Abstract
The roles of the NADPH phagocyte oxidase (phox) and inducible nitric oxide synthase (iNOS) in host resistance to virulent Salmonella typhimurium were investigated in gp91phox −/−, iNOS −/−, and congenic wild-type mice. Although both gp91phox −/− and iNOS −/− mice demonstrated increased susceptibility to infection with S. typhimurium compared with wild-type mice, the kinetics of bacterial replication were dramatically different in the gp91phox −/− and iNOS −/− mouse strains. Greater bacterial numbers were present in the spleens and livers of gp91phox −/− mice compared with C57BL/6 controls as early as day 1 of infection, and all of the gp91phox −/− mice succumbed to infection within 5 d. In contrast, an increased bacterial burden was detected within reticuloendothelial organs of iNOS −/− mice only beyond the first week of infection. Influx of inflammatory CD11b+ cells, granuloma formation, and serum interferon γ levels were unimpaired in iNOS −/− mice, but the iNOS-deficient granulomas were unable to limit bacterial replication. The NADPH phagocye oxidase and iNOS are both required for host resistance to wild-type Salmonella, but appear to operate principally at different stages of infection.
Keywords: Salmonella, virulence, innate immunity, oxidative, nitrosative
Introduction
Salmonella infections afflicting animals and humans pose a serious medical and veterinary problem worldwide. Despite considerable progress in understanding the genetic determinants of Salmonella virulence, rational vaccine design is still hampered by an inadequate understanding of the essential elements of host resistance to Salmonella. Much of the current knowledge of mechanisms of natural resistance and acquired immunity in salmonellosis has been generated in the mouse typhoid model 1 2 3, in which polymorphonuclear and mononuclear phagocytes play a critical role by limiting initial bacterial replication 4 5 6 7 8 9 10. In immunocompetent mice, bacterial growth in the reticulendothelial system is suppressed by the end of the first week of infection, resulting in a plateau phase 11. This requires an influx of bone marrow–derived mononuclear cells and coincides with the onset of hepatosplenomegaly and granuloma formation, but does not require functional CD4+ or CD8+ T cells 11. Soluble factors including TNF-α, IFN-γ, IL-12, and IL-18 are essential for the suppression of bacterial growth during sublethal Salmonella infections 9 12 13 14 15 16 17 18 19 20 21 22, and are specifically required for granuloma formation 9 12 17 and macrophage activation 14 20. Secondary infection in immunized mice additionally involves Salmonella-immune CD4+ and CD8+ T cells and Salmonella-specific antibodies 3 17.
The mechanisms by which phagocytes inhibit or kill virulent salmonellae are incompletely characterized, but the NADPH phagocyte oxidase has been strongly implicated 23 24. The NADPH phagocyte oxidase catalyzes the production of superoxide (O2·−) that can be metabolized to a variety of toxic reactive oxygen species (ROS). Patients deficient in the NADPH phagocyte oxidase are susceptible to recurrent microbial infections, including salmonellosis 25, in a clinical condition known as chronic granulomatous disease (CGD; for a review, see reference 26). The gp91phox gene encoding an essential component of the NADPH phagocyte oxidase has been targeted in mice, resulting in the elimination of oxidative burst activity in neutrophils and macrophages and an increased susceptibility to microorganisms including Salmonella typhimurium 24, Aspergillus fumigatus, Staphylococcus aureus 26, and Mycobacterium tuberculosis 27.
The role of the inducible nitric oxide (NO) synthase (iNOS) in the ability of macrophages to inhibit or kill Salmonella has been somewhat less clear 28 29. Dimeric iNOS catalyzes the conversion of l-arginine to l-citrulline with the production of NO·, which can be further metabolized to a variety of congeners (reactive nitrogen species [RNS]) with antimicrobial activity 30. Activated macrophages display increased expression of iNOS and elevated production of NO· derivatives (for a review, see reference 30). The role of NO· derivatives in host resistance to microbes has been documented in vitro and in vivo in a large number of infection models (for a review, see reference 31).
Generation of O2·− and NO· derivatives in response to Salmonella infection has been documented both in vivo 20 32 and in vitro 24 33 34 35. The activity of iNOS is positively regulated by several cytokines (IL-12, IFN-γ, TNF-α) known to be essential for host resistance to Salmonella 12 20 30. Evidence obtained using metabolic inhibitors and immunodeficient knockout mice suggests that oxygen radicals mediate resistance to Salmonella in mice and are required for maximal bacterial killing by macrophages 24 29. Furthermore, data obtained using NO synthase (NOS) inhibitors L-NMMA or aminoguanidine support a role for NO· derivatives in host resistance to Salmonella 32 33 36. However, some evidence from in vitro models has suggested that the respiratory burst oxidase, but not iNOS, is essential to kill virulent Salmonella, whereas both ROS and RNS are involved in the killing of some mutant Salmonella strains 24 28 29. Moreover, observations using the NOS-inhibitor aminoguanidine have suggested that iNOS may regulate infiltration of inflammatory cells in the tissues, rather than exert direct antimicrobial activity against Salmonella 36.
Many of the published studies examining phox-dependent and iNOS-dependent host defenses in salmonellosis have used attenuated bacterial strains and innately susceptible mice, making it somewhat difficult to ascertain the role of these defenses in infections with virulent Salmonella 29 32 33 36. The goal of this study was to clarify the roles of the NADPH phagocyte oxidase and iNOS in limiting bacterial replication during experimental infection with virulent S. typhimurium, using gp91phox −/− mice in a Salmonella-susceptible C57BL/6 (Nramp1s) genetic background, and iNOS −/− mice in both C57BL/6- (Nramp1s) and Salmonella-resistant 129Sv (Nramp1r) genetic backgrounds. Sublethal inocula of wild-type Salmonella were administered to facilitate the detection of phox-dependent and iNOS-dependent antimicrobial actions.
Materials and Methods
Animals.
C57BL/6 mice (Nramp1s) and 129Sv mice (Nramp1r) were purchased from Harlan Olac, Ltd. Mice homozygous for a targeted mutation in the gp91 subunit of the NADPH oxidase (gp91phox −/−) on a C57BL/6 background 26 were bred at the University of Colorado School of Medicine Center for Laboratory Animal Care. Mice homozygous for a targeted mutation in the iNOS gene (iNOS −/− [37, 38]) on a C57BL/6 or 129Sv background were bred at either BαK Universal, Ltd. or the Imperial College animal unit.
Bacteria.
S. typhimurium M525P, a variant of S. typhimurium M525 18, is a wild-type strain of intermediate virulence. S. typhimurium C5 is a highly virulent strain 6. For intravenous inoculation, bacteria were grown at 37°C as stationary overnight cultures in Luria-Bertani (LB) broth (Difco). Aliquots were snap frozen and stored in liquid nitrogen. The inoculum was diluted in PBS and injected in a lateral tail vein. The number of viable bacteria in each inoculum was checked by dilution and pour plating onto LB agar plates.
Bacterial Enumeration in Organ Homogenates.
Mice were killed by cervical dislocation. Spleens and livers were aseptically removed and homogenized in a Colworth Stomacher in 10 ml of cold distilled water 11. Viable counts were determined using pour plates of LB agar.
LD50 Determinations.
Groups of mice were injected intravenously with 10-fold decreasing doses of S. typhimurium M525P or S. typhimurium C5. Parallel groups of age-matched uninfected mice were observed in parallel during each experiment. Mortality was scored over a 30-d period. LD50 values were calculated according to the method of Reed and Muench 39.
IFN-γ ELISA.
Mice were bled from a lateral tail vein. Sera were collected and stored at −70°C. IFN-γ was measured by capture ELISA using antibody pairs and cytokine standards purchased from BD PharMingen.
For IFN-γ determinations, 96 multiwell ELISA plates (MaxiSorp Immuno Plate; Nunc) were coated overnight at 4°C with 50 μl/well of a rat anti–mouse IFN-γ IgG1 monoclonal capture antibody (clone R4-6A2) in 0.1 M NaHCO3 buffer, pH 9.5 at 2 μg/ml. After blocking with PBS supplemented with 10% FCS at 37°C for 1 h, twofold serum dilutions were loaded in 50 μl in triplicate, and the plates were incubated at 37°C for 2 h. Serial twofold dilutions of rIFN-γ ranging from 20 ng/ml to 40 pg/ml were included as standards. Biotinylated rat anti–mouse IFN-γ IgG1 mAb (clone XMG1.2; 100 μl/well) at 1 μg/ml in PBS supplemented with 10% FCS was added for 1 h at 37°C, after which 100 μl/well of peroxidase-labeled streptavidin at 2.5 μg/ml (Sigma-Aldrich) in PBS supplemented with 10% FCS was added for 45 min at room temperature. Ortho-phenylenediamine (OPD; 1 mg/ml in 0.2 M Na2HPO4, 0.1 M citrate buffer) in the presence of H2O2 was used to develop the plates. The reaction was stopped by adding 15 μl/well of 3 M H2SO4. OD was read at 490 nm. IFN-γ values (ng/ml) were determined by comparison with the standard curve. 80 pg/ml was considered to be the lower limit of sensitivity of this IFN-γ ELISA test.
Histology.
8-μm thick sections were prepared from tissues fixed by immersion in 10% formalinized saline, and were stained with hematoxylin and eosin. The number of focal lesions in several nonserial liver sections was counted at low-power magnification (×10) using a Nikon Labophot 2 microscope. The results were obtained by counting 15 fields per organ.
Staining for Flow Cytometry Analysis.
Single cell suspensions prepared from mouse spleens were washed once in Ca2+- and Mg2+-free Dulbecco's PBS containing 2 mM EDTA (Sigma-Aldrich) by centrifugation at 300 g and incubation in Gey's solution to lyse the red cells. White cells were washed twice more before being resuspended at a concentration of 107/ml in PBS containing 1% FCS, 0.1% NaN3, and 2 mM EDTA. All further steps were performed on ice. Antibodies for flow cytometry were purchased from BD PharMingen, with the exception of the anti–I-Ab MHC class II FITC-conjugated antibody, which was obtained from Caltag Laboratories. FcγIII/II receptors were blocked by incubating cells with the rat anti–mouse CD16/CD32 (IgG2b; clone 2.4G2) mAb for 10 min. The following antibodies were used: FITC–anti-CD4 (IgG2a; clone RM4-4), FITC–anti-CD8 (IgG2a; clone 53-6.7), PE–anti-CD11b (IgG2b; clone M1/70), FITC–anti-B220 (IgG2a; clone RA3-6B2), PE–anti-CD25 (IgG1; clone PC61), and FITC–anti–I-Ab MHC class II (IgM; clone 28-16-8S). Similarly conjugated isotype control antibodies were used in parallel. Cells were stained for 30 min with the relevant antibodies, washed with PBS, fixed with 1% paraformaldehyde, and kept in the dark until use. 1,000 events were acquired on a Becton Dickinson FACScan™.
Statistical Analysis.
Student's t test was used to determine the significance of differences between controls and experimental groups. Differences between experimental groups were considered significant for P values <0.05.
Results
Mortality of S. typhimurium Infection in Wild-type, gp91 phox−/−, and iNOS−/− Mice.
gp91phox −/− mice and congenic C57BL/6 wild-type control mice were infected intravenously with ∼600 CFU of S. typhimurium M525P. All gp91phox −/− mice were dead by day 4–5 of infection, despite the modest inoculum, whereas no deaths were observed among the C57BL/6 controls (Fig. 1 A). Thus, NADPH phagocyte oxidase–dependent antimicrobial mechanisms are required for host resistance in the early stages of Salmonella infection in mice.
Figure 1.
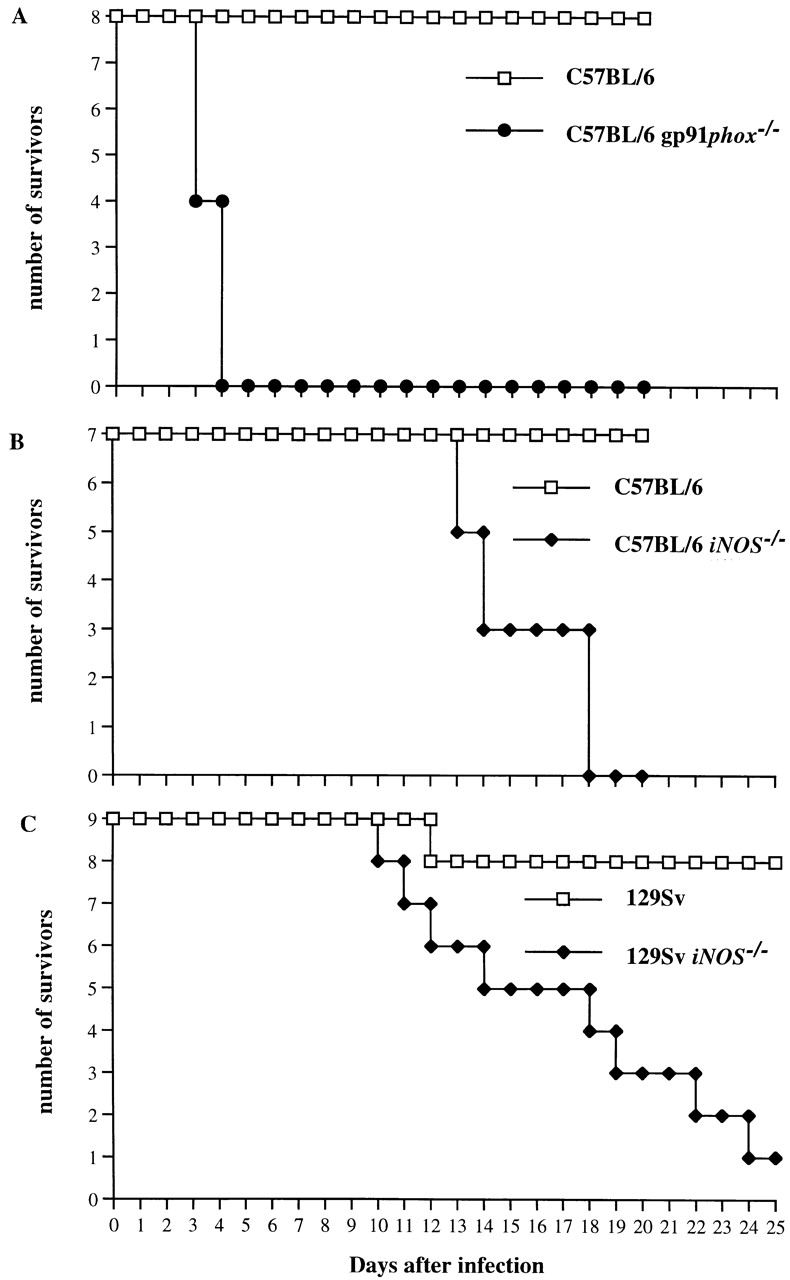
Survival of gp91phox −/−, iNOS −/−, and congenic wild-type mice infected with S. typhimurium. (A) Eight gp91phox −/− mice and eight congenic C57BL/6 controls (Nramp1s, Salmonella susceptible) were infected intravenously with ∼600 CFU of S. typhimurium M525P, a strain of intermediate virulence. (B) Seven iNOS −/−mice and seven congenic C57BL/6 controls (Nramp1s) were infected intravenously with ∼105 CFU of S. typhimurium M525P. (C) Nine iNOS −/− mice and nine congenic 129Sv controls (Nramp1 r, Salmonella resistant) were infected with ∼105 CFU of the highly virulent S. typhimurium C5 strain. Results are expressed as number of survivors at times after infection.
Groups of five to seven iNOS −/− mice on the Salmonella-susceptible C57BL/6 or Salmonella-resistant 129Sv strain backgrounds were infected intravenously with 10-fold decreasing doses ranging from 107–103 CFU of moderately virulent S. typhimurium M525P or the highly virulent S. typhimurium C5 strain, respectively. Congenic wild-type C57BL/6 or 129Sv control mice were infected in parallel experiments. Log10 LD50 values 1 mo after infection were found to be 3.35 ± 0.06 in C57BL/6 iNOS −/− mice and 4.29 ± 0.19 in 129Sv iNOS −/− mice, compared with 5.42 ± 0.22 and 5.28 ± 0.07 in the respective control groups. Thus, iNOS −/− mice show increased susceptibility to Salmonella, but can still survive infection with low bacterial inocula over a 30-d observation period. This contrasts with the gp91phox −/− mice, for whom even a very modest inoculum was rapidly lethal.
On the basis of the LD50 determinations, seven C57BL/6 iNOS −/− mice and seven congenic wild-type controls were infected intravenously with ∼105 CFU of S. typhimurium M525P (Fig. 1 B). Similarly, nine 129Sv iNOS −/− mice and nine congenic wild-type mice were infected with ∼105 CFU of S. typhimurium C5 (Fig. 1 C). All of the C57BL/6 iNOS −/− mice died within 18 d after inoculation (with deaths beginning on day 13), whereas all of the C57BL/6 wild-type mice survived the infection. Similarly, eight out of nine (89%) iNOS −/− mice in the 129Sv background died within 24 d after inoculation (with deaths beginning on day 10), whereas only one of nine (11%) wild-type control mice died throughout the course of the experiment. Thus, iNOS −/− mice infected with S. typhimurium show increased susceptibility to infection that leads to death of the animals during the second and third week of infection. No deaths were observed in parallel groups of five uninfected iNOS −/− or wild-type mice of either strain.
S. typhimurium Organism Burden After Infection of Wild-type, gp91phox−/−, and iNOS−/− Mice.
Hepatic and splenic bacterial burden was quantified in gp91phox −/− and congenic C57BL/6 wild-type control mice after intravenous infection with ∼600 CFU of S. typhimurium M525P. Bacterial counts by day 1 of infection were already significantly higher in the NADPH phagocyte oxidase–deficient mice compared with control animals, and were dramatically higher by day 3 of infection (Fig. 2A and Fig. B).
Figure 2.
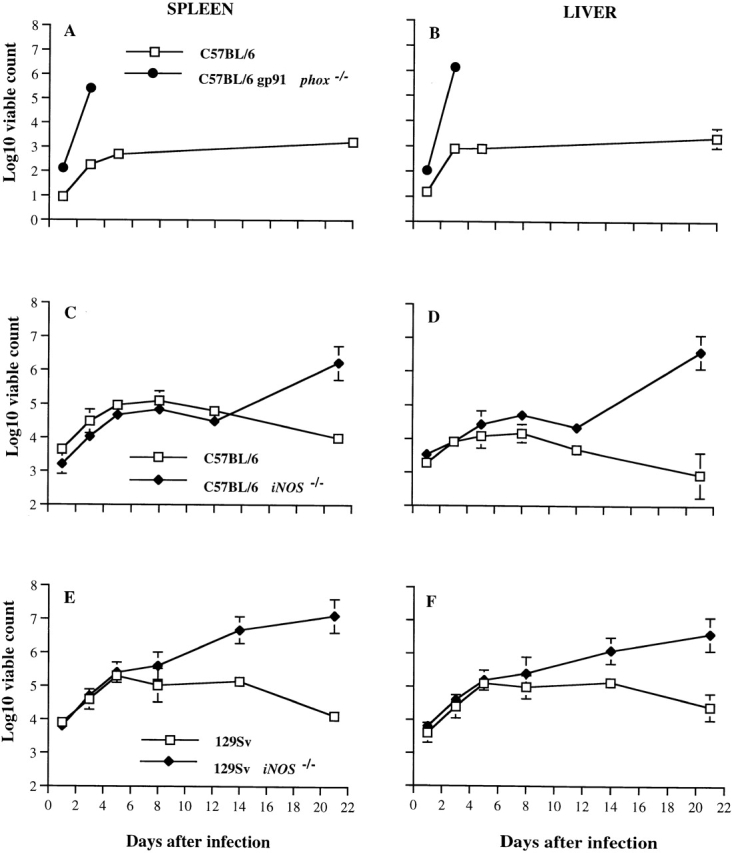
Bacterial counts in liver and spleen of gp91phox −/−, iNOS −/−, and congenic wild-type mice infected with S. typhimurium. (A and B) gp91phox −/− mice and congenic C57BL/6 controls (Nramp1s) were infected intravenously with ∼600 CFU of S. typhimurium M525P, a wild-type strain of intermediate virulence. All Salmonella-infected gp91phox −/− mice were dead by day four. (C and D) iNOS −/− mice and congenic C57BL/6 controls (Nramp1s) were infected with 6.5 × 104 CFU of S. typhimurium M525P. (E and F) iNOS −/− mice and congenic 129Sv controls (Nramp1r) were infected with 8.5 × 104 CFU of the highly virulent wild-type S. typhimurium C5 strain. Spleen and liver counts were determined at time points thereafter. The results are expressed as Log10 viable count (mean ± SD) obtained from groups of four mice per data point.
In parallel experiments, iNOS −/− and congenic C57BL/6 wild-type mice were infected with 6.5 × 104 CFU of S. typhimurium M525P, whereas iNOS −/− and congenic 129Sv wild-type controls were infected with 8.5 × 104 CFU of S. typhimurium C5. Fig. 2C and Fig. D shows that spleen and liver bacterial counts were similar in C57BL/6- and iNOS −/−- derivative mice during the first 5 d of infection, despite the administration of higher inocula than those administered to the gp91phox −/− mice. However, by days 8 and 12, the counts in the livers of iNOS −/− mice were significantly higher than those of C57BL/6 control mice; by day 21, the bacterial counts in both spleens and livers of C57BL/6 iNOS −/− mice were considerably higher in comparison to controls. The course of infection and differences in bacterial counts between mutant and wild-type mice were similar when a lower inoculum (6.5 × 103 CFU) was administered, except for lower absolute values and an absence of deaths observed throughout the 30-d experiment (data not shown). Fig. 2E and Fig. F shows the course of the infection with S. typhimurium C5 in 129Sv and congenic iNOS −/− mice. Bacterial counts in iNOS −/− mutant and wild-type mice were similar until day 8 of the infection. Thereafter, both spleen and liver counts were significantly higher in the iNOS −/− mice. Repeat experiments gave similar results (data not shown).
Histopathology of S. typhimurium Infection in Wild-type, gp91phox−/−, and iNOS−/− Mice.
Spleen weight was monitored at specified times in the experiments described in Fig. 2. The spleens of infected gp91phox −/− increased in size more rapidly (P < 0.01) than those of congenic C57BL/6 wild-type control mice (Fig. 3 A) after infection with 600 CFU S. typhimurium M525P, in parallel with organism burden. As shown in Fig. 3 B, a similar increase in spleen weight was observed during the first 8 d of the infection in C57BL/6 iNOS −/− mice and C57BL/6 mice infected with 6.5 × 104 CFU of S. typhimurium M525P. The mean spleen weight was slightly higher in C57BL/6 mice than in C57BL/6 iNOS −/−on day 12 (P = 0.02), but no significant differences in spleen weight between iNOS −/− mutant and wild-type mice were observed by day 21 of the infection. A similar experiment performed with a lower challenge dose (6.5 × 103 CFU per mouse) showed similar spleen weights in iNOS −/− mutant and C57BL/6 wild-type mice at all time points (data not shown). Fig. 3 C shows that significant (P = 0.02) differences in spleen weight between iNOS −/− mice and congenic 129Sv wild-type mice infected with 8.5 × 104 CFU of S. typhimurium C5 appeared by day 8 after infection and persisted until at least day 14.
Figure 3.
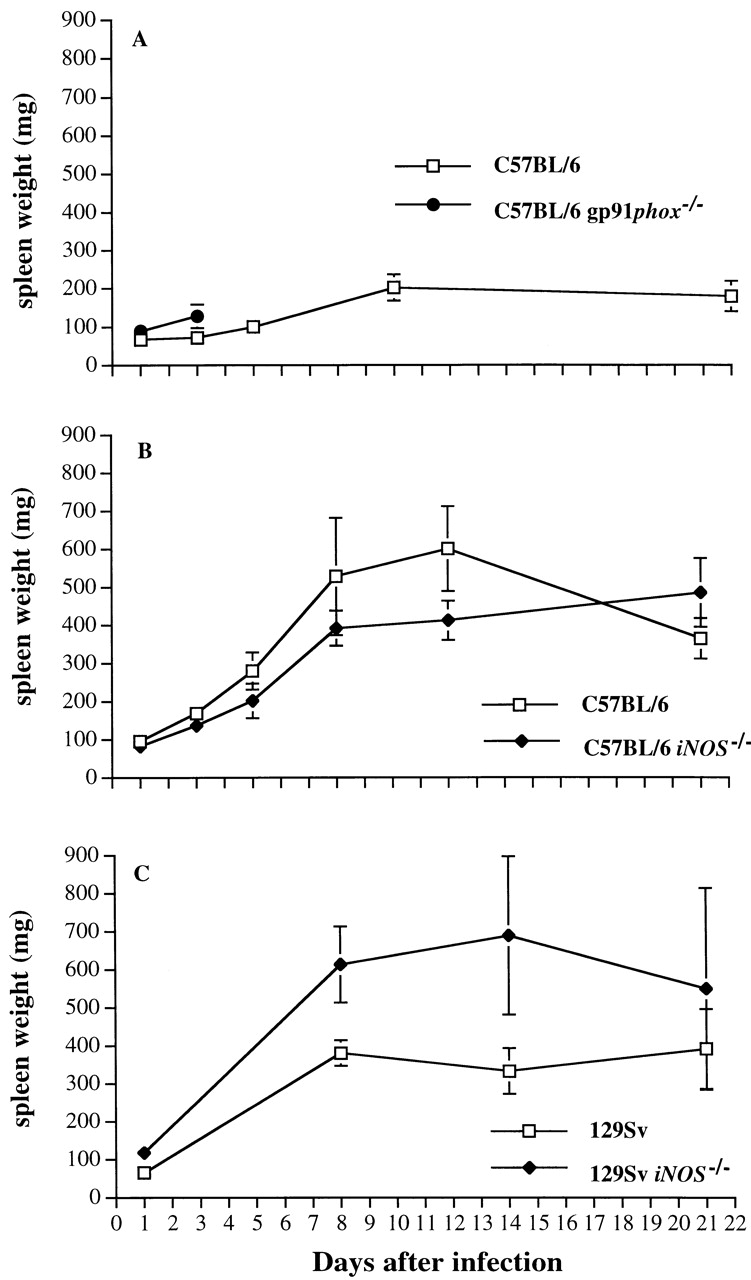
Spleen weights of gp91phox −/−, iNOS −/−, and congenic wild-type mice infected with S. typhimurium. Mice were infected as described in the legend to Fig. 2. Spleen weights were measured from (A) infected gp91phox −/− and congenic C57BL/6 mice, (B) iNOS −/− and congenic C57BL/6 mice, and (C) iNOS −/− mice and congenic 129Sv mice at designated time points. Results are expressed as mean spleen weight ± SD for four mice per data point.
Microscopic examination of livers of C57BL/6 and congenic gp91phox −/− and iNOS −/− mice revealed the early formation of microabscesses rich in polymorphonuclear neutrophils by day 3 of infection (Fig. 4a Fig. b Fig. c), with more extensive lesions noted in the gp91phox −/− animals. On days 8, 11, and 14, the focal lesions in the spleens and livers of the infected C57BL/6 and iNOS −/− mice consisted mainly of mononuclear cells and were surrounded by normal tissue. This evolution of the inflammatory response 10 18 occurred similarly in both iNOS −/− mice (Fig. 4d and Fig. f) and C57BL/6 mice (Fig. 4e and Fig. g), showing no iNOS dependence on the formation of mononuclear cell–rich granulomas. In several iNOS −/− mice it was visually evident that the average size of focal granulomas was larger than in congenic wild-type control mice. On day 21 of infection, granulomatous lesions present in the livers and spleens of iNOS −/− mice in the C57BL/6 background showed a variable degree of central necrosis (Fig. 4h Fig. i Fig. j Fig. k); macroscopically visible abscesses were present in the livers and occasionally in the spleens. The histological picture in organs of wild-type 129Sv and congenic iNOS −/− mice infected with S. typhimurium C5 was similar to that described in the C57BL/6 strain background, except for the absence of central necrosis in the hepatic and splenic lesions.
Figure 4.
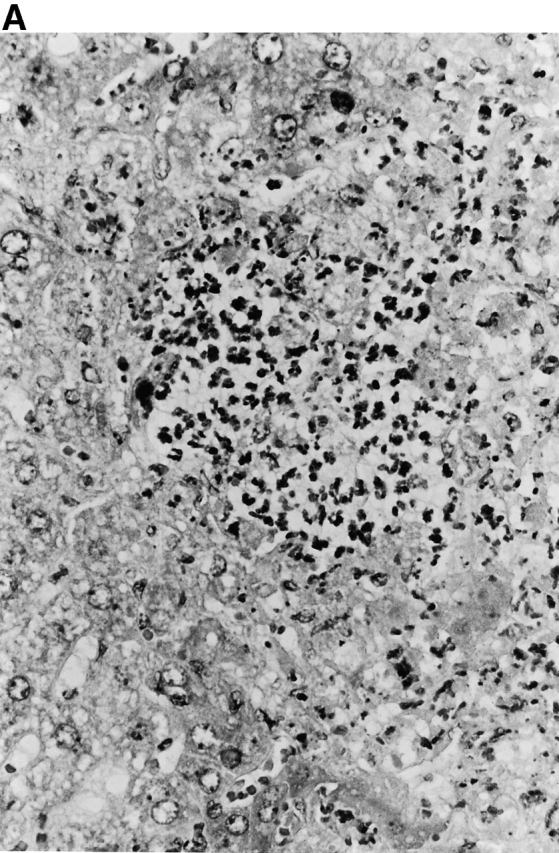
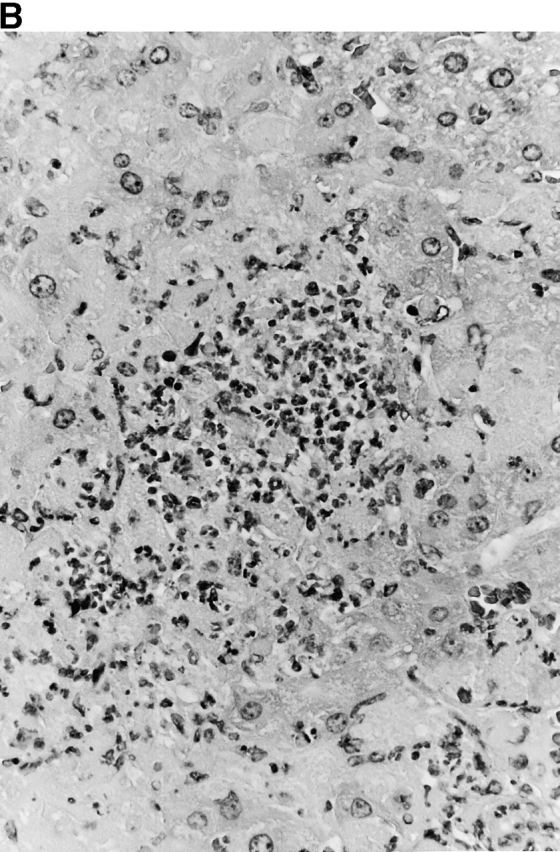
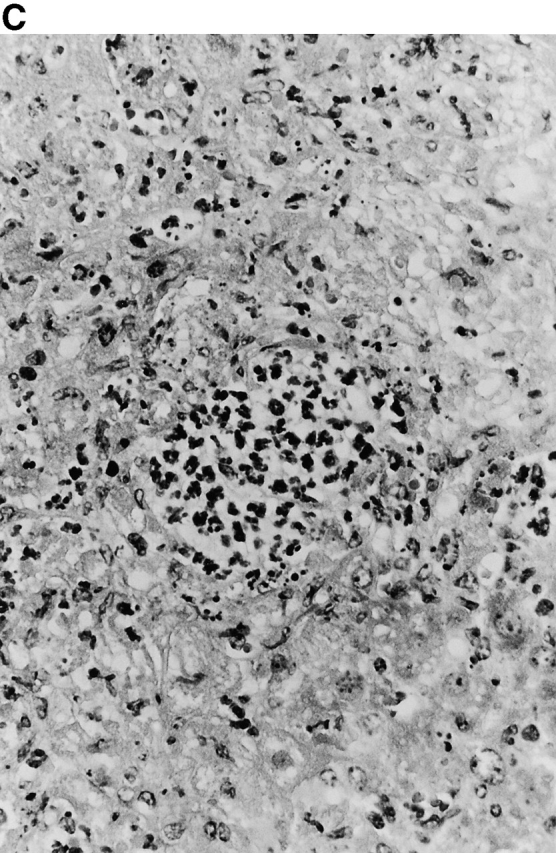
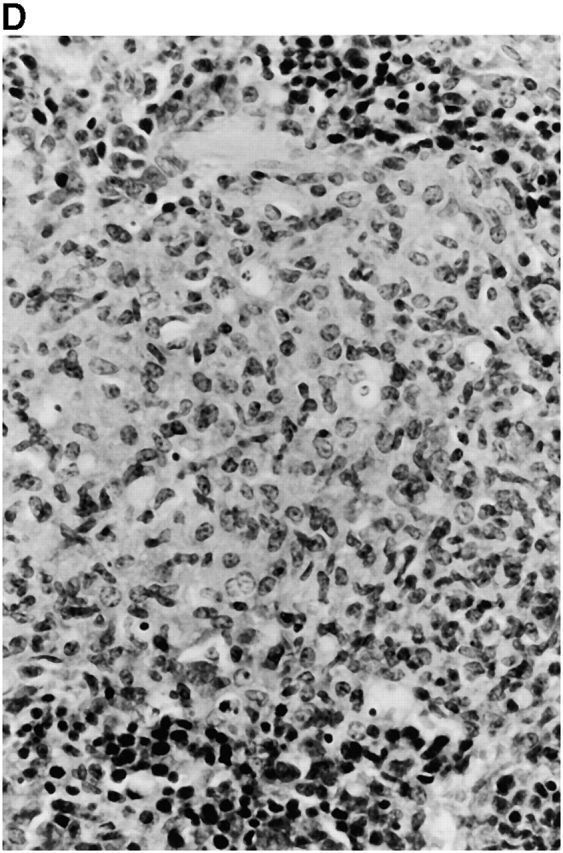
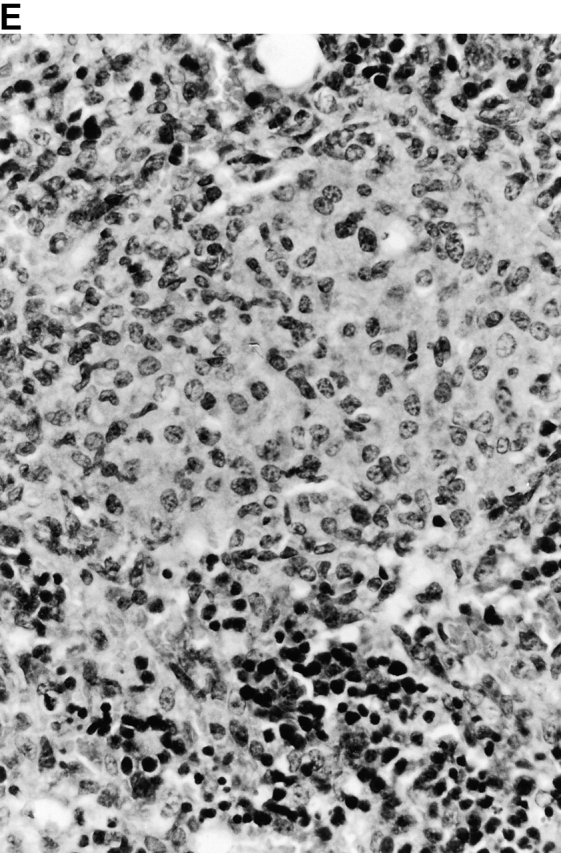
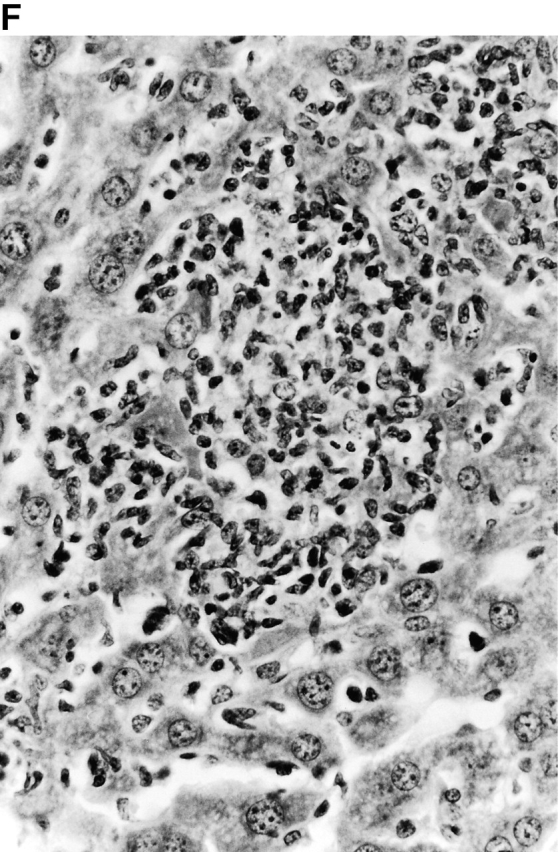
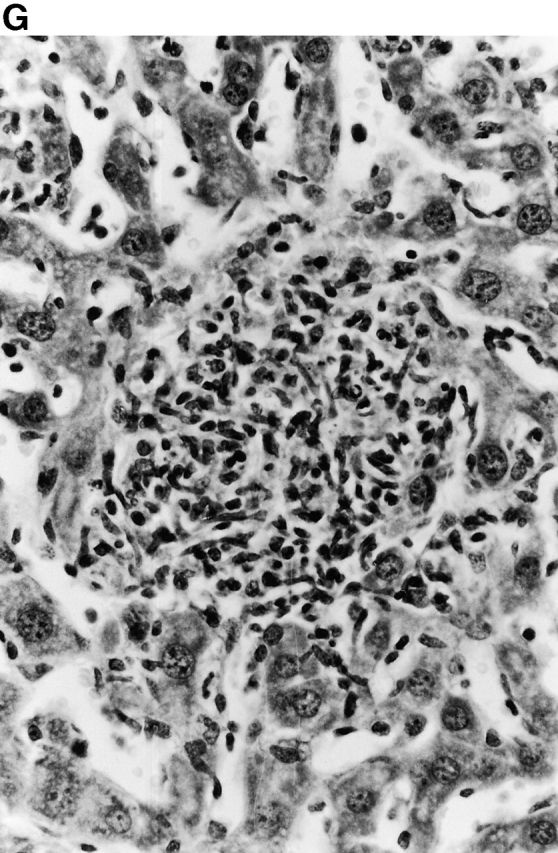
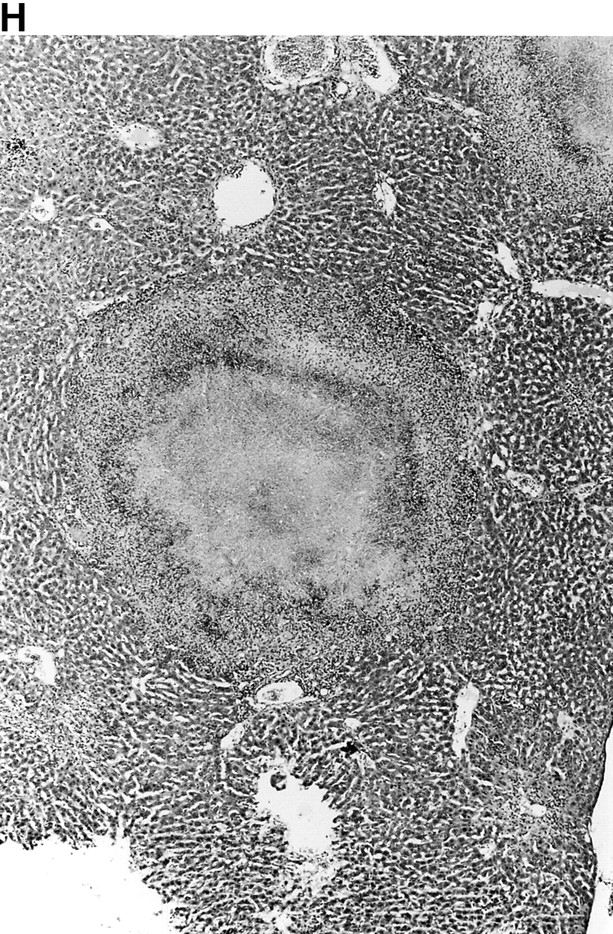
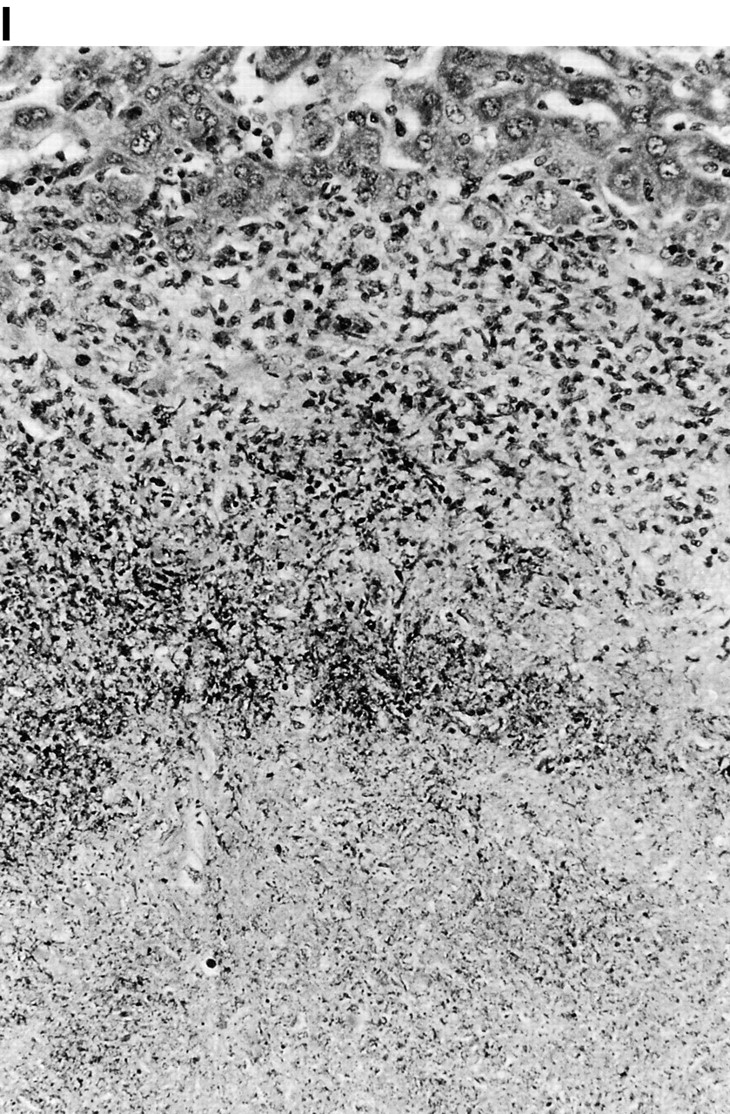
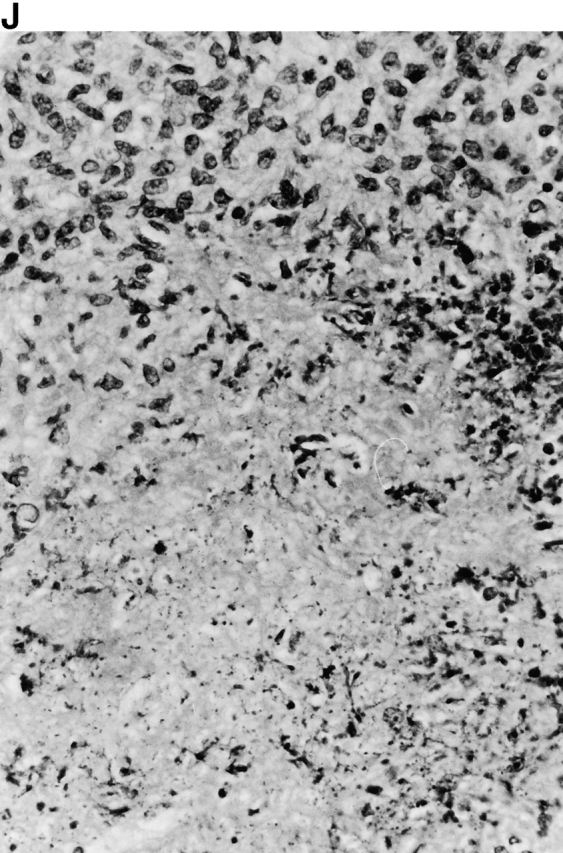
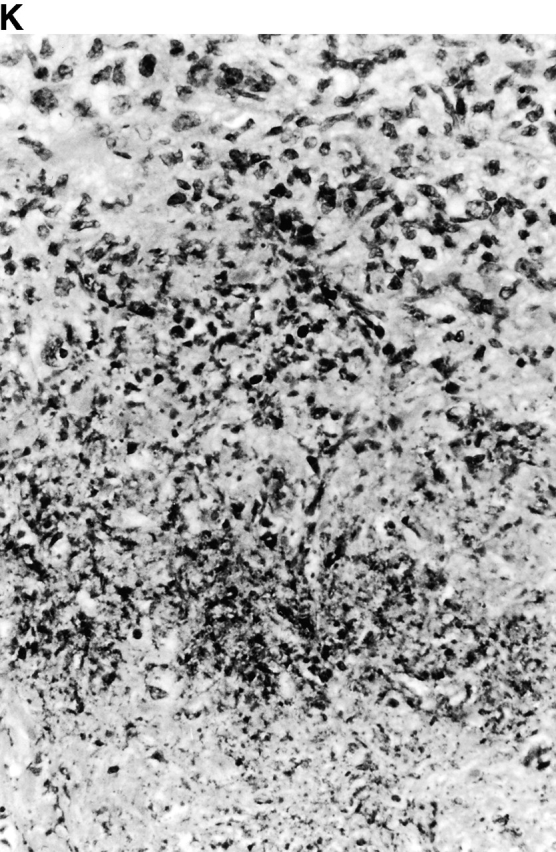
(continues on facing page). Microscopic appearance of livers and spleens from gp91phox −/−, iNOS −/−, and congenic wild-type mice infected with S. typhimurium. C57BL/6, gp91phox −/−, and iNOS −/− were infected as described in the legend to Fig. 2. Hematoxylin and eosin–stained sections were prepared from spleens and livers harvested at time points thereafter. Microabscesses were seen in the livers of (A) gp91phox −/−, (B) iNOS −/−, and (C) wild-type animals on day 3. Granulomatous lesions were seen within the (D and E) spleens and (F and G) livers of (D and F) iNOS −/− and (E and G) wild-type C57BL/6 mice on day 8 (original magnification: ×200). The histopathology from days 11 and 14 closely resembled that observed on day 8. Necrotic lesions were detected in the (H and I) livers and (J and K) spleens of iNOS −/− mice on day 21. Approximate original magnifications: A–G, ×400; H, ×60; I, ×200; and J–K, ×400.
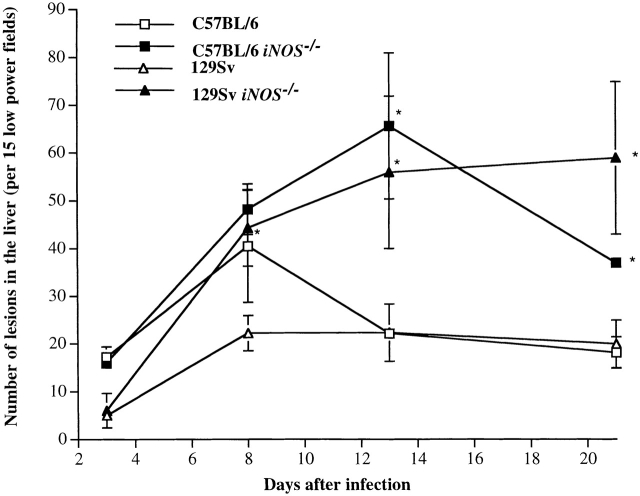
The focal lesions in several liver sections were counted at low-power magnification. As shown in Fig. 5, similar numbers of inflammatory foci were present in the livers of iNOS −/− and congenic C57BL/6 mice on day 3 (predominantly neutrophils) and day 8 (predominantly mononuclear cells) of infection, but the numbers of mononuclear cell–rich lesions were significantly higher in C57BL/6 iNOS −/− mice by days 12 and 21. Similar numbers of inflammatory foci were found within the livers of iNOS −/− and congenic 129Sv mice on day 3 of infection, but thereafter the number of lesions was significantly higher in the livers of the iNOS −/− mice. Thus, both iNOS −/− mice and congenic wild-type mice develop splenomegaly and form granulomas in the spleen and liver during experimental salmonellosis.
Figure 5.
Quantitation of focal hepatic lesions. Lesions were enumerated from iNOS −/− and congenic C57BL/6 mice infected with S. typhimurium M525P or iNOS −/−, and from congenic 129Sv mice infected with S. typhimurium C5 as described in the legend to Fig. 2. Results are shown as numbers of lesions in 15 low-power fields (mean ± SD from groups of four mice). *P ≤ 0.01.
Flow Cytometry of Splenocyte Populations in Salmonella-infected Wild-type and iNOS−/− Mice.
Studies in mice treated with iNOS inhibitors have suggested that iNOS might be required for the recruitment of inflammatory cells to the spleen 36. The percentages of CD11b+ cells and I-Ab+ CD11b+ cells were determined on days 3, 8, and 15 or 16 in the spleens of Salmonella-infected wild-type and iNOS −/− mice in experiments parallel to those depicted in Fig. 2. CD11b+ cells and I-Ab+CD11b+ cells were recruited to infected spleens of iNOS −/− mice and wild-type mice to a similar degree and, at some time points, higher percentages of inflammatory cells were present in the spleens of iNOS −/− mice compared with wild-type mice (Table ). The percentages of CD4+ T cells, CD8+ T cells, CD25+ cells, and B220+ cells in the spleens of wild-type and iNOS −/− mice were also comparable (not shown). The results shown are representative of three individual experiments that yielded similar results. Thus, the greater bacterial burden observed in iNOS −/− mice (Fig. 2C–F) cannot be attributed to an impairment in the infiltration of inflammatory cells.
Table 1.
Flow Cytometry
| CD11b+ | I-Ab+CD11b+ | |
|---|---|---|
| Day 3 | ||
| C57BL/6 | 12.45 | 5.0 |
| C57BL/6 iNOS−/− | 6.4 | 5.5 |
| 129 | 8.82 | 5.3 |
| 129 iNOS−/− | 12.8 | 8.2 |
| Day 8 | ||
| C57BL/6 | 12.11 | 7.69 |
| C57BL/6 iNOS−/− | 15.1 | 7.3 |
| 129Sv | 14.4 | 11.3 |
| 129Sv iNOS−/− | 25.1 | 22.7 |
| Days 15–16 | ||
| C57BL/6 | 12.8 | 6.83 |
| C57BL/6 iNOS−/− | 21.3 | 9.05 |
| 129Sv | 14.0 | 8.6 |
| 129Sv iNOS−/− | 23.0 | 12.7 |
Percentages of CD11b+ and CD11b+I-Ab+ double positive cells in the spleens of iNOS −/− and congenic C57BL/6 mice infected with S. typhimurium M525P, or iNOS−/− and congenic 129Sv mice infected with S. typhimurium C5. Mice were infected as described in the legend to Fig. 2. Results are expressed as percentages of total cells. The data are representative of three individual experiments that yielded similar results.
Serum IFN-γ Levels in Salmonella-infected Wild-type and iNOS−/− Mice.
Serum IFN-γ was measured by ELISA in the sera of six Salmonella-infected wild-type and six congenic iNOS −/− mice at days 3, 7, 11, 16, and 21 of infection. No statistically significant differences (P > 0.05) in serum IFN-γ levels between iNOS −/− and C57BL/6 wild-type mice after infection with S. typhimurium M525P were detected on days 3, 7, and 11. Serum IFN-γ levels at later time points were statistically higher (P < 0.05) in iNOS −/− mice (243 ± 109 pg/ml on day 16; 430 ± 44 pg/ml on day 21) than in C57BL/6 wild-type controls (82 ± 62 pg/ml on day 16; 75 ± 9 pg/ml on day 21). Similarly, no differences in serum IFN-γ levels between iNOS −/− and congenic 129Sv wild-type mice infected with S. typhimurium C5 were detected on days 3, 7, and 11, but IFN-γ levels on days 16 and 21 of infection were statistically higher (P < 0.05) in the iNOS −/− mice (500 ± 99 pg/ml on day 16; 710 ± 144 pg/ml on day 21) than in the 129Sv wild-type controls (156 ± 130 pg/ml on day 16; 200 ± 49 pg/ml on day 21). Thus, iNOS deficiency does not impair circulating levels of IFN-γ during Salmonella infection. Higher levels of IFN-γ at later time points are probably attributable to increased organism burden, but may also reflect regulatory effects of NO· on cytokine production.
Discussion
Detailed study of the course of Salmonella infection in gp91phox −/− or iNOS −/− mice has revealed major differences in the temporal contribution of the NADPH phagocyte oxidase and iNOS to host defense in salmonellosis. Enhanced bacterial proliferation was observed in gp91 phox −/− mice as early as day 1 after infection. In contrast, iNOS −/− mice effectively controlled bacterial growth in the tissues during the first week of infection, but a lethal increase in bacterial burden appeared later in the course of infection.
Early killing of bacteria by resident phagocytes and acute inflammatory cells during the first 24 h of infection appears to be heavily dependent on the presence of an intact NADPH phagocyte oxidase. These in vivo observations correlate well with in vitro studies, suggesting a crucial role for the oxidative burst in the rapid killing of ingested salmonellae by macrophages 28 29 35. The accelerated replication of Salmonella in gp91phox −/− mice that already possess an innately susceptible Nramp1s (G169D) allele 40 reveals a degree of independence between Nramp1 and oxidative burst–dependent host defenses, and shows that Nramp1s mice still retain potent antimicrobial mechanisms during the early phases of Salmonella infection. Indeed, Nramp1 (Ity) is believed to limit bacterial growth rather than enhance bacterial killing in vivo 41, whereas NADPH-mediated oxidative mechanisms have been shown to mediate killing of Salmonella by mononuclear cells 28 29 35. iNOS expression in the tissues can be detected very early in the course of a Salmonella infection (within 24 h) (32 34; our unpublished observations), raising the possibility of synergistic antimicrobial interactions between ROS and RNS, as have been shown for Escherichia coli and S. typhimurium in vitro 35 42 and for sodC-deficient S. typhimurium in vivo 24. However, such interactions are unlikely to be essential for the early killing of wild-type S. typhimurium in this experimental model, because iNOS was dispensable for the control of bacterial replication during the initial days of infection.
The early bactericidal role of the NADPH phagocyte oxidase followed by a late essential bacteriostatic role of iNOS mirrors our observations in elicited peritoneal phagocytes 35. Although high levels of superoxide production during initial phagocyte–pathogen interactions (with or without the presence of smaller quantities of NO·) can generate a variety of oxidant species, the subsequent predominant production of NO· over time would be predicted to lead to a progressive conversion to nitrosative chemistry 35 43. The tenuous stability of the activated NADPH phagocyte oxidase complex 44, and perhaps even its direct inhibition by nitrogen oxides 45, might contribute to the evolution of the host response from ROS to RNS dependence. This temporal progression of host defense may also be accounted for by the greater reliance of acute inflammatory neutrophils and resident mononuclear cells on oxidative killing mechanisms, succeeded by an influx of NO·-producing activated macrophages. Although oxidative bacterial killing by neutrophils is highly effective 46, organisms persisting within mononuclear cells 10 may require slower iNOS-dependent mechanisms for their eventual clearance.
In murine typhoid, iNOS is positively modulated by IFN-γ and TNF-α 12 20; therefore, it could be hypothesized that IFN-γ and TNF-α mediate the suppression of bacterial growth in the tissues of infected mice via NO·-dependent mechanisms. However, there are important distinctions among the courses of infection in cytokine-deficient, gp91phox −/−, and iNOS −/− mice. In contrast to iNOS −/− mice, whole body X-irradiated mice, IFN-γ−/− mice, IFN-γR−/− mice, TNFR55−/− mice, and mice treated with anti–IFN-γ or anti–TNF-α neutralizing antibodies fail to suppress the early growth of salmonellae in the reticuloendothelial system (11 12 13 16; our unpublished observations), and succumb within 7–8 d after challenge with wild-type Salmonella under experimental conditions similar to those used in this study (our unpublished observations). These observations indicate that IFN-γ and TNF-α do not exclusively enhance innate immunity via NOS activation. Later stages of Salmonella infection are controlled by T cell–mediated immunity, and mouse strains lacking T cells such as nu/nu mice, TcR-α/β−/− mice, recombination activating gene (rag)1 −/− mice, or scid mice fail to clear Salmonella and succumb to infection despite initial suppression of early bacterial replication (13 47; our unpublished observations). At this time, we cannot exclude that some degree of impairment in T cell responses to Salmonella might occur in iNOS −/− gene-targeted mutant mice, but recent evidence from mouse models of Trypanosoma brucei and Mycobacterium avium infection shows that iNOS-deficient mice can mount effective T cell responses despite the absence of high-output NO· production 48 49.
Early histopathological examination of Salmonella-infected mice revealed microabscesses containing neutrophils in all strains, but lesions were most extensive with surrounding necrosis in gp91phox −/− animals. iNOS −/− and wild-type mice progressed to develop macrophage-rich granulomas by day 8, but all gp91phox −/− had succumbed to uncontrolled infection by this time point. We observed the development of central necrosis in some granulomas during later stages of infection in Nramp1s C57BL/6 iNOS −/− mice but not in wild-type mice or Nramp1r129Sv iNOS −/− mice, possibly reflecting increased bacterial proliferation leading to the death of the phagocytic and parenchymal cells in the more susceptible mouse strain. Dysregulation of cytokine production in the absence of RNS may also be contributing to the development of necrosis 50 51.
Results obtained after chemical iNOS inhibition during Salmonella infection have suggested that impaired RNS production can lead to a reduction of macrophage tissue infiltration and granuloma formation 32 36. Nitrogen oxides are known to play a role in the positive regulation of macrophage inhibitory protein (MIP)-1α, a powerful chemoattractant for macrophages 51, and have additional important immunoregulatory properties 52. However, in this study we found the morphology of granulomatous lesions to be very similar in the tissues of iNOS −/− and congenic wild-type mice infected with S. typhimurium. In fact, focal lesions and numbers of CD11b+ cells were even more numerous in iNOS −/− mice during late stages of infection compared with wild-type mice. Increased granuloma formation, which has also been noted in iNOS −/− mice infected with M. avium 53, may reflect inhibitory effects of NO· on leukocyte recruitment 54. We have also shown that the influx of inflammatory cells in the spleen as measured by flow cytometry, the development of splenomegaly, the elevation of serum IFN-γ levels, and the activation of inflammatory cells as measured by I-Ab expression 12 55 are not impaired in iNOS −/− mice. Elevation of serum IFN-γ levels has also been described in iNOS −/− mice infected with T. brucei or Leishmania major 38 56. The discrepant findings in iNOS −/− mice and animals administered iNOS inhibitors may reflect NOS-independent actions of inhibitors such as aminoguanidine 57, or residual or compensatory mechanisms in the mice 58. Our results indicate that NO· production plays an important antimicrobial effector role during salmonellosis. This conclusion is further supported by observations in murine listeriosis, in which iNOS inhibition exacerbates infection and bacterial burden without apparent effects on hepatosplenic macrophage influx and granuloma formation 59.
In conclusion, the course of experimental salmonellosis in congenic mice lacking a functional NADPH phagocyte oxidase or iNOS has revealed critical temporal differences in the roles of these host immune effector mechanisms. The early induction of the NADPH phagocyte oxidase results in rapid bacterial killing, but this phase is rapidly succeeded by a prolonged plateau phase with eventual bacterial clearance dependent upon the induction of NO· production. This coordinated response may maximize antimicrobial actions of the innate immune system while limiting collateral tissue injury from exposure to reactive oxidants.
Acknowledgments
This work was supported by grants from The Wellcome Trust, the Biotechnology and Biological Sciences Research Council, the National Institutes of Health (AI39557, AI44486, and AI10181), and the James Biundo Foundation.
Footnotes
Abbreviations used in this paper: iNOS, inducible nitric oxide synthase; LB, Luria-Bertani; NO, nitric oxide; NOS, nitric oxide synthase; RNS, reactive nitrogen species; ROS, reactive oxygen species.
References
- Collins F.M. Vaccines and cell mediated immunity. Bacteriol. Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C.E., Khan C.M.A., Mastroeni P., Villarreal B., Dougan G., Chatfield S.N. Salmonella vaccinesmechanisms of immunity and their use as carriers of recombinant antigens. In: Ala D., Aldeen D., Hormaeche C.E., editors. Molecular and Clinical Aspects of Vaccine Development. John Wiley & Sons; Chichester, UK: 1994. pp. 19–153. [Google Scholar]
- Mastroeni P., Harrison J.A., Hormaeche C.E. Natural resistance and acquired immunity to Salmonella . Fund. Clin. Immunol. 1994;2:83–95. [Google Scholar]
- Fields P.I., Swanson R.V., Haidaris C.G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within macrophages are avirulent. Proc. Natl. Acad. Sci. USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S.K., Stocker B.A.D. Aromatic dependent Salmonella typhimurium are non virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hormaeche C.E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979;37:311–318. [PMC free article] [PubMed] [Google Scholar]
- Lissner C.R., Swanson R.N., O'Brien A.D. Genetic control of the innate resistance of mice to Salmonella typhimuriumexpression of the Ity gene in peritoneal and splenic macrophages isolated in vitro . J. Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- Maskell D.J., Hormaeche C.E., Harrington K.E., Joysey H.S., Liew F.Y. The initial suppression of bacterial growth in a Salmonella infection is mediated by a localised rather than a systemic response. Microb. Pathog. 1987;2:295–305. doi: 10.1016/0882-4010(87)90127-6. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Skepper J.N., Hormaeche C.E. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect. Immun. 1995;63:3674–3682. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Dahlfors A., Buchan A.M.J., Finlay B. Murine salmonellosis studied by confocal microscopySalmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo . J. Exp. Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C.E., Mastroeni P., Arena A., Uddin J., Joysey H.S. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 1990;70:247–250. [PMC free article] [PubMed] [Google Scholar]
- Everest P., Roberts M., Dougan G. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect. Immun. 1998;66:3355–3364. doi: 10.1128/iai.66.7.3355-3364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Ladel C., Miko D., Kaufmann S.H.E. Salmonella typhimurium aroA − infection in gene targeted immunodeficient mice. J. Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect. Immun. 1989;57:609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincy-Cain T., Clements J.D., Bost L. Endogenous and exogenous interleukin-12 augment protective immune response in mice orally challenged with Salmonella dublin . Infect. Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Arena A., Costa G.B., Liberto M.C., Bonina L., Hormaeche C.E. Serum TNFα in mouse typhoid and enhancement of a Salmonella infection by anti-TNFα antibodies. Microb. Pathog. 1991;11:33–38. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Role of T-cells, TNFα and IFNγ in recall of immunity to oral challenge with virulent salmonellae in mice immunised with live attenuated aroA Salmonella vaccines. Microb. Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Effect of late administration of anti-TNFα antibodies on a Salmonella infection in the mouse model. Microb. Pathog. 1993;14:473–480. doi: 10.1006/mpat.1993.1046. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Harrison J.A., Chabalgoity J.A., Hormaeche C.E. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect. Immun. 1995;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Harrison J.A., Robinson J.H., Clare S., Khan S., Maskell D.J., Dougan G., Hormaeche C.E. Interleukin 12 is required for the control of the growth of attenuated aromatic-compound dependent salmonellae in BALB/c micerole of gamma interferon and macrophage activation. Infect. Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Clare S., Khan S., Harrison J.A., Hormaeche C.E., Okamura H., Kurimoto M., Dougan G. IL18 (IGIF) contributes to host resistance and IFNγ production in mice infected with virulent S. typhimurium . Infect. Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J.P., Dougan G., Chatfield S.N. The involvement of tumor necrosis factor in immunity to Salmonella infection. J. Immunol. 1991;147:3161–3164. [PubMed] [Google Scholar]
- Buchmeier N., Lipps C.J., So M.H.Y., Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol. Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- De Groote M.A., Ochsner U.A., Shiloh M.U., Nathan C., McCord J.M., Dinauer M.C., Libby S.J., Vazquez-Torres A., Xu Y., Fang F. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouy R., Fischer A., Vilmer E., Seger R., Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J. Pediatr. 1989;114:555–560. doi: 10.1016/s0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- Pollock J.D., Williams D.A., Gifford M.A.C., Li L.L., Du X., Fisherman J., Orkin S.H., Doershuck C.M., Dinauer M.C. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Adams L.B., Dinauer M.C., Morgenstern D.E., Krahenbuhl J.K. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber. Lung. Dis. 1997;78:237–246. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- Shiloh M.U., Ruan J., Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect. Immun. 1997;65:3193–3198. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh M.U., MacMiking J.D., Nicholson S., Brause J.E., Potter S., Marino M., Fang F., Dinauer M., Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Fang F. Perspectives serieshost/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroote M.A., Fang F.C. Antimicrobial properties of nitric oxide. In: Fang F.C., editor. Nitric Oxide and Infection. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 231–261. [Google Scholar]
- Umezawa K., Akaike T., Fujii S., Suga M., Setoguchi K., Ozawa A., Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against S. typhimurium infection in mice. Infect. Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote M.A., Testerman T., Xu Y., Stauffer G., Fang F.C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium . Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- Khan S.A., Everest P., Servos S., Foxwell N., Zahringer U., Brade H., Rietschel E.T., Dougan G., Charles I.G., Maskell D.J. A lethal role for lipid A in Salmonella infections . Mol. Microbiol. 1998;29:571–579. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Jones-Carson J., Mastroeni P., Ischiropoulous H., Fang F.C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane A.S., Schwacha M.G., Eisenstein T.K. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 1999;67:891–898. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach V.E., Shesely E.G., Smithies O., Sherman P.A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Charles I.G., Smith A., Ure J., Feng G., Huang F., Xu D., Muller W., Moncada S., Liew F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Vidal S.M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasitesisolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Hormaeche C.E. The in vivo division and death rates of Salmonella typhimurium in the spleens of naturally resistant and susceptible mice measured by the superinfecting phage technique of Meynell. Immunology. 1980;41:973–979. [PMC free article] [PubMed] [Google Scholar]
- Pacelli R., Wink D.A., Cook J.A., Krishna M.C., DeGraff W., Friedman N., Tsokos M., Samuni A., Mitchell J.B. Nitric oxide potentiates hydrogen peroxide–induced killing of Escherichia coli . J. Exp. Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D.A., Cook J.A., Kim S.Y., Vodovotz Y., Pacelli R., Krishna M.C., Russo A., Mitchell J.B., Jourd'heuil D., Miles A.M., Grisham M.B. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. Chemical aspects involved in the balance between oxidative and nitrosative stress. J. Biol. Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- Tamura M., Takeshita M., Curnutte J.T., Uhlinger D.J., Lambeth J.D. Stabilization of human neutrophil NADPH oxidase activated in a cell-free system by cytosolic proteins and by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide. J. Biol. Chem. 1992;267:7529–7538. [PubMed] [Google Scholar]
- Iha S., Orita K., Kanno T., Utsumi T., Sato E.F., Inoue M., Utsumi K. Oxygen-dependent inhibition of neutrophil respiratory burst by nitric oxide. Free Radic. Res. 1996;25:489–498. doi: 10.3109/10715769609149071. [DOI] [PubMed] [Google Scholar]
- Vassiloyanakopoulos A.P., Okamoto S., Fierer J. The crucial role of polymorphonuclear leukocytes in resistance to Salmonella dublin infections in genetically susceptible and resistant mice. Proc. Natl. Acad. Sci. USA. 1998;95:7676–7681. doi: 10.1073/pnas.95.13.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K.A., Mastroeni P., Harrison J., de Hormaeche R.D., Hormaeche C.E. Salmonella typhimurium aroA, htrA and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect. Immun. 1997;65:1566–1569. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M.S., Florido M., Pais T.F., Appelberg R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase. J. Immunol. 1999;162:6734–6739. [PubMed] [Google Scholar]
- Millar A.E., Stenberg J., McSharry C., Wei X.Q., Liew F.Y., Turner C.M. T-cell responses during Trypanosoma brucei infections in mice deficient in inducible nitric oxide synthase. Infect. Immun. 1999;67:3334–3338. doi: 10.1128/iai.67.7.3334-3338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everest P., Allen J., Papacostantinopoulou A., Mastroeni P., Roberts M., Dougan G. Salmonella typhimurium infections in mice deficient in interleukin-4 productionrole of IL-4 in infection associated pathology. J. Immunol. 1997;159:1820–1827. [PubMed] [Google Scholar]
- Muhl H., Dinarello C.A. Macrophage inflammatory protein-1α production in lipopolysaccharide-stimulated human adherent blood mononuclear cells is inhibited by the nitric oxide synthase inhibitor NG-monomethyl-l-arginine. J. Immunol. 1997;159:5063–5069. [PubMed] [Google Scholar]
- McInnes I.B., Liew F.Y. Immunomodulatory actions of nitric oxide. In: Fang F.C., editor. Nitric Oxide and Infection. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 199–213. [Google Scholar]
- Ehlers S., Kutsch S., Benini J., Cooper A., Hanhn C., Gerdes J., Orme I., Martin C., Rietschel E.T. NOS2-derived nitric oxide regulates the size, quantity and quality of granuloma formation in Mycobacterium avium-infected mice without affecting bacterial loads. J. Immunol. 1999;98:313–323. doi: 10.1046/j.1365-2567.1999.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M.J., Sharkey K.A., Sihota E.G., Reinhardt P.H., Macmicking J.D., Nathan C., Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr. Opin. Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- Hertz C.J., Mansfield J.M. IFN-gamma-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell. Immunol. 1999;192:24–32. doi: 10.1006/cimm.1998.1429. [DOI] [PubMed] [Google Scholar]
- Wray G., Thiemermann C. Nitric oxide in sepsis. In: Fang F.C., editor. Nitric Oxide and Infection. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 265–280. [Google Scholar]
- Niedbala W., Wei X.Q., Piedrafita D., Xu D., Liew F.Y. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 1999;29:2498–2505. doi: 10.1002/(SICI)1521-4141(199908)29:08<2498::AID-IMMU2498>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Boockvar K.S., Granger D.L., Poston R.M., Maybody M., Washington M.K., Hibbs J.B., Kurlander R.L. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 1994;62:1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]