Abstract
Tumor necrosis factor α (TNF-α) is the key mediator of superantigen-induced T cell lethal shock. Here, we show that nuclear factor of activated T cells transcription factor, NFATp, controls susceptibility to superantigen-induced lethal shock in mice through its activation of TNF-α gene transcription. In NFATp-deficient mice, T cell stimulation leads to delayed induction and attenuation of TNF-α mRNA levels, decreased TNF-α serum levels, and resistance to superantigen-induced lethal shock. By contrast, after lipopolysaccharide (LPS) challenge, serum levels of TNF-α and susceptibility to shock are unaffected. These results demonstrate that NFATp is an essential activator of immediate early TNF-α gene expression in T cells and they present in vivo evidence of the inducer- and cell type–specific regulation of TNF-α gene expression. Furthermore, they suggest NFATp as a potential selective target in the treatment of superantigen-induced lethal shock.
Keywords: tumor necrosis factor α, staphylococcal enterotoxin B, lipopolysaccharide, transcription, toxic shock
Introduction
T cell production of TNF-α is critical in a number of disease states, including superantigen-induced lethal shock. In the case of toxic shock caused by staphylococcal enterotoxin B (SEB), the superantigen directly binds MHC class II and is recognized by Vβ8+ T cells in mice 1 2 3 4 5. In humans, it is recognized by Vβ3+, Vβ12+, Vβ14+, Vβ15+, Vβ17+, and Vβ20+ T cells (for review see reference 6). The subsequent burst of TNF-α released by these T cells initiates the cascade ultimately leading to cardiovascular collapse and death 7. The lethal shock syndrome caused by LPS stimulation of macrophages is also mediated by TNF-α 8 9 and is phenotypically indistinguishable from the superantigen-induced lethal shock syndrome 7.
TNF-α gene expression in T cells stimulated through the TCR is calcineurin dependent and is blocked by pretreatment of cells with the calcineurin inhibitor cyclosporin A (CsA; references 10, 11). More recent studies have shown that upon T cell activation the human TNF-α gene is regulated through the recruitment of nuclear factor of activated T cells (NFAT) and ATF-2/Jun proteins to their cognate binding sites in the TNF-α enhancer complex 12 13 14. However, regulation of TNF-α by LPS in macrophages results in the assembly of a different enhancer complex than is assembled in activated T cells. In the case of LPS-stimulated TNF-α gene expression, formation of the enhancer complex does not include NFAT, but rather is dependent upon the recruitment of Sp1 and Ets proteins 15.
The NFAT family of transcription factors contains five members (NFATp, NFATc, NFAT3, NFAT4, and NFAT5; references 16 17 18 19), which are expressed in a tissue-specific manner. NFATp, NFATc, and NFAT5 are expressed in lymphoid tissues, whereas NFAT4, which is abundant in skeletal muscle is only expressed in thymus, and NFAT3 is not expressed in any lymphoid tissues 16 19. NFAT proteins are translocated to the nucleus where they participate in gene regulation upon a calcineurin-mediated dephosphorylation step that can be blocked by CsA 20 21.
Here, using mice deficient in NFATp (NFATp−/−), we demonstrate that NFATp is a critical and irreplaceable activator of immediate early TNF-α gene expression in T cells. Furthermore, we show that NFATp controls susceptibility to lethal shock caused by superantigen but not by LPS. These studies thus provide in vivo evidence of the stimulus- and cell type–specific transcriptional regulation of the TNF-α gene and identify the transcriptional mechanism that influences the pathogenesis of lethal shock due to superantigen.
Materials and Methods
Animals.
Two pairs of NFATp+/− mice (129Sv/J1 background backcrossed to Balb/c for seven generations; gift from L.H. Glimcher (Harvard School of Public Health, Boston, MA) were bred, and NFATp−/− and wild-type (NFATp+/+) littermates were genotyped by PCR as previously described 22. Mice were maintained and bred in pathogen-free conditions and were used for experiments at the age of 8–14 wk. Their care was in accordance with institutional guidelines.
Cell Culture and Plasmids.
The Ar-5 T cell clone was maintained, transfected, and analyzed in the presence or absence of CsA as previously described 10. Purified splenocytes were stimulated in vitro at 1–3 x 107 cells/ml with 1 μg/ml of plate-bound anti-CD3 (2C11; PharMingen) for the indicated time points. The −982, −475 and −200 murine TNF-α CAT reporter genes have been described previously 10.
Electrophoretic Mobility Shift Assays and DNaseI Footprinting.
Nuclear extracts were prepared from splenocytes and the electrophoretic mobility shift assays were performed as previously described 10. The sequence of the −76 NFAT oligonucleotide is: 5′-AGGAAGTTTTCCGAGGGT-3′. The anti-NFATp antibody was a gift from Dr. A. Rao (Center for Blood Research, Boston, MA) and the anti-NFATc antibody was purchased from Affinity BioReagents, Inc.
DNaseI footprinting of the human TNF-α promoter was performed using recombinant NFATp (a gift from D. Thanos, Columbia University, New York, NY) as described previously 14.
RNase Protection Assay.
Cytokine mRNA levels were analyzed by multiprobe RNase Protection assay. RNA was hybridized overnight to 32P-labeled antisense RNA probes for TNF-α, IL-2, IL-4, and L-32, which had been synthesized in vitro from separate templates according to standard techniques 10.
Endotoxin and Superantigen Shock.
Female NFATp−/− and NFATp+/+ littermate mice were injected intraperitoneally with D-Gal (D[+]-Galactosamine hydrochloride, G-1639; Sigma-Aldrich) alone, D-Gal plus LPS (L-3012; Sigma-Aldrich), D-Gal plus SEB (BT202; Toxin Technology Inc.), or D-Gal plus recombinant murine TNF-α (13250-012; GIBCO BRL) solubilized in 0.5 ml of pyrogen-free NaCl 0.9%. Control animals were injected with the same volume of diluent. SEB was tested for LPS contamination at Toxin Technology Inc. and was certified to be LPS-free with the amount of LPS <10–100 EU/mg of SEB (1.8 EU = 1 ng LPS).
Lethal shock was determined on the basis of rough fur, responsiveness to cage motion, ataxia, and immobility. Mice were monitored every 30 min after injection and were killed when they were unresponsive to cage motion.
ELISA.
Serum cytokine levels were determined using Quantikine M mouse TNF-α and IL-2 ELISA kits from R&D Systems according to the manufacturer's instructions. Serum samples were analyzed at a dilution of 1:5.
Results and Discussion
The Role of NFATp in Murine TNF-α Gene Regulation.
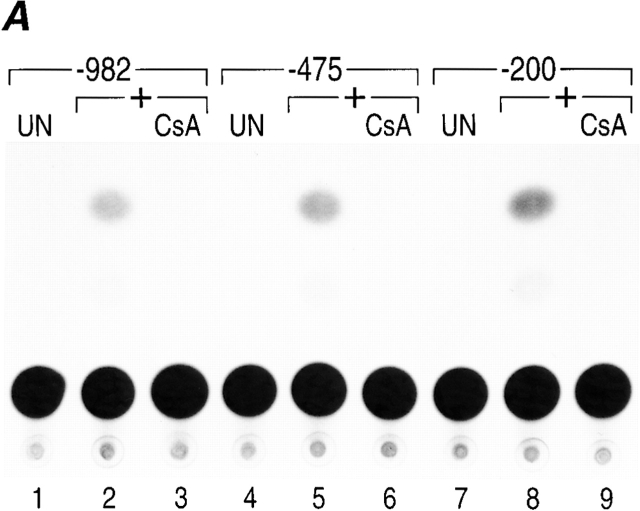
To determine whether murine TNF-α gene expression was dependent upon NFAT proteins in activated T cells similar to the human TNF-α gene, we first determined the minimal promoter region necessary for regulation of the murine TNF-α gene. We transfected murine TNF-α CAT reporter genes containing −982, −475, or −200 nucleotides upstream of the TNF-α mRNA cap site into a T cell clone and stimulated the cells with anti-CD3 antibody in the presence or absence of CsA. As shown in Fig. 1 A, −200 nucleotides upstream of the murine TNF-α mRNA cap site are sufficient for maximal anti-CD3 inducibility and CsA sensitivity of the TNF-α gene, similar to results obtained using the human TNF promoter 10 12.
Figure 1.
Murine TNF-α gene expression is dependent upon NFAT. (A) −200 nucleotides upstream of the TNF-α mRNA cap site are sufficient for CsA-sensitive anti-CD3 induction of TNF-α in T cells. The Ar-5 T cell clone was transfected with CAT reporters linked to murine TNF-α promoters containing −200, −475, or −982 nucleotides upstream of the mRNA cap site and treated with anti-CD3 antibody and pretreated with CsA for 10 min as indicated. A representative CAT assay of three independent transfections is shown. Transfections included CMV–β-gal as a control and CAT activity was normalized to β-galactosidase activity. (B) NFATp binds to six sites in the TNF-α promoter. Quantitative DNaseI footprinting using the murine TNF-α promoter (−200 to +87 nucleotides relative to the transcription start site) and increasing concentrations of recombinant NFATp (100 ng, 400 ng, and 2 μg) or BSA at 2 μg (–). The NFATp binding sites and the ATF-2/Jun binding site (CRE) are indicated in the drawing at the right.
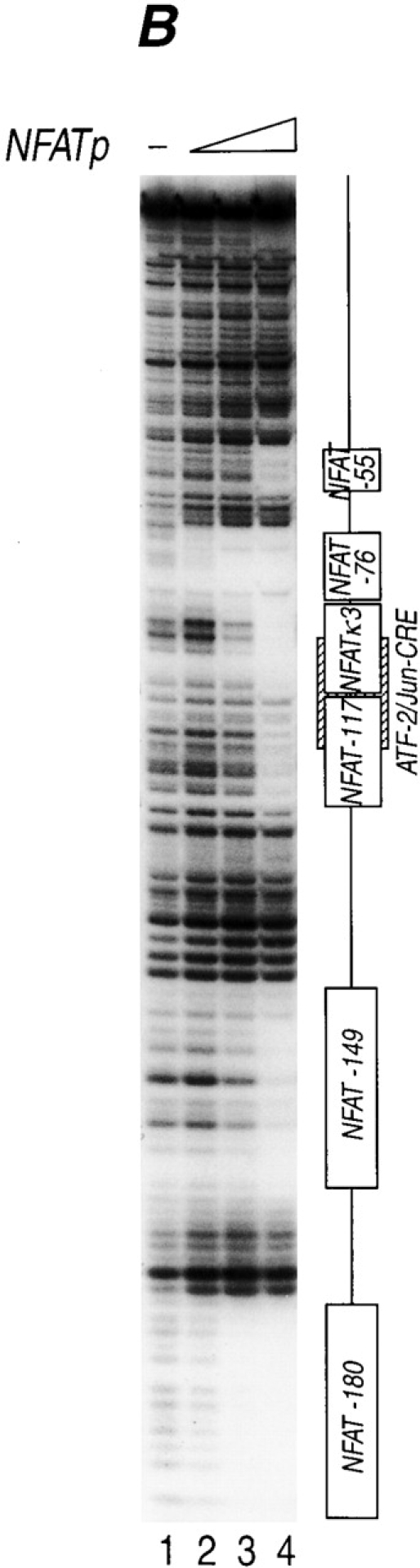
There are six NFAT binding motifs with different affinities for NFATp in the human TNF-α promoter (Tsytsykova, A.V., and A.E. Goldfeld, manuscript in preparation; reference 14). To determine if the pattern of binding of NFATp to corresponding sites in the murine TNF-α promoter was similar to its binding to the human TNF-α promoter, we next performed a quantitative DNAse I footprinting analysis. Using recombinant NFATp and a murine TNF-α promoter fragment spanning from −200 to +87 relative to the TNF-α transcription start site, we detected six sites in the murine TNF-α promoter that bind NFATp with different affinities (Fig. 1 B). Taken together, these data suggested that NFATp plays a critical role in the regulation of the murine TNF-α gene in T cells similar to its role in human TNF-α gene regulation 10 11 23.
Previous studies have demonstrated that T cell–derived TNF-α is the key mediator of lethal shock caused by the superantigen SEB 7. Antibodies to TNF-α abrogate this syndrome, and SCID mice, which lack lymphocytes, are resistant to shock, whereas SCID mice whose T cells have been reconstituted are sensitive to shock caused by SEB 7. Intriguingly, this cascade can be aborted by pretreatment of mice with the calcineurin inhibitor CsA 7, suggesting a role for calcineurin-dependent NFAT-driven TNF-α gene transcription in T cell lethal shock. Given that NFAT5 is not calcineurin dependent 19, and given the restricted tissue distribution of NFAT3 and NFAT4 16, we speculated that NFATp and/or NFATc might play a critical role in the initial transcriptional events leading to superantigen-induced lethal shock. To investigate this hypothesis we next tested the effect of SEB challenge in NFATp−/− mice and their NFATp+/+ littermates because NFATc-deficient mice are not viable 24 25.
Mice Deficient in NFATp Are Resistant to Superantigen-induced but Not to LPS-induced Lethal Shock.
We used the well-characterized D-Gal–sensitized mouse model 26, to evaluate the effect of SEB challenge in NFATp−/− and NFATp+/+ mice. Injection of 5 μg of SEB triggered a lethal shock syndrome in D-Gal–sensitized NFATp+/+ but not in D-Gal–sensitized NFATp−/− mice (Table ). The first signs of illness in NFATp+/+ mice became apparent 4 h after injection of SEB and by 8–10 h after injection NFATp+/+ mice became immobile and unresponsive and were killed. Titration of SEB in the NFATp+/+ mice showed 100 and 50% lethality with injection of 5 and 2 μg of SEB, respectively (Table ). Strikingly, NFATp−/− mice showed very little or no signs of illness at all and completely recovered within 24 h when injected with the same concentrations of SEB that caused death in their NFATp+/+ littermates. Thus, NFATp is a critical mediator in SEB-induced lethal shock.
Table 1.
NFATp−/− Mice Are Resistant to SEB-induced T Cell–mediated Lethal Shock, but Not to LPS-stimulated Lethal Shock
| D-Gal | LPS | SEB | TNF-α | Lethality | |
|---|---|---|---|---|---|
| NFATp+/+ | NFATp−/− | ||||
| mg/mouse | μg/mouse | dead/total | |||
| 20 | – | – | – | 0/3 | |
| – | 100 | – | – | 0/3 | |
| 20 | 10 | – | – | 5/5 | 5/5 |
| 20 | 1 | – | – | 5/5 | 3/3 |
| 20 | 0.1 | – | – | 8/12 | 6/9 |
| 20 | 0.01 | – | – | 0/3 | 0/3 |
| – | – | 200 | – | 0/3 | |
| – | – | 100 | – | 0/3 | |
| 20 | – | 100 | – | 3/3 | 3/3 |
| 20 | – | 10 | – | 4/4 | 3/4 |
| 20 | – | 5 | – | 14/14 | 0/9 |
| 20 | – | 2 | – | 4/7 | 0/6 |
| – | – | – | 1 | 0/3 | 0/3 |
| 20 | – | – | 1 | 4/4 | 4/4 |
| 20 | – | – | 0.1 | 3/6 | 4/6 |
| 20 | – | – | 0.01 | 0/6 | 0/6 |
Female mice received the indicated dosage of D-Gal, LPS, SEB, or recombinant murine TNF-α intraperitoneally in 0.5 ml pyrogen-free 0.9% NaCl. Surviving mice were monitored for 1 wk after injection. None of the mice that survived for 24 h after injection died later.
To demonstrate the specificity of the upstream signaling events leading to TNF-α gene transcription and protein production, and to demonstrate that this defect was not due to an inability to respond to TNF-α in the NFATp−/− mice, groups of D-Gal–sensitized NFAT+/+ and NFATp−/− mice were injected with increasing amounts of recombinant murine TNF-α protein. Both groups of mice had comparable sensitivity to recombinant TNF-α with ∼50% lethality at 0.1 μg/mouse (Table ). Thus, responsiveness to TNF-α is not compromised in the NFATp−/− mice.
LPS also causes a TNF-α–mediated lethal shock syndrome, which is phenotypically indistinguishable from SEB-induced TNF-α–mediated lethal shock in the mouse 7. By contrast to SEB, which triggers T cell secretion of TNF-α, macrophages are the source of TNF-α in shock caused by LPS 7. Notably, TNF-α gene regulation by LPS in monocytes/macrophages is not dependent upon NFAT and LPS-stimulated TNF-α mRNA levels from NFATp+/+ and NFATp−/− splenocytes are equivalent 15. Consistent with this observation, LPS triggered an identical lethal shock syndrome in both NFATp+/+ and NFATp−/− D-Gal–sensitized mice with 100 and 67% lethality at 1 and 0.1 μg of LPS, respectively (Table ). Thus, NFATp−/− mice are sensitive to LPS-mediated lethal shock in contrast to their resistance to SEB-induced lethal shock.
Decreased TNF-α Serum Levels in NFATp−/− Mice after SEB Challenge.
We next measured serum levels of TNF-α in NFATp+/+ and NFATp−/− mice after injection with SEB (5 μg/mouse) or LPS (1 μg/mouse). As shown in Fig. 2, TNF-α levels in NFATp+/+ mice peak between 1 and 2 h after injection with SEB or LPS and decrease sharply at later time points. However, after injection of NFATp−/− mice with SEB, the levels of TNF-α protein are markedly decreased (approximately fivefold) as compared with those levels obtained in NFATp+/+ littermates (Fig. 2 A). By contrast, serum TNF-α levels from NFATp+/+ and NFATp−/− animals injected with LPS are virtually identical (Fig. 2 B). Taken together, our results indicate that NFATp−/− mice are resistant to otherwise lethal doses of SEB due to dramatically decreased TNF-α production by SEB-responsive T cells.
Figure 2.
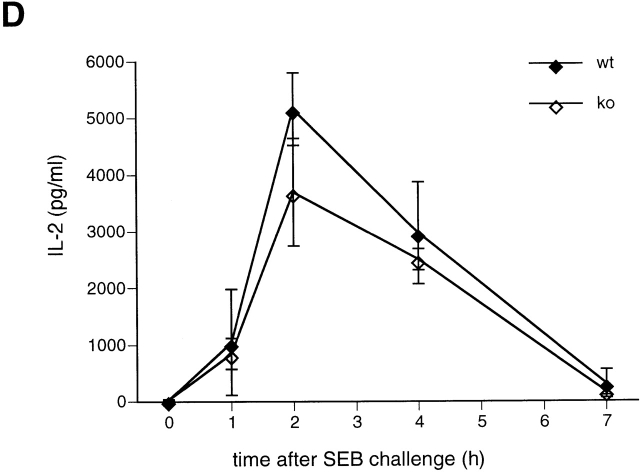
TNF-α protein levels are reduced in NFATp−/− mice after SEB but not after LPS injection. At each time point after injection with SEB (A, C, D) or LPS (B) two NFATp+/+ (♦) and two NFATp−/− (⋄) mice were killed and serum TNF-α (A, B, C) or IL-2 (D) levels were evaluated by ELISA. The error bars represent standard deviation. *P < 0.02 for protein levels in NFATp−/− mice versus their NFATp+/+ littermates.
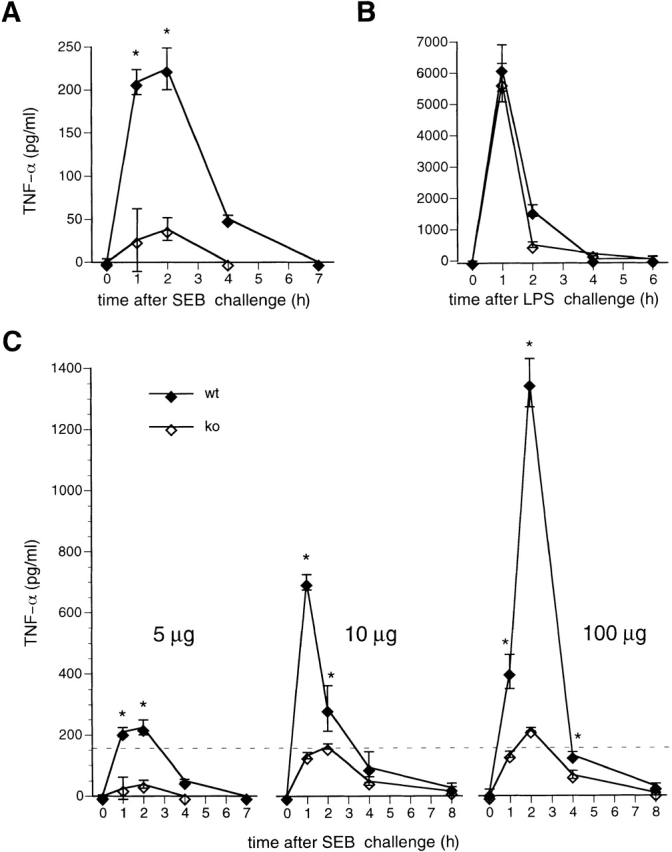
Notably, increasing amounts of SEB caused death in NFATp−/− mice, achieving 100% lethality with the injection of 100 μg (Table ). We next measured serum TNF-α levels in mice injected with 10 and 100 μg of SEB. TNF-α levels increase in both genotypes in a SEB dose-dependent manner, but stay dramatically lower in knockout animals as compared with their NFATp+/+ littermates (Fig. 2 C). As a control for the specificity of this effect upon TNF-α, we measured IL-2 levels after injection of a lethal dose of SEB in NFATp−/− mice and NFATp+/+ littermates and found no difference (Fig. 2 D). Thus, NFATp−/− mice are significantly compromised in their ability to produce TNF-α after SEB challenge at all doses; however, at higher doses of SEB the threshold lethal level of TNF-α (Fig. 2 C, dotted line) is achieved in NFATp−/− animals.
Delayed Induction of TNF-α mRNA Levels in Splenic T Cells from NFATp−/− Mice.
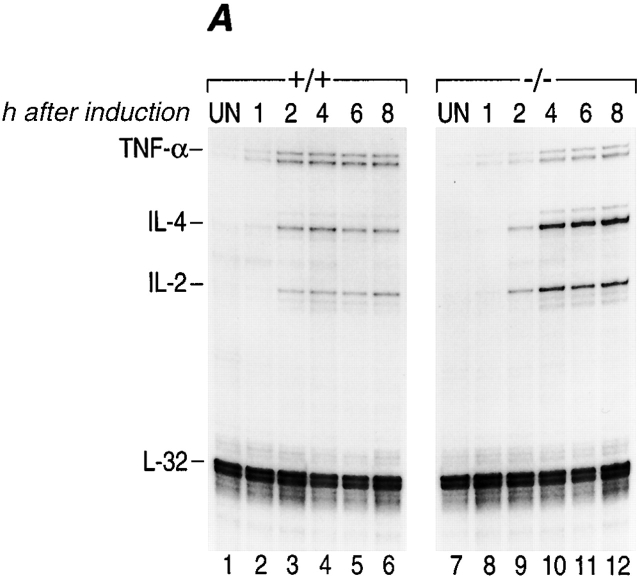
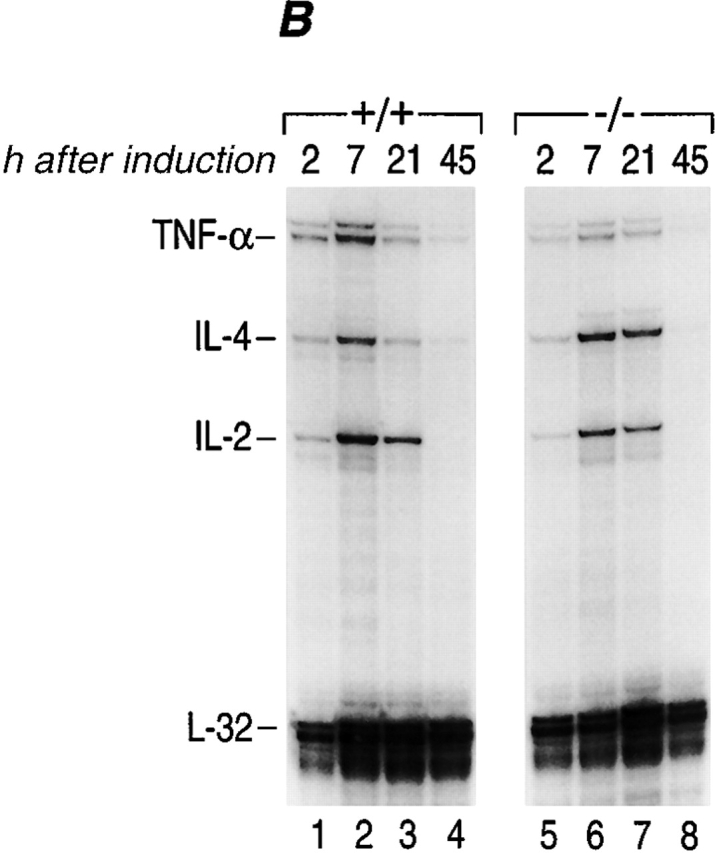
TNF-α is one of the earliest genes transcribed in an activated murine T cell 10. We thus speculated that NFATp plays a unique role as a transcriptional activator in immediate early TNF-α gene expression in activated T cells. As shown in Fig. 3 A, the kinetics of induction of TNF-α mRNA levels in NFATp−/− T cells are delayed ∼2 h compared with NFATp+/+ T cells (compare lanes 1–4 to lanes 7–10). Furthermore, these levels remain lower (∼50%) than those in NFATp+/+ mice for the first 8 h after stimulation (Fig. 3 A) and become equivalently low at 21 h after induction (Fig. 2 B, compare lane 3 to lane 7). As a control we measured IL-2 and IL-4 mRNA levels and found that the kinetics of induction of mRNA for both of these genes was similar in both genotypes (Fig. 3 A). Although IL-4 mRNA levels in NFATp−/− T cells were initially relatively lower (at 2 h), they were increased at all other timepoints compared with NFATp+/+ levels (Fig. 2A and Fig. B), consistent with previous reports 27. We conclude that the immediate early induction of TNF-α gene expression and the achievement of maximal mRNA levels in activated T cells requires NFATp.
Figure 3.
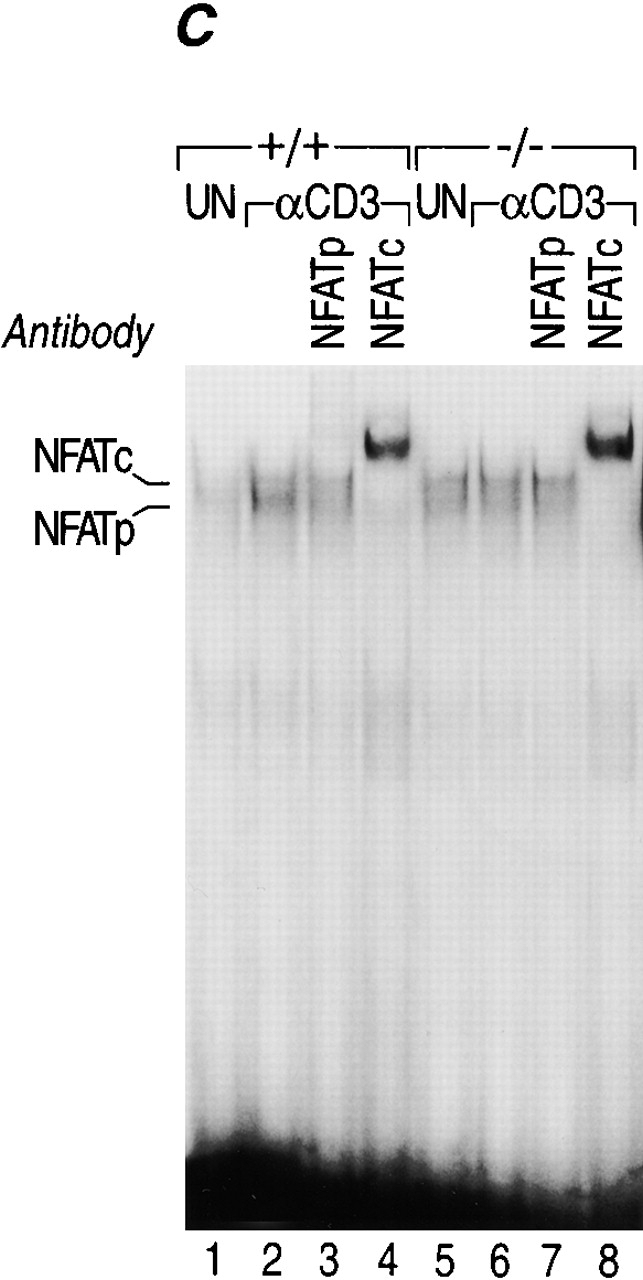
Kinetics of induction of cytokine mRNAs during primary in vitro stimulation of NFATp+/+ and NFATp−/− splenocytes. (A and B) The kinetics of induction of TNF-α by anti-CD3 are delayed in NFATp−/− mice. Spleen cells isolated from NFATp+/+ (+/+) and NFAT−/− (−/−) mice were unstimulated (UN) or stimulated up to 45 h with plate-bound anti-CD3 as indicated. At the indicated times, cells were harvested and the total cellular RNA was analyzed for TNF-α, IL-4, IL-2, and L-32 mRNA levels by RNase Protection assay. (C) Lack of binding of NFATp to DNA in NFATp−/− splenocytes. Nuclear extracts were prepared from purified splenocytes from NFATp+/+ (+/+) and NFAT−/− (−/−) mice that were unstimulated (UN) or stimulated with plate-bound anti-CD3 antibody for 1 h. An oligonucleotide probe matching the −76 NFAT binding site was used and the binding assay was performed in the presence or absence of the NFATp or NFATc antibodies as indicated.
The Absence of NFATp Binding Does Not Affect Binding of NFATc to the TNF-α Promoter.
We next investigated whether binding of NFATc was affected in the NFATp−/− mice and performed electrophoretic mobility shift assays using nuclear extracts from unstimulated or anti-CD3–activated splenocytes derived from NFATp−/− and NFATp+/+ littermate mice using the high affinity TNF-α −76 NFAT site as a probe. As shown in Fig. 3 C, NFATc binding is not affected in NFATp−/− splenocytes. Furthermore, we examined the kinetics of NFATc binding to the −76 NFAT site up to 48 h after anti-CD3 induction in both NFATp+/+ and NFATp−/− mice and found no difference (data not shown). Thus, the absence of NFATp does not impair NFATc binding to the TNF-α promoter.
Lethal shock in D-Gal sensitized mice is characterized by fulminant hepatitis 7 26. We next performed histopathological analysis of livers taken from treated animals 8 h after D-Gal, SEB plus D-Gal, or TNF-α plus D-Gal administration. As shown in Fig. 4, sections from livers taken from NFATp+/+ mice coinjected with D-Gal and SEB (5 μg) showed signs of fulminant hepatitis with massive hemorrhage and hepatocyte death with swelling of the cytoplasm and loss of nuclei. By contrast, sections from livers of NFATp−/− mice injected with the same dose of D-Gal and SEB showed only signs of nonspecific hepatotoxicity comparable to sections from mice injected with D-Gal alone or mice given no injection (Fig. 4). Liver sections from both NFATp+/+ and NFATp−/− mice treated with TNF-α and D-Gal showed identical damage consistent with fulminant hepatitis and massive hemorrhage and cell death (Fig. 4). Taken together, the SEB unresponsiveness of the NFATp−/− mice is not due to impairment of the TNF-α signaling pathway.
Figure 4.
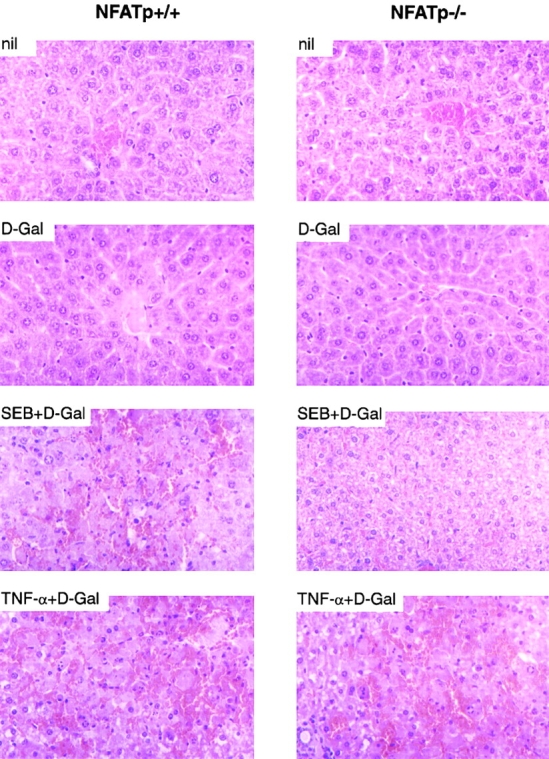
Histological analysis of liver sections after superantigen or TNF-α challenge. Liver was removed from NFATp+/+ and NFATp−/− mice 8 h after intraperitoneal injection of 0.9% NaCl (nil), D-Gal (20 mg), SEB (5 μg) plus D-Gal, or recombinant TNF-α (1 μg) plus D-Gal as indicated. Tissues were directly transferred into 10% formalin solution. Sections were stained with hematoxylin and eosin. Original magnification: approximately ×100.
The TNF-α gene is a unique example of a gene that is regulated through the assembly of inducer- and cell type–specific enhancer complexes 12 14 15. In the case of T cells activated through the TCR or by calcium flux, in vivo recruitment of NFATp is correlated with TNF-α gene expression 10 12 23. Our studies using NFATp−/− mice demonstrate that NFATp cannot be replaced by another NFAT family member in the TNF-α enhancer in activated T cells. Although the NFAT family of proteins share a rel-like DNA binding domain, the transcriptional activation domains of these proteins are distinct 16 19. Thus, it is likely that in the initial induction of TNF-α transcription, NFATp uniquely interacts with the other components in the TNF-α enhancer complex assembled in activated T cells.
NFATp−/− mice remain sensitive to LPS-mediated lethal shock consistent with the observation that LPS-stimulated TNF-α gene expression is not impaired in splenocytes from NFATp−/− mice, and that NFATp is not recruited to the LPS-stimulated TNF-α enhancer in macrophages 15. These results present in vivo evidence of the inducer- and cell type–specific regulation of TNF-α gene expression that has previously been established in cell culture and in in vitro model systems 12 13 14.
To our knowledge, NFATp is the first transcription factor to be identified that controls susceptibility to superantigen-induced lethal shock through its activation of immediate early TNF-α gene transcription. Therapeutic approaches that globally inhibit TNF-α have been limited by their lack of specificity 28 29. The results presented here demonstrate that through the manipulation of the cell type– and inducer-specific assembly of TNF-α enhancer complexes, TNF-α can be selectively inhibited and disease outcome can be dramatically influenced. Moreover, susceptibility or resistance to disease where TNF-α plays a role may be directly linked to the presence or the levels of expression of a single transcription factor.
Acknowledgments
We are indebted to L. Glimcher for the generous gift of two breeding pairs of heterozygote NFATp+/− mice, D. Thanos for the gift of the NFATp protein, A. Rao for the gift of the anti-NFATp antibody, and R. Barthel for generous assistance with all aspects of histopathological analyses of the mice. We thank F. Tucker for assistance in mouse care, P. Pesavento for performing the murine TNF-α CAT assay, and E. Tsitsikov for critical reading of the manuscript.
This work was supported by the National Institutes of Health (GM-56492) and an Established Investigator Award from the American Heart Association to A.E. Goldfeld. Critical support was also provided by Dr. F.S. Rosen and the Center for Blood Research.
References
- Schlievert P.M., Shands K.N., Dan B.B., Schmid G.P., Nishimura R.D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr., Yagi J., Conrad P.J., Katz M.E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol. Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J. Exp. Med. 1988;167:1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Herman A., Pullen A.M., Kubo R., Kappler J.W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin Bstimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Kuby J. Immunology 1997. W.H. Freeman and Company; New York: pp. 664 [Google Scholar]
- Miethke T., Wahl C., Heeg K., Echtenacher B., Krammer P.H., Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin Bcritical role of tumor necrosis factor. J. Exp. Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M.A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect. Immun. 1986;51:891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J., Fong Y., Hesse D.G., Manogue K.R., Lee A.T., Kuo G.C., Lowry S.F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Goldfeld A.E., McCaffrey P.G., Strominger J.L., Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J. Exp. Med. 1993;178:1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld A.E., Tsai E., Kincaid R., Belshaw P.J., Schrieber S.L., Strominger J.L., Rao A. Calcineurin mediates human tumor necrosis factor α gene induction in stimulated T and B cells. J. Exp. Med. 1994;180:763–768. doi: 10.1084/jem.180.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo J.V., Uglialoro A.M., Brinkman B.N.M., Merika M., Parekh B.S., King H.C., Tsai E.Y., Morielli A.D., Peralta E.G., Maniatis T., Thanos D., Goldfeld A.E. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor-α promoter. Mol. Cell Biol. 2000;20:2239–2247. doi: 10.1128/mcb.20.6.2239-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai E.Y., Jain J., Pesavento P.A., Rao A., Goldfeld A.E. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol. Cell. Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai E.Y., Yie J., Thanos D., Goldfeld A.E. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol. Cell. Biol. 1996;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai E.Y., Falvo J.V., Tsytsykova A.V., Barczak A.K., Reimhold A., Glimcher L., Fenton M.J., Gordon D.C., Dunn I.F., Goldfeld A.E. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, CREB binding protein and p300 is recruited to the tumor necrosis factor α promoter in vivo. Mol. Cell. Biol. 2000;20:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey T., Sun Y.L., Williamson K., Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- McCaffrey P.G., Luo C., Kerppola T.K., Jain J., Badalian T.M., Ho A.M., Burgeon E., Lane W.S., Lambert J.N., Curran T. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- Northrop J.P., Ho S.N., Chen L., Thomas D.J., Timmerman L.A., Nolan G.P., Admon A., Crabtree G.R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez C., Aramburu J., Rakeman A.S., Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl. Acad. Sci. USA. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G.R. Generic signals and specific outcomessignaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Rao A., Luo C., Hogan P.G. Transcription factors of the NFAT familyregulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Hodge M.R., Ranger A.M., Charles de la Brousse F., Hoey T., Grusby M.J., Glimcher L.H. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- McCaffrey P.G., Goldfeld A.E., Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-α gene transcription. J. Biol. Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- Yoshida H., Nishina H., Takimoto H., Marengere L.E., Wakeham A.C., Bouchard D., Kong Y.Y., Ohteki T., Shahinian A., Bachmann M. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- Ranger A.M., Oukka M., Rengarajan J., Glimcher L.H. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–635. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M.A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine–treated mice. J. Exp. Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A., Viola J.P., Lichtman A.H., Rao A. Down-regulation of IL-4 gene transcription and control of Th2 cell differentiation by a mechanism involving NFAT1. Immunity. 1997;7:849–860. doi: 10.1016/s1074-7613(00)80403-3. [DOI] [PubMed] [Google Scholar]
- Fisher C.J., Jr., Agosti J.M., Opal S.M., Lowry S.F., Balk R.A., Sadoff J.C., Abraham E., Schein R.M., Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- Grau G.E., Maennel D.N. TNF inhibition and sepsis — sounding a cautionary note. Nat. Med. 1997;3:1193–1195. doi: 10.1038/nm1197-1193. [DOI] [PubMed] [Google Scholar]