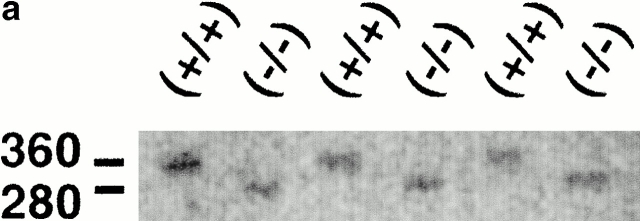Abstract
Neutrophils migrate to infected mucosal sites that they protect against invading pathogens. Their interaction with the epithelial barrier is controlled by CXC chemokines and by their receptors. This study examined the change in susceptibility to urinary tract infection (UTI) after deletion of the murine interleukin 8 receptor homologue (mIL-8Rh). Experimental UTIs in control mice stimulated an epithelial chemokine response and increased chemokine receptor expression. Neutrophils migrated through the tissues to the epithelial barrier that they crossed into the lumen, and the mice developed pyuria. In mIL-8Rh knockout (KO) mice, the chemokine response was intact, but the epithelial cells failed to express IL-8R, and neutrophils accumulated in the tissues. The KO mice were unable to clear bacteria from kidneys and bladders and developed bacteremia and symptoms of systemic disease, but control mice were fully resistant to infection. The experimental UTI model demonstrated that IL-8R–dependent mechanisms control the urinary tract defense, and that neutrophils are essential host effector cells. Patients prone to acute pyelonephritis also showed low CXC chemokine receptor 1 expression compared with age-matched controls, suggesting that chemokine receptor expression may also influence the susceptibility to UTIs in humans. The results provide a first molecular clue to disease susceptibility of patients prone to acute pyelonephritis.
Keywords: urinary tract infection, chemokine receptor, mucosal immunity, lipopolysaccharide, knockout mice
Introduction
Human hosts are continuously exposed to a diverse environmental microflora including a variety of pathogens. In most cases health is maintained through the coordinate action of specific immunity and nonimmune, or “innate,” defense mechanisms. Specific lymphocyte populations and their products are known to be essential for certain infections and for vaccine-induced protection, but the innate defenses have received less attention.
Urinary tract infections (UTIs) provide an excellent model to study resistance mechanisms of the mucosa. The urinary tract is open to microbial attack, but in most cases the urine remains sterile, indicating the presence of a highly efficient defense. Early studies suggested that innate rather than specific immunity was essential to maintain the sterility of the mucosa, but the cellular and molecular mechanisms of resistance were not identified. Nude 1, X-linked immunodeficient (xid), and SCID 2 mice with defective T lymphocyte, immunoglobulin, or B and T lymphocyte function were fully resistant to UTIs 3, but C3H/HeJ mice, then designated as LPS hyporesponders due to their impaired reactivity to LPS, 4 were highly susceptible to experimental UTIs 5 6 7. Their increased susceptibility was accompanied by defective neutrophil recruitment 8, suggesting that the signals from the epithelium to the neutrophils were impaired. Later studies showed that neutrophil depletion rendered normal mice as susceptible to UTIs as C3H/HeJ mice 9.
Epithelial cells are the first to encounter mucosal pathogens and form tight barriers that exclude invading microbes. In addition, they respond to microbial challenge by producing a variety of proinflammatory mediators, and set the stage for the subsequent activation of other cell types, including neutrophils. The signals involved in neutrophil migration across the mucosal barrier of the urinary tract have been defined in vitro 10. Chemokines are formed by uroepithelial cells in response to infection, and IL-8 has been shown to support neutrophil migration across infected epithelial cell layers 11. IL-8 responses occur in patients with UTIs and show a correlation to urine neutrophil numbers in individual patients 12. More recently, infection was also shown to augment CXC chemokine receptor (CXCR)1 and CXCR2 expression by human epithelial cell lines, and anti-CXCR1 Abs were shown to impair transmigration 13.
These in vitro studies postulated that inhibition of IL-8 or CXCR1 function should also impair neutrophil migration across the mucosa in vivo. As a consequence, the neutrophil-dependent antimicrobial defense should be disrupted. This hypothesis was tested using murine IL-8 receptor homologue (mIL-8Rh) knockout (KO) mice, in which the gene encoding the sole functional IL-8R homologue has been deleted 14. The receptor deletion approach was preferred over the more complex procedure of eliminating all potential IL-8 homologues in the mouse, as the chemokine response to UTIs involves several murine CXC chemokines, e.g., macrophage inflammatory protein (MIP)-2, cytokine-induced neutrophil chemoattractant (KC), and epithelial neutrophil activating peptide (ENA)-78 15. The results demonstrate a profound influence of IL-8R expression on neutrophil epithelial cell interactions, and on the resistance to UTIs.
Materials and Methods
Reagents
Tryptic soy agar and FCS were from Difco. Polyclonal rabbit anti–rat Ab, FITC-conjugated mouse IgG negative control mAb, FITC-conjugated goat anti–mouse IgG F(ab′)2 Ab, and FITC-streptavidin were from Dako. Mouse anti-hCXCR1 mAb (clone 42705.111; MAB330 and FAB330F) and mouse anti-hCXCR2 mAb (clone 48311.211; MAB331 and FAB331F) were from R&D Systems. RB6-8C5 neutrophil-specific mAb was a gift from Dr. A. Sjöstedt (Umea University, Umea, Sweden), Dr. W. Conlan (Trudeau Institute, Saranac Lake, NY), and Dr. R. Coffman, (DNAX Research Institute, Palo Alto, CA). RPMI 1640 and gentamycin were from Flow. Polymorphprep was from Nycomed Pharma. Trizol reagent was from Life Technologies. RiboQuant™ multiprobe protection assay system and hybridization buffer were from Becton Dickinson. The acrylamide gel was from National Diagnostics.
Bacteria
Escherichia coli 1177 of serotype O1:K1:H7 was isolated from a child with acute pyelonephritis. The strain was maintained by passage on tryptic soy agar plates (Difco). For experiments, Luria broth was inoculated with colonies from the tryptic soy agar plates and incubated overnight at 37°C. Under these conditions, E. coli 1177 expressed both P and type 1 fimbriae. Bacteria were harvested by centrifugation at 3,000 rpm for 10 min (RP centrifuge; Hettich Rotanta). The pellet was resuspended in 0.01 M of PBS, pH 7.2, to a concentration of 1–2 × 109 CFU/ml. The bacterial concentration was confirmed by viable counts.
Mice
Breeding pairs of Balb/c-Cmkar2tm1Mwm(mIL-8Rh KO) mice and congenic control Balb/cJ mice were purchased from The Jackson Laboratory. The Balb/c-Cmkar2tm1Mwmmice carry a targeted mutation in the mIL-8Rh gene. The mutation was introduced in the Balb/c background from the B6;129S-Cmkar2tm1Mwmdonor strain. The recipient Balb/cJ strain was backcrossed for 10 generations. At this level, the degree of similarity between unlinked loci is estimated to be >99.9%. Relevant control mice used in the study were congenic Balb/cJ mice used to create the backcross.
The genotype was confirmed by PCR on DNA extracted from tail clippings of individual mice. Validated primers specific for the wild-type mIL-8Rh gene (5′-ggT CgT ACT gCg TAT CCT gCC TCA g-3′ and 5′-Tag CCA TgA TCT TgA gAA gTCC Atg-3′) and for the inserted neomycin gene (5′-CTT ggg Tgg AgA ggC TAT TC-3′ and 5′-Agg TgA gAT gAC Agg AgA TC-3′) were used.
Experimental UTI
Female mice were used at an age of 9–16 wk. E. coli 1177 were injected into the mouse urinary tract as described previously 16. In brief, 0.1 ml of the bacterial suspension was installed into the bladder of anesthetized mice through a soft polyethylene catheter (outer diameter 0.61 mm; Clay Adams). Mice were killed by cervical dislocation under anesthesia at 2, 6, or 24 h, or 7 d after inoculation. Kidneys, bladders, and spleens were removed aseptically and homogenized in 5 ml of PBS in sterile disposable plastic bags with a Lab Stomacher® 80 homogenizer (Seward Ltd.). Infection was quantified by viable counts on serial dilutions of tissue homogenates.
Neutrophils in urine were quantified by microscopic counts before infection and killing using a hemocytometer chamber. Mice with >50 × 104 leukocytes/ml in a preinoculation sample or with a positive preinoculation urine culture were excluded.
Histology and Immunohistochemistry
Kidney and bladder tissues were rapidly frozen in liquid nitrogen and kept at −80°C until sectioned 15. In brief, sections for hematoxylin and eosin staining were fixed in 4% paraformaldehyde, rinsed in PBS, and air dried. Sections for MIP-2 staining were treated with 0.1% saponin (Sigma-Aldrich) in PBS to allow entry of the anti–MIP-2 Ab. Samples were counterstained with hematoxylin. Sections used for neutrophil staining and detection of mIL-8Rh receptor expression samples were fixed in 50 and 100% acetone and stained with a 1:200 dilution of the RB6-8C5 mAb or with anti–mIL-8Rh Abs (Santa Cruz Biotechnology, Inc.) in a 1:40 dilution for 3 h. An FITC-conjugated goat anti–rat Ab was used for detection. Sections were examined in a fluorescence microscope (Nikon) equipped with a 100 W mercury lamp (Osram) and Ploem-pac with the filter set for FITC. To prevent UV light quenching, buffered glycerol (ACO) containing 2% 1,4-diazabicyclo-(2,2,2)octane (Sigma-Aldrich) was used as a mounting medium.
Patients and Controls
Children with recurrent UTIs and acute pyelonephritis were prospectively enrolled in this study. There were 12 children (2 boys and 10 girls) with 16 documented episodes of acute pyelonephritis. Their ages were 6 mo–9 yr (median 3 yr) at the time of their first infection and 2–12 yr (median 6 yr) at the time of sampling.
Pyelonephritis was defined as a febrile infection (≥38.5°C) with a significant bacteriuria, C-reactive protein ≥20 g/liter, and with no symptoms of respiratory tract or other infections. Significant bacteriuria was defined by growth of a single strain (≥105 CFU/ml) in a midstream urine sample, or any growth in a suprapubic bladder aspirate. The patients were investigated by voiding urethrocystography, and in seven children vesicoureteric reflux was detected. On 99mTc-dimercaptosuccinic acid scintigraphy, all had renal polar uptake defects typical of pyelonephritis.
Samples for this study were obtained from each child during an infection-free interval. Control samples were obtained from children attending the pediatric outpatient clinic or admitted to the pediatric hospital for elective surgery. The 12 controls used to quantify neutrophil CXCR1 and CXCR2 expression were given the following diagnoses: asthma, pneumonia, hematuria, hydronephrosis, previous infectious mononucleosis, malignant astrocytoma, anometry, nephrotic syndrome, inguinal hernia, gastroscopy, and anal atresia. None of these children had documented episodes of UTIs. There were six boys and six girls in this control group. At the time of sampling, they were 1–11 yr (median 5.5 yr) old.
The eight controls used to quantify chemokine receptor mRNA were seen for the following diagnoses: obstipation, anal fissure, hypospadias, phimosis, and explorative laparatomy. At the time of sampling, they were 4–11 yr (median 6 yr) old. Samples for chemokine receptor mRNA analysis were obtained ∼1 yr after the first sample, again during an infection-free interval.
The study was approved by the Ethics Committee of the Lund University.
Neutrophil CXCR1 and CXCR2 Expression
Blood samples for neutrophil analysis were obtained at follow-up in the outpatient clinic during an infection-free interval. Neutrophils were purified from heparinized whole blood on a Polymorphprep density gradient (Nycomed Pharma) according to the manufacturer's instructions. The resulting cell suspension contained >97% neutrophils as determined by Wright's Giemsa stain and was 98% viable as determined by trypan blue exclusion.
Surface expression of the two IL-8Rs CXCR1 and CXCR2 was detected by confocal microscopy and flow cytometry. For confocal microscopy, cells were spun down on glass slides in a cytospin® 3 centrifuge (Shandon) at 500 rpm for 5 min and fixed in 4% paraformaldehyde. Slides were incubated with anti-hCXCR1, anti-hCXCR2 (10 μl/ml), or control mouse IgG Ab for 3 h at room temperature in a humid chamber. The slides were washed, incubated with FITC-conjugated goat anti–mouse Ab for 30 min, and examined in an MRC-1024 confocal equipment (Bio-Rad Laboratories) attached to a Nikon Eclipse E800 upright microscope.
For flow cytometry analysis neutrophils were incubated with FITC-conjugated mouse anti-hCXCR1, anti-hCXCR2, or mouse IgG Ab for 45 min on ice in the dark. The neutrophils were washed twice in PBS, and the receptor density was quantified in an EPICS profile II instrument (Coulter) with 5,000 cells counted for each sample. Instrumental settings were calibrated before each experiment using Flow-Set™ FITC-conjugated plastic beads (Coulter). Receptor expression by neutrophils in patient and control samples was compared with an adult standard run at the same time.
Neutrophil CXCR1 mRNA Levels
Blood samples for neutrophil CXCR1 mRNA quantification were obtained 12 mo after sampling for neutrophil CXCR1 and CXCR2 expression.
Total RNA was extracted from isolated neutrophils using the Trizol reagent according to the manufacturer's instructions (Life Technologies). In brief, the cells were treated with Trizol (1 ml TRIzol/5 × 106 cells) and homogenized. The cell lysates were treated with chloroform (0.2 ml/ml Trizol) and centrifuged (3,800 g for 45 min at 4°C). Total mRNA was precipitated from the aqueous phase by mixing with isopropyl alcohol (0.5 ml/ml Trizol) and centrifugation (3,800 g for 40 min at 4°C). Precipitated mRNA was washed once with 75% ethanol (1 ml/ml Trizol) and centrifuged (7,500 g for 5 min at 4°C). The pellets were air dried for 10 min and suspended in 20 μl hybridization buffer.
Detection and Quantitation of mRNA
Probe Synthesis.
Antisense RNA probes for the CXCR1 chemokine receptor (360 bp) and the two housekeeping genes L32 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were prepared according to the manufacturer's instructions. In brief, 1 μl of the template was mixed with RNasin, nucleotide GACU pool (dGTP, dATP, dCTP, and dUTP), dithiothreitol, 5× transcription buffer, [α-32P]UTP, and T7 polymerase in an Eppendorf tube. The mixture was incubated for 1 h at 37°C and the reaction was terminated by the addition of DNase and a 30-min incubation at 37°C. The RNA antisense was washed twice in an equal amount of phenol/chloroform/isoamyl alcohol (50:1), resuspended in ammonium acetate/ethanol (1:4), and incubated at −80°C for 30 min. The pellet was centrifuged at 14,000 g for 15 min, resuspended in 20 μl hybridization buffer, and used as a probe.
Sample Hybridization.
Aliquots of neutrophil mRNA were mixed with the antisense probe, and the samples were placed in a heat block prewarmed to 90°C. The temperature was allowed to slowly ramp down to 56°C and the samples were incubated for 16 h. The temperature was lowered to 37°C for 15 min before addition of 2.4 μl RNase. After RNase digestion, the samples were washed with phenol/chloroform and double-stranded RNA was extracted from the aqueous phase by addition of ammonium acetate/ethanol and incubation at −80°C for 30 min. The samples were centrifuged at 14,000 g for 5 min, and the pellets were air dried and resuspended in 5 μl 1× loading buffer.
Gel Resolution of Protected Probes.
The RNase digestion resulted in the degradation of single-stranded RNA and excess probe. The double-stranded target RNA species were protected and could be resolved by denaturing PAGE at 50 W for 3 h with 1× TBE (89 mM Tris, 89 mM boric acid, 2 mM EDTA, pH 8.0) as running buffer. The hybridization product was visualized using a PhosphorImager® (Storm® 840; Molecular Dynamics). An unprotected radiolabeled probe was loaded on the gel and used as a marker. The unprotected and protected mRNAs migrate differently but in a predictable manner that allows for the identification of individual products.
Statistical Analysis
The Mann-Whitney U test (two-tailed) and Wilcoxon's test were used. Differences were considered significant for P < 0.05.
Results
Infection Upregulates Chemokine Receptor Expression in Control Mice but Not in mIL-8Rh KO Mice.
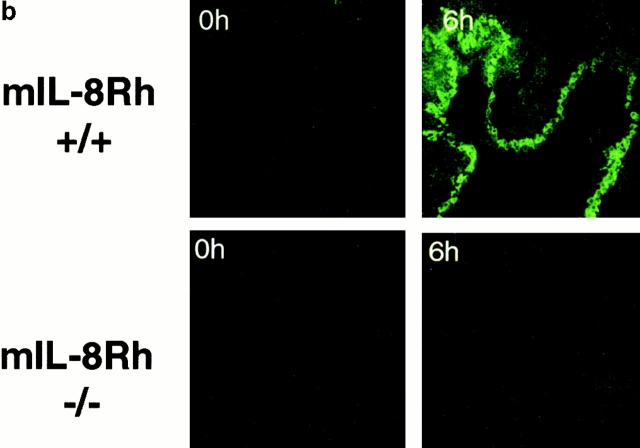
The IL-8R genotype of the KO or control mice was confirmed by PCR using primers specific for the structural gene or the neomycin cassette (Fig. 1 a). The IL-8R phenotype was detected by immunohistochemistry, using anti–mIL-8Rh mAbs to stain tissue sections obtained at various times after infection. Epithelial chemokine receptor expression was shown to increase after intravesical infection of control mice with a peak after 6 h (Fig. 1 b). Receptor expression was negative in mIL-8Rh KO mice before and at all times after infection.
Figure 1.
mIL-8Rh genotype and epithelial mIL-8Rh expression in normal and mIL-8Rh KO mice. (a) The wild-type mIL-8Rh allele (360 bp) in Balb/c mice (+/+) and the neomycin gene (280 bp) in mIL-8Rh KO mice (−/−) were detected by PCR. (b) mIL-8Rh expression in renal pelvic epithelium in control mice (+/+). There was no detectable mIL-8Rh expression in the KO mice (−/−). Kidney sections were obtained before and 6 h after infection and stained with anti–mIL-8Rh Ab. Original magnifications: ×160.
Experimental UTI Triggers a Mucosal CXC Chemokine Response.
Infection was shown to trigger an epithelial chemokine response in both control and mIL-8Rh KO mice (Fig. 2). MIP-2 staining was detected by immunohistochemistry after 2 h, with a peak intensity in control mice after 6 h. The epithelial cells were the first to respond, followed by inflammatory cells. There was no difference in the intensity of MIP-2 staining after 6 h between control and mIL-8Rh KO mice. At later times, the staining decreased in the control mice. In contrast, staining continued to increase in the KO mice, with a spread from the epithelium into underlying tissues.
Figure 2.
Epithelial chemokine secretion in normal and mIL-8Rh KO mice. MIP-2 expression in bladder sections from Balb/c (+/+) or mIL-8Rh KO (−/−) mice. A rapid epithelial MIP-2 response occurred in both mouse strains. Sections obtained before or 6 or 24 h, or 7 d after infection were stained with anti–MIP-2 Ab followed by phosphatase-conjugated goat anti–rabbit Ab and developed with Fast red substrate. Tissues were counterstained with hematoxylin and eosin. Original magnifications: ×200.
mIL-8Rh KO Mice Show Dysfunctional Neutrophil Recruitment in Response to UTI.
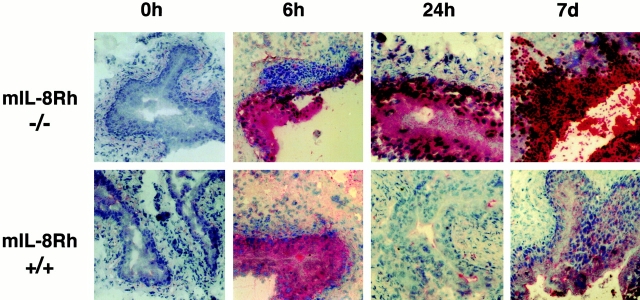
Neutrophil recruitment was quantified in tissue sections obtained at 2, 6, and 24 h, and 7 d. Infection of control mice triggered a rapid influx of neutrophils into the tissues with a peak after 6 h (Fig. 3). Neutrophils were evenly distributed from the blood vessels throughout the submucosa to the basolateral side of the bladder epithelium and were seen crossing the epithelium into the bladder lumen. By 24 h the neutrophil influx had subsided, and by 7 d no neutrophils were detected.
Figure 3.
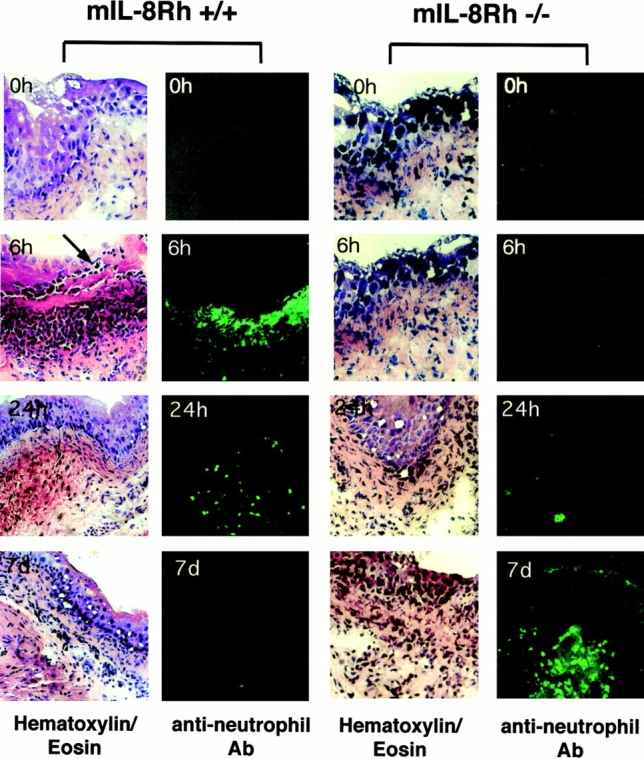
Difference in neutrophil recruitment between mIL-8Rh KO mice and control mice. Bladder sections were obtained before and 6 or 24 h, or 7 d after infection and stained with hematoxylin and eosin (left panels) and with the RB6-8C5 antineutrophil Ab (right panels). Infection of control mice caused a rapid influx of neutrophils into the bladder mucosa, and neutrophils were seen crossing into the urinary tract lumen (arrow). In mIL-8Rh KO mice, the neutrophil influx was delayed and neutrophils failed to traverse the epithelium and eventually accumulated in subepithelial tissue. Original magnifications: ×120.
Infection of mIL-8Rh KO mice caused a neutrophil response, but the neutrophil influx was delayed compared with the control mice, with detectable numbers after 24 h followed by a gradual increase until day 7 (Fig. 3). The neutrophils were trapped under the epithelium, apparently unable to cross the epithelial barrier into the urinary tract lumen. Thus, by day 7, massive numbers of neutrophils had accumulated on the basolateral side of the epithelial barrier.
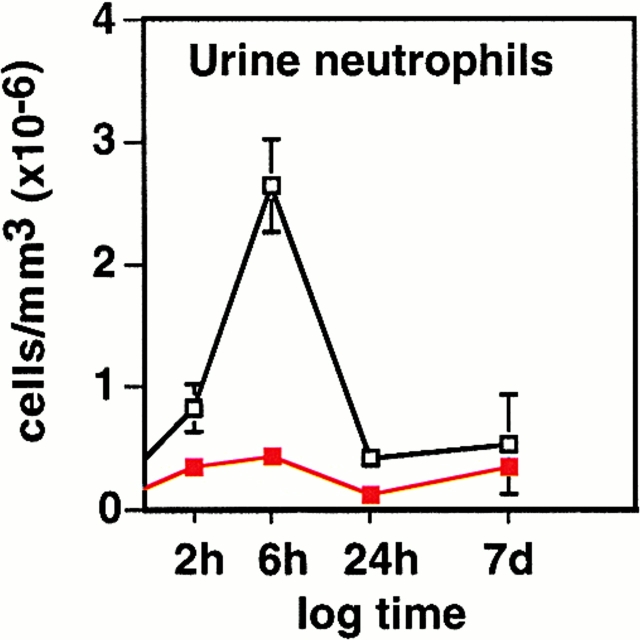
Urine neutrophil counts corroborated these findings (Fig. 4). In control mice, urine neutrophil numbers had started to increase by 2 h, reached a peak after 6 h, and returned to background levels at 24 h. In mIL-8Rh KO mice, urine neutrophil numbers remained low throughout infection (P 2h = 0.0179 and P 6h < 0.001).
Figure 4.
Impaired neutrophil recruitment in mIL-8Rh KO mice. Urine samples were obtained from the Balb/c (□) or mIL-8Rh KO mice (▪) before and at different times after infection. The neutrophil response in the control mice peaked at 6 h after infection. There was no detectable increase in PMN counts in the mIL-8Rh KO mice. Numbers are cells/ml, means ± SEM of 2 experiments; 6–10 mice per group.
These results demonstrate that the mIL-8Rh KO mutation disrupts neutrophil migration across the mucosal barrier, resulting in the entrapment of neutrophils in subepithelial tissue compartments. The mutant mice had an intact mucosal chemokine response to infection but lacked the chemokine receptors on both epithelial cells and neutrophils. As a result, neutrophil influx was delayed and transmigration across the epithelium was abrogated.
mIL-8Rh KO Mice Are Highly Susceptible to UTI and Develop Bacteremia.
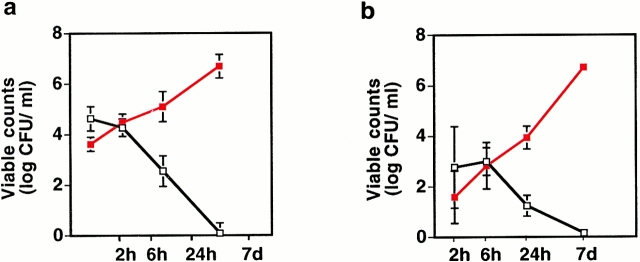
After infection of control mice with 108 CFU/ml, there was a rapid initial decline in bacterial numbers followed by a continued decline until day 7 when infection had cleared (Fig. 5). In the mIL-8Rh KO mice, bacterial numbers increased from 4.3 × 103 CFU/ml in bladders and 2.9 × 101 CFU/ml in kidneys after 2 h to 107 CFU/ml by day 7.
Figure 5.
Increased susceptibility to UTIs in mIL-8Rh KO mice. Balb/c (□) or mIL-8Rh KO (▪) mice were inoculated with E. coli 1177, and infection was monitored by viable counts of kidney and bladder homogenates after 2, 6, or 24 h, or 7 d. The mIL-8Rh KO mice failed to clear the bacterial inoculum. Numbers are CFU/ml, means ± SEM of 2 experiments; 6–10 mice per group.
In parallel with the increase in bacterial numbers, the mIL-8Rh KO mice developed symptoms of disease. They stopped moving around the cage, had ruffled fur, shivered, and were lethargic. Blood cultures became positive, indicating systemic spread of bacteria. Cultures from livers and spleens of mIL-8Rh mutant mice were positive on day 7. Cultures from livers and spleens of control mice were sterile.
These findings demonstrated that IL-8R expression influences the susceptibility to E. coli UTI.
Decreased Neutrophil CXCR1 Expression in Children Prone to Acute Pyelonephritis.
The susceptibility to UTI is known to vary between individuals, but no molecular mechanisms have been identified that explain this variation. The pronounced effect on disease susceptibility and pathology of the mIL-8Rh KO mutation in the KO mice suggested that similar dysfunctions might influence the neutrophil response and disease susceptibility in the human urinary tract. For this reason, we examined neutrophils from children with recurrent UTIs for the surface expression of the two high affinity IL-8 receptors CXCR1 and CXCR2, and the CXCR1 mRNA. The patients were compared with age-matched controls. While the patients had a history of recurrent UTI, they were free of infection at the time of sampling.
Neutrophils were obtained from the patients on two occasions with ∼1-yr interval. On the first occasion, surface expression of the CXCR1 or CXCR2 proteins was quantified by flow cytometry, using specific Abs. The morphology of the stained cells was inspected by confocal microscopy. On the second occasion, chemokine receptor–specific mRNA was quantified in the cells. Cells from the age-matched controls were treated in the same manner, with different control individuals on the first (nos. 1–12, Table ) and the second (see nos. 13–20 in Table ) sampling occasions.
Table 1.
Decreased CXCR1 Expression in Children with Acute Pyelonephritis
| Patientno. | Patient age atfirst pyelonephritis | Age at testing | ▵CXCR1 adult | Controlno. | Control ageat testing | ▵CXCR1 adult |
|---|---|---|---|---|---|---|
| yr | yr | yr | ||||
| 1 | 1 | 2 | −1.72 | 1 | 1 | −0.28 |
| 2 | 1 | 2 | −6.52 | 2 | 2 | −0.55 |
| 3 | 2 | 3 | −1.68 | 3 | 2 | 0.37 |
| 4 | 3 | 4 | 0.99 | 4 | 3 | 0.17 |
| 5 | 1.5 | 5 | −0.23 | 5 | 5 | 0.05 |
| 6 | 0.5 | 6 | −0.43 | 6 | 5 | 2.15 |
| 7 | 5 | 6 | 0.47 | 7 | 6 | 0.12 |
| 8 | 5 | 7 | −1.81 | 8 | 6 | 0.16 |
| 9 | 5 | 7 | 1.04 | 9 | 7 | −0.67 |
| 10 | 9 | 10 | −1.44 | 10 | 9 | 0.27 |
| 11 | 8 | 10 | −4.40 | 11 | 9 | 1.26 |
| 12 | 1 | 12 | −0.18 | 12 | 12 | 0.96 |
| Mean | −1.33 | P < 0.03 | 0.33 | |||
CXCR1 expression on neutrophils from patients with a history of acute pyelonephritis and age-matched controls as determined by flow cytometry.
Table 2.
Decreased CXCR1 mRNA in Children with Acute Pyelonephritis
| Patient no. | Patient ageat testing | mRNACXCR1/GAPDH | Control no. | Control ageat testing | mRNACXCR1/GAPDH |
|---|---|---|---|---|---|
| yr | yr | ||||
| 2 | 2 | 2.07 | 13 | 4 | 7.34 |
| 4 | 4 | 1.08 | 14 | 8 | 7.14 |
| 6 | 6 | 3.87 | 15 | 6 | 4.37 |
| 7 | 6 | 2.57 | 16 | 5 | 4.67 |
| 9 | 7 | 3.79 | 17 | 6 | 5.85 |
| 10 | 7 | 0.64 | 18 | 11 | 7.65 |
| 11 | 10 | 3.75 | 19 | 6 | 6.77 |
| 12 | 10 | 3.79 | 20 | 10 | 11.2 |
| Mean | 3.16 | P < 0.001 | 6.96 | ||
CXCR1 mRNA in neutrophils from patients with a history of acute pyelonephritis or age-matched controls.
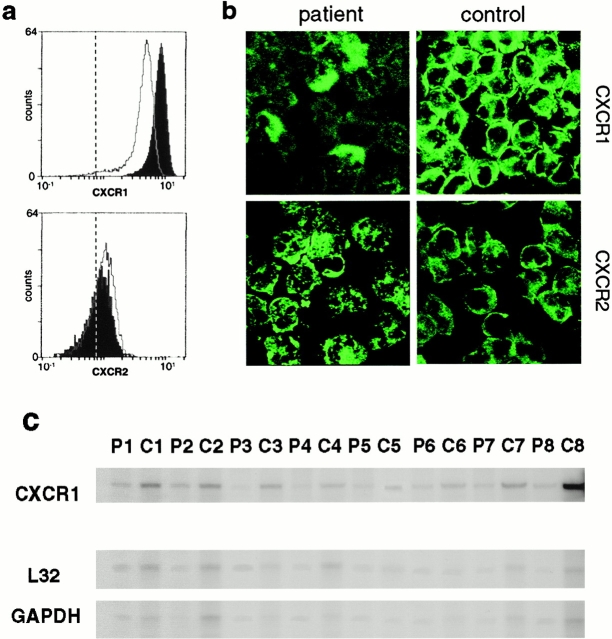
Neutrophil CXCR1 receptor expression was low in children with pyelonephritis compared with the age-matched controls (P < 0.03). This is exemplified in Fig. 6 a for patient 1 and control 1. By confocal microscopy, fewer CXCR1+ cells were observed in the patients than in the controls (Fig. 6 b), and flow cytometry showed reduced CXCR1 fluorescence intensity of the patient neutrophils compared with the controls. Pairwise analysis of CXCR1 expression on neutrophils from 12 patients with recurrent pyelonephritis and 12 age-matched controls showed consistently lower CXCR1 expression in the patients (P = 0.0269; Table ).
Figure 6.
Defective CXCR1 surface expression on neutrophils from children with acute pyelonephritis. (a) CXCR1 receptor expression, quantified by flow cytometry, was reduced in patient 1 (open peak) compared with control 1 (filled peak; for fluorescence intensity see Table ). CXCR2 receptor expression did not differ between the patient and control samples. The dotted line shows the background fluorescence of the IgG control Ab. (b) Confocal images illustrating the difference in neutrophil CXCR1 expression between neutrophils from patient 1 (top left) and control 1 (top right). There was no difference in CXCR2 expression between the patient and the control cells (bottom panels). (c) Decreased CXCR1 mRNA in neutrophils from children with acute pyelonephritis. CXCR1 mRNA was quantified by the RNA protection assay. Samples from patients (P1, P2–P8) or controls (C1, C2–C8) were loaded in alternate wells. CXCR1 mRNA was reduced in all of the patients compared with the controls. The bottom two lanes show mRNA for the housekeeping genes L32 and GAPDH. Data are summarized in Table .
The results demonstrated heterogeneity in CXCR1 expression among children with a history of acute pyelonephritis and showed that these patients have a large fraction of neutrophils with low surface CXCR1 expression. This difference was restricted to CXCR1; there was no difference in CXCR2 staining between patients and controls.
Difference in CXCR1 mRNA Levels between Patients and Controls.
Neutrophils were obtained ∼1 yr later from eight of the patients in Table and from eight new age-matched control children. Total mRNA was extracted and CXCR1 mRNA was amplified using specific primers. The message was quantified in relation to two different housekeeping genes. A pairwise comparison of mRNAs from patients and controls is shown in Fig. 6/Table . In every case the patient cells contained lower CXCR1 mRNA levels than the controls (P < 0.001).
Stability of the Low CXCR1 Expression Phenotype.
As chemokine receptor expression has been shown to vary over time, we addressed the stability of the CXCR1low phenotype of the patients. An individual with low CXCR1 expression was analyzed biweekly over a 1-yr period and was compared with an individual with normal CXCR1 levels. The low expression of the CXCR1 receptor was shown to be stable over time (data not shown). Furthermore, the low CXCR1 expression by the patients was detected at least twice with a 1-yr interval.
Discussion
The susceptibility to experimental UTI was examined in mIL-8Rh KO mice lacking the sole functional human IL-8Rh. A distinct and highly interesting phenotype was detected. The mice were extremely susceptible to UTI, apparently unable to eliminate the bacterial inoculum. They developed bacteremia and symptoms of severe disease. In addition, a dysfunctional neutrophil response was observed. Neutrophils were recruited to the site of infection but were unable to cross the epithelial barrier and were trapped in the tissues. The accumulation of neutrophils caused severe tissue destruction, and surviving mice developed renal scarring. This dramatic phenotype in the KO mouse, and the obvious parallels to human disease, encouraged us to examine CXCR expression in children with documented susceptibility to UTI. Patients with a history of acute pyelonephritis and recurrent UTIs were enrolled and their neutrophils were examined for CXCR expression. Both cell surface CXCR1 expression and CXCR1-specific mRNA levels were low in the patient neutrophils compared with age-matched controls. The findings suggest a human counterpart to the deficiency of the mIL-8Rh KO mice, and provide a first molecular handle on disease susceptibility of patients with acute pyelonephritis and renal scarring.
Mucosal infections give rise to a rapid neutrophil response 8. Pathogens activate mucosal chemokine production, resulting in the recruitment of neutrophils to the epithelial barrier and in neutrophils crossing the epithelium into the lumen 17 18. The molecular determinants of this process have been studied in vitro. Human uroepithelial cells secrete IL-8, and this chemokine supports neutrophil migration across infected epithelial layers 11. Chemokines exert their action by coupling to G protein–anchored receptors. While multiple classes of chemokine receptors have been described, the CXCRs are most essential for neutrophil recruitment. Urinary tract pathogens were recently shown to also enhance the expression of chemokine receptors, and neutrophil migration across epithelial cell layers was found to be CXCR1 dependent 13.
Mice have a single functional receptor for IL-8. This mIL-8Rh shares 69 and 71% amino acid identity with the human IL-8 receptors CXCR1 and CXCR2 19. Cacalano et al. deleted the single gene encoding mIL-8Rh by insertion of a neomycin cassette, and generated the mIL-8Rh KO mouse 14. Neutrophil chemotaxis in response to CXC chemokines (human IL-8 and MIP-2) was selectively abrogated in these mice, but responses to non-CXC ligands are retained. In this study we showed that epithelial CXCR expression increases after infection of normal mice. Infection of the mIL-8Rh KO mice failed to stimulate a CXCR response and neutrophil migration across the epithelial barrier was completely abrogated. These results demonstrate that chemokine receptor expression determines the fate of neutrophils at the mucosal barrier. It may be speculated that chemokine receptors influence neutrophil migration at other mucosal sites, and that epithelial chemokine receptors prevent activated neutrophils from accumulating in the tissues by directing them into the lumen.
The mIL-8Rh KO mice had intact chemokine production, but with time, a difference in tissue distribution of MIP-2 was noticed compared with the control mice. The epithelial MIP-2 response and the secretion of MIP-2 into the urine were similar in mIL-8Rh KO and control mice during the first hours after infection, showing that the receptor deficiency did not influence the chemokine response per se. At later times, the MIP-2 response decreased in the controls, but the mIL-8Rh KO mice continued to produce MIP-2, suggesting impaired downregulation and/or continued stimulation of this response. In view of the impaired bacterial clearance in the mIL-8Rh KO mice, we propose that the bacteria provide a continuing stimulus for production of these chemokines.
The drastic difference in susceptibility to infection between the control mice and the mIL-8Rh KO mice was seen using several different end points. Tissue counts from control mice showed a gradual decrease in bacterial numbers, with complete clearance after 7 d. The mice did not have positive blood cultures and appeared asymptomatic. Furthermore, they did not develop tissue pathology. The KO mice, in contrast, showed gradually increasing bacterial counts in kidneys and bladders until day 7. They became septic, developed symptoms of systemic disease, and had to be killed. Surviving mice developed tissue pathology resembling patients with renal scarring. These observations emphasize that the deletion of the mIL-8R gene has fundamental effects on the susceptibility to bacterial infection of the urinary tract, and that the KO mouse is an excellent model for studies of acute pyelonephritis and renal scarring. Earlier studies have shown that germ-free mIL-8 KO mice have increased susceptibility to Candida infection after peroral challenge and develop acute systemic candidiasis after systemic challenge 20. While the mIL-8Rh KO mice were more resistant during the early stage of Listeria monocytogenes infection, they later demonstrated evidence of chronic infection 21. These findings illustrate the importance of chemokine receptors and directed neutrophil migration for host resistance to different microbial infections.
In addition, the results reemphasized the importance of neutrophils as the crucial effector cells of the urinary tract. This was first proposed after studies in LPS hyporesponder mice 4. C3H/HeJ mice are highly susceptible to UTI and other gram-negative infections 5 6 7. Their increased susceptibility to UTI coincided with a defective neutrophil response 8, suggesting that these cells are important for bacterial clearance from the urinary tract. Subsequent studies confirmed the role of neutrophils for the urinary tract defense 9. Neutrophil depletion of resistant mice was shown to render them susceptible to infection, with impaired bacterial clearance from bladders or kidneys during the early phase of infection. This study showed that susceptibility may be caused not only by the absence of neutrophils, but by the chemotactic process. The chemokine receptor deficiency did not abrogate neutrophil recruitment into the tissues but aborted their migration to the site where bacteria were to be killed.
Urinary tract infections are among the most prevalent bacterial infections in humans. Asymptomatic bacteriuria occurs in 1% of schoolgirls, 2% of pregnant women, and 15–20% of elderly men and women 22. Symptomatic infections are exceedingly common, with acute cystitis being more frequent than acute pyelonephritis. Before the antibiotic era, acute pyelonephritis caused substantial mortality, and even today UTI continues to be a major clinical problem in terms of both prevalence and long-term consequences. Failure to resolve the local phase of acute infection may cause renal scarring in children and gram-negative septicemia in adults.
The marked individual differences in susceptibility to UTI have been recognized for decades. Defects of the urine flow and epithelial receptor expression are the two main host variables proposed to account for these differences. Urodynamic defects open up the urinary tract and allow bacteria of low virulence to establish. For example, vesicoureteric reflux increases the severity of kidney infection in some, but not all patients 23. Epithelial receptor expression influences the adhesive phenotype of the infecting strain 24. For example, the expression of epithelial cell receptors for P fimbriae is influenced by the P blood group 23 25 26. However, urodynamic defects do not explain the basic difference in susceptibility to acute pyelonephritis between individual hosts, and the variation in epithelial receptor expression can help select the infecting strain, but does not fundamentally alter the susceptibility to UTI.
This study proposes that the neutrophil-mediated defense is essential for resistance to UTI, and that the functional state of the neutrophils is influenced by chemokine receptor expression in the urinary tract. The enrolled children had experienced several episodes of UTI with at least one documented episode of acute pyelonephritis, and had dimercaptosuccinic acid–verified renal involvement. In spite of the small numbers, we found significant differences in receptor expression between patients and controls, suggesting that these differences are highly relevant. It may be discussed that the low receptor expression in the children was secondary to the influence of infection, or was a preexisting susceptibility factor of the individual. The samples were obtained during an infection-free interval, at least 6 mo after termination of antibiotic treatment resulting in clinical and microbiological cure, suggesting that there should be no influence on chemokine receptor expression of bacterial products like LPS and host response molecules like TNF-α. By repeating the analysis of receptor expression with a 1-yr interval, and by using two independent techniques to quantify the phenotype, we could show that the low expression was stable over time and was not caused by ongoing infection. Additionally, biweekly sampling of an individual with low levels of CXCR1 over a 1-yr period revealed that the CXCR1low phenotype was stable.
The results suggest that children with recurrent pyelonephritis have a reduced expression of CXCR1 that is stable over time. We propose that susceptibility is caused by inefficient bacterial clearance due to impairment of the innate host defense, and identify low chemokine receptor expression as a mechanism underlying this effect.
Acknowledgments
This study was supported by grants from The Swedish Medical Research Council (K97-06X-07934), The Crafoord, Wallenberg (97.123), and Österlund Foundations, and The Royal Physiographic Society, and by an Unrestricted Grant from Bristol-Myers Squibb. B. Frendéus was supported by the Swedish Foundation for Strategic Research, Infection, and Vaccinology program (grant 125/97).
Footnotes
Abbreviations used in this paper: CXCR, CXC chemokine receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KO, knockout; mIL-8Rh, murine IL-8 receptor homologue; MIP, macrophage inflammatory protein; UTI, urinary tract infection.
B. Frendéus and G. Godaly contributed equally to this work.
References
- Flanagan S.P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- Bosma G.C., Custer R.P., Bosma M.J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Svanborg-Edén C., Hagberg L., Briles D., McGhee J., Michalek S. Susceptibility to Escherichia coli urinary tract infection and LPS responsiveness. In: Skamene E., editor. Genetic Control of Host Resistance to Infection and Malignancy, Vol. 3. Alan R. Liss, Inc.; New York: 1985. pp. 385–398. [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M.Y., Huffel C.V., Du X., Birdwell D., Alejos E., Silva M., Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr micemutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Sultzer B.M. Genetic control of leucocyte responses to endotoxin. Nature. 1968;219:1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- O'Brien A.D., Rosenstreich D.L., Scher I., Campbell G.H., MacDermott R.P., Formal S.B. Genetic control of susceptibility to Salmonella typhimurium in micerole of Lps gene. J. Immunol. 1980;124:20–24. [PubMed] [Google Scholar]
- Hagberg L., Briles D., Svanborg-Edén C. Evidence for separate genetic defects in C3H/HeJ and C3HeB/FeJ mice that affect the susceptibility to gram-negative infections. J. Immunol. 1985;134:4118–4122. [PubMed] [Google Scholar]
- Shahin R.D., Engberg I., Hagberg L., Svanborg-Eden C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- Haraoka M., Hang L., Frendéus B., Godaly G., Burdick M., Strieter R., Svanborg C. Neutrophil recruitment and resistance to mucosal infection with uropathogenic Escherichia coli . J. Infect. Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- Parkos C., Delp C., Arnout M.A., Madara J. Neutrophil migration across cultured intestinal epithelium. J. Clin. Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaly G., Proudfoot A.E.I., Offord R.E., Svanborg C., Agace W. Role of epithelial interleukin-8 (IL-8) and neutrophil IL-8 receptor A in Escherichia coli-induced transuroepithelial neutrophil migration. Infect. Immun. 1997;65:3451–3456. doi: 10.1128/iai.65.8.3451-3456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agace W., Hedges S., Ceska M., Svanborg C. IL-8 and the neutrophil response to mucosal gram negative infection. J. Clin. Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaly G., Hang L., Frendéus B., Svanborg C. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knock out mice. J. Immunol. 2000;In press doi: 10.4049/jimmunol.165.9.5287. [DOI] [PubMed] [Google Scholar]
- Cacalano G., Le J., Kikly K., Ryan A.M., Pitts-Meek S., Hultgren B., Wood W.I., Moore M.W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- Hang L., Haraoka M., Agace W.W., Burdick M., Strieter R., Svanborg C. MIP-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J. Immunol. 1999;162:3037–3044. [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg-Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agace W., Hedges S., Andersson U., Andersson J., Ceska M., Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli . Infect. Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agace W., Connell H., Svanborg C. Host resistance to urinary tract infection. In: Mobley H., Warren J., editors. Urinary Tract InfectionMolecular Pathogenesis and Clinical Management. American Society for Microbiology; Washington, DC: 1995. pp. 221–243. [Google Scholar]
- Lee J., Cacalano G., Camerato T., Toy K., Moore M.W., Wood W.I. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Balish E., Wagner R.D., Vazquez-Torres A., Jones-Carson J., Pierson C., Warner T. Mucosal and systemic candidiasis in IL-8Rh−/− BALB/c mice. J. Leukoc. Biol. 1999;66:144–150. doi: 10.1002/jlb.66.1.144. [DOI] [PubMed] [Google Scholar]
- Czuprynski C.J., Brown J.F., Steinberg H., Carroll D. Mice lacking the murine interleukin-8 receptor homologue demonstrate paradoxical responses to acute and chronic experimental infection with Listeria monocytogenes . Microb. Pathog. 1998;24:17–23. doi: 10.1006/mpat.1997.0169. [DOI] [PubMed] [Google Scholar]
- Kunin C. Detection, Prevention and Management of Urinary Tract Infections 1987. Lea & Febiger; Philadelphia, PA: pp. 419 pp [Google Scholar]
- Lomberg H., Hanson L., Jacobsson B., Jodal U., Leffler H., Svanborg-Edén C. Correlation of P blood group phenotype, vesicoureteral reflux and bacterial attachment in patients with recurrent pyelonephritis. N. Engl. J. Med. 1983;308:1189–1192. doi: 10.1056/NEJM198305193082003. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipids as receptors for Escherichia coli lectins or adhesins. In: Mirelman D., editor. Microbial Lectins. John Wiley & Sons; New York: 1986. pp. 84–96. [Google Scholar]
- Stapleton A., Nudelman E., Clausen H., Hakomori S., Stamm W.E. Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on histo-blood group secretor status. J. Clin. Invest. 1992;90:965–972. doi: 10.1172/JCI115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Winberg J., Lundblad S., Svensson S., Cedergren B. The Pk antigen as a receptor for the hemagglutination of pyelonephritogenic Escherichia coli . FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 1980;7:297–302. [Google Scholar]