Natural killer (NK) T cells are a subset of mature T lymphocytes that express an α/β TCR together with markers characteristic of NK cells such as NK1.1 1 2. In contrast to conventional T cells, which recognize small peptides bound to polymorphic MHC class I or class II molecules, TCRs on NK T cells recognize glycolipids associated with the monomorphic MHC-like CD1d molecule. As expected from their restricted specificity, most NK T cells utilize a limited TCR repertoire comprising an invariant Vα14–Jα281 chain paired preferentially to Vβ8.2 (in the mouse) or the homologous invariant Vα24–JαQ chain paired to Vβ11 in humans.
At present, the physiological CD1d-associated ligands recognized by the semiinvariant TCR on NK T cells are not known. However a synthetic glycolipid, α-galactosylceramide (α-GalCer) has been shown to selectively activate NK T cells expressing the Vα14/Vβ8.2 (or Vα24/Vβ11) TCR complex 3. As α-GalCer is normally found in marine sponges (but not mammalian cells), it is generally believed that the physiological ligand of the semiinvariant TCR on NK T cells is an as yet unidentified glycolipid with a high degree of structural homology to α-GalCer.
A striking feature of NK T cells is their ability to rapidly produce large amounts of cytokines upon TCR engagement. Although cytokine production by NK T cells was initially believed to follow a Th2 pattern (dominated by IL-4; reference 4), it is now generally accepted that NK T cells can also secrete large quantities of IFN-γ and thus do not readily fit the Th1/Th2 paradigm. As expected from their phenotype, NK T cells also share functional properties with NK cells such as the ability to kill certain target cells such as tumor cells and immature thymocytes.
Despite their relatively well characterized functional properties in vitro, the physiological role of NK T cells in vivo remains to be established. In this respect, NK T cells are protective in immune responses to certain tumors and parasites and appear to play an important regulatory role in inhibiting the development of autoimmune disorders. Clearly the identification of physiological CD1d-associated glycolipid ligands (of either endogenous or exogenous origin) will be required to put NK T cells into a better defined context in homeostasis or pathology.
A major problem in NK T cell biology to date has been the lack of appropriate reagents to selectively identify CD1d-dependent NK T cells among heterogeneous lymphoid populations in vivo. In the mouse, the NK1.1 marker itself (a member of the NKR-P1 gene family) is polymorphic and only expressed by a very limited number of inbred mouse strains. Moreover, there is recent evidence that some NK1.1+ T cells do not express the canonical Vα14/Vβ8.2 TCR and in fact recognize CD1d-independent ligands 5 6 7. Although these problems could in theory be largely overcome by mAbs specific for the semiinvariant TCR chains on NK T cells, this is not possible in practice, as anti-Vβ8.2 antibodies stain a large fraction of conventional T cells and the only published anti-Vα14 mAb 8 is of controversial specificity 1. The situation in humans is somewhat different, as mAbs against both Vα24 and Vβ11 exist 9. Nevertheless, only a fraction of all Vα24+ or Vβ11+ T cells are NK T cells, and even a double staining procedure to selectively identify Vα24+Vβ11+ cells cannot exclude conventional T cells that rearrange Vα24 to J regions other than JαQ.
To circumvent these problems, Matsuda et al. 10 in this issue (as well as Benlagha et al. 11 in the June 5 issue) have succeeded in producing tetrameric CD1d–glycolipid complexes that bind selectively to NK T cells expressing the semiinvariant Vα14/Vβ8.2 or Vα24/Vβ11 TCR.
CD1d–α-GalCer Tetramers.
Tetramer technology was initially devised as a way of visualizing CD8+ T cells expressing TCRs specific for a given MHC class I–peptide complex. Although the specific peptides seen by a wide variety of TCRs on CD8+ cells had been known for some time, the interactions of monomeric MHC class I–peptide complexes with the TCR were of too low an affinity to result in stable binding. This problem was solved by Altman et al. 12, who engineered a unique bacterial biotinylation substrate at the COOH terminus of recombinant MHC class I molecules. Refolding of biotinylated heavy chains with specific peptides and subsequent incubation with fluorescent avidin resulted in the formation of tetrameric MHC class I–peptide complexes that bound stably and specifically to the appropriate TCR. This method has subsequently been extended to produce fluorescent tetramers of peptides bound to MHC class Ib molecules such as Qa-1b and HLA-E (which bind to specific receptors on subsets of NK cells) as well as MHC class II–peptide tetramers, which bind to TCR on CD4+ T cells 13 14 15.
Despite recent reports of fluorescent tetramers incorporating MHC class Ib molecules, the successful production of CD1d–glycolipid tetramers by Matsuda et al. 10 and Benlagha et al. 11 was somewhat unexpected. In the first place, all previous MHC heavy chains had been refolded with well characterized short peptides that could be produced in large quantity and high purity, and in many cases the crystal structure of the relevant MHC–peptide complex was known. In contrast, although the crystal structure of recombinant mouse CD1d had been solved 16, very little was known about the potential physical interactions between glycolipids and CD1d molecules. Even worse, preliminary attempts to measure the strength of interactions between purified CD1d molecules and α-GalCer by plasmon resonance technology had indicated a very short half-life 17, thereby suggesting that the production of stable tetramers might be difficult if not impossible. Fortunately, these concerns turned out to be premature, and it appears in retrospect that fluorescent CD1d–α-GalCer tetramers are relatively stable. Whatever the explanation for this discrepancy (and both papers give their versions), it is probably instructive in highlighting the limits of plasmon resonance measurements, particularly when nonpeptidic ligands are involved.
NK T Cell Subsets.
Arguably the most important experimental finding emanating from this first description of fluorescent CD1d–α-GalCer tetramers is a clearer definition of the heterogeneity of the NK T cell population itself. As mentioned previously, prototypic NK T cells in the mouse (as defined in an appropriate inbred strain such as C57BL/6) express NK1.1 together with semiinvariant Vα14/Vβ8.2 TCRs and are selected by CD1d during their development. Nevertheless, recent studies from several groups had suggested that some NK1.1-expressing T cells arise independently of CD1d and apparently do not express the semiinvariant TCRs 5 6 7. Conversely, indirect evidence supporting the existence of a CD1d-specific but NK1.1− T cell subset expressing a semiinvariant TCR had also been reported 18.
This confusion has now been clearly resolved by the studies of Matsuda et al. 10 and Benlagha et al. 11. Both groups provide definitive evidence for the presence of three distinct T cell subsets defined by simultaneous staining with NK1.1 and CD1d–α-GalCer tetramers (Table ). Prototypic NK T cells (NK1.1+, tetramer positive) express the semiinvariant TCR and are absent in CD1d-deficient mice. Furthermore, as predicted by earlier studies 5 6 7, NK1.1+ tetramer-positive cells localize preferentially to thymus and liver and are mainly of the CD4+ or (to a lesser extent) CD4−CD8− phenotype.
Table 1.
NK T Cell Subsets Defined by CD1d: α-GalCer Tetramers and NK1.1
| Property | NK1.1+ tetramer positive | NK1.1+ tetramer negative | NK1.1− tetramer positive |
|---|---|---|---|
| TCR repertoire | Vα14/Vβ8.2 | Diverse | Vα14/Vβ8.2 |
| Selecting ligand | CD1d | ? (not CD1d) | CD1d |
| Coreceptor expression | CD4 or CD4−CD8− | CD8 or CD4−CD8− | CD4 or CD4−CD8− |
| Tissue preference | Thymus, liver | Spleen, bone marrow | Intestine, lymph node |
A second T cell subpopulation expresses NK1.1 but does not stain with fluorescent CD1d–α-GalCer tetramers (Table ). Consistent with their tetramer-negative phenotype, these cells do not express the semiinvariant TCR (indeed they appear to express TCR Vα and Vβ domains at random) and do not depend upon CD1d for their development. NK1.1+ tetramer-negative cells are relatively more abundant in spleen and bone marrow (as opposed to thymus and liver), and most are of the CD8+ or CD4−CD8− phenotype. Again, these conclusions are largely consistent with the predictions of other earlier studies 5 6 7.
The third (and potentially most novel) T cell subset defined by Matsuda et al. 10 and Benlagha et al. 11 are NK1.1− but tetramer positive (Table ). Despite the absence of NK1.1 expression, these cells are CD1d dependent and express semiinvariant TCRs, as would be expected from their ability to bind CD1d–α-GalCer tetramers. The CD4/CD8 phenotype of NK1.1− tetramer-positive cells is very similar to that of prototypic NK T cells (mostly CD4+ with some CD4−CD8−), indicating that coreceptor expression is tightly linked to selection by CD1d. Of particular interest is the fact that NK1.1− tetramer-positive cells have a unique tissue distribution with respect to the other two subsets. Although present to some extent in typical organs where NK T cells localize (thymus, liver, bone marrow, and spleen), these cells represent the vast majority of tetramer-positive cells in lymph nodes and in the small intestine. Importantly, NK T cells have traditionally been considered to be absent from these latter tissues. Further experiments will be required to assess the physiological significance of NK1.1− tetramer-positive cells in the gut epithelium. In this regard, it is noteworthy that the NK1.1− (but not NK1.1+) subset of tetramer-positive cells expresses the CD49d (α4 integrin) homing receptor 11 and that the gut epithelium has previously been reported to express high levels of CD1d 19.
NK T Cell Development.
The developmental origin of prototypic NK T cells expressing the semiinvariant Vα14/Vβ8.2 TCR remains enigmatic. Although a number of early studies suggested an important role for extrathymic NK T cell development (particularly in the liver), more recent reports strongly suggest that most CD1d-dependent NK T cells are thymus derived 20 21. Assuming this to be the case, an important issue is whether thymic NK T cells arise as a byproduct of conventional T cell development or alternatively differentiate along an independent intrathymic NK T cell lineage. According to the first scenario (mainstream model), T cell precursors that randomly rearrange their TCR-α genes and express the semiinvariant TCR at the CD4+CD8+ stage of development would acquire the NK T cell phenotype, presumably as a consequence of unique aspects of TCR signaling mediated by CD1d–glycolipid recognition. In the second model, a separate lineage of committed NK T precursor cells would be rescued from cell death upon expression of the semiinvariant TCR and recognition of CD1d. As current evidence does not support a directed Vα14 rearrangement mechanism in NK T cells 22, the mainstream model is easier to reconcile with the extremely low probability (estimated to be ∼1:50,000) of randomly rearranging the semiinvariant TCR during development 1.
Any model of NK T cell development must account for the peculiar coreceptor expression pattern of mature CD1d-dependent NK T cells. As described previously 5 6 7 and emphasized by the current detailed analysis of CD1d–α-GalCer tetramer–positive cells 10 11, CD1d-dependent NK T cells in all tissues analyzed (irrespective of NK1.1 expression) consist of a majority (60–80%) of CD4+ cells, with the remaining cells being CD4−CD8−. Essentially no CD8+ tetramer-positive cells can be detected. If the mainstream model for NK T cell development is correct, this in turn implies that CD4+CD8+ precursors expressing the semiinvariant TCR can give rise to either CD4+or CD4−CD8− (but not CD8+) progeny upon CD1d recognition.
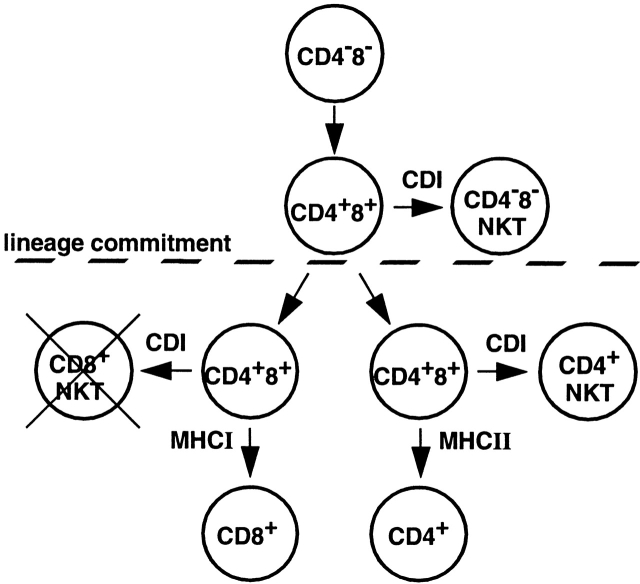
A speculative version of the mainstream model for CD1d-dependent NK T cell development is presented in Fig. 1. This model is similar to that proposed earlier by Bendelac et al. 1 in the sense that it assumes that CD8+ NK T cells cannot fully mature (presumably because CD8 expression contributes to the avidity of CD1d recognition by immature NK T cells and thus leads to negative selection). However, the model differs from earlier versions in that it proposes a critical stage of thymus development at which mainstream CD4+CD8+ precursor cells become irreversibly committed to the CD4 lineage. According to this model, CD4+CD8+ precursors that interact productively with CD1d before lineage commitment will downregulate both CD4 and CD8 and emerge as CD4−CD8− mature NK T cells. In contrast, CD4+CD8+ precursors that recognize CD1d only after lineage commitment has occurred will follow a predetermined developmental program involving selective CD8 downregulation and emerge as CD4+ mature NK T cells.
Figure 1.
Speculative model of thymic NK T cell development. The model attempts to account for the unusual coreceptor expression pattern of CD1d-dependent NK T cells as a byproduct of mainstream T cell development. The hatched line represents a putative stage where CD4+CD8+ thymocytes become irreversibly committed to the CD4 or CD8 lineage.
Two observations arising from the current studies of CD1d–α-GalCer tetramers are potentially relevant to the mainstream model of NK T cell development outlined in Fig. 1. First, Matsuda et al. 10 show that a small proportion of tetramer-positive cells in the thymus have a CD4+CD8+ phenotype. If confirmed, this finding would provide the first direct quantitative estimate (∼1/2,000) for the frequency of CD1d–α-GalCer–binding cells within the CD4+CD8+ immature thymocyte population. Such cells would be obvious candidate precursors of mature NK T cells. Second, Benlagha et al. 11 show that the coreceptor phenotype of mature tetramer-positive NK T cells is radically altered in transgenic mice overexpressing the invariant Vα14 chain (and containing elevated numbers of tetramer-positive cells). Instead of the usual predominance of CD4+ cells observed in the tetramer-positive subset in normal mice, tetramer-positive cells in Vα14-transgenic mice are almost exclusively CD4−CD8−. In the context of the model proposed in Fig. 1, these latter data could be interpreted to mean that premature expression of the semiinvariant TCR in the thymi of Vα14-transgenic mice leads to preferential selection of CD1d-dependent NK T cells before the lineage commitment checkpoint and consequently an enrichment for the CD4−CD8− phenotype. Another independent finding that is consistent with the model depicted in Fig. 1 is that CD4−CD8− NK T cells arise before CD4+ NK T cells during neonatal ontogeny (Lees, R.K., and H.R. MacDonald, unpublished data).
NK T Cell Homeostasis.
Another aspect of NK T cell biology that is amenable to analysis with CD1d–α-GalCer tetramers is homeostasis. In this respect, it was recently reported that NK T cells in the liver (and to a lesser extent the spleen) are extremely sensitive to activation-induced cell death upon TCR engagement by low doses of anti-CD3 antibodies 23. Matsuda et al. 10 have now confirmed and extended these findings by showing that treatment of mice with α-GalCer induces a rapid disappearance of tetramer-positive cells that is most marked in the liver. In apparent contrast to earlier data 23, regeneration of tetramer-positive cells in the liver after α-GalCer treatment seems to be relatively slow. Further experiments monitoring CD1d–α-GalCer tetramer–positive cells in vivo after diverse stimuli should provide novel insights into the dynamics and homeostasis of NK T cells.
Future Perspectives.
Aside from their obvious importance in studying NK T cell development and homeostasis in normal mice, CD1d–α-GalCer tetramers should prove to be extremely useful for monitoring NK T cells in a wide spectrum of immune responses in vivo as well as in pathological situations such as autoimmune disorders. A major strength of the tetramer technology is that CD1d molecules are sufficiently conserved between mice and humans so that CD1d–α-GalCer tetramers cross-react efficiently with the semiinvariant TCRs of the two species 11. This conservation should facilitate the optimization of CD1d–α-GalCer tetramer production so that NK T cells can be efficiently monitored in blood (or tissue biopsies) of patients during the course of treatment for tumors or autoimmune diseases. In the mouse, CD1d–α-GalCer tetramers should prove indispensable for monitoring NK T cells in the vast majority of inbred strains where the NK1.1 allele is not expressed. Importantly, the list of NK1.1− strains to be analyzed with tetramers includes BALB/c (the prototype strain for Th2 responses) and the nonobese diabetic mouse, where a role for NK T cells in the control of autoimmune diabetes has been indirectly established 24 25.
Finally, it is perhaps pertinent to speculate on the broader implications of fluorescent tetramer technology as it applies to CD1–lipid interactions. In this context, it should be noted that mouse CD1d can apparently bind and present lipids distinct from α-GalCer (such as phospholipids) to T cells not expressing the semiinvariant TCR 26. Moreover, in contrast to mice, humans (and most other mammals analyzed to date) express multiple members of the CD1 family in addition to CD1d. Importantly, presentation of glycolipids appears to be a general property of CD1 molecules. Indeed it is well established that glycolipid components of mycobacterial cell walls such as mycolic acid and lipoarabinomannan are presented to T cells via human CD1b 27, and recent evidence suggests that human CD1c may also bind and present isoprenoid glycolipids of mycobacterial origin 28. An obvious future goal of the tetramer technology ushered in by Matsuda et al. 10 and Benlagha et al. 11 will be to produce novel tetramers composed of other glycolipids bound to different CD1 molecules. If successful, this approach may revolutionize our perspective of the scope and importance of the CD1 antigen presentation pathway.
References
- Bendelac A., Rivera M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- MacDonald H.R. NK1.1+ T cell receptor-α/β+ cellsnew clues to their origin, specificity, and function. J. Exp. Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Paul W.E. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G., Lees R., Smiley S.T., Taniguchi M., Grusby M.J., MacDonald H.R. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- Hammond K.J., Pelikan S.B., Crowe N.Y., Randle-Barrett E., Nakayama T., Taniguchi M., Smyth M.J., van Driel I.R., Scollay R., Baxter A.G. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Zeng D., Gazit G., Dejbakhsh-Jones S., Balk S.P., Snapper S., Taniguchi M., Strober S. Heterogeneity of NK1.1+ T cells in the bone marrowdivergence from the thymus. J. Immunol. 1999;163:5338–5345. [PubMed] [Google Scholar]
- Ito T., Ishibashi K., Imai K., Koseki H., Ra C.S., Fernandez E., Kantake M., Saito T., Taniguchi M. Monoclonal antibody against murine T cell receptor Vα14 cross-reacts with human CD3 epsilon and detects disulfide-linked dimeric form. Int. Immunol. 1991;3:991–995. doi: 10.1093/intimm/3.10.991. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Padovan E., Casorati G., Brockhaus M., Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J.L., Naidenko O.V., Gapin L., Nakayama T., Taniguchi M., Wang C.-R., Koezuka Y., Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–753. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K., Weiss A., Beavis A., Teyton L., Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 2000;191:1895–1904. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Vance R.E., Kraft J.R., Altman J.D., Jensen P.E., Raulet D.H. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J. Exp. Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braud V.M., Allan D.S., O'Callaghan C.A., Soderstrom K., D'Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Crawford F., Kozono H., White J., Marrack P., Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Castano A.R., Segelke B.W., Stura E.A., Peterson P.A., Wilson I.A. Crystal structure of mouse CD1an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- Naidenko O.V., Maher J.K., Ernst W.A., Sakai T., Modlin R.L., Kronenberg M. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J. Exp. Med. 1999;190:1069–1080. doi: 10.1084/jem.190.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Paul W.E. A population of CD62Llow NK1.1− CD4+ T cells that resembles NK1.1+ CD4+ T cells. Eur. J. Immunol. 1998;28:3172–3182. doi: 10.1002/(SICI)1521-4141(199810)28:10<3172::AID-IMMU3172>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bleicher P.A., Balk S.P., Hagen S.J., Blumberg R.S., Flotte T.J., Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- Tilloy F., Di Santo J.P., Bendelac A., Lantz O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur. J. Immunol. 1999;29:3313–3318. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Coles M.C., Raulet D.H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- Shimamura M., Ohteki T., Beutner U., MacDonald H.R. Lack of directed Vα14-Jα281 rearrangements in NK1+ T cells. Eur. J. Immunol. 1997;27:1576–1579. doi: 10.1002/eji.1830270638. [DOI] [PubMed] [Google Scholar]
- Eberl G., MacDonald H.R. Rapid death and regeneration of NKT cells in anti-CD3ε- or IL-12-treated micea major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- Hammond K.J.L., Poulton L.D., Palmisano L.J., Silveira P.A., Godfrey D.I., Baxter A.G. α/β-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehuen A., Lantz O., Beaudoin L., Laloux V., Carnaud C., Bendelac A., Bach J.F., Monteiro R.C. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumperz J.E., Roy C., Makowska A., Lum D., Sugita M., Podrebarac T., Koezuka Y., Porcelli S.A., Cardell S., Brenner M.B. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- Porcelli S.A., Segelke B.W., Sugita M., Wilson I.A., Brenner M.B. The CD1 family of lipid antigen-presenting molecules. Immunol. Today. 1998;19:362–368. doi: 10.1016/s0167-5699(98)01289-4. [DOI] [PubMed] [Google Scholar]
- Moody D.B., Ulrichs T., Muhlecker W., Young D.C., Gurcha S.S., Grant E., Rosat J.P., Brenner M.B., Costello C.E., Besra G.S. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]