Summary
A defining feature of centromeres is the presence of the histone H3 variant CENP-ACnp1. It is not known how CENP-ACnp1 is specifically delivered to, and assembled into, centromeric chromatin. Through a screen for factors involved in kinetochore integrity in fission yeast, we identified Sim3. Sim3 is homologous to known histone binding proteins NASPHuman and N1/N2Xenopus and aligns with Hif1S. cerevisiae, defining the SHNi-TPR family. Sim3 is distributed throughout the nucleoplasm, yet it associates with CENP-ACnp1 and also binds H3. Cells defective in Sim3 function have reduced levels of CENP-ACnp1 at centromeres (and increased H3) and display chromosome segregation defects. Sim3 is required to allow newly synthesized CENP-ACnp1 to accumulate at centromeres in S and G2 phase-arrested cells in a replication-independent mechanism. We propose that one function of Sim3 is to act as an escort that hands off CENP-ACnp1 to chromatin assembly factors, allowing its incorporation into centromeric chromatin.
Keywords: DNA
Introduction
The centromere is the chromosomal locus where the kinetochore is assembled to coordinate accurate chromosome segregation. The site of kinetochore assembly is dependent on the deposition of an unusual form of chromatin containing CENP-A. CENP-A (known as CID, HCP-3, Cse4, and Cnp1 in Drosophila, C. elegans, S. cerevisiae, and S. pombe, respectively) is a histone H3 variant that replaces H3 in specialized nucleosomes found only at active, but not inactive, centromeres in all eukaryotes (reviewed Cleveland et al., 2003).
Human centromeres are normally found at repetitive arrays of α-satellite DNA and can be assembled de novo on introduced α-satellite DNA (reviewed in Cleveland et al., 2003; Sullivan et al., 2001). However, CENP-A chromatin assembly and propagation are remarkably plastic, as it can assemble and direct kinetochore proteins to assemble on noncentromeric sequences. (Lo et al., 2001; Heun et al., 2006; Castillo et al., 2007). Such observations suggest that assembly of CENP-A chromatin—at regional centromeres composed of arrays of CENP-A nucleosomes—is governed by epigenetic processes. Once assembled at a site, a propagation mechanism must ensure CENP-A chromatin recognition and replenishment during or after each round of DNA replication (Cleveland et al., 2003; Henikoff and Dalal, 2005; Sullivan et al., 2001; Sullivan, 2001). Mechanisms must also operate to ensure that newly made, free CENP-A is delivered only to centromeres for assembly and is excluded from noncentromeric chromatin. This could be achieved by strict control of CENP-A levels; S. cerevisiae CENP-ACse4 is regulated by ubiquitin-dependent proteolysis, and overexpression of a nondegradable form results in its broad distribution (Collins et al., 2004). In Drosophila and human cells, overexpression of CENP-A allows its assembly at ectopic sites (Heun et al., 2006).
Analyses in metazoan cells indicate that centromeres replicate asynchronously and that CENP-A levels peak in G2 (Shelby et al., 2000; Sullivan and Karpen, 2001). This suggests that there is not, as previously thought, tight coupling between the timing of centromeric DNA replication and CENP-A synthesis and raises the possibility that CENP-A might be deposited after centromere replication, either by replacement of H3 or by filling of chromatin gaps (Furuyama et al., 2006; Shelby et al., 2000; Sullivan, 2001).
Little is known about how CENP-A is delivered to, or assembled on, centromeric DNA and not at other sites in the genome. Human RbAp46/48 and the fission yeast ortholog Mis16 contribute to the localization of CENP-A at centromeres, and RbAp48 can mediate assembly of CENP-A into chromatin in vitro (Furuyama et al., 2006; Hayashi et al., 2004). The human Mis18 complex accumulates at human centromeres between telophase and G1 and is required for the deposition of newly synthesized CENP-A at centromeres. The Mis16/RbAp46/48 and Mis18 proteins appear to regulate the acetylation status of centromeric histones, which in turn affects CENP-A recruitment. Recent analyses indicate that in human cells CENP-A is incorporated in G1 and this requires passage through mitosis (Jansen et al., 2007). Mis18 and associated proteins may prime this by their prior recruitment to centromeres in telophase (Fujita et al., 2007; Maddox et al., 2007). However, neither Mis16 nor Mis18 has been shown to associate with CENP-A. It is important to understand how CENP-A is delivered to and incorporated specifically at centromeres because the assembly of more than one kinetochore on a chromosome can lead to genome instability and chromosomal rearrangements (Sullivan and Willard, 1998).
Analyses in mammalian cells indicate that the histone fold domain is essential for targeting of CENP-A to centromeres (Black et al., 2007; Sullivan et al., 1994). In addition, proteins that associate with human CENP-A—but not H3—nucleosomes have been identified, including two subunits of the FACT chromatin remodeling complex (Foltz et al., 2006; Obuse et al., 2004). However, despite the identification of such CENP-A-associated proteins, little is known about factors such as chaperones that might ensure the safe passage of CENP-A to, or its assembly at, active centromeres and centromeres alone. Recently the S. cerevisiae Scm3 protein has been found to be required to assemble a specialized centromeric nucleosome that lacks H2A-H2B (Camahort et al., 2007; Mizuguchi et al., 2007; Stoler et al., 1995). However, it is not known how widespread such an unusual CENP-A nucleosome is in eukaryotes. It is likely that an escort of some type exists for CENP-A because Asf1, CAF1, HIRA, and NASP/N1-N2 act to chaperone H3-H4, with HIRA being specifically required to mediate H3 replacement with H3.3 (reviewed in Loyola and Almouzni, 2004). Moreover, the chaperones NAP1 and Chz1 both bind H2A-H2B and variant H2AZ-H2B dimers; Chz1 shows a preference for H2AZ-H2B, whereas FACT is required for transcription-coupled disassembly of H2A-H2B from nucleosomes (Loyola and Almouzni, 2004; Luk et al., 2007).
The three centromeres of fission yeast are 35–110 kb and contain two distinct chromatin domains (Figure 1A; reviewed in Pidoux and Allshire, 2004): outer repeat heterochromatin and central domain CENP-ACnp1 chromatin (Partridge et al., 2000; Takahashi et al., 2000). Marker genes inserted within either domain are transcriptionally silenced (Allshire et al., 1994; Allshire et al., 1995). Several proteins have been shown to affect the association of CENP-A with chromatin, including Ams2, Mis6, 15, 16, 17, 18, and Sim4, yet none of these have been reported to bind CENP-A (Chen et al., 2003; Hayashi et al., 2004; Pidoux et al., 2003; Takahashi et al., 2000, 2005). Sim4 and Mis6 form a complex, Mis6 is required for the deposition of newly synthesized CENP-A at centromeres in G2 phase (Pidoux et al., 2003; Takahashi et al., 2000, 2005), and vertebrate Mis6, CENP-I, is also required for the incorporation of newly synthesized CENP-A at centromeres (Okada et al., 2006).
Figure 1.
The NASP-Related Protein Sim3 Is Required for Central Core Silencing at Centromeres and Normal Chromosome Segregation
(A) S. pombe centromeres consist of a central core domain surrounded by outer repeat regions. The arg3+ insertion at cnt1 allows monitoring of central core silencing (Pidoux et al., 2003).
(B) Left, serial dilution to monitor silencing at central core (cnt1:arg3+), outer repeat heterochromatin (otr2:ura4+), and telomeres (tel1L:his3+), assayed by growth on indicated media. sim3-143, sim3-205, cnp1-76, and mis6-302 mutants specifically alleviate central core silencing. Outer repeat and telomeric silencing is alleviated in the heterochromatin mutant rik1Δ. Right, serial dilution assay to assess growth and viability of sim3 mutants compared to wild-type at 25°C and 36°C on YES + phloxine B; darker pink colonies contain more dead cells. Strains are FY3027, 6154, 5496, 4462, 5691, and 3606.
(C) Chromosome segregation defects in sim3-143. Wild-type and sim3-143 cells were shifted to 36°C for 6 hr before fixation and immunolocalization with antibodies to α-tubulin (microtubules; green) and DAPI staining (DNA; red). Scale bar, 5 μm.
(D) Quantification of chromosome segregation patterns. Numbers are percentages of each pattern in early (short spindles) and late mitosis (elongated spindles) in cultures grown at 36°C (6 hr).
Silencing within the central domain of fission yeast centromeres is dependent on kinetochore integrity (reviewed in Pidoux and Allshire, 2004). Previously we utilized a sensitized marker gene to identify mutants that specifically alleviate central domain silencing (Pidoux et al., 2003). In addition to the kinetochore protein Sim4, we identified mutations in the histone fold domain of CENP-ACnp1. This suggests that silencing within the central domain is dependent on the assembly of CENP-ACnp1 chromatin and that other mutants might identify factors more directly involved in the delivery of CENP-ACnp1 to centromeres and assembly of CENP-ACnp1 chromatin. Here we identify the Sim3 protein, homologous to metazoan histone binding proteins NASP and N1/N2, as being required for kinetochore integrity. Our analyses suggest that one function of Sim3 is to escort CENP-ACnp1 for assembly into the specialized chromatin that underlies the kinetochore.
Results
Sim3 Encodes a NASP-Related Protein Required for Central Core Silencing and Normal Chromosome Segregation
The arg3+ gene inserted within the central core (cnt1:arg3+) of fission yeast centromere 1 (cen1) is transcriptionally silent, resulting in slow growth on plates lacking arginine (−ARG) (Figures 1A and 1B). Two alleles of sim3 (-143 and -205) that allow faster growth on −ARG plates were identified (Pidoux et al., 2003) (Figure 1B). Sim3 is specifically involved in silencing within the kinetochore domain, as neither allele affects silencing of marker genes placed within the outer repeats (otr2:ura4+) or adjacent to a telomere (tel1L:his3+) (Figure 1B). Both sim3 mutants exhibit impaired growth at all temperatures (Figure 1B). sim3-143 and sim3-205 mutants display a variety of abnormal mitotic phenotypes, including hypercondensed chromatin, lagging chromosomes in anaphase, and unequal segregation of chromosomes (Figures 1C and 1D).
The gene encoding Sim3 was identified by complementation with a high-copy genomic library. Complementing plasmids contained the ORF SPBC577.15c. Sequencing of ORF SPBC577.15c PCR-amplified from sim3-143 and sim3-205 cells in multiple mutant progeny from crosses revealed the missense mutations G81E and E207K (Figure 2B), respectively. We conclude that ORF SPBC577.15c encodes the Sim3 protein. Consistent with this, cells lacking the sim3+ gene are dead at 18°C and display moderate to severe growth impairment at higher temperatures. Furthermore, sim3Δ cells display a similar repertoire of phenotypes as sim3-143 and sim3-205 cells (Figure S1 available online). This suggests that other unidentified pathways must provide some cover for Sim3 functions.
Figure 2.
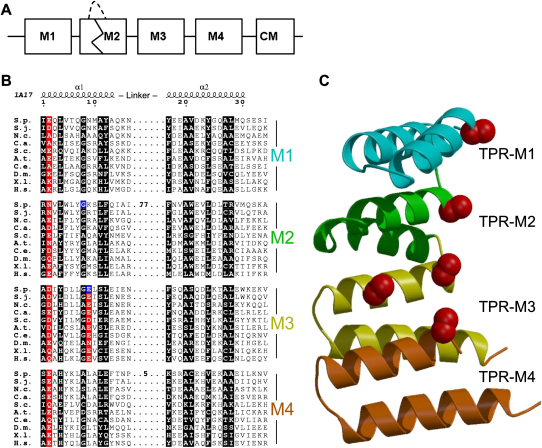
Sim3 Has Interrupted TPR Motifs
(A) Motif arrangement in the SHNi-TPR family: four TPR-related motifs (M1–M4) are predicted (see [B]), and a carboxy-terminal motif (CM) also shares similarity.
(B) Global alignment of amphipathic helices in motifs –M4 against TPR motif. S. pombe Sim3 (S.p.) was aligned with related proteins from other species (see Supplemental Data). The secondary structure from TPR1 of PP5 (PDB code 1A17) is shown above the alignment. Motifs M1, M3, and M4 are predicted to be TPR-like motifs in Human NASP. Global alignment shows that M1, M2, M3, and M4 can be structurally aligned with a TPR motif if insertions are allowed between the amphipathic helices. These insertions are sparse in hydrophobic residues and are likely to be unstructured. The hydrophobic residues that define the TPRs are shown as white-on-black boxes. The positions of mutations in Sim3 are highlighted in blue. Residue 2 of all four SHNi-TPR motifs and residue 9 of M3 are highlighted in red.
(C) A model of a four-TPR structure extrapolated from the structure of the three TPRs of PDB code 1fch. Insertions could be accommodated in the links between helices and the links between TPR modules. The Ca and Cb carbons of Residue 2 (M1–M4) and residue 9 of M3 are shown as red spheres. The protein is shown in blue to red graded by sequence number from N to C terminus.
Sim3 can be structurally aligned with histone binding proteins from fungi to mammals with tetratricopeptide repeats (TPR) (Figure 2). S. cerevisiae Hif1 acts as a chaperone for H3/H4 (Ai and Parthun, 2004). N1/N2 is complexed with stored histone H3 and H4 in Xenopus oocytes, whereas NASP copurifies with both histone H3 and the H3 replacement variant H3.3 from HeLa cells and has been shown to bind histone H1 (Dilworth et al., 1987; Kleinschmidt and Seiter, 1988; Richardson et al., 2000; Tagami et al., 2004). Although S. cerevisiae was reported to lack a NASP-related protein (Aravind et al., 2000), our analyses show that Hif1 aligns with other family members.
The family, which we call SHNi-TPR (Sim3-Hif1-NASP interrupted TPR), shares a similar organization: TPR-related motifs M1–M4 and a charged C-terminal motif (Figure 2A). For M1, M3, and M4, at least one SHNi-TPR member has a canonical TPR of 34 residues, whereas other members align to the amphipathic helices in the repeat but have different length unstructured insertions between the two helices (e.g., 77 and 5 residues in M2 and M4 of Sim3; Figure 2B). The global alignment of M1 to M4 shows that large insertions can be accommodated in the linker between the M2 helices. The sim3-143 and sim3-205 mutations, G81E and E207K, both occur in the middle of first amphipathic helices of predicted SHNi-TPR repeats (M2 and M3). These bulkier residues, which also change the charge, are expected to destabilize the protein fold. A predicted model is shown in Figure 2C (see Discussion).
CENP-ACnp1 Levels at Centromeres Are Reduced in sim3 Mutants
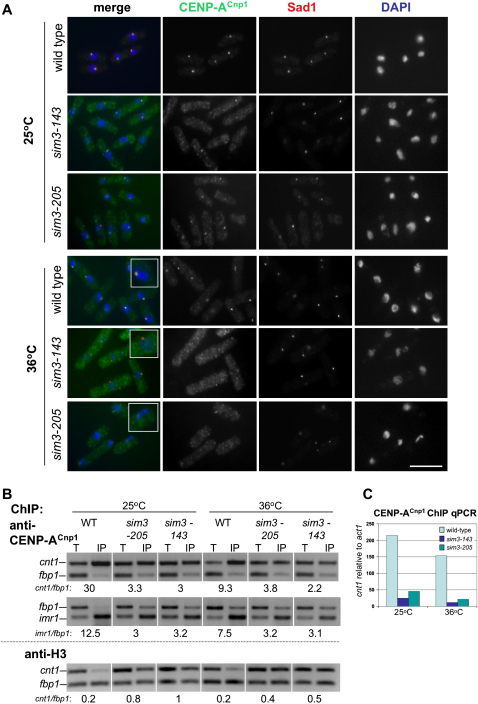
As sim3 and cnp1 mutants alleviate silencing at the central kinetochore domain where the CENP-ACnp1 H3 variant chromatin is exclusively assembled, we determined if the level of CENP-ACnp1 at centromeres is altered in sim3 mutants. Anti-CENP-ACnp1 antiserum was used to localize CENP-ACnp1 in wild-type and mutant cells at 25°C and 36°C. In wild-type G2 fission yeast, all centromeres cluster adjacent to the spindle pole body (SPB) at the nuclear periphery, resulting in a single CENP-ACnp1 signal. However, in sim3-143 and sim3-205, the CENP-ACnp1 signal is reduced or lost, particularly at 36°C, with few cells showing a bright signal adjacent to the SPB (marked by Sad1 staining; Figure 3A). Centromeres remain associated with the SPB in sim3 mutants, and thus the reduced CENP-ACnp1 localization cannot be explained by loss of centromere-SPB clustering (Figure S2).
Figure 3.
CENP-ACnp1 Levels at Centromeres Are Reduced in sim3 Mutants
(A) CENP-ACnp1 localization in wild-type and sim3 strains at 25°C and 36°C. Strains were grown at 25°C or shifted to 36°C for 6 hr before fixation and processing for immunolocalization with anti-CENP-ACnp1 (green), anti-Sad1 (red), and DAPI (blue). Scale bar, 10 μm.
(B) Top, ChIP of CENP-ACnp1 in wild-type and sim3 mutants at 25°C and 36°C (6 hr). Immunoprecipitated DNA was analyzed by multiplex PCR. cnt1 or imr1 enrichment is measured relative to the fbp1 euchromatic control and normalized to the input (T, Total) PCR. Bottom, ChIP of histone H3 in wild-type and sim3 mutants at 25°C and 36°C. The kinetics of fixation differ at 25°C and 36°C, and so ChIPs at the two temperatures are not directly comparable.
(C) ChIP of CENP-ACnp1 in wild-type and sim3 mutants at 25°C and 36°C. In a separate experiment from (B), the level of cnt1 and act1 DNA in the input and anti- CENP-ACnp1-immunoprecipitated chromatin was determined by quantitative real-time PCR.
The association of CENP-ACnp1 with centromeres was examined by chromatin immunoprecipitation (ChIP; Figure 3B). In agreement with the immunolocalization data, CENP-ACnp1 association with both cnt and imr regions of the central domain of cen1 was reduced in both sim3 mutants (note: ChIPs at 25°C and 36°C are not comparable due to different fixation kinetics). qPCR quantification confirmed loss of centromeric cnt1 enrichment relative to the euchromatic act1+ gene in the sim3 mutants at 25°C and 36°C (Figure 3C). As less CENP-ACnp1 associates with the central domain in sim3 mutants, it is possible that its place is taken by histone H3 in this defective central domain chromatin. Histone H3 is normally underrepresented in the central domain in wild-type cells, and in sim3 mutants, concomitant with the observed decrease in CENP-ACnp1, elevated levels of H3 can be detected in the central domain (Figure 3B). This is consistent with the observation that cells with mutant CENP-ACnp1 also have elevated levels of H3 within the central domain and suggests that persistence of H3 may be the default when CENP-ACnp1 chromatin assembly is defective (Castillo et al., 2007). A trivial explanation is that the levels of CENP-ACnp1 are reduced in sim3 cells, resulting in less CENP-ACnp1 being available for incorporation. The anti-CENP-ACnp1 antiserum is unable to detect CENP-ACnp1 by western; however, the levels of myc-tagged CENP-ACnp1 (expressed from the native promoter as the only source of CENP-A) detected in wild-type, sim3-143, and sim3-205 cells were similar at 25°C and 36°C (Figure S3), as were the levels of H3 (Figure S4).
Previously we have shown that an increased dose of CENP-ACnp1 suppresses sim3-143 and sim3-205 phenotypes (Pidoux et al., 2003). Reciprocal to this, overexpression of H3 antagonizes viability of sim3 mutant cells at 32°C, whereas overexpression of H4 improves viability of both mutants (Figure S5). This again suggests that the defect in central domain chromatin observed in sim3-143 and -205 mutants (a high ratio of H3:CENP-ACnp1) is exacerbated by overexpression of histone H3, whereas the provision of more wild-type CENP-A or H4 facilitates CENP-ACnp1 deposition by a defective Sim3 chaperone or by the Sim3-independent pathway implied by sim3Δ viability.
These analyses indicate that there is reduced CENP-ACnp1 and increased histone H3 at the central core in cells with defective Sim3. Thus, Sim3 is required to ensure that central domain chromatin is composed mainly of CENP-ACnp1 rather than H3 nucleosomes and suggests that obstructions in the CENP-ACnp1 assembly pathway result in H3 nucleosomes remaining or taking their place. Thus, Sim3 could act to promote CENP-ACnp1 delivery and incorporation in place of H3, or it might act to prevent H3 deposition in the central domain and thereby promote CENP-ACnp1 incorporation instead.
The Sim3 Protein Is Distributed throughout the Nucleus
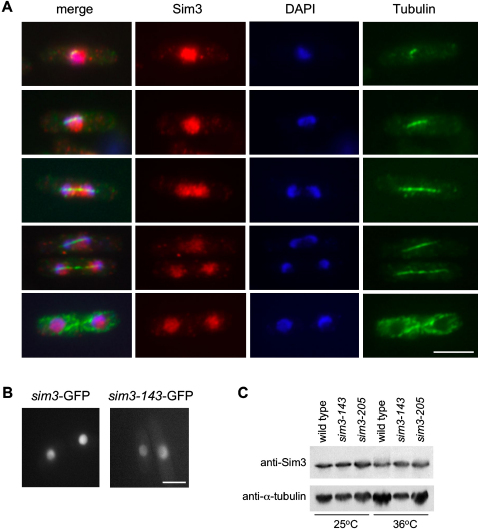
Other proteins involved in kinetochore function and CENP-ACnp1 association with the central kinetochore domain are themselves located at the central domain. This includes Mis6, 15, 16, 17, and Sim4, though Mis16 is more dispersed over chromatin and Ams2 is only in the nucleus at the onset of S phase and lost in early G2, whereas Mis18 is recruited to centromeres in late mitosis (Chen et al., 2003; Fujita et al., 2007; Hayashi et al., 2004; Pidoux et al., 2003). Anti-Sim3 antibodies were used to localize Sim3 in cells costained with anti-α-tubulin as an indicator of cell-cycle stage. Sim3 localizes throughout the entire nucleus at all cell-cycle stages with no indication of a concentration at centromeres (Figure 4A). In live cells expressing functional GFP-tagged Sim3 (as the only source of Sim3 expressed from the native promoter at the endogenous locus), an even distribution throughout the nucleus was observed. Staining of fixed Sim3-GFP cells with anti-Sim3 or anti-GFP produced a slightly punctate pattern (data not shown), but comparison with the Sim3-GFP pattern in live cells indicates that this is a consequence of fixation (Figure 4B). Costaining with anti-CENP-ACnp1, cell permeabilization and extraction of Sim3-GFP, and ChIP provided no evidence that Sim3 is preferentially localized at centromeres (data not shown), and we conclude that it is a soluble nuclear protein. Although Mis16 and Ams2 are not centromere specific, they have both been shown to be concentrated at centromeres by ChIP (Chen et al., 2003; Hayashi et al., 2004). Western analyses of wild-type and sim3 mutant extracts from cells grown at either 25°C or 36°C indicate that similar levels of Sim3 protein are present (Figure 4C), suggesting that mutant Sim3 proteins are not labile. In addition, Sim3 localization in cells expressing mutant Sim3-143-GFP or Sim3-GFP appears very similar (Figure 4B).
Figure 4.
Sim3 Is Distributed throughout the Nucleus
(A) Localization of Sim3 to the nucleus throughout the cell cycle. Immunofluorescence of wild-type cells using affinity-purified anti-Sim3 antibody (red), anti-tubulin (microtubules; green), and DAPI (DNA; blue). Merged images are shown on the left. Scale bar, 10 μm.
(B) Fluorescence images of live interphase cells expressing Sim3-GFP (FY6326) and Sim3-143-GFP (FY6308).
(C) Western analysis using anti-Sim3 antibody to detect levels of Sim3 in wild-type and sim3 mutants at 25°C and 36°C (6 hr) or anti-tubulin as a loading control.
CENP-ACnp1 Physically Interacts with Sim3
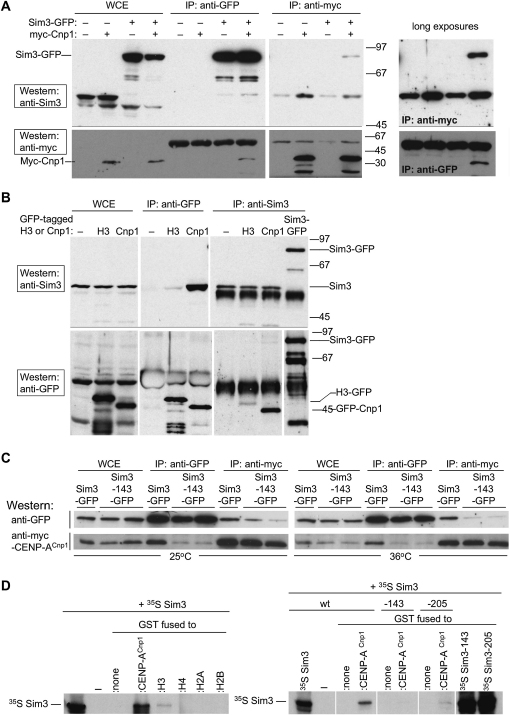
Sim3 is distributed throughout the nucleus, affects CENP-ACnp1 levels at centromeres, and has a similar domain organization as proteins known to bind histones. Thus, although Sim3 is not concentrated at centromeres, one role for it might be to escort CENP-ACnp1 to the central domain of centromeres and therefore Sim3 would be expected to interact with CENP-ACnp1. To determine if Sim3 is associated with CENP-ACnp1, immmunoprecipitations were performed with strains containing different combinations of tagged Sim3 and CENP-ACnp1 (Figure 5 and Figure S6). myc-CENP-ACnp1 was coimmunoprecipitated with Sim3-GFP only when both tags were present. Reciprocally, Sim3-GFP was coimmunoprecipitated with myc-CENP-ACnp1 (Figure 5A). In addition, in strains overexpressing GFP-CENP-ACnp1, immunoprecipitation with anti-GFP antibody pulls down GFP-CENP-ACnp1 and Sim3, and GFP-CENP-ACnp1 is detected in anti-Sim3 immunoprecipitates (Figure 5B). We also examined whether H3 can associate with Sim3. Attempts to detect H3 or H4 in Sim3 immunoprecipitates with available anti-H3/H4 antisera were mainly unsuccessful (data not shown), and we therefore utilized strains expressing tagged-H3 or H4 to increase sensitivity. Our analyses indicate that HA-tagged CENP-ACnp1, H3, and H4 can coimmunoprecipiate with Sim3 in cells overexpressing these HA-tagged proteins (nmt41x-HA-CENP-ACnp1; inv1-H3-HA; inv1-H4-HA) (Figure S6). To address whether Sim3 has a preference for CENP-ACnp1 or H3, we compared the level of GFP-tagged-CENP-ACnp1 or -H3 that coimmunoprecipitated with Sim3 in cells expressing approximately equivalent levels of either GFP-tagged histone (Figure 5B). These experiments may indicate that more CENP-ACnp1 than H3 can associate in vivo with Sim3. In reciprocal experiments, less Sim3 was detected in anti-H3-GFP than anti-GFP-CENP-ACnp1 immunoprecipitates (Figure 5B). This suggests that Sim3 may have a preference for CENP-ACnp1 over H3, but potential caveats are that the GFP-tagged histones are present at different levels relative to their respective endogenous histones, and the GFP tag may interfere differentially with the binding of these distinct histones to Sim3.
Figure 5.
Association of CENP-ACnp1 and H3 with Sim3
(A) Sim3-GFP and myc-CENP-ACnp1 coimmunoprecipitate. Extracts were prepared from cells in which Cnp1 and Sim3 were untagged (FY1645) or which expressed Sim3-GFP (FY6322), or myc-CENP-ACnp1 (FY5927), or both Sim3-GFP and myc-CENP-ACnp1 (FY6374). Immunoprecipitations were performed with sheep anti-GFP or mouse anti-myc (9E10). Immunoprecipitates (IP) and whole-cell extracts (WCE) were analyzed by western blot with rabbit anti-GFP or rabbit anti-myc antibodies as indicated. Positions of Sim3-GFP and Myc-CENP-ACnp1 proteins and molecular weight standards are indicated. Long exposures of two panels are shown on the right for comparison.
(B) GFP-CENP-ACnp1 and histone H3-GFP coimmunoprecipitate with Sim3. Extracts were prepared from strains expressing no tagged proteins (FY1645), GFP-CENP-ACnp1 (FY5205), histone H3-GFP (FY6443, a gift from Mohan Balasubramanian), or Sim3-GFP (FY6322). Immunoprecipitations were performed with sheep anti-GFP or rabbit anti-Sim3. IPs and WCEs were analyzed by western blot with rabbit anti-Sim3 or rabbit anti-GFP antibodies as indicated. Positions of Sim3, Sim3-GFP, H3-GFP, and GFP-CENP-ACnp1 proteins and molecular weight standards are indicated. Different length exposures are shown for different panels to allow relevant bands to be seen clearly.
(C) Reciprocal coimmunoprecipitations from extracts of cells expressing Sim3-GFP and myc-CENP-ACnp1 (FY6374) or Sim3-143-GFP and myc-CENP-ACnp1 (FY6368/9), grown at 25°C or 36°C (6 hr). Antibodies as in (A).
(D) Sim3 and CENP-ACnp1 interact in vitro. Left, 35S-labeled Sim3 produced by in vitro transcription and translation was incubated with GST fusion proteins, and pull downs were analyzed by SDS-PAGE and fluorography. 35S-labeled Sim3 (1/5th) was run in the left lane. Right, binding of mutant 35S-labeled Sim3-143 and Sim3-205 to GST-CENP-ACnp1 was compared to wild-type in the in vitro binding assay.
As sim3 mutants display decreased levels of CENP-ACnp1 at cnt1, we investigated if mutant Sim3 protein is defective in its association with CENP-ACnp1. Coimmununoprecipitates from extracts of cells containing Sim3-GFP and myc-CENP-ACnp1 were compared with those from cells in which mutant Sim3 was tagged with GFP (i.e., Sim3-143-GFP and myc-CENP-ACnp1). Although similar levels of both myc-CENP-ACnp1 and GFP-tagged protein were seen in wild-type versus mutant extracts, reciprocal coimmunoprecipitations indicated that lower amounts of the proteins were together in a complex in sim3 mutants compared to wild-type (Figure 5C). Thus, CENP-ACnp1 can exist in a complex with Sim3, and this association is reduced in strains with G81E and E207K mutations in the SHNi-TPR repeats of Sim3.
We addressed whether Sim3 directly interacts with CENP-ACnp1 and/or H3. Initially we assessed the ability of GST-Sim3 to pull down recombinant H3-H4-H2A-H2B octamers, H3-H4 tetramers, or soluble histones and found that Sim3 has affinity for histones (Figure S7). In addition, yeast two-hybrid assays indicated that Sim3 interacts with both CENP-ACnp1 and H3 in one configuration (Figure S7). Thus, Sim3 may chaperone both H3 and CENP-ACnp1 in different contexts. We next performed in vitro binding experiments (Figure 5D). 35S-labeled Sim3 produced by in vitro transcription-translation was tested for binding to GST or GST fused to CENP-ACnp1, H2A, H2B, H3, and H4. It is possible that a protein in the in vitro transcription-translation reaction mediates the interaction observed; however, substantially more Sim3 associated with GST-CENP-ACnp1 compared with GST-H3 and little or no Sim3 associates with GST, GST-H2A, -H2B, or -H4. Binding of 35S-labeled mutant proteins Sim3-143 and Sim3-205 to GST-CENP-ACnp1 was dramatically reduced compared with wild-type Sim3. This is consistent with an interaction between Sim3 and CENP-ACnp1 that is compromised by altered residues in M2 and M3 of the SHNi-TPRs (Figure 2C).
The findings that Sim3 binds CENP-ACnp1 in vitro and may associate preferentially with CENP-ACnp1 relative to H3 in cell extracts are consistent with Sim3 acting as an escort for CENP-ACnp1. By delivering CENP-ACnp1 to putative chromatin assembly factors, Sim3 would ensure that CENP-A is incorporated into the centromeric chromatin that underlies the kinetochore. This does not exclude the possibility that Sim3 also acts to chaperone histone H3.
Sim3 Is Required for the Incorporation of Newly Synthesized CENP-ACnp1 at Centromeres
It has been proposed that CENP-ACnp1 is deposited by both replication-coupled and replication-independent mechanisms (Takahashi et al., 2005). To further investigate CENP-ACnp1 deposition at different phases of the cell cycle and to address the involvement of Sim3 in these processes, we set up a system to rapidly induce GFP-CENP-ACnp1. A GFP-CENP-ACnp1 construct under the control of the invertase (inv1) promoter was introduced into wild-type (cnp1+) cells (Figure 6A). The inv1 promoter has the advantage of being induced within 30–90 min of switching from glucose to sucrose-rich medium and can be induced during cell-cycle arrests (Iacovoni et al., 1999). Northern and western analyses indicate that the GFP-CENP-ACnp1 transcript and protein are induced, reaching maximum levels within 60 min after switching to sucrose (Figure 6B). Analysis by fluorescence microscopy indicated that very few cells (<5%) showed a GFP-CENP-ACnp1 signal under repressed conditions (glucose) (Figure 6C). Upon induction of inv1-GFP-CENP-ACnp1 for 60 min, all cells contained the characteristic CENP-ACnp1 spot (Figure 6D). Staining with an anti-Sad1 confirmed that this signal is adjacent to the SPB and thus represents centromeres (Figure S8).
Figure 6.
Sim3 Mediates the Incorporation of Newly Synthesized GFP-CENP-ACnp1 at Centromeres
(A) Schematic of strain with inducible GFP-CENP-ACnp1 expressed from inv1 promoter.
(B) Northern and western time-course analysis showing induction of GFP-CENP-ACnp1 transcript and protein from the invertase promoter (inv1) in wild-type (FY8481), sim3-143 (FY8482), and mis6-302 (FY8519) at 25°C. adh1 was used as a loading control for northern analysis. Levels of induced GFP-CENP-ACnp1 protein were determined by anti-GFP western analysis, and Bip1 was used as a loading control. R, repressed conditions; times of induction are given in minutes.
(C) Cells grown in repressed conditions (10% glucose) at 25°C were fixed and analyzed by fluorescence microscopy; very few cells showed a GFP-CENP-ACnp1 signal (<5%).
(D) Incorporation of newly synthesized GFP-CENP-ACnp1 in wild-type, sim3-143, sim3-205, and mis6-302 at 25°C and 36°C. Cultures were grown at 25°C or shifted to 36°C for 5 hr under repressed conditions, then inv1-GFP-CENP-ACnp1 was induced by switching to sucrose media for a further hour at either 25°C or 36°C (for details see text). Cells were fixed and analyzed by fluorescence microscopy, and the presence of the characteristic CENP-ACnp1 signal was scored for each strain and condition (percentage indicated; n = 200).
(E) Anti-GFP ChIP on wild-type and sim3-143 strains containing inv1-GFP-CENP-ACnp1 at 25°C, grown under repressed or induced (1 hr) conditions. ChIPs were analyzed by PCR: cnt1 enrichment is measured relative to the fbp1 euchromatic control and normalized to the input.
To determine whether Sim3 is required for the incorporation of newly synthesized CENP-ACnp1 into centromeric chromatin, expression of inv1-GFP-CENP-ACnp1 was induced in wild-type, sim3-143, and sim3-205 cells at 25°C and 36°C (Figure 6D). After 60 min induction at 25°C, we consistently observed a GFP dot in all wild-type nuclei. In contrast, less than 11% of sim3-143 or sim3-205 cells displayed a GFP-CENP-ACnp1 signal, which was substantially weaker than that seen in wild-type cells. Northern and western analyses indicate that GFP-CENP-ACnp1 is produced in sim3 mutants (Figure 6B). For analyses at 36°C, cells were shifted to 36°C for 5 hr, prior to induction of inv1-GFP-CENP-A at 36°C for an additional hour. Most (99%) of the wild-type cells exhibited a strong GFP-CENP-ACnp1 dot in the nucleus after induction, but less than 5% of sim3 cells exhibited GFP-CENP-ACnp1 at centromeres. As a control, the incorporation of GFP-CENP-ACnp1 was monitored in a mis6-302 mutant, which has previously been shown to be defective in incorporation of CENP-ACnp1-GFP induced from the nmt1 promoter (Takahashi et al., 2000). As expected, no GFP-CENP-ACnp1 centromeric signal was observed in most mis6-302 cells at 25°C or 36°C (Figure 6D). However, in a proportion of mis6-302 cells (13.5% and 8.5%), a very strong signal was observed at centromeres and GFP-CENP-ACnp1 appeared to fill the nucleus. This confirms that Mis6 is required for the deposition of new CENP-ACnp1 at centromeres in most cells. It is not known why GFP-CENP-ACnp1 overaccumulates in a proportion of mis6-302 cells.
Anti-GFP ChIP was performed on wild-type and sim3-143 cells before and after induction of GFP-CENP-ACnp1 at 25°C. In wild-type cells, little GFP-CENP-ACnp1 was found to associate with cnt1 prior to induction, but after 60 min induction, robust enrichment of cnt1 relative to fbp1 was observed (Figure 6E). In sim3-143 cells, little GFP-CENP-ACnp1 associates with cnt1 after induction. These analyses indicate that, in wild-type cells, newly made GFP-CENP-ACnp1 is efficiently deposited in the central domain chromatin. However, although GFP-CENP-ACnp1 is induced, it is inefficiently incorporated at centromeres in sim3 mutant cells. This demonstrates that one function of Sim3 is to allow newly synthesized CENP-ACnp1 to be incorporated into centromeric chromatin.
The localization of induced inv1-HA-tagged histone H3 to chromatin was unaffected in sim3 mutants and was identical to that observed in wild-type cells, therefore sim-143 and sim3-205 do not appear to affect the deposition of new histone H3 in this relatively crude assay (Figure S9). Additionally, the fact that H3 is detected in the central domain in place of CENP-A in sim3 mutants suggests that the ability to assemble H3 into chromatin is not compromised in sim3 mutants (Figure 3). If Sim3 plays a major role in the deposition of H3, cells expressing defective Sim3 would be expected to have a general defect in chromatin integrity, which might affect gene expression. It is also possible that the mutations in sim3 indirectly affect centromeres through changes in expression of centromere components. To address these concerns, expression profiling of sim3-143 and sim3-205 cells was performed (see Table S1). mRNA levels in logarithmically growing cultures of sim3 mutant cells were compared to those in wild-type. Lists of misregulated genes in sim3 mutants relative to wild-type were established. Using a standard 2-fold cutoff for changes in gene expression, only 20 genes were >2-fold up- or downregulated by the stronger sim3-205 allele and no genes were affected by the weaker sim3-143 allele. Using a less stringent 1.5-fold cutoff, 43 genes were affected by sim3-143 and 113 genes were affected by sim3-205. Surprisingly, only one gene (SPAC1F8.04c) was similarly affected by both sim3 alleles. Thus, we conclude that relatively few genes and distinct sets of genes were affected in the two sim3 mutant alleles. The annotation of the affected genes indicates that no gene products known to be involved in centromere function are affected, thus arguing against indirect effects of sim3 on CENP-A chromatin assembly. The fact that so few genes are affected in sim3 mutants indicates that Sim3 is not required for general chromatin integrity and suggests that one of its main functions is to escort CENP-ACnp1. However, other roles for Sim3 in H3 chromatin integrity may remain to be uncovered.
Sim3 Is Required to Aid the Deposition of CENP-ACnp1 during S and G2
During S phase, newly synthesized histones are deposited on DNA by a chromatin assembly process that is tightly coupled to DNA synthesis at the replication fork. However, replacement histones such as H3.3 and H2AZ are deposited in a replication-independent manner and require distinct assembly factors (Mizuguchi et al., 2004; Tagami et al., 2004).
Human CENP-A is synthesized in G2, and its incorporation at centromeres is not synchronized with their replication (Shelby et al., 2000; Sullivan and Karpen, 2001). CENP-ACnp1-GFP is also incorporated at centromeres in fission cells blocked in G2 (Takahashi et al., 2005). As ∼70% of cells in an asynchronous culture are in G2, the data presented above (Figure 6) suggest that, like the Mis6 (control), Sim3 participates in a CENP-ACnp1 chromatin assembly process in interphase. However, to test more rigorously if new CENP-ACnp1 produced from inv1-GFP-CENP-ACnp1 is incorporated during G2 or other cell-cycle stages, this construct was combined with the temperature-sensitive cdc10 (G1 phase arrest), cdc25 (G2 phase arrest) mutations, or analyzed cells in the presence of hydroxyurea (HU; S phase arrest).
Cdc10 is required for the initiation of S phase; after 4 hr at 36°C, cdc10-129 cells are elongated, indicative of a G1/S cell-cycle arrest (Forsburg and Nurse, 1991). cdc10-129 cells were incubated at 36°C for 3 hr and then inv1-GFP-CENP-ACnp1 was induced by switching to sucrose for an additional 60 min incubation at 36°C (4 hr total). Only weak GFP-CENP-ACnp1 foci were visible in a few cells in cdc10-129-arrested cells (Figure 7A), suggesting that newly produced CENP-ACnp1 is not efficiently incorporated at centromeres in cells arrested in G1. Thus the CENP-ACnp1 assembly pathway may be downregulated in G1.
Figure 7.
Sim3 Aids the Deposition of GFP-CENP-ACnp1 during S and G2
(A) Wild-type and cdc10-129 mutant (G1 arrest) cells were incubated at 36°C for 3 hr followed by induction of inv1-GFP-CENP-ACnp1 for 60 min in sucrose medium (4 hr total at 36°C). Cells were fixed and analyzed for presence of GFP-CENP-ACnp1 spot by fluorescence microscopy.
(B) Wild-type, sim3-143, sim3-205, and mis6-302 cells were arrested in S phase by the addition of 25 mM hydroxyurea (HU) for 4 hr at 25°C, followed by induction of inv1-GFP-CENP-ACnp1 in sucrose media containing HU for a further 1 hr (5 hr total). Cells were analyzed as in (A).
(C) Incorporation of newly synthesized GFP-CENP-ACnp1 in cdc25-22 (FY8518), sim3-143 cdc25-22 (FY8717), and sim3-205 cdc25-22 (FY8718) strains at 36°C. Cultures grown at 25°C were shifted to 36°C for 3 hr in repressed conditions, then inv1-GFP-CENP-ACnp1 was induced for a further 1 hr at 36°C. Cells were fixed, DAPI stained, and analyzed by fluorescence microscopy for the presence of GFP-CENP-ACnp1 signal (n = 200 for each strain). Scale bar, 10 μm.
(D) Model: Sim3 (C shape) acts a classic chaperone, directly binding CENP-ACnp1 (white triangle), escorting it to the centromere, and handing it over to centromere-associated chromatin assembly factors (white crescent, X) that incorporate CENP-ACnp1 in place of histone H3. CENP-ACnp1 and H3 nucleosomes are shown as white cubes and gray cylinders, respectively. Evicted H3 may also be received by Sim3.
To determine whether Sim3-dependent CENP-ACnp1 deposition operates during S phase, wild-type, sim3-143, and sim3-205 cells were arrested in early S phase by the addition of HU. After 4 hr in HU, new GFP-CENP-ACnp1 was induced (1 hr in HU + sucrose), and GFP-CENP-ACnp1 foci were clearly formed in wild-type, but not sim3 or most mis6 (control), cells (Figure 7B). Thus CENP-ACnp1 can be deposited in S phase without ongoing replication.
Cdc25 is required to activate Cdc2 cyclin-dependent kinase and allow cells to enter mitosis (Forsburg and Nurse, 1991). After 4 hr at 36°C, cdc25-22 cells are blocked at G2/M as indicated by elongated cell morphology. After 3 hr incubation at 36°C, inv1-GFP-CENP-ACnp1 was induced in cdc25-22 cells for 60 min at 36°C. All cdc25-22 cells displayed a GFP-CENP-ACnp1 dot (Figure 7C), indicating that GFP-CENP-ACnp1 produced in G2-arrested cells is deposited at centromeres. However, in parallel experiments, a centromeric GFP-CENP-ACnp1 signal was not detectable in sim3-143 cdc25-22 and sim3-205 cdc25-22 cells. This indicates that the NASP-related protein Sim3 is required for the efficient replication-independent deposition of CENP-ACnp1 at fission yeast centromeres during G2.
Thus, Sim3 is required in both S- and G2-arrested cells to allow CENP-ACnp1 incorporation at centromeres. Although distinct mechanisms of CENP-ACnp1 deposition have been found to operate during S and G2 phases of the cell cycle (Takahashi et al., 2000, 2005), it appears that Sim3 is required to escort nascent CENP-ACnp1 in both S and G2 phase to CENP-ACnp1 chromatin assembly factors for incorporation at centromeres (Figure 7D).
Discussion
The sim3 mutants were identified through their alleviation of silencing within the central kinetochore domain of fission yeast centromeres. Here, we have shown that the sim3 mutations reside in an ORF that can be aligned with the known histone binding proteins N1/N2, NASP, and Hif1. We have demonstrated that Sim3 is required for normal levels of CENP-ACnp1 at the central domain of centromeres and probably interacts directly with CENP-ACnp1 in vitro. In addition, our analyses indicate that Sim3 can associate with H3, suggesting that Sim3 may have other roles in chromatin assembly and integrity. However, expression profiling indicates that Sim3 does not act generally to maintain chromatin. In addition, immunoprecipitations from fission yeast extracts suggest that Sim3 may preferentially associate with CENP-ACnp1 compared to histone H3.
The two sim3 mutants isolated have altered residues in the conserved interrupted TPR-like repeats (SHNi-TPR), and both disrupt Sim3-CENP-ACnp1 complex formation in vitro and in vivo. The Sim3 protein is not a kinetochore protein but is distributed throughout the nucleus. Cells with defective Sim3 are unable to incorporate newly synthesized CENP-ACnp1 at centromeres in S or G2 phases. Together, these data are consistent with a model in which Sim3 acts as an escort for CENP-ACnp1, ensuring that it is delivered to centromeres. We suggest that Sim3 hands off CENP-ACnp1 to other assembly factors located at centromeres (Figure 7D). In this way, Sim3 might also contribute to the specificity of incorporation and prevent the inappropriate assembly of CENP-ACnp1 into noncentromeric chromatin. Centromere-associated proteins such as Ams2, Mis6, 15, 16, 17, 18, and Sim4 are known to affect CENP-ACnp1 incorporation in the central domain and are candidates for the putative CENP-ACnp1 acceptors. However, to date, none of these kinetochore proteins have been shown to associate with CENP-ACnp1. Indeed, to the best of our knowledge, Sim3 is the first protein in fission yeast, which has regional rather than point centromeres, that has been shown to associate with CENP-ACnp1. Hence, Sim3 may contribute to the propagation of CENP-ACnp1 chromatin at a specific locus by only surrendering CENP-ACnp1 to factors exclusively associated with active centromeres. Such factors might include the ortholog of S. cerevisiae Scm3 (Camahort et al., 2007; Mizuguchi et al., 2007; Stoler et al., 2007), but this remains to be tested.
It is well established that canonical histone H3 can be evicted and replaced by the H3.3 variant in metazoan cells. This provides the paradigm for replication-independent histone replacement (reviewed in Henikoff and Ahmad, 2005). The replacement of H3 by H3.3 is induced by transcription and, because it can occur in interphase cells, is uncoupled from replication. H3.3 complexes with the chaperones HIRA and ASF1A, which are required to allow its assembly into chromatin—interestingly, NASP also associated with H3.3 (Tagami et al., 2004). The Swr1 complex promotes the replacement of core histone H2A with the variant H2AZ, and its Swc2 subunit directly binds H2AZ and is required for H2A-H2AZ exchange (Mizuguchi et al., 2004; Wu et al., 2005). Chz1 passes H2AZ to Swr1 but does not participate in H2AZ-H2A replacement (Luk et al., 2007). Our analyses detected elevated levels of histone H3 in the central kinetochore domain in cells with defective Sim3. One explanation is that histone H3 is deposited at centromeres by default during replication and is subsequently evicted and replaced with the kinetochore-specific H3 variant CENP-ACnp1 in a manner similar to that described for replacement of H3 by H3.3 during transcription (Ahmad and Henikoff, 2002; McKittrick et al., 2004; Mito et al., 2005). Such a process may be reliant on a priming step mediated by Mis18 in late metaphase (Fujita et al., 2007; Maddox et al., 2007). Indeed, the detected Sim3-H3 association might be indicative of Sim3 being required to receive evicted H3 after an H3-to-CENP-A exchange event. A complete understanding of such CENP-A histone replacement/exchange factors awaits further investigation. Regardless of the exact CENP-A assembly mechanism, we envisage that fission yeast Sim3 acts ahead of such chromatin assembly or histone exchange factors as a classic chaperone, ensuring that CENP-ACnp1 is handed over to centromere-associated CENP-A assembly factors, but unlike S. cerevisiae Scm3, it is not a component of the final structure itself.
As newly synthesized CENP-ACnp1 is deposited at centromeres in both G2- and S phase-arrested cells, CENP-ACnp1 deposition does not appear to be mandatorily coupled to ongoing replication, even during S phase. As CENP-ACnp1 levels decline and histone H3 levels increase at centromeres in sim3 mutants, it is possible that H3 is initially deposited in S phase and subsequently replaced by nascent CENP-ACnp1 during S and G2. Another possibility is that by binding to CENP-ACnp1 Sim3 acts to prevent its promiscuous incorporation into noncentromeric chromatin. The order of events, players, and specific interactions in such a complex exchange or remodeling event may differ in details between species so that in some organisms CENP-A may be incorporated in G1 after priming in late mitosis (Fujita et al., 2007; Jansen et al., 2007), whereas in other organisms, related events may occur at different cell-cycle stages.
The structural alignment indicates that Sim3 and other SHNi-TPR family members contain a reiterated sequence motif that is an interrupted form of TPR repeat (Figure 2). TPR motifs are normally found in tandem arrays, and structures show that these helical hairpins form a head-to-tail zigzag structure to create a convex face and a concave surface inside the superhelix (reviewed in D'Andrea and Regan, 2003). In some structures, a peptide is shown to bind within the cavity formed by the TPR motifs (Scheufler et al., 2000). In the SHNi-TPR family, position 2 of each repeat has a negatively charged side chain or an amidated version. Moreover, position 9 of M3 is often negatively charged. Intriguingly, these residues line the concave face in a model of four SHNi-TPR repeats and may form an ion binding site or a recognition site for a positively charged section of proteins such as histones (Figure 2C). The mutations identified in Sim3, which affect both in vitro and in vivo interactions with CENP-ACnp1, are predicted to disrupt this putative recognition site.
Thus, Sim3 is related to NASP that has been shown to copurify with both H3 and H3.3 from mammalian cells (Tagami et al., 2004). Curiously, S. cerevisiae was reported to lack a NASP protein (Aravind et al., 2000). This might reflect the fact that S. cerevisiae kinetochores contain a single CENP-A nucleosome, whereas centromeres in other organisms (including the yeast C. albicans) have arrays of CENP-A nucleosomes at each centromere (Baum et al., 2006), and in humans, at least, these contain H2A-H2B (Foltz et al., 2006). In fact, although the S. cerevisiae protein Hif1 shares only weak similarity to NASP, N1/N2, and Sim3, it can be aligned with them and has been shown to bind H3/H4 and contribute to their deposition.
It is not known if NASP proteins are required for the deposition of histones in metazoa, but NASP associates with H3 and H3.3 in HeLa cells. It is also not known if NASP associates with CENP-A in these cells. Different organisms may put different emphases on particular mechanisms that restrict CENP-A to centromeres; these may include proteolysis and/or targeting (Black et al., 2007; Collins et al., 2004). In fission yeast, the Sim3 NASP-related protein appears to act as a CENP-ACnp1 chaperone but it may also have other unidentified roles in aiding H3 dynamics, as indicated by its association with H3. It will be interesting to determine if other NASP-like proteins are involved in shepherding CENP-A to centromeres.
Experimental Procedures
Induction and Analysis of Newly Synthesized GFP-CENP-ACnp1
GFP-CENP-ACnp1 was placed under the control of the inv1 promoter and integrated at the ura4 locus (strain FY8481). The inv1 promoter was repressed in PMG with 10% glucose and induced by switching to PMG with 4% sucrose as the sole carbon source for 1 hr. To block DNA replication, cells were treated with 25 mM HU for 4 hr at 25°C. For FACS analysis (BD FACSCalibur), cells were fixed in 70% ethanol before staining with propidium iodide.
Other Methods
In vitro binding assays are described in the Supplemental Data. Microscopy and ChIP were performed as previously described (Pidoux et al., 2003). Immunoprecipitations were performed as previously described (Millband and Hardwick, 2002). Modifications and details of antibodies are given in the Supplemental Data.
Acknowledgments
We thank members of the Allshire Lab for helpful advice and discussions. We thank K. Gull, I. Hagan, K. Hardwick, and K. Samejima for antibodies; M. Balasubramanian, S. MacNeill, B. Mellone, and M. Yanagida for strains; C. Shimoda for providing the pAL genomic library; Eun Shik Choi for plasmids and help with qPCR; and T. Owen-Hughes for histone octamers and tetramers. We thank M. Vogelauer, I. Stancheva, K. Hardwick, and G. Almouzni for critical input on the manuscript. E.M.D. was supported by a Wellcome Trust Studentship. A.L.P. was supported in part by the AICR and the Wellcome Trust. W.R. was supported by the MRC UK. M.M. was supported by the Erasmus Exchange programme. This work was initiated with core funding from the MRC UK and subsequently the Wellcome Trust (065061/Z). R.C.A. is a Wellcome Trust Principal Research Fellow.
Published: December 27, 2007
Footnotes
Supplemental Data include Supplemental Experimental Procedures, Supplemental References, nine figures, and three tables and can be found with this article online at http://www.molecule.org/cgi/content/full/28/6/1029/DC1/.
Contributor Information
Alison L. Pidoux, Email: alison.pidoux@ed.ac.uk.
Robin C. Allshire, Email: robin.allshire@ed.ac.uk.
Accession Numbers
The Gene Expression omnibus (GEO) submission series for the sim3 microarray data is GSE7560 at http://www.ncbi.nlm.nih.gov/geo/.
Supplemental Data
References
- Ahmad K., Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell. 2002;9:1191–1200. doi: 10.1016/s1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- Ai X., Parthun M.R. The nuclear Hat1p/Hat2p complex: a molecular link between type B histone acetyltransferases and chromatin assembly. Mol. Cell. 2004;14:195–205. doi: 10.1016/s1097-2765(04)00184-4. [DOI] [PubMed] [Google Scholar]
- Allshire R.C., Javerzat J.P., Redhead N.J., Cranston G. Position effect variegation at fission yeast centromeres. Cell. 1994;76:157–169. doi: 10.1016/0092-8674(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Allshire R.C., Nimmo E.R., Ekwall K., Javerzat J.P., Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Aravind L., Watanabe H., Lipman D.J., Koonin E.V. Lineage-specific loss and divergence of functionally linked genes in eukaryotes. Proc. Natl. Acad. Sci. USA. 2000;97:11319–11324. doi: 10.1073/pnas.200346997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M., Sanyal K., Mishra P.K., Thaler N., Carbon J. Formation of functional centromeric chromatin is specified epigenetically in Candida albicans. Proc. Natl. Acad. Sci. USA. 2006;103:14877–14882. doi: 10.1073/pnas.0606958103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B.E., Jansen L.E., Maddox P.S., Foltz D.R., Desai A.B., Shah J.V., Cleveland D.W. Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol. Cell. 2007;25:309–322. doi: 10.1016/j.molcel.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Camahort R., Li B., Florens L., Swanson S.K., Washburn M.P., Gerton J.L. Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol. Cell. 2007;26:853–865. doi: 10.1016/j.molcel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Castillo A.G., Mellone B., Partridge J.F., Richardson W., Hamilton G.L., Allshire R.C., Pidoux A.L. Plasticity of fission yeast CENP-A chromatin driven by relative levels of histone H3 and H4. PLOS Genetics. 2007;3:e121. doi: 10.1371/journal.pgen.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E.S., Saitoh S., Yanagida M., Takahashi K. A cell cycle-regulated GATA factor promotes centromeric localization of CENP-A in fission yeast. Mol. Cell. 2003;11:175–187. doi: 10.1016/s1097-2765(03)00011-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D.W., Mao Y., Sullivan K.F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Collins K.A., Furuyama S., Biggins S. Proteolysis contributes to the exclusive centromere localization of the yeast Cse4/CENP-A histone H3 variant. Curr. Biol. 2004;14:1968–1972. doi: 10.1016/j.cub.2004.10.024. [DOI] [PubMed] [Google Scholar]
- D'Andrea L.D., Regan L. TPR proteins: the versatile helix. Trends Biochem. Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Dilworth S.M., Black S.J., Laskey R.A. Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell. 1987;51:1009–1018. doi: 10.1016/0092-8674(87)90587-3. [DOI] [PubMed] [Google Scholar]
- Foltz D.R., Jansen L.E., Black B.E., Bailey A.O., Yates J.R., 3rd, Cleveland D.W. The human CENP-A centromeric nucleosome-associated complex. Nat. Cell Biol. 2006;8:458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- Forsburg S.L., Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu. Rev. Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Hayashi T., Kiyomitsu T., Toyoda Y., Kokubu A., Obuse C., Yanagida M. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev. Cell. 2007;12:17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Furuyama T., Dalal Y., Henikoff S. Chaperone-mediated assembly of centromeric chromatin in vitro. Proc. Natl. Acad. Sci. USA. 2006;103:6172–6177. doi: 10.1073/pnas.0601686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Ahmad K. Assembly of variant histones into chromatin. Annu. Rev. Cell Dev. Biol. 2005;21:133–153. doi: 10.1146/annurev.cellbio.21.012704.133518. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Dalal Y. Centromeric chromatin: what makes it unique? Curr. Opin. Genet. Dev. 2005;15:177–184. doi: 10.1016/j.gde.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Heun P., Erhardt S., Blower M.D., Weiss S., Skora A.D., Karpen G.H. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev. Cell. 2006;10:303–315. doi: 10.1016/j.devcel.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovoni J.S., Russell P., Gaits F. A new inducible protein expression system in fission yeast based on the glucose-repressed inv1 promoter. Gene. 1999;232:53–58. doi: 10.1016/s0378-1119(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Jansen L.E., Black B.E., Foltz D.R., Cleveland D.W. Propagation of centromeric chromatin requires exit from mitosis. J. Cell Biol. 2007;176:795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J.A., Seiter A. Identification of domains involved in nuclear uptake and histone binding of protein N1 of Xenopus laevis. EMBO J. 1988;7:1605–1614. doi: 10.1002/j.1460-2075.1988.tb02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A.W., Magliano D.J., Sibson M.C., Kalitsis P., Craig J.M., Choo K.H. A novel chromatin immunoprecipitation and array (CIA) analysis identifies a 460-kb CENP-A-binding neocentromere DNA. Genome Res. 2001;11:448–457. doi: 10.1101/gr.167601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola A., Almouzni G. Histone chaperones, a supporting role in the limelight. Biochim. Biophys. Acta. 2004;1677:3–11. doi: 10.1016/j.bbaexp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Luk E., Vu N.D., Patteson K., Mizuguchi G., Wu W.H., Ranjan A., Backus J., Sen S., Lewis M., Bai Y., Wu C. Chz1, a nuclear chaperone for histone H2AZ. Mol. Cell. 2007;25:357–368. doi: 10.1016/j.molcel.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Maddox P.S., Hyndman F., Monen J., Oegema K., Desai A. Functional genomics identifies a Myb domain-containing protein family required for assembly of CENP-A chromatin. J. Cell Biol. 2007;176:757–763. doi: 10.1083/jcb.200701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick E., Gafken P.R., Ahmad K., Henikoff S. Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc. Natl. Acad. Sci. USA. 2004;101:1525–1530. doi: 10.1073/pnas.0308092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millband D.N., Hardwick K.G. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell. Biol. 2002;22:2728–2742. doi: 10.1128/MCB.22.8.2728-2742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito Y., Henikoff J.G., Henikoff S. Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 2005;37:1090–1097. doi: 10.1038/ng1637. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G., Shen X., Landry J., Wu W.H., Sen S., Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G., Xiao H., Wisniewski J., Smith M.M., Wu C. Nonhistone Scm3 and histones CenH3–H4 Assemble the core of centromere-specific nucleosomes. Cell. 2007;129:1153–1164. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Obuse C., Yang H., Nozaki N., Goto S., Okazaki T., Yoda K. Proteomics analysis of the centromere complex from HeLa interphase cells: UV-damaged DNA binding protein 1 (DDB-1) is a component of the CEN-complex, while BMI-1 is transiently co-localized with the centromeric region in interphase. Genes Cells. 2004;9:105–120. doi: 10.1111/j.1365-2443.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- Okada M., Cheeseman I.M., Hori T., Okawa K., McLeod I.X., Yates J.R., 3rd, Desai A., Fukagawa T. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat. Cell Biol. 2006;8:446–457. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- Partridge J.F., Borgstrom B., Allshire R.C. Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev. 2000;14:783–791. [PMC free article] [PubMed] [Google Scholar]
- Pidoux A.L., Allshire R.C. Kinetochore and heterochromatin domains of the fission yeast centromere. Chromosome Res. 2004;12:521–534. doi: 10.1023/B:CHRO.0000036586.81775.8b. [DOI] [PubMed] [Google Scholar]
- Pidoux A.L., Richardson W., Allshire R.C. Sim4: a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation. J. Cell Biol. 2003;161:295–307. doi: 10.1083/jcb.200212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R.T., Batova I.N., Widgren E.E., Zheng L.X., Whitfield M., Marzluff W.F., O'Rand M.G. Characterization of the histone H1-binding protein, NASP, as a cell cycle-regulated somatic protein. J. Biol. Chem. 2000;275:30378–30386. doi: 10.1074/jbc.M003781200. [DOI] [PubMed] [Google Scholar]
- Scheufler C., Brinker A., Bourenkov G., Pegoraro S., Moroder L., Bartunik H., Hartl F.U., Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Shelby R.D., Monier K., Sullivan K.F. Chromatin assembly at kinetochores is uncoupled from DNA replication. J. Cell Biol. 2000;151:1113–1118. doi: 10.1083/jcb.151.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S., Keith K.C., Curnick K.E., Fitzgerald-Hayes M. A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev. 1995;9:573–586. doi: 10.1101/gad.9.5.573. [DOI] [PubMed] [Google Scholar]
- Stoler S., Rogers K., Weitze S., Morey L., Fitzgerald-Hayes M., Baker R.E. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc. Natl. Acad. Sci. USA. 2007;104:10571–10576. doi: 10.1073/pnas.0703178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B., Karpen G. Centromere identity in Drosophila is not determined in vivo by replication timing. J. Cell Biol. 2001;154:683–690. doi: 10.1083/jcb.200103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.F. A solid foundation: functional specialization of centromeric chromatin. Curr. Opin. Genet. Dev. 2001;11:182–188. doi: 10.1016/s0959-437x(00)00177-5. [DOI] [PubMed] [Google Scholar]
- Sullivan B.A., Willard H.F. Stable dicentric X chromosomes with two functional centromeres. Nat. Genet. 1998;20:227–228. doi: 10.1038/3024. [DOI] [PubMed] [Google Scholar]
- Sullivan K.F., Hechenberger M., Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J. Cell Biol. 1994;127:581–592. doi: 10.1083/jcb.127.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.A., Blower M.D., Karpen G.H. Determining centromere identity: cyclical stories and forking paths. Nat. Rev. Genet. 2001;2:584–596. doi: 10.1038/35084512. [DOI] [PubMed] [Google Scholar]
- Tagami H., Ray-Gallet D., Almouzni G., Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Chen E.S., Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288:2215–2219. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Takayama Y., Masuda F., Kobayashi Y., Saitoh S. Two distinct pathways responsible for the loading of CENP-A to centromeres in the fission yeast cell cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:595–606. doi: 10.1098/rstb.2004.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.H., Alami S., Luk E., Wu C.H., Sen S., Mizuguchi G., Wei D., Wu C. Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat. Struct. Mol. Biol. 2005;12:1064–1071. doi: 10.1038/nsmb1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.