Abstract
Leukocyte recruitment to target tissue is initiated by weak rolling attachments to vessel wall ligands followed by firm integrin-dependent arrest triggered by endothelial chemokines. We show here that immobilized chemokines can augment not only arrest but also earlier integrin-mediated capture (tethering) of lymphocytes on inflamed endothelium. Furthermore, when presented in juxtaposition to vascular cell adhesion molecule 1 (VCAM-1), the endothelial ligand for the integrin very late antigen 4 (VLA-4, α4β1), chemokines rapidly augment reversible lymphocyte tethering and rolling adhesions on VCAM-1. Chemokines potentiate VLA-4 tethering within <0.1 s of contact through Gi protein signaling, the fastest inside-out integrin signaling events reported to date. Although VLA-4 affinity is not altered upon chemokine signaling, subsecond VLA-4 clustering at the leukocyte-substrate contact zone results in enhanced leukocyte avidity to VCAM-1. Endothelial chemokines thus regulate all steps in adhesive cascades that control leukocyte recruitment at specific vascular beds.
Keywords: adhesion, integrin, endothelium, chemokine, shear flow
Introduction
Leukocyte recruitment to inflamed tissue requires rapid activation of integrin-dependent arrest of the leukocyte on the target endothelium as a checkpoint for subsequent diapedesis to the extravascular tissue 1 2. These processes are triggered by Gi protein–coupled receptors (GPCRs) to chemokines, chemoattractive regulators of hematopoietic cell migration 3, which are displayed on endothelial sites of hematopoietic cell extravasation 4 5 6. Chemokines signal through seven spanner receptors linked to the α subunit of heterotrimeric Gi proteins 7 8. Circulating leukocytes must loosely tether to and roll on vessel endothelium through specific primary adhesion molecules to facilitate their encounter of stimulatory signals leading to rapid conversion of rolling behavior to firm integrin-dependent arrest on the endothelium 7 9 10 11. Primary leukocyte adhesions to endothelium, namely, tethering and rolling, are mediated by specialized lectins, primarily selectins, as well as by leukocyte integrins sharing an α4 subunit, such as very late antigen 4 (VLA-4; α4β1), and the mucosal homing receptor, α4β7. Firm integrin-dependent leukocyte arrest on vascular endothelium depends on rapid modulation of integrin avidity to ligand. Elucidating the mechanisms of integrin activation by chemokines at confined leukocyte–endothelium contact zones under shear flow is crucial for understanding how these cytokines regulate leukocyte trafficking to target sites. To delineate how integrin avidity can be modulated rapidly by endothelial chemokines, we used videomicroscopy in order to follow in real time chemokine-activation of VLA-4. VLA-4 is the major vascular integrin receptor for vascular cell adhesion molecule 1 (VCAM-1), a key adhesion molecule conferring endothelial adhesiveness of mononuclear leukocytes, eosinophils, and hematopoietic progenitor cells (HPCs) at sites of inflammation or allergy and within the bone marrow vasculature 12 13. We show here that immobilized chemokines can augment reversible VLA-4-integrin–mediated tethering and rolling of leukocytes on VCAM-1 before and independent of firm integrin-mediated arrest on the endothelial ligand. Chemokine-triggered Gi protein signaling coupled to VLA-4 clustering events takes place within subseconds of leukocyte contact with VCAM-1 and requires juxtaposition of the integrin ligand and the chemokine. This is the first demonstration that endothelial chemokines may function at an earlier stage than was previously realized in augmenting primary reversible leukocyte interactions with vascular endothelium preceding cell arrest on vessel walls under physiological flow conditions.
Materials and Methods
Antibodies and Reagents.
The function-blocking anti–VLA-4 integrin mAb HP1/2 and the nonblocking VLA-4 mAb B5G10 (both directed against the α4 integrin subunit; references 14, 15), the VCAM-1–blocking mAb 4B9, and the L-selectin blocking mAb DREG-200 (provided by Dr. T.K. Kishimoto, Boehringer-Ingelheim Pharmaceuticals, Ridgefield, CT), as well as the anti CXCR4 mAb 12g5 (PharMingen) were all used as purified Igs. BSA (fraction V), Ca2+- and Mg2+-free HBSS, Ficoll-Histopaque 1077, and the kinase inhibitors wortmannin and genestein were obtained from Sigma-Aldrich. EDTA and Hepes were from Merck. Human serum albumin (HSA; fraction V), pertussis toxin (PTX), and bis-(O-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) were from Calbiochem-Novabiochem. The chemokines SDF-1 (stromal-derived factor 1), RANTES (regulated on activation, normal T cell expressed and secreted), SLC (secondary lymphoid tissue chemokine), EBI1 ligand chemokine (ELC), IFN-γ–inducible protein 10 (IP-10), thymus and activation-regulated chemokine (TARC), monocyte chemoattractant protein 1 (MCP-1), and eotaxin were obtained from R&D Systems. SDF-1 and its mutant P2G were produced as described previously 16. Different preparations of SDF-1 exhibited similar preadhesive activities when coated at identical densities onto the various adhesive substrates (data not shown). An eight-residue peptide containing the tripeptide motif (leucine-aspartate-valine, LDV), EILDVPST, derived from the CS-1 region of fibronectin, and its control peptide EIDVLPST were prepared by solid phase peptide synthesis using an ABIMED AMS-422 automated peptide synthesizer.
Cells.
Human peripheral blood lymphocytes (obtained from healthy donors) were isolated from citrate-anticoagulated whole blood by dextran sedimentation and density separation over Ficoll-Histopaque. The mononuclear cells thus obtained were washed and further purified on nylon wool and plastic adherence as previously described 17. The resulting purified peripheral blood lymphocytes consisted of >90% CD3+ T lymphocytes (termed herein PBTLs) and were cultured in LPS-free RPMI/10% FCS for 15–18 h before use. Memory and naive CD3+ T lymphocyte subsets (CD45RO+ and CD45RA+, respectively) were isolated by negative selection using magnetic cell separation 17. Lymphocyte subset purity was verified by FACS® staining and was >95%. Jurkat cells were maintained in RPMI 1640 (Sigma-Aldrich) supplemented with 10% heat inactivated FCS (Biological Industries), 2 mM l-glutamine, and penicillin/streptomycin (Bio Lab). Human cord blood CD34+ progenitors (>95% pure) were isolated by standard Ficoll separation followed by positive selection using a magnetic bead separation kit (mini MACS; Miltenyi Biotec) according to the manufacturer's instructions. Chinese hamster ovary (CHO) cells transfected with full-length human VCAM-1 were maintained in α-MEM, supplemented with 10% dialyzed FCS, 4 mM l-glutamine, and 200 nM methotrexate (Sigma-Aldrich). Human umbilical cord endothelial cells (HUVECs) were isolated from umbilical cord veins as previously described 6, pooled, and established as primary cultures in M199 containing 10% FCS, 8% pooled human serum, 50 μg/ml endothelial cell growth factor (Sigma-Aldrich), 10 U/ml porcine intestinal heparin (Sigma-Aldrich), and antibiotics. Primary cultures were serially passaged (1:3 split ratio) and passages 3–4 were taken for adhesion experiments.
Fluorocytometry and Confocal Microscopy.
Ligand-induced binding site (LIBS) expression was determined by cytofluorometry of T cells immunostained with the LIBS reporter mAb 15/7 (a gift of Dr. T. Yednock, Elan Pharmaceuticals, Inc., South San Francisco, CA), in the presence of 1–500 μM of EILDVPST peptide or its analogue, EIDVLPST. Cell staining was performed in binding medium (HBSS containing 2 mg/ml BSA, 10 mM Hepes, pH 7.4, and Ca2+ and Mg2+ at 1 mM each) at 24°C as previously described 18 19. Dose dependence of LIBS induction by the octapeptide ligand was sensitive to VLA-4 affinity 18 19. VLA-4 clustering was analyzed by confocal laser scanning microscopy. SDF-1 (2 μg/ml) or HSA (2 μg/ml) was coated on 3-μm polystyrene beads (Sigma-Aldrich) under identical conditions used for preparing the adhesive substrates in the flow chamber experiments. T cells were suspended in binding medium at 24°C with chemokine or HSA beads (0.5–5 × 106 cells; 10 beads/cell) for 15 min, or with phorbol myristate acetate (PMA; 100 ng/ml, 2 min), washed, fixed with 0.5% paraformaldehyde and immobilized on polylysine coated glass slides. VLA-4 distribution on fixed cells was probed with the α4 subunit specific mAb B5G10, followed by FITC-labeled secondary mAb as described 20. Samples were analyzed at 488 nm with a krypton/argon laser confocal microscope (Bio-Rad Laboratories).
Shear Flow Experiments.
The flow chamber assays have been described in detail elsewhere 19. Soluble purified seven-domain human VCAM-1, sVCAM-1 21 was mixed in coating medium (PBS buffered with 20 mM sodium bicarbonate pH, 8.5) with a fixed amount of carrier (2 μg/ml HSA) and adsorbed as 10-μl spots on polystyrene plates (Becton Dickinson) for 2 h at 37°C, alone or with the indicated amounts of intact or heat-inactivated chemokines. Plates were washed and blocked with HSA (20 mg/ml). VCAM-1 site densities were assessed using 125I-labeled anti–VCAM-1 mAb, 4B9, as previously described 9. VCAM-1 coating densities were comparable when the ligand was coimmobilized with either heat-inactivated or intact chemokine (data not shown). CHO–VCAM-1 or HUVECs were preseeded on fibronectin-coated plates (Falcon Tissue Culture Plates; Becton Dickinson) for 24 h before treatment with stimulatory cytokines. HUVECs were stimulated for 18 or 40 h with heparin-free culture media supplemented with TNF-α (2 ng/ml, 50 U/ml; R&D Systems). Cell monolayers and VCAM-1/chemokine-coated substrates were assembled as the lower wall of the flow chamber (260-μm gap) and extensively washed with binding medium. The flow chamber was mounted on the stage of an inverted phase contrast microscope (Diaphot 300; Nikon). All flow experiments were conducted at 37°C. Cells were perfused at 106cells/ml through the chamber at desired flow rate generated with an automated syringe pump (Harvard Apparatus). The entire periods of cell perfusion were recorded on a videotape with a long integration LIS-700 CCD video camera (Applitech) and a Time Lapse SVHS-Video recorder (AG-6730; Panasonic). All cellular interactions with the adhesive substrates were determined by manually tracking the motions of individual cells along 0.9-mm field paths for 1 min. Cellular interactions with VCAM-1–bearing surfaces were >95% α4 integrin dependent, i.e., could be blocked with the α4 subunit blocking mAb HP1/2. Different categories of tethered cells were defined according to their subsequent motion (transient, rolling, rolling followed by arrest, and immediate arrest) as previously described 19. In each experiment all events were normalized to a constant cell population flowing in immediate proximity with the substrate. Frequency of each category of tethers was expressed in percentage of units (event × cell−1 × 102); 1% unit measured at 0.5, 1, and 1.5 dyn/cm2 corresponded to tethering rate of 1.5 × 10−3, 3 × 10−3, and 4.5 × 10−3 events × cell−1mm−1s−1, respectively. To selectively block high affinity VLA-4 subsets on perfused T cells, cells were preincubated for 5 min in binding medium with 0.75 mM EILDVPST and perfused unwashed over the VCAM-1–containing substrates. Control peptide had no inhibitory effect under identical conditions. For inhibition of intracellular signaling, PBTLs were preincubated in binding medium with inhibitors of protein tyrosine kinase (genestein at 100 μM) or PI-3 kinase (wortmannin at 100 nM) for 30 min at 37°C and perfused unwashed over the VCAM-1/chemokine–coated substrate. To chelate intracellular Ca2+ in PBTLs, lymphocytes (106/ml of H/H medium [HBSS/10 mM Hepes, pH 7.4, supplemented with 2 mg/ml BSA]) were preloaded for 30 min at 37°C with the cell-permeable Ca2+ chelator, BAPTA-AM (dissolved at 25 mM in DMSO stock solution and used at 1:1,000 dilution). Loaded cells were washed and immediately perfused over the adhesive substrates. Transient tethers were determined as previously described 22 at a resolution of 0.02 s in a digital still playback mode (AG-7355; Panasonic). All tethering events were set to start at t = 0 and the natural log of the tethers that remained bound after initiation of tethering was plotted against tether duration to yield a characteristic slope = −k off. Cell displacements were determined by computerized motion analysis (Galai) that tracked cell positions every 40 ms at a horizontal resolution of 0.6 μm in the flow direction. A rolling cell was considered to pause on the substrate if moving <0.6 μm between successive positions. This gave optimal correlation between computerized motion analysis and manual determination of pauses directly from the video monitor (data not shown). Tethering frequency data are expressed as the mean ± range or SD. Statistical comparison of means was performed by a two-tailed unpaired Student's t test.
Results and Discussion
To study chemokine modulation of PBTL adherence to endothelial cells (ECs) under flow, we used a monolayer of TNF-α–activated HUVECs as model ECs. As TNF-activated HUVECs display only minute levels of functional lymphocyte chemokines on their apical surface 23, the monolayer was overlaid with the pleiotropic lymphocyte chemokine SDF-1α 24. PBTL capture by and rolling on TNF-activated HUVECs was largely mediated by both E-selectin and VCAM-1 (Fig. 1 A, top). Surprisingly, EC-bound SDF-1 could dramatically augment the frequency of cells initiating primary capture events (tethers) to TNF-activated HUVECs, in addition to its ability to stimulate firm integrin-dependent arrest of PBTLs already captured and rolling on the ECs (Fig. 1 A). SDF-1 also increased by twofold the frequency of VLA-4–dependent PBTL capture by selectin-blocked TNF-activated HUVECs (Fig. 1 A, top), without altering VCAM-1 expression on these cells (data not shown). SDF-1 also dramatically increased PBTL tethering and firm arrest of lymphocytes on HUVECs activated with TNF-α for a prolonged period, which lacked endothelial selectin activity (Fig. 1 A, bottom, and data not shown). Cell surface–bound SDF-1 could also augment the frequency of PBTLs initiating primary capture events to VCAM-1–transfected CHO cells, and stimulated firm integrin-dependent arrest of nearly all lymphocytes captured on the cell monolayer (Fig. 1 B). Moreover, SDF-1 coimmobilized with purified VCAM-1 coated on polystyrene substrate enhanced PBTL tethering by more than fourfold, along with triggering rapid arrest of tethered lymphocytes on the adhesive substrate (Fig. 2 A). Complete blocking of chemokine-triggered or spontaneous PBTL tethering to VCAM-1 with β1 integrin mAb suggested an exclusive role for VLA-4, rather than the α4β7 integrin in SDF-1–triggered PBTL tethering to VCAM-1 (data not shown).
Figure 1.

Cell surface–bound SDF-1 augments VLA-4–mediated capture and arrest of lymphocytes on endothelial VCAM-1 under physiological shear flow. (A) The frequency of PBTLs perfused at a shear stress of 1.5 dyn/cm2 over HUVECs stimulated for either 18 h (top) or 40 h (bottom) are capable of tethering transiently, or tether and roll or arrest on the cell monolayers. These different categories are depicted in stacked bars. SDF-1 (1 μg/ml in binding medium) was overlaid for 10 min on each monolayer and washed extensively before PBTL perfusion. Indicated cell monolayers were pretreated for 10 min with the E-selectin blocking mAb BB11 (at 10 μg/ml). Indicated PBTL samples were pretreated with the α4-integrin subunit mAb, HP1/2, to block their VLA-4–dependent interactions with endothelial VCAM-1. Where indicated, PBTLs were perfused over the endothelial monolayers in the presence of 1 mM EDTA. Standard deviation of total tethering values between multiple experiments on the different TNF-activated HUVECs was <10% of the mean. Asterisk indicates that chemokine-dependent augmentation in total tethering to activated HUVECs was highly significant (n = 5, P < 0.001). (B) Frequency of different categories of tethers initiated by PBTLs on VCAM-1–expressing CHO cells at 1.5 dyn/cm2. SDF-1 (1 μg/ml) was overlaid on the CHO monolayer as described for panel A. Data shown in B are representative of four independent experiments.
Figure 2.
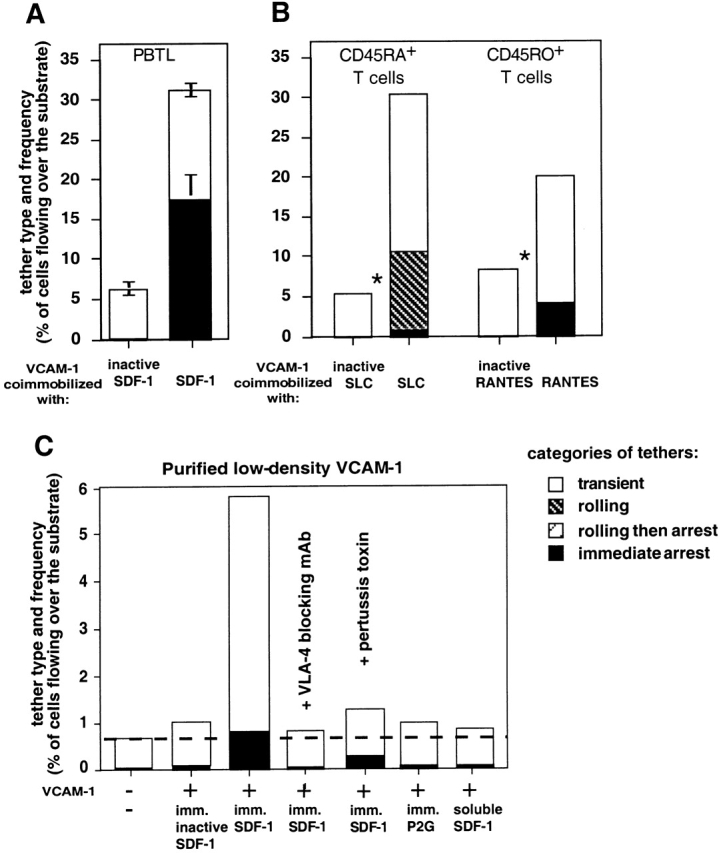
Immobilized chemokines augment VLA-4–mediated capture and arrest of T lymphocytes to purified VCAM-1 under shear flow. (A) Frequency of PBTL tethers to purified sVCAM-1 coated at 1.5 μg/ml on polystyrene surface with functional or heat-inactivated SDF-1 (2 μg/ml). The different tether categories were determined in two fields at 1 dyn/cm2 and results are an average and range of each tether category. (B) Frequency and type of tethers formed by naive (CD45RA+) and memory (CD45RO+) subsets of peripheral blood CD3+ T lymphocytes interacting at 1 dyn/cm2 with sVCAM-1 (1.5 μg/ml) coated together with the indicated intact or heat-inactivated chemokines (each at 2 μg/ml). Chemokine-dependent augmentation in total tethering was highly significant (*P < 0.001 and 0.004, for experiments performed on the CD45RA+ CD45RO+ subsets, respectively). (C) Frequency of VLA-4–mediated tethers of PBTLs to sVCAM-1 coated at 0.5 μg/ml, alone, or with inactivated SDF-1, SDF-1, or the non-signaling SDF-1 mutant P2G 16 (each at 2 μg/ml), determined at 0.5 dyn/cm2. VCAM-1 coating density on all substrates was identical as verified by radioimmunodetermination. For PTX treatment, PBTLs were cultured for 15 h with 100 ng/ml of the toxin. In A and D, where indicated, PBTLs were pretreated with the VLA-4 blocking mAb, HP1/2 (10 μg/ml), and were perfused unwashed over the VCAM-1–bearing surfaces. Data in A–C are representative of four independent experiments.
The ability to augment α4 integrin tethering to ligand was not restricted to SDF-1 nor to a specific subset of lymphocytes: α4-dependent tethering of both naive CD45RA+ and memory CD45RO+ T cell subsets was markedly augmented by the prototypic chemokines SLC and RANTES, respectively (Fig. 2 B). In spite of their lower constitutive levels of VLA-4–mediated tethering to VCAM-1, VLA-4 on naive T cells responded with high efficiency to immobilized SLC, consistent with the high level expression of the SLC receptor, CCR7, on these lymphocytes 25. In contrast, only slight augmentation of VLA-4–mediated PBTL tethering to VCAM-1 could be induced by immobilized MCP-1 or eotaxin under identical conditions (data not shown), consistent with low level expression of receptors to these chemokines on resting PBTLs 26. Notably, PBTLs did not have to arrest on the adhesive surface in order to respond to the immobilized chemokine, which augmented reversible VLA-4 tethers to low density VCAM-1 under flow (Fig. 2 C). Thus, immobilized SDF-1 increased by 15-fold the rate of PBTL tethers to low density VCAM-1, even though the vast majority of SDF-1–triggered VLA-4 tethers were reversible and transient (Fig. 2 C). Moreover, at a fixed chemokine density on the adhesive substrate, lower VCAM-1 density resulted in a greater chemokine-induced increase in VLA-4 tethering under shear flow (Fig. 2B and Fig. C). However, immobilized SDF-1 lacked intrinsic adhesive activity towards PBTLs, since PBTL tethering was completely inhibited upon α4 integrin blocking (Fig. 2 C). This is different from the endothelial chemokine fractalkine, which captures leukocyte subsets in an integrin-independent manner 27. Chemokine-triggered PBTL tethers, but not spontaneous VLA-4 tethers to VCAM-1 mediated by constitutively functional VLA-4 subsets on PBTLs, were completely inhibited by PTX inactivation of the Gi-α subunit of PBTLs (Fig. 2 C and data not shown). Furthermore, an SDF-1 mutant, P2G, with retained affinity to the SDF-1 receptor, CXCR4, but defective Gi-protein signaling activity 16 failed to augment VLA-4 tethers to VCAM-1 (Fig. 2 C). However, all chemokines tested failed to augment any T cell tethering to immobilized E- or P-selectins or to the CD44 ligand, hyaluronan (Grabovsky, V., O. Dwir, and R. Alon, manuscript in preparation). SDF-1 was also able to augment the rate of VLA-4–dependent cell capture to VCAM-1 before triggering firm integrin adhesion under flow in fresh human CD34+ HPCs: the frequency of HPC tethering to low density VCAM-1 was augmented by at least twofold by immobilized SDF-1 at 1–1.25 dyn/cm2 (n = 4).
Surprisingly, chemokines had to be copresented with VCAM-1 in the same adhesive surface to stimulate VLA-4 tethering; saturating levels of soluble chemokines failed to trigger VLA-4 tethers of PBTLs to low or high density VCAM-1 under shear flow (Fig. 2 C and data not shown). Thus, global occupancy of chemokine receptors with ligand, which enhances integrin-dependent adhesion and motility in extravascular compartments, is insufficient to trigger rapid VLA-4 tethering to ligand under shear flow. In addition, prior T cell exposure to immobilized SDF-1 during rolling on P-selectin failed to augment VLA-4 tethering to a downstream chemokine-free VCAM-1 substrate (data not shown). This result suggested that juxtaposition of the chemokine and integrin ligand is necessary to stimulate integrin-mediated tethering under flow. Although integrin-dependent adhesion can be stimulated by soluble chemokines, such stimulation predominates in static adhesive processes, which take minutes to complete 17 28. In physiological settings, chemoattractants are presented to tethered or rolling leukocytes at endothelial adhesive zones through specific associations with endothelial proteoglycans or membranal moieties 29 30 31. The failure of soluble chemokines to modulate α4 adhesiveness in T cells or HPCs (this study and reference 6) is consistent with an exclusive physiological role for surface-bound as opposed to serum-based chemokines in the rapid triggering of VLA-4 adhesions of these cells to endothelial ligands under shear flow.
The coating density of chemokine at a VCAM-1–containing contact site dictated the dynamic property of the VLA-4 tether. At high coating density, the majority of SDF-1–triggered VLA-4 tethers resulted in immediate lymphocyte arrest on cell-surface or isolated VCAM-1 (Fig. 1 and Fig. 3 B). However, at medium density SDF-1 augmented VLA-4 tethering without triggering arrests (Fig. 3 A). Instead, SDF-1, as well as other lymphocyte chemokines such as SLC, thymus and activation-regulated chemokine, IP-10, or RANTES triggered rolling interactions of PBTLs on VCAM-1 (Fig. 3 B), which consisted of closely spaced reversible tethers (Fig. 3 C), and were generally not followed by arrests (Fig. 3A and Fig. B). In contrast, PMA, a direct activator of protein kinase C and a general integrin stimulant, did not enhance VLA-4 tethering or rolling on VCAM-1, but rather, converted weak VLA-4 tethers into firm interactions with VCAM-1 (Fig. 3 A and reference 32). VLA-4–VCAM-1 interactions are mediated under shear flow by preexistent high and low affinity VLA-4 subsets, but only the high affinity interactions can be selectively inhibited by soluble VLA-4 ligand 19. Interestingly, the majority of SDF-1–triggered rolling and transient tethers were not susceptible to soluble LDV-containing, fibronectin-derived peptide (Fig. 3 A), suggesting they were mediated by low affinity VLA-4 subsets. The small fraction of SDF-1–triggered tethers followed by cellular arrests on VCAM-1 was entirely inhibited by the soluble VLA-4 ligand, suggesting these arrests were mediated exclusively by the high affinity VLA-4 subset that was preexistent on intact PBTLs interacting with VCAM-1 alone (Fig. 3 A). Chemokine triggering of VLA-4 tethering therefore is not associated with de novo elevation of VLA-4 affinity to ligand. Indeed, PBTL exposure to bead-immobilized SDF-1 also did not increase VLA-4 affinity to soluble ligand, and when probed by measuring expression of the ligand-induced integrin epitope 15/7, triggered by increasing levels of monovalent VLA-4 ligand, namely, the fibronectin-derived LDV peptide (reference 18 and data not shown).
Figure 3.
Effect of chemokines on VLA-4-mediated rolling of PBTLs on VCAM-1. (A) Lymphocytes were perfused at a shear stress of 1 dyn/cm2 on VCAM-1 (3 μg/ml) coimmobilized with inactivated or intact SDF-1 (1 μg/ml). The frequency of different categories of tethers is depicted in stacked bars. The contribution of high affinity VLA-4 subsets on perfused lymphocytes to each category was assessed by selective blocking of these subsets with EILDVPST peptide (at 0.75 mM). Data shown is representative from one of four experiments using different donors. (B) Chemokine triggering of VLA-4–dependent capture events followed by rolling or immediate arrests of PBTLs on VCAM-1, determined at 1.5 dyn/cm2 on VCAM-1 (3 μg/ml) coimmobilized with inactive SDF-1 or different intact chemokines (each at 2 μg/ml). (C) Instantaneous velocities of representative PBTLs interacting with VCAM-1 (3 μg/ml) coimmobilized with either inactive SDF-1 or active SDF-1 or IP-10 (1 and 2 μg/ml, respectively). The instantaneous velocities are plotted from the downstream entry of each cell into the field.
Transient tethers to very low density ligand are quantal adhesive units and their kinetics of formation and dissociation provide key insights into receptor function at nascent adhesive contact sites 22 33. We first studied chemokine-modulation of these quantal adhesive units in a homogenous population of Jurkat cells expressing high levels of both VLA-4 and CXCR4 19 34. The majority of VLA-4 tethers of Jurkat cells dissociated from low VCAM-1 (18 sites/μm2) with first order dissociation kinetics (Fig. 4 A), with a k off independent of VCAM-1 density (not shown) suggesting that these tethers represented quantal VLA-4–VCAM-1 adhesive units. Identical VCAM-1 density coimmobilized with SDF-1 supported a threefold higher frequency of VLA-4 tethers, with lifetimes similar to spontaneous VLA-4 tethers to VCAM-1 (Fig. 4 A). Notably, the majority of VLA-4 tethers triggered by SDF-1 lasted <0.1 s, suggesting that the signaling event triggered by the immobilized chemokine must have operated on VLA-4 within this short time frame. In sharp contrast to SDF-1, PMA treatment of Jurkat did not enhance the frequency of VLA-4 tethers formed on VCAM-1, but dramatically prolonged the lifetime of these tethers (Fig. 4 B). Fresh PBTLs tethered transiently to medium or low density VCAM-1, respectively, at two to sixfold higher frequencies in the presence of coimmobilized SDF-1 (Fig. 4 C). Similar to Jurkat T cells, the majority of the PBTLs dissociated from VCAM-1 with first order dissociation kinetics with a k off independent of VCAM-1 density (Fig. 4 C). The k off of chemokine-triggered tethers was comparable to that of spontaneous VLA-4–VCAM-1 tethers and the majority of tethers lasted <0.2 s, a time frame suitable for adhesive cellular contacts to support rolling adhesions 35. It is notable that higher VCAM-1 densities were required to promote transient VLA-4–dependent tethers in PBTLs than in Jurkat cells to VCAM-1 alone or to VCAM-1 coimmobilized with chemokine, consistent with the higher VLA-4 expression on the T cell line than on PBTLs 36. Nevertheless, transient VLA-4 tethers of Jurkat and PBTLs under a given shear stress had similar lifetimes (Fig. 4). Chemokine signaling therefore appeared to propagate within a similarly short time frame in both PBTLs and Jurkat T cells. Taken together, these dynamic studies also indicate that chemokines upregulate VLA-4 avidity to VCAM-1 through entirely different pathways than those implicated in VLA-4 stimulation by phorbol ester triggering of protein kinase C. In contrast to the numerous short-lived VLA-4–VCAM-1 tethers triggered by chemokines, which allow optimal rolling to take place on VCAM-1 under shear flow, phorbol ester stimulation of VLA-4 avidity prolongs tether duration without triggering new tethers (Fig. 3 and Fig. 4). This integrin agonist can therefore stabilize firm adhesion to VCAM-1, but fails to enhance tethering and rolling to the endothelial VLA-4 ligand under physiological flow.
Figure 4.

Immobilized chemokine and PMA modulate VLA-4 tether properties through distinct mechanisms. Kinetics of formation and dissociation of transient VLA-4–mediated tethers on very low density VCAM at a shear stress of 0.5 dyn/cm2. Effect of immobilized SDF-1 (A) or soluble PMA (B) on tether frequency and lifetime. (A) The duration of all tethers formed by equal number of Jurkat cells perfused for 1 min over sVCAM-1 (18 sites/μm2) alone or cocoated with SDF-1 at 2 μg/ml was determined, and the natural log of the tethers that remained bound after initiation of tethering was plotted against tether duration. (B) Effect of PMA treatment of Jurkat on the frequency and duration of Jurkat tethers to sVCAM-1 (36 sites/μm2). In A and B a first order dissociation fitting of tether duration is indicated by white symbols. Filled symbols denote longer tethers with high order dissociation kinetics. (C) Effect of immobilized SDF-1 and VCAM-1 density on frequency and duration of tethers formed by PBTLs interacting at a shear stress of 0.5 dyn/cm2 with the indicated densities of sVCAM-1 coimmobilized together with either inactivated or intact SDF-1 at 2 μg/ml. Least square analysis values of linear plots are depicted in r2 in A–C. Background tethering to HSA-coated substrate was 0.5% in A–C. Results are representative of three to four independent experiments.
The quantal VLA-4 tethers forming on VCAM-1 appeared to consist of multivalent integrin–VCAM-1 bonds, since their frequency of formation diminished below a threshold VCAM-1 density (180 and 10 sites/μm2 for PBTLs and Jurkat cells, respectively, at a shear stress of 0.5 dyn/cm2; Fig. 5 A). Tether frequency also increased by an order of magnitude with VCAM-1 dimerization (Grabovsky, V., S. Feigelson, R. Lobb, and R. Alon, manuscript in preparation), suggesting that immobilized chemokines may increase VLA-4 tethering by driving VLA-4 clustering at adhesive contact zones. Indeed, robust VLA-4 clustering could be triggered by surface-bound SDF-1, but not by PMA or soluble chemokine (Fig. 5 B and data not shown), even in the absence of VCAM-1. The failure of PMA to trigger VLA-4 clustering appeared to be a specific property of this integrin, because similar PMA treatment of freshly isolated PBTLs induces LFA-1 clustering 37. The ability of a surface-bound chemokine to induce VLA-4 clustering was a restricted process in that chemokine-coated beads did not induce clustering of a nonintegrin adhesion receptor, L-selectin, under similar conditions (Grabovsky, V., S. Feigelson, R. Lobb, and R. Alon, manuscript in preparation). To further demonstrate that chemokines can trigger VLA-4–mediated tethering independent of conformational alterations in VLA-4 structure, leading to enhanced affinity to ligand, SDF-1 was coimmobilized with a VLA-4–specific mAb coated at a density below that needed to capture VLA-4–expressing cells from the flow 19. PBTL tethering to the VLA-4 mAb was enhanced by up to fourfold by coimmobilized SDF-1 (Fig. 5 C) as well as by other chemokines including SLC and ELC (data not shown), but not by the SDF-1 mutant, P2G (Fig. 5 C). This and the ability of PTX-pretreatment of PBTLs to eliminate chemokine-triggered tethering to VLA-4–specific mAb (Fig. 5 C) are consistent with a GPCR-mediated signaling event triggering the rapid VLA-4 clustering that takes place on the surface of the PBTLs tethered to the VLA-4–specific mAb. Chemokine triggering of PBTL tethering to VLA-4 mAb occurred within <1 s, and was not seen with mAbs to PBTL adhesion receptors such as L-selectin (Fig. 5 C). Consistent with its inability to induce VLA-4 tethering to VCAM-1 (Fig. 4 B and 5 B), PMA lacked any augmenting effect on lymphocyte tethering to α4-specific mAb (Fig. 5 C). These results support the idea that chemokine induction of VLA-4–mediated lymphocyte tethering involves subsecond alteration of integrin clustering at the cell-substrate contact site, which can not be recapitulated by PMA activation. Interestingly, immobilized SDF-1 could also augment transient PBTL tethering to mAbs directed against its cognate receptor, CXCR4 (Fig. 5 C), suggesting that chemokine receptors may also get clustered upon lymphocyte contact with their immobilized ligands.
Figure 5.
Immobilized chemokine induces rapid clustering of VLA-4 at adhesive contact zones. (A) Effect of VCAM-1 density on frequency of PBTL tethering, measured at a shear stress of 0.5 dyn/cm2. All tethers measured on the indicated VCAM-1 densities were transient and diminished below a threshold VCAM-1 density (180 sites/μm2). (B) Confocal microscopy analysis of immunofluorescence staining of VLA-4 on PBTLs briefly incubated with control (HSA-coated) or SDF-1–coated beads (control and SDF-1, respectively), washed, fixed, and stained with the nonblocking VLA-4–specific mAb, B5G10. For PMA stimulation, cells were incubated with PMA for 2 min before VLA-4 staining. (C) Microclustering of VLA-4 and CXCR4 is enhanced within subsecond contact of PBTLs with surface-bound mAbs in the presence of immobilized SDF-1 leading to increased lymphocyte tethering to the surface. Tethering frequency of PBTLs measured at 0.75 dyn/cm2 to the VLA-4–, CXCR4-, or L-selectin–specific mAbs (HP1/2, 12G5, and DREG200, respectively), coated onto the substrates at 0.2 μg/ml, together with inactive SDF-1 (−), intact SDF-1, or the P2G SDF-1 mutant (+), or the control chemokine ELC, each at 2 μg/ml. The frequency of transient tethers and of tethers resulting in immediate arrests is depicted. The majority of transient tethers lasted <1 s. The non-PBTL binding mAb 4B9 (anti–VCAM-1) served as negative control. SDF-1–dependent augmentation in total tethering to anti–VLA-4 mAb coimmobilized with intact SDF-1 was significant compared with tethering measured in the presence of P2G (n = 4, P < 0.01). PTX pretreatment of PBTLs abolished 90 ± 5% of SDF-1–triggered PBTL tethering to the anti–VLA-4 mAb HP1/2 coimmobilized with intact SDF-1. (D) Effect of inhibitors to major integrin or GPCR signaling effectors on SDF-1–triggered tethering to VCAM-1. PBTLs were preincubated for 30 min with the PTK inhibitor (genestein; 100 μM), the PI-3K inhibitor (wortmannin; 100 nM), or with control DMSO solution (0.1%). VLA-4–dependent tethers were determined at 1 dyn/cm2 on VCAM-1 (1.5 μg/ml) coimmobilized with inactive or active SDF-1 (2 μg/ml, left). The effect of Ca2+ chelation on chemokine augmentation of VLA-4 tethering was tested on VCAM-1 (2 μg/ml) coimmobilized with SDF-1 (2 μg/ml) at shear stress of 1.5 dyn/cm2 (right). To chelate [Ca2+], PBTLs were preloaded with BAPTA-AM (at 25 μM) or control DMSO solution as described in Materials and Methods. The experiments shown in A–D are each representative of four independent experiments.

Once arrested on endothelium, adherent leukocytes not only upregulate avidity of other integrins like LFA-1 in response to endothelial chemokines 10, but may respond to additional chemokine signals which activate Rho family GTPases and recruit integrin avidity modulators to the plasma membrane 38 39 40. However, these events are unlikely to take place within subseconds of cell contact with immobilized chemokine under shear flow. Indeed, chemokine-triggered VLA-4 tethering was not susceptible to inhibitors of protein tyrosine kinase or to blockers of PI-3 kinase activity, implicated in GPCR signaling (Fig. 5 D). Ca2+ mobilization by chemokines, a hallmark of chemokine signaling, was also found unnecessary for chemokine-triggered VLA-4 tethering to VCAM-1, since chelation of intracellular Ca2+ did not abrogate chemokine-triggered VLA-4 tethering or arrest on VCAM-1 under flow (Fig. 5 D). These results suggest that chemokine signaling to leukocyte integrins under shear flow, although Gi-protein dependent, is not only exceptionally faster than that which occurs during chemokine-triggered cytoskeleton remodeling and cell chemotaxis 34 41 42, but is likely to involve effector molecules distinct from those regulating cell spreading and motility 43.
Taken together, our results suggest that endothelium-displayed, but not soluble, chemokines trigger leukocyte VLA-4 tethers by rapidly increasing the effective concentration of VLA-4 within VCAM-1–containing adhesive contact sites of tethered leukocytes. As VCAM-1 is a relatively inefficient tethering ligand and supports leukocyte tethering at threshold densities 10–100-fold higher than that of selectin-mediated tethering 44, chemokines may be required to rapidly cluster VLA-4 receptors and thereby to lower the threshold VCAM-1 density necessary for tether formation under shear flow. This uniquely fast GPCR modulation of VLA-4 avidity allows the participation of the integrin in reversible rolling interactions of tethered leukocytes. Tethering is followed by establishment of stationary, firm, VLA-4–dependent adhesion to endothelial VCAM-1 and subsequent GPCR modulation of additional integrin interactions at the site of final leukocyte arrest. Thus, rolling and arrest appear to be distinct quantitative manifestations of chemokine-triggered tether formation; rolling is supported by sequential fast formation and breaking of singular tethers at continuously translated contact zones, whereas arrest is probably promoted by a high number of simultaneously formed tethers at a single contact site.
Integrin adhesiveness is often induced by alterations of surface clustering without changes in affinity to ligand 45 46 47 48. Chemokines rapidly increase the avidity of leukocyte integrins such as α4β7, LFA-1, and Mac-1 5 10 49 50, but it is not clear whether avidity modulation of these integrins involves clustering, alterations in affinity, or both. The study presented here suggests that chemokines alter VLA-4 avidity in lymphocytes without altering the intrinsic affinity state of the integrin or the lifetime of its tether bonds. The ability of selectins to mediate tethering and rolling has been attributed to high rates of bond formation and dissociation under flow as well as to mechanical stability of tether bonds 44. Likewise, chemokine-stimulated formation of fast breaking VLA-4 tethers to VCAM-1 appear to fulfill these conditions. In contrast to VLA-4, chemokine-triggered avidity of LFA-1 or Mac-1 enhances only firm leukocyte adhesion to endothelial ligands without augmenting tethering or rolling 10 49. Thus, the ability of chemokines to augment VLA-4–mediated rolling appears to depend on the intrinsic ability of this integrin to engage in reversible tethers with its endothelial ligand VCAM-1 under shear flow even in the absence of chemokine stimulation 32 51. Since the VLA-4 homologue, α4β7, can engage in rolling tethers with its mucosal ligand MadCAM-1 51, it is possible that α4β7-mediated tethering and rolling of lymphocyte subsets within mucosal vascular beds are also upregulated by endothelial chemokines 50.
α4 integrins, but not LFA-1 or Mac-1, are predominately expressed on leukocyte-microvilli, preferential sites of leukocyte–endothelial contacts under shear flow 52 53. It is possible that proximity between GPCR and α4 integrins at these surface projections may facilitate the subsecond coupling of GPCR signals to modulation of integrin clustering at adhesive contact sites under shear flow. Chemokine-triggered VLA-4 avidity may also require the segregation of the GPCR and integrin to specific lipid microdomains, enriched with signaling molecules implicated in integrin function 54. Our results indicate that SDF-1 efficiently triggers VLA-4 avidity both in PBTLs and Jurkat cells, in spite of the different VLA-4 levels and adhesive activity in these cell types. This suggests that the type and expression level of the GPCRs, rather than the cell type upon which VLA-4 is expressed or the activation state of the integrin, dictates the ability of that GPCR to enhance VLA-4 tethering under shear flow. Indeed, a wide spectrum of VLA-4 activity states in distinct subsets of PBTLs, lymphoblastoid Jurkat cells, and CD34+ HPCs can all undergo subsecond avidity changes in response to chemokine signaling through respective GPCRs.
The ability of VLA-4 to form fast breaking bonds within milliseconds at leukocyte-endothelium contact sites suggests that rather than a buildup of Gi protein signals transmitted to the rolling leukocytes 55, individual VLA-4 clustering events are independently triggered at multiple leukocyte–EC contact sites. This rapid chemokine stimulation of integrin activity under shear flow introduces a new regulatory step of lymphocyte adherence to vascular endothelium; rather than functioning subsequent to leukocyte tethering to vascular endothelium, we show for the first time that surface-bound chemokines can signal to and modulate the activity of a leukocyte integrin during its initial contact with its vascular ligand. The demonstration that chemokines displayed on the endothelium can modulate integrin activity during the very early phases of adhesive cascades between leukocytes and endothelium suggests that these chemoattractants play a far more versatile role in regulating leukocyte trafficking to target endothelial sites than previously was realized.
Acknowledgments
We wish to thank Drs. T. Yednock and T. Kishimoto for kindly providing reagents; S. Franitza and O. Dwir for assistance in PBTL isolation and VCAM-1 density determinations; and Dr. S. Shwarzbaum for editorial assistance. Special thanks to Drs. I. Pecht and R. Seger for helpful discussions.
R. Alon is the Incumbent of The Tauro Career Development Chair in Biomedical Research. Parts of this work were supported by the Israel Science Foundation, the Minnerva foundation, Germany, and the Crown Endowment Fund (to R. Alon).
Footnotes
Abbreviations used in this paper: CHO, Chinese hamster ovary; EC, endothelial cell; ECL, EBI1 ligand chemokine; GPCRs, Gi protein–coupled receptors; HPC, hematopoietic progenitor cell; HSA, human serum albumin; HUVEC, human umbilical cord endothelial cell; IP-10, IFN-γ–inducible protein 10; PBTL, peripheral blood T lymphocyte; PTX, pertussis toxin; RANTES, regulated on activation, normal T cell expressed and secreted; SDF-1, stromal derived factor 1; SLC, secondary lymphoid tissue chemokine; VCAM, vascular cell adhesion molecule; VLA-4, very late antigen 4.
References
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Broxmeyer H.E. Chemokinessignal lamps for trafficking of T and B cells for development and effector function. J. Leukoc. Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann K., Tam C., Cyster J.G., Rosen S.D., Williams L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D.P., Warnke R., Ruffing N., Kassam N. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Peled A., Grabovsky V., Habler L., Sandbank J., Arenzana-Seisdedos F., Petit I., Ben-Hur H., Lapidot T., Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargatze R.F., Butcher E.C. Rapid G protein–regulated activation event involved in lymphocyte binding to high endothelial venules. J. Exp. Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Qin S., Bacon K.B., Mackay C.R., Butcher E.C. Biology of chemokine and classical chemoattractant receptorsdifferential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J.Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M.B., Springer T.A. Leukocytes roll on a selectin at physiologic flow ratesdistinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Hedrick J., Zlotnik A., Siani M.A., Thompson D.A. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Warnock R.A., Askari S., Butcher E.C., von Andrian U.H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R.R., Hemler M.E. The pathophysiologic role of alpha 4 integrins in vivo. J. Clin. Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo I.B., von Andrian U.H. Adhesion and homing of blood-borne cells in bone marrow microvessels. J. Leukoc. Biol. 1999;66:25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- Hemler M.E., Huang C., Takada Y., Schwarz L., Strominger J.L., Clabby M.L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J. Biol. Chem. 1987;262:11478–11485. [PubMed] [Google Scholar]
- Lobb R.R., Antognetti G., Pepinsky R.B., Burkly L.C., Leone D.R., Whitty A. A direct binding assay for the vascular cell adhesion molecule-1 (VCAM1) interaction with alpha 4 integrins. Cell Adhes. Commun. 1995;3:385–397. doi: 10.3109/15419069509081293. [DOI] [PubMed] [Google Scholar]
- Crump M.P., Gong J.H., Loetscher P., Rajarathnam K., Amara A., Arenzana-Seisdedos F., Virelizier J.L., Baggiolini M., Sykes B.D., Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M.W., Alon R., Springer T.A. The C-C chemokine MCP-1 differentially modulates the avidity of beta 1 and beta 2 integrins on T lymphocytes. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- Yednock T.A., Cannon C., Vandevert C., Goldbach E.G., Shaw G., Ellis D.K., Liaw C., Fritz L.C., Tanner L.I. Alpha 4 beta 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J. Biol. Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- Chen C., Mobley J.L., Dwir O., Shimron F., Grabovsky V., Lobb R.L., Shimizu Y., Alon R. High affinity VLA-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J. Immunol. 1999;162:1084–1095. [PubMed] [Google Scholar]
- Lub M., van Vliet S.J., Oomen S.P., Pieters R.A., Robinson M., Figdor C.G., van Kooyk Y. Cytoplasmic tails of beta 1, beta 2, and beta 7 integrins differentially regulate LFA-1 function in K562 cells. Mol. Biol. Cell. 1997;8:719–828. doi: 10.1091/mbc.8.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R., Chi-Rosso G., Leone D., Rosa M., Newman B., Luhowskyj S., Osborn L., Schiffer S., Benjamin C., Dougas I. Expression and functional characterization of a soluble form of vascular cell adhesion molecule 1. Biochem. Biophys. Res. Commun. 1991;178:1498–1504. doi: 10.1016/0006-291x(91)91063-i. [DOI] [PubMed] [Google Scholar]
- Alon R., Hammer D.A., Springer T.A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Marfaing-Koka A., Devergne O., Gorgone G., Portier A., Schall T.J., Galanaud P., Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J. Immunol. 1995;154:1870–1878. [PubMed] [Google Scholar]
- Bleul C.C., Fuhlbrigge R.C., Casasnovas J.M., Aiuti A., Springer T.A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Schubel A., Breitfeld D., Kremmer E., Renner-Muller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Uguccioni M., Bordoli L., Baggiolini M., Moser B. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- Fong A.M., Robinson L.A., Steeber D.A., Tedder T.F., Yoshie O., Imai T., Patel D.D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J. Exp. Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Alon R., Moser B., Springer T.A. Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by CC chemokines in monocytesimplications for transendothelial chemotaxis. J. Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant D.E., Patel K.D., McIntyre T.M., McEver R.P., Prescott S.M., Zimmerman G.A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombina juxtacrine system for adhesion and activation of neutrophils. J. Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Adams D.H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Middleton J., Neil S., Wintle J., Clark-Lewis I., Moore H., Lam C., Auer M., Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Alon R., Kassner P.D., Carr M.W., Finger E.B., Hemler M.E., Springer T.A. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Benoliel A.M., Bongrand P. Measuring the lifetime of bonds made between surface-linked molecules. J. Biol. Chem. 1995;270:26586–26592. doi: 10.1074/jbc.270.44.26586. [DOI] [PubMed] [Google Scholar]
- Sotsios Y., Whittaker G.C., Westwick J., Ward S.G. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J. Immunol. 1999;163:5954–5963. [PubMed] [Google Scholar]
- Chen S., Springer T.A. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 1999;144:185–200. doi: 10.1083/jcb.144.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley J.L., Ennis E., Shimizu Y. Isolation and characterization of cell lines with genetically distinct mutations downstream of protein kinase C that result in defective activation-dependent regulation of T cell integrin function. J. Immunol. 1996;156:948–956. [PubMed] [Google Scholar]
- Lub M., van Kooyk Y., van Vliet S.J., Figdor C.G. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol. Biol. Cell. 1997;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C., Campbell J.J., Butcher E.C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Laudanna C., Mochly-Rosen D., Liron T., Constantin G., Butcher E.C. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J. Biol. Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- Del Pozo M.A., Vicente-Manzanares M., Tejedor R., Serrador J.M., Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur. J. Immunol. 1999;29:3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ganju R.K., Brubaker S.A., Meyer J., Dutt P., Yang Y., Qin S., Newman W., Groopman J.E. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J. Biol. Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., del Pozo M.A. Leukocyte polarization in cell migration and immune interactions. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G., Weiner O.D., Herzmark P., Balla T., Sedat J.W., Bourne H.R. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R., Chen S., Puri K.D., Finger E.B., Springer T.A. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J. Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov Y.N., Juliano R.L. Phorbol ester modulation of integrin-mediated cell adhesiona post receptor event. J. Cell Biol. 1989;108:1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faull R.J., Kovach N.L., Harlan J.M., Ginsberg M.H. Affinity modulation of integrin alpha 5 beta 1regulation of the functional response by soluble fibronectin. J. Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski A., Rosa M.D., Bixler S., Lobb R., Burkly L.C. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes. Commun. 1995;3:131–142. doi: 10.3109/15419069509081282. [DOI] [PubMed] [Google Scholar]
- Stewart M.P., Cabanas C., Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J. Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- Weber C., Katayama J., Springer T.A. Differential regulation of beta 1 and beta 2 integrin avidity by chemoattractants in eosinophils. Proc. Natl. Acad. Sci. USA. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachynski R.K., Wu S.W., Gunn M.D., Erle D.J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin alpha 4 beta 7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 1998;161:952–956. [PubMed] [Google Scholar]
- Berlin C., Bargatze R.F., Campbell J.J., von Andrian U.H., Szabo M.C., Hasslen S.R., Nelson R.D., Berg E.L., Erlandsen S.L., Butcher E.C. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Hasslen S.R., Nelson R.D., Erlandsen S.L., Butcher E.C. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Abitorabi M.A., Pachynski R.K., Ferrando R.E., Tidswell M., Erle D.J. Presentation of integrins on leukocyte microvillia role for the extracellular domain in determining membrane localization. J. Cell Biol. 1997;139:563–571. doi: 10.1083/jcb.139.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M.E. Integrin associated proteins. Curr. Opin. Cell. Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Kunkel E.J., Chomas J.E., Ley K. Role of primary and secondary capture for leukocyte accumulation in vivo. Circ. Res. 1998;82:30–38. doi: 10.1161/01.res.82.1.30. [DOI] [PubMed] [Google Scholar]




