Abstract
The prevailing paradigm is that production of the interleukin (IL)-12 p70 heterodimer, a critical T helper cell type 1 (Th1)–inducing cytokine, depends on the induced transcription of the p40 subunit. Concordant with this paradigm, we found that dendritic cells (DCs) produced IL-12 p70 only after at least 2–4 h of stimulation with lipopolysaccharide plus interferon γ. However, using several complementary experimental approaches, including electron and confocal microscopy, we now show that resting murine and human myeloid cells, including macrophages/DCs and DC-rich tissues, contain a novel source of bioactive IL-12 that is preformed and membrane associated. These preformed, membrane-associated IL-12 p70 stores are released within minutes after in vitro or in vivo contact with Leishmania donovani, an intracellular pathogen. Our findings highlight a novel source of bioactive IL-12 that is readily available for the rapid initiation of Th1 host responses to pathogens such as Leishmania species.
Keywords: IL-12 p70, dendritic cells, Th1/Th2, Leishmania, membrane
Introduction
IL-12 is central to the orchestration of both innate and acquired immune responses 1. IL-12 is produced primarily by phagocytic cells and, among its multiple activities, it is a potent inducer of IFN-γ from T and NK cells 1. As such, it is critical for the development of Th1 responses that are required for generating protective cell-mediated inflammatory responses against invading intracellular microorganisms. The importance of IL-12 in generating protective Th1 responses is illustrated by the finding that humans and experimental murine models with genetic mutations that block IL-12 or IFN-γ–dependent signaling pathways have marked sensitivity to intracellular infections such as the Mycobacterium and Leishmania species 2 3 4 5.
There are two general paradigms regarding the mechanisms that control IL-12 production by APCs such as dendritic cells (DCs) and macrophages. First, in all systems where IL-12 regulation has been molecularly defined, it has been found to be heavily transcriptionally dependent 1 6 7. Biologically active IL-12 (p70) is a heterodimeric protein composed of the covalently linked products of p40 and p35, two separate genes that are regulated independently 1. Although both p35 and p40 are highly regulated proteins, there is general consensus that new synthesis of IL-12 p70 depends primarily on the induced transcription of the p40 gene 1 6 7 8. Notably, the induced transcription of the p40 gene is delayed compared with other proinflammatory cytokines such as TNF-α; stimulation of phagocytic cells with LPS plus IFN-γ or Staphylococcus aureus (both potent inducers of IL-12) leads to the accumulation of IL-12 p40 mRNA within ∼2–4 h 1. Because the accumulation of p40 mRNA occurs only after at least 2 h of APC activation, the general consensus is that bioactive IL-12 is available to induce Th1 differentiation only after this time period 1.
A second emerging paradigm is that certain intracellular pathogens may exploit a mechanism of IL-12 regulation that involves cross-linking of surface receptors on macrophages 9. Through receptor-mediated mechanisms, microorganisms such as the measles virus, HIV, and Leishmania major have been shown to inhibit the production of IL-12, whereas others, such as Mycobacterium tuberculosis, Cryptococcus neoformans, and Toxoplasma gondii, induce IL-12 production 9 10 11 12 13. The basis for this differential host–microbe interaction remains unclear. Nevertheless, the studies that have examined the Leishmania-mediated suppression of IL-12 production from APCs have generally been restricted to the determination of IL-12 production after 2 h of contact between the microbe and a distinct subset of APCs, namely macrophages/monocytes 10 11 12.
DCs differ from monocytes/macrophages in their ability to serve as potent activators of naive T cells, thus acting as critical initiators of the primary specific immune response 14. Given the central role of DC-derived IL-12 in inducing Th1 differentiation and the notion that the factors that influence Th1–Th2 skewing should be operative soon after contact with a pathogen 14 15 16 17 18, we hypothesized that there is a preformed store of IL-12 that is readily available for the rapid initiation of a Th1 immune response shortly after contact with specific microorganisms.
Materials and Methods
Materials.
All reagents for cell culture were obtained from Life Technologies; recombinant growth factors were from R&D Systems; ELISA reagents and antibodies were from PharMingen and R&D Systems; and chemicals were from Sigma-Aldrich, unless stated otherwise.
Murine DCs.
DCs were differentiated from 4–6-wk-old murine (BALB/c) bone marrow cells in the presence of growth factors GM-CSF (50 ng/ml) and IL-4 (1 ng/ml) as previously described 19. By FACS™ analysis, the cells had phenotypic characteristics of DCs: they expressed abundant MHC class II, CD80, CD40, CD11b, and DEC-205. Cell-surface markers for contaminating macrophages (F4/80+), B cells (B220+), and T cells (CD3+) were detected on < 1% of cells.
Parasites.
6–70-d-old stationary phase Leishmania donovani 1S strain (MHOM/SD/001S-2D) were maintained in Medium 199 with 20% FBS and were washed three times in DMEM before use. In some experiments, metacyclic promastigotes or opsonized/nonopsonized forms of L. donovani and L. major parasites were also used 20 21. Stationary-phase Leishmania promastigotes were inactivated by exposure to UV light (12 h), formalin (10% for 5 h), trypsin (1% for 5 h), or heat (65°C for 15 min). In some experiments, parasites were treated with cytochalasin D (30 μg/ml) for 1 h at 37°C; these parasites had reduced motility (due to polymerization of the cell-wall actin; reference 22) but remained viable (data not shown). In some experiments, stationary-phase Leishmania promastigotes were labeled with the membrane-stain PKH26 red fluorescent cell linker (Sigma-Aldrich). The PKH-labeled promastigotes were fluorescent, viable in vitro, and induced infection in vivo (data not shown).
DC Infection, Stimulation, or Treatment.
DCs were rested for 4 h before addition of L. donovani promastigotes (107/ml) or stimulation with LPS (10 μg/ml) plus IFN-γ (20 U/ml). DCs (2–5 × 106/ml) were placed in polypropylene tubes, and live stationary-phase L. donovani promastigotes were added at a 10:1 multiplicity of infection and mixed gently, then aliquots were transferred immediately to 24-well plates and cultured at 37°C in 5% CO2. Duplicate wells were dedicated for each time point of analysis. At various time intervals, the supernatants were harvested (duplicate samples) and the IL-12 p70 levels were determined by ELISA (anti–mouse IL-12 p35/p70 and p40/p70 were used as primary and secondary antibodies, respectively). The detection range for murine IL-12 p70 was between 12.3 pg/ml and 9,000 pg/ml. IL-12 levels that fell below the limit of detection are designated as zero (0). In some experiments, before addition of parasites or LPS plus IFN-γ, DCs were pretreated with monensin (2 μM for 4 h), brefeldin A (1 μg/ml for 4 h), actinomycin D (10 μg for 1 h), or cytochalasin D (10 μg for 1 h; all from Sigma-Aldrich). In some experiments, opsonized or nonopsonized latex beads (2.97, 1.094, and 0.76 μm; Sigma-Aldrich) or Histoplasma capsulatum G217 strain 23 were also added to the DCs. Methods for opsonization are as described in reference 21.
Bioassay.
To determine the biological activity of the preformed stores of IL-12, the ability of IL-12 to induce Ag-specific IFN-γ production was determined by a bioassay. Ag-primed splenocytes were obtained from vaccinated or infected mice as previously described 19. In brief, BALB/c mice were vaccinated intravenously with bone marrow–derived DCs pulsed with soluble L. donovani–derived Ag. 14 d later, the spleens were harvested, and single-cell suspensions (106/ml) were used in the bioassay. The splenocytes were cultured with Ag, i.e., soluble L. donovani–derived Ag (25 μg/ml) ± sample supernatants (50% vol/vol of supernatants of DCs alone or DCs that had been in contact with L. donovani for 10 min) ± recombinant IL-12 p70 or IL-12–blocking antibody (clone 17.8). At the concentration used (4 μg/ml), the IL-12 blocking antibody blocks IL-12–induced IFN-γ production by 50%. IFN-γ was measured by ELISA after 48 h of addition of the DC supernatants to the splenocytes.
DC Labeling and Confocal Microscopy.
DCs were labeled with the indicated antibodies at 4°C or room temperature with or without cell permeabilization. A commercially available anti–IL-12 p70 Ab (R&D Systems) was used to localize IL-12 p70 with rat anti–mouse IL-12 p70 IgG1 followed by FITC-conjugated mouse anti–rat IgG1. Nonspecific binding was blocked using 5% mouse serum or 10% fetal bovine serum. As control for IL-12 p70, the biotinylated rat anti–mouse IgG1 isotype standard plus mouse anti–rat IgG1-FITC was used. Saponin (0.1%) solution was used to permeabilize cells. Labeled DCs were analyzed on an Olympus-Fluoview confocal laser scanning microscope (480-argon laser and 568-krypton laser).
Electron Microscopy.
All steps were performed on ice and each wash was repeated three times with TBS (pH 7.6), unless indicated otherwise. Murine DCs were cultured in 24-well plates, supernatants were removed, and the adherent cells were washed, incubated with primary (anti–IL-12 p70) or isotype control Ab (rat IgG1) for 1 h, washed again, and then incubated with secondary Ab (biotinylated anti-IgG1) for 1 h. DCs were washed, incubated with streptavidin 10-nm gold particles (PharMingen) for 1 h, washed again, and fixed with electron microscopy (EM) fixative for 15 min (2% paraformaldehyde/0.5% glutaraldehyde) and then with 1% osmium tetroxide for 30 min, and then rinsed with veronal-acetate buffer. To dehydrate the cells, a graded series of ethanol (10 min incubation at each increasing concentration) followed by a mixture of absolute ethanol and Polybed 812 resin (15 min) was used. The DCs were covered with resin for 15 min; after draining the resin, resin-filled Beem capsules were placed over the cells and incubated at 58°C overnight. The polymerized resin capsules with adherent DCs were removed from the plate and incubated at 85°C for 1 h. Thin sections were cut from each block, stained with uranyl acetate and lead citrate, and viewed using a Phillips electron microscope.
DC Lysates.
Murine DCs (107) were resuspended in complete lysis buffer composed of 700 μl of 1× Relaxation buffer-EGTA (1,000 mM KCL, 30 mM NaCl, 35 mM MgCl2, 10 mM ATP[NA]2, 12.5 mM EGTA, 100 mM Pipes, pH 6.8; reference 24) prepared in distilled water, 100 μl of 1 mg/ml leupeptin, 100 μl of 1 mg/ml of pepstatin (all Sigma-Aldrich), 100 μl of 5% NP-40 (Polysciences), and 10 μl of 100 mM PMSF (Sigma-Aldrich) in isopropanol. The DC lysates were centrifuged at 2,200 rpm at 4°C. Supernatants were collected and used in an ELISA for IL-12 p70.
DC Plasma Membrane Extraction.
The methods of Maeda et al. 25 were used to isolate plasma membranes from 5 × 107 bone marrow–derived DCs. In brief, DCs were resuspended in 5 ml of the homogenization buffer described by Maeda et al. and homogenized using a Polytron homogenizer (Ika Labortechnics). The homogenate was layered over 10 ml of 41% sucrose in the homogenization buffer and centrifuged at 95,000 g for 1 h in a Beckman TL-100 ultracentrifuge. The white band at the interface was collected, centrifuged, washed, and resuspended in the complete lysis buffer described above and then used as the plasma membrane fraction 25. After sucrose gradient centrifugation, the pellet below the interface was used as the nonplasma membrane fraction 25.
Protein Extraction from Tissues.
Mouse and human spleens, thymus, and lymph nodes, and mouse ears were homogenized. Ears from BALB/c mice were dissected, the dermal and epidermal sheets were separated and cut into small pieces with scissors, and the pieces were then homogenized between the frosted ends of two microscope slides. The homogenized ear tissue was sonicated for 15 s on ice and rested for 1–2 min on ice, and the sonication was repeated three more times. Single-cell suspensions were prepared from mouse spleen, thymus, and lymph nodes by homogenizing the tissue between the frosted ends of two microscope slides. Erythrocytes in these cell suspensions were lysed with red cell lysis buffer (Sigma-Aldrich); the cell pellet was washed twice with PBS and then resuspended in 500 μl of complete lysis buffer (described above). The cell lysates from the aforementioned tissues were centrifuged at 2,200 rpm at 4°C, and an ELISA was used to measure IL-12 p70 in these supernatants. Frozen human tissues (courtesy of Dr. D. Troyer, Pathology Core of the San Antonio Cancer Institute, San Antonio, TX) were homogenized and resuspended in the complete lysis buffer and centrifuged, and IL-12 p70 levels in the supernatants were measured by ELISA. All of the aforementioned procedures were conducted at 4°C or on ice.
Human Leukocyte Isolation and Cell Lysates.
Leukocytes were isolated from 50 ml of heparinized venous blood obtained from normal volunteers. Equal volumes of blood and a solution containing 3% dextran (Amersham Pharmacia Biotech) and 0.85% NaCl were mixed, and erythrocytes were sedimented for 18 min at 4°C, followed by centrifugation at 1,800 rpm for 5 min at 4°C. Osmotic lysis (at 4°C) was used to remove the remainder of the erythrocytes. The leukocyte pellet was washed two times with cold PBS and then resuspended in 500 μl of complete lysis buffer. The cell lysates were centrifuged at 2,200 rpm at 4°C, and IL-12 p70 was measured in the supernatants. PBMCs and neutrophils were prepared as described previously 26. PBMCs were resuspended in RPMI and 10% FBS and cultured in tissue culture flasks for 2 h. The nonadherent cells (lymphocyte fraction) were aspirated and the adherent cells (monocytes) were collected using cell scrapers.
Animals and Infection.
L. donovani promastigotes (2–3 × 107/2 ml of PBS) were injected into the peritoneal cavity of 6-wk-old BALB/c mice. After 20 min, the peritoneal fluid was removed, the cells were pelleted by centrifugation at 2,200 rpm for 5 min, and the supernatants were snap frozen and stored at −70°C until measurement of IL-12 p70. The local review boards of the University of Texas Health Science Center at San Antonio approved all protocols.
Results
Preformed, Membrane-associated IL-12 p70.
Based on the homology between the IL-12 p40 subunit and the extracellular domain of the IL-6 receptor as well as the finding that p35 encodes an NH2-terminal sequence resembling that found in some membrane-associated proteins, a theoretical argument was made for a membrane-bound p70-like complex that could initiate responses on cell–cell contact 27. Using a heterodimer-specific anti–IL-12 mAb, Fan et al. 28 provided flow cytometry evidence suggestive of IL-12 on the cell surface of a human monocytic and a murine macrophage cell line. However, direct evidence for a functional membrane-associated, preformed pool of IL-12 is lacking. Given the central role of the DC–IL-12 axis in host immune functions, our initial efforts were focused on determining whether there is a membrane-associated form of IL-12 p70 in this cell type.
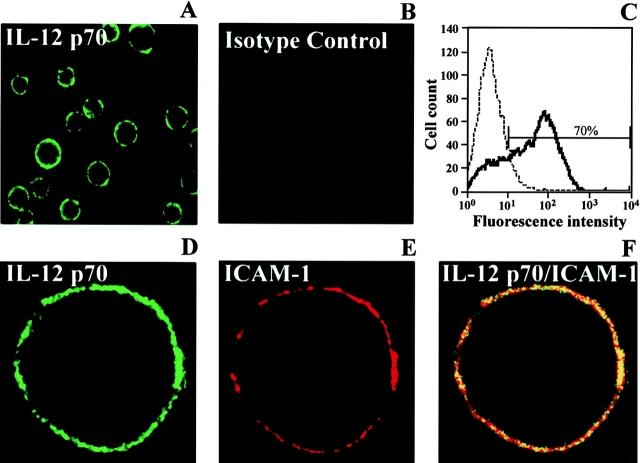
By confocal microscopy, IL-12 p70 immunoreactivity was localized to the periphery of nonpermeabilized DCs (Fig. 1A and Fig. B). By FACS™ analysis, 70% of the identical population of DCs used for confocal microscopy stained for IL-12 p70 (Fig. 1 C), and after permeabilization there was no increase in fluorescence intensity of IL-12 p70 and only a 4–6% increase in the number of positive-staining cells (data not shown). The immunoreactivity for IL-12 p70 colocalized with that for intercellular adhesion molecule (ICAM)-1, a well-characterized plasma membrane–associated molecule (29 30; Fig. 1D–F). In additional experiments, immunoreactivity for IL-12 p40 or p35 was also detected on the periphery of nonpermeabilized DCs (data not shown).
Figure 1.
Plasma membrane localization of immunoreactive IL-12 p70 on resting nonpermeabilized murine DCs. DCs were stained with IL-12 p70 (A) or rat IgG1 isotype Ab (B) and visualized by confocal microscopy. (C) FACS™ analysis showing that ∼70% of the DC population shown in A has surface staining for IL-12 p70. (D–F) Fluorescence for IL-12 p70-FITC (D, green), ICAM-1-PE (E, red), or colocalization of IL-12 p70 and ICAM-1 (F, orange). One representative experiment out of three is shown.
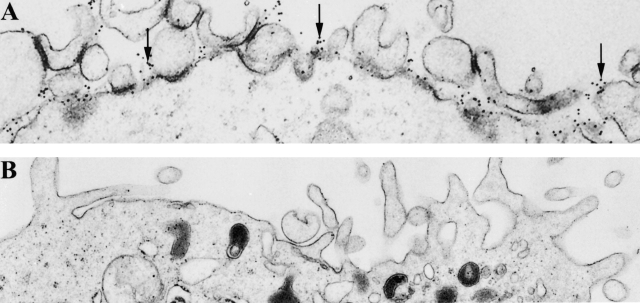
The peripheral localization of immunoreactive IL-12 p70 on DCs was confirmed by EM studies (Fig. 2A and Fig. B). Immunogold reactivity for IL-12 p70 but not the isotype control was present along the entire surface of the DC plasma membrane. The p40 subunit of the IL-12 was also detected on the cell surface by EM (data not shown).
Figure 2.
EM photomicrographs showing plasma membrane localization of IL-12 p70 (arrows) in DCs. DCs were labeled with IL-12 p70 (A) and isotype control Ab (B) and visualized by EM. One representative experiment out of three is shown.
We also used cell fractionation of permeabilized and nonpermeabilized cells to determine the distribution of IL-12 p70 in the plasma membrane and nonplasma membrane fractions of murine DCs. Based on both biochemical and immunologic criteria, plasma membranes prepared by the methods of Maeda et al. yield high-purity plasma membrane fractions 25. Using this method for cell fractionation in three separate experiments, we found that the ratio of the IL-12 p70 content in DC plasma membranes/nonplasma membranes was generally 5:1. For example, in a typical experiment, the IL-12 p70 content in the plasma membrane fraction was 50 pg/μg of protein, whereas in the nonplasma membrane fraction it was 11 pg/μg of protein. In the absence of NP-40 in the complete lysis buffer, IL-12 p70 was not detected in any of the samples examined. Thus, these data complement the microscopic studies (Fig. 1 and Fig. 2), and collectively indicate that the bulk of the preformed stores of IL-12 p70 in unstimulated DCs is restricted primarily to the plasma membrane.
Biological Relevance of Preformed, Membrane-associated Stores of IL-12 p70.
Given the importance of IL-12 in mediating rapid host responses to invading organisms, we asked the following questions: is this membrane-associated immunoreactive IL-12 p70 released from the cell surface, and is it biologically active? We also surmised that in the context of host–parasite interactions, such as that between L. donovani, an intracellular parasite, and DCs, this source of preformed IL-12 should be available to initiate host immune responses soon after contact with the parasite. For this reason, we limited our analyses to determining the bioavailability of this source of IL-12 shortly after DCs come into contact with L. donovani.
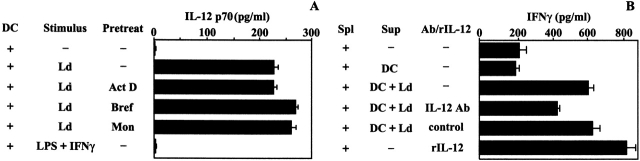
The IL-12 p70 produced by unstimulated DCs was below the detection limits of the ELISA; however, after as little as 10 min of contact with stationary phase (or purified metacyclic) L. donovani promastigotes, murine DCs released IL-12 p70 (Fig. 3 A). This release was independent of de novo IL-12 synthesis, as pretreatment of DCs with actinomycin D (inhibitor of transcription) or monensin or brefeldin A (inhibitors of Golgi transport) had no effect on the amounts of IL-12 p70 released from DCs (Fig. 3 A).
Figure 3.
Rapid release of bioactive, preformed IL-12 p70 after 10 min of contact with L. donovani (Ld) promastigotes. (A) IL-12 p70 was detected in the supernatants of DCs in contact with L. donovani for 10 min but not in the supernatants of DCs stimulated for the same duration with LPS plus IFN-γ. Pretreating (pretreat) DCs with actinomycin (Act), brefeldin A (Bref), or monensin (Mon) had no effect on the amounts of IL-12 p70 released from DCs in contact with L. donovani. (B) Supernatants (Sup) from unstimulated DCs or DCs in contact with L. donovani (DC + Ld) was added to Ag-reactive splenocytes (Spl). A blocking IL-12 Ab but not isotype control Ab reduced the amounts of IFN-γ that is released after addition of supernatants from DC–Leishmania cocultures to Ag-reactive splenocytes. The amounts of IFN-γ released from splenocytes after addition of supernatants from DC–Leishmania cocultures to Ag-reactive splenocytes was similar to that released after addition of recombinant IL-12 p70 (300 pg/ml).
In contrast to these effects of L. donovani, there was no detectable IL-12 p70 in the DC supernatants stimulated for 10 min with LPS plus IFN-γ (Fig. 3 A). However, consistent with the time required to induce IL-12 p40 transcription 1 6 7, the release of IL-12 p70 from DCs after stimulation with LPS plus IFN-γ was detected only after 2–4 h (∼100–300 pg/ml), and this was inhibited by pretreatment of DCs with actinomycin D, monensin, or brefeldin A (data not shown).
Although the ELISA used to measure the IL-12 released from DCs detects the bioactive form for IL-12 p70, we used a bioassay to confirm this (Fig. 3 B). Addition of supernatants from DCs in contact with L. donovani increased the production of IFN-γ from Ag-primed splenocytes, and this increase was partially inhibited by an IL-12–blocking Ab (Fig. 3 B). The increase in IFN-γ that occurred after addition of supernatants from DC–parasite cocultures was similar in magnitude to that observed after addition of rIL-12 p70 to splenocytes. Collectively, these findings indicate that biologically active levels of IL-12 p70 were present in the supernatants of DC–L. donovani cocultures.
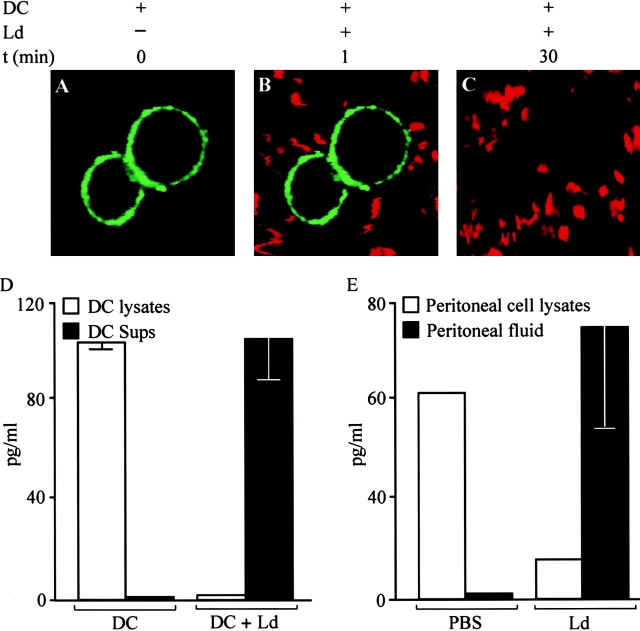
We next postulated that if the parasites indeed trigger the rapid release of membrane-associated IL-12, then the peripherally localized immunoreactivity for IL-12 p70 should diminish shortly after DCs come into contact with L. donovani. Concordant with this, membrane-bound immunoreactivity for IL-12 p70 was detected before (Fig. 4 A) and immediately after (Fig. 4 B) addition of L. donovani promastigotes but not after 10 or 30 min (Fig. 4 C) of contact between DCs and parasites. The timing at which this complete loss of immunoreactivity occurred depended on the temperature at which DCs had been labeled. For DCs labeled at room temperature it was after 10 min of contact with parasites (data not shown), whereas for DCs labeled at 4°C the loss was partial at 10 min but complete at 30 min (Fig. 4A–C). In contrast, there was no loss in IL-12 p70 immunoreactivity in DCs that had been stimulated with LPS plus IFN-γ for 10 min, nor if the DCs were exposed to L. donovani parasites pretreated with cytochalasin D (data not shown).
Figure 4.
Contact between DCs and L. donovani (Ld) promastigotes leads to a rapid decrease in the membrane-associated immunoreactivity for IL-12 p70. (A) Resting nonpermeabilized DCs were stained for IL-12 p70 at 4°C. Using confocal microscopy, the IL-12 p70–labeled green fluorescent cells were visualized in unstimulated nonpermeabilized DCs (t = 0 min). (B) PKH-labeled red fluorescent parasites were added after 1 min (t = 1 min). (C) Loss of green fluorescence, i.e., IL-12 p70 immunoreactivity was not observed after 30 min of contact with parasites (t = 30 min). Note that the same field is being visualized in A–C. One representative experiment out of three is shown. (D) Inverse relationship between the amount of plasma membrane–bound IL-12 p70 and the amount of IL-12 p70 released into the supernatant after DC–parasite contact in vitro. The amount of membrane-bound IL-12 p70 was lower in the lysates of DCs in contact with the parasite compared with lysates from unstimulated DCs. In contrast, the amounts of IL-12 p70 in the supernatants (Sups) from DC–Leishmania cocultures was higher than that in supernatants of resting DCs. One representative experiment out of three is shown. (E) L. donovani promastigotes induced the rapid in vivo release of IL-12. L. donovani stationary phase (2–3 × 107/2 ml of PBS) or PBS was injected into the peritoneal cavity of BALB/c mice, and after 20 min the peritoneal fluid was collected and centrifuged. IL-12 p70 in the supernatants and the lysates of the cell pellet was determined. The amount of IL-12 p70 in peritoneal fluid of mice injected with PBS was low, whereas that present in the peritoneal cell pellet lysates was high. In contrast, in mice injected with L. donovani, the amount of IL-12 p70 in the peritoneal fluid was high, whereas that present in the peritoneal cell pellet lysates was low. The experiment was performed three times (n = four mice/group), with similar results.
Concomitant with this loss in the membrane-bound immunoreactivity for IL-12 p70 (Fig. 4A–C), there should be a corresponding decrease in the total content of IL-12 p70 in DCs that were in contact with parasites. Fig. 4 D shows that the IL-12 p70 content in the lysates of DCs that were in contact with parasites was significantly lower than in unstimulated DCs. The converse was observed in the supernatants of these DC cultures: the levels of IL-12 p70 were significantly higher in the supernatants of DC–Leishmania cocultures than in unstimulated DCs (Fig. 4 D). Taken together, the confocal microscopy findings and the pattern of IL-12 content in the supernatants and DC lysates before and after addition of parasites is consistent with the presence of a preformed pool of membrane-associated IL-12 p70 that is biologically active and is readily released after contact with L. donovani.
To extend these findings, observed in an in vitro setting of DC–parasite contact, we determined whether IL-12 p70 is released rapidly in vivo in response to an infectious challenge. In this in vivo model, L. donovani promastigotes or PBS was introduced into the peritoneal cavity of BALB/c mice and the peritoneal fluid was recovered after 20 min. IL-12 p70 could be detected in the fluid recovered from the peritoneal cavity of mice after a challenge with L. donovani promastigotes (Fig. 4 E), but not in the peritoneal fluid collected after the injection of PBS. In contrast, the amounts of IL-12 p70 in the cell lysates of cells collected from the peritoneal cavity after PBS challenge was higher than that found in the cell lysates of cells collected after the parasite challenge. These in vivo findings mirrored the in vitro findings shown in Fig. 4A–D. Because macrophages and not DCs are the more common cell type in the peritoneal cavity, it is likely that these APCs are the major contributor of the preformed IL-12 detected.
In Vitro Determinants of IL-12 p70 Release.
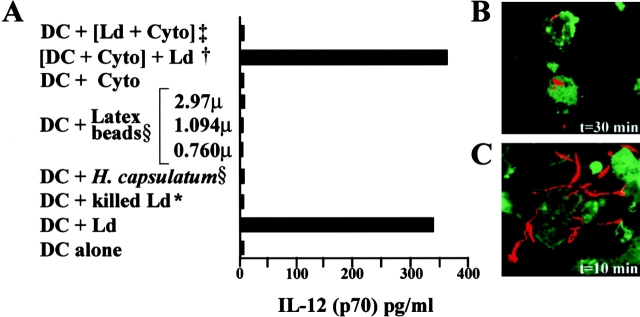
What are the L. donovani determinants that contribute to the rapid release of bioactive IL-12? Is contact with the parasite sufficient, or is phagocytosis required for IL-12 release? Does contact with innate objects or nonmotile organisms also induce the release of IL-12? To address these questions, we conducted a series of experiments (Fig. 5). Parasites inactivated by heat or other inactivating agents (UV light, formalin, or trypsin), Histoplasma capsulatum (an intracellular nonmotile fungal organism), or latex beads of varying diameters, did not induce the release of IL-12 p70 from DCs. Infection with Leishmania involves two processes: parasite-dependent attachment and host-dependent internalization, and pretreatment of Leishmania promastigotes with cytochalasin is known to reduce significantly the ability of the parasites to attach to cells 22. We found that Leishmania promastigotes immobilized (but not killed) with cytochalasin D did not induce IL-12 release (Fig. 5 A). In contrast, pretreatment of DCs with cytochalasin D, an inhibitor of phagocytosis, reduced DC infection by 50% but did not inhibit IL-12 p70 release from DCs in contact with Leishmania species. Furthermore, phagocytosed intracellular parasites were only visualized after 30 min (Fig. 5 B), but not after 10 min of contact with DCs (Fig. 5 C). Collectively, these findings indicated that an important determinant for the very early release of preformed membrane-associated DC stores of IL-12 is contact with motile live L. donovani parasites but not phagocytosis.
Figure 5.
Determinants of the IL-12 p70 release by DCs. (A) 10 min after adding infectious or noninfectious agents to resting or cytochalasin D–-pretreated murine DCs, IL-12 p70 was measured in the supernatants of DCs by ELISA. *, L. donovani inactivated by different methods; §, opsonized or nonopsonized latex beads or H. capsulatum. The findings were similar with opsonized and nonopsonized latex beads, and were also the same regardless of the method used to inactivate the parasites (see Materials and Methods), and for this reason a single bar is used to represent these findings. †, DCs pretreated with cytochalasin D for 1 h and then cocultured with L. donovani; ‡, L. donovani pretreated with cytochalasin D (Cyto) for 1 h and then added to the DCs. Brackets indicate pretreatment of DCs or parasites with cytochalasin D. Results are from one of three representative experiments. (B–C) The PKH26 red fluorescent cell linker kit was used to label L. donovani parasites. DCs were stained with FITC. The interaction between labeled parasites (red) and the DCs (green) was visualized at different time points. After 30 min (B) parasites were found within some DCs, whereas they were still extracellular at 10 min (C). Data from one of three representative experiments is shown.
Distribution of Preformed IL-12 p70 in Murine and Human Leukocytes and Tissues.
We next determined whether this preformed membrane-associated IL-12 p70 was specific to murine DCs/macrophages or were they also present in other murine and human cells/tissues. As described in Materials and Methods, special care was taken to avoid stimulating the cells while processing these samples. IL-12 p70 (pg/μg of protein; mean ± SD; n = 12 mice) was present in the cell lysates of skin (4.0 ± 2.8), lymph node (4.9 ± 1.3), spleen (2.6 ± 0.4), and thymus (3.8 ± 1.6) of BALB/c mice; the cell lysates from RAW and J774 murine cell lines also contained IL-12 p70.
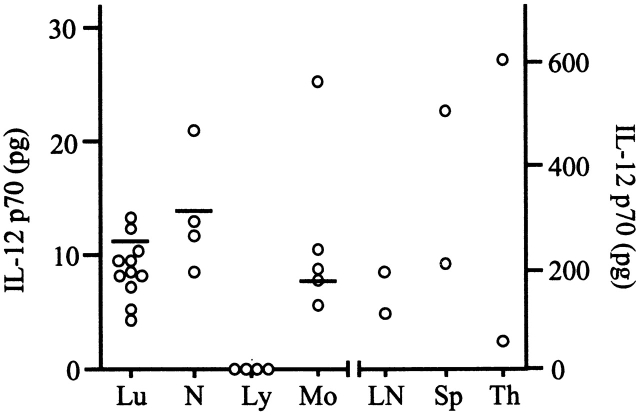
Preformed IL-12 p70 was also present in the cell lysates of several human tissues including lymph nodes, spleen, and thymus, and myeloid cells such as monocytes and granulocytes (Fig. 6). Notably, there was a wide interindividual variation in the amounts of preformed IL-12 p70 present in the total leukocyte population (Fig. 6).
Figure 6.
Distribution of IL-12 p70 in human cells/tissues. IL-12 p70 (in pg) detected in protein lysates of total leukocytes (Lu) isolated from whole blood (values normalized to pg/ml of blood); neutrophils (N), lymphocytes (Ly), or monocytes (Mo) (values normalized to pg/106 cells); lymph node (LN), spleen (Sp), or thymus (Th) (values normalized to pg/g tissue). Note that the scales of the left and right y axes are different and are for the values to the left and right, respectively, of the tick marks on the x axis.
Discussion
In support of our hypothesis, we discovered a bioactive IL-12 p70 in myeloid cells, including DCs that are readily available for rapid release after contact with an intracellular parasite such as L. donovani. This readily mobilizable source of IL-12 is independent of the induced transcription of IL-12 p40 and is membrane associated. IL-12 p70 represents the covalent association between p40 and p35, and hence the finding of p70 in the plasma membranes of unstimulated, native phagocytes would suggest a preassembled store of this cytokine.
The complete repertoire of microbial agents and the precise microbe- or host-dependent factors that trigger the rapid release of membrane-associated preformed IL-12 are not known. In addition to the data presented here for L. donovani, L. major and Listeria monocytogenes—highly motile microorganisms—also induced the early release of IL-12 p70 from splenocytes and DCs after 10 min of contact (data not shown). We found that as early as 10 min after DC–L. donovani contact, NF-κb proteins were translocated (data not shown), but whether this signaling cascade initiated after cell contact is required for the rapid release of preformed IL-12 stores is currently under investigation.
Our studies provide a mechanism for the observation that IL-12 is released rapidly from DCs after in vivo infection with L. major 31 or L. donovani 10, and perhaps other organisms such as Toxoplasma 13. Our in vitro and in vivo studies would also suggest that preformed stores of bioactive IL-12 are also available for rapid release from monocytes and macrophages. The rapid release of these IL-12 stores appears to be dependent on contact between DCs and parasites but independent of phagocytosis. Based on studies by other investigators, there is evidence that once the parasite is internalized, the transcription-dependent synthesis of IL-12, at least in monocytes/macrophages, is impaired 10 11 12.
Given the central role of IL-12 in host defenses, the swiftness with which preformed bioactive IL-12 was mobilized after DC–parasite contact should not be surprising. It is striking that evolutionarily conserved systems are also in place for the rapid release via preformed stores of other proinflammatory cytokines such as TNF-α 32 33. It is conceivable that the rapid release of IL-12 from DCs may act in an autocrine fashion to further stimulate DCs via constitutively expressed IL-12 receptors 34, and/or serve as a first line of defense by activating T cells or NK cells in the immediate vicinity 35. In this scenario, the immediate release of even low levels of membrane-associated IL-12 could trigger a host of critical immune responses, including upregulation of the IL-12 receptor 36 and a consequent increase in cell responsiveness to IL-12, and the induction of an early IFN-γ response. This is highly relevant in the context that immunity against intracellular protozoan Leishmania species is highly dependent on the rapid development of a Th1-biased immune response 17 37. Indeed, the very rapid release of bioactive IL-12 after parasite–DC contact is consistent with the hypothesis that IL-12 serves as a “match” to ignite the immune response in L. major infection 38. Furthermore, several studies using recombinant IL-12 and anti–IL-12 antibody have documented that the early IL-12 response to infection is critical to the final outcome 39 40. Whether the amounts of preformed membrane-associated IL-12 in humans are genetically controlled remains to be established, but variability in the amounts stored and/or released could be an important determinant of host susceptibility to various infections.
Acknowledgments
We thank R.A. Clark for insightful discussions; B. Cherniak for helpful suggestions; N. Venkataprasad for initial experiments; V. Frolich for assistance with confocal microscopy; D. Pearson, D. King, and Nancy Ransom for technical assistance; and A.S. Ahuja for forbearance. M. Quinones acknowledges the mentorship of Dr. E. Garavito, University of San Martin, Colombia during his undergraduate studies.
This work would not have been possible without the strong support of the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (to S.S. Ahuja and S.K. Ahuja). This work was supported by a Veterans' Administration (VA) Career Development Award and a VA Merit Award (to S.S. Ahuja), and a VA Merit Award (to P.C. Melby).
Footnotes
Abbreviations used in this paper: DC, dendritic cell; EM, electron microscopy; ICAM-1, intercellular adhesion molecule 1.
References
- Trinchieri G. Interleukin-12a cytokine at the interface of inflammation and immunity. Adv. Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- Altare F., Lammas D., Revy P., Jouanguy E., Doffinger R., Lamhamedi S., Drysdale P., Scheel-Toellner D., Girdlestone J., Darbyshire P. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Invest. 1998;102:2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altare F., Jouanguy E., Lamhamedi S., Doffinger R., Fischer A., Casanova J.L. Mendelian susceptibility to mycobacterial infection in man. Curr. Opin. Immunol. 1998;10:413–417. doi: 10.1016/s0952-7915(98)80114-3. [DOI] [PubMed] [Google Scholar]
- de Jong R., Altare F., Haagen I.A., Elferink D.G., Boer T., van Breda Vriesman P.J., Kabel P.J., Draaisma J.M., van Dissel J.T., Kroon F.P. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- Mattner F., Magram J., Ferrante J., Launois P., Di Padova K., Behin R., Gately M.K., Louis J.A., Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N.M., Chehimi J., Kubin M., Aste M., Chan S.H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Chow J.M., Gri G., Carra G., Gerosa F., Wolf S.F., Dzialo R., Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babik J.M., Adams E., Tone Y., Fairchild P.J., Tone M., Waldmann H. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J. Immunol. 1999;162:4069–4078. [PubMed] [Google Scholar]
- Mosser D.M., Karp C.L. Receptor mediated subversion of macrophage cytokine production by intracellular pathogens. Curr. Opin. Immunol. 1999;11:406–411. doi: 10.1016/s0952-7915(99)80068-5. [DOI] [PubMed] [Google Scholar]
- Gorak P.M., Engwerda C.R., Kaye P.M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Carrera L., Gazzinelli R.T., Badolato R., Hieny S., Muller W., Kuhn R., Sacks D.L. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow–derived macrophages from susceptible and resistant mice. J. Exp. Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Butcher B., Sacks D.L. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell levelselective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C., Yap G., Schulz O., Rogers N., Schito M., Aliberti J., Hieny S., Sher A. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–647. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Launois P., Ohteki T., Swihart K., MacDonald H.R., Louis J.A. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1. Eur. J. Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- Ingulli E., Mondino A., Khoruts A., Jenkins M.K. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbach W., Laskay T. The host response to Leishmania infection. Adv. Immunol. 2000;74:275–317. doi: 10.1016/s0065-2776(08)60912-8. [DOI] [PubMed] [Google Scholar]
- Laskay T., Diefenbach A., Rollinghoff M., Solbach W. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 1995;25:2220–2227. doi: 10.1002/eji.1830250816. [DOI] [PubMed] [Google Scholar]
- Ahuja S.S., Reddick R.L., Sato N., Montalbo E., Kostecki V., Zhao W., Dolan M.J., Melby P.C., Ahuja S.K. Dendritic cell (DC)-based anti-infective strategiesDCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J. Immunol. 1999;163:3890–3897. [PubMed] [Google Scholar]
- Sartori A., Oliveira M.A., Scott P., Trinchieri G. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J. Immunol. 1997;159:2849–2857. [PubMed] [Google Scholar]
- Sacks, D.L., and P.C. Melby. 1998. Animal models for the analysis of immune responses to Leishmaniasis. In Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeek, D.H. Marguiles, E.M. Shevach, and W. Stober, editors. John Wiley & Sons, Inc., New York. 19.12.11–19.12.20.
- Wyler D.J. In vitro parasite-monocyte interactions in human leishmaniasis. Evidence for an active role of the parasite in attachment. J. Clin. Invest. 1982;70:82–88. doi: 10.1172/JCI110606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja S.S., Mummidi S., Malech H.L., Ahuja S.K. Human dendritic cell (DC)-based anti-infective therapyengineering DCs to secrete functional IFN-gamma and IL-12. J. Immunol. 1998;161:868–876. [PubMed] [Google Scholar]
- Borregaard N., Heiple J.M., Simons E.R., Clark R.A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidasetranslocation during activation. J. Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S.Q. A simple and rapid method for the preparation of plasma membranes. Biochim. Biophys. Acta. 1983;731:115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- Ahuja S.K., Murphy P.M. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- Gearing D.P., Cosman D. Homology of the p40 subunit of natural killer cell stimulatory factor (NKSF) with the extracellular domain of the interleukin-6 receptor. Cell. 1991;66:9–10. doi: 10.1016/0092-8674(91)90131-h. [DOI] [PubMed] [Google Scholar]
- Fan X., Sibalic V., Niederer E., Wuthrich R.P. The proinflammatory cytokine interleukin-12 occurs as a cell membrane-bound form on macrophages. Biochem. Biophys. Res. Commun. 1996;225:1063–1067. doi: 10.1006/bbrc.1996.1295. [DOI] [PubMed] [Google Scholar]
- De Panfilis G., Manara G.C., Ferrari C., Torresani C. Adhesion molecules on the plasma membrane of epidermal cells. II. The intercellular adhesion molecule-1 is constitutively present on the cell surface of human resting Langerhans cells. J. Invest. Dermatol. 1990;94:317–321. doi: 10.1111/1523-1747.ep12874444. [DOI] [PubMed] [Google Scholar]
- De Panfilis G., Manara G.C., Ferrari C., Torresani C., Lonati A. Adhesion molecules on the plasma membrane of epidermal cells. IV. Immunolocalization of the intercellular adhesion molecule-1 (ICAM-1, CD54) on the cell surface of a small subpopulation of keratinocytes freshly isolated from normal human epidermis. Reg. Immunol. 1992;4:119–129. [PubMed] [Google Scholar]
- Vieira L.Q., Hondowicz B.D., Afonso L.C., Wysocka M., Trinchieri G., Scott P. Infection with Leishmania major induces interleukin-12 production in vivo. Immunol. Lett. 1994;40:157–161. doi: 10.1016/0165-2478(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Decoster E., Vanhaesebroeck B., Vandenabeele P., Grooten J., Fiers W. Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J. Biol. Chem. 1995;270:18473–18478. doi: 10.1074/jbc.270.31.18473. [DOI] [PubMed] [Google Scholar]
- Lacy P., Mahmudi-Azer S., Bablitz B., Hagen S.C., Velazquez J.R., Man S.F., Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- Grohmann U., Belladonna M.L., Bianchi R., Orabona C., Ayroldi E., Fioretti M.C., Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- Hassan-Zahraee M., Wu J., Gordon J. Rapid synthesis of IFN-gamma by T cells in skin may play a pivotal role in the human skin immune system. Int. Immunol. 1998;10:1599–1612. doi: 10.1093/intimm/10.11.1599. [DOI] [PubMed] [Google Scholar]
- Jones D., Elloso M.M., Showe L., Williams D., Trinchieri G., Scott P. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major . Infect. Immun. 1998;66:3818–3824. doi: 10.1128/iai.66.8.3818-3824.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell D.J., Locksley R.M. Leishmania major infection of inbred miceunmasking genetic determinants of infectious diseases. Bioessays. 1999;21:510–518. doi: 10.1002/(SICI)1521-1878(199906)21:6<510::AID-BIES7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Fowell D.J., Wakil A.E., Locksley R.M. Interleukin-12 in murine leishmaniasis—match, flame or fuel? Res. Immunol. 1995;146:566–575. doi: 10.1016/0923-2494(96)83033-1. [DOI] [PubMed] [Google Scholar]
- Heinzel F.P., Schoenhaut D.S., Rerko R.M., Rosser L.E., Gately M.K. Recombinant interleukin 12 cures mice infected with Leishmania major . J. Exp. Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F.P., Rerko R.M., Ahmed F., Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J. Immunol. 1995;155:730–739. [PubMed] [Google Scholar]