Abstract
We studied the role of CD43 (leukosialin/sialophorin), the negatively charged sialoglycoprotein of leukocytes, in the binding of mycobacteria to host cells. CD43-transfected HeLa cells bound Mycobacterium avium, but not Salmonella typhimurium or Shigella flexneri. Quantitative bacteriology showed that macrophages (Mφ) from wild-type mice (CD43+/+) bound M. avium, Mycobacterium bovis (bacillus Calmette-Guérin), and Mycobacterium tuberculosis (strain H37Rv), whereas Mφ from CD43 knockout mice (CD43−/−) did not. Fluorescence microscopy demonstrated that the associated M. avium had been ingested by the CD43+/+ Mφ. The inability of CD43−/− Mφ to bind M. avium could be restored by addition of galactoglycoprotein (Galgp), the extracellular mucin portion of CD43. The effect of Galgp is not due to opsonization of the bacteria, but required its interaction with the Mφ; other mucins had no effect. CD43 expression by the Mφ was also required for optimal induction by M. avium of tumor necrosis factor (TNF)-α production, which likewise could be reconstituted by Galgp. In contrast, interleukin (IL)-10 production by M. avium–infected Mφ was CD43 independent, demonstrating discordant regulation of TNF-α and IL-10. These findings describe a novel role of CD43 in promoting stable interaction of mycobacteria with receptors on the Mφ enabling the cells to respond specifically with TNF-α production.
Keywords: mycobacteria, CD43, macrophages, tumor necrosis factor α, interleukin 10
Introduction
Mycobacteria are among the most important infectious agents in the world. Mycobacterium tuberculosis, which is spread by airborne transmission, causes more deaths globally than any other infectious agent. Estimates indicate 90 million people infected with M. tuberculosis during the current decade, 30 million of whom will die as a result of infection 1. In the United States, Mycobacterium avium complex has emerged as the most prevalent opportunistic infection among patients with advanced HIV-1 infection. M. avium infections in patients with AIDS and other immunodeficiencies typically manifest with widespread visceral organ involvement and high-grade bacteremia; the patients suffer considerable morbidity and shortened life span 2 3 4 5.
Macrophages (Mφ), the predominant host cells, are the first line defense against spread of mycobacterial infection. In the successful process, Mφ eliminate mycobacterial infection by a complex network of events involving TNF-α production 6 and leading to apoptosis and elimination of the host Mφ containing endocytosed microorganisms 7 8. Pathogenic mycobacteria have evolved mechanisms to survive and replicate within endosomes of Mφ 9 after entering the cells by one of several Mφ receptor pathways. These include complement receptors CR1, CR3, and CR4, mannose receptor, CD14, Fc receptors, scavenger receptors, and one or more receptors for pulmonary surfactant protein A (SP-A) 10 11. To some extent, the outcome of mycobacterial infection is influenced by the receptor, in that uptake via Fc receptors, but not complement receptors, induces reactive oxygen metabolites, a process believed to decrease survival of endocytosed mycobacteria 12.
This study aims to understand the role of nonreceptor surface molecules in regulating binding and uptake of mycobacteria by Mφ. CD43, a cell surface mucin on macrophages, was considered as a candidate regulatory molecule because of its known ability to interfere with adhesion processes. For example, CD43-transfected HeLa cells, unlike wild-type HeLa cells, display decreased intracellular adhesion molecule (ICAM)-mediated adhesion 13, and targeted disruption of the CD43 gene in mice increased adhesiveness of splenic T cells 14. Therefore, we used CD43-transfected and wild-type HeLa cells as well as Mφ from CD43 knockout and wild-type mice to determine whether the presence of CD43 diminishes binding of mycobacteria to Mφ and, by doing so, diminishes or abrogates important downstream events such as cytokine production.
Materials and Methods
Animals.
CD43 knockout mice were generated by homologous recombination via embryonic stem cell chimeras 14. The CD43−/− mice and control wild-type mice (in C57 × SVJ 129 background) were maintained under specific pathogen-free conditions at the Tufts University School of Medicine. For all experiments, 6–8-wk-old mice matched for sex were used. In select experiments, gene status was verified by flow cytometric analysis of cells stained with PE-conjugated S7 rat anti–mouse CD43 mAb (BD PharMingen).
Microorganisms.
Shigella flexneri and Salmonella typhimurium were provided by Dr. J. Cohen, Imperial College of Science, Technology and Medicine (London, UK). BCG (M. bovis strain bacillus Calmette-Guérin) was obtained from the Trudeau Institute, Saranac Lake, NY. The M. tuberculosis strain H37Rv was a gift of Dr. Hardy Kornfeld, Boston University School of Medicine. M. avium serovar 4, isolated from an AIDS patient and typed by the Mycobacterial Culture Collection (National Jewish Hospital, Denver, CO), was grown to log phase in Middlebrook 7H9 broth with 5 mg/ml albumin, 1 mg/ml dextrose, and 3 μg/ml catalase (OADC; Difco). The mycobacteria were harvested by centrifugation (2,000 g), washed twice in HBSS, suspended in Middlebrook 7H9 broth, sonicated for 10 s at 500 W, and stored in aliquots at −70°C. M. avium, BCG, and M. tuberculosis were thawed as needed, sonicated for 20 s to disperse the bacteria, and quantified by plate counting. 10-fold dilutions (5 replicates per dilution) were plated on Middlebrook 7H11 agar with albumin-dextrose-catalase enrichment. The plates were incubated at 37°C in 90% humidity for 10 d to 4 wk, and colonies were counted. M. avium colonies were exclusively of the smooth, transparent morphotype 15 and appeared homogeneously dispersed.
HeLa Cell Lines.
The generation of HeLa cell CD43 transfectants was described previously 13. Wild-type HeLa cells and HeLa cells transfected with vector (pSV2Neo) were used as controls. The cells in RPMI 1640 with 10% heat-treated (56°C for 30 min) FCS and 25 mM Hepes were propagated by plating 2 × 106 cells/ml in 24-well culture plates (Costar) and passaging by EDTA treatment. Immediately before infection, confluent HeLa cells were washed three times with HBSS, and fresh medium was added.
Live M. avium, S. flexneri, and S. typhimurium were stained with carboxyfluorescein diacetate (CFDA; Molecular Probes). The bacteria at 107/ml were incubated with 4 μM CFDA in DMEM at 37°C for 30 min. After extensive washing, the CFDA-stained microorganisms were used to challenge confluent cultures of CD43-transfected, wild-type, and vector-transfected HeLa cells by coculture at 1, 5, or 10 microorganisms/cell for 2–4 h. The cells were washed three times in HBSS and analyzed by fluorescence microscopy.
Murine Spleen Mφ.
Murine spleens were removed sterilely and teased, and the released cells were washed three times in HBSS and suspended in RPMI 1640 with 10% heat-treated (56°C for 30 min) FCS, 25 mM Hepes, and 2 × 10−5 M 2-ME (Sigma-Aldrich). All media tested negative for LPS (<0.05 ng/ml) in the Limulus amebocyte lysate assay (BioWhittaker). Spleen cells were plated at 2 × 106 cells/well in 24-well plates (Costar) for 4–6 d in 5% CO2 at 37°C. Immediately before infection, nonadherent cells were discarded, and the Mφ populations, which were 97–99% pure by esterase stain, were washed four times in HBSS, and fresh medium was added.
Triplicate wells of adherent Mφ (∼1.0 × 105 cells/well) were inoculated with M. avium, BCG, or M. tuberculosis strain H37Rv at 2, 10, or 20 microorganisms/cell and cultured for 1–4 h for mycobacteria binding assays or 4–48 h for cytokine measurements. To quantify bound mycobacteria, the adherent Mφ were washed four times with HBSS, lysed by addition of SDS (500 μl of 0.2%), and the effect of SDS was terminated with FCS (500 μl 50%). Cell lysates (100 μl) were cultured at 37°C in 5-ml vials containing culture broth and [14C]palmitic acid (Bactec vials; Becton Dickinson). Accumulated 14CO2 was quantified at 24-h intervals using the Bactec model 460TB system (Becton Dickinson). Plots of accumulated 14CO2 versus time of culture yielded T-100 values, defined as time needed to reach 14CO2 accumulation value of 100 on a scale of 0–999 16. Linear correlation was found between T-100 and the log of bacterial number over the range 101 to 107 organisms, and assay results, expressed as number of mycobacteria associated with Mφ, were determined from a calibration plot. Calibration was provided by periodic parallel assay of mycobacteria that had been quantified by plate counting.
Light Microscopy.
Monolayers of adherent Mφ were prepared by plating 2 × 106 murine spleen cells on 13-mm-diameter plastic coverslips (Thermanox™, no. 174950; Nunc); these were incubated with M. avium, harvested and washed as described above, and dried. The coverslips were stained with TB carbolfuchsein KF and counterstained with TB brilliant green K following the manufacturer's instructions (Kinyoun acid-fast staining procedure for mycobacteria; Difco). Mφ containing >10 detectable bacteria were scored as positive. Quantitation is based on visual scoring of 3–5 fields with 30–80 cells each.
Fluorescence Microscopy.
Monolayers of adherent Mφ on plastic coverslips as described above were incubated with M. avium, harvested, washed, and fixed with 4% paraformaldehyde in PBS. The mycobacteria in the fixed monolayers were stained with the rhodamine-auramine TB Fluorescent stain kit (no. 4312521; Becton Dickinson) according to the manufacturer's directions. The stained cells were examined by phase–contrast and fluorescent microscopy with a Nikon microscope.
Galactoglycoprotein and Other Mucins.
Galactoglycoprotein (Galgp; provided by Dr. Karl Schmid, Department of Biochemistry, Boston University School of Medicine, and Dr. H. Gerhard Schwick, Behringwerke AG, Marburg/Lahn, Germany), was purified from pooled normal human plasma by anion exchange chromatography of the supernatant of Cohn fraction V followed by gel filtration 17 with the additional step of solid-phase immunoabsorption using antiserum raised in a rabbit that had been immunized with Galgp but that produced antibodies only against the trace contaminants 18. Galgp appeared as a single ∼120-kD band on SDS gels stained with Alcian blue, a glycoprotein stain 19, and no component was detected when overloaded gels (50 μg samples) were stained with the protein stain Coomassie blue (data not shown).
Antifreeze fish glycoprotein (AFGP) 20 isolated from Northern cod (mol wt of ∼3,000) was provided by A/F Protein, Inc. (Waltham, MA). Purified porcine submaxillary gland mucin (PSM) was reduced, carboxymethylated, and treated with trypsin to generate a monomeric mucin species (PSM-RT) 21. The mucins were added to Mφ cultures at the same time as M. avium, unless otherwise noted.
Measurement of TNF-α and IL-10.
Murine spleen Mφ were isolated, cultured in 24-well plates, and inoculated with M. avium as described above. At 4–48 h after infection, supernatants were harvested and the concentrations of TNF-α and IL-10 were determined by ELISA using matched antibody pairs and cytokine standards (Endogen).
Phagocytosis.
Monolayers of murine spleen Mφ were prepared by plating 2 × 106 murine spleen cells on plastic coverslips as described for microscopy experiments. 4–6 d later, the adherent monolayers were washed and polystyrene (latex) fluorescent microspheres of 1-μm diameter (Fluoresbrite YG Microspheres; Polysciences, Inc.) were added to triplicate wells at a ratio of 200 beads/Mφ and incubated for 30 min. The coverslips were washed five times in HBSS at 37°C and air-dried. The Mφ were examined in a Nikon microscope; cells with at least two phagocytized beads were scored as positive.
Statistical Analysis.
The results are expressed as the mean ± SEM. Statistical differences were determined using SigmaStat Statistical Software (Jandel Scientific), using the t test for normally distributed data with equal variances and the Mann-Whitney rank sum test for data populations with nonnormal distributions and/or unequal variances.
Results
CD43 Is Required for Optimal Binding of Mycobacteria to Mφ.
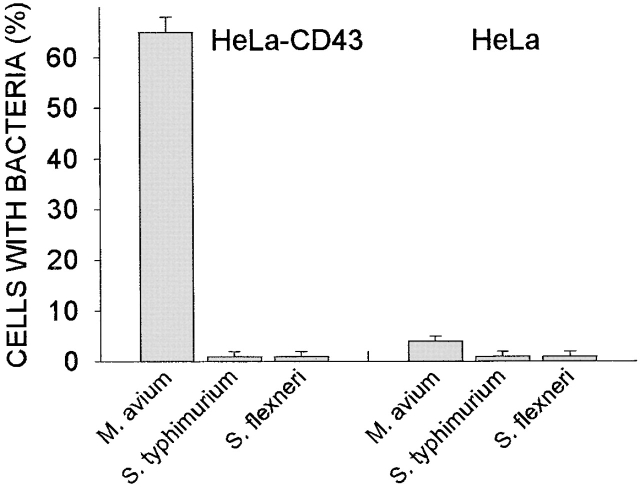
As our initial approach, we incubated CD43-transfected HeLa cells and control HeLa cells with M. avium, S. typhimurium, and S. flexneri. After 4 h exposure to the pathogen, we quantified the cells that had bound bacteria. Contrary to expectations, M. avium were found to be stably associated with the CD43-transfected HeLa cells (Fig. 1, left) and were not associated with control HeLa cells, both wild-type (data not shown) and vector-transfected cells (Fig. 1, right). The binding of M. avium to CD43-expressing HeLa cells appeared to be specific, since neither of the other tested bacterial species, S. typhimurium and S. flexneri, bound to CD43-transfected (or control) HeLa cells (Fig. 1).
Figure 1.
Specific association of M. avium with CD43-transfected HeLa cells. HeLa cells transfected with CD43 (HeLa-CD43) and vector-transfected cells (HeLa) were incubated as indicated with stained live M. avium, S. typhimurium, and S. flexneri at 10 organisms/cell for 4 h. Cells were washed, and the infected cells were counted using fluorescence microscopy (see Materials and Methods). Shown is a representative experiment. The number of CD43-transfected HeLa cells that scored positive for M. avium binding was significantly different than the number of vector-transfected cells (n = 3, P < 0.0001).
The surprising finding that M. avium bind to CD43+ HeLa cells prompted us to examine the role of CD43 in binding of mycobacteria by Mφ, their normal host cell. Monolayers of splenic Mφ were prepared from wild-type (CD43+/+) and CD43 gene–deleted (CD43−/−) mice. When these were challenged with M. avium, quantitative bacteriology showed that the CD43+/+ Mφ bound significant numbers of M. avium (Fig. 2 A). In contrast, CD43−/− Mφ failed to bind M. avium or bound minimal numbers of the bacteria. The binding of M. avium to CD43+/+ Mφ was dependent on bacterial dose (shown below) and time of coincubation (Fig. 2 A). An exposure time of 4 h was chosen for further experiments because, at this time, binding of the M. avium to the Mφ was substantial and bacterial growth was not yet observed.
Figure 2.
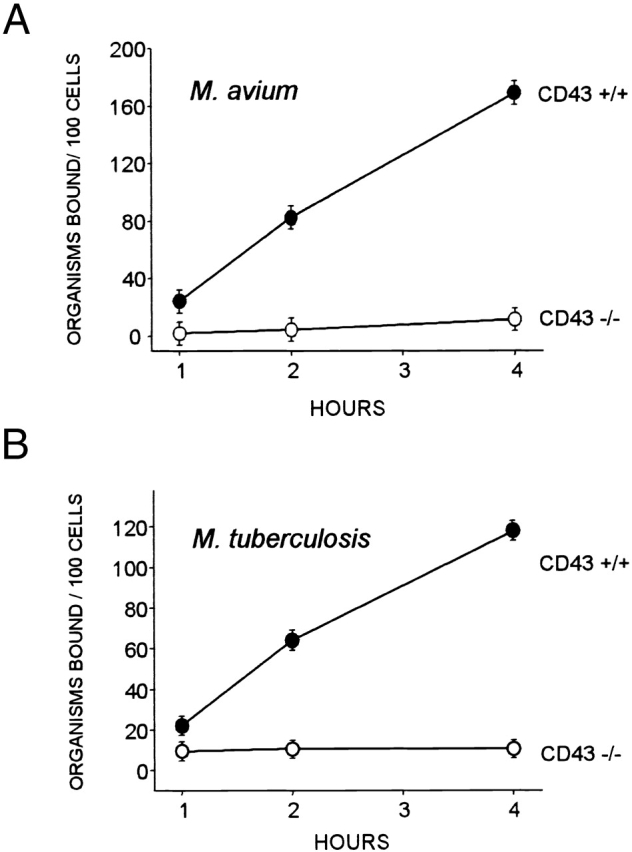
Binding of M. avium and M. tuberculosis to murine CD43+/+ and CD43−/− Mφ examined by quantitative bacteriology. (A) M. avium serovar 4 were incubated with adherent splenic Mφ (∼1 × 105) at a bacteria/cell ratio of 2:1 for the indicated time; the Mφ were extensively washed, and the adherent bacteria were quantified. (B) M. tuberculosis strain H37Rv were similarly analyzed. Shown are mean values ± SEM for three mice of each group. The number of M. avium and M. tuberculosis associated with CD43−/− and CD43+/+ Mφ are significantly different at all time points (P < 0.0001). Although the absolute numbers of bound mycobacteria varied, similar differences between CD43+/+ and CD43−/− Mφ were observed in two additional independent experiments.
To examine whether other mycobacterial species are also dependent on CD43 for uptake, splenic mouse Mφ were challenged with M. tuberculosis H37Rv, a virulent strain. Whereas substantial binding was seen for CD43+/+ Mφ, CD43−/− Mφ failed to bind M. tuberculosis (Fig. 2 B). Similar results, i.e., binding to CD43+/+ Mφ and minimal or absent binding to CD43−/− Mφ, were obtained also for BCG (data not shown). Collectively, these findings strongly indicate a positive role for CD43 in mycobacterial binding.
In an independent approach, Mφ incubated with M. avium were evaluated by light microscopy after acid-fast staining of the mycobacteria. On infection with M. avium at a 20:1 ratio, 57 ± 6% of CD43+/+ Mφ had >10 bacteria detectable by staining compared with 8 ± 3% of CD43−/− Mφ (n = 4).
In other experiments, Mφ incubated with M. avium were examined by fluorescence microscopy after staining of the mycobacteria with rhodamine-auramine. When incubated at a 20:1 ratio, fluorescent micrographs showed multiple mycobacteria associated with all or most of the CD43+/+ Mφ (Fig. 3 A, left) and negligible association of mycobacteria with CD43−/− Mφ (right). Higher magnification micrographs showed that the mycobacteria associated with CD43+/+ Mφ are localized predominantly within the cells (Fig. 3 B, left). In contrast, in the rare cases where mycobacteria were found associated with CD43−/− Mφ, the microorganisms were localized to the periphery of the cells (Fig. 3 B, right).
Figure 3.
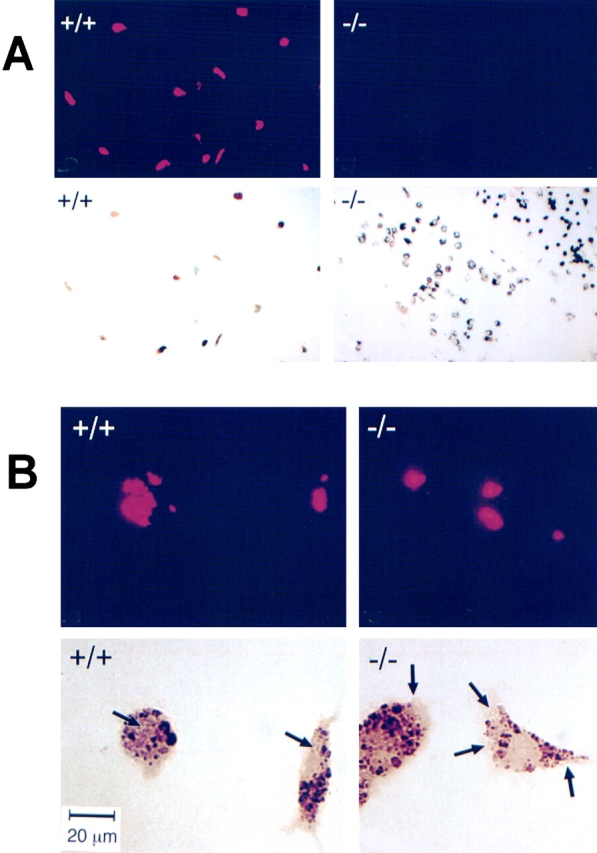
Association of M. avium with CD43+/+ and CD43−/− Mφ examined by fluorescence microscopy. M. avium were incubated with Mφ at a ratio of 20:1 for 4 h. After washing and fixing, mycobacteria associated with the cell monolayers were stained with auramine-rhodamine (TB staining reagents), and the preparations were examined by microscopy. (A) Shown are fluorescent micrographs (top) and phase–contrast images of the same frames (bottom) of CD43+/+ (left) and CD43−/− (right) Mφ. Note that almost all of the CD43+/+ Mφ have multiple associated mycobacteria; in contrast, negligible numbers of mycobacteria are associated with CD43−/− Mφ. (B) Higher magnification images. Description as in A. The arrows on the phase–contrast micrographs indicate the regions of the cells with positive signals in the corresponding fluorescent micrographs. Note that mycobacteria associated with CD43+/+ Mφ are localized within the cells. In contrast, in the very rare CD43−/− Mφ with associated mycobacteria, the organisms are localized to the periphery of the cell. Bar, (B) 20 μm.
To test whether the diminished association of mycobacteria with CD43−/− Mφ is due to a global defect in phagocytosis, monolayers of wild-type and CD43−/− Mφ were incubated with nonopsonized inert latex fluorospheres (200 spheres/cell) for 30 min 22. Uptake of the latex beads examined by fluorescence microscopy showed that 76 ± 5% of CD43+/+ and 73 ± 5% of CD43−/− Mφ contained fluorescent beads. The finding that CD43−/− Mφ do not take up less beads than CD43+/+ Mφ (n = 3, P < 0.6) suggests that the cells are not globally defective in phagocytosis.
Galgp Restores M. avium Binding to CD43−/− Mφ.
The extracellular mucin region of CD43 was identified as a normal component of human plasma, given the name Galgp, and isolated 17. We reasoned that if CD43 functions as a receptor for mycobacteria, addition of soluble extracellular CD43 to wild-type Mφ might abrogate mycobacterial binding by competing with cell surface CD43. However, addition of Galgp to CD43+/+ Mφ resulted in enhanced mycobacterial binding (not shown). More importantly, addition of 100 μg/ml Galgp to CD43−/− Mφ at the time of infection restored the time-dependent (Fig. 4 A) and bacterial dose–dependent (Fig. 4 B) association of M. avium with CD43−/− Mφ. Restoration of M. avium binding to CD43−/− Mφ by Galgp (100 μg/ml) was also seen when the cells were evaluated by acid-fast staining (data not shown). These findings suggest that Mφ CD43, rather than serving as a receptor for mycobacteria, functions by promoting or stabilizing binding or uptake of mycobacteria.
Figure 4.
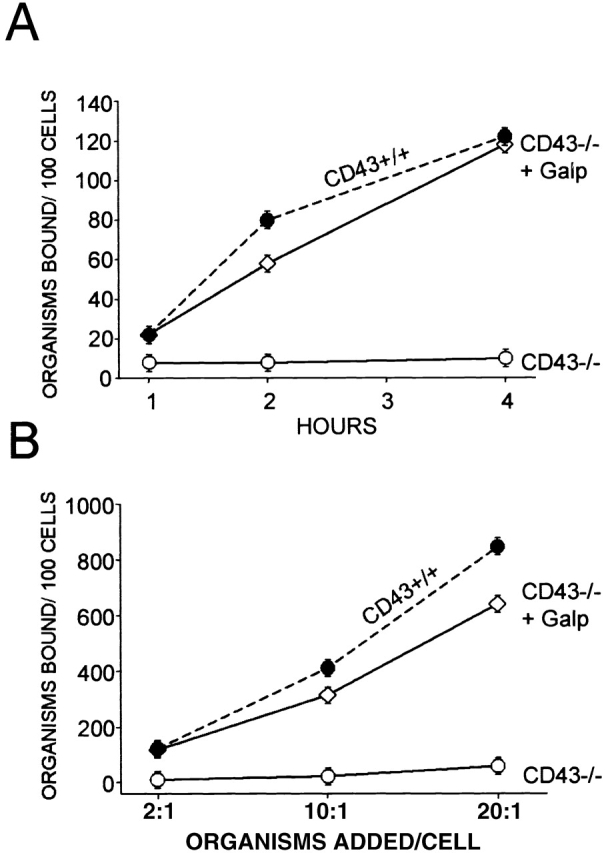
Addition of Galgp restores binding of M. avium by CD43−/− Mφ. (A) M. avium were incubated for varying times with CD43−/− Mφ in the absence (○) and presence (⋄) of 100 μg/ml Galgp. Bacteria/cell ratio was 2:1. The Mφ were harvested and extensively washed, and adherent bacteria were quantified. The binding of M. avium to CD43+/+ Mφ in the absence of Galgp is shown for comparison (•, dashed lines). (B) M. avium at varying bacteria/cell ratios as indicated were incubated with CD43−/− Mφ for 4 h in the absence (○) and presence (⋄) of 100 μg/ml Galgp. Shown are mean values ± SEM for three mice of each group. The number of mycobacteria associated with CD43−/− Mφ in the presence and absence of 100 μg/ml Galgp was significantly different in all conditions (P < 0.0001). Comparable effects of Galgp addition were observed in two additional experiments. Galgp at 200 μg/ml produced similar results (data not shown).
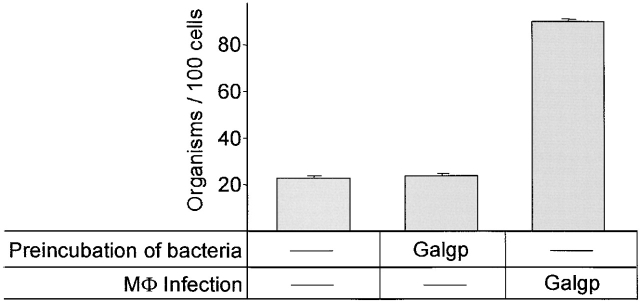
The proposed collaborative role of CD43 in enabling mycobacterial association with Mφ was further tested by preincubating M. avium with Galgp for 4 h, washing the bacteria extensively, and adding them to CD43−/− Mφ for an additional 4-h incubation. Whereas coincubation of Galgp, M. avium, and Mφ enhanced the binding of mycobacteria to Mφ, no enhancement was observed when mycobacteria were preincubated with Galgp, washed, and then added to the cells (Fig. 5), indicating that Galgp does not opsonize the mycobacteria.
Figure 5.
Preincubation of M. avium with Galgp does not enhance their binding to CD43−/− Mφ. M. avium were preincubated for 4 h with 100 μg/ml Galgp or without additive, as indicated, and then extensively washed. The bacteria were used to infect CD43−/− Mφ (infection ratio of two organisms per cell). Galgp (100 μg/ml) or saline was added, as indicated at the time of infection. After extensive washing, M. avium associated with Mφ were quantified. Shown are mean values ± SEM for cells from three mice. The number of M. avium associated with Mφ were significantly different for mycobacteria preincubated with Galgp compared with M. avium infected in the presence of Galgp (P < 0.0001). Similar effects of Galgp treatment were seen in one additional experiment.
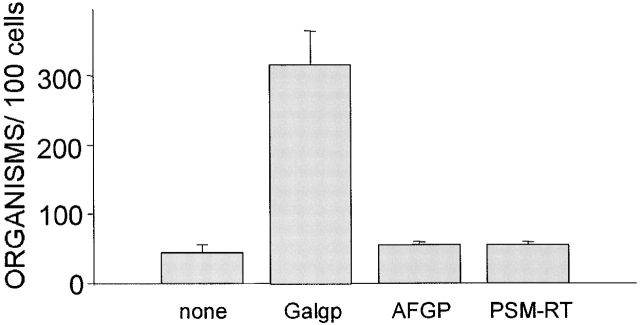
We next determined whether other mucins are also able to restore the association of M. avium with CD43−/− Mφ. M. avium were added to CD43−/− Mφ together with 100 μg/ml of Galgp or AFGP 20 or PSM that had been reduced, carboxymethylated, and trypsin-treated to generate linear monomeric mucin molecules (PSM-RT) 21. After 4 h, association of bacteria with Mφ was assessed. M. avium binding to CD43−/− Mφ was enhanced only by the presence of Galgp. AFGP and PSM-RT failed to enhance association of M. avium with Mφ (Fig. 6).
Figure 6.
Galgp, but not other mucins, enables CD43−/− Mφ to bind M. avium. M. avium at a bacteria/cell ratio of 2:1 were incubated with CD43−/− Mφ in the absence (none) or presence of Galgp, AFGP, or PSM reduced and trypsin-treated (PSM-RT) at 100 μg/ml. After 4 h, the cells were washed and the mycobacteria associated with cells were quantified. Shown are mean values ± SEM for cells from three mice. The number of M. avium associated with the CD43−/− Mφ incubated with the mycobacteria in the presence of Galgp, AFGP, or PSM were significantly different (P < 0.0001). Similar effects of mucin treatments were seen in one additional experiment.
In contrast, addition of Galgp did not enhance association of M. avium with wild-type (CD43−) HeLa cells. On infection with a 1:1 ratio, the number of bacteria associated per 100 cells was 5, 7, and 40, respectively, for vector-transfected HeLa, vector-transfected HeLa with Galgp (100 μg/ml), and CD43-transfected HeLa. At an infection ratio of 5:1, the corresponding results were 10, 10, and 70 bacteria bound per 100 cells. Similar results were obtained when M. avium–challenged HeLa cells (infection ratio of 15:1) were evaluated by acid-fast staining and light microscopy. 70% of CD43-transfected HeLa cells, but <10% of vector-transfected HeLa and vector-transfected HeLa with Galgp, scored positive for bacterial association (≥5 bacteria/cell). In the case of CD43-transfected HeLa cells, the associated mycobacteria were clearly located outside the cell perimeter as anticipated for these nonphagocytic cells 23.
In Contrast to CD43+/+ Mφ, CD43−/− Mφ Fail to Produce TNF-α in Response to M. avium and This Deficiency Is Corrected by Galgp.
Early after inoculation with mycobacteria, wild-type Mφ respond by producing the proinflammatory cytokine TNF-α 24, which plays a central role in defense against microbial organisms including mycobacteria 25 26. To investigate whether M. avium–dependent TNF-α production is altered in CD43−/− Mφ, we measured TNF-α levels produced by CD43−/− and CD43 +/+ Mφ over the 4–48-h period after inoculation. M. avium inoculation of CD43+/+ Mφ resulted in the production of 1,150 ± 200 ng/ml TNF-α at 8 h. In contrast, CD43−/− Mφ inoculated with the same M. avium/cell ratio produced <200 ng/ml TNF-α (Fig. 7 A). The possibility of a TNF-α expression defect in CD43−/− Mφ was ruled out by finding that CD43−/− and wild-type Mφ produced comparable levels of TNF-α when stimulated for 8 h with LPS (100 ng/ml; data not shown), suggesting that the defective induction of TNF-α production is M. avium specific. Importantly, the inability of CD43−/− Mφ to produce TNF-α in response to M. avium was overcome by the addition of Galgp during inoculation (Fig. 7 B), and the extent of the restorative effect was dependent on Galgp concentration.
Figure 7.
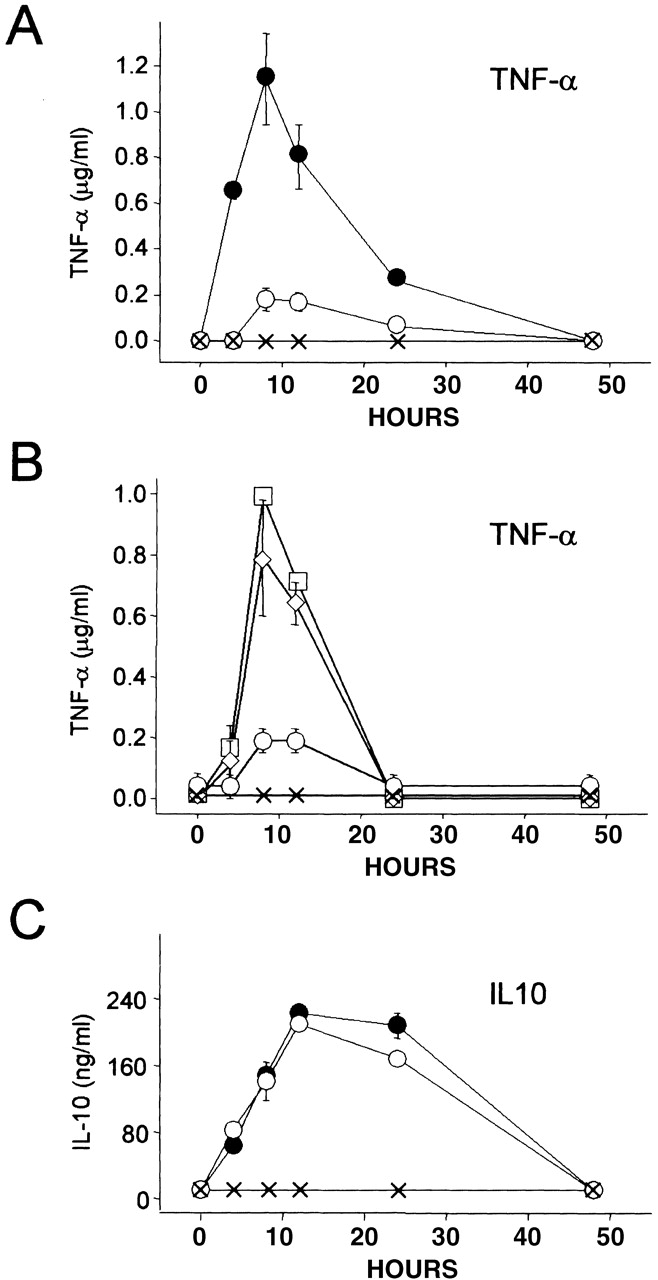
TNF-α and IL-10 production examined in CD43+/+ and CD43−/− Mφ challenged with M. avium. (A) M. avium–induced TNF-α production is significantly impaired in CD43−/− Mφ. Mφ from CD43+/+ (•) and CD43−/− (○) mice were inoculated with M. avium (two bacteria per Mφ), and the supernatants harvested at the indicated times were assayed by ELISA. The levels of TNF-α produced by M. avium–challenged CD43+/+ and CD43−/− Mφ are significantly different at 4, 8, and 12 h (P < 0.001, 0.007, and 0.01, respectively). In the absence of mycobacterial challenge, neither CD43+/+ (×) nor CD43−/− Mφ (not shown) produced TNF-α. (B) Impaired TNF-α production by M. avium–challenged CD43−/− Mφ is restored by Galgp. CD43−/− Mφ were inoculated with M. avium (two bacteria per Mφ) in the absence (○) and presence of 100 (⋄) and 200 (□) μg/ml Galgp. Galgp was tested also at 50 μg/ml, and the resulting TNF-α levels were indistinguishable from levels produced in the absence of added Galgp (○). Note that 200 μg Galgp increases TNF-α to levels of CD43+/+ cells at 8 and 12 h (compare with A). The levels of TNF-α are significantly different for M. avium–challenged CD43−/− Mφ in the absence and presence of 100 and 200 μg/ml Galgp at 8 and 12 h (P < 0.02). Note also that Mφ cultured with Galgp (200 μg/ml) in the absence of mycobacterial challenge (×) did not produce TNF-α. (C) M. avium–dependent IL-10 production is not impaired in CD43−/− Mφ. The Mφ supernatants from A were assayed for IL-10 by ELISA. The levels of IL-10 produced by CD43+/+ (•) and CD43−/− (○) Mφ after inoculation with M. avium are not significantly different at any time point (P values range from 0.06 to 0.85). Neither CD43+/+ (×) nor CD43−/− Mφ (not shown) produced IL-10 in the absence of mycobacterial challenge. Values are mean ± SEM of replicate assays of fractions from three mice of each group.
Incubation of wild-type Mφ with mycobacteria also induces production of the immunosuppressive cytokine IL-10, which counteracts the effects of TNF-α 27. Incubation with M. avium of CD43−/− and CD43+/+ Mφ induced comparable levels of IL-10 (Fig. 7 C), indicating that the lesser interaction of mycobacteria with CD43−/− Mφ is sufficient to induce IL-10 production. The discordant regulation of TNF-α and IL-10 demonstrates that the failure to produce TNF-α in response to mycobacterial challenge is a specific defect of CD43−/− macrophages.
Discussion
Mycobacterial diseases, some of which are spread by airborne transmission, are a serious global public health problem 3. In this communication, we provide the first evidence identifying a new component of apparent importance for the uptake of mycobacteria by their primary host cell, the Mφ. This component, CD43, is the predominant sialoglycoprotein on leukocytes. We show that the presence of CD43 is obligatory for firm association of mycobacteria with Mφ. Mφ from wild-type mice and CD43-transfected HeLa cells bound high numbers of M. avium. In contrast, negligible numbers of M. avium associated with untransfected HeLa cells and with Mφ from CD43 gene–deleted mice. The role of CD43 in the binding of mycobacteria is specific because other bacteria, S. typhimurium and S. flexneri, did not bind to CD43 + HeLa cells. Also, Mφ from CD43+/+ mice bound two other species of mycobacteria, M. tuberculosis and M. bovis, and these also failed to bind to CD43−/− Mφ. Fluorescence microscopy of Mφ incubated with M. avium showed that the associated mycobacteria had been ingested by the CD43+/+ Mφ. CD43 was also required for the production of TNF-α, but not of IL-10, by Mφ challenged with M. avium, strongly suggesting a functional link between the action of CD43 in mycobacterial binding and/or uptake and induction of TNF-α production.
CD43, also called sialophorin or leukosialin, is a prevalent sialoglycoprotein on monocytes, neutrophils, and T lymphocytes 28 29. On T cells, CD43 extends 45 nm from the phospholipid bilayer, making it the largest glycoprotein on the cell surface 30. One function of CD43 is that of a repulsive or barrier molecule restricting cell to cell contact 13 14 31 due to its negative charge, prevalence, size, and rigidity. In addition, in vitro binding of CD43 mAb transduces intracellular signals that lead to T lymphocyte proliferation (e.g., 32) and activation of macrophages, increasing homotypic adhesion and hydrogen peroxide production 32 33. At the mechanistic level, evidence suggests that ligation of CD43 induces tyrosine phosphorylation reactions 34 35, and ligated CD43 itself binds the actin filament linker proteins, moesin and ezrin, which function in cytoskeletal reorganization 36.
The inability of Mφ that lack CD43 to bind mycobacteria and produce TNF-α was corrected by the addition of Galgp, a purified mucin glycoprotein equivalent to the extracellular region of CD43. Galgp, which is 76% carbohydrate, was originally identified as a plasma component. Its high content of galactose and N-acetylgalactosamine suggested its identity with the extracellular region of CD43, and this relationship was confirmed by amino acid sequencing 18. Isolated plasma Galgp is heterogeneous; all molecules have the NH2-terminal CD43 sequence, but they have varying COOH termini. The longest species encompasses 226 of the 235 extracellular CD43 amino acids. It is believed that Galgp originates as leukocyte surface CD43 primarily because the single copy (human) CD43 gene encodes a single polypeptide that includes a transmembrane and a cytoplasmic region, missing from Galgp (discussed in reference 18). Relative to other plasma glycoproteins that originate as leukocyte surface molecules, the levels of Galgp are extremely high, >10 μg/ml of normal plasma 37. It is not known whether Galgp is released by proteolysis from intact cells or arises by another mechanism, e.g., as a stable residue remaining after leukocyte death.
Because Galgp was obtained by chromatography (from a human plasma Cohn fraction), we cannot eliminate the possibility that the active component that enhances mycobacterial adherence is a contaminating species. This scenario is unlikely because contaminants were not seen on overloaded SDS gels stained for glycoprotein and protein. Also, trace proteinaceous materials remaining after conventional chromatographic purification were removed by a final immunoabsorption step. Importantly, addition of Galgp to uninfected CD43−/− Mφ did not induce TNF-α or IL-10 (Fig. 7A–C), indicating that the action of Galgp is not due to induction of cytokines by trace contaminants.
Two other monomeric mucins, AFGP, which consists of tandem repeats of an O-glycosylated tripeptide, and PSM-RT, a derivative of PSM, which consists of tandem repeats of 81 residues, failed to mediate adherence of M. avium with Mφ (Fig. 6), indicating that the action of Galgp is specific. Whereas AFGP lacks sialic acid, PSM-RT, like Galgp, is heavily sialylated, bearing N-glycolylneuraminic acid on 40–50% of its O-glycans 21. These findings indicate that the effect of Galgp is not simply due to the presence of sialylated O-glycans.
The finding that Galgp, a soluble species, enables CD43−/− Mφ to bind mycobacteria indicates that CD43 does not by itself function as a surface receptor for mycobacteria. Clues to the mode of action of CD43/Galgp might be found by reference to other functionally interchangeable membrane protein/soluble protein pairs. These include the IL-6 receptor (IL-6R), which exists in an integral membrane and a solubilized form elaborated by cleavage from the cell surface 38. Soluble IL-6R is able to bind IL-6 and associate with the IL-6R coreceptor gp130, thereby mediating signaling in IL-6R–negative cells 39. Similar behavior is observed for the membrane and soluble forms of CD14. On binding LPS, soluble CD14, like cell surface CD14, can trigger intracellular signaling by interacting with its coreceptor toll-like receptor 2 (TLR-2) 40 41.
Previous studies have shown that binding and phagocytosis of mycobacteria by Mφ can be mediated by several surface receptors, including complement receptors CR1, CR3, and CR4, Fc receptors, CD14, mannose receptor, receptors for SP-A, and scavenger receptors (for a review, see reference 11). In light of this plethora of receptors, the near absence of mycobacterial uptake in CD43−/− Mφ suggests that CD43 functions as a coreceptor or facilitator and/or cofactor of the mycobacterial uptake process. The finding that soluble Galgp restores M. avium binding to CD43−/− Mφ but not to CD43− HeLa cells also attests to the contribution of Mφ components other than CD43/Galgp in the interaction with mycobacteria.
The action of Galgp to enhance mycobacteria binding and/or uptake resembles the action of SP-A. Addition of SP-A, a member of the collectin (collagen-like lectin) family of multimeric innate defense glycoproteins, increases the adherence and phagocytosis of M. tuberculosis by Mφ 42. Like Galgp, SP-A must be present during the interaction of mycobacteria with Mφ. Indeed, reference to earlier studies suggests a candidate Mφ molecule that may functionally interact with both CD43/Galgp and SP-A. This molecule, a surface glycoprotein called C1qRp, binds SP-A 43, and the binding of SP-A to C1qRp enhances the Mφ capacity for phagocytosis of opsonized targets 44. Since it was also shown that CD43 and C1qRp copurify and coimmune precipitate 45, Galgp/CD43 and C1qRp may be components of a multimeric Mφ complex that enhances mycobacteria binding and uptake.
Inoculation of wild-type Mφ with mycobacteria induces the proinflammatory cytokine TNF-α 46, which is known to decrease the survival of M. avium 6 by promoting apoptosis of the infected host Mφ 47, an apparent innate defense mechanism that prevents systemic spread of infection 8. M. avium–induced TNF-α production failed to occur in CD43−/− Mφ and was restored by addition of Galgp, strongly suggesting that the action of CD43 in mycobacterial binding and/or uptake and TNF-α induction are functionally linked.
On the other hand, M. avium–dependent induction of IL-10, an antiinflammatory cytokine and TNF-α antagonist 27, was not impeded in CD43−/− Mφ, strengthening existing evidence 48 that mycobacteria induce separate or divergent pathways for TNF-α and IL-10 production.
In summary, this study identifies the surface mucin CD43 as a component essential for robust binding and/or uptake of mycobacteria by Mφ and for mycobacteria-induced TNF-α production, but not for production of IL-10. Further studies are warranted to identify the Mφ molecules that cooperate with CD43 and the mechanism that enhances mycobacterial binding.
Acknowledgments
We thank Karl Schmid, Department of Biochemistry, Boston University School of Medicine, and H. Gerhard Schwick and Heinz Haupt, Behringwerke AG, Marburg, Germany, for purified Galgp; Michael Erisman, A/F Protein, Inc., Waltham, MA, and Garth Fletcher, A/F Protein Canada, St. John's, Newfoundland, Canada, for antifreeze protein; Hardy Kornfeld, Boston University School of Medicine, for M. tuberculosis strain H37Rv; and John Cohen, Imperial College of Science, Technology and Medicine, London, UK, for S. flexneri and S. typhimurium. Daniel Friend, Brigham and Women's Hospital and Harvard Medical School, is gratefully acknowledged for help with fluorescence microscopy.
This study was supported by National Institutes of Health grants AI31006, AI41710, AI29880, and AI39574 and National Institutes of Health Clinical Investigator award HL02881 (to Manjunath N.).
Footnotes
Abbreviations used in this paper: AFGP, antifreeze fish glycoprotein; BCG, bacillus Calmette-Guérin; Galgp, galactoglycoprotein; CFDA, carboxyfluorescein diacetate; Mφ, macrophage(s); PSM, porcine submaxillary gland mucin; SP-A, surfactant protein A.
References
- Snider D.E., Raviglione M., Kochi A. Global burden of tuberculosis. In: Bloom B.R., editor. TuberculosisPathogenesis, Protection, and Control. American Society for Microbiology; Washington, DC: 1994. pp. 1–11. [Google Scholar]
- Collins F.M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin. Microbiol. Rev. 1989;2:360–377. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B.R., Murray C.J.L. Tuberculosiscommentary on an emergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Fenton M.J., Vermeulen M.W. Immunopathology of tuberculosisroles of macrophages and monocytes. Infect. Immun. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M.A., Hopewell P.C., Yajko D.M., Hadley W.K., Lazarus E., Mohanty P.K., Modin G.W., Feigal D.W., Cusick P.S., Sande M.A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J. Infect. Dis. 1991;164:994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- Bermudez L.E., Young L.S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 1988;140:3006–3013. [PubMed] [Google Scholar]
- Molloy A., Laochumroonvorapong P., Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J. Exp. Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratazzi C., Arbeit R.D., Carini C., Remold H.G. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- Russell D.G. Mycobacterium and Leishmaniastowaways in the endosomal network. Trends Cell Biol. 1995;12:4073–4082. doi: 10.1016/s0962-8924(00)88963-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger L.S., Horwitz M.A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J. Clin. Invest. 1990;85:1304–1314. doi: 10.1172/JCI114568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J.D. Macrophage receptors for Mycobacterium tuberculosis . Infect. Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D., Silverstein S.C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardman B., Sikorski M.A., Staunton D.E. CD43 interferes with T-lymphocyte adhesion. Proc. Natl. Acad. Sci. USA. 1992;89:5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N., Correa M., Ardman M., Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995;377:535–538. doi: 10.1038/377535a0. [DOI] [PubMed] [Google Scholar]
- Meylan P.R., Richman D.D., Kornbluth R.S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect. Immun. 1990;58:2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderlied C.B., Young L.S., Yamada J.K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob. Agents Chemother. 1987;31:1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Mao S.K.Y., Kimura A., Hayashi S., Binette J.P. Isolation and characterization of a serine-threonine-rich galactoglycoprotein from normal human serum. J. Biol. Chem. 1980;255:3221–3226. [PubMed] [Google Scholar]
- Schmid K., Hediger M.A., Brossmer R., Collins J.H., Haupt H., Marti T., Offner G.D., Schaller J., Takagaki K., Walsh M.T. Amino acid sequence of human plasma galactoglycoproteinidentity with the extracellular region of CD43 (sialophorin) Proc. Natl. Acad. Sci. USA. 1992;89:663–667. doi: 10.1073/pnas.89.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardi A.H., Michos G.A. Alcian blue staining of glycoproteins in acrylamide disc electrophoresis. Anal. Biochem. 1972;49:607–609. doi: 10.1016/0003-2697(72)90472-1. [DOI] [PubMed] [Google Scholar]
- Hays L.M., Feeney R.E., Crowe L.M., Crowe J.H., Oliver A.E. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. USA. 1996;93:6835–6840. doi: 10.1073/pnas.93.13.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken T.A., Owens C.L., Pasumarthy M. Determination of the site-specific O-glycosylation pattern of the porcine submaxillary mucin tandem repeat glycopeptide. Model proposed for the polypeptide:galnac transferase peptide binding site. J. Biol. Chem. 1997;272:9709–9719. doi: 10.1074/jbc.272.15.9709. [DOI] [PubMed] [Google Scholar]
- Oda T., Maeda H. A new simple fluorometric assay for phagocytosis. J. Immunol. Methods. 1986;88:175–183. doi: 10.1016/0022-1759(86)90004-9. [DOI] [PubMed] [Google Scholar]
- Meresse S., Steele-Mortimer O., Finlay B.B., Gorvel J.P. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman G.W., Gan H.X., McCarthy P.L., Jr., Remold H.G. Survival of human macrophages infected with Mycobacterium avium intracellulare correlates with increased production of tumor necrosis factor-alpha and IL-6. J. Immunol. 1991;147:3942–3948. [PubMed] [Google Scholar]
- Kindler V., Sappino A.P., Grau G.E., Piguet P.F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kaneko H., Yamada H., Mizuno S., Udagawa T., Kazumi Y., Sekikawa K., Sugawara I. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab. Invest. 1999;79:379–386. [PubMed] [Google Scholar]
- Moore K.W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T.R. Interleukin-10. Annu. Rev. Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Rosen F.S. Sialophorin (CD43) and the Wiskott-Aldrich syndrome. Immunodefic. Rev. 1990;2:151–174. [PubMed] [Google Scholar]
- Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991;1:347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Shotton D.M., Williams A.F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostberg J.R., Barth R.K., Frelinger J.G. The Roman god Janusa paradigm for the function of CD43. Immunol. Today. 1998;19:546–550. doi: 10.1016/s0167-5699(98)01343-7. [DOI] [PubMed] [Google Scholar]
- Sperling A.I., Green J.M., Mosley R.L., Smith P.L., DiPaolo R.J., Klein J.R., Bluestone J.A., Thompson C.B. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J. Exp. Med. 1995;182:139–146. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y.H., Remold-O'Donnell E., LeBien T.W., Remold H.G. A monoclonal antibody to sialophorin (CD43) induces homotypic adhesion and activation of human monocytes. J. Exp. Med. 1989;170:259–267. doi: 10.1084/jem.170.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N., Ardman B. CD43 regulates tyrosine phosphorylation of a 93-kD protein in T lymphocytes. Blood. 1995;86:4194–4198. [PubMed] [Google Scholar]
- Pedraza-Alva G., Merida L.B., Burakoff S.J., Rosenstein Y. CD43-specific activation of T cells induces association of CD43 to Fyn kinase. J. Biol. Chem. 1996;271:27564–27568. doi: 10.1074/jbc.271.44.27564. [DOI] [PubMed] [Google Scholar]
- Serrador J.M., Nieto M., Alonso-Lebrero J.L., del Pozo M.A., Calvo J., Furthmayr H., Schwartz-Albiez R., Lozano F., Gonzalez-Amaro R., Sanchez-Mateos P., Sanchez-Madrid F. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood. 1998;91:4632–4644. [PubMed] [Google Scholar]
- Schwick H.G., Haupt H. Human plasma protein of unknown function. In: Putnam F.W., editor. The Plasma Proteins. Structure, Function and Genetic Control. Academic Press; New York: 1984. pp. 167–220. [Google Scholar]
- Mullberg J., Schooltink H., Stoyan T., Gunther M., Graeve L., Buse G., Mackiewicz A., Heinrich P.C., Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur. J. Immunol. 1993;23:473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- Romano M., Sironi M., Toniatti C., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- Kirschning C.J., Wesche H., Merrill Ayres T., Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.B., Mark M.R., Gray A., Huang A., Xie M.H., Zhang M., Goddard A., Wood W.I., Gurney A.L., Godowski P.J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- Gaynor C.D., McCormack F.X., Voelker D.R., McGowan S.E., Schlesinger L.S. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J. Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- Nepomuceno R.R., Henschen-Edman A.H., Burgess W.H., Tenner A.J. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- Tenner A.J., Robinson S.L., Borchelt J., Wright J.R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J. Biol. Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- Guan E.N., Burgess W.H., Robinson S.L., Goodman E.B., McTigue K.J., Tenner A.J. Phagocytic cell molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J. Biol. Chem. 1991;266:20345–20355. [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Keane J., Balcewicz-Sablinska M.K., Remold H.G., Chupp G.L., Meek B.B., Fenton M.J., Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcewicz-Sablinska M.K., Keane J., Kornfeld H., Remold H.G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]