1% NP-40 and immunoprecipitated with Sepharose-PA–coupled (Amersham Pharmacia Biotech) mAbs. Samples were analyzed by discontinuous SDS-PAGE either undigested or digested with N-glycosidase F (Boehringer) and transferred to Immobilon P (Millipore). After staining with neutravidin (Pierce Chemical Co.), the Renaissance Chemiluminescence Kit (NEN Life Science Products) was used for detection.
Analysis of the 2B4 Signal Transduction Pathway.
Cells (108) were stimulated or not with 100 μM sodium pervanadate 27 28 29, and 1% NP-40 lysates were immunoprecipitated with mAbs and rabbit antiserum coupled to Sepharose-PA or Sepharose-CNBR (Amersham Pharmacia Biotech). Samples were analyzed in discontinuous SDS-PAGE, transferred to Immobilon-P (Millipore), and probed with the following: (a) antiphosphotyrosine mAb (PY20-HRPO; Transduction Laboratories), (b) anti–SHP-2 phosphatase (PTP1D; Transduction Laboratories) or anti–SHP-1 phosphatase mAbs (PTP1C; Transduction Laboratories) followed by rabbit anti–mouse horseradish peroxidase (HRPO; Dako), and (c) anti-SH2D1A rabbit antiserum followed by donkey anti–rabbit HRPO (Amersham Pharmacia Biotech). The Renaissance Chemiluminescence Kit (NEN Life Science Products) was used for detection.
The anti-SH2D1A rabbit antiserum was produced by Eurogentec. The 12–amino acid KLH-conjugated C-QGTTGIREDPDV peptide was used.
Reverse Transcriptase PCR Analysis of 2B4 and SH2D1A Transcripts in XLP-NK Cells.
Total RNA was extracted from the polyclonal NK population and clones of XLP patients using RNA-CLEAN (TIB MOLBIOL). cDNA synthesis was performed using oligo-dT priming. Primers used for cDNA amplification of complete human 2B4 open reading frame (ORF; 1197 bp) were the following: 5′-CCTGGCCCCTCTGTCCTG (PP35 ORF up) and 5′-TCCTGTGCCGTCATCCACTG (PP35 ORF down). Amplification was performed for 30 cycles (30 s at 94°C, 30 s at 60°C, and 30 s at 72°C), followed by a 7-min incubation at 72°C. The SH2D1A cDNA (632 bp) was amplified using the following primers: 5′-CAgCggCATCTCCCTTg (SH2D1A-3 ORF forward) and 5′-TTTCAAAgCTCCTCACTATg (SH2D1A-4 ORF reverse). Amplification was performed for 30 cycles (30 s at 94°C, 30 s at 55°C, and 30 s at 72°C), followed by a 7-min incubation at 72°C. Both PCR reactions were performed using AmpliTaq (PE Biosystems). PCR product obtained from NK cells was subcloned into pcDNA 3.1 vector (Invitrogen) and sequenced. DNA sequencing was performed using a d-Rhodamine Terminator Cycle Sequencing Kit and a 377 Applied Biosystems Automatic Sequencer (PE Biosystems).
Results
Characterization of SH2D1A Mutations in Two XLP Patients.
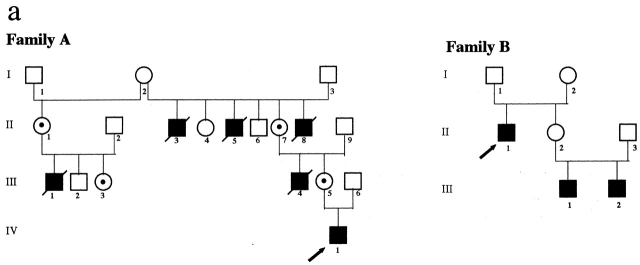
Two XLP families have been analyzed (Fig. 1 a). In family A, the proband (hereafter referred to as patient A) is a 3-yr-old, EBV-seronegative child who was identified as genotypically affected by a mutation at the SH2D1A locus represented by a G to T nucleotide change at the translation initiation codon (ATG to ATT), leading to a methionine to isoleucine amino acid change. The same mutation was identified in XLP obligate carriers from the same family, in which several males died of fulminant hepatitis or leukemia after EBV infection. The immunological phenotype in patient A was normal apart from slightly reduced IgG1 and IgG3 serum levels.
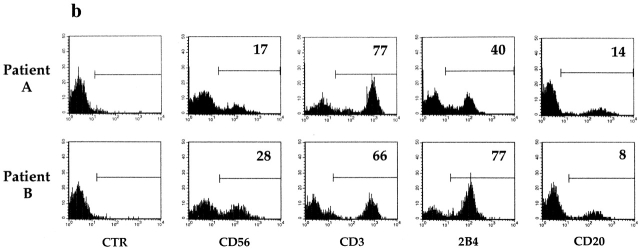
Figure 1.
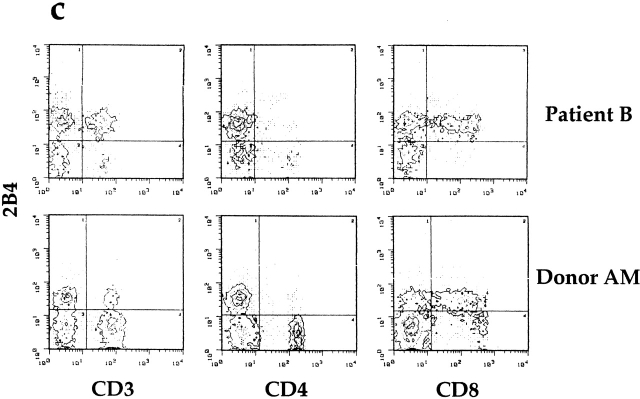
Family pedigrees and cytofluorimetric analysis of PBLs from two XLP patients. (a) Pedigrees of family A and family B with XLP. The arrows indicate index cases. Mutation analysis at the SH2D1A locus confirmed the diagnosis of XLP in patient IV,1 from family A and in patients II,1, III,1, and III,2 from family B. In both pedigrees, dotted circles identify carrier females, as diagnosed by mutation analysis at the SH2D1A locus. (b) Freshly isolated PBLs derived from XLP patients A and B were analyzed by immunofluorescence and FACS® analysis using mAbs specific for the indicated molecules, followed by isotype-specific PE-conjugated goat anti–mouse second reagent. Cells incubated with the second reagent alone represent the negative control (CTR). Percentages of positive cells are indicated in each square. (c) PBLs derived from XLP patient B and from a healthy donor (A.M.) were analyzed by two-color immunofluorescence and FACS® analysis with anti-2B4 mAb in combination with anti-CD3, anti-CD4, or anti-CD8 mAbs. Isotype-specific FITC- or PE-conjugated goat anti–mouse was used as second reagent. The contour plots were divided into quadrants representing unstained cells (lower left), cells with only red fluorescence (upper left), cells with red and green fluorescence (upper right), and cells with only green fluorescence (lower right).
In family B, the proband is an EBV-seropositive 56-yr-old male who was treated for a histiocytic lymphoma at 14 years of age and developed severe hepatitis at 21 years. Because of recurrent episodes of pneumonia and sinusitis, at 36 years of age he was further investigated and found to be hypogammaglobulinemic. Mutation analysis at the SH2D1A locus revealed a C to T nucleotide change at position 163, leading to premature termination at codon 55. Of patient B's two nephews, the elder developed severe hepatitis at 27 years of age, and the younger was diagnosed with EBV-related non-Hodgkin lymphoma at 3 years of age, and since then suffered from recurrent upper and lower respiratory tract infections. Both patient B nephews carry the same mutation at codon 55.
PBLs of the two patients were analyzed by indirect immunofluorescence and FACS® analysis for the expression of cell surface markers including CD3, CD56, CD20, and 2B4. Although in patient A the percentage of positive cells was within a normal range 13 15, in patient B the percentage of 2B4+ cells was markedly higher than in control individuals (Fig. 1 b). Double fluorescence analysis of PBLs derived from patient B revealed that most CD3+ T cells were 2B4+. All of these CD3+2B4+ cells were CD8+, whereas the small CD3+2B4− subset (<5%) was CD4+. Remarkably, all CD8+ cells (including both CD8bright T cells and CD8dull NK cells) were 2B4+. It is of note that in normal individuals (see the representative individual A.M. in Fig. 1 c), a fraction of CD8bright cells is consistently 2B4− 13 14 15. Note also that in donor A.M., unlike in patient B, a low percentage of CD3+ cells expressed 2B4.
Expression and Function of Triggering Surface Molecules in NK Cells from XLP Patients.
To assess the transcription of SH2D1A gene in NK cells isolated from the XLP patients A and B, we performed reverse transcriptase PCR analysis of RNA extracted from polyclonal NK cell populations. The sequence analysis of cloned PCR products showed the presence of two cDNAs, one corresponding to the full-length SH2D1A transcript and the other to the previously described coding sequence lacking 55 nucleotides in exon 3, termed SH2D1AΔ55 or hSAPΔ55 9. Both transcripts carried the expected mutations (described above; data not shown). Using a similar approach, we analyzed NK cells derived from healthy donors. Both unmutated SH2D1A and SH2D1AΔ55 transcripts could be identified.
The same polyclonal NK cell populations were analyzed for the surface expression of 2B4 and of several other NK cell markers. Expression of the HLA class I–specific inhibitory receptors, including killer inhibitory receptors (KIRs) and CD94/NKG2A, was comparable to that of healthy donors (not shown). Similarly, analysis of triggering receptors including CD16 and NCRs (NKp46, NKp44, and NKp30 [23–29]) as well as 2B4 did not reveal substantial differences compared with normal controls (not shown). These data are in agreement with those obtained in fresh PBLs, and suggest that the lack of SH2D1A protein does not affect the surface expression (or density) of 2B4 in human NK cells.
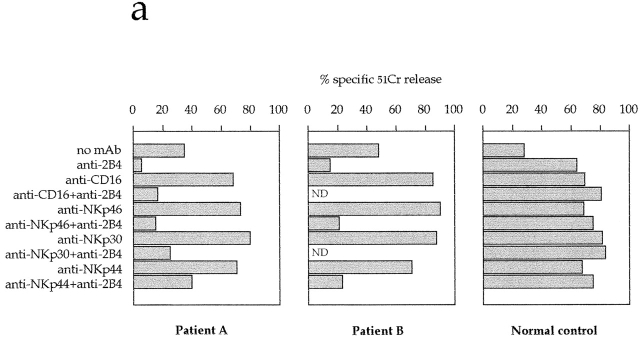
XLP-NK cells were then assessed for cytolytic activity in a redirected killing assay against murine (FcγR+) P815 target cells. As shown in Fig. 2 a, the NK-mediated spontaneous lysis of P815 was comparable to that mediated by NK cells derived from healthy donors. Moreover, addition of anti-CD16 or anti-NCR mAbs resulted in the enhancement of cytolytic activity 25 27 29. On the other hand, unlike in normal NK cells 15 21, both the spontaneous and the CD16- or NCR-induced lysis of P815 was sharply inhibited by anti-2B4 mAb in XLP-NK cells (Fig. 2 a). These results indicate that in XLP-NK cells, cross-linking of 2B4 molecules results in inhibition of cytolytic activity induced via different triggering receptors.
Figure 2.
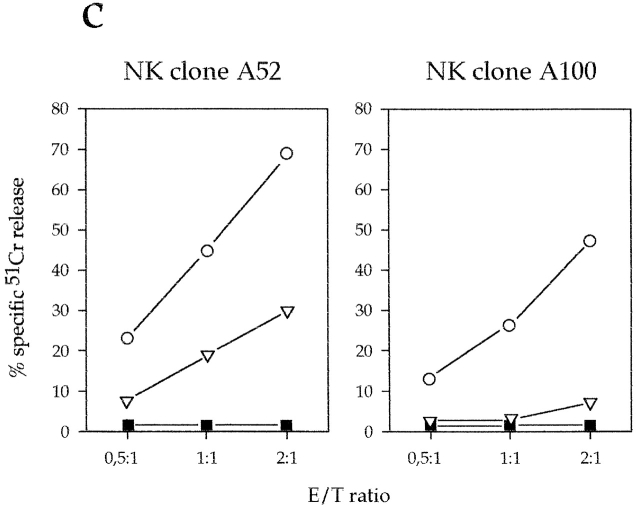
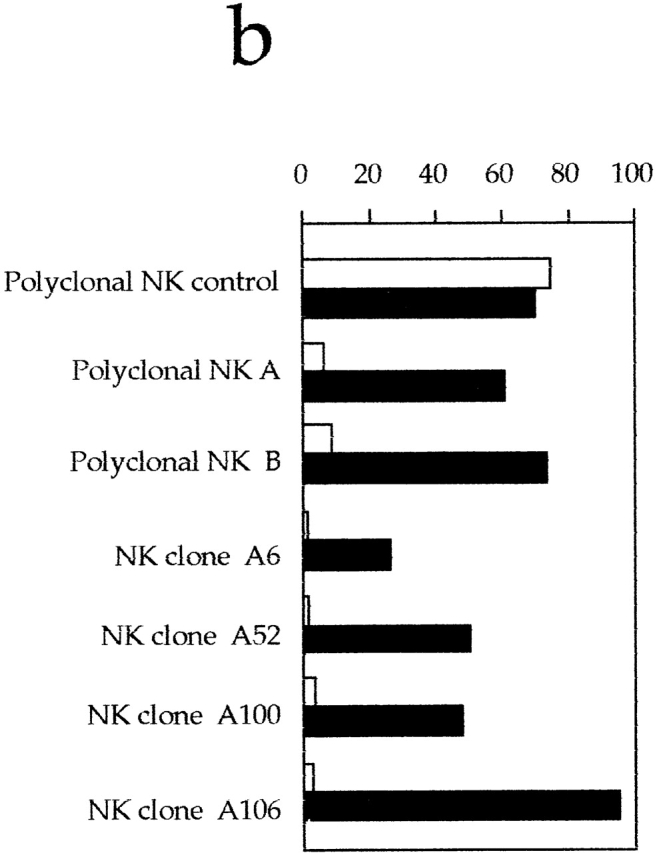
Engagement of 2B4 inhibits the NK-mediated cytolytic activity in XLP patients. (a) Polyclonal NK cell populations derived from XLP patients A and B or from a normal control were analyzed for cytolytic activity in a redirected killing assay against the FcγR+ P815 target cell line in the absence or presence of mAbs specific for the indicated molecules. The E/T ratio used was 8:1. All the mAbs used in this experiment were of the IgG1 isotype. (b) Polyclonal NK cell populations derived from a normal control or from XLP patients A and B and four representative NK cell clones derived from XLP patient A were analyzed against the EBV+CD48+HLA class I− LCL 721.221 target cell line in the absence (white bars) or presence (black bars) of anti-2B4mAb. The E/T ratio used was 3:1. (c) Two representative NK cell clones derived from XLP patient A were analyzed against the EBV+HLA class I− LCL 721.221 target cell line in the absence of mAb (▪) or in the presence of anti-2B4 mAb alone (○), or in combination with anti-NKp46 mAb (▿). The E/T ratios used are indicated.
2B4 Inhibits the NK-mediated Killing of EBV B Cell Lines in XLP Patients.
A panel of >50 NK cell clones derived from patient A and two polyclonal NK cell populations derived from patients A and B were analyzed for their ability to lyse the LCL 721.221 EBV cell line and the Daudi Burkitt lymphoma 31. It is of note that both of these target cells are HLA class I− and express large amounts of CD48 (i.e., the ligand for 2B4 [14, 18–20]). Because of the lack of HLA class I expression, both targets were lysed by NK cells from healthy donors (Fig. 2 b), but they were resistant to lysis by XLP-NK cells. However, upon mAb-mediated masking of 2B4 molecules, an efficient restoration of lysis of both LCL 721.221 (Fig. 2 b) and Daudi (not shown) cells could be detected. These data suggested that in XLP patients, the 2B4-mediated recognition of CD48 on target cells may induce a strong inhibitory effect on the NK cell–mediated cytotoxicity. In agreement with this concept, the cytolytic activity of XLP-NK cell clones was also restored by mAb-mediated masking of CD48 molecules (not shown). On the other hand, XLP-NK cells could efficiently lyse some NK-susceptible tumor cell lines, including the HLA class I− SMMC (hepatocarcinoma [31]) and the MEL15 (melanoma [31]; data not shown). Remarkably, unlike LCL 721.221 or Daudi, these tumors do not express CD48 molecules (not shown). Taken together, these data imply that CD48 also represents a ligand for the “inhibitory” 2B4 molecules of XLP-NK cells. In addition, they show that upon disruption of the 2B4–CD48 inhibitory interactions (either by masking the receptor or the ligand), NK cells from XLP patients display a cytolytic activity comparable to that of normal NK cells.
2B4 Inhibits Killing of EBV-infected Cells by Interfering with NKp46-mediated NK Cell Activation.
Previous studies indicated that in healthy individuals, lysis of LCL 721.221 target cells is primarily mediated by triggering signals delivered via the NKp46 NCR 31. In agreement with these data, killing of LCL 721.221 cells by normal NK cell clones was sharply inhibited by mAb-mediated masking of NKp46. As reported above, NKp46 also displays a normal triggering capability in XLP-NK cells. Thus, we further evaluated whether lysis of LCL 721.221 target cells (detectable upon mAb-mediated masking of 2B4) was also dependent upon NKp46 in XLP. To this end, XLP-NK cells were assessed for cytotoxicity against LCL 721.221 target cells either in the presence of anti-2B4 mAb or in the presence of both anti-2B4 and anti-NKp46 mAbs. As shown in Fig. 2 c, upon masking of both receptors, target cell lysis was sharply inhibited.
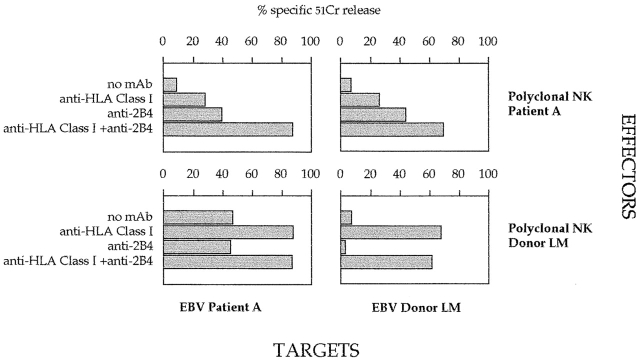
The availability of an LCL from XLP patient A allowed us to analyze the ability of NK cells to kill autologous EBV+ target cells. Unlike LCL 721.221 or Daudi, patient A LCLs expressed HLA class I molecules. Therefore, the cytolytic activity of XLP-NK cells was evaluated in the presence of appropriate anti–HLA class I mAbs (of IgM isotype; [30]). As shown in Fig. 3, under these experimental conditions, we could not detect restoration of lysis, as it occurs with normal NK cells. These data suggested the existence of additional inhibitory signals, possibly mediated by the interaction between 2B4 and CD48. Indeed, lysis of autologous LCLs could be efficiently reconstituted only when both the 2B4–CD48 and KIR–HLA class I interactions were simultaneously disrupted by the addition of specific mAbs (Fig. 3). In conclusion, these data indicate that in XLP patients, the altered function of 2B4 deeply affects the ability of NK cells to kill both autologous and allogeneic EBV-transformed B cells.
Figure 3.
Analysis of XLP-NK cell–mediated cytolytic activity against autologous LCLs. Polyclonal NK cell populations derived from XLP patient A or from a healthy donor (LM) were analyzed against LCLs derived from patient A or from donor LM in the absence or presence of mAbs specific for the indicated molecules. The E/T ratio used was 5:1.
Molecular Characterization of 2B4 in XLP-NK Cells.
Recently, Southern blot analysis of human DNA indicated that human 2B4 is encoded by a single gene of ∼25 kb 16. This finding suggests that the inhibitory function of 2B4 in XLP-NK cells is not consequent to the transcription of a different 2B4-encoding gene. To confirm this hypothesis, the expression of the 2B4 transcript was analyzed by reverse transcriptase PCR in polyclonal NK cells derived from the XLP patients A and B or from healthy donors. Sequence analysis of the amplified cDNAs revealed, in all samples analyzed, the presence of two major products: the first corresponded to the full-length 2B4 molecule 16 17 18 20 21, and the second to a sequence identical to 2B4 but lacking the IgC2 domain (1D-2B4; sequence data available from EMBL/GenBank/DDBJ under accession no. AJ245376). Remarkably, COS7 cells transiently transfected with the 1D-2B4 construct were not stained by any of the five available anti-2B4 mAbs (not shown). Finally, a poorly represented transcript referred to as sh2B4 (sequence data available at EMBL/GenBank/DDBJ under accession no. AJ245377) was detected in NK cell clones derived from both XLP patients and healthy donors. The sh2B4 is characterized by an insertion of 140 bp and encodes a polypeptide of 329 amino acids identical to 2B4 in the first 320 amino acids. This cDNA is likely to result from a splicing error, as the extra 140-bp sequence contains a consensus sequence for an acceptor splice site. Being detectable also in normal donors, sh2B4 is unlikely to be responsible for the inhibitory functions of 2B4 in XLP patients. Along this line, previous studies indicated that unlike in mice 32, in humans only a single 2B4 molecule is expressed at the cell surface 16.
Biochemical Characterization of 2B4 Molecular Complex in Normal and XLP-NK Cells.
The cytoplasmic tail of 2B4 is characterized by four tyrosine-based motifs (TxYxxI/V; reference 14). 2B4 tyrosine phosphorylation upon pervanadate treatment has been demonstrated previously in both normal NK cells 14 and cell transfectants 17. Experiments performed in 2B4 cell transfectants suggested that it may associate with SH2D1A and SHP-2 17. Moreover, it has also been suggested that SH2D1A competes with SHP-2 for binding to 2B4. However, in normal NK cells, neither SH2D1A nor SHP-2 association to 2B4 could be detected 14. Thus, controversial information existed on the molecular mechanisms that account for 2B4 activating function. We therefore performed a comparative analysis of 2B4 and associated molecules in normal or XLP-NK cells.
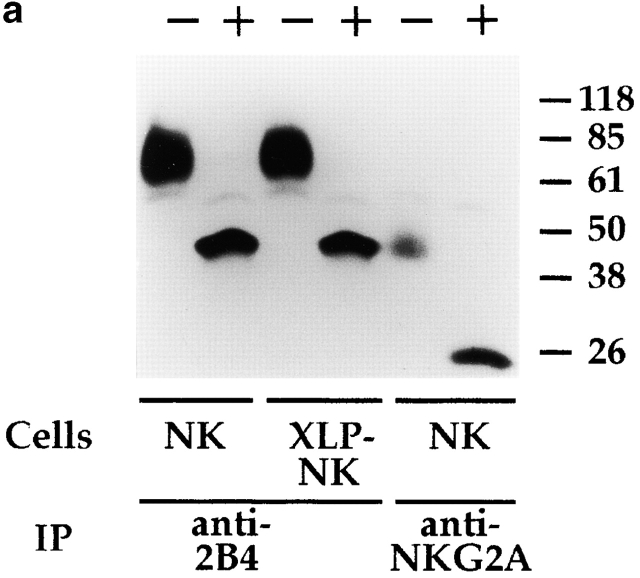
To this end, polyclonal NK cell populations were first surface labeled with biotin, and cell lysates were immunoprecipitated with anti-2B4 mAb. 70-kD surface molecules 14 15 16 17 18 were immunoprecipitated from both normal and XLP-NK cells. Moreover, treatment with N-glycosidase revealed an identical protein backbone of ∼40 kD (Fig. 4 a).
Figure 4.
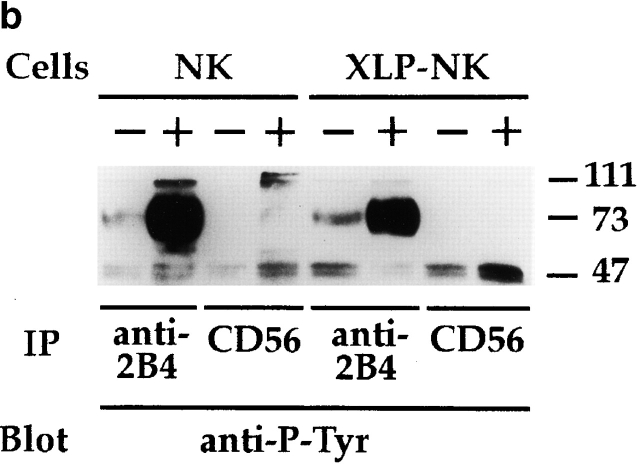
Biochemical analysis of 2B4 molecules in normal and XLP-NK cells. (a) Polyclonal NK cell populations derived from healthy donors (NK) or XLP-patients (XLP-NK) were surface labeled with biotin and immunoprecipitated with anti-2B4 (S39 mAb) or anti-NKG2A (Z199 mAb). Samples were treated (+) or not (−) with N-glycosidase F and analyzed in an 8% SDS-PAGE under reducing conditions. (b) Anti-2B4 or anti-CD56 (FS280 mAb, negative control) immunoprecipitates were obtained from NK or XLP-NK cell populations either untreated (−) or treated (+) with sodium pervanadate. Samples were analyzed in an 11% SDS-PAGE under reducing conditions and probed with antiphosphotyrosine (anti-P-Tyr) mAb. Molecular mass markers (kD) are indicated on the right.
Next, 2B4 molecules were immunoprecipitated from cells treated or not with sodium pervanadate. Samples were analyzed in SDS-PAGE and probed with antiphosphotyrosine mAb. As shown in Fig. 4 b, under the experimental conditions used, a low degree of 2B4 tyrosine phosphorylation was consistently detected in IL-2–cultured polyclonal NK cell populations derived from both healthy donors and XLP patients. Notably, sodium pervanadate treatment led to strong increments of 2B4 tyrosine phosphorylation both in normal and XLP-NK cells, although in XLP-NK cells phosphorylation appeared to be of lower density.
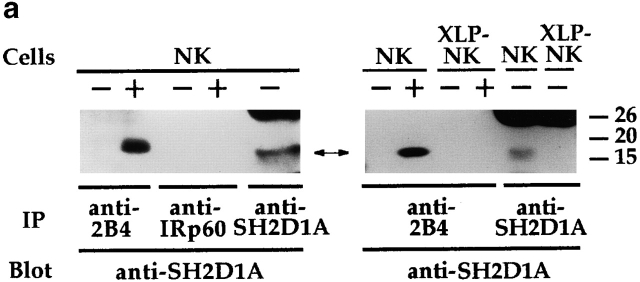
2B4 immunoprecipitates, obtained as described in the legend to Fig. 4 b, were probed with anti-SH2D1A antiserum. As shown in Fig. 5 a and in line with previous reports on cell transfectants 17, treatment with pervanadate led to the association of the 2B4 receptor with the SH2D1A molecule in normal NK cells. On the other hand, in XLP-NK cells, neither SH2D1A protein nor 2B4–SH2D1A association could be detected, as expected.
Figure 5.
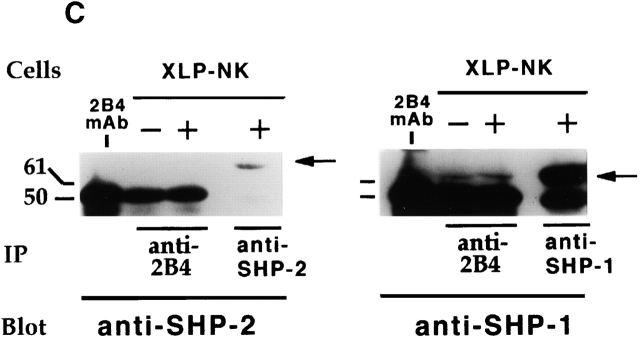
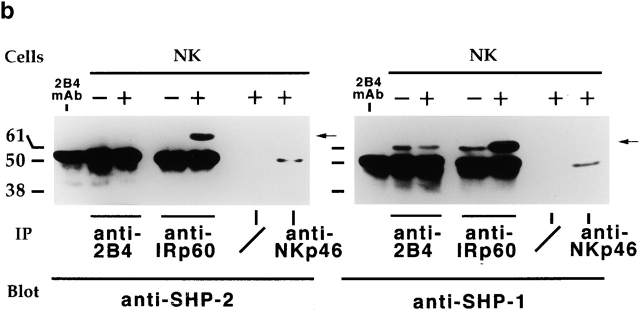
Biochemical characterization of 2B4-associated molecules in normal and XLP-NK cells. (a) NK or XLP-NK cell populations, either untreated (−) or treated (+) with sodium pervanadate, were immunoprecipitated with anti-2B4 (S39 mAb), anti-IRp60 (P192 mAb, isotype-matched negative control), or anti-SH2D1A antiserum. Samples were analyzed in a 14% SDS-PAGE under reducing conditions and probed with anti-SH2D1A antiserum. (b) 2B4, IRp60 (positive control), and NKp46 (BAB281 mAb, negative control) molecules were sequentially immunoprecipitated from normal polyclonal NK cell populations either untreated (−) or treated (+) with sodium pervanadate. Identical samples were divided, analyzed in 7% SDS-PAGE under reducing conditions, and probed with either anti–SHP-2 (left) or anti–SHP-1 (right) mAbs. Sepharose-PA preclearing on treated cells (/) is also shown. (c) Anti-2B4, anti–SHP-2, or anti–SHP-1 immunoprecipitates were derived from XLP-NK cell populations either untreated (−) or treated (+) with sodium pervanadate. Identical samples were divided, analyzed in 7% SDS-PAGE under reducing conditions, and probed with either anti–SHP-2 (left) or anti–SHP-1 (right) mAbs. Molecular mass markers (kD) are indicated. Arrows indicate SH2D1A, SHP-2, and SHP-1 molecules. The first lanes in b and c were loaded with the S39 (2B4-specific) mAb alone as control.
2B4 immunoprecipitates, obtained as indicated above, were subsequently probed with mAb specific for SHP-2 or SHP-1 cytoplasmic phosphatases. Negative and positive controls were represented by NK cell lysates immunoprecipitated with mAbs specific for NKp46 25 or IRp60 33 molecules, respectively. NKp46 is an NK-specific receptor that transduces activating signals via the association with the CD3ζ/FcεRIγ molecules 23 27 28. IRp60 is an immunoreceptor tyrosine-based inhibition motif (ITIM)-bearing inhibitory receptor, expressed by all human NK cells, that associates with SHP-1 and SHP-2 33. No 2B4–SHP-2 association could be detected in normal and XLP-NK cells (Fig. 5b and Fig. c). However, SHP-1 phosphatase was detected in 2B4 immunoprecipitates obtained from both untreated and treated cells. Remarkably, both in normal and in XLP-NK cells, sodium pervanadate treatment did not result in increments of the 2B4-associated SHP-1 (Fig. 5b and Fig. c) whereas in control IRp60 immunoprecipitates, this treatment led to a strong increment of IRp60-associated SHP-1. The finding that SHP-1 also associates with 2B4 in untreated cells could be justified by the low degree of 2B4 tyrosine phosphorylation detected in IL-2–cultured normal or XLP-NK cells used in these experiments (Fig. 4 b).
Discussion
In this study, we show that an altered function of 2B4 molecules may contribute to the inability of XLP patients to control EBV infections. In these patients, 2B4 functions as an inhibitory rather than activating receptor, and this altered function causes a severe impairment of the NK cell–mediated cytotoxicity against EBV-infected cells.
Previous studies using cell transfectants 17 provided evidence that human 2B4 can associate to SH2D1A molecules. Because XLP disease is characterized by mutations in the SH2D1A-encoding gene 8 9 10, the aim of this study was to analyze whether 2B4 molecules had an altered function in XLP patients. In agreement with previous data obtained in cell transfectants, we show that in NK cells derived from healthy donors, SH2D1A associates with phosphorylated 2B4 molecules. In contrast, in XLP-NK cells, SH2D1A molecules were missing, and consequently they did not associate with 2B4. In patient A, the lack of functional SH2D1A proteins was consequent to a mutation in the ATG initiation codon, whereas in patient B, a nonsense mutation at codon 55 resulted in a truncated product characterized by an incomplete SH2 domain. Cytofluorimetric analysis indicated that lack of SH2D1A did not affect the levels of surface expression of 2B4 molecules. Both biochemical and molecular analysis revealed that 2B4 molecules isolated from normal or XLP-NK cells were identical. Importantly, 2B4 was found to associate with the SHP-1 phosphatase in both normal and XLP-NK cells. Whether SHP-1 is responsible for delivering the 2B4-mediated inhibitory signals in XLP-NK cells lacking SH2D1A molecules is under investigation. A direct demonstration that the signaling pathway initiated by 2B4 is dramatically altered in XLP-NK cells was obtained by several functional studies. Thus, cross-linking of 2B4 resulted in the generation of inhibitory signals that blocked the NK-mediated cytolysis. This inhibitory effect affected not only the spontaneous cytotoxicity but also the NK cell activation induced via CD16 or NCRs. These data suggested that 2B4 molecules could be involved in the inability of XLP patients to mount an efficient immune response against EBV infections. More direct evidence that this may represent a major physiopathological mechanism in XLP was provided by the analysis of EBV-infected LCLs. LCLs express relatively high amounts of CD48 (i.e., the natural ligand of 2B4 molecules [18–20]). We hypothesized that 2B4-mediated recognition of CD48 molecules could result in inhibition of the XLP-NK cell–mediated killing of EBV-infected B cells. Indeed, XLP-NK cells were unable to kill the HLA class I− EBV B cell lines LCL 721.221 and Daudi. This was consequent to a 2B4-mediated inhibitory effect, as cytolytic activity could be restored upon mAb-mediated disruption of the 2B4–CD48 interaction. The cytolytic activity of XLP-NK cells could also be analyzed against an autologous EBV+ LCL expressing HLA class I molecules. In this case, only the simultaneous mAb-mediated disruption of both HLA–KIR and 2B4–CD48 interactions allowed a complete recovery of cytotoxicity. Downregulation of HLA class I during EBV infection has been reported previously in different studies 34 35 36. In normal individuals, HLA class I downregulation would allow NK cells to kill EBV-infected cells 30. However, in XLP patients, clearance of HLA-deficient EBV-infected cells would be impaired because of the occurrence of inhibitory rather than activating interactions between 2B4 and CD48. Thus, it appears conceivable that the altered 2B4 function may contribute to the inefficient control of EBV+ B cells in XLP-patients. Along this line, although not shown, preliminary data suggest that in XLP patients, 2B4 may also negatively regulate T cell–mediated responses. Therefore, it is possible that specific CTL responses against EBV+ B cells may also be impaired by a molecular mechanism similar to that responsible for NK cell dysfunction. Altogether, the altered function of 2B4 may account for a general inability of different cytolytic effector cells to control EBV infections. This will occur both when infected cells express HLA class I (because of an impairment of CTLs) and when downregulation of HLA class I had occurred (because of an impairment of NK cells).
Our data may have relevant therapeutic implications. XLP has a dismal prognosis: although bone marrow transplantation represents the only form of permanent cure, it gives optimal results only if performed before EBV infection. In contrast, antiviral drugs, intravenous immunoglobulins, and B cell–specific mAbs have been used with modest results in the treatment of overt lymphoproliferative disease in XLP patients. Our data suggest that administration of anti-2B4 antibody could represent a novel form of treatment that could help to control lymphoproliferation while preparing for bone marrow transplantation.
Acknowledgments
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità, Ministero della Sanità, Ministero dell'Università e della Ricerca Scientifica e Tecnologica, Consiglio Nazionale delle Ricerche, Progetto Finalizzato Biotecnologie, and by National Institutes of Health grant HD17427 (to H.D. Ochs). The financial support of Telethon-Italy (grants E.0892 and E.668) is gratefully acknowledged.
Footnotes
Abbreviations used in this paper: HRPO, horseradish peroxidase; KIR, killer inhibitory receptor; LCL, lymphoblastoid cell line; NCR, natural cytotoxicity receptor; ORF, open reading frame; SH2D1A, Src homology 2 domain–containing protein; SHP, Src homology 2 domain–containing phosphatase; XLP, X-linked lymphoproliferative disease.
S. Parolini and C. Bottino contributed equally to this work.
References
- Purtilo D.T., Cassel C.K., Yang J.P.S. LetterFatal infectious mononucleosis in familial lymphohistiocytosis. N. Engl. J. Med. 1974;201:736. doi: 10.1056/nejm197410032911415. [DOI] [PubMed] [Google Scholar]
- Schuster V., Kreth H.W. X-linked lymphoproliferative disease. In: Ochs H.D., Smith C.I.E., Puck J.M., editors. Primary Immunodeficiency Diseases, a Molecular and Genetic Approach. Oxford University Press; New York: 1999. pp. 222–232. [Google Scholar]
- Seemayer T.A., Gross T.G., Egeler R.M., Pirruccello S.J., Davis J.R., Kelly C.M., Okano M., Lanyi A., Sumegi J. X-linked lymphoproliferative diseasetwenty-five years after the discovery. Pediatr. Res. 1995;38:471–478. doi: 10.1203/00006450-199510000-00001. [DOI] [PubMed] [Google Scholar]
- Sullivan J.L., Woda B.A. X-linked lymphoproliferative syndrome. Immunodefic. Rev. 1989;1:325–347. [PubMed] [Google Scholar]
- Lindsten T., Seeley J.K., Ballow M., Sakamoto K., St S., Onge J., Yetz P., Aman, Purtilo D.T. Immune deficiency in the X-linked lymphoproliferative syndrome. II. Immunoregulatory T cell defects. J. Immunol. 1982;129:2536–2540. [PubMed] [Google Scholar]
- Okano M., Pirruccello S.J., Grierson H.L., Johnson D.R., Thiele G.M., Purtilo D.T. Immunovirological studies of fatal infectious mononucleosis in a patient with X-linked lymphoproliferative syndrome treated with intravenous immunoglobulin and interferon-alpha. Clin. Immunol. Immunopathol. 1990;54:410–418. doi: 10.1016/0090-1229(90)90054-t. [DOI] [PubMed] [Google Scholar]
- Donhuijsen-Ant R., Abken H., Bornkamm G., Donhuijsen K., Grosse-Wilde H., Neumann-Haefeli D., Westerhausen M., Wiegand H. Fatal Hodgkin and non-Hodgkin lymphoma associated with persistent Epstein-Barr virus in four brothers. Ann. Intern. Med. 1988;109:946–952. doi: 10.7326/0003-4819-109-12-946. [DOI] [PubMed] [Google Scholar]
- Coffey A.J., Brooksbank R.A., Brandau O., Oohashi T., Howell G.R., Bye J.M., Cahn A.P., Durham J., Heath P., Wray P. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- Sayos J., Wu C., Morra M., Wang N., Zhang X., Allen D., van Schaik S., Notarangelo L., Geha R., Roncarolo M.G. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- Nichols K.E., Harkin D.P., Levitz S., Krainer M., Kolquist K.A., Genovese C., Bernard A., Ferguson M., Zuo L., Snyder E. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks B.G., Chang C.C., Garballido J., Yssel H., de Vries J.E., Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- Mathew P.A., Garni-Wagner B.A., Land K., Takashima A., Stoneman E., Bennett M., Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J. Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- Valiante N.M., Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J. Exp. Med. 1993;178:1397–1406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Cella M., Langen H., Friedlein A., Colonna M. Activating interactions in human NK cell recognitionthe role of 2B4-CD48. Eur. J. Immunol. 1999;29:1676–1683. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Moretta A., Bottino C., Tripodi G., Vitale M., Pende D., Morelli L., Augugliaro R., Barbaresi M., Ciccone E., Millo R., Moretta L. Novel surface molecules involved in human NK cell activation and triggering of the lytic machinery. Int. J. Cancer Suppl. 1992;7:6–10. [PubMed] [Google Scholar]
- Boles K.S., Nakajima H., Colonna M., Chuang S.S., Stepp S.E., Bennett M., Kumar V., Mathew P.A. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27–34. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Lazetic S., Woollatt E., Sutherland G.R., Lanier L.L., Phillips J.H. Human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J. Immunol. 1999;162:6981–6985. [PubMed] [Google Scholar]
- Brown M.H., Boles K., van der Merwe P.A., Kumar V., Mathew P.A., Barclay A.N. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 1998;188:2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y., McKay P.F., Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J. Immunol. 1998;161:5809–5812. [PubMed] [Google Scholar]
- Kubin M.Z., Parsley D.L., Din W., Waugh J.Y., Davis-Smith T., Smith C.A., Macduff B.M., Armitage R.J., Chin W., Cassiano L. Molecular cloning and biological characterization of NK cell activation-inducing ligand, a counterstructure for CD48. Eur. J. Immunol. 1999;29:3466–3477. doi: 10.1002/(SICI)1521-4141(199911)29:11<3466::AID-IMMU3466>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sivori S., Parolini S., Falco M., Marcenaro E., Biassoni R., Bottino C., Moretta L., Moretta A. 2B4 functions as a co-receptor in human natural killer cell activation. Eur. J. Immunol. 2000;30:787–793. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bolhuis R.L.H., Roozemond R.C., Van de Griend R.J. Induction and blocking of cytolysis in CD2+, CD3− NK and CD2+, CD3+ cytotoxic T lymphocytes via CD2 50 KD sheep erythrocyte receptor. J. Immunol. 1986;136:3939–3944. [PubMed] [Google Scholar]
- Moretta A., Biassoni R., Bottino C., Mingari M.C., Moretta L. Natural cytotoxicity receptors that trigger human NK-mediated cytolysis. Immunol. Today. 2000;21:228–234. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- Bottino C., Biassoni R., Millo R., Moretta L., Moretta A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum. Immunol. 2000;61:1–6. doi: 10.1016/s0198-8859(99)00162-7. [DOI] [PubMed] [Google Scholar]
- Sivori S., Vitale M., Morelli L., Sanseverino L., Augugliaro R., Bottino C., Moretta L., Moretta A. p46, a novel natural killer cell–specific surface molecule that mediates cell activation. J. Exp. Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessino A., Sivori S., Bottino C., Malaspina A., Morelli L., Moretta L., Biassoni R., Moretta A. Molecular cloning of NKp46a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M., Bottino C., Sivori S., Sanseverino L., Castriconi R., Marcenaro E., Augugliaro R., Moretta L., Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex–restricted tumor cell lysis. J. Exp. Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni C., Bottino C., Vitale M., Pessino A., Augugliaro R., Malaspina A., Parolini S., Moretta L., Moretta A., Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende D., Parolini S., Pessino A., Sivori S., Augugliaro R., Morelli L., Marcenaro E., Accame L., Malaspina A., Biassoni R. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone E., Pende D., Vitale M., Nanni L., Di Donato C., Bottino C., Morelli L., Viale O., Amoroso A., Moretta A., Moretta L. Self class I molecules protect normal cells from lysis mediated by autologous natural killer cells. Eur. J. Immunol. 1994;24:1003–1006. doi: 10.1002/eji.1830240434. [DOI] [PubMed] [Google Scholar]
- Sivori S., Pende D., Bottino C., Marcenaro E., Pessino A., Biassoni R., Moretta L., Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human natural killer cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Stepp S.E., Schatzle J.D., Bennett M., Kumar V., Mathew P.A. Gene structure of the murine NK cell receptor 2B4presence of two alternatively spliced isoforms with distinct cytoplasmic domains. Eur. J. Immunol. 1999;29:2392–2399. doi: 10.1002/(SICI)1521-4141(199908)29:08<2392::AID-IMMU2392>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cantoni C., Bottino C., Augugliaro R., Morelli L., Marcenaro E., Castriconi R., Vitale M., Pende D., Sivori S., Millo R. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human natural killer cells. Eur. J. Immunol. 1999;29:3148–3159. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Masucci M.G., Torsteindottir S., Colombani B.J., Brautbar C., Klein E., Klein G. Down-regulation of class I HLA antigens and of the Epstein-Barr virus (EBV)-encoded latent membrane protein (LMP) in Burkitt lymphoma lines. Proc. Natl. Acad. Sci. USA. 1987;84:4567–4571. doi: 10.1073/pnas.84.13.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler R., Eissner G., Meissner P., Uebel S., Tampe R., Lazis S., Hammerschmidt W. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood. 1997;90:2390–2397. [PubMed] [Google Scholar]
- Rowe M., Khanna R., Jacob C.A., Argaet V., Kelly A., Powis S., Belich M., Croom-Carter D., Lee S., Burrows S.R. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur. J. Immunol. 1995;25:1374–1384. doi: 10.1002/eji.1830250536. [DOI] [PubMed] [Google Scholar]