Abstract
This report shows that cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) plays a key role in T cell–mediated dominant immunologic self-tolerance. In vivo blockade of CTLA-4 for a limited period in normal mice leads to spontaneous development of chronic organ-specific autoimmune diseases, which are immunopathologically similar to human counterparts. In normal naive mice, CTLA-4 is constitutively expressed on CD25+CD4+ T cells, which constitute 5–10% of peripheral CD4+ T cells. When the CD25+CD4+ T cells are stimulated via the T cell receptor in vitro, they potently suppress antigen-specific and polyclonal activation and proliferation of other T cells, including CTLA-4–deficient T cells, and blockade of CTLA-4 abrogates the suppression. CD28-deficient CD25+CD4+ T cells can also suppress normal T cells, indicating that CD28 is dispensable for activation of the regulatory T cells. Thus, the CD25+CD4+ regulatory T cell population engaged in dominant self-tolerance may require CTLA-4 but not CD28 as a costimulatory molecule for its functional activation. Furthermore, interference with this role of CTLA-4 suffices to elicit autoimmune disease in otherwise normal animals, presumably through affecting CD25+CD4+ T cell–mediated control of self-reactive T cells. This unique function of CTLA-4 could be exploited to potentiate T cell–mediated immunoregulation, and thereby to induce immunologic tolerance or to control autoimmunity.
Keywords: CTLA-4, autoimmune disease, regulatory T cell, CD25, self-tolerance
Introduction
CTL-associated antigen 4 (CTLA-4 [CD152]) is a CD28 homologue expressed on activated T cells and, upon interaction with CD80 or CD86 on APCs, exerts a downregulatory or attenuating effect on T cell–mediated immune responses 1 2. Accumulating evidence indicates that it also plays an important role in immunologic self-tolerance in the periphery. CTLA-4–deficient mice, for example, develop a severe lymphoproliferative disorder and die from autoimmune-like disease within 1 mo after birth 3 4. Administration of anti–CTLA-4 Ab exacerbated autoimmune responses in a murine model of multiple sclerosis or insulin-dependent diabetes mellitus (IDDM [5–7]), enhanced tumor immunity 8, or prevented the induction of immunologic tolerance to concurrently administered non–self-antigens 9. However, it is uncertain whether these findings can be explained by the current notion that CTLA-4 transduces negative signals to activated effector T cells 1 2 10. In this report, we show another possible role of CTLA-4 in immunologic self-tolerance.
There is accumulating evidence that, in addition to clonal deletion or anergy, T cell–mediated dominant control of self-reactive T cells represents one mechanism for maintaining immunologic self-tolerance 11. For example, removal of a subpopulation of CD4+ T cells leads to spontaneous development of various T cell–mediated autoimmune diseases (e.g., thyroiditis, gastritis, and insulin-dependent diabetes mellitus) in otherwise normal rodents, and reconstitution of the removed population prevents the autoimmune development 12 13. Elimination of CD25+CD4+ T cells (which constitute 5–10% of peripheral CD4+ T cells in normal naive mice), for example, elicits various autoimmune diseases in genetically susceptible strains of mice 14 15 16 17 18 19 20. The elimination also evokes potent tumor immunity in otherwise nonresponding animals 17. These in vivo and in vitro findings, especially similar effects of CTLA-4 blockade and CD25+ T cell elimination on autoimmunity and tumor immunity, have led us to examine possible involvement of CTLA-4 in T cell–mediated immunoregulation.
Here, we demonstrate that CTLA-4 is indeed a key costimulatory molecule for activating CD25+CD4+ regulatory T cells to exert suppression and thereby to control self-reactive T cells, and that in vivo blockade of CTLA-4 expressed on the regulatory T cells suffices to break natural self-tolerance and elicit pathological autoimmunity.
Materials and Methods
Mice.
8-wk-old BALB/c, BALB/c athymic nude, or C57BL/6 (B6) mice were purchased from SLC. DO11.10 transgenic mice expressing transgenic TCR specific for an OVA peptide were the gift of Dr. D.Y. Loh (Roche Japan, Kamakura, Japan). CTLA-4– or CD28-deficient mice on the B6 background were described previously 3 21. All these mice were maintained in our animal facility and cared for in accordance with the institutional guidelines for animal welfare.
In Vivo Injection of mAb.
The hybridoma cells secreting hamster anti–CTLA-4 mAb (UC10-4F10-11) were a gift from Dr. J. Bluestone (University of Chicago, Chicago, IL [22]). The Ab was purified from ascites in SCID mice using protein A columns 22 23. Rat anti-CD25 mAb (PC61) was purified from ascites in SCID mice by 40% ammonium sulfate precipitation twice as described previously 17.
Serologic Analysis.
For flow cytometric analysis, 106 cells were incubated with FITC- or PE-labeled or biotinylated mAbs, with RPE–CyChrome 5–streptavidin (Dako) as the secondary reagent for biotinylated Abs, and analyzed by flow cytometry (Epics-XL; Beckman Coulter). FITC-labeled or biotinylated anti-CD25 mAb (7D4), FITC–anti-CD4 mAb (H129.19), PE-labeled anti–CTLA-4 mAb (UC10-4F10-11), and anti-FcR mAb (2.4G2) were purchased from BD PharMingen.
Cell Sorting.
Spleen and lymph node cell suspensions prepared from 8-wk-old BALB/c, DO11.10, or CD28-deficient mice or 2–3-wk-old CTLA-4–deficient mice were stained with FITC–anti-CD25 mAb (7D4) and PE–anti-CD4 mAb (H129.19; BD PharMingen) and sorted by a FACS® (Epics-Elite; Beckman Coulter), as described previously 18 19. Purity of the CD25+ or CD25−CD4+ population was >90% and ∼99%, respectively.
In Vitro Proliferation Assay.
Lymph node and spleen T cells (2.0–2.5 × 104), sorted as described above, and red blood cell–lysed, x-irradiated (20 Gy) BALB/c spleen cells (5 × 104) as APCs were cultured for 3 d in 96-well round-bottomed plates (Costar) in RPMI 1640 medium supplemented with 10% FCS (GIBCO BRL), penicillin (100 U/ml), streptomycin (100 μg/ml), and 50 μM 2-ME. Anti–CD3 mAb (145-2C11; Cedarlane Laboratories) at a final concentration of 10 μg/ml or Con A at 1 μg/ml were added to the culture for stimulation 18 19. Incorporation of 3H-TdR (1 μCi/well; Dupont) by proliferating lymphocytes during the last 6 h of the culture was measured 18 19. Fab fragments of anti–CTLA-4 mAb or normal hamster IgG (Cortex Biochem, Inc.) were prepared by digesting these Abs with immobilized papain (Pierce Chemical Co.) according to the manufacturer's instruction.
Histology and Serology.
Stomachs and other organs were fixed with 10% formalin and processed for hematoxylin and eosin staining. Serum titers of autoantibodies specific for the gastric parietal cells were assayed by ELISA 14 15. Gastritis was graded 0–2+ depending on macroscopic and histological severity, as described previously 12 14 15.
Results and Discussion
Constitutive Expression of CTLA-4 on CD25+CD4+ T Cells in Normal Naive Mice.
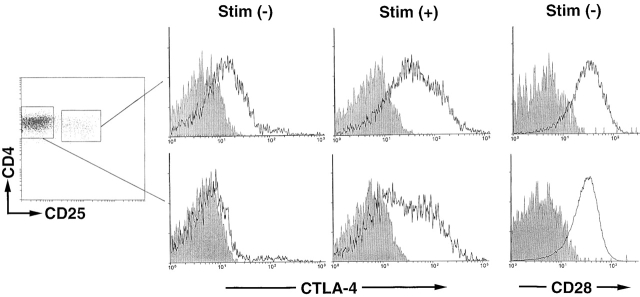
CTLA-4 is supposed to be expressed on T cells only after activation 1 2. However, in the peripheral lymphoid organs of normal naive mice, flow cytometric analysis of cell surface as well as cytoplasmic expression of CTLA-4 revealed that the molecule was constitutively expressed on/in CD25+CD4+ T cells but not on/in CD25−CD4+ T cells (or CD8+ T cells; data not shown) whether the mice were raised in a specific pathogen-free condition or not (Fig. 1). Both populations expressed high levels of CTLA-4 upon in vitro T cell stimulation (for example, with Con A). The two populations showed no difference in their expression levels of CD28, which appears to be constitutively expressed on all murine T cells 24.
Figure 1.
Constitutive expression of CTLA-4 on CD25+CD4+ T cells in normal naive mice. Lymph node and spleen cell suspensions prepared from a 2-mo-old BALB/c mouse were stained with FITC–anti-CD4 Ab and biotinylated anti-CD25 Ab with RPE-CyChrome 5–conjugated streptavidin as the secondary reagent. Cells enclosed with rectangles were sorted as CD25+ or CD25−4+ T cells. The sorted cells were stained for 2 h at 37°C with PE-conjugated anti–CTLA-4 Ab or PE-conjugated anti–human CD4 Ab as a staining control, and are shown as histograms (solid lines or shaded areas, respectively; the abscissa shows the staining intensity on a logarithmic scale and the ordinate shows the number of cells on an arbitrary scale) (reference 28). The sorted cells were also cultured with Con A (1 μg/ml) at 37°C for 24 h and incubated for the last 2 h with PE–anti–CTLA-4 Ab. CD28 expression on CD25+ or CD25−4+ T cells is shown as histograms by staining the cells with PE–anti-CD28 Ab. A representative result of five independent experiments is shown. Stim., stimulation.
Induction of Autoimmune Disease in Normal Mice by Anti–CTLA-4 Ab Treatment.
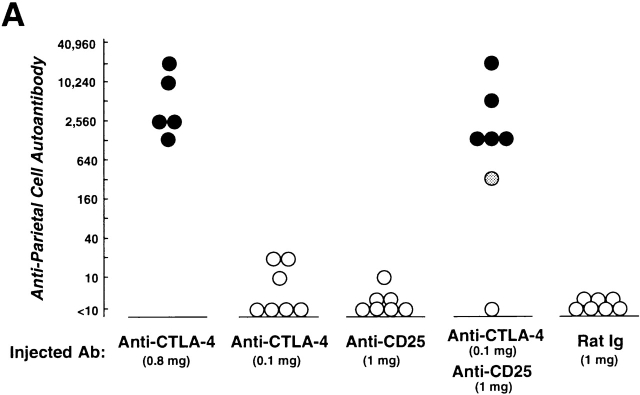
To determine the role of CTLA-4 expressed on CD25+CD4+ T cells in self-tolerance and autoimmunity, we administered purified anti–CTLA-4 mAb (0.8 or 0.1 mg), anti-CD25 mAb (1 mg), or the mixture of anti–CTLA-4 mAb (0.1 mg) and anti-CD25 mAb (1 mg) once a week three times to normal BALB/c mice from the age of 10 d, and examined the mice at 3 mo of age (17 25; Fig. 2). All the mice treated with high doses (0.8 mg/injection) of anti–CTLA-4 mAb spontaneously developed histologically and serologically evident autoimmune gastritis and some other autoimmune diseases (i.e., oophoritis or mild sialoadenitis, in one mouse each) (12 14 15; Fig. 2a Fig. b Fig. c). Low doses of anti–CTLA-4 mAb or anti-CD25 mAb alone induced only low titers of autoantibody without histological evidence of autoimmune disease, whereas treatment with the mixture of the two mAbs produced autoimmune gastritis in the majority (∼85%) of mice.
Figure 2.
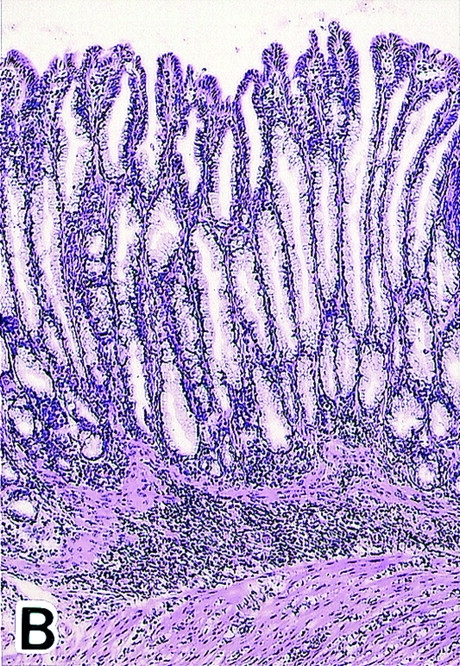
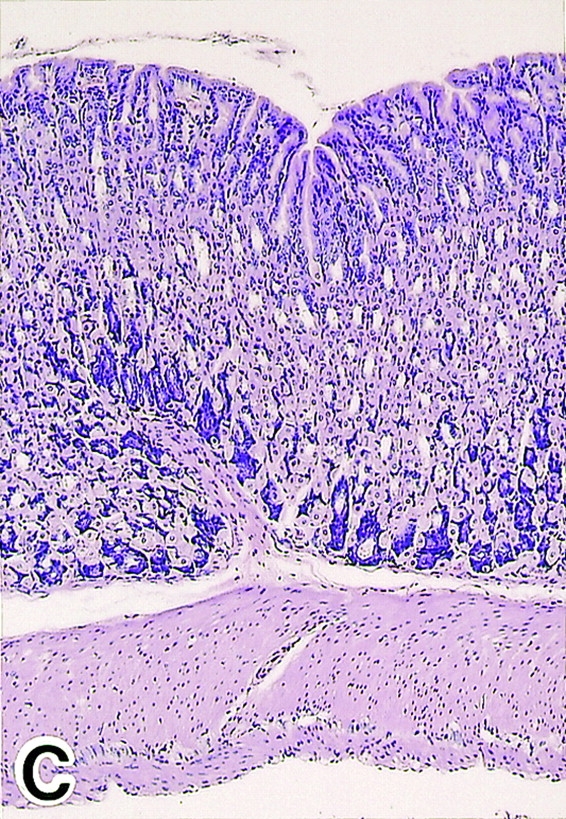
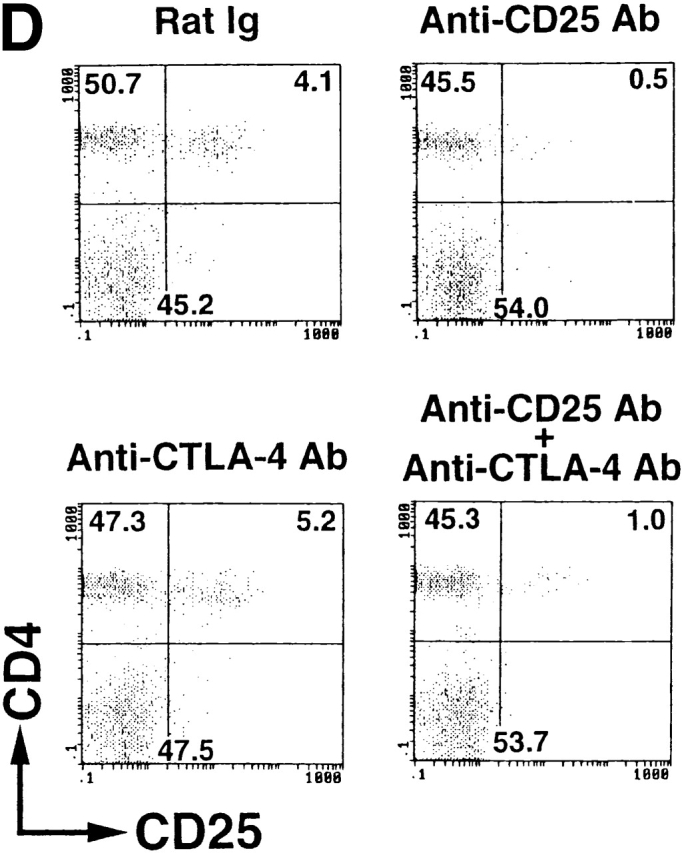
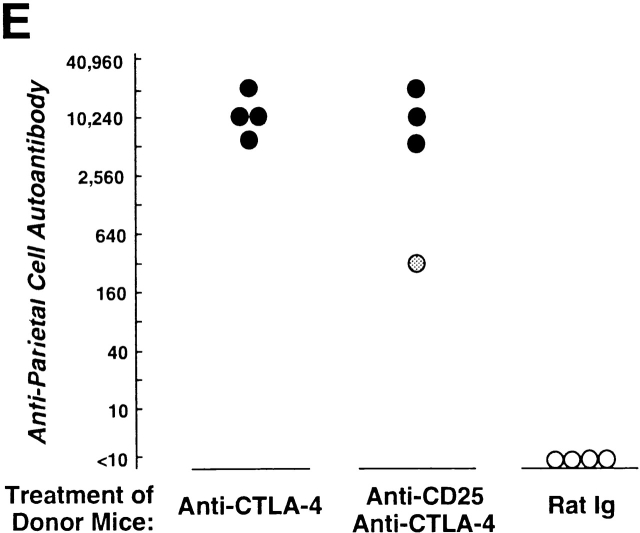
Induction of autoimmune disease in BALB/c mice by administration of anti–CTLA-4 Ab with or without anti-CD25 Ab. (A) BALB/c mice were injected intraperitoneally with indicated doses of purified anti–CTLA-4 mAb, anti-CD25 mAb, a mixture of the two mAbs, or control rat Ig once a week three times from the age of 10 d, and were examined at 3 mo of age for histological and/or serological development of autoimmune disease. The gastric lesion was histologically graded as macroscopically and histologically evident grade-2 gastritis (black circle), histologically evident grade-1 gastritis (gray circle), or intact gastric mucosa (white circle; for detailed grading, see references 12, 14, 15). Titers of antiparietal cell autoantibodies were assessed by ELISA. The gastric mucosa of a grade-2 gastritis in a mouse treated with anti–CTLA-4 mAb is shown in B, and the intact gastric mucosa of a mouse treated with rat Ig is shown in C. Samples were stained with hematoxylin and eosin (original magnification: ×25). (D) Composition of T cell subpopulations defined by the expression of CD4 and CD25 7 d after the last Ab treatment. Lymph node cells were stained with PE–anti-CD4 mAb and FITC–anti-CD25 mAb, 7D4, another anti-CD25 mAb with different epitope specificity, as described previously (reference 18). A representative staining of three independent experiments is shown. (E) Spleen cells (5 × 107) from anti–CTLA-4 mAb– or mixed mAb–treated mice with gastritis or from rat Ig–treated mice were transferred intravenously to BALB/c athymic nude mice, which were histologically and serologically examined 2 mo later. Black circle, macroscopically and histologically evident grade-2 gastritis; gray circle, histologically evident grade-1 gastritis; white circle, intact gastric mucosa. A representative result of more than three independent experiments is shown. The means of duplicate cultures are shown, and the SEM was within 10% of the mean for each experiment.
Administration of anti-CTLA-4 mAb did not significantly alter the number of CD25+CD4+ T cells or the ratio of CD25+ T cells among CD4+ T cells, whereas administration of anti-CD25 mAb or the mixed mAbs reduced CD25+CD4+ T cells to 10–30% of the controls (Fig. 2 D). For example, 1 wk after the last treatment, percentages of CD25+ cells among the CD4+ cells in the lymph nodes were 7.2 ± 0.3% in the control mice, 10.1 ± 0.30% and 9.6 ± 0.26% in the high- or low-dose anti–CTLA-4 mAb–treated mice, respectively, 0.93 ± 0.15% in the anti–CD25 mAb–treated mice, and 2.1 ± 0.1% in the mixed mAb–treated mice (n = 3 for each).
The affected gastric mucosa was characterized by loss and damage of the parietal cells and the chief cells, compensatory hyperplasia of mucous-secreting cells, and a massive infiltration of mononuclear cells (Fig. 2b and Fig. c). The lesion was immunopathologically similar to human autoimmune gastritis with pernicious anemia 26. Furthermore, the gastritis thus induced could be adoptively transferred to naive BALB/c athymic nude mice by spleen cells from gastritis-bearing BALB/c mice, indicating that the lesion was a result of a bona fide autoimmune disease (Fig. 2 E).
Thus, autoimmune diseases similar to those produced by elimination of CD25+CD4+ T cells could be elicited in normal mice by blockade of CTLA-4 with high-dose anti–CTLA-4 Ab, or reduction of CD25+CD4+ T cells accompanied by CTLA-4 blockade on the residual T cells with low-dose anti–CTLA-4 Ab 14 15. In the latter treatment, it is plausible that reduction of CD25+CD4+ T cells might have partially triggered the activation of autoimmune effector T cells, which might then be further activated by the blockade of CTLA-4 expressed on them. Nevertheless, successful autoimmune induction by CTLA-4 blockade alone without reduction of CD25+CD4+ T cells (Fig. 2 D), together with the constitutive expression of CTLA-4 on normal CD25+CD4+ T cells (Fig. 1), indicates that the blockade of CTLA-4 on the CD25+CD4+ T cells is most likely the triggering mechanism of this autoimmunity.
Role of CTLA-4 in CD25+CD4+ T Cell–mediated Suppression In Vitro.
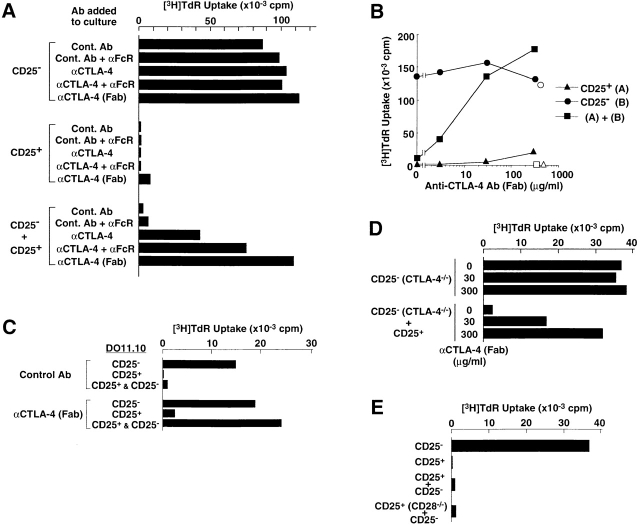
CD25+CD4+ T cells present in normal naive mice exhibited no proliferative response to TCR stimulation, whereas, upon TCR stimulation, they potently suppressed the activation and proliferation of other T cells, as reported previously (18 19 20 27; Fig. 3). To examine the possible role of CTLA-4 in this natural anergy and suppression, we added anti–CTLA-4 Ab, its Fab fragments, or control hamster Ab to 3-d cultures of CD25+CD4+ T cells or CD25−CD4+ T cells (purified as shown in Fig. 1), or a mixture of an equal number of the two populations, and stimulated the cultures with Con A (Fig. 3 A) or soluble anti-CD3 mAb (Fig. 3 B) along with APCs. Anti-FcγII/III receptor (FcR [CD16/CD32]) mAb was also added to some cultures to inhibit anti–CTLA-4 mAbs from attaching to FcRs on APCs, thereby preventing the Abs from cross-linking CTLA-4 and transducing signals 22 23. In these experiments, intact anti–CTLA-4 mAb, especially in the presence of anti-FcR mAb, significantly neutralized CD25+CD4+ T cell–mediated suppression of the activation and proliferation of CD25−CD4+ T cells (Fig. 3 A). Although others reported no significant effect of anti–CTLA-4 mAb in a similar cell culture system 20, the discrepancy could be attributed to the concentration of anti–CTLA-4 mAb in the culture; i.e., with a high dose (100 μg/ml) of anti–CTLA-4 mAb, as shown here, the majority of CTLA-4 molecules could be blocked rather than cross-linked by the Ab attached to FcR on APCs. This blocking effect was further supported by the result that Fab–anti–CTLA-4 mAb, which is presumably able to block but unable to cross-link CTLA-4 22 23, more efficiently abrogated the suppression in a dose-dependent fashion (Fig. 3A and Fig. B). In addition to these polyclonal T cell activations, the Fab fragments also neutralized CD25+CD4+ T cell–mediated control of antigen-specific activation and proliferation of OVA-specific T cells from DO11.10 transgenic mice (18 19; Fig. 3 C). On the other hand, intact or Fab–anti–CTLA-4 mAb did not significantly affect the responses of CD25−CD4+ T cells or break the anergic state of CD25+CD4+ T cells (Fig. 3A–C).
Figure 3.
Key role of CTLA-4 expressed on CD25+CD4+ regulatory T cells in downregulating the activation and proliferation of other T cells. (A) Anti–CTLA-4 mAb (100 μg/ml) or control hamster Ab with or without anti-FcR mAb (10 μg/ml) or Fab–anti-CTLA-4 mAb (100 μg/ml) was added to the culture of CD25+ or CD25−CD4+ T cells purified from BALB/c spleen and lymph node cells (as shown in Fig. 1), or these two populations were mixed at a 1:1 ratio. The culture was stimulated for 3 d with Con A (1 μg/ml) along with irradiated BALB/c spleen cells as APCs. Incorporation of 3H-TdR during the last 6 h of the culture is shown as the degree of proliferation. Background counts in the wells with APCs alone were always <1,500 cpm in these experiments and those below. α, anti-. (B) CD25+ or CD25−CD4+ T cells or a 1:1 mixture of the two populations were stimulated with anti-CD3 mAb (10 μg/ml) in the presence of Fab–anti–CTLA-4 mAb at various concentrations (black symbols) or control Fab–hamster IgG at 300 μg/ml (white symbols). (C) CD25+ or CD25−CD4+ T cells or two populations mixed at an equal ratio, purified from the spleens of DO11.10 transgenic mice, were cultured for 3 d with 0.1 μM OVA-P(323–339) in the presence of Fab–anti–CTLA-4 mAb or control Fab–hamster IgG at 100 μg/ml. α, anti-. (D) CD25−CD4+ T cells prepared from 3-wk-old CTLA-4–deficient (CTLA-4−/−) B6 mice or a 1:1 mixture of these cells with CD25+CD4+ T cells from 2-mo-old normal B6 mice was stimulated with anti-CD3 mAb for 3 d in the presence of Fab–anti–CTLA-4 mAb at indicated concentrations. The counts of CTLA-4–deficient CD25−CD4+ T cells cultured without stimulation were background levels (i.e., <2,000 cpm). α, anti-. (E) CD25+CD4+ T cells prepared from CD28-deficient (CD28−/−) B6 mice or normal B6 mice were mixed with an equal number of CD25−CD4+ T cells from normal B6 mice and stimulated with anti-CD3 mAb for 3 d. A representative result of more than three independent experiments is shown. The means of duplicate cultures are shown, and the SEM was always with 10% of the mean for each experiment.
To determine more definitely the role of CTLA-4 on CD25+CD4+ T cells, we used CTLA-4–deficient mice. CD25+CD4+ T cells from normal mice efficiently suppressed CD25−CD4+ T cells from CTLA-4–deficient mice, and Fab–anti-CTLA-4 mAb abrogated this suppression in a dose-dependent fashion (Fig. 3 D). In the reciprocal combination, CTLA-4–deficient CD25+CD4+ T cells also exhibited weak suppressive activity (∼50% in percentage suppression compared with >95% by normal CD25+ CD4+ T cells at 1:1 mixing with CD25−CD4+ T cells). The CD25+ T cells constituted up to 30% of CD4+ T cells in the enlarged spleens of CTLA-4–deficient mice and appeared to be different from normal CD25+CD4+ T cells in including a large number of abnormally activated T cells 3. It remains to be determined whether this weak suppressive activity could be peculiar to CTLA-4–deficient CD25+CD4+ T cells (e.g., because of compensatory activation of other genes encoding molecules concerned with the suppression). Indeed, blockade of certain other cell surface molecules could abrogate the suppressive activity of CTLA-4–deficient as well as normal CD25+CD4+ T cells (our unpublished results). Despite this possibility, the results in Fig. 3A–D, when taken together, indicate that CTLA-4 expressed on CD25+CD4+ regulatory T cells is critically involved in this in vitro T cell–mediated immunoregulation.
Activation of T cells usually requires a costimulatory signal through CD28 in addition to the TCR signal 24. However, CD25+CD4+ T cells from CD28-deficient mice exhibited a potent suppressive activity (Fig. 3 E). This indicates that CD28 on CD25+CD4+ T cells (Fig. 1) is dispensable for the CD25+CD4+ T cell–mediated suppression, even if CD28 deficiency might affect the development of regulatory T cells (e.g., the number of CD25+ CD4+ T cells in CD28-deficient B6 mice reduced to ∼20% of normal B6 mice; our unpublished data) or their level of CTLA-4 expression 28 29. On the other hand, we have shown previously that ligation of the CD28 molecule with anti-CD28 Ab, in conjunction with TCR stimulation, can break the naturally anergic state of CD25+ CD4+ T cells and simultaneously abolish their suppressive activity 18 19. These findings collectively imply unique roles for CTLA-4 and CD28 in CD25+CD4+ regulatory T cells; i.e., costimulation via CTLA-4 may activate the regulatory T cells to exert suppression, whereas costimulation via CD28 may attenuate their suppressive activity. The balance between the two signals in a particular CD25+CD4+ regulatory T cell may be critical in determining its activation threshold and/or its suppressive activity. Furthermore, the balance between regulatory T cells activated through CTLA-4 and effector T cells activated through CD28 may contribute to determining the threshold or the magnitude of a particular immune response.
It remains to be determined how CD25+CD4+ regulatory T cells suppressively control other T cells upon stimulation via CTLA-4 and TCR. Although TGF-β possibly secreted by TCR- and CTLA-4–stimulated T cells may contribute to attenuating activated T cells, as shown by others 30, anergic/suppressive CD25+CD4+ T cells stimulated via CTLA-4 and TCR did not secrete detectable amounts of TGF-β, and the suppression was not abrogated by adding neutralizing anti–TGF-β Ab to the culture (18 20; our unpublished data). As a possible mechanism of the suppression, CD25+CD4+ T cells stimulated via CTLA-4 and TCR may physically interfere with the interaction of other T cells with APCs by competing for the costimulatory molecules on APCs, as they are predominantly expressing CTLA-4 (which has a higher affinity for CD80/CD86 than CD28 [22]) and various adhesion molecules (such as intercellular adhesion molecule [ICAM]-1, LFA-1, and CD2) at high levels 18 19 20. Alternatively, the signals via TCR and CTLA-4 may activate CD25+CD4+ T cells to deliver to other T cells a negative signal for activation and proliferation. These possibilities are currently under investigation.
There are accumulating demonstrations that CTLA-4 deficiency or blockade induces or exacerbates autoimmunity, enhances tumor immunity, or prevents induction of immunologic tolerance 4 5 6 7 8 9. These effects of CTLA-4 deficiency or blockade have been largely explained as a hindrance of CTLA-4–transduced negative signals to activated T cells mediating immune responses 1 2. However, our present in vivo and in vitro results indicate that the effects can also be attributed, at least in part, to the blockade of CTLA-4 molecules expressed on CD25+CD4+ regulatory T cells and consequent interference with T cell–mediated immunoregulation. CTLA-4 also appears to play a similar role in the function of regulatory T cells that control inflammatory bowel disease 31. Exploitation of this unique role of CTLA-4 in immunologic self-tolerance would help to develop novel strategies for treating or preventing autoimmune disease, enhancing immunity to autologous tumor cells, or inducing tolerance to allotransplants.
Acknowledgments
We thank Drs. K.S.K. Tung and T. Sakihama for critically reading the manuscript, Dr. J.A. Bluestone for providing us with the hybridoma-secreting anti–CTLA-4 Ab, Ms. E. Moriizumi for preparing histology, and Ms. Sue Plyte for taking care of sending mice abroad.
This work was supported by grants-in-aid from the Ministry of Human Welfare and the Organization for Pharmaceutical Safety and Research of Japan.
References
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Bluestone J.A. Is CTLA-4 a master switch for peripheral T cell tolerance? J. Immunol. 1997;158:1989–1993. [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Karandikar N.J., Vanderlugt C.L., Walunas T.L., Miller S.D., Bluestone J.A. CTLA-4a negative regulator of autoimmune disease. J. Exp. Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin P.J., Maldonado J.H., Davis T.A., June C.H., Racke M.K. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- Luhder F., Hoglund P., Allison J.P., Benoist C., Mathis D. Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J. Exp. Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Perez V.L., Van Parijs L., Biuckians A., Zheng X.X., Strom T.B., Abbas A.K. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Kohler G., Ecabert B., Mak T.W., Kopf M. Lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 1999;163:1128–1131. [PubMed] [Google Scholar]
- Moller G. Dominant immunological tolerance. Immunol. Rev. 1996;149:1–243. [Google Scholar]
- Sakaguchi S., Fukuma K., Kuribayashi K., Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerancedeficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 1985;161:72–85. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathologyprevention by OX-22low subset. J. Exp. Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25)breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Asano M., Toda M., Sakaguchi N., Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri-Payer E., Amar A.Z., Thornton A.M., Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Shimizu J., Yamazaki S., Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cellsa common basis between tumor immunity and autoimmunity. J. Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cellsinduction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. Thymus and autoimmunityproduction of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Thornton A., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K.P., Kundig T.M., Kishihara K., Wakeham A., Kawai K., Ohashi P.S., Thompson C.B., Mak T.W. Differential T cell co-stimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects of the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C.H., Bluestone J.A., Nadler L.M., Thompson C.B. The B7 and CD28 receptor families. Immunol. Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kearney E.R., Walunas T.L., Karr R.W., Morton P.A., Loh D.Y., Bluestone J.A., Jenkins M.K. Antigen-dependent clonal expansion of a trace population of antigen specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- Gleeson P.A., Toh B.H. Molecular targets in pernicious anemia. Immunol. Today. 1991;12:233–238. doi: 10.1016/0167-5699(91)90036-S. [DOI] [PubMed] [Google Scholar]
- Read S., Mauze S., Asseman C., Bean A., Coffman R., Powrie F. CD38+ CD45RBlow CD4+ T cellsa population of T cells with immune regulatory activities in vitro. Eur. J. Immunol. 1998;28:3435–3447. doi: 10.1002/(SICI)1521-4141(199811)28:11<3435::AID-IMMU3435>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Alegre M.L., Noel P.J., Eisfelder B.J., Chuang E., Clark M.R., Reiner S.L., Thompson C.B. Regulation of surface and intracellular expression of CTLA-4 on mouse T cells. J. Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- Fallarino F., Fields P.E., Gajewski T.F. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J. Exp. Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jin W., Wahl S.M. Engagement of cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) induces transforming growth factor β (TGF-β) production by murine CD4+ T cells. J. Exp. Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]