Abstract
We have studied the role of secreted immunoglobulin (Ig)M in protection from infection with influenza virus and delineated the relative contributions of B-1 versus B-2 cell–derived IgM in this process. Mice deficient in secreted IgM but capable of expressing surface IgM and secreting other Ig classes show significantly reduced virus clearance and survival rates compared with wild-type controls. Irradiation chimeras in which only either B-1 or B-2 cells lack the ability to secrete IgM show mortality rates similar to those of mice in which neither B-1 nor B-2 cells secrete IgM. Dependence on both sources of IgM for survival is partially explained by findings in allotype chimeras that broadly cross-reactive B-1 cell–derived natural IgM is present before infection, whereas virus strain–specific, B-2 cell–derived IgM appears only after infection. Furthermore, lack of IgM secreted from one or both sources significantly impairs the antiviral IgG response. Reconstitution of chimeras lacking B-1 cell–derived IgM only with IgM-containing serum from noninfected mice improved both survival rates and serum levels of virus-specific IgG. Thus, virus-induced IgM must be secreted in the presence of natural IgM for efficient induction of specific IgG and for immune protection, identifying B-1 and B-2 cell–derived IgM antibodies as nonredundant components of the antiviral response.
Keywords: B cells, immunoglobulin M, immune protection, CD5+ B cell, respiratory tract
Introduction
Two types of IgM can be found in circulation in normal mice. Natural IgM, secreted mainly by (CD5+) B-1 cells in the apparent absence of antigen stimulation, constitutes most of the circulating IgM 1 2 3 4 and tends to be polyreactive to both foreign antigens and self-components. In contrast, antigen-induced IgM is mostly produced by conventional B (B-2) cells only after antigen stimulation 4. Both natural and induced IgM are polymeric and as such have the ability to bind multimeric antigen and to efficiently activate the classical complement cascade. In addition, due to their polymeric structure, IgM can be transported via the poly-Ig receptor onto mucosal surfaces to provide protection from pathogenic invasion 5 6. These unique properties, and the fact that IgM is the first class of Ig produced during an infection, allow both natural and antigen-induced IgM to act as an early defense mechanism against mucosal and systemic pathogens. Indeed, by creating gene-targeted mice whose B cells cannot secrete IgM but can express surface IgM and IgD and secrete other classes of Igs (secreted [s]IgM−/−), we have previously demonstrated a protective role for natural (B-1 cell–derived) IgM in systemic bacterial infection 7 8. These sIgM−/− mice succumb in greatly increased numbers to cecal ligation and puncture-induced acute peritonitis during the first 32 h after infection compared with wild-type controls 8.
The roles of natural and pathogen-induced IgM in immune protection from viral infection are controversial. Studies with vesicular stomatitis virus (VSV) indicated that virus-induced IgM, secreted early after infection in a T cell–independent manner, can provide protection from acute primary VSV infection 9 10. In contrast, in studies in which monoclonal IgM antibodies specific for influenza virus were passively transferred into SCID mice, Palladino et al. 11 showed that the transferred IgM antibodies were protective when given before but not after virus infection. Thus, the VSV data suggest a role for virus-induced IgM in immune protection from primary viral infection, whereas the data from the influenza studies with SCID mice suggest a protective role only during recall responses. We have shown previously that only B-2 cells are induced to respond to influenza infection by producing antiviral antibodies but that considerable levels of natural antibodies to influenza are present in the sera of mice before an infection, which are produced by B-1 cells 4. As SCID mice lack both types of IgM, virus-induced and natural, together the data suggest that both B-1 and B-2 cell–derived IgM antibodies might have to be present to provide immune protection against a primary viral infection.
To unequivocally determine the roles of natural and virus-induced IgM in protection from a primary virus infection, we have examined the survival rates and antibody responses after influenza virus infection in sIgM−/− mice and in irradiation chimeras that lack sIgM from either B-1 cells or B-2 cells. Our findings demonstrate that both B-1 and B-2 cell–derived IgM antibodies are necessary for optimal immune protection from infection and that one mechanism by which sIgM functions is by positively regulating the magnitude of the virus-specific IgG response.
Materials and Methods
Mice and Virus Infection.
Mice that lack the ability to secrete IgM due to a targeted mutagenesis that disrupts expression of the secreted but not membrane-bound form of IgM (sIgM−/−) have been described previously 7. Mutant mice on the 129/Sv background and wild-type 129/Sv mice (sIgM+/+) as well as C57BL/6 and B6.C20 mice were bred and maintained in the Animal Facility at Stanford University. The reassortant influenza virus strain Mem71 bearing the hemagglutinin of A/Memphis/1/71 (H3) and the neuraminidase of A/Bellamy/42 (N1) was harvested and stored as described 12. Unless otherwise stated, anesthetized mice were infected intranasally at a virus dose of 1.6 × 106 PFU per mouse. The infective dose was determined in preliminary experiments to be the highest virus dose that did not cause mortality in 3-mo-old 129/Sv wild-type mice.
Irradiation Chimera.
Irradiation chimeras were constructed using 2-mo-old lethally irradiated (850 rads) recipients, bone marrow as source for B-2 cells, and peritoneal cavity wash-out (PerC) cells as source of B-1 cells 13. To make chimeras containing IgM-secreting B-1 cells but sIgM−/− B-2 cells, 5 × 106 PerC cells from sIgM+/+ mice and 3 × 106 bone marrow cells from sIgM−/− mice were transferred into irradiated sIgM−/− recipients. To construct chimeras containing IgM-secreting B-2 cells but sIgM−/− B-1 cells, 3 × 106 bone marrow cells from sIgM+/+ mice and 5 × 106 PerC cells from sIgM−/− mice were transferred into irradiated sIgM−/− mice. Control chimeras were also generated by transferring both bone marrow and PerC cells from the same donor, i.e., from sIgM−/− or sIgM+/+ mice. Identical protocols were used to generate B-1/B-2 allotype-chimeric mice. For this, lethally irradiated C57BL/6 mice were used as recipients of 3 × 106 C57BL/6 (Igh-b) bone marrow cells and 5 × 106 PerC cells from congenic B6.C20 (Igh-a) mice. Cell suspensions were prepared according to standard methods and injected intravenously via the tail vein. Chimeras were analyzed or infected 2–3 mo after cell transfers.
Virus Plaque Assay.
Virus plaque assay was performed according to Tannock et al. 14, using Mardin-Darby canine kidney (MDCK) cells and lung homogenates obtained from mice at different days after influenza virus infection. In brief, fresh lung homogenates were added at various dilutions in duplicate to semiconfluent layers of MDCK cells in 6-well tissue culture plates containing RPMI 1640, 5% FCS, and 5 × 10−4 M l-glutamine. Virus infection was allowed to proceed for 45 min at 37°C, and then cells were overlayed with 0.9% agarose in L-15 medium (GIBCO BRL) supplemented with 100 U of penicillin, 100 μg/ml streptomycin, 0.01 M Hepes buffer, pH 6.8, and 0.1% trypsin-l-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK; Worthington Biochemical Corp.). The culture was incubated for 3 d in a humidified incubator at 37°C containing 5% CO2. Cell layers were fixed with 5% formaldehyde and stained with a solution of 0.5% crystal violet in methanol, and plaques were counted for those dilutions of the lung homogenate at which the number of plaques was between 10 and 200.
ELISA.
Serum levels of influenza virus–specific antibodies were determined by ELISA as described 4. Arbitrary units of virus-specific Mem71 Ig titers were calculated by comparison to those of a hyperimmune serum. For comparison of IgM titers specific for virus strains A/Mem71 (H3N1), A/Guangdong (H3N2), A/PR8 (H1N1), and B/Panama, ELISAs were carried out in a similar manner using purified virus antigen from the various virus strains. Serum titers are expressed in Fig. 6 as reciprocal dilution of the sera that gave an OD (490 nm) reading of 0.1 (roughly fivefold above background). Total serum IgM was measured by ELISA using myeloma IgM as standard 4.
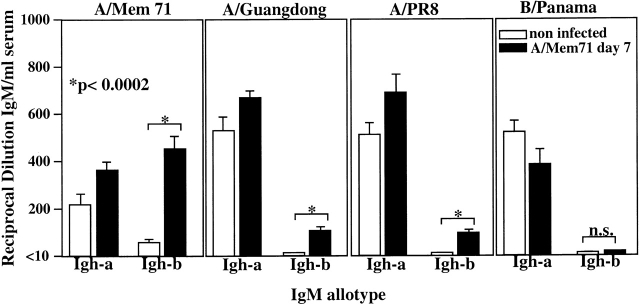
Figure 6.
Reactivity of B-1 and B-2 cell–derived IgM antibodies before and after infection with influenza virus A/Mem71. Sera from B-1/B-2 allotype-chimeric mice, generated as outlined in the Fig. 5 legend, were taken before and 7 d after infection with influenza virus A/Mem71 to determine the titers of B-1 (Igh-a) and B-2 (Igh-b) cell–derived antibodies able to bind four different influenza virus strains as indicated. Indicated are antiviral titers, determined by ELISA as reciprocal of the serum dilution that resulted in an OD 490 nm reading significantly higher than that of the background. n.s., not significant.
10-Color FACS® Analysis and Sort.
Single-cell suspensions from spleens and PerC cells of chimeras 2 mo after adoptive cell transfer were stained simultaneously with antibody conjugates specific to CD21–FITC (mAb 7G6) (PharMingen); CD43–PE (S7); CD5–biotin (53-7.3); CD23–Alexa-594 (B3B4); CD11b–allophycocyanin (M1/70); IgM Cy7–allophycocyanin (331); IgMa Cy7–allophycocyanin (DS-1); IgMb Cy7–allophycocyanin (AF6.78); IgD Cy7–PE (1126); IgDa Cy7–PE (AMS9); IgDb Cy7–PE (AF6.122); CD3–Cascade Blue (CB) or –Cascade Yellow (CY); CD4–CY or –CB (GK1.5); CD8–CY or –CB (53.6.7); macrophage–CY or –CB (F4/80); and CD19–Cy5.5–allophycocyanin. Streptavidin–Cy5–PE was used as second step reagent. Noncommercial conjugates and tandem dyes were prepared as outlined previously 15 16 17. The novel fluorochrome Cy5.5–allophycocyanin was prepared and conjugated to the anti-CD19 mAb with a method similar to that described for Cy7–allophycocyanin 17. Propidium iodide was added at 0.25 μg/ml. Cells were assayed using a modified triple laser Cytomation/Becton Dickinson hybrid FACS® (described in references 18 and 19), and data were analyzed with the FlowJo software (TreeStar Inc.).
CD3+CD4+CD19− T cells from single-cell suspensions of mediastinal lymph nodes from day 7 influenza virus–infected sIgM−/− and sIgM+/+ mice were sorted after staining with CD3–FITC, CD4–PE, and CD19–allophycocyanin on the Cytomation/Becton Dickinson hybrid FACS®. Purity of sorted cells was >96% as assessed by FACS® immediately after sorting.
Proliferation Assay.
FACS®-purified mediastinal lymph node CD4+ T cells from sIgM−/− and wild-type mice were cultured at indicated numbers in DMEM/10% FCS/5 × 10−5 M β-ME, 216 mg/liter l-glutamine, and antibiotics in the presence of 2.5 × 105 irradiated splenic feeder cells per well. Splenic feeder cells were pulsed with 200 hemagglutinin units of Mem71 or with PBS for 90 min before irradiation. Cells were incubated for 24 h at 37°C in 7.5% CO2/92.5% air and then pulsed with 0.5 μCi [methyl-3H]thymidine. Cells were harvested after a further 8-h culture period onto filter mats, and incorporated radioactivity was measured using a liquid scintillation counter.
Reconstitution of Chimeric Mice with Polyclonal IgM.
Wild-type mice and sIgM−/− mice were bled via the tail vein, and serum was stored in aliquots at −20°C until used. Groups of chimeric animals reconstituted with wild-type bone marrow and PerC cells from sIgM−/− mice were injected intraperitoneally with 0.5 ml of wild-type serum 1 h before influenza virus infection to restore polyclonal IgM (n = 11). Serum injections were repeated daily for 5 d after infection. Control mice were given the same amount of serum from sIgM−/− mice.
Statistical Analysis.
Statistical analyses were carried out using the nonparametric Wilcoxon/Kruskal/Wallis rank test or two-tailed Student's t test when appropriate. Survival data were analyzed by Log Rank Test with the Kaplan-Meier method. Data are considered statistically significant when P < 0.05.
Results
Reduced Survival of sIgM−/− Mice after Influenza Virus Infection.
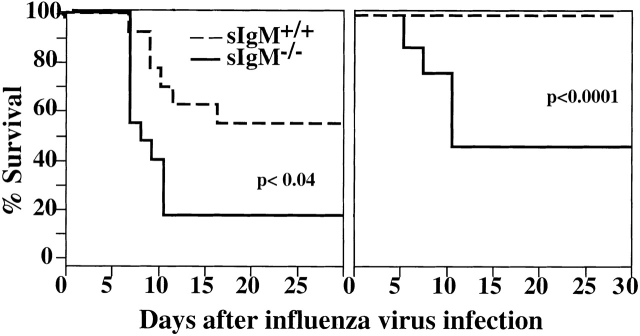
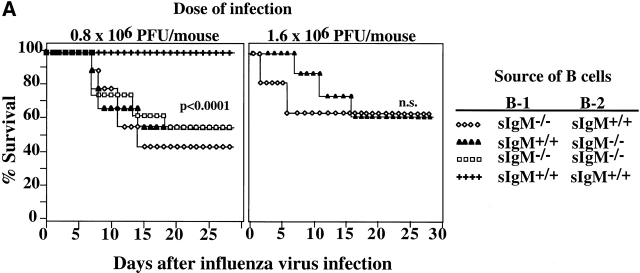
To determine whether sIgM provides immune protection during acute influenza virus infection, sIgM−/− mice and wild-type controls at 2 and 3 mo of age were infected intranasally with 1.6 × 106 PFU of influenza virus Mem71. This virus dose was chosen because preliminary studies had shown that it was the maximal virus dose that did not cause deaths in 3-mo-old wild-type mice (data not shown). As shown in Fig. 1, the same virus dose caused 50% mortality of sIgM−/− mice (P < 0.0001). At a younger age (2 mo), when mice were more susceptible to influenza virus infection, still significantly higher percentages of sIgM−/− mice died from infection than wild-type mice (80 vs. 40%, respectively; P < 0.04). Most deaths occurred between days 6 and 10 after infection. Secretion of IgM is only one immune mechanism that contributes to the clearance of influenza virus infection, however, as infections with lower doses of virus (0.2, 0.4, and 0.8 × 106 PFU per mouse) did not cause deaths in 3-mo-old sIgM−/− mice nor in age-matched wild-type controls (data not shown).
Figure 1.
Increased influenza virus–induced mortality in mice lacking sIgM. Groups of 2- (n = 14; left panel) and 3-mo-old (n = 10; right panel) sIgM−/− and sIgM+/+ mice were infected with influenza virus Mem71, and survival was monitored daily. Percentages of survival are shown as a function of time.
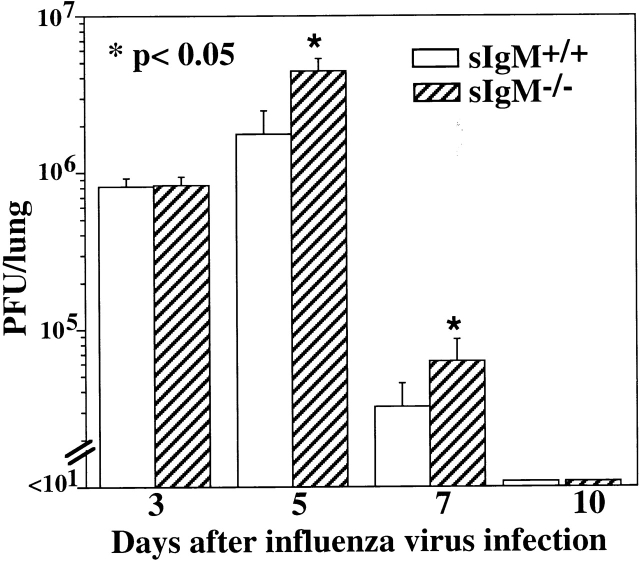
Increased Viral Load in Lungs of sIgM −/− Mice.
Lung virus titers from groups of sIgM−/− and wild-type mice at days 3, 5, 7, and 10 after influenza virus infection were determined to assess whether the increased mortality of sIgM−/− mice was associated with a higher viral load at the site of virus replication. Whereas the viral titers in lungs of sIgM−/− and control mice were comparable early after infection (day 3), at days 5 and 7 after infection there was a significant (P < 0.05) increase in lung virus load of sIgM−/− mice that survived until that time compared with wild-type controls (Fig. 2). Those sIgM−/− mice that survived the infection cleared the virus around day 10, similar to wild-type mice. Thus, the absence of sIgM is associated with a significant increase in viral load in the respiratory tract shortly before virus-induced deaths are observed. As we could perform MDCK assays only on the lungs of surviving mice, it is possible that at time points later than day 5, mice with even higher viral loads died preferentially and thus that these measurements underestimate the differences in the viral loads of these mice.
Figure 2.
Reduced virus clearance in the lungs of sIgM−/− mice. Groups of 3-mo-old sIgM−/− and sIgM+/+ mice (10–12 per time point) were infected with influenza virus Mem71, and lung homogenates were prepared at indicated times after infection. Lung virus titers were determined by MDCK cell plaque assay. Statistical analysis was determined using the Wilcoxon Rank Test.
Impaired Antiviral IgG Responses in the Absence of sIgM.
To determine the antibody responses to influenza virus infection, serum levels of anti–influenza virus-specific Igs in wild-type and sIgM−/− mice were assayed by ELISA. As expected, considerable levels of virus-binding natural IgM antibodies were present in wild-type mice before infection, and virus-specific IgM levels were further induced by infection (Fig. 3). No virus-specific IgM was detected in the sIgM−/− mice. The sIgM−/− mice responded to influenza virus infection, but the levels of virus-specific IgG2a were significantly reduced compared with wild-type controls (Fig. 3). By day 22 after infection, the levels of virus-specific IgG2a were comparable between sIgM−/− mice and wild-type controls. In addition, the levels of virus-specific IgG1 in sIgM−/− mice were significantly reduced on day 7 after infection compared with control mice. As IgG2a is the predominant IgG isotype produced in response to virus infection 20 21, these results suggest that one mechanism by which sIgM protects the host from influenza virus infection is by promoting an efficient neutralizing IgG antibody response.
Figure 3.
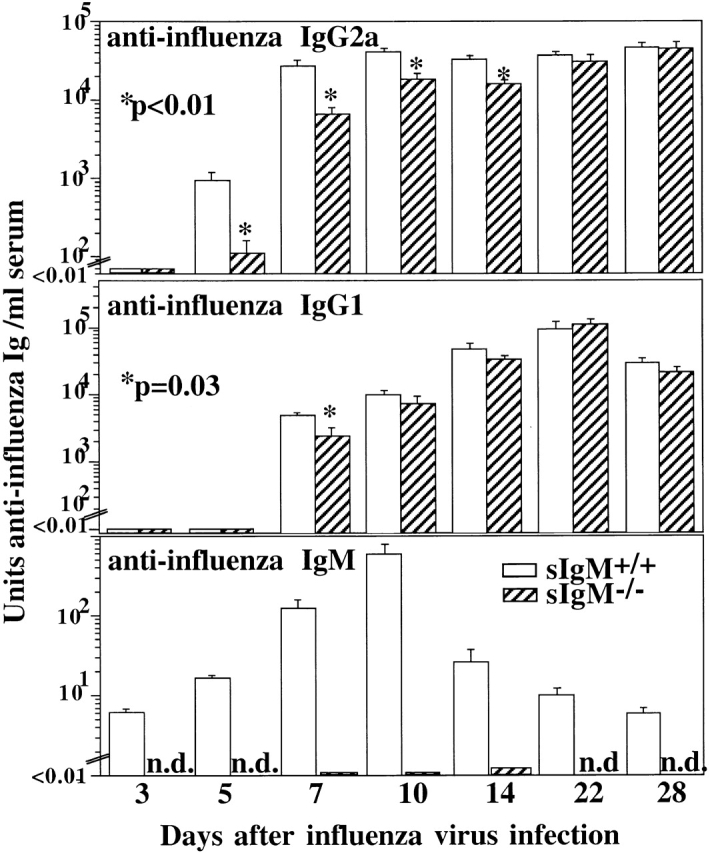
Impaired virus-specific IgG response in the absence of sIgM. Groups of 3-mo-old sIgM−/− and sIgM+/+ mice (n = 10) were infected with influenza virus Mem71. Mice were bled at indicated times, and the titers of influenza virus–specific IgM, IgG1, and IgG2a were measured by ELISA. Arbitrary units of antivirus Ig per milliliter were determined by comparison to a standard hyperimmune serum.
Lack of sIgM Does Not Affect the Height of the Virus-specific CD4+ Th Cell Response.
As cell-mediated immune mechanisms are known to mediate protection from influenza virus infection 22, we investigated whether the lack of IgM affects virus-induced T cell responses. The overall cellularity of mediastinal lymph nodes and spleen and lung parenchyma before and at day 7 after infection, the peak of the cellular response 23, was comparable in these mice (data not shown). Extensive FACS® analysis did not reveal significant differences in the number of T cells, the ratio of CD4+/CD8+ T cells, or the frequency of activated T cells accumulating in lung parenchyma of sIgM−/− and wild-type mice before and at day 7 of infection (data not shown).
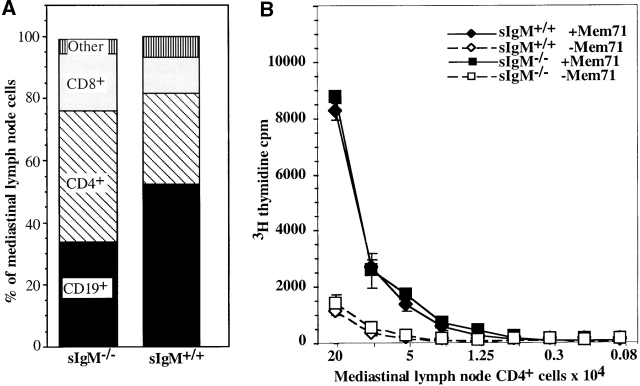
In the draining mediastinal lymph nodes that become visible only after influenza virus infection 12, the frequency of CD19+ B cells was reduced and CD3+ T cell numbers were increased in sIgM−/− mice compared with wild-type controls (Fig. 4 A). The ratio of CD4/CD8 was similar, ∼3:1 in both groups of mice. Thus, CD4+ T cell recruitment/accumulation in the draining lymph nodes is not negatively affected by the lack of sIgM.
Figure 4.
Lack of sIgM does not affect the T helper response in sIgM−/− mice. Respiratory tract draining mediastinal lymph nodes were isolated from sIgM−/− and sIgM+/+ mice 7 d after infection with influenza A/Mem71. (A) Frequencies of B cell (CD19+) and CD4+ and CD8+CD3+ T cell populations in the mediastinal lymph nodes were assessed by FACS®. (B) Indicated numbers of FACS®-purified CD3+CD4+ T cells from sIgM−/− and sIgM+/+ mice were cultured in the presence of Mem71 virus–pulsed (closed symbols) or nonpulsed (open symbols) irradiated splenic APCs. Incorporation of [3H]thymidine was determined as a measure of T cell proliferation.
FACS®-purified CD4+ T cells from mediastinal lymph nodes 7 d after influenza virus infection were stimulated in vitro with virus-pulsed APCs, and proliferation was determined by measuring [3H]thymidine incorporation. The results, shown in Fig. 4 B, demonstrate that CD4+ T cells from both types of mice responded to virus-pulsed APCs with a roughly eightfold increase in proliferation compared with nonpulsed APCs, suggesting that similar frequencies of influenza-virus specific CD4+ T cells are induced in the draining lymph nodes of wild-type and sIgM−/− mice and therefore that the presence of sIgM does not affect the magnitude of the antigen-reactive CD4+ T cell response. Thus, disturbance of the humoral immune response alone in the presence of an apparently normal T cell response leads to an increase in mortality rates after acute influenza virus infection.
B-1 and B-2 Cell–derived IgM Antibodies Differ in Virus Recognition.
We previously showed that influenza virus–binding sIgM is provided by two sources, B-1 cells and B-2 cells 4. To delineate the contributions of B-1 and B-2 cell–derived IgM on protection from influenza virus infection, we generated irradiation chimeras using allotype-congenic wild-type C57BL/6 (Igh-b) and B6.C20 (Igh-a) mice. In these mice, B-1 and B-2 cells and their sIgs can be distinguished with allotype- and isotype-specific mAbs. In recipients of Igh-b–expressing bone marrow cells and Igh-a–expressing PerC cells 2–3 mo after transfer, at least 85–90% of B-1 cells in the peritoneal cavity are PerC cell donor derived, whereas more than 99% of B-2 cells are bone marrow derived (Fig. 5). Similar results were found for the spleen (data not shown). In these recipients, about two-thirds of serum IgM is PerC cell derived and one-third is bone marrow donor derived (Igh-a, 570 μg/ml; Igh-b, 240 μg/ml; mean titers, n = 10).
Figure 5.
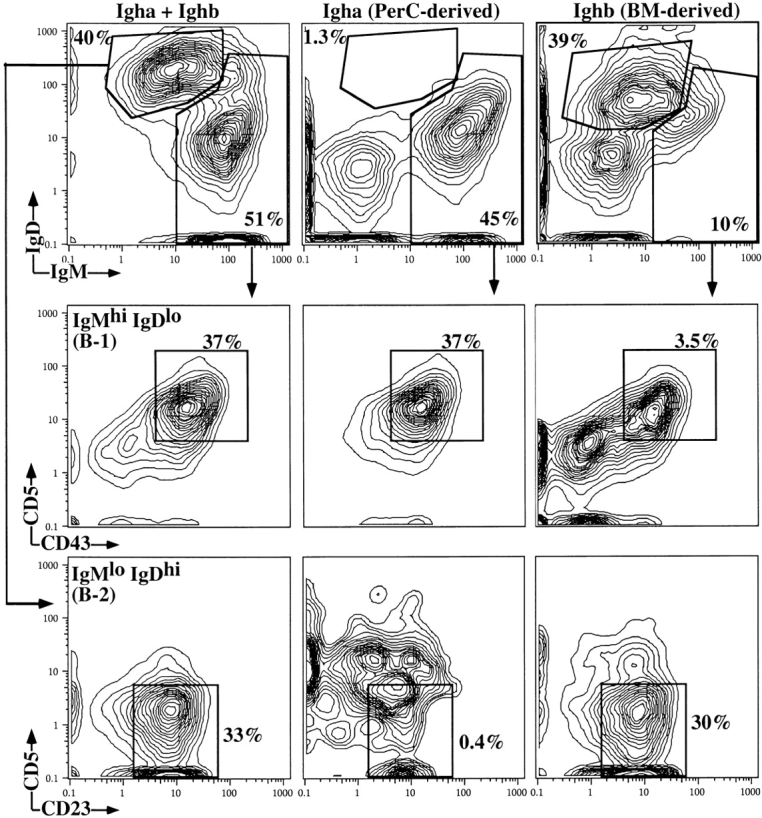
Adoptive cell transfer of bone marrow and PerC cells results in B-1/B-2 chimerism. Lethally irradiated C57BL/6 mice were reconstituted with bone marrow cells from C57BL/6 (Ighb) and PerC cells from congenic B6.C20 (Igha) mice. 2 mo after cell transfer, PerC cells were taken from the recipients, stained with indicated antibodies, and analyzed using 10-color flow cytometry. Shown are 5% contour plots of cells gated for exclusion of propidium iodide and expression of the pan-B cell marker CD19. B-1 and B-2 cells were distinguished by differences in their expression of total and allotype-specific IgM and IgD. B-1 cells were further distinguished from B-2 cells by their expression of CD11b (MAC-1) and their low expression of CD21 (data not shown). Percentages indicate frequencies of cells in gate of all PerC (CD19+) B cells.
To determine the contributions of B-1 and B-2 cells on virus-specific serum IgM levels, we determined by ELISA the titers of a- and b-allotype IgM that can react with a panel of four influenza virus strains before and 7 d after infection with influenza virus A/Mem71. The results, summarized in Fig. 6, reveal large differences in both titers and binding properties of IgM antibodies derived from these two different cell populations. Before infection with influenza virus, considerable titers of virus-binding IgM antibodies are found in the sera that can bind to at least four different influenza virus strains (A/Mem71, A/Guangdong, A/PR8, and B/Panama). Consistent with our previous findings 4, these presumably cross-reactive IgM antibodies are derived mainly from B-1 (Igh-a) cells. B-2 cell–derived virus-binding IgM antibodies, present at very low levels before infection, are induced significantly after infection with influenza A/Mem71. In contrast to the B-1 cell–derived IgM, however, these infection-induced B-2 cell–derived antibodies show greatly reduced reactivity with the other influenza A virus strains and, importantly, they show virtually no cross-reactivity with the influenza B virus strain B/Panama (Fig. 6). Thus, B-1 and B-2 cell–derived IgM antibodies recognize different antigens on the virus. The broad specificity of the B-1 cell–derived antibodies might suggest their binding to carbohydrate moieties on the virus, whereas the more restricted recognition pattern of the B-2 cell–derived IgM antibodies could suggest recognition of virus-specific peptide determinants.
Survival of Influenza Virus Infection Is Dependent on the Presence of Both Natural and Virus-induced IgM.
To assess the effects of the presence of natural and virus-induced IgM on the survival from influenza virus infection, we constructed irradiation chimeras that contained natural IgM–secreting B-1 cells but sIgM−/− B-2 cells or vice versa using a protocol identical to that used to generate the allotype chimeras. After infection with 0.8 × 106 PFU per mouse, chimeras that lacked either natural IgM or virus-induced IgM died at a rate (∼50%) similar to that of chimeras lacking both natural and virus-induced IgM (Fig. 7 A). In contrast, none of the chimeras that had received both natural IgM–secreting B-1 cells and virus-induced IgM–secreting B-2 cells died from the infection (P < 0.0001). When challenged with 1.6 × 106 PFU per mouse, chimeras that lacked natural IgM died considerably earlier (at day 3) than chimeras that lacked virus-induced IgM, although their final survival rates were similar (Fig. 7 A). Taken together, the data demonstrate that natural and induced IgM antibodies derived from B-1 and B-2 cells, respectively, represent nonredundant immune mechanisms that provide protection against influenza virus infection. Natural IgM appears to function early, before virus-induced IgM becomes available.
Figure 7.
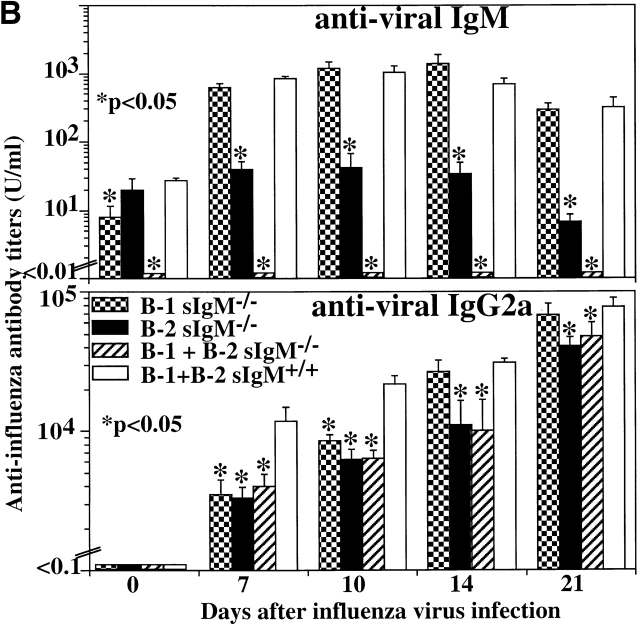
IgM antibodies secreted by both B-1 and B-2 cells are necessary for survival from influenza virus infection and for maximal antiviral IgG2a response. (A) Irradiation chimeras containing B-1 and B-2 cells from indicated sources were constructed as described in Materials and Methods. 2 mo after reconstitution, groups of chimeras (n = 9) were infected with influenza Mem71 at the indicated virus doses, and survival was monitored daily after infection. Similar results were obtained in two independent experiments after infection with 1.6 × 106 PFU per mouse. (B) Chimeras infected with influenza virus Mem71 at 0.8 × 106 PFU per mouse were bled at indicated times after influenza virus infection, and serum levels of virus-specific IgM and IgG2a antibodies were determined by ELISA. Arbitrary units of antivirus Ig per milliliter are shown.
Both B-1 and B-2 Cell–derived IgM Antibodies Are Required for Optimal Antiviral IgG2a Response.
2 mo after cell transfer, chimeras containing IgM-secreting B-1 cells, B-2 cells, or both B-1 and B-2 cells had similar levels of total serum IgM (991 ± 134 μg/ml, 1,167 ± 74 μg/ml, and 1,274 ± 113 μg/ml, respectively). Consistent with the data from the allotype-chimeric mice (Fig. 6), chimeras containing IgM-secreting B-1 but lacking IgM-secreting B-2 cells had normal levels of virus-binding IgM. The levels of natural virus-binding IgM did not increase significantly in these mice after infection (Fig. 7 B). In contrast, the levels of virus-specific IgM were significantly induced after influenza virus infection in chimeras containing either IgM-secreting B-2 cells and sIgM−/− B-1 cells, or both B-1 and B-2 IgM-secreting cells.
Within the first 10 d of virus infection, serum levels of virus-specific IgG2a were significantly lower in chimeras in which either only one type of B cell or none of the B cells was capable of secreting IgM as compared with chimeras with IgM-secreting B-1 and B-2 cells (Fig. 7 B). By day 14 after infection, antiviral IgG2a titers in chimeras that contained IgM-secreting B-2 cells but sIgM−/− B-1 cells reached the same levels as in chimeras with IgM-secreting B-1 and B-2 cells. In contrast, in the absence of B-2 cell–derived IgM but with B-1 cell–derived IgM, the IgG2a titers remained significantly lower even at day 21 after infection. Thus, in the absence of either B-1 cell–derived natural IgM or B-2 cell–derived virus-induced IgM, antiviral IgG2a responses are impaired, but B-2 cell–derived virus-induced IgM appears to have a stronger overall effect.
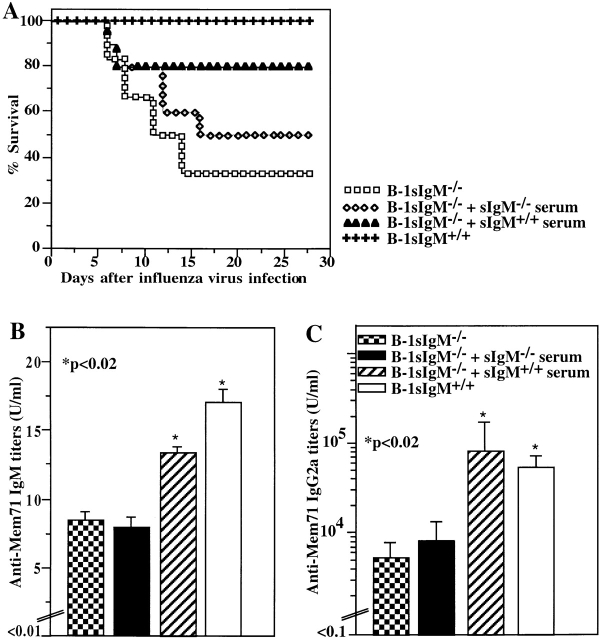
Application of Normal Serum IgM Alters the Outcome of Infection in sIgM−/− Mice.
The requirement for both natural and virus-induced IgM in optimal protection from influenza virus infection was further tested with chimeric mice that contained wild-type B-2 cells and sIgM−/− B-1 cells. To reconstitute natural IgM in these mice, normal serum was injected 1 h before infection and daily thereafter for 5 d. A control group received serum from sIgM−/− mice. Injection of wild-type serum significantly increased virus-binding natural IgM levels; however, the levels did not reach those in control mice reconstituted with wild-type B-1 and B-2 cells (Fig. 8 B). This partial reconstitution of virus-binding natural IgM increased the levels of survival (Fig. 8 A) compared to controls injected with sIgM−/− serum and to irradiation chimeras lacking both B-1 and B-2 cell–derived IgM. Consistent with the overall protective effect of administered natural IgM, the virus-specific IgG2a response on day 8 after infection in reconstituted animals was significantly increased compared with that seen in the control mice and reached levels similar to those in chimeras reconstituted with IgM-secreting B-1 and B-2 cells. Thus, the presence of natural IgM alone at the time of infection is insufficient to rescue sIgM−/− mice from death or to affect the height of the virus-specific IgG2a response. However, in conjunction with the early virus-induced IgM, full immune protection is achieved.
Figure 8.
Reconstitution of natural IgM–deficient chimeras results in increased survival and increased antiviral IgG2a serum titers after influenza virus infection. Lethally irradiated recipients were reconstituted with bone marrow from wild-type mice and PerC cells from sIgM−/− mice (B-1 sIgM−/−). Chimeras received daily injections of 0.5 ml of normal serum (sIgM+/+ serum; n = 10) or IgM-deficient serum (sIgM−/− serum; n = 10) immediately before infection and daily for 5 d after infection. Control mice received both bone marrow and PerC cells from sIgM+/+ mice (B-1 sIgM+/+). (A) Survival from infection with influenza A/Mem71 was monitored daily for 28 d. (B) Mem71-binding serum IgM titers were determined by ELISA in groups (n = 9) of noninfected chimeras after 3 d of daily injections with the indicated type of serum or in controls. Arbitrary units of antivirus Ig per milliliter are shown. (C) Mem71-specific IgG2a titers in the sera of indicated groups of irradiation chimeras were determined at day 8 after infection with influenza A/Mem71 by ELISA. Arbitrary units of antivirus Ig per milliliter are shown.
Discussion
Humoral responses to influenza virus infection in mice are provided by two discrete B cell populations: B-1 cells, which provide virtually all of the preexisting natural IgM antibodies, and B-2 cells, which secrete almost all of the infection-induced antiviral IgM and IgG2a antibodies (Fig. 6) 4. In this study, we demonstrate that IgM antibodies derived from both B-1 and B-2 cells can provide immune protection against influenza virus infection provided that they are both present. The lack of sIgM from either of the two B cell populations results in enhanced mortality similar to that seen in mice that lack both B-1 and B-2 cell–derived IgM. Furthermore, replenishment of mice lacking only B-1 cell–derived antibodies with normal serum IgM increases their rates of survival after infection. Thus, the effects of B-1 and B-2 cell–derived IgM are nonredundant and act in concert to provide full immune protection.
The requirement for IgM from both B-1 and B-2 cells suggests that IgM from the two cell types may function via discrete cooperating mechanisms to confer protection or that the antibodies might act at different times and/or locations after infection. Chimeras containing virus-induced IgM from B-2 cells but lacking natural IgM from B-1 cells appear to die sooner after infection than chimeras with natural IgM but no virus-induced IgM (Fig. 7 A). The preexisting, broadly reactive, B-1 cell–derived IgM may be required to provide initial protection against a novel viral pathogen before a stronger and more specific B-2 cell–derived IgM response is induced (Fig. 6). In the absence of natural IgM, very few antiviral antibodies are present when infection first occurs. Although the B-2 antibody response is induced, it may not be induced rapidly enough to confer optimal protection. Conversely, in the absence of virus-induced B-2 cell–derived IgM, the levels of natural IgM do not significantly increase (Fig. 6 and Fig. 7 B) and may not be sufficient to control the increasing viral load.
Our data demonstrate a clear role for B-1 cells in early protection from an acute viral infection. Together with our earlier demonstration that death from acute cecal puncture and ligation-induced sepsis is prevented by administration of B-1 cell–derived phosphatidyl choline–specific IgM antibodies 8, we have come closer to understanding the role of this cell population in immune protection. Protection from influenza virus infection by B-1 cell–derived IgM antibodies does not entail significant increases in their normal serum levels after infection (Fig. 6; reference 4), suggesting that clonal expansion and increased IgM secretion is not initiated in vivo after infection. This is consistent with observations in vitro that B-1 cells do not respond to cross-linking of the B cell receptor with proliferation, possibly due to the expression of inhibitory coreceptors such as CD5 24. However, B-1 cells can respond to certain T cell–dependent antigens with increased antibody production 25, leaving open the possibility that increased focal production of antiviral IgM might occur only locally in the respiratory tract that binds to antigen and thus can not be detected in the serum.
A number of studies have demonstrated that passive transfer of immune serum or monoclonal antiviral antibodies can provide protection from influenza virus infection 6 11 26 27 28 29. In addition, B cell–deficient mice were shown to have reduced survival rates after influenza virus infection 6 30. Our findings are consistent with these observations, confirming that B cells and B cell products are part of the protective immune responses to influenza virus infection. Furthermore, by demonstrating that both natural and virus-induced IgM are required for protection, our findings explain the apparently normal clearance of influenza virus in the absence of CD4+ T cells 31 32. Although nude mice and mice deficient in CD4+ T cells lack the ability to mount T cell–dependent antibody responses, they should have normal levels of natural IgM and can mount T cell–independent IgM responses 10. Together with other immune defense mechanisms, such as the virus-specific CD8+ T cell response, these IgM responses are apparently sufficient to provide the observed immune protection.
Our findings also help to explain the apparent discrepancy concerning the role of sIgM during acute primary infection with VSV and influenza virus. Studies on VSV showed that the early induction of IgM antibodies confers immune protection during primary infection 9 10. In contrast, transfer of monoclonal anti–influenza virus-specific IgM antibodies into SCID mice was shown to be protective only when antibodies were given before infection 11, indicating that IgM antibodies are protective only during recall responses. Similar to the transfer of antibodies into SCID mice after initiation of infection, chimeras that lack natural IgM from B-1 cells can mount a normal B-2 cell antibody response but are still susceptible to infection (Fig. 7 A). The failure to protect SCID mice by transfer of monoclonal virus-specific IgM antibodies after initiation of infection is probably because SCID mice lack preexisting B-1 cell–derived natural IgM antibodies. Consistent with this notion, we show here (Fig. 8 A) that transfer of natural IgM–containing serum into mice deficient only in natural IgM can increase survival after influenza virus infection. Thus, the outcome of a primary infection with influenza virus, similar to that of a primary infection with VSV, is critically influenced by the presence of sIgM.
A question that arises from our studies is how sIgM provides immune protection against a viral infection. Binding of virus by IgM may neutralize viral infectivity 33 or may lead to complement activation and promote clearance by phagocytic cells via complement receptor–mediated uptake 34. Our studies also reveal a previously unsuspected mechanism by which sIgM can confer protection against virus infection: by promoting an efficient antiviral IgG response (Fig. 3). Consistent with the requirement for both natural and virus-induced IgM for maximal survival from an acute viral infection, both IgM antibodies are required for efficient induction of the IgG response, in particular the induction of virus-specific IgG2a (Fig. 7 B). Thus, sIgM protects from viral infection at least in part by promoting an efficient IgG response to the virus. The isotype most strongly affected by the lack of IgM depends on the type of antigen encountered. The induction of IgG2a, the predominant isotype produced in response to influenza virus infection 20 21, is most severely decreased in sIgM−/− mice (Fig. 3). Other studies had shown that IgG1, the predominant isotype response to KLH 35, is most severely reduced in sIgM−/− mice after KLH immunization 7 36. The presence of sIgM therefore maximizes the induction and/or secretion of antigen-specific IgG (Fig. 3).
The requirement for sIgM in the antiviral IgG response is consistent with our previous observations showing that sIgM is required for efficient IgG antibody response to suboptimal doses of T cell–dependent antigens 7 36. However, the respective roles of pre-existing natural IgM and antigen-induced IgM in the ensuing IgG response were not delineated in those previous studies. The impaired IgG responses in chimeras lacking either natural or virus-induced IgM clearly show that both IgM are required, although antigen-induced IgM from B-2 cells appears to have a stronger effect (Fig. 7 B). The lack of sIgM seems to directly affect the B cell and not the helper T cell response. B cell accumulation and/or recruitment into the respiratory tract draining lymph nodes is reduced after influenza virus infection in sIgM−/− mice compared with controls, whereas the CD4+ T cell response appears normal (Fig. 4).
Although the precise mechanisms by which IgM enhances the B cell response are not known, because complement and complement receptors are crucial for the induction of normal antibody responses 37 38 39, previous studies proposed that natural IgM antibodies augment IgG responses by activating complement to form immune complexes 7 36. These complexes may then activate B cells by cross-linking B cell receptors and/or be trapped on follicular dendritic cells for efficient germinal center reaction. The fact that the newly induced IgM is more effective at promoting IgG response than natural IgM suggests that focal secretion of virus-specific IgM immediately after B cell activation may directly activate the IgM-secreting B cells for IgG production. As discussed more extensively elsewhere 40, sIgM might act directly on the B cells in an autocrine fashion through engagement of their complement receptors, which are known to provide important costimulatory signals 39. Such engagement of complement receptors could lead to increased B cell activation and proliferation and therefore an increase in the size of the initial B cell response. Alternatively, polymeric IgM might enhance antigen-mediated Ig receptor triggering by cross-linking surface Ig receptors on B cells that have bound the antigen, thus also affecting clonal burst size. The reduced number of B cells found in the draining lymph nodes of the respiratory tract 7 d after influenza virus infection in sIgM−/− mice compared with control mice (Fig. 4 A) further supports the notion that sIgM enhances early clonal expansion of antigen-specific B cells.
In summary, we provide clear evidence for a critical role for sIgM, and therefore for the humoral immune response, in immune protection from acute influenza virus infection. We also show that, perhaps because of temporal and/or spatial constraints, IgM secreted by both B-1 and B-2 cells is required for optimal protection against infection. B-1 cell–derived natural IgM appears to provide initial defense, whereas B-2 cell–derived, virus-stimulated IgM provides additional protection and functions as an autocrine regulator that, in concert with B-1 cell–derived IgM, boosts efficient IgG responses to the virus.
Acknowledgments
We thank Marianne Boes and Michiko Nakajima for technical assistance.
This work was supported in part by National Institutes of Health grant AI34762-34 (to L.A. Herzenberg) and AI41762 (to J. Chen).
Footnotes
Abbreviations used in this paper: MDCK, Mardin-Darby canine kidney; s, secreted; VSV, vesicular stomatitis virus.
References
- Coutinho A., Kazatchkine M.D., Avrameas S. Natural autoantibodies. Curr. Opin. Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Natural autoantibodiesfrom ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol. Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Casali P., Schettino E.W. Structure and function of natural antibodies. Curr. Top. Microbiol. Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- Baumgarth N., Herman O.C., Jager G.C., Herzenberg L.A., Herzenberg L.A. Innate and acquired immunities to influenza virus are provided by distinct B cells. Proc. Natl. Acad. Sci. USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr. Top. Microbiol. Immunol. 1989;146:13–24. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Mozdzanowska K., Furchner M., Washko G., Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Boes M., Esau C., Fischer M.B., Schmidt T., Carroll M., Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- Boes M., Prodeus A.P., Schmidt T., Carroll M.C., Chen J. A critical role of natural immunoglobulin M in immediate defense against systemical bacterial infection. J. Exp. Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T., Bachmann M.F., Bluethmann H., Kikutani H., Hengartner H., Zinkernagel R.M. T-independent activation of B cells by vesicular stomatitis virusno evidence for the need of a second signal. Cell. Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Zinkernagel R.M. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- Palladino G., Mozdzanowska K., Washko G., Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Brown L., Jackson D., Kelso A. Novel features of the respiratory tract T-cell response to influenza virus infectionlung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J. Virol. 1994;68:7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor A., Stall A., Adams S., Watanabe K., Herzenberg L. De novo development and self-replenishment of B cells. Int. Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- Tannock G.A., Paul J.A., Barry R.D. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA.6/60-ca, and parental strains with similar surface antigens. Infect. Immun. 1984;43:457–462. doi: 10.1128/iai.43.2.457-462.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R.R. Purification and characterization of monoclonal antibodies Weir D.M. Handbook of Experimental Immunology, Vol. 1 1986. 13 Blackwell Scientific Publications; Palo Alto, CA: 1–13.9. [Google Scholar]
- Anderson M.T., Baumgarth N., Haugland R.P., Gerstein R.M., Tjioe I., Herzenberg L.A., Herzenberg L.A. Pairs of violet-light-excited fluorochromes for flow cytometric analysis. Cytometry. 1998;33:435–444. doi: 10.1002/(sici)1097-0320(19981201)33:4<435::aid-cyto7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Roederer M., Kantor A.B., Parks D.R., Herzenberg L.A. Cy7PE and Cy7APCbright new probes for immunofluorescence. Cytometry. 1996;24:191–197. doi: 10.1002/(SICI)1097-0320(19960701)24:3<191::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Roederer M., DeRosa S., Gerstein R., Anderson M., Bigos M., Stovel R., Nozaki T., Parks D., Herzenberg L., Herzenberg L. 8-Color, 10-parameter flow cytometryelucidation of complex leukocyte heterogeneity. Cytometry. 1997;29:1–12. doi: 10.1002/(sici)1097-0320(19971201)29:4<328::aid-cyto10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Bigos M., Baumgarth N., Jager G.C., Herman O.C., Nozaki T., Stovel R.T., Parks D.R., Herzenberg L.A. Nine color eleven parameter immunophenotyping using three laser flow cytometry. Cytometry. 1999;36:36–45. doi: 10.1002/(sici)1097-0320(19990501)36:1<36::aid-cyto5>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Coutelier J.P., van der Logt J.T., Heessen F.W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocart M.J., Mackenzie M.J.S., Steward G.A. The IgG subclass responses induced by wild-type, cold-adapted and purified hemagglutinin from influenza virus A/Queensland/6/72 in CBA/CaH mice. J. Gen. Virol. 1988;69:1873–1882. doi: 10.1099/0022-1317-69-8-1873. [DOI] [PubMed] [Google Scholar]
- Doherty P.C., Topham D.J., Tripp R.A., Cardin R.D., Brooks J.W., Stevenson P.G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Baumgarth N., Brown L., Jackson D., Kelso A. Novel features of the T cell response to influenza infectionT cells increase levels of IFN-γ and maintain high levels of IL-5 and IL-10. J. Virol. 1994;68:7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikah G., Carey J., Ciallella J.R., Tarakhovsky A., Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- Taki S., Schmitt M., Tarlinton D., Forster I., Rajewsky K. T cell-dependent antibody production by Ly-1 B cells. Ann. NY Acad. Sci. 1992;651:328–335. doi: 10.1111/j.1749-6632.1992.tb24632.x. [DOI] [PubMed] [Google Scholar]
- Kris R.M., Yetter R.A., Cogliano R., Ramphal R., Small P.A. Passive serum antibody causes temporary recovery from influenza virus infection of the nose, trachea and lung of nude mice. Immunology. 1988;63:349–353. [PMC free article] [PubMed] [Google Scholar]
- Renegar K.B., Small J.P.A. Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- Virelizier J.L. Host defenses against influenza virusthe role of antihemagglutinin antibody. J. Immunol. 1975;115:434–439. [PubMed] [Google Scholar]
- Scherle P.A., Palladino G., Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 1992;148:212–217. [PubMed] [Google Scholar]
- Bot A., Isobe H., Bot S., Schulman J., Yokoyama W.M., Bona C.A. Cellular mechanisms involved in protection and recovery from influenza virus infection in immunodeficient mice. J. Virol. 1996;70:5668–5672. doi: 10.1128/jvi.70.8.5668-5672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp R.A., Sarawar S.R., Doherty P.C. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2IAb gene. J. Immunol. 1995;155:2955–2959. [PubMed] [Google Scholar]
- Allan W., Tabi Z., Cleary A., Doherty P.C. Cellular events in the lymph node and lung of mice with influenza. Consequence of depleting CD4+ T cells. J. Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- Taylor H.P., Dimmock N.J. Mechanisms of neutralization of influenza virus by IgM. J. gen . Virol. 1985;66:903–907. doi: 10.1099/0022-1317-66-4-903. [DOI] [PubMed] [Google Scholar]
- Caroll M.C. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Kelso A., Groves P., Troutt A.B., Pech M.H. Rapid establishment of a stable IL-4/IFN-gamma production profile in the antigen-specific CD4+ T cell response to protein immunization. Int. Immunol. 1994;6:1515–1523. doi: 10.1093/intimm/6.10.1515. [DOI] [PubMed] [Google Scholar]
- Ehrenstein M.R., O'Keefe T.L., Davies S.L., Neuberger M.S. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croix D.A., Ahearn J.M., Rosengard A.M., Han S., Kelsoe G., Ma M., Carroll M.C. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J. Exp. Med. 1996;183:1857–1864. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H., Holers V.M., Li B., Fang Y.-F., Mariathasan S., Goellner J., Strauss-Schoenberger J., Karr R.W., Chaplin D.D. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA. 1996;96:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T.F., Inaoki M., Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- Baumgarth N. A two phase model of B cell activation. Immunol. Rev. 2000;In press doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]