Abstract
The cellular receptor for murine thymic stromal lymphopoietin (TSLP) was detected in a variety of murine, but not human myelomonocytic cell lines by radioligand binding. cDNA clones encoding the receptor were isolated from a murine T helper cell cDNA library. TSLP receptor (TSLPR) is a member of the hematopoietin receptor family. Transfection of TSLPR cDNA resulted in only low affinity binding. Cotransfection of the interleukin 7 (IL-7)Rα chain cDNA resulted in conversion to high affinity binding. TSLP did not activate cells from IL-7Rα−/− mice, but did activate cells from γc−/− mice. Thus, the functional TSLPR requires the IL-7Rα chain, but not the γc chain for signaling.
Keywords: cytokine receptors, interleukin 2 receptor, interleukin 7 receptor, cytokine, DNA sequence
Introduction
The regulation of lymphopoiesis is orchestrated by a complex, overlapping network of soluble and membrane-bound molecules, among which IL-7 has been shown to play a central role 1. The effects of IL-7 are mediated by interaction with a heteromeric receptor complex consisting of a specific IL-7 binding chain (the α subunit) and the γc chain common to the IL-2, IL-4, IL-9, and IL-15 receptor complexes 2. This group of receptors, related by their common use of the γc chain, are otherwise distinct both in structure and in the range of biological activities induced by their ligands. The signaling pathways initiated by ligand binding to these receptor complexes share significant similarities, however, activating overlapping sets of kinases and other signaling molecules 3. To date, IL-4 is the only member of this group that has been demonstrated to mediate a biological signal in the absence of the γc chain, suggesting that there are both γc-dependent and -independent classes of functional IL-4 receptors 4.
As described by Sims et al. in this issue 5, we have recently identified and cloned a molecule termed thymic stromal lymphopoietin (TSLP) that shares a closely overlapping, but distinct, profile of biological activities with IL-7. Given this close overlap, we initiated studies to characterize the cellular receptor for TSLP with particular attention to the possibility that these two cytokines might share common receptor components. In this paper we describe the binding characteristics of the TSLPR complex on lymphoid cells and the cloning of a TSLP-specific binding protein. We demonstrate that the IL-7Rα subunit is obligatory for formation of a high affinity, signaling competent TSLPR complex, whereas the γc chain is not required.
Materials and Methods
Cell Lines.
The TSLP-dependent NAG8/7 cell line was maintained as described previously 6. BAF/BO3 cells were also maintained under these conditions, except that parental BAF/BO3 cells were supplemented with 100 ng/ml murine IL-3, and BAF/BO3 cells expressing murine IL-7 receptor were supplemented with 100 ng/ml human IL-7.
Binding Assays.
Recombinant murine TSLP was expressed in mammalian cells (CV-1/EBNA) and purified as described by Sims et al. in this issue 5. Purified TSLP and murine IL-7 were radiolabeled using the enzymobead radioiodination reagent (Bio-Rad Laboratories) as described previously 7. Radiolabeled stocks were stored at 4°C in RPMI 1640 containing 2% BSA, 20 mM Hepes, and 0.2% sodium azide, pH 7.2. Binding assays were performed by a phthalate oil separation method as described previously 7. For binding assays in which TSLPR cDNA was transfected into CV-1/EBNA cells, control experiments demonstrated no difference in binding of TSLP to CV-1/EBNA cells either as adherent monolayers or in suspension; hence, all binding assays were performed with cell suspensions. CV-1/EBNA cells were transfected with receptor cDNA using DEAE-dextran followed by chloroquine treatment as described previously 8, and assayed for binding 2 d later. Nonspecific binding was assessed by including either a 1,000-fold molar excess of unlabeled TSLP and IL-7, or polyclonal rabbit anti–murine IL-7 (at a concentration previously determined to inhibit 125I–IL-7 binding) into the binding mixture.
Affinity Cross-Linking.
Cell suspensions were incubated with binding medium containing 0.5–1 nM 125I-TSLP or 125I–IL-7 for 3 h at 4°C. Nonspecific binding and cross-competition were determined by using 1,000-fold molar excess of unlabeled cytokines. After binding, cells were washed three times with PBS at 4°C, and cross-linking reagent bis-(sulfosuccinimidyl) suberate was added to a final concentration of 0.1 mg/ml; no bis-(sulfosuccinimidyl) suberate was added to control samples. Cells were cross-linked for 30–45 min at 25°C, washed once with PBS, and extracted in PBS/1% Triton containing 1 mM PMSF, 10 μM pepstatin A, 10 μM leupeptin, 2 mM O-phenanthroline, 1 mM EGTA, 0.5 mM EDTA, and 0.02% sodium azide for 20–30 min at 4°C. Lysed cells were centrifuged at 12,000 g for 15 min at 4°C, and supernatants were retained. Supernatants were then analyzed by autoradiography after 8–16% gradient SDS–PAGE as described previously 9.
BAF/BO3 Transfection and Proliferation Assays.
BAF/BO3 cells were transfected with murine IL-7Rα cDNA by electroporation as described 10. In brief, 8 × 106 cells were washed in RPMI 1640, 10 μg murine IL-7Rα cDNA was added 11, electroporated at 280 V and 960 μF using a Bio-Rad Laboratories gene pulser, and selected in 100 ng/ml human IL-7. For proliferation assays, parental and transfected BAF/BO3 cells were cultured in 96-well flat-bottomed plates (1 × 104 cells/well) with serial threefold dilutions of murine IL-3, TSLP, or human IL-7. The starting concentration of cytokines was 10 μg/ml; background was measured by adding cells to assay medium alone. Cells were cultured for 2 d, pulsed for 5 h with 3H-TdR, harvested onto glass fiber filters, and incorporation was determined.
cDNA Cloning.
An oligo-dT–primed cDNA library from the murine T helper cell clone 7B9 12 was transfected into CV1/EBNA cells and screened with 125I-TSLP essentially as described 13. A chloroquine-mediated DEAE-dextran method 8 was used to transfect subconfluent monolayers of CV-1/EBNA cells grown in pronectin-treated chamber slides, with miniprep DNA from pools of 1,000 clones from the 7B9 library. Since the 7B9 library was in an expression vector that contained the SV40 origin of replication but lacked the Epstein-Barr virus origin, the library plasmids (2.5 μg per slide) were cotransfected with pSV3neo 14 (0.5 μg) as a source of T antigen to allow the plasmid to replicate and thereby increase both the transfection efficiency and the expression level 15. After 2 d of expression, slides of transfected cells were incubated with 1 nM 125I-TSLP in binding medium containing 5% nonfat dry milk for 1 h at 25°C. The slides were then washed with PBS, fixed with 2.5% glutaraldehyde in PBS, washed, air-dried overnight, and dipped in liquid photographic emulsion. After 3 d of exposure in the dark at 25°C, the slides were developed and examined at ×10–20 magnification for the presence of silver grains over cells that have bound to 125I-TSLP. Positive pools were broken down and single clones were isolated as described 13. TSLPR sequence data are available from EMBL/GenBank/DDBJ under accession no. AF232936.
RNA Hybridization.
PolyA+ RNA (5 μg) was run on agarose gels, transferred to a nylon membrane, and hybridized to a 32P-labeled antisense riboprobe derived from the entire insert of clone 7a as described 13. The final wash of the filters was for 60 min at 63°C in 0.1× SSC.
Interspecific Mouse Backcross Mapping.
Interspecific backcross progeny were generated by mating (C57BL/6J × Mus spretus)Fl females and C57BL/6J males as described 16. A total of 205 N2 mice were used to map the Tslpr locus. DNA isolation, restriction enzyme digestion, agarose gel electrophoresis, Southern blot transfer, and hybridization were performed essentially as described 17. All blots were prepared with Hybond-N+ nylon membrane (Amersham Pharmacia Biotech). The probe, a 1.35-kb EcoRl/Hindlll fragment of mouse cDNA, was labeled with [α-32P]dCTP using a random primed labeling kit (Stratagene); washing was done to a final stringency of 0.25× SSCP, 0.1% SDS, 65°C. A major fragment of 3.3 kb was detected in Pvull-digested C57BL/6J DNA and a major fragment of 3.7 kb was detected in Pvull-digested M. spretus DNA. The presence or absence of the 3.7-kb Pvull M. spretus–specific fragment was followed in backcross mice.
A description of the probes and RFLPs for the loci linked to Tslpr including Bmp3, Gfi 1, and Adrbk2 has been reported previously 18 19. Recombination distances were calculated using Map Manager, v2.6.5. Gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns.
Whitlock-Witte Culture Analysis.
Livers were isolated from newborn IL-7Rα−/− and wild-type C57BL/6 mice. The derivation and description of mice with a targeted disruption of the IL-7Rα gene have been described 20. Cultures were set up with a modification of the method of Whitlock and Witte 21. In brief, livers were disrupted into a single cell suspension and cultured in RPMI 1640 plus 5% charcoal filtered FCS (Hyclone) containing human IL-7 (10 ng/ml), or murine stem cell factor (SCF) (1 μg/ml), or murine TSLP (100 ng/ml), or SCF plus TSLP in 60-mm tissue culture dishes (Costar) and incubated at 37°C for 9 d. Phenotypic analysis was performed with FITC-conjugated RA3-6B2, GR-1, and Mac-1 Abs. Samples were analyzed on a FACScan™ (Becton Dickinson) using either a lymphoid- or myeloid-specific gate as determined by forward versus side light scatter profiles. The lymphoid gate was defined by the light scatter of the cells that grew out under IL-7 stimulation, and the myeloid gate was defined by the light scatter of the cells that grew out under SCF stimulation.
Thymocyte and Bone Marrow Proliferation Assays.
Unfractionated thymocytes from mice deficient in γc 22 or wild-type controls were cultured in 96-well plates (2 × 105 cells/well). Cells were stimulated with ConA (5 μg/ml) alone or in combination with IL-7 (10 ng/ml), or TSLP (10 and 0.4 ng/ml). Cells were cultured for 72 h and were pulsed with 3H-TdR for the last 6 h. Mice deficient in both γc and recombination activating gene (RAG)-2 23 were obtained through intercrossing, and mutants were identified by PCR using genomic DNA derived from tail snips. Bone marrow was flushed from femurs and tibias and the cells were stained with PE-conjugated anti-B220 Ab. B220+ B cell lymphoid precursors were isolated by cell sorting using a FACStarPLUS™ (Becton Dickinson). B cell cultures were initiated by plating 106 sorted B220+ cells on mitomycin C–treated ST2 stromal cells 24 supplemented with TSLP (100 ng/ml). After a period of 10 d, growth of clusters of round lymphoid cells was observed which were found to consist of >95% B220+ lymphocytes. Nonadherent cells (5 × 104) were washed and replated in 96-well round-bottomed plates in 200 μl of RPMI 1640 supplemented with 10% FCS, 50 μM 2-ME, nonessential amino acids, and antibiotics, and the following growth factor combinations: muIL-7 (50 ng/ml), TSLP (100 ng/ml), and SCF (100 ng/ml). Cells were cultured for 2 d and pulsed with 3H-TdR for the last 12 h.
Results
Characterization of TSLP Receptors on Lymphoid Cell Lines.
Purified recombinant murine TSLP was iodinated and shown to exhibit specific binding to both B and T lymphoid cell lines. Typical equilibrium binding data for 125I-TSLP at 37°C to the murine pre-B cell line 70Z/3 and the murine T cell line 7B9 is shown in Fig. 1A and Fig. B. Conditions chosen for binding reflected preliminary experiments that showed that binding was saturable and required <60 min to reach equilibrium at either 37 or 4°C. In both cases, Scatchard analysis of the data indicated a single class of high affinity binding sites for TSLP 25. For 70Z/3 cells, the calculated affinity constant (K a) was 7.1 ± 1.2 × 109 M−1 with 1,200 ± 900 sites per cell (average of 12 experiments), and for 7B9 cells, the K a was 1.1 ± 0.1 × 1010 M−1 with 1,200 ± 700 sites per cell (average of 2 experiments). Binding experiments with 70Z/3 cells were also performed at 4°C and produced binding constants very similar to those obtained at 37°C (data not shown). These results are in contrast to those previously obtained with murine IL-7 where binding to the pre-B cell line IxN/2b produced complex biphasic curves at either 4 or 37°C, indicative of both high and low affinity IL-7 binding sites on these cells 9.
Figure 1.
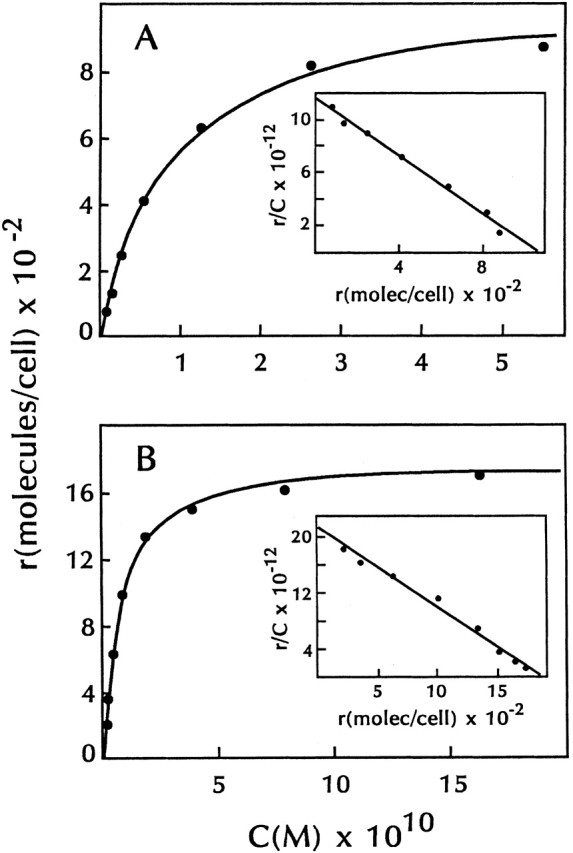
Equilibrium binding of 125I-TSLP to lymphoid cell lines. 70Z/3 cells (A) and anti-CD3–stimulated 7B9 cells (B) were incubated with various concentrations of 125I-TSLP for 30 min at 37°C. Binding was assayed as described in Materials and Methods. All data were corrected for nonspecific binding. Insets show Scatchard representations of specific binding from each panel.
TSLPR expression was evaluated on a variety of murine and human cell lines by radioligand binding (Table ). TSLP binding by murine cell lines of both lymphoid and myelomonocytic origin was detected at relatively low levels. Most cell lines that bound 125I-TSLP exhibited a single high affinity binding site with a K a between 0.4 and 1 × 1010 M−1. Interestingly, in some murine cell lines (LSTRA, NS1.1, P815), a low affinity binding component was also detected. This affinity was difficult to measure accurately and was <4 × 108 M−1. These results suggest that the TSLPR complex may consist of a low affinity binding subunit that is converted to high affinity when complexed with an additional receptor chain, as has been previously shown for many other cytokine receptors 2. Interestingly, cell lines that bound TSLP were also known to bind murine IL-7 9. However, unlike murine IL-7, which is capable of binding to IL-7 receptors on human cells, none of the human lines tested was found to bind murine TSLP. Given that these lines represented a panel of both lymphoid and myelomonocytic cell types quite analogous to the murine lines that bind TSLP, these data would suggest quite strongly that murine TSLP is incapable of cross-reacting with a putative human TSLPR.
Table 1.
Distribution of TSLPRs
| TSLP bound (molecules/cell) | |||
|---|---|---|---|
| Cell line | Characteristic | High K a | Low K a |
| Murine cells | |||
| Nag8/7 | TSLP-dependent pre-B cell | 500–1,700 | nd |
| 70Z3 | Pre-B cell | 300–3,000 | nd |
| IxN/2B | IL-7–dependent pre-B cell | 700–1,500 | nd |
| NFS-25-C3 | Pre-B lymphoblast | 440 | nd |
| NFS-5-C1 | Pre-B lymphoblast | 480 | nd |
| BAF/BO3 | Pre-B cell | nd | 250–2,500 |
| Wehi 279 | B cell | nd | nd |
| 7B9 | T cell clone (helper) | 500–1,800 | nd |
| CTLL | IL-2–dependent T cell | nd | nd |
| EL-4 | T cell thymoma | nd | nd |
| LSTRA | T lymphocytic leukemia | 35 | 7,500 |
| FDCP-2 | Myeloid | nd | nd |
| FDCP-1 | Promyeloid | nd | 210 |
| PU5-1.8 | Macrophage | 1,000 | nd |
| P388D1 | Macrophage | nd | nd |
| J774 | Macrophage | nd | 300 |
| Raw 264.7 | Monocytic | nd | nd |
| NS1.1 | Monocytic | 100 | 1,400 |
| P815 | Mastocytoma | 20 | 1,300 |
| MC-6 | Mast cell | nd | 540–2900 |
| DA-1 | Mast cell | nd | nd |
| Human cells | |||
| JM-1 | Pre-B cell | nd | nd |
| EU.1 | Pre-B cell | nd | nd |
| Daudi | B cell lymphoma | nd | nd |
| Raji | B cell lymphoma | nd | nd |
| CB23 | B cell lymphoma | nd | nd |
| MP-1 | B cell lymphoma | nd | nd |
| THP-1 | Monocytic | nd | nd |
| U937 | Monocytic | nd | nd |
| MO7E | Premegakaryocytic | nd | nd |
| TF-1 | Proerythrocytic | nd | nd |
| KG-1 | Myelogenous leukemia | nd | nd |
| W126 | Lung fibroblast | nd | nd |
| IMTLH | Stromal | nd | nd |
Binding experiments were conducted as described in Materials and Methods. Molecules bound per cell were determined by Scatchard analysis of complete sets of binding data and represent total sites per cell.
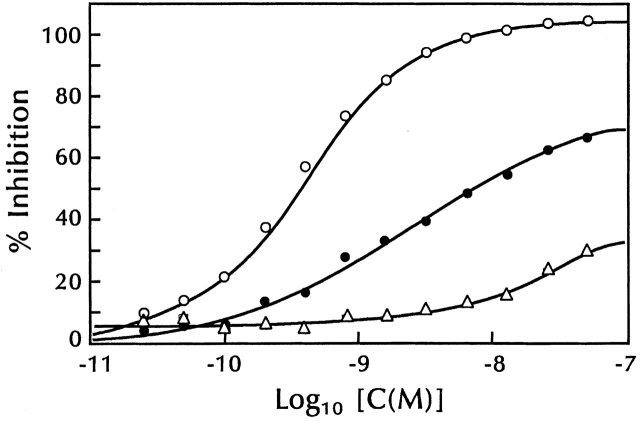
To explore the possibility that TSLP might interact with subunits of the IL-7 receptor, inhibition studies were performed in which unlabeled TSLP, murine IL-7, and human IL-7 were tested for their ability to inhibit binding of 125I-TSLP to 70Z/3 cells. Both murine and human IL-7 were capable of inhibiting 125I-TSLP binding, although not as effectively as unlabeled TSLP (Fig. 2), which exhibited an inhibition constant of 9.8 × 109 M−1. Also, murine IL-7 was more effective than human IL-7 at competing for TSLP binding. These data suggest that TSLP was able to interact with at least one component of the IL-7 receptor complex. To gain further understanding of the nature of the TSLPR, we undertook to clone the cDNA for a specific TSLP binding chain.
Figure 2.
Inhibition of 125I-TSLP binding by unlabeled TSLP and IL-7. 70Z/3 cells (5 × 107 cells) were incubated with 125I-TSLP (2.6 × 10−10 M) and varying concentrations of unlabeled TSLP (○), murine IL-7 (•), and human IL-7 (▵) for 30 min at 37°C. Binding was assayed as described in Materials and Methods. The continuous curves were calculated from either a one-site (TSLP) or two-site (murine and human IL-7) competitive inhibition equation using a K a value for 125I-TSLP of 8.8 × 109 M−1. All data were corrected for nonspecific binding.
Isolation of a cDNA Clone Encoding a TSLP Binding Chain.
Cloning of a cDNA encoding the TSLPR was accomplished by direct expression in mammalian cells 26. A cDNA library was generated in an expression vector using mRNA from a murine T helper cell clone (7B9) that binds radiolabeled TSLP. Pools of cDNA clones were transfected into CV-1/EBNA cells and 3 d later the cells were incubated with 125I-TSLP, with the expectation that if a pool contained a functional TSLPR cDNA, expression of that clone would result in TSLP binding to some of the transfected CV-1/EBNA cells. Two positive pools were found after screening 50,000 cDNA clones, which were then broken down into successively smaller subpools until a single cDNA clone encoding a TSLPR was isolated from each (clones 7a and 19). The inserts from the two clones were sequenced and found to be identical in the extensive region of overlap.
The longer of the two TSLPR clones, clone 7a, had a single open reading frame of 359 amino acids (Fig. 3 A). The sequence predicts a type I transmembrane protein with a hydrophobic signal peptide at the NH2 terminus and a single membrane-spanning segment. The extracellular portion is a member of the hematopoietin receptor family 27 that includes the receptors for many other cytokines. The TSLPR lacks the second of the four conserved cysteines present in other hematopoietin family members, and in place of the hallmark WSXWS motif contains the sequence WTAVT. There are two potential sites for N-linked glycosylation. The cytoplasmic portion is predicted to be 106 amino acids in length. It contains the “box 1” region 28 that is common to all hematopoietin family receptors and is thought to be a binding site for signal transducing molecules. In addition, the TSLPR shows a more extended homology with the cytoplasmic regions of the erythropoietin receptor, the IL-9 receptor, and the β chain of the IL-2 receptor (Fig. 3 B). There is a formal possibility that the natural TSLPR protein is larger than shown in Fig. 3 A, since no in-frame stop codons are present in the 5′ untranslated region of clone 7a. If that is the case, however, the additional NH2-terminal amino acids would seem to be unnecessary for proper cell surface membrane expression and TSLP binding activity of the molecule.
Figure 3.
Murine TSLPR cDNA. (A) The cDNA and predicted amino acid sequence of the murine TSLPR clone 7a. Signal peptide cleavage is shown by the arrow as occurring after Ala 21, although cleavage after Ala 15 would be nearly as preferred (reference 37). The transmembrane region is underlined; asterisks mark the cysteines conserved in hematopoietin family members; a double underline indicates the sequence WTAVT, present here in place of the more typical WSXWS; the box 1 sequence of Murakami et al. is boxed (reference 28). A second functional cDNA clone isolated in the expression screen, clone 19, extends from nucleotide 80 through the polyA stretch at the 3′ end. The initiating Met-Ala-Trp sequence at the NH2 terminus of clone 7a is replaced in clone 19 by Met-Gly, encoded in the adapter used to insert the cDNA into the vector. (B) Alignment of the membrane-proximal segment of the murine TSLPR with membrane-proximal segments of the murine erythropoietin receptor (EpoR), the murine IL-2 receptor β chain, and the murine IL-9 receptor, with regions of amino acid conservation highlighted.

Expression of TSLPR mRNA in Murine Tissues and Cell Lines.
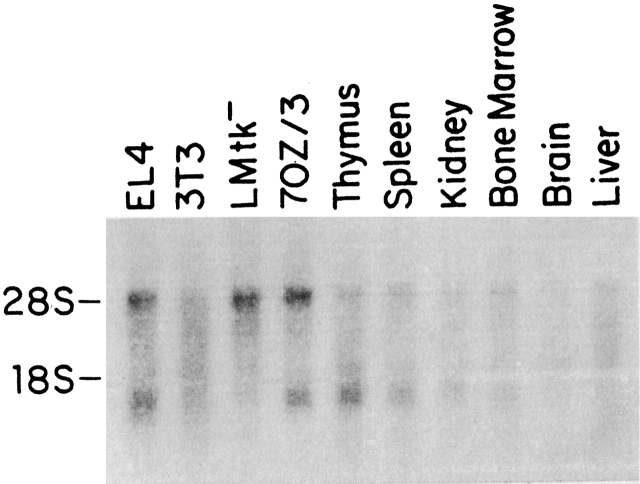
Northern blots reveal a single hybridizing RNA species that migrates somewhat faster than 18S rRNA (Fig. 4), consistent with the mRNA size of ∼1,300 nucleotides (excluding polyA) estimated from the cDNA clones. TSLPR mRNA was detected in EL/4 and 70Z/3 cells, thymus, spleen, and weakly in kidney and bone marrow. It was not detected in 3T3 cells, LMtk− cells, brain, and liver (Fig. 4). Expressed sequence tags derived from TSLPR mRNA have been found in mouse cDNA libraries made from placenta, embryo, embryonic stem cells, testes, small intestine, brain, and macrophages.
Figure 4.
Northern blot analysis of TSLPR mRNA. PolyA+ RNA from various cell lines and tissues was hybridized to an antisense TSLPR riboprobe as described in Materials and Methods. The band at 28S reflects the cross-hybridization of the TSLPR probe to residual rRNA present in the samples.
Chromosomal Location of Tslpr.
The mouse chromosomal location of Tslpr was determined by interspecific backcross analysis using progeny derived from matings of (C57BL/6J × M. spretus, Fl × C57BL/6J) mice 16. The mapping results indicated that Tslpr is located in the central region of mouse chromosome 5 linked to Bmp3, Gfi 1, and Adrbk2 (data not shown). The most likely gene order is: centromere - Bmp3 - Gfi 1 - Tslpr - Adrbk2.
Expression and Characterization of TSLPR cDNA.
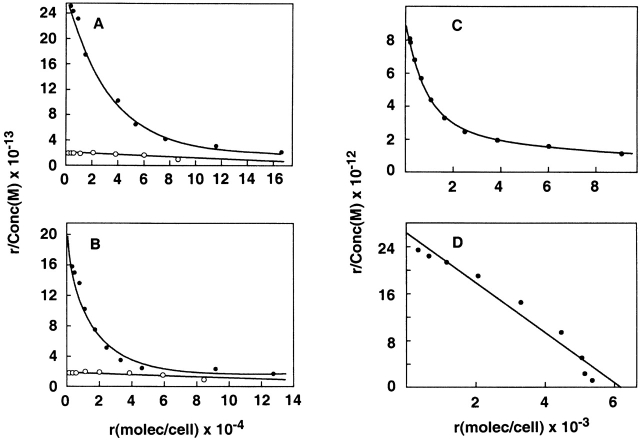
Evidence that the isolated TSLPR clone 7a encoded a functional TSLP binding protein is shown in Fig. 5A and Fig. B. TSLPR clone 7a expressed alone in transfected CV-1/EBNA cells was found to bind 125I-TSLP with low affinity only, displaying a single low affinity site with a K a of 1.3 ± 1.2 × 108 M−1 (average of six experiments). This affinity is similar to that observed for 125I-TSLP binding to a limited subset of murine cell lines such as BAF/BO3 and MC6. To determine if the IL-7Rα subunit might be a component of the TSLPR complex that would increase the affinity of TSLP binding, TSLPR cDNA was cotransfected into CV-1/EBNA cells with either murine (Fig. 5 A) or human (Fig. 5 B) IL-7Rα cDNA clones. Binding analysis with 125I-TSLP revealed that cells coexpressing the TSLPR and either murine or human IL-7Rα proteins exhibited biphasic binding characteristics with both high and low affinity binding sites. The new high affinity site had a K a of 1.8 ± 1.1 × 1010 M−1 (average of five experiments), which is similar to that observed for 125I-TSLP binding to lymphoid and myelomonocytic cells, suggesting that the native high affinity TSLPR complex might be comprised of just these two components.
Figure 5.
Binding characteristics of TSLPR with the IL-7Rα chain. (A and B) Binding of 125I-muTSLP to CV-1/EBNA cells transfected with murine TSLPR with (•) or without (○) murine IL-7Rα (A) or human IL-7Rα (B). (C and D) Binding of 125I–muIL-7 (C) or 125I-muTSLP (D) to BAF/BO3 cells transfected with the murine IL-7Rα. In all cases binding was done for 30 min at 37°C and assayed as described in Materials and Methods.
This hypothesis was further explored by taking advantage of the observation that BAF/B03 cells demonstrate only low affinity binding for 125I-TSLP (K a < 4 × 108 M−1) and lack the IL-7Rα chain as they do not bind 125I-IL-7 (data not shown). Additionally, this indicates that IL-7 does not bind to TSLPR. These cells were stably transfected with murine IL-7Rα cDNA as described above (see Materials and Methods), and the binding affinities for 125I–muIL-7 and 125I-TSLP were measured (Fig. 5C and Fig. D). 125I–IL-7 binding was very similar to that described previously for other murine cell lines, exhibiting a biphasic Scatchard plot 9. The curve shown in Fig. 5 C had 1,100 high affinity sites with a K a of 7 × 109 M−1, and a large number of low affinity sites (>20,000) with an affinity of <1 × 108. In contrast, binding of 125I-TSLP to these transfected cells (Fig. 5 D) showed a single high affinity site with a K a of 3.9 ± 0.4 × 109 M−1 with 4,500 ± 3,100 sites/cell (average of four experiments). This affinity is in the same range as that measured for TSLP binding on the various lymphoid and myelomonocytic cell lines shown in Table . In addition, it is apparent from the 125I–IL-7 binding curve that the IL-7Rα chain is expressed in apparent excess to the number of endogenous TSLPR chains. This ratio would explain why all the low affinity TSLPR chains are effectively converted to high affinity TSLP binding complexes by the presence of the IL-7Rα chain, and suggests that in most of the cell lines we tested for TSLP binding, the IL-7Rα is also expressed in excess relative to the TSLPR chain.
Characterization of the TSLP Receptor by Affinity Cross-Linking.
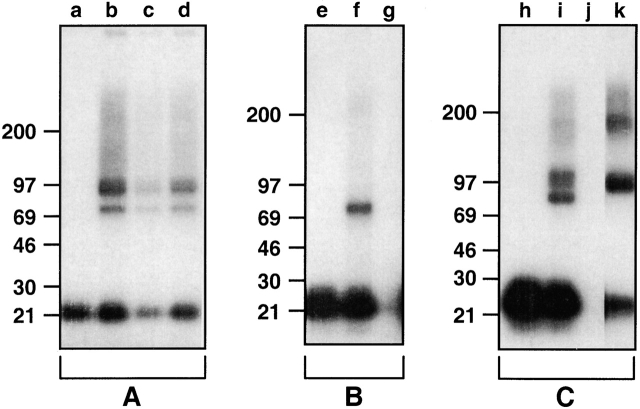
To further characterize the TSLPR complex, we performed affinity cross-linking studies on a cell line (70Z/3) containing endogenous high affinity TSLPRs, on CV-1/EBNA cells that had been transfected with only the TSLPR clone 7a, and on BAF/BO3 cells (which express endogenous TSLPR chain) transfected with the murine IL-7R cDNA. On 70Z/3 cells (Fig. 6 A), cross-linking with 125I-TSLP revealed two major species with molecular masses of ∼100 and 75 kD. Cross-linking of 125I-TSLP in both of these bands is relatively evenly reduced in the presence of unlabeled murine or human IL-7 (Fig. 6 A, lanes c and d). Incubation in the presence of unlabeled TSLP totally eliminates the presence of both cross-linked species (data not shown). After subtraction of the molecular mass of TSLP (major species of 23 kD, with minor species of 18 kD due to differential glycosylation), the cross-linked species would correspond to membrane proteins of ∼75 and 50 kD, respectively. The larger species corresponds closely to the size of the murine IL-7R chain as previously measured by affinity cross-linking, suggesting that the smaller species may represent the TSLPR chain 9 11. This hypothesis is substantiated by the results obtained from cross-linking TSLP to CV-1/EBNA cells transfected with the TSLPR clone 7a alone (Fig. 6 B). A single cross-linked species is observed that corresponds in size to the smaller of the two species seen on the 70Z/3 cells (Fig. 6 B, lane f), and cross-linking of 125I-TSLP to this species is completely inhibited by the inclusion of unlabeled TSLP in the incubation mixture (Fig. 6 B, lane g). Cross-linking to BAF/BO3 cells that contain endogenous TSLPR and transfected recombinant murine IL-7Rα produced a pattern of cross-linking with 125I-TSLP essentially identical to that seen on the 70Z/3 cells (Fig. 6 C, lane i). Cross-linked complexes of ∼100 and 75 kD are observed, which are competitively inhibited by unlabeled TSLP (Fig. 6 C, lane j). To elucidate the nature of the higher molecular mass cross-linked species, these cells were also cross-linked with 125I–muIL-7. As shown in Fig. 6 C, lane k, two main cross-linked species were observed, one of ∼100 kD and a larger species of ∼180 kD. This pattern is very similar to what was previously observed for 125I–IL-7 cross-linking 9, where we proposed that the higher molecular mass species may correspond to cross-linking of 125I–IL-7 to a dimer of IL-7Rα chains. The 100-kD cross-linked species corresponds closely in size to the larger of the two species cross-linked with 125I-TSLP. Taken together, these data provide strong evidence that the expressed natural and recombinant TSLPR chains have a molecular mass of ∼50 kD, and that in cells expressing both the TSLPR and IL-7Rα chains, TSLP is capable of interacting with both.
Figure 6.
Characterization of the TSLPR by affinity cross-linking. (A) 70Z/3 cells were incubated with 125I-TSLP in the absence of unlabeled competitor (a and b), or in the presence of unlabeled murine IL-7 (c) or human IL-7 (d) and then treated with (b–d) or without (a) cross-linker. (B) CV-1 cells transfected with TSLPR clone 7a were incubated with 125I-TSLP in the absence of unlabeled competitor (e and f) or in the presence of unlabeled TSLP (g) and then treated with (f and g) or without (e) cross-linker. (C) BAF/BO3 cells transfected with muIL-7Rα were incubated with 125I-TSLP in the absence of unlabeled competitor (h and i) or in the presence of unlabeled TSLP (j), or with 125I–IL-7 (k) and then treated with (i–k) or without (h) cross-linker. Cross-linking was performed as described in Materials and Methods and samples were run on SDS-PAGE under reducing conditions using 8–16% gradient gels and analyzed by autoradiography.
Signal Transduction Mediated by TSLPR Complexes.
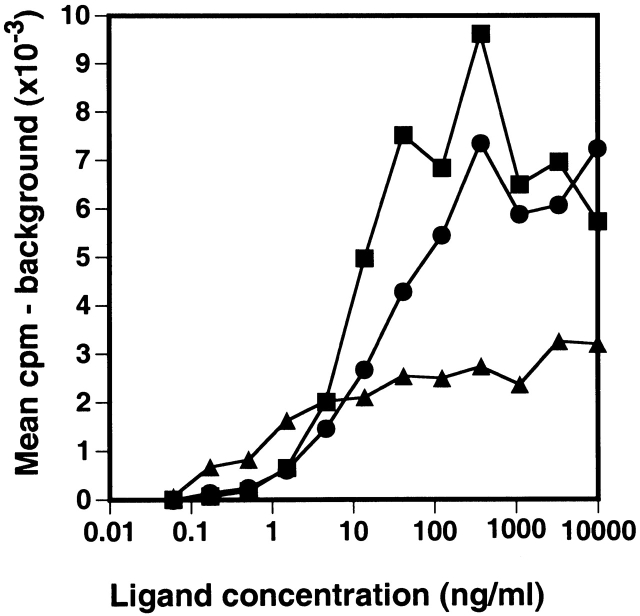
Having shown that the TSLPR is a low affinity TSLP binding chain that can be converted to a high affinity binding complex in the presence of the IL-7Rα chain, we next wanted to examine the signaling capabilities of these two forms of the TSLPR. BAF/BO3 cells (which express TSLPR and γc) that were transfected with the IL7Rα were tested for their response to IL-3, IL-7, and TSLP. Parental BAF/BO3 cells that proliferated in response to IL-3 were incapable of responding to either human IL-7 or TSLP even when these factors were used at very high concentrations (data not shown). BAF/BO3 cells transfected with the IL-7Rα now proliferated to both IL-7 and TSLP (Fig. 7). Although these cells proliferated to a greater extent to IL-7, their response to TSLP was significantly greater than background (cells with no added growth factor, P < 0.05) at all concentrations of TSLP >1.5 ng/ml. It therefore appears, at least in this cell population, that the IL-7Rα chain is required for generation of a functional TSLPR complex.
Figure 7.
Proliferative response of BAF/BO3 cells transfected with the muIL7Rα chain. BAF/BO3 cells expressing murine IL-7Rα chain (2 × 105 cells/ml) were cultured in medium alone, murine IL-3 (▪), human IL-7(•), or murine TSLP (▴) for 2 d. Cells were then pulsed for 5 h with 3H-TdR and harvested. The background cpm (cells in medium alone) averaged 184 cpm.
Given that the functional IL-7Rα complex has also been shown to contain the γc chain 29, it was necessary to determine if the functional TSLPR complex requires the γc chain and thus exists as a heterotrimer with TSLPR and IL-7Rα, or whether the TSLPR and IL-7Rα chains are sufficient for signaling. BAF/BO3 cells endogenously express the γc chain, so the proliferative results in the IL-7Rα–transfected BAF/BO3 line were not informative. To determine the requirement for the IL-7Rα and γc chains in formation of a functional TSLPR, we therefore examined the ability of TSLP to mediate biological activities on cells from mice that were deficient in either the IL-7Rα chain or the γc chain.
Analysis of TSLP Activity in Whitlock-Witte Cultures Derived from IL-7Rα–deficient Mice.
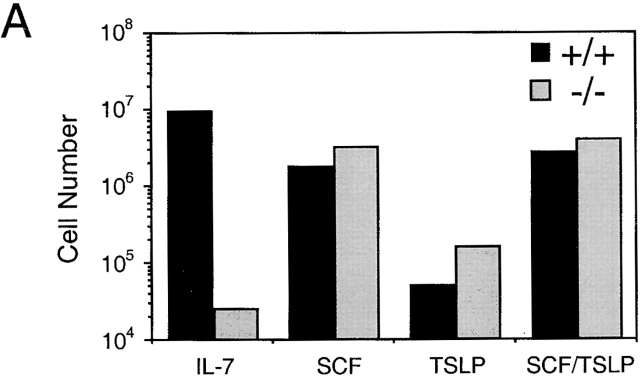
Whitlock-Witte cultures were established using murine neonatal liver as the source of hematopoietic cells from wild-type or IL-7Rα−/− mice 30 31. These cells were cultured with TSLP, IL-7, SCF, or TSLP plus SCF for 9 d. Nonadherent cells were then harvested, counted, and analyzed by immunoflourescent staining and flow cytometry.
As can be seen in Fig. 8 A, compared with IL-7 or SCF, TSLP did not stimulate significant growth of either wild-type or IL-7Rα–deficient cells in this culture system. However, the nonadherent cell yield in response to TSLP by both wild-type and IL-7Rα–deficient cells is greater than in cultures with no added cytokine in which only an adherent cell layer develops. The combination of TSLP and SCF did not result in a greater degree of nonadherent cell growth than with SCF alone in either wild-type or IL-7Rα–deficient cells. Interestingly, phenotypic analysis of these cultures showed that cells grown in SCF or SCF plus TSLP had a roughly similar distribution of myeloid cells (Fig. 8 B, myeloid gate, Mac-1 and Gr-1 staining). However, wild-type cells grown in SCF plus TSLP had a significant population of B220+ cells (Fig. 8 B, lymphoid gate) that was not present in cultures grown in SCF alone (an insignificant number of events was present in the lymphoid gate to accumulate data; Fig. 8 B). IL-7Rα−/− cells cultured with SCF and TSLP resulted in only myeloid cell growth (an insignificant number of events was present in the lymphoid gate to accumulate data; Fig. 8 B). Thus, these data demonstrate that TSLP alone does not stimulate significant growth in Whitlock-Witte culture conditions, but in the presence of SCF results in significant lymphoid growth, compared with SCF alone which stimulates only myeloid growth. This lymphoid population is not observed in cultures established with IL-7R−/− cells, further supporting the hypothesis that TSLP-mediated signaling requires the IL-7Rα chain.
Figure 8.
Analysis of TSLP activity in Whitlock-Witte cultures derived from wild-type or IL-7Rα−/− mice. (A) Livers from IL-7Rα−/− or wild-type newborn mice were grown under Whitlock-Witte culture conditions for 9 d in the presence of either IL-7 (10 ng/ml), SCF (1 μg/ml), TSLP (100 ng/ml), or SCF plus TSLP. Enumeration of nonadherent cells was determined by trypan blue exclusion. (B) Immunofluorescent profiles of nonadherent cells isolated from IL-7Rα−/− and wild-type mice. Lymphoid and myeloid gates were established by the forward versus side light scatter profiles of cells grown in IL-7 alone or SCF alone, respectively. Myeloid gated cells were analyzed for Mac-1 and Gr-1 expression after growth in SCF (gray histogram) or SCF plus TSLP (white histogram). Lymphoid gated cells were analyzed for B220 expression after growth in IL-7 (hatched histogram) or SCF plus TSLP (white histogram). Black histogram represents IgG1 isotype control staining of cells grown in IL-7.
Analysis of TSLP Activity on Cells from γc-deficient Mice.
To determine if the γc chain was involved in the TSLPR signaling complex, the proliferative response of wild-type and γc−/− lymphocytes in response to TSLP was assessed. It has been previously demonstrated that γc−/− thymocytes proliferate poorly in response to ConA, and that this response cannot be augmented by the addition of exogenous IL-2, IL-4, or IL-7 22. As can be seen in Table , the addition of TSLP to ConA resulted in a significant increase in proliferation of γc−/− thymocytes, suggesting that γc is not required for TSLP-mediated signaling. This observation was confirmed using B cell precursors from RAG-2–deficient mice 23. These mice accumulate B cell precursors due to an inability to rearrange Ig genes precisely at the point where they are most responsive to the combination of IL-7 and SCF. Thus, long term cultures of RAG-2–deficient B cell progenitors can be maintained in vitro with IL-7 and SCF 32. B220+ bone marrow cells from RAG-2−/− or γc−/−RAG-2−/− double mutants were cultured on ST2 stromal cells supplemented with TSLP. The nonadherent lymphoid cells that expanded expressed B220 and IL-7Rα and were negative for surface IgM, Gr-1, and Mac-1 (data not shown). As can be seen in Table , these enriched B220+ B cell precursors from γc−/−RAG-2−/− mice were able to proliferate in response to TSLP in the presence of SCF, but not to IL-7 in the presence of SCF. These data demonstrate that the γc is not required for the response to TSLP by thymocytes and B cell precursors under these culture conditions.
Table 2.
Proliferative Response of Thymocytes and B220+ Cells from γc−/− Mice to TSLP
| γc−/− | γc+/+ | |
|---|---|---|
| cpm × 10−3 | ||
| Thymocytes | ||
| ConA | 4.7 | 7.2 |
| ConA + IL-7 | 4.6 | 25.6 |
| ConA + TSLP 10 ng/ml | 11.5 | 15.3 |
| ConA + TSLP 0.4 ng/ml | 11.2 | 10.9 |
| PMA/ionomycin | 49.5 | 52.4 |
| PMA/IL-4 | 0.2 | 44.1 |
| B220+ cells (RAG-2−/−) | ||
| SCF | 0.2 | 0.5 |
| IL-7 | 0.4 | 11.2 |
| IL-7/SCF | 0.5 | 20.3 |
| TSLP | 1.7 | 1.6 |
| TSLP/SCF | 11.6 | 5.6 |
Enriched B220+ cells are from γc−/−RAG-2−/− and γc+/+RAG-2−/− mice (see Materials and Methods). The values are the arithmetic mean of triplicate wells and are from a single representative experiment.
Discussion
TSLP is a cytokine that shares a closely overlapping, but distinct, profile of biological activities with IL-7 6. In Sims et al. in this issue, we have reported the cloning and further characterization of this molecule 5. In this report, we utilize purified recombinant-derived murine TSLP to characterize the cell surface receptor complex to which this cytokine binds, and report cloning of a cDNA encoding a specific murine TSLP binding chain. Equilibrium binding studies with radiolabeled TSLP demonstrated that a variety of murine lymphoid, myeloid, and monocytic cell lines were capable of binding TSLP, in most cases exhibiting a single high affinity binding site. However, there were a few cell lines that exhibited both a high and low affinity site, and three lines that exhibited low affinity binding only. This pattern of binding is reminiscent of several other cytokine receptors that contain two or more subunits, in particular those that contain a specific low affinity binding chain, and an additional converting subunit that generates a high affinity, signal tranduction competent complex.
Given the apparent biological relationship of TSLP to IL-7, it was also interesting to note that all the cell lines that were found to bind TSLP had been previously demonstrated to bind IL-7. This suggested the possibility that the receptors for TSLP and IL-7 might share one or more common subunits. Subsequent binding inhibition and cross-linking studies substantiated this hypothesis and led to our efforts to clone a receptor subunit that might specifically bind TSLP. A cDNA clone encoding a TSLP binding chain (TSLPR) was isolated by direct expression from a murine 7B9 T helper cell clone cDNA library. The sequence predicts a type I transmembrane protein that is clearly a member of the hematopoietin receptor family, but with interesting variations. The TSLPR lacks the second of the four conserved cysteines present in other hematopoietin family members, and in place of the hallmark WSXWS motif it contains the sequence WTAVT. The cytoplasmic portion contains the “box 1” region that is common to all hematopoietin family receptors and is thought to be a binding site for signal transducing molecules 28. In addition, the TSLPR sequence shows extended homology with the cytoplasmic regions of the erythropoeitin receptor, the IL-9R, and the β chain of the IL-2R.
Following up on the possibility that TSLP and IL-7 might share common receptor subunits, the binding of TSLP to TSLPR, both in the presence and absence of IL-7Rα, was examined by expressing these subunits in CV-1/EBNA cells. The results showed that the TSLPR subunit alone bound TSLP with low affinity whereas the combination of TSLPR and IL-7Rα generated a high affinity site. The affinities of both these sites matched those previously measured in murine cell lines. Interestingly, both the murine and human IL-7Rα subunits were equally effective in combining with the murine TSLPR subunit to generate a high affinity murine TSLP binding complex. This was somewhat surprising since extensive binding studies failed to identify any human cell lines capable of binding murine TSLP, indicating that this molecule exhibits strict species specificity. In contrast, both murine and human IL-7 are capable of binding to receptors on cells of the opposite species.
Further characterization of TSLPR-mediated binding and signal transduction was done by using the BAF/B03 cell line, which expresses endogenous TSLPR and γc, but no IL-7Rα. This line binds TSLP with low affinity only, does not bind IL-7, and is incapable of proliferating in response to either cytokine. When a stable transfectant of this line was generated that expressed the IL-7Rα subunit as well, the cells were now found to bind both IL-7 and TSLP with high affinity and to proliferate in response to these cytokines. This result indicated that, at a minimum, the TSLPR and IL-7Rα subunits were required to generate a functional TSLPRcomplex. This conclusion was further substantiated by the observation that in Whitlock-Witte cultures established from IL-7Rα−/− mice, TSLP was incapable of stimulating cell growth. TSLP did show activity in this culture system, however. When combined with SCF, TSLP stimulated growth of wild-type B220+ cells that were absent in cultures grown in SCF alone.
Signal transduction in the IL-7 receptor complex requires the γc chain, first described for the IL-2 receptor complex 29 33. The possibility that the γc might be involved in TSLP signal transduction was tested functionally by studying the in vitro proliferative response of thymocytes and pre-B cells from γc−/− mice to IL-7 and TSLP. In each case, there was an insignificant response of γc−/− cells to IL-7, as expected; however, γc−/− cells were still competent to respond to TSLP. In total, these data indicate that high affinity binding and biological activity of TSLP require the IL-7Rα chain, but do not require the γc chain of the IL-2R complex.
The relationship of TSLP and IL-7 to their cellular receptors is analogous to the IL-13–IL-4 cytokine–receptor system in which IL-13–mediated signaling through the IL-13R utilizes the IL-4Rα chain but not the γc chain 34 35. Interestingly, both IL-7 and TSLP have overlapping biological activities as well as unique properties. Both IL-7 and TSLP promote growth of B cell and T cell precursors; however, the unique biological responses generated through binding of TSLP to the TSLPR–IL-7Rα complex have yet to be well understood. A recent report describes the ability of TSLP and IL-7 to induce tyrosine phosphorylation of the signal transducer and activator of transcription (Stat)5 transcription factor. TSLP did not induce activation of Janus kinase 1 and Janus kinase 3, whereas IL-7–mediated signaling did so in the same cell line 36. This evidence indicates that TSLP will have biological effects that are not common to IL-7, and these remain to be elucidated in detail.
Footnotes
Abbreviations used in this paper: K a, affinity constant; RAG, recombination activating gene; SCF, stem cell factor; TSLP, thymic stromal lymphopoietin.
References
- Komschlies K.L., Grzegorzewski K.J., Wiltrout R.H. Diverse immunological and hematological effects of interleukin-7implications for clinical application. J. Leukoc. Biol. 1995;58:623–633. doi: 10.1002/jlb.58.6.623. [DOI] [PubMed] [Google Scholar]
- Di Santo J.P., Kühn R., Müller W. Common cytokine receptor γ chain (γc)-dependent cytokinesunderstanding in vivo functions by gene targeting. Immunol. Rev. 1995;148:19–34. doi: 10.1111/j.1600-065x.1995.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Ihle J.N., Witthuhn B.A., Quelle F.W., Yamamoto K., Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- Chomarat P., Banchereau J. An update on Interleukin-4 and its receptor. Eur. Cytokine Netw. 1997;8:333–344. [PubMed] [Google Scholar]
- Sims J.E., Williams D.E., Morrissey P.J., Garka K., Foxworthe D., Price V., Friend S., Farr A.G., Bedell M., Jenkins N.A., Copeland N.G., Grabstein K., Paxton R.J. Molecular cloning and biological characterization of a novel lymphoid growth factor. J. Exp. Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S.L., Hosier S., Nelson A., Foxworthe D., Williams D.E., Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- Park L.S., Friend D., Gillis S., Urdal D.L. Characterization of the cell surface receptor for granulocyte-macrophage colony stimulating factor. J. Biol. Chem. 1986;261:4177–4187. [PubMed] [Google Scholar]
- Cosman D., Cerretti D.P., Larson A., Park L.S., March C., Dower S.K., Gillis S., Urdal D. Cloning, sequence and expression of the human interleukin-2 receptor. Nature. 1984;312:768–770. doi: 10.1038/312768a0. [DOI] [PubMed] [Google Scholar]
- Park L.S., Friend D.J., Schmierer A.E., Dower S.K., Namen A.E. Murine interleukin 7 (IL-7) receptor. Characterization on an IL-7–dependent cell line. J. Exp. Med. 1990;171:1073–1079. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S.F., Tough T.W., Franklin T.L., Armitage R.J., Alderson M.R. Induction of macrophage inflammatory protein-1β gene expression in human monocytes by lipopolysaccharide and IL-7. J. Immunol. 1991;147:2234–2239. [PubMed] [Google Scholar]
- Goodwin R.G., Friend D., Ziegler S.F., Jerzy R., Falk B.A., Gimpel S., Cosman D., Dower S.K., March C.J., Namen A.E., Park L.S. Cloning of the human and murine interleukin-7 receptorsdemonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Gruss H.J., Davis T., Anderson D., Farrah T., Baker E., Sutherland G.R., Brannan C.I., Copeland N.G., Jenkins N.A. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- McMahan C.J., Slack J.L., Mosley B., Cosman D., Lupton S.D., Brunton L.L., Grubin C.E., Wignall J.M., Jenkins N.A., Brannan C.I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R.C., Berg P. Selection for animal cells that express the Escherichia coli gene for xanthine-guanine phosphoribosyltransferase. Proc. Natl. Acad. Sci. USA. 1981;78:2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chasseval R., de Villartay J.P. High level transient gene expression in human lymphoid cells by SV40 large T antigen. Nucleic Acids Res. 1992;20:245–250. doi: 10.1093/nar/20.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N.G., Jenkins N.A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991;7:113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Jenkins N.A., Copeland N.G., Taylor B.A., Lee B.K. Organization, distribution and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus . J. Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J.L., Onorato J.J., Arriza J.L., Stone W.C., Lohse M., Jenkins N.A., Gilbert D.J., Copeland N.G., Caron M.G., Lefkowitz R.J. Cloning, expression, and chromosomal location of β-adrenergic receptor kinase 2. J. Biol. Chem. 1991;266:14939–14946. [PubMed] [Google Scholar]
- Bell D.W., Taguchi T., Jenkins N.A., Gilbert D.J., Copeland N.G., Gilks C.B., Zweidler-McKay P., Grimes H.L., Tsichlis P.N., Testa J.R. Chromosomal localization of a gene, GFI1, encoding a novel zinc finger protein reveals a new syntenic region between man and rodents. Cytogenet. Cell Genet. 1995;70:263–267. doi: 10.1159/000134048. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Morissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C.A., Witte O.N. Long term culture of B lymphocytes and their precursors. Proc. Natl. Acad. Sci. USA. 1982;79:3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo J.P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor γ chain. Proc. Natl. Acad. Sci. USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M., Alt F.W. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangements. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nishikawa S., Ikuta K., Yamamura F., Naito M., Takahashi K., Nishikawa S.I. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2B cell progenitor develops first in embryonal body rather than in yolk sac. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:1337–1347. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatchard G. The attraction of small molecules and ions. Ann. NY Acad. Sci. 1949;51:660–672. [Google Scholar]
- Sims J.E., March C.J., Cosman D., Widmer M.B., MacDonald H.R., McMahan C.J., Grubin C.E., Wignall J.M., Jackson J.L., Call S.M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin gene superfamily. Science. 1988;241:585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Cosman D. The hematopoietin receptor superfamily. Cytokine. 1993;5:95–106. doi: 10.1016/1043-4666(93)90047-9. [DOI] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamagughi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc. Natl. Acad. Sci. USA. 1991;88:11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S.M., Ziegler S.F., Tsang M., Cao X., Leonard W.J. Interleukin-2 receptor γ chaina functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Barker J.E. Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev. Biol. 1968;18:14–21. doi: 10.1016/0012-1606(68)90020-1. [DOI] [PubMed] [Google Scholar]
- Nicola N.A., Metcalf D., von Mechner H., Burgess A.W. Isolation of murine fetal hematopoietic progenitor cells and selective fractionation of various erythroid precursors. Blood. 1981;58:367–377. [PubMed] [Google Scholar]
- Yasunga M., Wang F., Kunisada T., Nishikawa S., Nishikawa S.I. Cell cycle control of c-kit+ IL-7R+ B precursor cells by two distinct signals derived from IL-7 receptor and c-kit in a fully defined medium. J. Exp. Med. 1995;182:315–323. doi: 10.1084/jem.182.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura M. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Hilton D.J., Zhang J.G., Metcalf D., Alexander W.S., Nicola N.A., Willson T.A. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc. Natl. Acad. Sci. USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A., Grunewald S.M., Duschl A., Fischer A., Di Santo J.P. Mouse macrophage development in the absence of the common gamma chaindefining receptor complexes responsible for IL-4 and IL-13 signaling. Eur. J. Immunol. 1997;27:1762–1768. doi: 10.1002/eji.1830270725. [DOI] [PubMed] [Google Scholar]
- Levin S.D., Koelling R.M., Friend S.L., Ziegler S.F., Perlmutter R.M., Farr A.G. Thymic stromal lymphopoieitin (TSLP)a cytokine that promotes the development of sIgM+ B cells in vitro and signals via a novel mechanism. J. Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]