Abstract
Increasing evidence indicates that dendritic cells (DCs) are the antigen-presenting cells of the primary immune response. However, several reports suggest that B lymphocytes could be required for optimal T cell sensitization. We compared the immune responses of wild-type and B cell-deficient (μMT) mice, induced by antigen emulsified in adjuvant or pulsed on splenic dendritic cells. Our data show that lymph node cells from both control and μMT animals were primed, but each released distinct cytokine profiles. Lymph node T cells from control animals secreted interferon (IFN)-γ, interleukin (IL)-2, and IL-4, whereas those from μMT mice produced IFN-γ and IL-2 but no IL-4. To test whether B cells may influence the T helper cell type 1 (Th1)/Th2 balance by affecting the function of DCs, we immunized mice by transferring antigen-pulsed DCs from wild-type or mutant mice. Injection of control DCs induced the secretion of IL-4, IFN-γ, and IL-2, whereas administration of DCs from μMT animals failed to sensitize cells to produce IL-4. Analysis of IL-12 production revealed that DCs from μMT mice produce higher levels of IL-12p70 than do DCs from wild-type animals. These data suggest that B lymphocytes regulate the capacity of DCs to promote IL-4 secretion, possibly by downregulating their secretion of IL-12, thereby favoring the induction of a nonpolarized immune response.
Keywords: T helper cell type 1/type 2 balance, primary response, interleukin 4, interleukin 10, dendritic–B cell interaction
Introduction
The specificity, amplitude, and character of an immune response are determined early on, at the stage of antigen presentation. Among the population of APCs, which includes dendritic cells (DCs), B lymphocytes, and macrophages, DCs have the unique capacity to sensitize naive T cells (for review see reference 1) and are considered the sentinels that switch on the immune system at the appropriate “danger” signal. This property correlates with several unique features, including high expression of MHC and costimulatory molecules, motility, efficient antigen capture and processing, specialization of function over time, production of T cell–activating cytokines, etc.
Injection of splenic DCs, pulsed extracorporeally with antigen, induces the development of Th cells secreting a large array of cytokines (IL-2, IFN-γ, IL-4, IL-5, and IL-10) in syngeneic animals. The capacity of DCs to direct the development of selected Th populations has been shown to be modulated by pathogens, cytokines, and other environmental factors 2. In particular, we have shown that DCs may induce the development of Th0 (or Th1 and Th2) lymphocytes in a neutral environment, and the differentiation of a polarized Th1 or Th2 population in the presence of IL-12 or IL-10, respectively 3.
The role of B cells (which represent the most abundant APC population) in T cell priming is still controversial. Several studies have revealed a critical role for B cells in the T cell response in vivo 4 5 6. These results were challenged by reports showing that the absence of B cells had little impact on T cell responsiveness 7 8. More recently, a few studies have suggested that B cells are not required for T cell sensitization but play an essential role in the induction of IL-4 gene expression by T lymphocytes 9 10.
In this study, we compared the development of antigen-specific responses in B cell–deficient and wild-type mice. Our data demonstrate that the development of IL-4–secreting cells was impaired in the absence of B lymphocytes. We further determined that DCs from B cell–deficient mice had a reduced capacity to induce IL-4 production. These observations suggest that B lymphocytes regulate the Th1/Th2 polarized effector function of DCs in vivo.
Materials and Methods
Mice.
C57BL/10, C57BL/6, C57BL/6, and 10-Igh-6tm1Cgn (B cell–deficient, or μMT, mice; reference 11) and C57BL/10-Il10tm1Cgn (IL-10–deficient; reference 12) mice were purchased from The Jackson Laboratory. Some C57BL/6 mice were purchased from Charles River Laboratories. All animals were maintained in our pathogen-free facility and used at 8–12 wk of age.
Culture Media.
The medium used for the isolation of DCs was RPMI 1640 (Seromed; Biochem KG) supplemented with 2% HY (Ultroser HY; Life Technologies) and additives. Lymph node cells from mice injected with KLH in CFA or KLH-pulsed DCs were cultured in Click's medium (Irvin Scientific) supplemented with 0.5% heat-inactivated FCS or mouse serum, respectively, and additives.
Antigen, Antibodies, and Cytokines.
The antigen used was KLH from Calbiochem-Novabiochem. The following antibodies were used in this study: anti-Thy 1.2 (HO134; American Type Culture Collection, or ATCC), anti-I-Ab (25.9.17; ATCC), anti–heat stable antigen (anti-HSA; ATCC), anti-FcγR (2.4G2; ATCC), anti-CD45R/B220 (RA3-6B2; PharMingen), rat anti–mouse IgG2a (LO-MG2a-7; provided by H. Bazin, Université Catholique de Louvain, Brussels, Belgium), 5D9 and 5C3 (rat anti–IL-12 p40; provided by Dr. Presky, Hoffmann-LaRoche, Nutley, NJ), and C17.8 (rat IgG2a anti–IL-12 p40). Murine recombinant IL-10 was purchased from PeproTech.
Purification of DCs.
DCs were purified from spleens as shown previously 13, except that spleen cells were digested with collagenase, further dissociated in Ca2+-free media in the presence of EDTA, separated into low and high density fractions on a Nycodenz gradient, and cultured overnight with KLH (50 μg/ml). In some experiments, DCs were incubated with 20 ng/ml murine recombinant IL-10. After overnight culture, nonadherent cells contained at least 90% of DCs, as assessed by morphology and specific staining, using anti-CD11c mAb, N418 14.
Injections.
DCs were purified from either untreated animals or mice treated with FLT3L, as indicated in the legend to Fig. 2 and Fig. 3, and to Fig. 4 D. For FLT3L treatment, mice were injected intraperitoneally with 10 μg of recombinant human FLT3L daily for 9 d. The immunization protocols were as follows: KLH (100 μg) in CFA or KLH-pulsed DCs (administered at a dose of 3 × 105 cells) were injected into the fore and hind footpads, according to a protocol described by Inaba et al. 15. Draining popliteal and axillary lymph nodes were harvested 5 or 6 d after injection, as indicated in the legend to Fig. 1, Fig. 2, Fig. 4 D, and 5.
Figure 2.
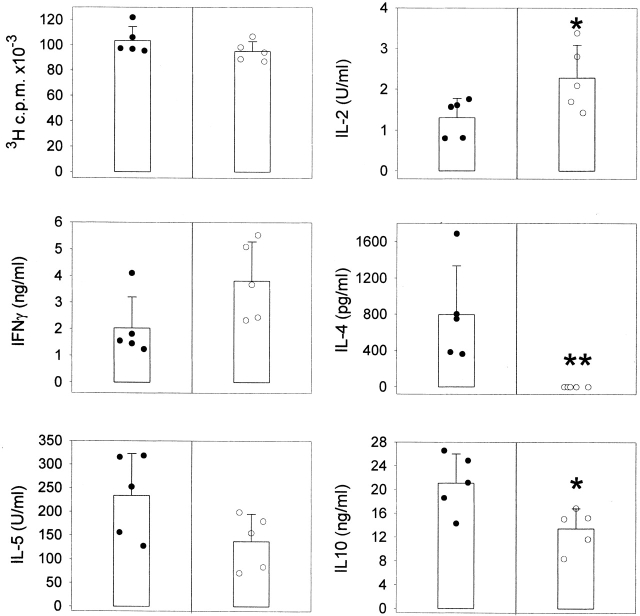
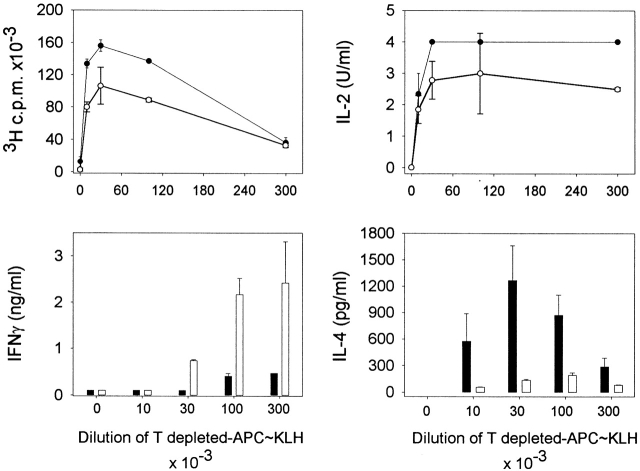
DCs from μMT mice do not prime for IL-4. C57BL/10J were injected with KLH-pulsed DCs from FLT3L-treated C57BL/10J (black symbols) or μMT (white symbols) mice into the hind and fore footpads. Lymph node cells were harvested 6 d later and cultured with KLH (5 μg/ml). Proliferative responses and cytokine secretion were measured as indicated in Materials and Methods. Each symbol represents the response of a single mouse and vertical bars are the mean results of five mice of each type (± SD). Five independent experiments were performed with similar results using DCs from untreated or FLT3L-treated mice. Limits of detection: for IL-2, 0.16 U/ml; IL-4 and IL-5, 62.5 pg/ml; IFN-γ, 0.3 ng/ml; IL-10, 1 ng/ml. The groups indicated by single and double asterisks are significantly different from control (Student's t test, P < 0.05 and 0.01, respectively; n = 5).
Figure 3.
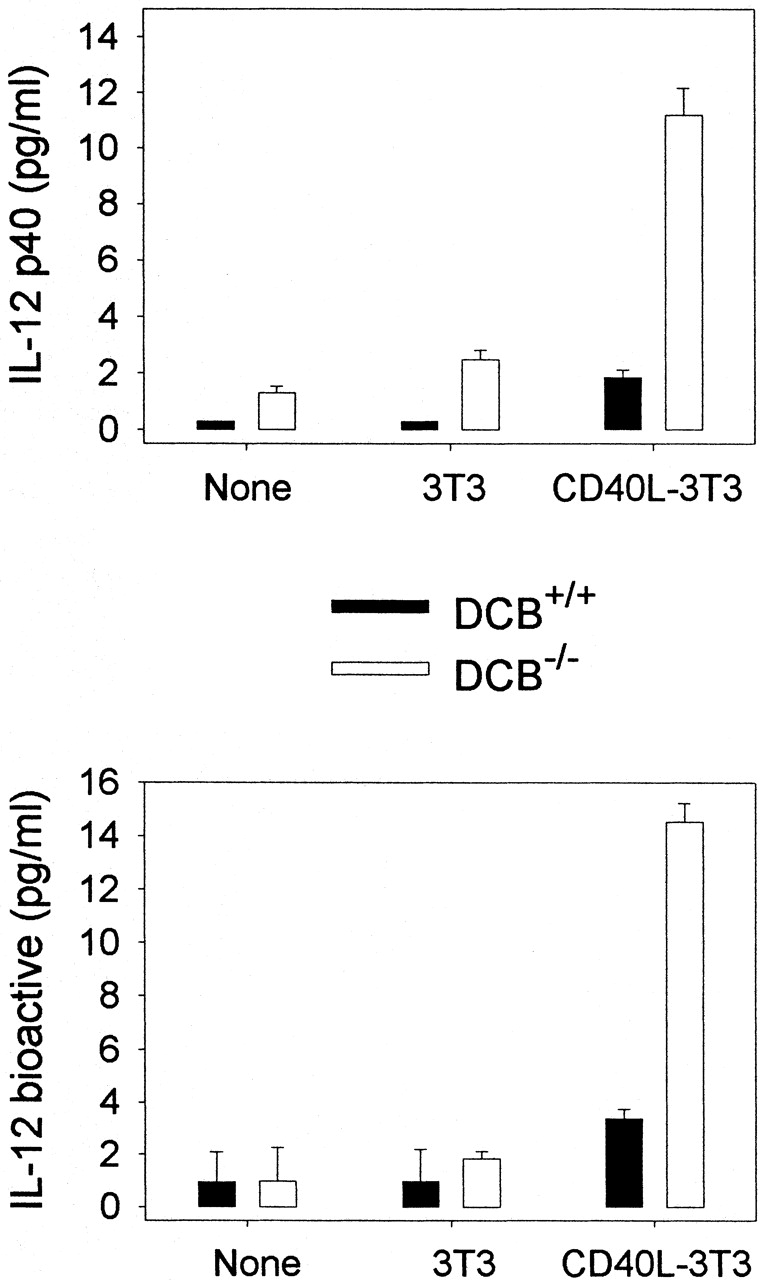
Production of IL-12 by DCs. DCs purified from untreated WT (DCB+/+) or μMT (DCB−/−) mice were cultured with medium alone (None) or with fibroblasts transfected (CD40L-3T3) or not (3T3) with CD40L. 48-h and 72-h supernatants were tested for IL-12 p40 and p70 content, respectively. Limits of detection: for p40, 0.3 pg/ml; p70, 0.13 pg/ml. Data shown are the mean ± SD of duplicate cultures. Three independent experiments were performed with similar results, using DCs purified from untreated or FLT3L-treated mice.
Figure 4.
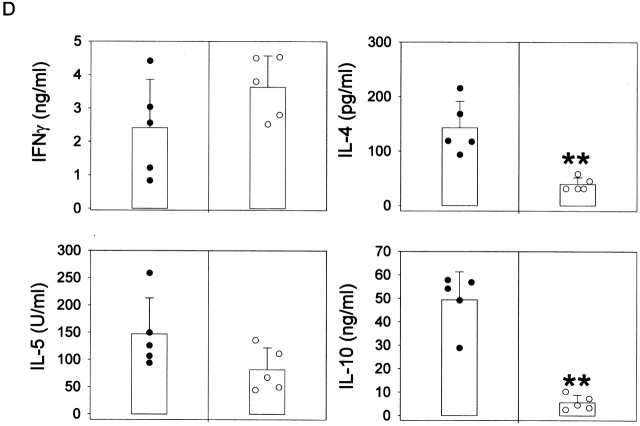
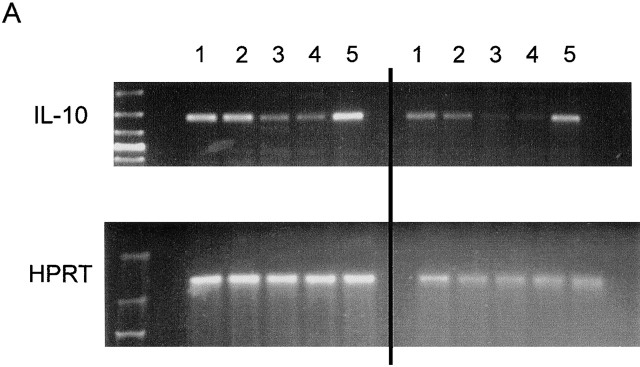
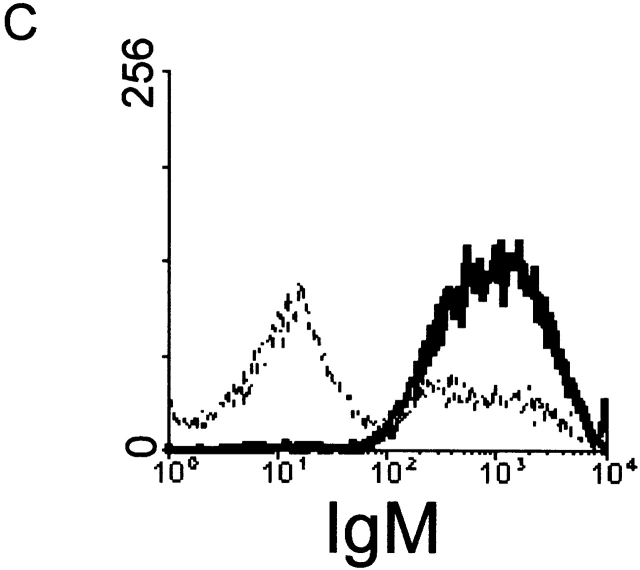
Role of IL-10. (A) RT-PCR analysis of IL-10 expression was performed in spleen cells from untreated wild-type (lanes 1 and 2) and μMT (lanes 3 and 4) mice, using IL-10 and HPRT primers. Anti-CD3–injected mice were used as positive control (lane 5). Left and right sides represent the amplification by PCR of 3λ and 1λ cDNA, respectively. Similar data were obtained in three independent experiments. (B) RT-PCR analysis of IL-10 expression was performed in unseparated spleen cells (lane 1) and splenic CD19+ cell population (lane 2), which contains 98% B lymphocytes as assessed by FACS™ analysis for IgM expression (C). The T cell hybridoma 3B4.15 was used as negative control (panel B, lane 3). (D) DCs were purified from C57BL/10 (black symbols) or IL-10 knockout (white symbols) mice that were treated for 9 d consecutively with FLT3L. KLH-pulsed DCs were injected into the hind and fore footpads of wild-type recipient animals. Lymph nodes were harvested 6 d later and cultured with KLH (5 μg/ml). The supernatants were collected and cytokine activities were determined by ELISA. Detection limits: for IFN-γ, 0.3 ng/ml; IL-4, 31.3 pg/ml; IL-5, 31.3 U/ml; IL-10, 1 ng/ml. Each symbol represents production of cytokine of individual animals, and vertical bars are the mean results of five mice. Two independent experiments were performed with similar results. The groups indicated by the double asterisks are significantly different from control (Student's t test, P < 0.01, n = 5).
Figure 1.
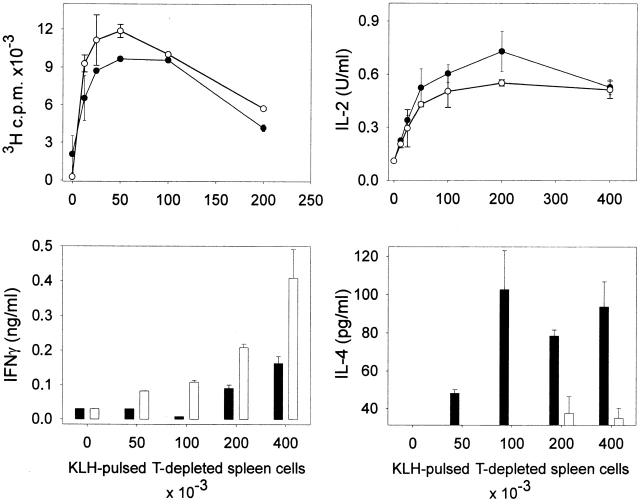
Responses of T cells in μMT and C57BL/6 mice to KLH. Wild-type (black symbols) or μMT (white symbols) mice were injected into the footpads with 100 μg KLH emulsified in CFA. Lymph nodes were harvested 5 d later and purified T cells (2 × 105/well) were incubated with graded numbers of irradiated, T-depleted spleen cells from C57BL/6 mice that had been pulsed with KLH (100 μg/ml). Proliferation and cytokine production were measured as indicated in Materials and Methods. Limits of detection: for IL-2, 0.16 U/ml; for IL-4, 31.25 pg/ml; for IFN-γ, 0.03 ng/ml. The data are representative of four independent experiments using pooled T cells from five C57BL/6 or μMT mice and shown as the mean of duplicate cultures ± SD.
FACS™ Analysis.
Low density spleen cells (see above) were double stained for CD11c expression using FITC-conjugated N418 and for CD8α expression using PE-conjugated anti-CD8α mAb (PharMingen). The cells were gated based on characteristic forward and light scatter, and analyzed on a FACSort™ (Becton Dickinson).
In Vitro Assays.
Unseparated lymph node cells (4 × 105) were cultured in graded doses of KLH. In some experiments, 2 × 105 purified T cells were incubated with graded doses of irradiated, KLH-pulsed spleen cells depleted of T lymphocytes by treatment with anti-Thy1.2 mAb and rabbit complement (BioMérieux s.a.). Lymph node T cells were purified by negative selection: in brief, pooled lymph node cells were incubated with anti–I-Ab (25-917), anti-HSA, anti-FcγR, anti-CD45R/B220, and rat anti–mouse IgG2a mAbs at 4°C, followed by goat anti–rat IgG-coated beads (BioMag; PerSeptive Biosystems) and further passed over a magnet. Reanalysis of the selected population confirmed purity of >95%. Proliferation was measured as thymidine incorporation during the last 16 h of a 3-d culture. Culture supernatants were assayed for IL-2 after 24 h of incubation, for IL-4 after 48 h, for IFN-γ after 72 h, and IL-5 and IL-10 after 96 h. IL-2 and IFN-γ were measured as previously described 3. IL-4, IL-5, and IL-10 were quantified by two-site ELISA from PharMingen.
PCR Analysis of IL-10 Gene Expression.
RNA was extracted from splenocytes from untreated C57BL/6, μMT, or anti-CD3–injected C57BL/6 mice, from B cell-enriched CD19+ C57BL/6 spleen cells, and from T cell hybridoma 3B14.15 using the TriPure reagents (Boehringer). The CD19+ cells were enriched from spleen cells by incubation with anti-CD19–coupled microbeads and positive selection over a MACS column (Miltenyi Biotec). After preparation of cDNA, PCR was performed essentially as previously described 16. Reactions were incubated in a PerkinElmer/Cetus DNA thermal cycler for 30 cycles for IL-10 gene expression and 24 cycles for housekeeping HPRT gene expression (denaturation: 30 s, 94°C; annealing: 1 min, 57°C; extension: 1 min, 72°C). Primers used were as follows: IL-10 sense primers 5′-TCAAACAAAGGACCAGCTGGACAACATACTGC-3′ and antisense 5′-CTGTCTAGGTCCTGGAGTCCAGCAGACTCAA-3′ (amplified fragment of 421 bp). HPRT sense primers: 5′-GTTGGTATACAGGCCAGACTTTGTTG-3′ and antisense 5′-GAATTTCAACTTGCGCTCATCTTAGGC-3′ (amplified fragment of 163 bp).
Production of IL-12 by DCs.
Purified DCs (see above) were cultured in medium alone (RPMI 1640 supplemented with 5% FCS) or in the presence of 3T3 fibroblasts transfected or not with CD40L. The supernatants were assayed for IL-12 p40 after 48 h using a two-site ELISA 3. IL-12 p70 production was monitored on 72 h supernatants by a bioassay based on the ability of IL-12 to induce IFN-γ production by spleen cells. In brief, culture supernatants were added to 5 × 105 spleen cells and incubated for 72 h. IFN-γ content from culture supernatants was assayed using two-site ELISA, as described above. A linear standard curve was generated by incubating spleen cells with serial dilutions of recombinant murine IL-12. Specificity of bioassay was confirmed by inhibiting IFN-γ production with neutralizing anti–IL-12 p40 mAb (C17.8, rat IgG2a).
Results
Responses of T Cells in μMT and B6 Mice to KLH.
We first compared the immune response of mice that are genetically deficient for B lymphocytes (μMT mice) and their control littermates. μMT and wild-type animals were injected in the footpads with KLH emulsified in CFA and the draining lymph nodes were harvested 5 d later. The data in Fig. 1 indicate that KLH-specific T cells were primed in both groups of mice, as assessed by KLH-dependent proliferation in culture. Of note, the analysis of the cytokines released by lymph node cells revealed a differential T helper development in μMT versus control mice. T lymphocytes from wild-type mice secreted IFN-γ and IL-4 when rechallenged with KLH in vitro, whereas T cells from μMT mice produced higher levels of IFN-γ but no detectable IL-4 in the same conditions. These observations indicate that B lymphocytes are dispensable for T cell priming but are required for the development of IL-4–secreting cells.
Impaired Ability of DCs from μMT Mice to Promote IL-4 Secretion.
There is increasing evidence that the cell that presents the antigen to T cell may influence the Th1/Th2 balance in vivo. In particular, injection of splenic DCs has been shown to induce the activation of T cells that secrete a large array of cytokines 3 17. Because DCs are the APCs of the primary immune response, we analyzed the adjuvant function of DCs purified from control and μMT mice. DCs were purified from spleens and pulsed with KLH during overnight culture, as previously described 17. Of note, expression of B7-1, B7-2, and CD40 molecules was similar on DCs from both strains of mice, whereas MHC class II was expressed at slightly higher levels on DCs from μMT animals (data not shown). 3 × 105 DCs were injected into the footpads of wild-type mice, and the lymph nodes were harvested 6 d later. The data in Fig. 2 show that DCs from wild-type and μMT mice differentially regulated the development of Th cells. Injection of DCs from μMT mice induced the differentiation of cells secreting IL-2, IFN-γ, IL-5, and IL-10, but no detectable IL-4, whereas DCs from control mice prime for IL-4 production in addition to other cytokines.
DCs from μMT Mice Produce Higher Levels of Bioactive IL-12.
Because IL-12 appears to be the dominant cytokine favoring the differentiation of Th1 cells over Th2 cells, we measured the production of IL-12 by DCs upon in vitro stimulation. The data in Fig. 3 show that DCs from μMT mice produced higher levels of IL-12 homodimer (p40) and heterodimer (p70) than did DCs from wild-type mice. This observation suggests that the lack of IL-4 priming upon immunization with DCs from B cell–deprived mice may result from increased production of IL-12 by transferred DCs. By contrast, DCs from wild-type mice release lower amounts of IL-12 and have the capacity to promote IFN-γ and IL-4 production upon adoptive transfer. We next compared the number of CD8α+ and CD8α− DCs in wild-type and μMT mice, since only the subset of DCs expressing CD8α has been shown to produce IL-12 18 19. The data in Table show that the proportion of DC subsets is similar in both strains of mice.
Table 1.
Analysis of DC Subsets
| Wild-type mice | μMT mice | |||
|---|---|---|---|---|
| CD8α2 | CD8α1 | CD8α2 | CD8α1 | |
| 1 | 64.8 | 35.2 | 67.8 | 32.2 |
| 2 | 60.0 | 40.0 | 75.0 | 25.0 |
| 3 | 80.6 | 19.4 | 78.1 | 21.9 |
| 4 | 83.0 | 17.0 | 70.7 | 29.3 |
Low density spleen cells from FLT3L-injected (1 and 2) or untreated (3 and 4) mice were double stained for CD8α expression using PE-conjugated anti-CD8α mAb and for CD11c expression using FITC-conjugated N418. The data represent the percentage of CD8α2 and CD8α1 cells among DCs (gated for N418 expression).
IL-10 Message Is Downregulated in Spleen Cells from μMT Mice.
IL-10 has been shown to downregulate IL-12 production, thereby favoring the onset of a Th2-type response. We measured the level of IL-10 mRNA in unstimulated spleen cells from both strains of mice. The data in Fig. 4 A indicate that IL-10 message was decreased in μMT mice, as compared with control animals, suggesting that B cells promote IL-10 secretion. Of note, splenic CD19+ lymphocytes (containing at least 98% B cells, as assessed by expression of IgM: Fig. 4 C) constitutively express mRNA specific for IL-10 (Fig. 4 B). To better define the role of IL-10 in the regulation of IL-12 secretion by DCs, we transferred DCs from IL-10–deficient mice into wild-type recipients. As shown in Fig. 4 D, DCs from IL-10 knockout animals induced an immune response characterized by the development of T cells secreting very low amounts of IL-4 and IL-10.
Role of the Microenvironment.
To test whether the microenvironment in which priming of T cells occurs may influence the character of the immune response, KLH-pulsed DCs from wild-type animals were transferred into control and B cell–deprived recipient mice. As shown in Fig. 5, immunization of μMT mice resulted in sensitization of cells producing high levels of IFN-γ and low levels of IL-4, whereas priming of wild-type mice induced lower IFN-γ and higher IL-4 production. Evidence that T cells from μMT mice have the capacity to produce IL-4 is provided by the observation that injection of Th2-prone, IL-10–treated DCs into μMT mice results in the development of IL-4–secreting cells (Table ).
Figure 5.
B cells from recipient animals are required for IL-4 priming. KLH-pulsed DCs from untreated C57BL/10 mice were injected into the hind and fore footpads of wild-type (black symbols) or μMT (white symbols) recipients. Lymph nodes were harvested 6 d later and purified T cells were cultured with various numbers of γ-irradiated, T cell–depleted spleen cells from C57BL/10 mice, which have been pulsed with KLH. Proliferation and lymphokine production were measured as indicated in Materials and Methods. Detection limits: for IL-2, 0.16 U/ml; IFN-γ, 0.3 ng/ml; IL-4, 16 pg/ml. The results represent the mean of duplicate cultures (± SD). Similar data were obtained in three independent experiments.
Table 2.
IL-10–treated DCs Induce T Cells from μMT Mice to Secrete IL-4
| Recipient: | Control mice | μMT mice | |
|---|---|---|---|
| Injectedwith: | UntreatedDCs | UntreatedDCs | IL-10–treatedDCs |
| Exp. 1 | |||
| 1 | 305.4 ± 3.1 | 80.6 ± 3.1 | 305.9 ± 52.4 |
| 2 | 187.7 ± 0.8 | Not detectable | 242.8 ± 17.8 |
| Exp. 2 | |||
| 1 | 593.3 ± 73.4 | 63.7 ± 35.6 | 318.5 ± 31.1 |
| 2 | 204.4 ± 8.3 | 45.3 ± 0.5 | 610.0 ± 52.3 |
Secretion of IL-4 (in pg/ml) by lymph node T cells. Untreated or IL-10–treated DCs from wild-type mice were pulsed in vitro with KLH (50 μg/ml) and injected into the fore and hind footpads of control or μMT mice. 6 d later, lymph node cells were harvested and cultured with 5 μg/ml KLH and the Il-4 content was assessed by ELISA. Exp. indicates the experiment number; the indented numbers refer to individual mice. Limit for detection is 31.25 pg/ml.
Discussion
The results reported here show that B lymphocytes have a profound regulatory effect on the antigen-presenting function of DCs in vivo. Indeed, DCs from B cell–deprived animals have an impaired capacity to induce antigen-specific differentiation of IL-4–secreting T cells when transferred in control animals. The diminished IL-4–promoting capacity of DCs correlates with an enhanced production of IL-12. These observations suggest that B lymphocytes interact with DCs and lower the level of IL-12 released by DCs, thereby leading to priming of both Th1 and Th2 lymphocytes. Of note, it has been shown that interactions between DCs and B cells may occur regularly during B cell recirculation. The interaction is thought to be confined to small B cells, is totally T cell and antigen independent, and is not MHC restricted 20. Furthermore, cooperation between DCs and B lymphocytes has been demonstrated by Dubois et al. 21, who showed that in vitro–generated DCs promote the proliferation of naive and CD40-activated B cells and produce factors that induce differentiation of activated B cells into plasma cells. Our observations show that a bidirectional regulation occurs upon DC–B cell interaction.
Although we do not have direct evidence, several features suggest that IL-10 may be involved in this immunoregulatory process: splenocytes from μMT mice express reduced levels of IL-10 mRNA than wild-type animals; DCs from IL-10 knockout mice display properties similar to DCs from μMT mice, i.e., they have lost the capacity to induce the development of IL-4–secreting cells; treatment of DCs from μMT mice with IL-10 restored the generation of IL-4–producing cells in vivo; and numerous reports have shown that IL-10 inhibits the production of IL-12 heterodimer by DCs in vitro and in vivo 3 10. Collectively, these observations suggest that the level of IL-12 released by DCs is regulated by B lymphocytes, presumably via the production of IL-10. Our data show that B lymphocytes may produce IL-10 constitutively. B cell–depleted spleen cells also express mRNA for IL-10 (data not shown), suggesting that other cell populations may indirectly control the level of IL-12.
There is evidence that the level of IL-12 released by transferred DCs determines the Th1/Th2 balance in vivo. DCs from wild-type animals produce intermediate levels of IL-12 and induce the development of T cells producing IL-2, IFN-γ, IL-4, IL-5, and IL-10 (this paper and reference 17). DCs, treated with IL-10 in culture, secrete decreased amounts of IL-12 and direct the differentiation of Th2-type cells 3, similar to the response induced by DCs from IL-12–deficient mice 3. Conversely, DCs from IL-10– or B cell–deficient mice release increased levels of IL-12 and do not promote IL-4 secretion (this paper). Coadministration of IL-12 with the wild-type DCs results in sensitization of Th1-type cells only 17. Therefore, the development of IL-4–secreting T lymphocytes appears as a default when T cells are sensitized in the presence of low levels of IL-12. Whether IL-12 directly or indirectly inhibits the development of Th2-type cells is still a matter of speculation.
Our observations indicate that DCs from B cell–deprived mice have lost the ability to prime for IL-4 production but have retained the capacity to induce the development of cells producing diminished but still significant amounts of IL-5 and IL-10. The lack of association of expression among IL-4, IL-5, and IL-10 has been recently reported by Kelso et al. 22, who analyzed the single-cell expression patterns in type 1– or type 2–polarized responses.
Our data also show that DCs from wild-type mice injected in a μMT host induce the development of T cells secreting IL-2 and IFN-γ, but not IL-4. Whether B lymphocytes limit IL-12 synthesis by transferred DC homing in the T cell area of lymph nodes remains to be determined. Additional studies, including injection of labeled DCs in the footpad and staining of lymph node sections for IL-12 p70, would help clarify this point. Interestingly, Wykes et al. 23 have shown that DCs can retain unprocessed antigen and directly interact with B lymphocytes to initiate antibody synthesis. Thus, B cells recognizing the antigen expressed by DCs form short-lived clusters and could give regulatory signals to the transferred DCs. Alternatively, direct B to T cell signaling may promote IL-4 synthesis in this experimental model. It is noteworthy that T cells from μMT mice have the capacity to produce IL-4, as T cells from μMT mice produced high amounts of IL-4 in response to Schistosoma mansoni eggs 8 or Onchocerca volvulus 24. The capacity of T cells from μMT mice to secrete IL-4 is further illustrated by the observation that injection of DCs that have been treated with IL-10 to enhance their capacity to promote Th2 development 3 induces the production of significant levels of IL-4 in μMT recipient mice (Table ).
We and others have reported that subclasses of DCs directed the development of distinct T helper cells in vivo 18 19. Thus, CD8α+ and CD8α− DCs, purified from spleens, have the potential to differentially regulate the Th1/Th2 balance: CD8α− DCs induced the activation of cells secreting high levels of IL-4, IL-5, and IL-10, and low levels of IL-2 and IFN-γ, whereas CD8α+ DCs sensitize cells producing IL-2 and IFN-γ, but little IL-4, IL-5, or IL-10. We therefore compared the numbers of DCs of either subset in μMT and wild-type mice. Little difference was found between these strains, suggesting that B lymphocytes did not alter the distribution of DC subsets (Table ). Of note, the number of DCs was consistently reduced (by approximately twofold) in B cell–deprived mice as compared with wild-type animals, an observation that may result from a lack of survival or maturation signals and/or chemoattractants factors such as B cell–derived chemokines macrophage inflammatory protein (MIP)-1α and MIP-1β 25.Our results are consistent with the observations of Stockinger et al. 10 that the B cell is the crucial APC for the development of Th2 responses. Stockinger et al. also recently have identified a feedback loop triggered by IL-12 that promotes Th2 differentiation 26. Thus, delivery of IL-12 by DCs during B cell activation induces the secretion of IL-6 and IL-10 by activated B cells. IL-6 and IL-10 confer the capacity to induce IL-4 expression in T cells to these B lymphocytes. These data were interpreted to indicate that DCs, through IL-12 secretion, enhance the ability of B cells to influence T cell differentiation toward Th2. Our data further extend these observations by showing that DCs themselves, after interaction with B cells, show higher IL-4–inducing activity.
Interestingly, our results may shed new light on the enigmatic observation that μMT mice showed enhanced CTL activity to tumor cells in comparison to their control littermates 27. Qin et al. reported that the presence of B cells in the priming phase resulted in disabled help for CTL-mediated tumor immunity, and interpreted these data by a competition between B cells and other APCs for antigen. Our data suggest that the absence of B cells may enhance the generation of tumor immunity by promoting a polarized Th1 response.
In conclusion, our observations suggest that at the steady state level, IL-10 released by B lymphocytes may control the level of IL-12 released by DCs, which upon encounter with an antigen would induce an unpolarized immune response. In addition, there is evidence that presentation of antigen by B lymphocytes may preferentially direct the development of Th2-type cells 28 29. Therefore, B cells may directly (upon interaction with T cells) or indirectly (by controlling IL-12 production via DCs) favor the development of Th2-type cells that provide helper activity for antibody synthesis, thereby promoting their own effector function.
Acknowledgments
We thank Oberdan Leo for interesting discussions and helpful suggestions; Drs. M. Goldman, O. Leo, M. Kapsenberg, and P. Kalinski for careful review of the manuscript; F. De Mattia, S. Fougeray, and D. Chaussabel for valuable help; and G. Dewasme, M. Swaenepoel, F. Tielemans, and P. Veirman for technical assistance.
The Laboratory of Animal Physiology was supported by grants of the Fonds National de la Recherche Scientifique (FNRS)/Télévie, by the Fonds de la Recherche Fondamentale Collective, by the European Commission (CEC TMR Network Contract FMRX-CT96-0053), and by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming. M. Moser is a Research Associate from the FNRS.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; μMT, B cell–deficient.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Kalinski P., Hilkens C.M.U., Wierenga E.A., Kapsenberg M.L. T-cell priming by type-1 and type-2 polarized dendritic cellsthe concept of a third signal. Immunol. Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- De Smedt T., Van Mechelen M., De Becker G., Urbain J., Leo O., Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivoa major role for B cells in presenting antigen to T cells in lymph nodes. J. Immunol. 1987;138:2848–2856. [PubMed] [Google Scholar]
- Kurt-Jones E.A., Liano D., HayGlass K.A., Benacerraf B., Sy M.S., Abbas A.K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J. Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- Liu Y., Wu Y., Ramarathinam L., Guo Y., Huszar D., Troustine M., Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int. Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- Ronchese F., Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J. Exp. Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M.M., Di Rosa F., Jankovic D., Sher A., Matzinger P. Successful T cell priming in B cell-deficient mice. J. Exp. Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay A.E., DeKruyff R.H., Umetsu D.T. Antigen-primed T cells from B cell-deficient JHD mice fail to provide B cell help. J. Immunol. 1998;160:1694–1700. [PubMed] [Google Scholar]
- Stockinger B., Zal T., Zal A., Gray D. B cells solicit their own help from T cells. J. Exp. Med. 1996;183:891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Lohler J., Rajewsky K., Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Sornasse T., Flamand V., De Becker G., Bazin H., Tielemans F., Thielemans K., Urbain J., Leo O., Moser M. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J. Exp. Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Witmer-Pack M.D., Agger R., Crowley M.T., Lawless D., Steinman R.M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Metlay J.P., Crowley M.T., Steinman R.M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D., Van Mechelen M., Zanin C., Doutrelepont J.-M., Velu T., Gérard C., Abramowicz D., Scheerlinck J.-P., De Baetselier P., Urbain J. Preferential activation of Th2 cells in chronic graft-versus-host reaction. J. Immunol. 1993;150:361–366. [PubMed] [Google Scholar]
- De Becker G., Moulin V., Tielemans F., De Mattia F., Urbain J., Leo O., Moser M. Regulation of T helper differentiation in vivo by soluble and membrane proteins provided by antigen-presenting cells. Eur. J. Immunol. 1998;28:3161–3171. doi: 10.1002/(SICI)1521-4141(199810)28:10<3161::AID-IMMU3161>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α+ and CD8α2 subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir N., Liu L., MacPherson G.G. Dendritic cells and resting B cells form clusters in vitro and in vivoT cell independence, partial LFA-1 dependence, and regulation by cross-linking surface molecules. J. Immunol. 1998;160:1774–1781. [PubMed] [Google Scholar]
- Dubois B., Vanbervliet B., Fayette J., Massacrier C., Van Kooten C., Brière F., Banchereau J., Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Groves P., Ramm L., Doyle A.G. Single-cell analysis by PCR reveals differential expression of multiple type 1 and 2 cytokine genes among cells within polarized CD4+ T cell populations. Int. Immunol. 1999;11:617–621. doi: 10.1093/intimm/11.4.617. [DOI] [PubMed] [Google Scholar]
- Wykes M., Pombo A., Jenkins C., MacPherson G.G. Dendritic cells interact with naive B lymphocytes to transfer antigen and initiate class switching in primary T-dependent response. J. Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- Hall L.R., Lass J.H., Diaconu E., Strine E.R., Pearlman E. An essential role for antibody in neutrophil and eosinophil recruitment to the corneaB cell-deficient (μMT) mice fail to develop Th2-dependent, helminth-mediated keratitis. J. Immunol. 1999;163:4970–4975. [PubMed] [Google Scholar]
- Crowley M.T., Reilly C.R., Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J. Immunol. 1999;163:4894–4900. [PubMed] [Google Scholar]
- Skok J., Poudrier J., Gray D. Dendritic cell-derived IL-12 promotes B cell induction of Th2 differentiationa feedback regulation of Th1 development. J. Immunol. 1999;163:4284–4291. [PubMed] [Google Scholar]
- Qin Z., Richter G., Schüler T., Ibe S., Cao X., Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat. Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- Gajewski T.F., Pinnas M., Wong T., Fitch F.W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J. Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- Denis O., Latinne D., Nisol F., Bazin H. Resting B cells can act as antigen presenting cells in vivo and induce antibody response. Int. Immunol. 1993;5:71–78. doi: 10.1093/intimm/5.1.71. [DOI] [PubMed] [Google Scholar]