Abstract
The contribution of the NADPH phagocyte oxidase (phox) and inducible nitric oxide (NO) synthase (iNOS) to the antimicrobial activity of macrophages for Salmonella typhimurium was studied by using peritoneal phagocytes from C57BL/6, congenic gp91phox −/−, iNOS −/−, and doubly immunodeficient phox −/−iNOS −/− mice. The respiratory burst and NO radical (NO·) made distinct contributions to the anti-Salmonella activity of macrophages. NADPH oxidase–dependent killing is confined to the first few hours after phagocytosis, whereas iNOS contributes to both early and late phases of antibacterial activity. NO-derived species initially synergize with oxyradicals to kill S. typhimurium, and subsequently exert prolonged oxidase-independent bacteriostatic effects. Biochemical analyses show that early killing of Salmonella by macrophages coincides with an oxidative chemistry characterized by superoxide anion (O2·−), hydrogen peroxide (H2O2), and peroxynitrite (ONOO−) production. However, immunofluorescence microscopy and killing assays using the scavenger uric acid suggest that peroxynitrite is not responsible for macrophage killing of wild-type S. typhimurium. Rapid oxidative bacterial killing is followed by a sustained period of nitrosative chemistry that limits bacterial growth. Interferon γ appears to augment antibacterial activity predominantly by enhancing NO· production, although a small iNOS-independent effect was also observed. These findings demonstrate that macrophages kill Salmonella in a dynamic process that changes over time and requires the generation of both reactive oxidative and nitrosative species.
Keywords: phagocyte, Salmonella, innate immunity, nitrosative, oxidative
Introduction
Salmonella pathogenesis commences in the ileal mucosa with invasion of M cells or ingestion by CD18-expressing phagocytes 1 2. For the duration of the infection, Salmonella can be found principally within mononuclear phagocytes 3, which can serve as a vehicle of extraintestinal dissemination 1 and as a protected site for intracellular bacterial replication 3. The capacity to survive within macrophages is an absolute requirement for Salmonella virulence in vivo 4. Macrophages contribute to resistance to Salmonella by forming granulomas and limiting bacterial growth 5, but the effector mechanisms by which mononuclear phagocytes combat this intracellular pathogen are incompletely understood.
Radicals generated by the NADPH oxidase and inducible nitric oxide (NO) synthase (iNOS) are cytotoxic for a variety of microorganisms as phylogenetically diverse as viruses, bacteria, protozoa, and fungi. The NADPH oxidase expressed by myeloid cells catalyzes the univalent reduction of molecular oxygen to O2·−. This radical has only limited membrane diffusibility and modest antibacterial activity, but also serves as a precursor to more toxic reactive oxygen species (ROS [6]). The critical role of the phagocyte oxidase (phox) is reflected by the enhanced susceptibility of patients with chronic granulomatous disease to a wide range of microbial pathogens, including Salmonella species 6 7 8. Immunodeficient gp91phox −/− mice, an animal model for X-linked chronic granulomatous disease (CGD), are accordingly very susceptible to experimental Salmonella typhimurium infections 9 10. The reduced killing capacity of cell lines impaired in their ability to sustain a respiratory burst further attests to the importance of the NADPH oxidase in the anti-Salmonella activity of macrophages 11.
NOS isoforms generate NO· in a complex reaction that consumes NADPH, oxygen, and l-arginine 12. NO· by itself posses only weak antimicrobial activity against Salmonella 13, but congeners resulting from NO· autooxidation such as NO2·, N2O3, and S-nitrosothiols 14 enhance its cytotoxic potential. In addition, concerted actions of the NADPH oxidase and iNOS can synergize to form highly potent antimicrobial species. For example, NO· reacts at a rate of 6.7 × 109 M/s with O2·− to form ONOO− 15 16, an oxidant capable of damaging lipids, proteins, and DNA; ONOO− has been associated with enhanced killing of S. typhimurium, Escherichia coli, and Candida albicans 13 17 18. NO· can also act in concert with H2O2 to kill E. coli in vitro by a mechanism that appears to be at least partially iron dependent 19.
The contribution of NO· is of particular significance in resistance to intracellular pathogens. Development of a Th1 immune response dominated by IFN-γ, IL-2, and IL-12 synthesis in combination with NO·-mediated effector functions has been correlated with resistance to Leishmania and mycobacterial infections 20 21. Similarly, IFN-γ, IL-12, and TNF-α production is associated with resistance to S. typhimurium 22 23. However, the contribution of NO· in innate immunity to Salmonella is still a matter of active debate. Evidence obtained from several laboratories using several nitrogen oxide donors such as S-nitrosoglutathione, acidified NO2 −, and ONOO− has unequivocally established that S. typhimurium is susceptible to reactive nitrogen species (RNS) in vitro 13 24 25 26. Furthermore, S. typhimurium deficient in DNA repair systems (e.g., umuC and recBC), small thiol molecules (e.g., homocysteine and glutathione), or detoxifying enzymes (e.g., flavohemoprotein or copper zinc superoxide dismutase) is hypersusceptible to NO· congeners in vitro, and shows reduced macrophage resistance and virulence in vivo 13 25 27 28 29. Yet, despite this compelling evidence favoring distinct NO· actions against Salmonella, some studies have failed to demonstrate that NO· plays a role in macrophage inhibition or killing of wild-type S. typhimurium 9 24 30 31.
In this work, we employed macrophages from C57BL/6 mice and their congenic iNOS −/−, gp91phox −/−, and doubly immunodeficient iNOS −/−gp91phox −/− derivatives to elucidate the contributions of the NADPH phagocyte oxidase and iNOS to antibacterial actions of macrophages for wild-type S. typhimurium.
Materials and Methods
Bacterial Strains.
Wild-type S. typhimurium strains American Type Culture Collection 14028s 1 and M525P 10 32 were used for this study. For immunofluorescence microscopy, rpsM::gfp was moved by P22-mediated transduction from strain SMO22 1 into S. typhimurium 14028s to yield S. typhimurium AF991 (gfp +).
Mice.
Wild-type C57BL/6 mice were purchased from The Jackson Laboratory. Congenic iNOS −/− 33, gp91phox −/− 34, and doubly immunodeficient iNOS −/−gp91phox −/− 9 mice were bred in our animal facility according to Institutional Animal Care and Use Committee guidelines. The iNOS −/− mice were the progeny (N3–N5) of mice backcrossed onto a C57BL/6 background for 10 generations (gift of C. Nathan, Cornell University, New York, NY). The gp91phox −/− and iNOS −/−gp91phox −/− mice were progeny (N4–N6) of mice described previously 9 34. gp91phox −/− and iNOS −/−gp91phox −/− mice were maintained on drinking water containing 15 mg/ml itraconazole, 0.2 mg/ml trimethoprim, and 40 mg/ml sulfamethoxazole up to 4 d before experimentation to prevent spontaneous infections.
Macrophages.
Peritoneal macrophages from C57BL/6 and congenic iNOS −/− 33, gp91phox −/− 34 and doubly immunodeficient iNOS −/−gp91phox −/− 9 mice were harvested 4 d after intraperitoneal inoculation of 1 mg/ml sodium periodate as described 25. The peritoneal exudate cells were resuspended in RPMI 1640 supplemented with 10% heat-inactivated FCS (Gemini Bioproducts), 1 mM sodium pyruvate, 10 mM Hepes, and 2 mM l-glutamine (all reagents from Sigma-Aldrich). The macrophages were selected by adherence in a 96-well plate and cultured for 48 h at 37°C in a 5%-CO2 incubator. Unless otherwise indicated, adherent macrophages were treated in vitro overnight with 20 U/ml of IFN-γ (Life Technologies) from a 105 U/ml stock containing 0.8 ng/ml LPS.
Macrophage Killing Assays.
Periodate-elicited macrophages were challenged with S. typhimurium opsonized with 10% normal mouse serum at a 10:1 multiplicity of infection, allowed to internalize the bacteria for 15 min, and washed with prewarmed medium containing 6 μg/ml gentamicin 25. At several time points after infection, the macrophages were lysed with 0.5% sodium deoxycholate, and surviving bacteria were enumerated on Luria-Bertani agar plates. The results are expressed as percentage survival.
Chemiluminescence.
Macrophage chemiluminescence was estimated by the reduction of 25 μM lucigenin (bis-N-methylacridinium) and the oxidation of 100 μM luminol (5-amino-2,3-dihydro-1,4-phthalazinedione; Sigma-Aldrich) with a Lumistar chemiluminometer (BMG Lab Technologies) in Microlite flat-bottomed microtiter plates (Dinex Technologies, Inc.). The macrophages were challenged with wild-type S. typhimurium American Type Culture Collection 14028s as described in the macrophage killing assays above. Extracellular bacteria were removed by washing and the addition of 6 μg/ml gentamicin. At specified time intervals, medium was harvested and replaced by fresh medium for performance of the assays. Lucigenin and luminol were used as indicators of O2·− and ONOO− production 35 36, respectively. 1 mM uric acid 37 38 inhibited ∼80% of luminol-dependent chemiluminescence generated by IFN-γ–activated periodate-elicited macrophages 1 h after bacterial challenge, whereas 1,000 U/ml catalase (gift of Dr. S. Libby, North Carolina State University, Raleigh, NC) inhibited only 20%. These observations, along with the dependence of chemiluminescence on both the NADPH oxidase and iNOS, indicate that the majority of this luminol chemiluminescence is mediated by ONOO−, whereas a minor proportion reflects H2O2. Periodate-elicited macrophages from wild-type, iNOS −/−, and phox −/− mice were compared.
Superoxide Anion Determination.
O2·− was quantified by the superoxide dismutase–inhibitable reduction of ferricytochrome c 39. At different time points after infection with Salmonella, medium was removed from cultured IFN-γ–treated 48 h–aged periodate-elicited macrophages and replaced with fresh medium containing 60 μM ferricytochrome c in phenol red–free Earle's balanced salt solution. After 1 h incubation in 5% CO2 at 37°C, the OD of the supernatants was determined spectrophotometrically at 550 nm. The concentration of O2·− was calculated by using an ε550 of 2.1 × 103 M−1 cm−1. All reagents were purchased from Sigma-Aldrich.
Hydrogen Peroxide Determination.
H2O2 was measured by the horseradish peroxidase–dependent oxidation of phenol red 39. The macrophages were challenged with Salmonella as described above, and extracellular bacteria were removed by washing and the addition of gentamicin before incubation in Earle's balanced salt solution containing 0.56 mM phenol red and 20 U/ml horseradish peroxidase. At indicated time intervals, medium was harvested and replaced with fresh medium. After 1 h incubation at 37°C in a 5%-CO2 atmosphere, the absorbance of the supernatants was read at 600 nm after mixing with 10 μl of 1 N NaOH per well. H2O2 was quantitated by comparison with a standard curve prepared with known concentrations of H2O2. All reagents were purchased from Sigma-Aldrich.
NOX Determination.
NO synthesis by periodate-elicited macrophages challenged with Salmonella as described above was estimated by measuring the accumulation of nitrite (NO2 −) and nitrate (NO3 −), stable metabolites of the reaction of NO with oxygen, using the Griess reaction. The NO2 − present in supernatants of Salmonella-infected macrophages was measured spectrophotometrically at 550 nm after mixing with an equal volume of Griess reagent (0.5% sulfanilamide and 0.05% N-1-naphthylethylenediamide hydrochloride in 2.5% acetic acid). The NO2 − concentration was determined from a standard curve prepared with NaNO2. The NO3 − accumulated in the supernatants was estimated by the Griess reaction after enzymatic reduction of NO3 − to NO2 − 40 41 in pH 7 sodium phosphate buffer containing 1.6 U/ml nitrate reductase, 16 U/ml glucose dehydrogenase, 10 μM NADPH, and 10 mM glucose-6-phosphate. NO3 − concentration was calculated as the difference between NO2 − accumulated in the presence and absence of nitrate reductase. At designated time points, the NOX concentrations were determined from S. typhimurium–challenged macrophages cultured for 1 h in serum-free IMDM (Sigma-Aldrich) supplemented with 7.5% minimal essential amino acid solution (Life Technologies), 1% minimal nonessential amino acid solution, 1.1 mM sodium pyruvate (Life Technologies), 1 mg/ml streptomycin sulfate (Sigma-Aldrich), 0.5 mg/ml gentamicin (Sigma-Aldrich), 0.6 mg/ml penicillin G (Life Technologies), 0.75% wt/vol dextrose (Sigma-Aldrich), 0.85% wt/vol NaHCO3 (Sigma-Aldrich), and 1% nutridoma-SP (Boehringer). Low background levels of nitrate (∼1 μM) detected in parallel wells were subtracted to exclude a contribution by residual levels of nitrate present in the culture medium.
Immunocytochemistry.
Macrophages plated onto sterile coverslips were incubated for 48 h at 37°C in a 5%-CO2 atmosphere. The macrophages were stimulated with IFN-γ during the last 20 h and challenged with S. typhimurium strain 14028s as described above. After 90 min of infection, the coverslips were washed with PBS, and the cells were fixed with 2% paraformaldehyde in PBS for 20 min. After extensive washing with 0.1% Tween in PBS, the macrophages were incubated with a 3% normal goat serum solution in Tris balanced solution, pH 7.3. The cells were stained with 5 μg/ml of a rabbit antinitrotyrosine polyclonal antibody 42 or a rabbit antidinitrophenol (Zymed Laboratories) polyclonal control antibody for 1 h, followed by a rhodamine-conjugated goat anti–rabbit polyclonal antibody (Jackson ImmunoResearch Laboratories) for 1 h. After washing, the coverslips were mounted with Vectashield® (Vector Laboratories) and examined with an Olympus IX70 inverted microscope, a Photometrics PXL camera with Kodak KAF1400 chip (6.7 × 6.7 μm physical pixels giving 67 nm per image pixel with a ×100 oil immersion objective), and a Silicon Graphic O2 computer with DeltaVision deconvolution software (Applied Precision).
Results
NADPH Oxidase– and iNOS-derived Chemical Species Contribute to the Antimicrobial Activity of Macrophages for Salmonella.
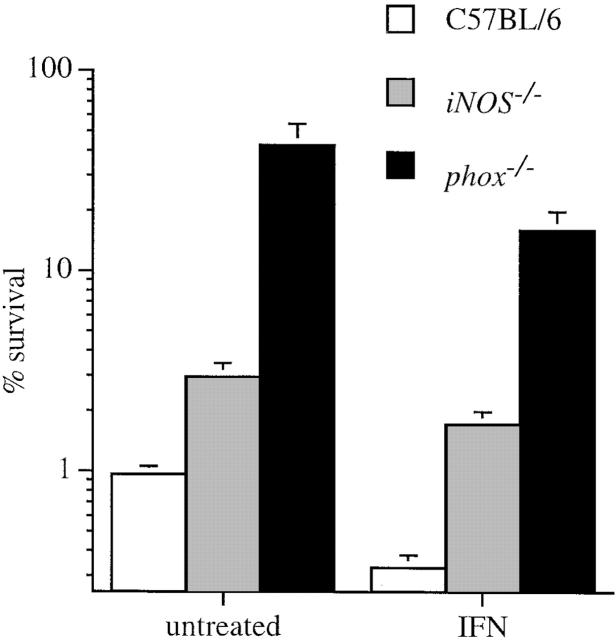
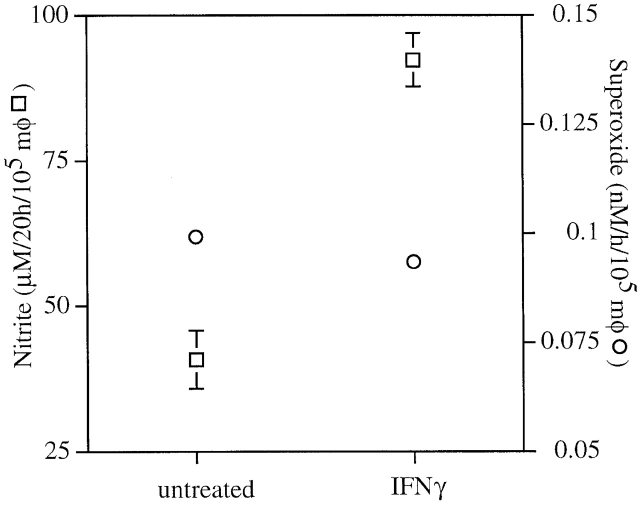
Macrophages from iNOS −/− or gp91phox −/− mice exerted less antimicrobial activity to S. typhimurium strain 14028s than macrophages from congenic wild-type control animals (Fig. 1). Macrophages lacking the NADPH oxidase were less effective in their ability to contain Salmonella than congenic cells lacking iNOS, suggesting that O2·− or its derivatives play a greater role than NO· congeners in Salmonella killing (Fig. 1). The addition of IFN-γ enhanced bactericidal activity of wild-type macrophages (Fig. 1), and this was correlated with enhanced NO· production (Fig. 2). IFN-γ did not increase O2·− production by wild-type macrophages as measured by reduction of cytochrome c (Fig. 2), and only very modestly increased the antimicrobial activity of iNOS −/− macrophages (Fig. 1). Similar results were obtained for wild-type S. typhimurium American Type Culture Collection strain 14028s and wild-type strain M525P (data not shown).
Figure 1.
Both ROS and RNS contribute to the antimicrobial activity of macrophages for Salmonella. Intracellular bacterial counts of untreated and IFN-γ–activated periodate-elicited macrophages from C57BL/6, iNOS −/−, and gp91phox −/− mice were compared. Viable intracellular bacteria were quantified by plating 20 h after the macrophages were challenged with S. typhimurium 14028s. The data are the mean ± SEM of 12–18 independent observations obtained on at least four separate days.
Figure 2.
Nitrite and superoxide production by untreated and IFN-γ–activated macrophages. NO2 − (□) accumulated by macrophages (mφ) from C57BL/6 mice in response to S. typhimurium 14028s was determined by the Griess reaction at 20 h after challenge, and O2·− (○) production was measured by the reduction of cytochrome c over a 1-h interval 20 h after challenge. The data are the mean ± SEM of six independent observations obtained on at least two separate days.
Temporal Differences in iNOS− and NADPH Oxidase–mediated Macrophage Cytotoxicity.
To study the relative contribution of iNOS and the NADPH oxidase to phagocyte-mediated killing of S. typhimurium in more detail, the cytotoxicity of IFN-γ–activated macrophages from wild-type, iNOS −/−, and gp91phox −/− mice was studied over a period of 14 h (Fig. 3). Macrophages from wild-type C57BL/6 mice exhibited pronounced bactericidal activity towards Salmonella during the first 6 h after challenge. In fact, these macrophages eliminated >99% of the original inoculum during the first 6 h of infection. At later time points, macrophages exhibited cytostatic behavior, confining the bacterial burden to a steady level. Although the NADPH oxidase contributed more than iNOS to overall macrophage antimicrobial activity, substantial temporal differences were observed in the contribution of these systems. Macrophages deficient in the NADPH oxidase did not reduce the original inoculum, but were still able to maintain the bacterial burden at a steady level over time (Fig. 3 A). In contrast, macrophages deficient in iNOS considerably reduced the initial inoculum but were unable to control bacterial replication at later time points (Fig. 3 B). The contribution of iNOS to macrophage antibacterial activity was detectable as early as 2 h after the initial challenge (P < 0.05), but became more substantial over time.
Figure 3.
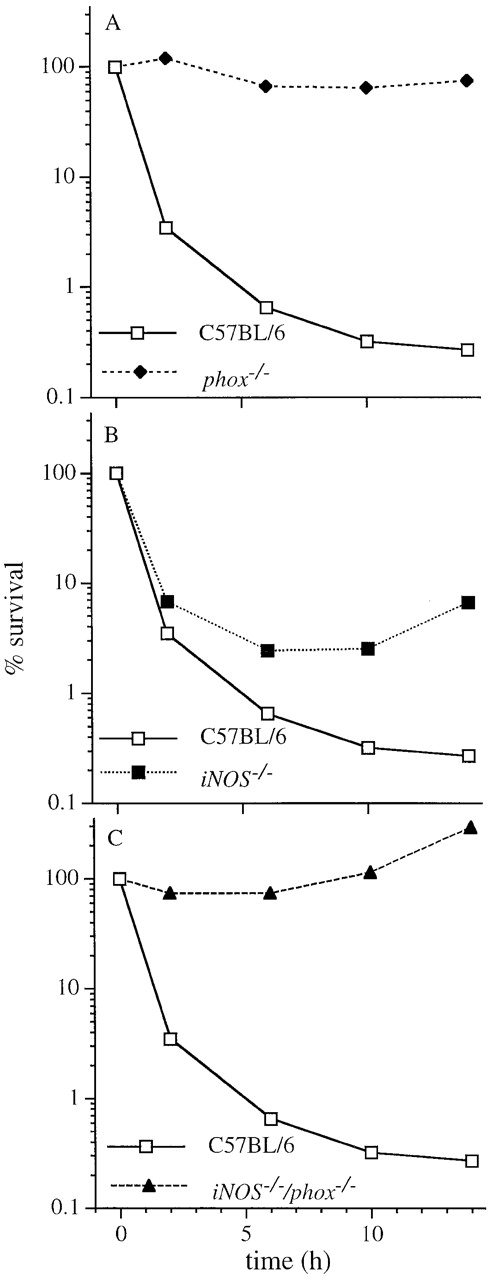
The contribution of iNOS and the NADPH phagocyte oxidase to the antimicrobial activity of macrophages for S. typhimurium varies over time. The killing activity of IFN-γ–activated, periodate-elicited macrophages from (A–C) C57BL/6, (A) phox −/−, (B) iNOS −/−, and (C) iNOS −/−gp91phox −/− mice was recorded over a 1-h period after challenge with wild-type S. typhimurium strain 14028s. The data are the mean ± SEM of 3–11 independent observations obtained on at least two separate days.
In accord with these observations, macrophages deficient in both the NADPH oxidase and iNOS did not reduce the inoculum at early time points nor achieve subsequent control of bacterial replication (Fig. 3 C). A comparison of macrophages from phox −/− and iNOS −/−gp91phox −/− mice revealed an NO·-dependent antimicrobial activity at later time points that is independent of the NADPH oxidase.
Production of ROS and RNS in Response to Salmonella.
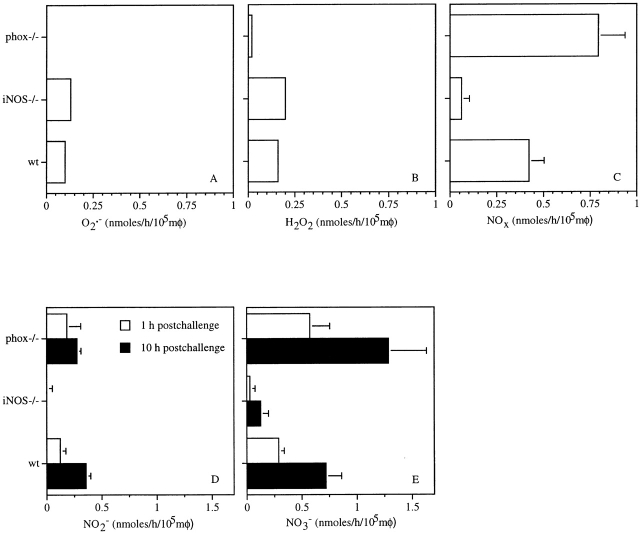
Production of ROS and RNS by IFN-γ–activated macrophages from wild-type, iNOS −/−, and gp91phox −/− mice was determined over a 10-h period by chemiluminescence using lucigenin and luminol, and by spectrophotometry using the Griess reagent (Fig. 4). Production of ROS by macrophages from wild-type mice was initiated immediately after phagocytosis, decreasing to undetectable levels by 6 h thereafter (Fig. 4 A). Lucigenin-dependent chemiluminescence was three times lower than that mediated by luminol (Fig. 4A and Fig. B), suggesting that a substantial proportion of the O2·− formed by the NADPH oxidase reacts with NO· to form ONOO−. As anticipated, macrophages from phox −/− mice exhibited neither lucigenin- nor luminol-dependent chemiluminescence. Macrophages from iNOS −/− mice showed a considerable diminution in luminol-dependent chemiluminescence. However, these macrophages generated a prolonged lucigenin-dependent chemiluminescence that persisted for the duration of the experiment.
Figure 4.
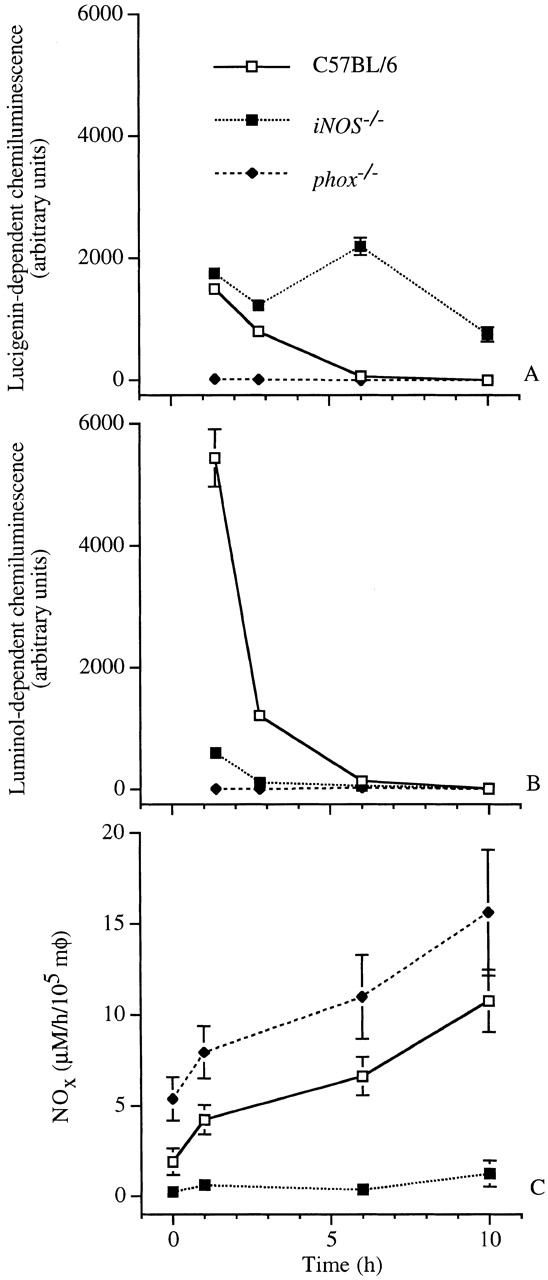
The lucigenin- and luminol-dependent chemiluminescence and NOX production of Salmonella-infected macrophages vary over time. The capacity of IFN-γ–activated macrophages (mφ) to produce ROS and RNS was measured at selected 1-h intervals over a 10-h period from independent wells. The respiratory burst and NOX production were measured as (A) lucigenin- and (B) luminol-dependent chemiluminescence, and (C) by the Griess reaction, respectively. The data are the mean ± SEM of 3–11 independent observations obtained on at least four separate days.
The rate of NOX production by macrophages from wild-type mice infected with S. typhimurium increased over time (Fig. 4 C). In agreement with previous observations 9, macrophages from gp91phox −/− mice consistently produced more NOX than wild-type controls, likely reflecting a lack of NO· scavenging by O2·−. Macrophages from iNOS −/− mice produced very low residual quantities of NOX, possibly reflecting constitutive NOS activity.
The relative abundance of ROS and RNS produced by macrophages during the first hour after challenge with S. typhimurium strain 14028s was characterized further (Fig. 5). Of the ROS measured, H2O2 was the most abundant, accounting for ∼0.15 ± 0.01 nmol/h/105 macrophages, closely followed by O2·− (0.10 ± 0.01 nmol/h/105 macrophages; Fig. 5A and Fig. B). Macrophages from iNOS −/− mice produced more O2·− and H2O2 in response to Salmonella infection than those from wild-type control animals (P < 0.05). Macrophages from iNOS −/− mice appeared to produce very low levels of NOx that almost exclusively consisted of NO3 − (Fig. 5C and Fig. E), suggesting that a significant proportion of the NO· attributed to constitutive NOS might react with O2·− to form ONOO− (Fig. 4 B). Macrophages from gp91phox −/− mice did not produce detectable quantities of O2·−, H2O2, or chemiluminescence.
Figure 5.
Macrophage production of ROS and RNS in response to Salmonella challenge is largely dependent on the NADPH oxidase and iNOS. (A) O2·−, (B) H2O2, and (C) NOX production were measured by reduction of cytochrome c, horseradish peroxidase–dependent oxidation of phenol red, and the Griess reaction, respectively. A–C represent metabolite production during the first hour after challenge. D and E represent NO2 − and NO3 − accumulated over a 1-h interval at 1 and 10 h after challenge. The data are the mean ± SEM of 3–10 independent observations obtained on at least two separate days. mφ, macrophages.
Even at the earliest time point, ∼0.4 nmol/h/105 macrophages of NOX was produced (Fig. 5 C). The majority of the NO· was oxidized to NO3 − (∼0.3 nmol/h/105 macrophages), although a third was metabolized to NO2 − (Fig. 5D and Fig. E). After 10 h of infection, the macrophages increased their production of both NO3 − and NO2 −. NO3 − production greatly exceeded that of NO2 − in wild-type, iNOS −/−, and gp91phox −/− macrophages. NO· synthesized by constitutive NOS appear to have a negligible contribution to macrophage antimicrobial activity, as the NOS inhibitor NG-monomethyl l-arginine did not reduce the inhibition of Salmonella by macrophages from iNOS −/− mice (data not shown).
Nitrotyrosine Formation in Salmonella-infected Macrophages.
To determine whether ONOO− produced by macrophages targets intracellular Salmonella, formation of nitrotyrosine, a product that can be formed from the reaction of ONOO− with tyrosine residues, was investigated by immunofluorescence microscopy (Fig. 6). In agreement with the biochemical data (Fig. 4), nitrotyrosine was present in wild-type macrophages infected with wild-type S. typhimurium, but absent from NADPH oxidase–deficient control macrophages (Fig. 6A and Fig. C). The presence of nitrotyrosine was markedly reduced, but not totally absent, in macrophages from iNOS −/− mice (Fig. 6 B). Nitrotyrosine labeling failed to colocalize with green fluorescent protein (GFP)-tagged Salmonella in any instance (Fig. 6A and Fig. B), suggesting that ONOO− is formed but may not contribute to bacterial killing. In further support of this notion, the scavenger uric acid did not diminish, but rather enhanced by twofold the bactericidal activity of wild-type macrophages (Fig. 7), suggesting that formation of ONOO− is actually detrimental to macrophage anti-Salmonella activity.
Figure 6.
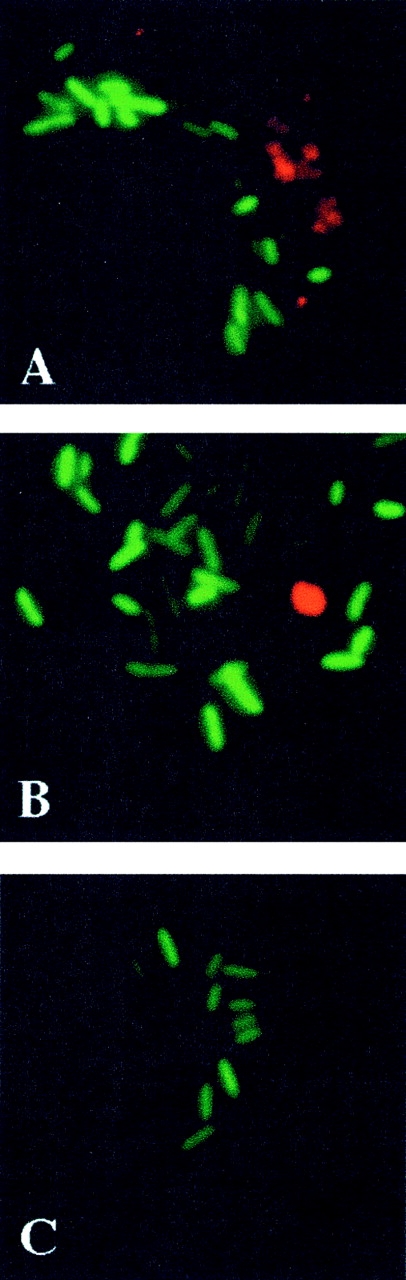
Nitrotyrosine staining fails to colocalize with bacteria in Salmonella-containing macrophages. The presence of nitrotyrosine (red) was examined by immunofluorescence microscopy of IFN-γ–activated macrophages from (A) C57BL/6, (B) iNOS −/−, and (C) gp91phox −/− mice that were challenged in vitro with GFP-expressing S. typhimurium (green). The pictures are representative of data obtained on two separate days.
Figure 7.
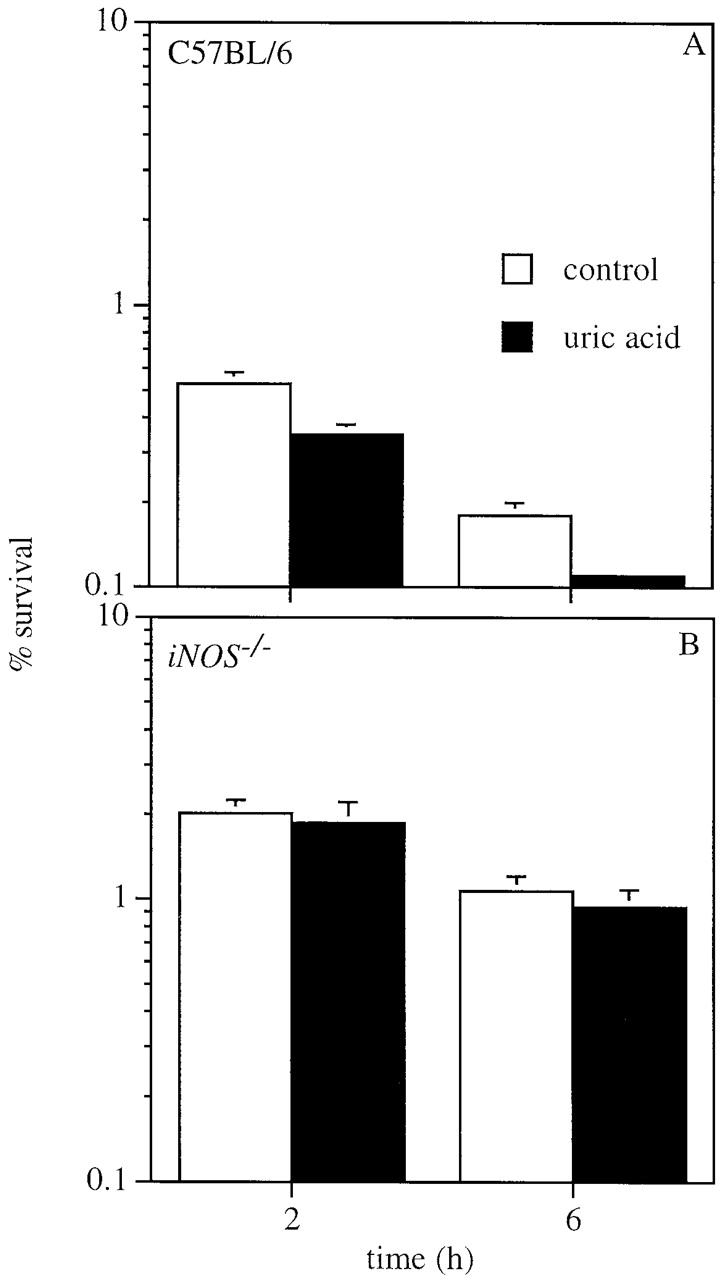
Uric acid improves killing of Salmonella by macrophages. 1 mmol uric acid enhances killing of Salmonella by (A) IFN-γ–activated macrophages, but this enhancement is iNOS dependent (B). The data are the mean ± SEM of six independent observations obtained on two separate days.
Discussion
Macrophages can kill or limit the replication of intracellular bacteria by producing antimicrobial peptides, lysosomal enzymes, ROS, and RNS. The importance of ROS for macrophage killing of S. typhimurium has been demonstrated 9 11 30; however, the participation of RNS has been less clear 9 24 30 31. In this work, we demonstrate that although the NADPH oxidase is more essential than iNOS for Salmonella killing by peritoneal macrophages, iNOS nevertheless contributes to macrophage antibacterial activity against Salmonella in distinct and important ways. The NADPH oxidase is required for rapid initial Salmonella killing by macrophages (Fig. 3 A), and iNOS provides a subsequent sustained bacteriostatic effect (Fig. 3 B). The failure of previous investigators to demonstrate macrophage NO·-dependent anti-Salmonella activity may be attributable to a reliance on phagocyte killing assays of relatively brief duration 9 24 30. Effector functions of macrophage-derived nitrogen oxides may explain why iNOS is required for resistance to Salmonella infection 10 43, despite the apparent immunosuppressive actions of NO· on T cells 43 44.
NO·-dependent anti-Salmonella activity was demonstrable in both untreated and IFN-γ–treated macrophages, suggesting that bacterial products such as LPS and DNA 45 can trigger sufficient NO· synthesis to exert antimicrobial activity, at least in partially activated peritoneal cells elicited by sodium periodate. Nevertheless, the addition of IFN-γ enhances macrophage anti-Salmonella activity predominantly by increasing NO· production (Fig. 1 and Fig. 2).
Although ROS and RNS can exert synergistic antimicrobial actions 14 17 18 19 25, they principally act in sequential fashion in assays of macrophages infected with Salmonella. An early phase of rapid oxidative killing occurs during the peak respiratory burst, followed by a nitrosative bacteriostatic phase (Fig. 3 and Fig. 4). The functional separation of these two phases of antimicrobial activity is illustrated by the capacity of macrophages deficient in the NADPH oxidase to maintain their bacterial load at a steady level despite an inability to reduce the original inoculum. In contrast, congenic iNOS-deficient macrophages retained early bacterial killing but were unable to maintain control of subsequent bacterial replication over time. These temporal differences in the antimicrobial behavior of macrophages were paralleled by the early detection of oxidative products followed by a later rise in nitrogen oxides (Fig. 4). The respiratory burst of infected macrophages peaked shortly after phagocytosis, decreasing rapidly thereafter. The brief duration of the respiratory burst may result in part from direct inhibition of NADPH oxidase assembly by NO· congeners 46 47 48 49, as macrophages from iNOS −/− mice sustained a more prolonged respiratory burst. Additionally, the increasing abundance of nitrogen oxides over time may be quenching the oxidative chemistry of O2·− and ONOO− 50 51.
The sequential, functionally distinct, and essential roles of ROS and RNS in Salmonella killing or inhibition by macrophages contrast with earlier studies of Leishmania, in which ROS did not appear to play a role 52. Studies of Listeria killing by macrophages have yielded somewhat conflicting results, with various investigators reporting that antilisterial activity is RNS independent 9 53, ROS dependent 9, or either RNS or ROS dependent depending on the timing of activation 54.
S. typhimurium killing by macrophages appears to be completely dependent upon the NADPH oxidase. H2O2 is likely to make a major contribution to bacterial killing, as it diffuses rapidly through membranes and reacts with transition metals to form highly toxic hydroxyl radicals. O2·− has also been implicated by observations that sodC mutant bacteria deficient in periplasmic Cu,Zn-superoxide dismutase are hypersusceptible to macrophage killing and exhibit reduced virulence 25 55 56.
NO· makes a relatively minor contribution to early macrophage oxidative killing (Fig. 3). ONOO−, a reactive molecule capable of mediating 1- and 2-electron oxidations 16 with potent in vitro microbicidal activity for E. coli, S. typhimurium, and C. albicans 17 18 25, might account for this activity. However, intracellular bacteria fail to colocalize with nitrotyrosine (Fig. 6), a molecular signature that can be associated with ONOO− synthesis 15 42, suggesting that ONOO− may not be responsible for early bactericidal effects. The scavenger uric acid actually potentiates Salmonella killing (Fig. 7), further indicating that ONOO− production by Salmonella-containing macrophages may be responsible for host cell autotoxicity rather than antimicrobial activity. Synergistic actions of metal ions, NO· redox congeners, and H2O2, or the production of singlet oxygen from the reaction of NO· and H2O2, are alternative mechanisms by which NO·-derived species may potentiate NADPH oxidase–dependent macrophage killing 19 57 58.
At this time, the identity of the nitrogen oxides responsible for the sustained NO·-dependent inhibition of bacterial growth are not known, but possibilities include NO· itself, N2O3, and S-nitrosothiols, which have been shown to exert bacteriostatic activity for S. typhimurium in vitro 13 14. The predominant accumulation of NO3 − rather than NO2 − at late time points in the macrophage assay when ONOO− is no longer detectable suggests that dioxygenase activity 59 might be involved in NO· oxidation.
Of the three NOS isoforms that can catalyze the enzymatic production of NO·, iNOS is most closely associated with antimicrobial activity. The contribution of cNOS to the antibacterial activity of macrophages in this study was negligible. The small quantity of NO· attributed to cNOS was oxidized to NO3 − (∼0.12 nmol/h/105 macrophages). A small amount of nitrotyrosine staining and NOx production detectable in iNOS-deficient macrophages infected with Salmonella suggests that NO· derived from cNOS can react with O2·−, as has been demonstrated in murine Mycoplasma pulmonis infection 60. These results might indicate that murine mononuclear phagocytes are able to express both inducible and constitutive NOS, as has been suggested by others 61 62, although functional cNOS has not yet been definitively demonstrated in primary murine peritoneal macrophages.
In conclusion, both the NADPH phagocyte oxidase and iNOS contribute to the ability of macrophages to inhibit or kill S. typhimurium. Analysis of murine peritoneal macrophages reveals a temporally coordinated action of ROS and RNS. Rapid bacterial killing coinciding with production of O2·− by the NADPH phagocyte oxidase is followed by a prolonged period of inhibition of bacterial growth associated with the production of iNOS-derived nitrosative species. The sequential roles of oxidative and nitrosative phagocyte antimicrobial mediators in vitro are mirrored by the temporal relationship of early NADPH oxidase–dependent and late iNOS-dependent antimicrobial effector mechanisms observed during murine Salmonella infection in vivo 10.
Acknowledgments
We thank B. Hybertson for providing access to a chemiluminometer, and W. Betz and S. Fadul for assistance with immunofluorescence microscopy. We are grateful to C. Nathan and D. Wink for their insightful review of this manuscript before submission.
This work was supported by National Institutes of Health (NIH) postdoctoral fellowship AI10181, NIH grants AI39557 and AI44486, the James Biundo Foundation, the Wellcome Trust, and Biotechnology and Biological Sciences Research Council.
Footnotes
Abbreviations used in this paper: iNOS, inducible nitric oxide synthase; NO, nitric oxide; NOS, nitric oxide synthase; ROS, reactive oxygen species; RNS, reactive nitrogen species.
References
- Vazquez-Torres A., Jones-Carson J., Baumler A.J., Falkow S., Valdivia R., Brown W., Le M., Berggren R., Parks W.T., Fang F.C. Extraintestinal dissemination of Salmonella via CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Fang F.C. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 2000;3:54–59. doi: 10.1016/s1369-5274(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Richter-Dahlfors A., Buchan A.M.J., Finlay B.B. Murine salmonellosis studied by confocal microscopySalmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P.I., Swanson R.V., Haidaris C.G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G.B. Cellular resistance to infection. J. Exp. Med. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.A., Britigan B.E. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J.T., Scott P.J., Babior B.M. Functional defect in neutrophil cytosols from two patients with autosomal recessive cytochrome-positive chronic granulomatous disease. J. Clin. Invest. 1989;83:1236–1240. doi: 10.1172/JCI114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouy R., Fischer A., Vilmer E., Seger R., Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J. Pediatr. 1989;114:555–560. doi: 10.1016/s0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- Shiloh M.U., MacMicking J.D., Nicholson S., Brause J.E., Potter S., Marino M., Fang F., Dinauer M., Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Vazquez-Torres A., Fang F.C., Xu Y., Khan S., Hormaeche C.E., Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 2000;192:237–247. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N.A., Lipps C.J., So M.Y., Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol. Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- MacMicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- De Groote M.A., Granger D., Xu Y., Campbell G., Prince R., Fang F.C. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F.C. Perspectives serieshost/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Pryor W.A., Squadrito G.L. The chemistry of peroxynitritea product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Zhu L., Gunn C., Beckman J.S. Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Jones-Carson J., Balish E. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect. Immun. 1996;64:3127–3133. doi: 10.1128/iai.64.8.3127-3133.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacelli R., Wink D.A., Cook J.A., Krishna M.C., DeGraff W., Friedman N., Tsokos M., Samuni A., Mitchell J.B. Nitric oxide potentiates hydrogen peroxide–induced killing of Escherichia coli . J. Exp. Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Röllinghoff M., Diefenbach A. Nitric oxide in Leishmaniasisfrom antimicrobial activity to immunoregulation. In: Fang F.C., editor. Nitric Oxide and Infection. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 361–377. [Google Scholar]
- Chan J., Flynn J. Nitric oxide in Mycobacterium tuberculosis infection. In: Fang F.C., editor. Nitric Oxide and Infection. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 281–310. [Google Scholar]
- Mastroeni P., Harrison J.A., Robinson J.H., Clare S., Khan S., Maskell D.J., Dougan G., Hormaeche C.E. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent Salmonellae in BALB/c micerole of gamma interferon and macrophage activation. Infect. Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Skepper J.N., Hormaeche C.E. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect. Immun. 1995;63:3674–3682. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S., Onozuka K., Shinomiya H., Nakano M. Sensitivity of bacteria to NaNO2 and to l-arginine-dependent system in murine macrophages. Microbiol. Immunol. 1991;35:325–329. doi: 10.1111/j.1348-0421.1991.tb01561.x. [DOI] [PubMed] [Google Scholar]
- De Groote M.A., Ochsner U.A., Shiloh M.U., Nathan C., McCord J.M., Dinauer M.C., Libby S.J., Vazquez-Torres A., Xu Y., Fang F.C. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xie Q.W., Nathan C. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol. Cell. 1998;1:795–805. doi: 10.1016/s1097-2765(00)80079-9. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Oda Y., Uozumi M. Induction of umuC gene expression by nitrogen dioxide in Salmonella typhimurium . Mutat. Res. 1985;142:99–102. doi: 10.1016/0165-7992(85)90047-8. [DOI] [PubMed] [Google Scholar]
- De Groote M.A., Testerman T., Xu Y., Stauffer G., Fang F.C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium . Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- Crawford M.J., Goldberg D.E. Role for the Salmonella flavohemoglobin in protection from nitric oxide. J. Biol. Chem. 1998;1273:12543–12547. doi: 10.1074/jbc.273.20.12543. [DOI] [PubMed] [Google Scholar]
- Shiloh M.U., Ruan J., Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect. Immun. 1997;65:3193–3198. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P., Saarinen M., He Q., Virtala M., Salmi M., Granfors K. Human monocytic U937 cells kill Salmonella in vitro by NO-independent mechanisms. Infect. Immun. 1999;67:3670–3673. doi: 10.1128/iai.67.7.3670-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Effect of late administration of anti-TNFα antibodies on a Salmonella infection in the mouse model. Microb. Pathog. 1993;14:473–480. doi: 10.1006/mpat.1993.1046. [DOI] [PubMed] [Google Scholar]
- MacMicking J.D., Nathan C., Hom G., Chartrain N., Fletcher D.S., Trumbauer M., Stevens K., Xie Q.W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Pollock J.D., Williams D.A., Gifford M.A., Li L.L., Du X., Fisherman J., Orkin S.H., Doerschuk C.M., Dinauer M.C. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhu H., Kuppusamy P., Roubaud V., Zweier J.L., Trush M.A. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J. Biol. Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- Radi R., Cosgrove T.P., Beckman J.S., Freeman B.A. Peroxynitrite-induced luminol chemiluminescence. Biochem. J. 1993;290:51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D.C., Spitsin S., Kean R.B., Champion J.M., Dickson G.M., Chaudhry I., Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Salzman A.L. Endogenous peroxynitrite is involved in the inhibition of mitochondrial respiration in immuno-stimulated J774.2 macrophages. Biochem. Biophys. Res. Commun. 1995;209:739–743. doi: 10.1006/bbrc.1995.1561. [DOI] [PubMed] [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Gilliam M.B., Sherman M.P., Griscavage J.M., Ignarro L.J. A spectrophotometric assay for nitrate using NADPH oxidation by Aspergillus nitrate reductase. Anal. Biochem. 1993;12:359–365. doi: 10.1006/abio.1993.1341. [DOI] [PubMed] [Google Scholar]
- Verdon C.P., Burton B.A., Prior R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995;224:502–508. doi: 10.1006/abio.1995.1079. [DOI] [PubMed] [Google Scholar]
- Crow J.P., Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteinsin vivo marker of peroxynitrite. Methods Enzymol. 1996;269:185–194. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- MacFarlane A.S., Schwacha M.G., Eisenstein T.K. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 1999;67:891–898. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Ramadi B.K., Meissler J.J., Jr., Huang D., Eisenstein T.K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur. J. Immunol. 1992;22:2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- Gao J.J., Zuvanich E.G., Xue Q., Horn D.L., Silverstein R., Morrison D.C. Cutting edgebacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J. Immunol. 1999;163:4095–4099. [PubMed] [Google Scholar]
- Clancy R.M., Leszczynska-Piziak J., Abramson S.B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iha S., Orita K., Utsumi T., Sato E.F., Inoue M., Utsumi K. Oxygen-dependent inhibition of neutrophil respiratory burst by nitric oxide. Free Radic. Res. 1996;25:489–498. doi: 10.3109/10715769609149071. [DOI] [PubMed] [Google Scholar]
- Forslund T., Sundqvist T. Nitric oxide reduces hydrogen peroxide production from human polymorphonuclear neutrophils. Eur. J. Clin. Invest. 1995;25:9–14. doi: 10.1111/j.1365-2362.1995.tb01518.x. [DOI] [PubMed] [Google Scholar]
- Fujii H., Ichimori K., Hoshiai K., Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J. Biol. Chem. 1997;272:32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- Wink D.A., Cook J.A., Kim S.Y., Vodovotz Y., Pacelli R., Krishna M.C., Russo A., Mitchell J.B., Jourd'heuil D., Miles A.M., Grisham M.B. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. Chemical aspects involved in the balance between oxidative and nitrosative stress. J. Biol. Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- Wink D.A., Vodovotz Y., Grisham M.B., DeGraff W., Cook J.C., Pacelli R., Krishna M., Mitchell J.B. Antioxidant effects of nitric oxide. Methods Enzymol. 1999;301:413–424. doi: 10.1016/s0076-6879(99)01105-2. [DOI] [PubMed] [Google Scholar]
- Assreuy J., Cunha F.Q., Epperlein M., Noronha-Dutra A., O'Donnell C.A., Liew F.Y., Moncada S. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major . Eur. J. Immunol. 1994;24:672–676. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- Leenen P.J., Canono B.P., Drevets D.A., Voerman J.S., Campbell P.A. TNF-alpha and IFN-gamma stimulate a macrophage precursor cell line to kill Listeria monocytogenes in a nitric oxide-independent manner. J. Immunol. 1994;153:5141–5147. [PubMed] [Google Scholar]
- Ohya S., Tanabe Y., Makino M., Nomura T., Xiong H., Arakawa M., Mitsuyama M. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect. Immun. 1998;66:4043–4049. doi: 10.1128/iai.66.9.4043-4049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F.C., DeGroote M.A., Foster J.W., Baumler A.J., Ochsner U., Testerman T., Bearson S., Giard J.C., Xu Y., Campbell G., Laessig T. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA. 1999;96:7502–7507. doi: 10.1073/pnas.96.13.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J.L., Sansone A., Canvin J.R., Pallen M.J., Langford P.R., Wallis T.S., Dougan G., Kroll J.S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol. Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- Noronha-Dutra A.A., Epperlein M.M., Woolf N. Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide–mediated killing. FEBS Lett. 1993;321:59–62. doi: 10.1016/0014-5793(93)80621-z. [DOI] [PubMed] [Google Scholar]
- Chazotte-Aubert L., Oikawa S., Gilibert I., Vianchini F., Kawanishi S., Ohshima H. Cytotoxicity and site-specific DNA damage induced by nitroxyl anion (NO−) in the presence of hydrogen peroxide. J. Biol. Chem. 1999;274:20909–20915. doi: 10.1074/jbc.274.30.20909. [DOI] [PubMed] [Google Scholar]
- Hausladen A., Gow A.J., Stamler J.S. Nitrosative stressmetabolic pathway involving the flavohemoglobin. Proc. Natl. Acad. Sci. USA. 1998;95:14100–14105. doi: 10.1073/pnas.95.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman-Davis J., Gibbs-Erwin J., Lindsey J.R., Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc. Natl. Acad. Sci. USA. 1999;96:4953–4958. doi: 10.1073/pnas.96.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.H., Warner T.D., Nakane M., Forstermann U., Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol. Pharmacol. 1992;41:615–624. [PubMed] [Google Scholar]
- Hecker M., Walsh D.T., Vane J.R. Characterization of a microsomal calcium-dependent nitric oxide synthase in activated J774.2 monocyte/macrophages J. Cardiovasc. Pharmacol 20Suppl. 121992. S139 S141 [DOI] [PubMed] [Google Scholar]