Abstract
Interleukin (IL)-12 may be secreted as a bioactive T helper type 1 (Th1) cell–inducing heterodimer, as a monomer, or as an antagonistic homodimer. We analyzed the IL-12 produced by mouse splenic dendritic cells (DCs), human thymic DCs, and cultured human monocyte-derived DCs. IL-12 production required both a microbial or T cell–derived stimulus and an appropriate cytokine milieu. The different IL-12 forms were differentially regulated by the cytokines present rather than the stimulus used. IL-4 alone or together with granulocyte/macrophage colony-stimulating factor or interferon γ effectively enhanced the production of the bioactive heterodimer and selectively reduced the antagonistic homodimer of IL-12. Therefore, IL-4, the major Th2-driving cytokine, provides a negative feedback causing DCs to produce the major Th1-inducing cytokine, bioactive IL-12.
Keywords: granulocyte/macrophage colony-stimulating factor, homodimeric interleukin 12, T helper type 1, T helper type 2, interferon γ
Introduction
IL-12 is a cytokine composed of two disulfide-linked subunits of 35 kD (p35) and 40 kD (p40), which are encoded by two separate genes. These associate to form the bioactive heterodimer of 70 kD (p70 1 2 3). IL-12 p70 acts on T cells and NK cells by inducing proliferation, enhancing cytotoxicity, and promoting IFN-γ production. It is the most important cytokine for induction of Th1 cells and plays a major role in resistance to bacterial, viral, and parasitic infections, as well as to tumors 4. Furthermore, as an inflammation-promoting cytokine, IL-12 is considered to be involved in the onset or progress of various autoimmune diseases 5.
Besides the biologically active heterodimer p70, IL-12 can also be secreted in vitro and in vivo in large excess as a p40 monomer or (p40)2 homodimer 6 7. In both mice and humans, (p40)2 was shown to bind to the IL-12 receptor and antagonize p70-induced T cell proliferation and IFN-γ production of NK and T cells 8 9 10. In addition, recombinant (p40)2 was able to ameliorate IL-12–dependent effects in autoimmune diabetes, endotoxin-induced shock, and rejection after transplantation 7 11 12 13. Similarly, expression of the IL-12 p40 gene in vivo led to prolonged syngeneic islet graft survival and impaired Th1 responses 14 15 16. Interestingly, the growth of certain hybridomas in vivo was accompanied by elevated levels of (p40)2 secretion and suppression of natural immunity 17. Thus, the biological activity of IL-12 may ultimately be determined by the ratio of p70 to (p40)2, as well as by the absolute levels of the p70 form.
Many APCs produce some IL-12, but dendritic cells (DCs) have been shown to be major producers, and their intimate contact with T cells during initiation of a primary immune response implicates them as major inducers of Th1 versus Th2 polarization 18 19. The control of DC IL-12 production is therefore of importance in immune system regulation. DCs must develop to a certain stage before they are capable of IL-12 production. The actual production of IL-12 is highly dependent on both an IL-12 stimulus and the cytokine milieu during stimulation. In DCs, the major stimuli for IL-12 production are of microbiological origin or are T cell derived, in the latter case mainly the CD40–CD40 ligand (CD40L) interaction 19 20 21 22. Of the cytokines, IFN-γ and GM-CSF are described as enhancers of IL-12 production, whereas IL-10, prostaglandins, and transforming growth factor β are described as potent inhibitors of IL-12 production 20 23 24 25 26. The role of IL-4 is less clear, since it is reported to have a positive priming effect on monocytes if used at least 1 d before the stimulation for IL-12 production 26 27 28, but if added simultaneously with the IL-12 stimulus, then IL-4 is reported to be inhibitory 20 26 27 29 30 31. Also, one report suggests that IL-4, if used together with CD40L, has an enhancing effect on IL-12 production, but if used together with LPS and IFN-γ, has an inhibiting effect 32. Not all of these reports considered the balance between the various IL-12 forms, and most used macrophages, blood monocytes, or culture-derived DCs. In addition, a role for IL-4 in priming or maturing DCs was not always separated from a role in the actual induction of IL-12 secretion.
In this study we have analyzed the IL-12 p40, p70, and (p40)2 production of freshly isolated and cultured mouse and human DCs in rapid response to various stimuli and under the influence of the cytokines IL-4, GM-CSF, and IFN-γ. To our surprise, IL-4 inhibited the antagonistic (p40)2 form but increased the bioactive p70 form of IL-12. By contrast, IFN-γ alone predominantly increased the antagonistic (p40)2 form. Furthermore, IL-4 and IFN-γ together showed a strong synergism in increasing p70, while maintaining the IL-4 block of production of the antagonistic (p40)2. Overall, this resulted in an optimal production of agonist p70 with minimal antagonist (p40)2. These effects of IL-4 and IFN-γ were observed with a large variety of microbiological or T cell–dependent stimuli in both mouse and human DCs, freshly isolated or cultured. This form of regulation leads to a multiple negative feedback model with DCs opposing the tendency towards T cell cytokine polarization.
Materials and Methods
Mice.
C57BL/6J wehi mice were bred under specific pathogen-free conditions in the animal facility of The Walter and Eliza Hall Institute.
Cytokines, Abs, and Reagents.
Murine rGM-CSF, murine rIL-4, human rGM-CSF, and human trimeric CD40L were gifts from Immunex Corporation (Seattle, WA). Human rIL-4 (used for the monocyte-derived DC [MoDC] experiments) was provided by Schering-Plough Research Institute (Kenilworth, NJ). Rat rIFN-γ (bioactive in mice), human rIFN-γ, and human rIL-4 (used for the human thymic DC experiments) were purchased from PeproTech. Human rIL-12 p70 and human rIL-12 p40 were purchased from BD PharMingen. Murine rIL-12 p70 and murine rIL-12 (p40)2 were purchased from R&D Systems. LPS and polyinosinic-polycytidylic acid (poly I:C) were purchased from Sigma-Aldrich. Flt3 ligand (Flt3-L) was produced in this laboratory from the CHO-flk2 cell line provided by Prof. Nic Nicola (The Walter and Eliza Hall Institute). Pansorbin (fixed and heat-killed Staphylococcus aureus Cowan I [SAC]) was purchased from Calbiochem-Novabiochem. An oligonucleotide containing a CpG motif (CpG) was synthesized by GeneWorks according to a published sequence (CpG1668 33). The hybridoma producing the mAb for human CD40 (G28.5) was obtained from the American Type Culture Collection. The hybridoma producing the mAb for mouse CD40 (FGK45.5) was provided by Dr. A. Rolink (Basel Institute for Immunology, Basel, Switzerland). The hybridoma producing a neutralizing mAb for mouse IL-4 (BVD4-1D11) was provided by Dr. J. Abrams (DNAX Research Institute, Palo Alto, CA). The fluorescence-conjugated Ab used for selecting DCs was FITC-conjugated anti-CD11c (N418). mAbs were purified and labeled as published elsewhere 34 35.
Mouse DC Preparation.
Pools of spleens were used for DC extraction as described in detail elsewhere 34. In brief, organs were chopped, digested with collagenase, and treated with EDTA. Light density cells were collected by a density centrifugation procedure. Non-DC lineage cells were depleted by coating them with a mixture of mAbs and then depleting the coated cells with magnetic beads coupled to anti–rat IgG 35. The DC-enriched preparations were then immunofluorescent stained with an anti-CD11c–FITC mAb. Propidium iodide was added in the final wash to label dead cells. For cytometric sorting, the cells were gated for DC characteristics, namely high forward and side scatter and bright staining for CD11c, with propidium iodide–labeled cells excluded. The purity of the sorted DCs was >98%.
Stimulation of Isolated Mouse DCs for IL-12 Production.
Sorted splenic mouse DCs (105) were cultured in 96-well round-bottomed plates in a final volume of 200 μl with an IL-12 stimulus (1 μg/ml LPS, 25 μg/ml anti-CD40 mAb, 10 μg/ml poly I:C, 250 nM CpG, 5–20 μg/ml SAC, or mixes containing all stimuli listed) in the presence or absence of IL-4 (100 U/ml or titrated), GM-CSF (200 U/ml or titrated), and IFN-γ (20 ng/ml or titrated). After an 18–23-h culture the supernatant was collected, separated from cells by centrifugation, and stored until analysis at −70°C.
Stimulation of Mice for IL-12 Production In Vivo.
Mice were administered LPS (10 μg) or CpG (10 nmol) with or without the addition of IL-4 (0.5 μg) via intraperitoneal injection. These reagents were administered in PBS containing 1% FCS. Control mice received intraperitoneal injections of PBS/FCS alone. Mice were killed after 4 h, blood was taken, and the serum was collected for IL-12 assay by ELISA.
Generation of Human MoDCs.
For MoDC generation, CD14+ monocytes were affinity purified using the MACS CD14 isolation kit (Miltenyi Biotec) and 5 × 105 cells were cultured in 1 ml RPMI 1640, 10% FCS, rGM-CSF (40 ng/ml), and rIL-4 (500 U/ml) in 24-well flat-bottomed plates. By day 7, when MoDCs represented >90% of cultured cells, the wells were pooled and cells were washed extensively to eliminate any IL-4 carryover. These MoDCs would be classed as relatively immature, expressing only moderate levels of HLA-DR and HLA-A, -B, or -C, only low levels of CD86, and no detectable CD80 or CD83.
Stimulation of Human MoDCs for IL-12 Production.
Washed MoDCs (104 or 105 per 200 μl) were plated with CD40L (1 μg/ml) or a mix of stimuli (1 μg/ml LPS, 25 μg/ml anti-CD40 mAb, 100 μg/ml poly I:C, 1 μM CpG) and the cytokines (10 ng/ml rIL-4, 50 ng/ml rGM-CSF, 20 ng/ml rIFN-γ as specified in the figure legends) and cultured for 1 or 3 d. The cell-free supernatants were stored until analysis at −70°C.
Isolation of Human Thymic DCs.
Human thymus samples were discarded tissue from newborn children undergoing corrective cardiac surgery. The isolation protocol was similar to the protocol used for mouse DCs. In brief, the tissue was cut into small fragments, suspended in 10 ml of RPMI 1640 containing 2% FCS, collagenase, and DNase, then digested with intermittent agitation for 15 min at 37°C followed by 5 min at room temperature with constant agitation. To disrupt DC–T cell complexes, EDTA was added to the digest, and incubation with agitation was continued for 5 min. The suspension was then passed through a stainless steel sieve to remove aggregates. All remaining procedures were performed at 0–4°C. The cells were recovered from the digest by centrifugation, then the pellet was immediately resuspended in 1.068 g/cm3 isoosmotic Nycodenz medium (Nycomed Pharma), and a low density fraction was collected after centrifugation at 1,700 g for 10 min. The low density fraction was diluted in balanced salt solution (BSS) containing EDTA, and the cells were recovered by centrifugation. The cells were then incubated for 25 min with a mixture of mAbs (all from American Type Culture Collection) including anti-CD3 (OKT3), anti-CD8 (OKT8), anti-CD7 (3A1), anti-CD15 (WEMG1), anti-CD19 (FMC-63), anti-CD20 (B1), and anti–glycophorin A (10F7MN), in EDTA-BSS containing 2% human serum. After incubation, the cells coated with mAbs were removed by two cycles of sheep anti–mouse Ig-coupled magnetic beads (Dynabeads; Dynal). The first cycle was at 3:1 and the second at 6:1 bead to cell ratio. The cells were then kept overnight at 4°C in EDTA-BSS–10% FCS. The next morning, the cells were incubated for 25 min at 4°C with Cy5-conjugated anti–HLA-DR (2.06; American Type Culture Collection) and biotinylated anti-CD11b (OKM1; American Type Culture Collection) in EDTA-BSS containing 2% human serum. After two washes, the cells were incubated with streptavidin–Texas red (Amersham Pharmacia Biotech). Mature DCs were then sorted on the basis of high HLA-DR and negative CD11b fluorescence, together with characteristic high forward and side light scatter, using a FACStarPLUS™ (Becton Dickinson). Purity was always >98% after reanalysis.
Stimulation of Human Thymic DCs for IL-12 Production.
Isolated thymic DCs (5 or 9 × 104) were cultured in 96-well round-bottomed plates in a final volume of 200 μl with human rGM-CSF (50 ng/ml), human rIFN-γ (20 ng/ml), and CD40L (1 μg/ml) in the presence or absence of human rIL-4 (20 ng/ml) for 2 or 3 d. The cell-free supernatants were stored until analysis at −70°C.
IL-12 Polypeptide Analysis by Western Transfer and Immunoblotting.
Aliquots of DC culture supernatants or mouse serum were subjected to SDS-PAGE (9% acrylamide) under nonreducing conditions. The electrophoresed proteins were transferred onto Immobilon-P membrane (Millipore) according to the manufacturer's instructions. Membranes were blocked with 5% BSA in PBS overnight at 4°C. IL-12 polypeptides were detected by incubation with biotinylated C17.8 (anti–IL-12 p40) mAb (0.5 μg/ml in 1% BSA, 0.05% Tween 20 in PBS) for 1 h at 4°C, followed by incubation with streptavidin–horseradish peroxidase conjugate (Amersham Pharmacia Biotech) dilution in 1% BSA, 0.05% Tween 20 in PBS for 1 h at 4°C. The membranes were then developed with Supersignal West Pico Chemiluminescent Substrate (Pierce Chemical Co.), according to the manufacturer's instructions.
Analysis of Mouse and Human IL-12 by ELISA.
Aliquots of DC culture supernatants or mouse serum were assayed by two site ELISA. In brief, 96-well polyvinyl chloride microtiter plates (Dynatech Laboratories) were coated with the appropriate purified capture mAb, namely R2-9A5 (anti–mouse IL-12 p70; American Type Culture Collection), 20C2 (anti–human IL-12 p70; BD PharMingen), C15.6 (anti–mouse IL-12 p40; BD PharMingen), or C8.3 (anti–human IL-12 p40; BD PharMingen). Cytokine binding was then detected with the appropriate biotinylated detection mAb, namely R1-5D9 (anti–mouse IL-12 p40; American Type Culture Collection), C8.6 (anti–human IL-12 p40; BD PharMingen), or C17.8 (anti–mouse IL-12 p40; hybridoma provided by L. Schofield, The Walter and Eliza Hall Institute). The readout was then obtained by using streptavidin–horseradish peroxidase conjugate (Amersham Pharmacia Biotech) and a substrate solution containing 548 μg/ml 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS; Sigma-Aldrich) and 0.001% hydrogen peroxide (Ajax Chemicals) in 0.1 M citric acid, pH 4.2, followed by scanning the optical density at 405–490 nm. Since the mouse IL-12 p40 ELISA used also detects mouse IL-12 p70, the amount of mouse IL-12 p70 obtained with the mouse IL-12 p70 ELISA was subtracted from the values obtained with the mouse IL-12 p40 ELISA.
Northern Analysis of IL-12 p40 and p35 mRNA.
For Northern analysis, groups of three mice were treated with Flt3-L (10 μg/d for 10 d) and the splenic DCs were isolated. The administration of Flt3-L to C57BL/6 mice greatly enhances the number of splenic DCs (by >30-fold), without altering their IL-12 response regulation. Cultures of these DCs (13 × 106 DCs/5 ml culture) were stimulated for 4 h with CpG (0.5 μM) alone or together with IFN-γ (20 ng/ml), IL-4 (50 U/ml), or a combination of the two cytokines. Total RNA isolated from ∼2 × 106 DCs using RNAagents (Promega) was fractionated on 1% formaldehyde-agarose gels and transferred onto Hybond-C membranes (Amersham Pharmacia Biotech). Filters were baked, prehybridized in 50% formamide, 5× SSC, 0.02% Ficoll, 0.02% polyvinylpyrrolidine, 0.02% albumin, and 500 μg/ml of denatured herring sperm DNA, and then hybridized for 18 h at 42°C with probes at a concentration of 2 × 106 counts/ml. Filters were washed in 0.2× SSC, 0.1% SDS at 65°C, and exposed to autoradiography at −70°C. For successive hybridizations, filters were first treated by boiling in 10 mM EDTA and 0.1% SDS to remove bound probe. The probes used were 1-kb EcoRI–HindIII murine p40 cDNA 36, a 0.66-kb EcoRI–KpnI fragment derived by PCR from the murine p35 cDNA 36, and a 1.1-kb PstI rat glyceraldehyde 3-phosphate dehydrogenase cDNA 37 insert. The probes were radiolabeled by random primer extension with [α-32P]dATP to specific activities ranging between 5 × 108 and 109 cpm/μg.
Results
IL-4 Enhancement of Bioactive IL-12 Production by Mouse Splenic DCs in Response to Microbiological and T Cell–dependent Stimuli.
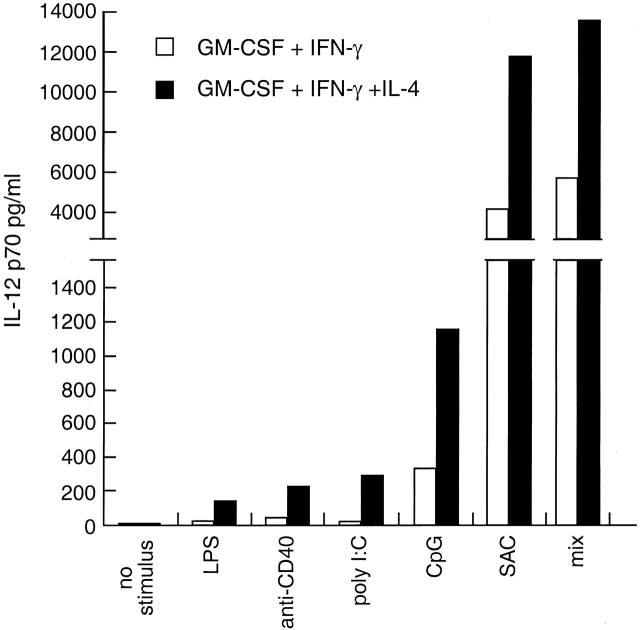
A large number of different stimuli is able to induce IL-12 production in DCs 4. We analyzed the production of IL-12 p70 by freshly isolated mouse spleen DCs exposed to a panel of stimuli in the presence of GM-CSF and IFN-γ alone or together with IL-4 (Fig. 1). Of the stimuli tested, LPS and heat-killed SAC are bacteria derived, poly I:C mimics virus-derived double stranded RNA, and the CpG motif–containing oligodeoxynucleotides are a stimulatory component of bacterial DNA. These can be grouped as stimuli of microbiological origin. Abs to CD40 or soluble CD40L represent a T cell–derived stimulus.
Figure 1.
IL-4 enhances IL-12 p70 production by mouse splenic DCs. Mouse splenic DCs were cultured with an IL-12 stimulus (LPS, anti-CD40 mAb, poly I:C, CpG, or SAC [10 μg/ml], or a mix of all stimuli together) in the presence of GM-CSF and IFN-γ with or without IL-4, for 18 h. The supernatants were assayed for IL-12 p70 content by ELISA. The result is typical of more than five experiments of this type.
Production of the bioactive p70 form of IL-12 required a microbial or T cell–derived stimulus (Fig. 1). However, IL-4, in the presence of IFN-γ and GM-CSF, enhanced IL-12 p70 production with all stimuli tested, irrespective of whether a microbiological or T cell–derived stimulus was used (Fig. 1 and data not shown). The profound effect of IL-4 on the production of IL-12 was most clearly seen when weak IL-12 stimuli were used (LPS, anti-CD40 mAb, poly I:C, or suboptimal doses of CpG); under these conditions, IL-4 induced up to sixfold more IL-12 p70 production (Fig. 1 and data not shown). In the presence of potent IL-12 stimuli (SAC, optimal concentration of CpG, CD40L, or mixes of various stimuli), IL-4 could induce a further 2.5-fold increase of IL-12 p70 production (Fig. 1, see Fig. 4 and Fig. 5, and data not shown).
Figure 4.
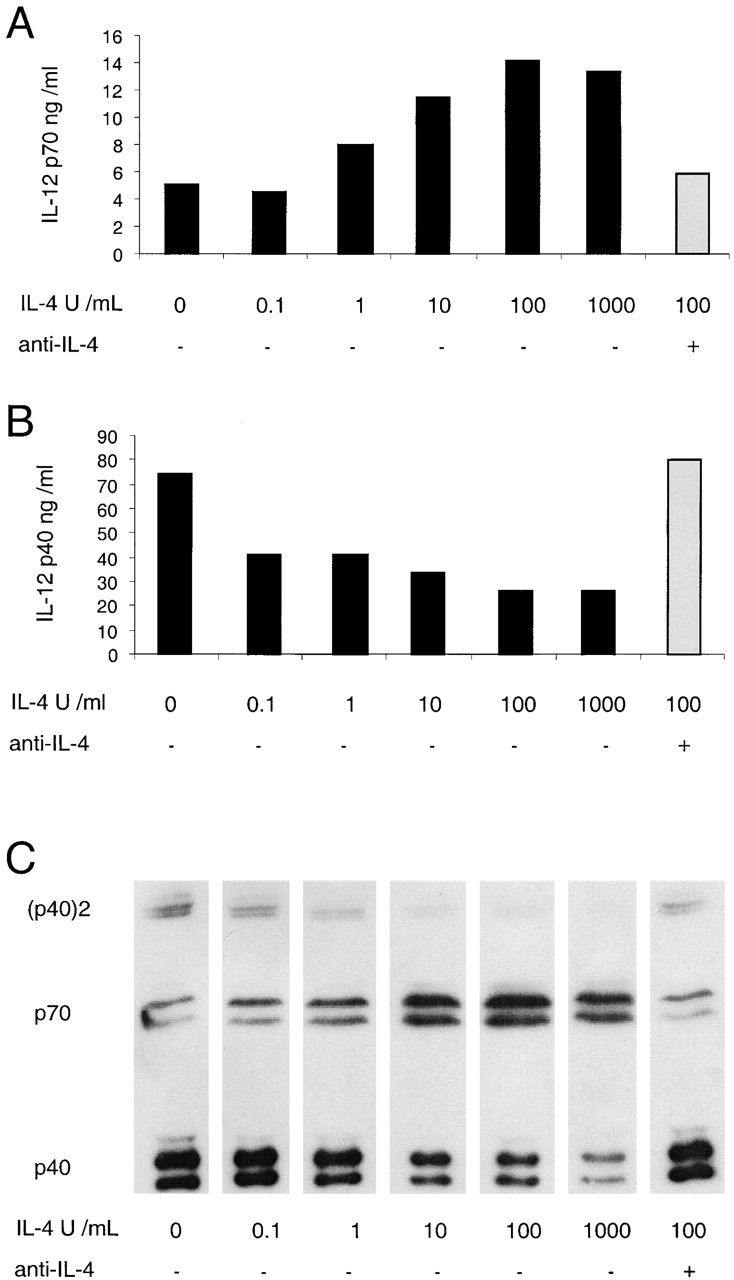
Titration of the effect of IL-4 on the production of IL-12 by mouse splenic DCs. Mouse splenic DCs were cultured with a mix of stimuli as used in Fig. 1 (but with SAC used at only 5 μg/ml), in the presence of IFN-γ and GM-CSF and titrated concentrations of IL-4, for 18 h. A neutralizing mAb to IL-4 (10 μg/ml) was included for one set of conditions as indicated (gray bar). The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The result is typical of three similar experiments with ELISA readouts and Western blotting.
Figure 5.
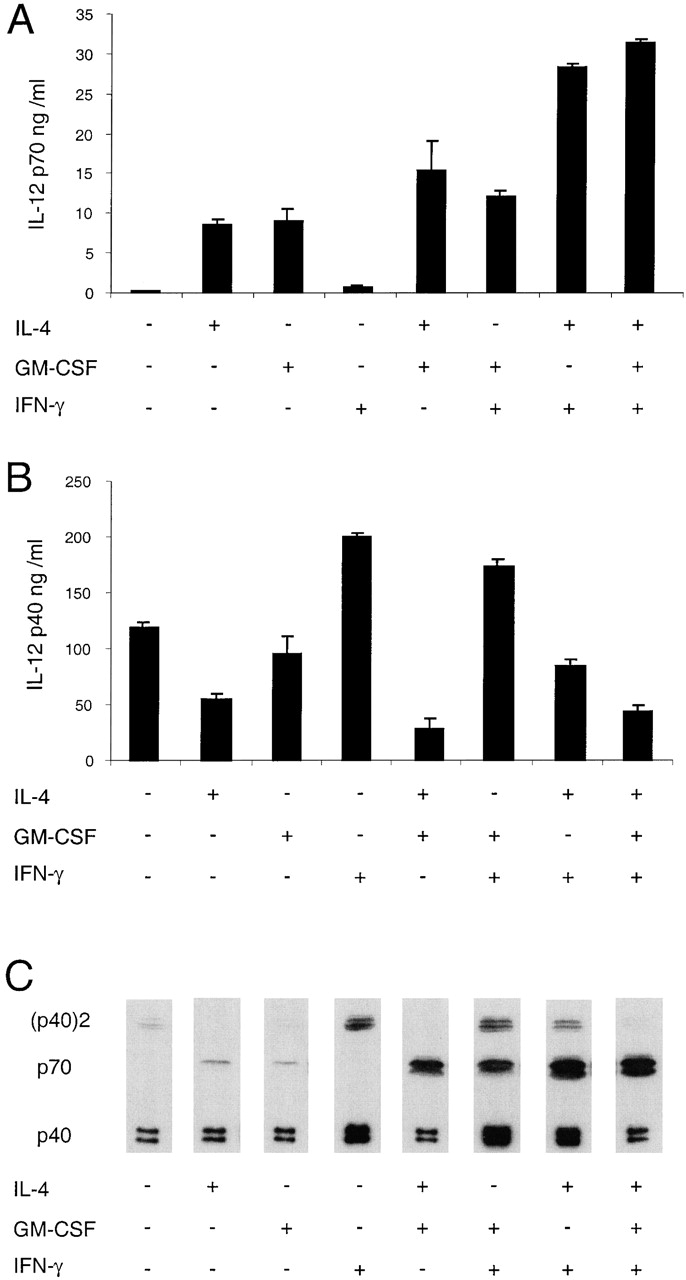
The effects of combinations of IL-4, GM-CSF, and IFN-γ on the IL-12 production by mouse splenic DCs. Mouse splenic DCs were cultured with a mix of stimuli as used in Fig. 1 in the presence or the absence of IL-4, IFN-γ, or GM-CSF, for 23 h. The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The result is typical of more than five similar experiments with ELISA readouts and Western blotting.
Titration of Cytokines Regulating IL-12 Production by Mouse Splenic DCs.
IL-12 production by DCs can be influenced by several cytokines during the stimulation 4. We analyzed the influence of IFN-γ, GM-CSF, and IL-4 on the production of the three forms of IL-12. In addition to the sensitive ELISA assay for the p70 and total p40 production, we used Western blots to directly visualize the level of the antagonistic (p40)2 form of IL-12.
Increasing the concentration of IFN- γ during IL-12 stimulation resulted in mouse DCs producing both more total IL-12 p40 and bioactive IL-12 p70 (Fig. 2A and Fig. B). However, even maximal concentrations of IFN-γ induced such low levels of bioactive IL-12 p70 that this could only be detected by sensitive ELISA and not by Western blot. By contrast, the IFN-γ–induced increase in the production of the antagonistic (p40)2 IL-12 was substantial, detected by Western blot, and increased in a dose-dependent manner (Fig. 2 C).
Figure 2.

Titration of the effect of IFN-γ on the production of IL-12 by mouse splenic DCs. Mouse splenic DCs were cultured with SAC (20 μg/ml) in the presence of titrated concentrations of IFN-γ, for 23 h. The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The error bars represent the variation within one typical experiment. The experiment has been repeated three times for the ELISA and two times for Western blots, with similar results.
The titration of GM-CSF (in the presence of IFN-γ) revealed that even relatively small concentrations of GM-CSF efficiently increased IL-12 p70 production by mouse DCs (Fig. 3 A). However, the production of IL-12 (p40)2 was only slightly reduced, as was the total IL-12 p40 (Fig. 3C and Fig. B).
Figure 3.
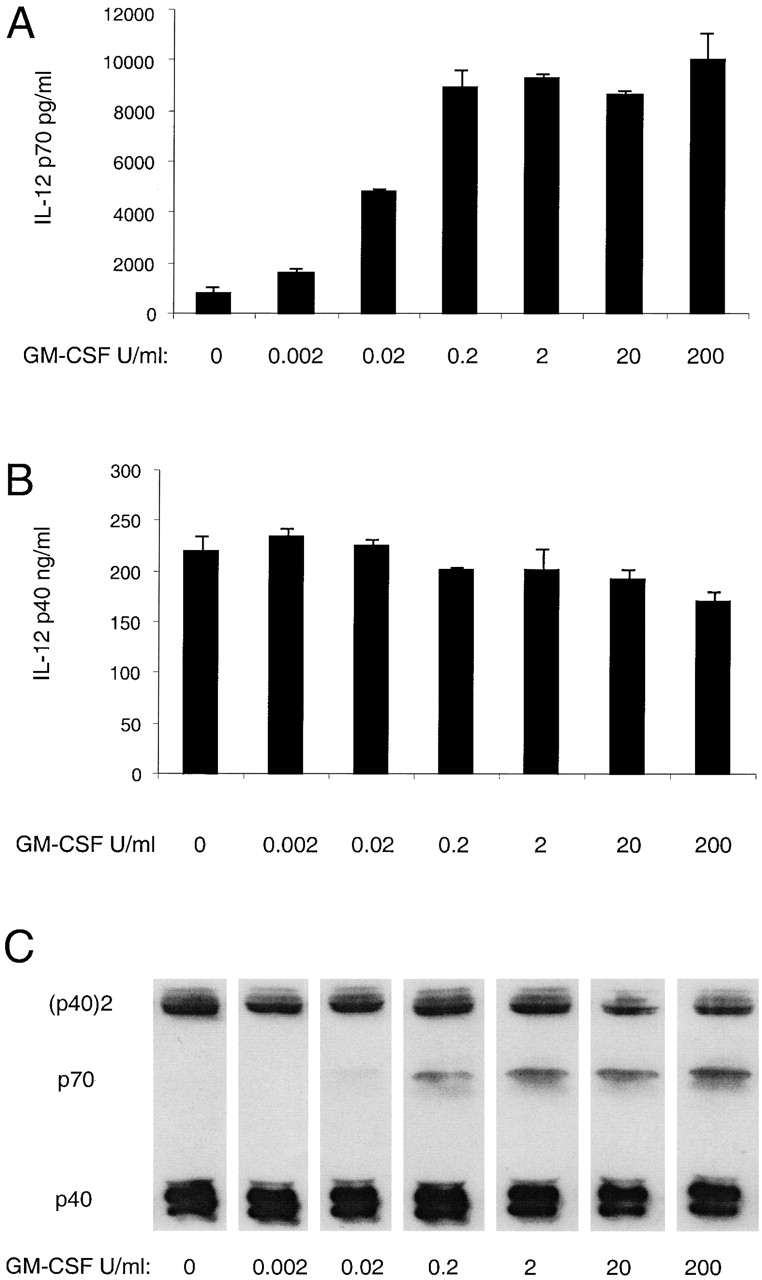
Titration of the effect of GM-CSF on the production of IL-12 by mouse splenic DCs. Mouse splenic DCs were cultured with SAC (20 μg/ml) in the presence of IFN-γ and titrated concentrations of GM-CSF, for 23 h. The supernatants were analyzed for IL-12 p70 and p40 by ELISA (A and B) and Western blotting (C). The result is typical of three experiments with ELISA readouts and Western blotting.
The titration of IL-4 (in the presence of IFN-γ and GM-CSF) showed a dose-dependent increase of IL-12 p70 (Fig. 4 A). Moreover, it showed a drastic decrease in total IL-12 p40, even in the presence of low concentrations of IL-4 (Fig. 4 B). Importantly, the reduction in total p40 was associated with a dose-dependent decrease of the antagonistic form of IL-12 (p40)2, which was reduced to near undetectable levels. The specificity of the effect was substantiated by the addition of an IL-4–neutralizing mAb that completely blocked the p70 increase and the p40 decrease induced by IL-4 (Fig. 4).
The Effects of Various Combinations of IL-4, GM-CSF, and IFN-γ on Mouse Splenic DC IL-12 Production.
Having determined optimal concentrations, we then analyzed the influence of IL-4, GM-CSF, and IFN-γ, alone or in various combinations, on the production of the three different forms of IL-12 by freshly isolated mouse splenic DCs (Fig. 5). An optimal combined stimulus was used with this study, but similar results were obtained with other strong stimuli used alone. Similar results were also obtained with splenic DCs isolated from Flt3-L–treated mice (data not shown).
Without any added cytokine, the stimulus used gave only marginal production of the bioactive IL-12 p70, but IL-12 p40 was produced in large excess and some of this was in the antagonistic homodimeric form (Fig. 5). The p40/(p40)2 ratio based on densitometry readings was 12.6. The cytokines were first tested alone. Addition of IL-4 alone increased the amount of IL-12 p70 by >30-fold. Concomitantly, the amount of total p40 was nearly halved and Western blot revealed that the remaining p40 was mainly the monomeric form. The p40/(p40)2 ratio was then 3,400. GM-CSF, like IL-4, increased the amount of bioactive IL-12, but had little effect on the total p40. Addition of IFN-γ during the stimulation led to an increase of all three forms of IL-12, although the increase of the antagonistic (p40)2 was most profound. The p40/(p40)2 ratio was then only 2.4. The excess of total p40 over p70 was ∼260-fold.
Various combinations of cytokines were then assessed. The combination of GM-CSF and IL-4, compared with the individual cytokines alone, resulted in an additive effect on the production of IL-12 p70 and a reduction of the IL-12 p40. As with IL-4 alone, no homodimeric p40 was detectable by Western blot. The combination of GM-CSF and IFN-γ resulted in a small increase of p70 compared with GM-CSF only and a small decrease in total IL-12 p40 compared with IFN-γ alone. The combination of IFN-γ and IL-4 had a synergistic effect on the production of IL-12 p70, resulting in 27-fold more IL-12 p70 compared with IFN-γ alone. In contrast, the IFN-γ–induced increase of total IL-12 p40 was much less in the presence of IL-4 (Fig. 5 B). The Western blots showed that some of this reduction in total p40 was due to a lower production of the (p40)2 homodimer, the p40/(p40)2 ratio changing from 2.4 to 9.1 (Fig. 5 C). The addition of GM-CSF to the mix of IFN-γ and IL-4 had little effect on p70 production but led to a further decrease of total IL-12 p40 production as detected by ELISA. The Western blot revealed an especially strong decrease in DC production of the antagonistic (p40)2 homodimer, with a change from a p40/(p40)2 ratio of 9.1 to a ratio of 161. Thus, the three cytokines used together gave the best possible agonist to antagonist ratio under the range of conditions tested.
The Independent Regulation of Mouse Spleen DC IL-12 p35 and p40 Gene Expression.
The marked changes in the IL-12 p70 to p40 ratio under the influence of IL-4 suggested there was independent control of the expression of genes for IL-12 p35 and IL-12 p40, rather than control of IL-12 being only at the level of the p40 gene. This was tested directly by Northern analysis of the levels of p35 and p40 mRNA (Fig. 6). To limit the number of mice used, mice were first pretreated with Flt3-L, which increases DC levels ∼30-fold 38. Whereas stimulation of mouse spleen DCs by CpG alone enhanced mainly the p40 mRNA levels, stimulation in the presence of IL-4 reduced this p40 mRNA and enhanced markedly the level of p35 mRNA. Thus, the effects of IL-4 on the p70 to p40 ratio reflected at least in part, if not entirely, differential regulation of p35 and p40 gene translation.
Figure 6.
The effects of IL-4 on p70 expression are mediated by the regulation of p35 mRNA. Mouse splenic DCs were cultured with CpG alone or with the addition of IFN-γ, IL-4, or both, for 4 h. RNA was extracted from the cells and subjected to Northern transfer analysis. The relative amounts of radioactive p35-specific or p40-specific probes bound to the mRNA samples as quantitated using a PhosphorImager® (Molecular Probes) are shown. A repeat experiment confirmed these findings.
Regulation of IL-12 Production In Vivo by IL-4.
To determine if the effects seen with pure mouse spleen DCs in culture could occur in vivo, mice were injected with either LPS or CpG, each either alone or together with IL-4. The level and form of IL-12 in the serum were tested by ELISA 4 h later (Table ). Both stimuli induced clearly detectable IL-12 in the serum. With LPS or CpG alone the p40 form predominated. In the presence of IL-4 the ratio of p70/p40 was markedly enhanced, exactly as seen with cultured DCs. The regulatory system seen in culture appeared to be functional in vivo.
Table 1.
The Influence of IL-4 on the Production of IL-12 p70 and p40 In Vivo
| Stimulus | p70 | p40 |
|---|---|---|
| pg/ml | ng/ml | |
| None (medium alone) | <60 | 4 ± 1 |
| LPS | 127 ± 23 | 162 ± 15 |
| LPS + IL-4 | 254 ± 73 | 74 ± 4 |
| CpG | 693 ± 150 | 369 ± 10 |
| CpG + IL-4 | 2,132 ± 47 | 226 ± 35 |
Mice were injected with the stimulus, killed 4 h later, then serum levels of p40 and p70 IL-12 were determined by ELISA. This experiment was repeated three times with similar results.
IL-4 Enhancement of Bioactive IL-12 Production by Human Thymic DCs.
The experiments using freshly isolated mouse DCs demonstrated that IL-4 alone or together with IFN-γ increased the production of IL-12 p70 and simultaneously reduced the production of IL-12 p40. It was important to test if this effect could also be observed with freshly isolated human DCs or was limited to one species. Accordingly, we isolated human thymic DCs and stimulated them with CD40L in the presence of GM-CSF and IFN-γ, with or without IL-4. As observed with the mouse splenic DCs, IL-4 increased IL-12 p70 and decreased IL-12 p40 production in freshly isolated human thymic DCs (Table ).
Table 2.
IL-12 p70 and p40 Production by Isolated Human Thymic DCs in the Presence or Absence of IL-4
| Experiment | |||
|---|---|---|---|
| 1 | p70 (pg/ml) | 260 | 542 |
| p40 (pg/ml) | 12,992 | 5,440 | |
| 2 | p70 (pg/ml) | 6 | 76 |
| p40 (pg/ml) | 906 | 391 | |
| IL-4 | − | + |
Human thymic DCs (9 × 104 DCs per 200 μl in experiment 1, or 5 × 104 DCs per 200 μl in experiment 2) were incubated with CD40L in the presence of GM-CSF and IFN-γ with or without IL-4 for 3 d (experiment 1) or 2 d (experiment 2). Supernatants were assayed for IL-12 p70 and p40 by ELISA.
The Effects of IL-4, GM-CSF, and IFN-γ on the IL-12 Production by Human Monocyte–derived DCs.
Since cultured human MoDCs are the most commonly used DC preparation due to their ready availability, we checked that the effects of IL-4, GM-CSF, and IFN-γ applied to these cells as well as to freshly isolated human DCs. This was especially important in view of the disparate reports of the effects of IL-4 on monocyte IL-12 production and the possibility that culture of DCs might alter their behavior. These relatively immature MoDCs expressed intermediate levels of HLA-DR and HLA-ABC, low levels of CD86, but no detectable CD80 or CD83; all of these markers could be upregulated after stimulation with CD40L (data not shown).
CD40L or a mix of stimuli induced in these MoDCs only very small amounts of IL-12 p70 but a large excess of IL-12 p40 if no cytokines were added (Table ). Addition of IL-4 alone increased the amount of IL-12 p70 in all three experiments and, concomitantly, reduced IL-12 p40. GM-CSF alone was less efficient in inducing IL-12 p70 compared with IL-4 and showed little effect on the amount of the IL-12 p40 produced. IFN-γ alone was as good or better than IL-4 alone in enhancing IL-12 p70, but consistent with the mouse data the amount of IL-12 p40 also increased, resulting in an excess of IL-12 p40 and an IL-12 p40/p70 ratio >300. The combination of IFN-γ and IL-4 had a synergistic effect on the production of IL-12 p70, resulting in a 4–17-fold increase of IL-12 p70 compared with IFN-γ alone. Concomitantly, the IFN-γ–induced increase of total IL-12 p40 dropped two- to threefold when combined with IL-4. The combination of IL-4, IFN-γ, and GM-CSF resulted in the optimal ratio of IL-12 p70 to total p40, in agreement with the results using freshly isolated human or mouse DCs (Fig. 5).
Table 3.
The Influence of IL-4, IFN-γ, and GM-CSF on the Production of IL-12 p70 and p40 of Human MoDCs
| Experiment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | p70 (pg/ml) | 0 | 420 | 90 | 430 | 740 | 760 | 4,200 | 3,920 |
| p40 (ng/ml) | 53 | 20 | 48 | 200 | 21 | 152 | 88 | 75 | |
| 2 | p70 (pg/ml) | 40 | 80 | 50 | 330 | 30 | 280 | 1,120 | 1,250 |
| p40 (ng/ml) | 18 | 12 | 19 | 100 | 11 | 94 | 45 | 42 | |
| 3 | p70 (pg/ml) | 9 | 171 | 84 | 1,062 | 290 | 3,110 | 17,100 | 20,200 |
| p40 (ng/ml) | 32 | 14 | 33 | 466 | 19 | 553 | 156 | 107 | |
| IL-4 | − | + | − | − | + | − | + | + | |
| GM-CSF | − | − | + | − | + | + | − | + | |
| IFN-γ | − | − | − | + | − | + | + | + |
Human MoDCs (104 DCs per 200 μl in experiments 1 and 2, 105 DCs per 200 μl in experiment 3) were incubated with CD40L (experiments 1 and 2) or a mix of stimuli containing LPS, poly I:C, CpG, and an mAb for CD40 (experiment 3), and cytokines as indicated for 2 d (experiments 1 and 2) or 1 d (experiment 3). The amount of IL-12 p70 and p40 produced was analyzed by ELISA.
Discussion
The polarization of T cell cytokine production patterns into the Th1 and Th2 types has become a basic laboratory manipulation for cellular immunologists. However, pushing the entire T cell immune system to these extremes would lead to immunopathologies in vivo. It is therefore likely that regulatory systems exist to maintain a more appropriate, balanced cytokine response in a normal animal. In this study we describe such a feedback system, mediated, surprisingly, by IL-4. As well as the well-established positive feedback by which IL-4, a Th2 cytokine, directly promotes Th2 differentiation from Th0 cells, we have found that IL-4 also has the potential to promote Th1 differentiation. However, this is an indirect effect, via the action of IL-4 on DCs to enhance the production and bioactivity of IL-12, the major Th1-inducing cytokine. This effect of IL-4 on immediate DC IL-12 production is separate from any effects IL-4 may have in promoting the development of certain DC populations. This effect on IL-12 production cannot be considered in isolation, and accordingly, we have studied the effect of IL-4 in conjunction with other cytokines and DC stimuli. We have verified that the results apply to both mouse and human DCs, freshly isolated or cultured, under a wide range of conditions. We have also demonstrated that this regulatory system is operative in vivo. Importantly, we have not just measured the total IL-12 p40, but also monitored the ratio between the bioactive p70 and the antagonistic (p40)2 IL-12 forms.
IL-4 as the only cytokine, but even more efficiently in synergy with IFN-γ and GM-CSF, increases the IL-12 p70 production of freshly isolated mouse splenic DCs, freshly isolated human thymic DCs, and cultured human MoDCs. Concomitantly, it decreases the amount of the total IL-12 p40 and of the antagonistic IL-12 (p40)2. These results point to an independent regulation of the p40 and p35 components of IL-12. We have confirmed by Northern analysis that the regulation is at the level of gene transcription, and that the p35 and p40 genes are under independent control (Fig. 6). The results also suggest a separate regulation of the formation of the antagonistic homodimer from the p40 monomer, but we have not examined this in more detail. It should be emphasized that the cytokines alone do not provide a sufficient signal for bioactive IL-12 production by DCs, but function together with microbial or T cell–dependent DC activation stimuli.
These findings are in contrast to some publications reporting an inhibitory effect of IL-4 on the ability of DCs to produce IL-12 p70 20 32. One of the studies used an mAb to CD40, and the other LPS and IFN-γ as stimuli. In our hands, IL-4 clearly enhances IL-12 p70 production of mouse splenic DCs if the same stimuli or a large panel of IL-12 stimuli are used (Fig. 1 and data not shown). This effect of IL-4 is not strain specific, since similar results were obtained with DCs isolated from BALB/c mice (data not shown). It also occurs in vivo (Table ). We also found the same effect with freshly isolated and cultured human DCs. Clearly, we are dealing with a general phenomenon.
Monocytes can be primed with IL-4 for higher IL-12 production if the cytokine is present at least 1 d before the contact with a stimulus 26. This effect might reflect the induction of monocyte differentiation towards a DC phenotype. In our studies we can rule out such a priming effect as the reason for the increase of IL-12 p70 in the presence of IL-4. First, in the case of the mouse splenic DCs and the human thymic DCs, we used freshly isolated DCs that already express surface class II MHC molecules and major costimulatory molecules (CD40, CD80, and CD86), as well as in the case of the human thymic DCs, the maturation marker CD83, all at a level typical of mature but not immature DCs. Second, kinetic studies with mouse splenic DCs showed that IL-12 production is a very rapid event, with IL-12 p70 being detected as early as 2 h after stimulation in the presence of IL-4 and reaching a plateau at 10 h (data not shown).
One reason for the failure to observe these effects of IL-4 previously could be the use of DCs cultured in the presence of IL-4 and GM-CSF. We have noted that unless these DCs are washed thoroughly there is sufficient carryover of IL-4 and GM-CSF to initiate IL-12 production without exogenous cytokine addition (data not shown). This simple technical problem can obscure the difference between a role of these cytokines in differentiating or priming the DCs and the role of these same cytokines during the actual stimulation of IL-12 production. In addition, the presence of endotoxin or other microbial products in antigens or culture media could obscure the requirement for an additional DC stimulus.
In this study we have used the total DC population, without separation into the distinct DC subpopulations 39. One subset, the CD8+ mouse spleen DCs, has been shown to have the highest potential to produce IL-12 in vitro and in vivo 40 41 42. We have confirmed that freshly isolated CD8+ DCs are the major IL-12 producers in our systems and verified that the effects of IL-4 and other cytokines on IL-12 production are also seen with purified CD8+ DCs (data not shown). However, although the absolute levels of IL-12 are lower, all subtypes of splenic DCs respond to IL-4 with increased IL-12 p70 and decreased IL-12 p40 production (data not shown). Furthermore, since the amounts of IL-12 p70 produced by the CD8− DCs are very low, often under the detection limit without the addition of IL-4, this leads to the impression that only CD8+ DCs produce IL-12.
Based on our findings, we propose a model integrating the regulatory effects of IL-4 and IFN-γ on the production of IL-12. DCs receiving appropriate stimuli (either microbiological or T cell derived) in a Th2 environment and thus in the presence of IL-4, will produce IL-12 p70, leading to IFN-γ production by T cells or NK cells. The strong synergy of IFN-γ and IL-4 promotes the production of high amounts of IL-12 p70, in turn enhancing IFN-γ production. This balances the direct effect of IL-4 in promoting Th2 development. Conversely, an environment with a high concentration of IFN-γ in the absence of IL-4 favors production of the antagonistic IL-12 (p40)2, concomitantly counterbalancing any exaggerated Th1 response. New primary T cells entering the system, perhaps responding to different antigens, would therefore be less likely to be immediately polarized to a Th1 or Th2 cytokine profile. In short, this model provides a mechanism for the downregulation of a typical Th2 response, mediated via the major protagonist of Th2 development, IL-4.
In light of a recent finding, a further factor promoting IL-12 production could be added to this model. IL-4 together with IL-12 p70 has been shown to induce IFN-γ production by DCs 43. This endogenously produced IFN-γ, together with IL-4, would then amplify the production of IL-12 p70, according to our findings. So even in an extreme Th2 situation, in which the production of T cell–derived IFN-γ is blocked, this production of IFN-γ by DCs could enhance their IL-12 production, which in turn could induce large amounts of IFN-γ from T cells and NK cells, thus overcoming the Th2 status.
Several findings with animal infection systems strengthen this model and argue against a general inhibitory role of IL-4 on IL-12 production. In experimental autoimmune uveoretinitis in rats, the addition of IL-4 augments the production of IFN-γ and other Th1-related effector molecules and aggravates the disease in vivo 44. An additional study showed that IL-4–deficient mice, in contrast to nondeficient mice, fail to achieve a protective Th1 status in response to Candida albicans in the late stage of infection 45.
In addition, our findings and the resulting model fit well with several observations obtained by Leishmania major infection of mice. Most inbred mouse strains are genetically resistant, whereas BALB/c mice are susceptible. The susceptibility of the BALB/c mice is caused by an aberrant Th2 response to the parasite, in contrast to the protective Th1 response of resistant strains 46. The critical role of IL-12 in mounting the protective Th1 response is well established, and DCs have been identified as the major source of IL-12 during L. major infection 47 48 49. A strain of mice displaying intermediate susceptibility to L. major infection develops a dominant Th2 type response during early weeks of infection, but later switches spontaneously to Th1 and resolves infection 50. If normally resistant mice are treated with neutralizing Abs to IL-12 or IFN-γ during the first 3 wk of L. major infection, they develop a Th2 response and become susceptible. However, during the Ab treatment they continue to produce IL-12 in spite of exhibiting a Th2 phenotype, and on discontinuing the Ab treatment they eventually switch from a Th2 to a Th1 response 51.
The findings presented here have several clinical implications. Vaccination strategies aimed at producing Th1 responses must now consider the proinflammatory effects of IL-4 on DCs as well as the antiinflammatory aspects of IL-4 on the T cells. On the other hand, the knowledge that IL-4 itself has the potential to counterbalance Th2 responses if DCs and an IL-12 stimulus are present could lead to new strategies for treating Th2-related diseases, such as allergies and some parasitic infections. Providing a strong IL-12 stimulus during an ongoing Th2 response might be sufficient to overcome the Th2 bias in some diseases. In fact, it was recently shown that the addition of a strong IL-12 stimulus (CpG) could reverse an established Th2 response when given as late as 20 d after lethal L. major infection 52.
Acknowledgments
We thank F. Battye, D. Kaminaris, V. Lapatis, and J. Chan for assistance with flow cytometric sorting. We thank F. Jaehrling for helpful discussion in establishing the ELISA systems.
This work was supported by the National Health and Medical Research Council, Australia. H. Hochrein is supported by a Deutsche Krebshilfe fellowship. S. Vandenabeele is supported by the Cooperative Research Center for Vaccine Technology, Brisbane, Australia.
Footnotes
Abbreviations used in this paper: BSS, balanced salt solution; DC, dendritic cell; MoDC, monocyte-derived DC; poly I:C, polyinosinic-polycytidylic acid; SAC, Staphylococcus aureus Cowan I.
References
- Kobayashi M., Fitz L., Ryan M., Hewick R.M., Clark S.C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S.F., Temple P.A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R.M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- Gubler U., Chua A.O., Schoenhaut D.S., Dwyer C.M., McComas W., Motyka R., Nabavi N., Wolitzky A.G., Quinn P.M., Familletti P.C. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12a cytokine at the interface of inflammation and immunity. Adv. Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- Gately M.K., Renzetti L.M., Magram J., Stern A.S., Adorini L., Gubler U., Presky D.H. The interleukin-12/interleukin-12-receptor systemrole in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N.M., Chehimi J., Kubin M., Aste M., Chan S.H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F.P., Hujer A.M., Ahmed F.N., Rerko R.M. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- Gillessen S., Carvajal D., Ling P., Podlaski F.J., Stremlo D.L., Familletti P.C., Gubler U., Presky D.H., Stern A.S., Gately M.K. Mouse interleukin-12 (IL-12) p40 homodimera potent IL-12 antagonist. Eur. J. Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- Ling P., Gately M.K., Gubler U., Stern A.S., Lin P., Hollfelder K., Su C., Pan Y.C., Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- Mattner F., Fischer S., Guckes S., Jin S., Kaulen H., Schmitt E., Rude E., Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur. J. Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- Rothe H., O'Hara R.M., Jr., Martin S., Kolb H. Suppression of cyclophosphamide induced diabetes development and pancreatic Th1 reactivity in NOD mice treated with the interleukin (IL)-12 antagonist IL-12(p40)2. Diabetologia. 1997;40:641–646. doi: 10.1007/s001250050728. [DOI] [PubMed] [Google Scholar]
- Mattner F., Ozmen L., Podlaski F.J., Wilkinson V.L., Presky D.H., Gately M.K., Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect. Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M.K., Carvajal D.M., Connaughton S.E., Gillessen S., Warrier R.R., Kolinsky K.D., Wilkinson V.L., Dwyer C.M., Higgins G.F., Jr., Podlaski F.J. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann. NY Acad. Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- Kato K., Shimozato O., Hoshi K., Wakimoto H., Hamada H., Yagita H., Okumura K. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc. Natl. Acad. Sci. USA. 1996;93:9085–9089. doi: 10.1073/pnas.93.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Nagata M., Arisawa K., Yoshida R., Fujihira K., Okamoto N., Moriyama H., Miki M., Saito I., Hamada H. Local expression of immunoregulatory IL-12p40 gene prolonged syngeneic islet graft survival in diabetic NOD mice. J. Clin. Invest. 1998;102:1807–1814. doi: 10.1172/JCI2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Wang C.R., Yoneto T., Waki S., Sunaga S., Komagata Y., Mitsuyama M., Miyazaki J., Nariuchi H. Reduced T helper 1 responses in IL-12 p40 transgenic mice. J. Immunol. 1998;160:588–594. [PubMed] [Google Scholar]
- Schmidt C., Brijs L., Cliquet P., De Baetselier P. Increased IL-12 P40 homodimer secretion by spleen cells during in vivo growth of the BW-19 T cell hybridoma accompanies suppression of natural immunity. Int. J. Cancer. 1998;77:460–466. doi: 10.1002/(sici)1097-0215(19980729)77:3<460::aid-ijc24>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Macatonia S.E., Hosken N.A., Litton M., Vieira P., Hsieh C.S., Culpepper J.A., Wysocka M., Trinchieri G., Murphy K.M., O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Heufler C., Koch F., Stanzl U., Topar G., Wysocka M., Trinchieri G., Enk A., Steinman R.M., Romani N., Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewein P., Janke K., Heufler C., Kampgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhasselt V., Buelens C., Willems F., De Groote D., Haeffner-Cavaillon N., Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cellsevidence for a soluble CD14-dependent pathway. J. Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- Kubin M., Chow J.M., Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor alpha, and IL-1 beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- Hayes M.P., Wang J., Norcross M.A. Regulation of interleukin-12 expression in human monocytesselective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- D'Andrea A., Ma X., Aste-Amezaga M., Paganin C., Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cellspriming for IL-12 and tumor necrosis factor alpha production. J. Exp. Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C.G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J.E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN- gamma or IL-10. J. Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- Marshall J.D., Robertson S.E., Trinchieri G., Chehimi J. Priming with IL-4 and IL-13 during HIV-1 infection restores in vitro IL-12 production by mononuclear cells of HIV-infected patients. J. Immunol. 1997;159:5705–5714. [PubMed] [Google Scholar]
- Snijders A., Hilkens C.M., van der Pouw Kraan T.C., Engel M., Aarden L.A., Kapsenberg M.L. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 1996;156:1207–1212. [PubMed] [Google Scholar]
- Bonder C.S., Finlay-Jones J.J., Hart P.H. Interleukin-4 regulation of human monocyte and macrophage interleukin-10 and interleukin-12 production. Role of a functional interleukin-2 receptor gamma-chain. Immunology. 1999;96:529–536. doi: 10.1046/j.1365-2567.1999.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen M.J., Miller M.A., Shinnick T.M., Ziegler H.K. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J. Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- Takenaka H., Maruo S., Yamamoto N., Wysocka M., Ono S., Kobayashi M., Yagita H., Okumura K., Hamaoka T., Trinchieri G., Fujiwara H. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J. Leukoc. Biol. 1997;61:80–87. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- Sparwasser T., Miethke T., Lipford G., Borschert K., Hacker H., Heeg K., Wagner H. Bacterial DNA causes septic shock. Nature. 1997;386:336–337. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- Vremec D., Zorbas M., Scollay R., Saunders D.J., Ardavin C.F., Wu L., Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleeninvestigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., Shortman K. Dendritic cell subtypes in mouse lymphoid organscross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Tone T., Thompson S.A., Babick J.M., Nolan K.F., Tone M., Raven C., Waldman H. Structure and chromosomal location of the mouse interleukin-12 p35 and p40 subunit genes. Eur. J. Immunol. 1996;26:1222–1227. doi: 10.1002/eji.1830260606. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J.M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde 3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984;12:6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E., Brasel K., Teepe M., Roux E.R., Lyman S.D., Shortman K., McKenna H.J. Dramatic increase in the numbers of functionally mature dendritic cells in mice treated with Flt3 ligandmultiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa C.R., Hieny S., Scharton-Kersten T., Jankovic D., Charest H., Germain R.N., Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α1 and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T., Matsuda S., Koyasu S. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J. Immunol. 2000;164:64–71. doi: 10.4049/jimmunol.164.1.64. [DOI] [PubMed] [Google Scholar]
- Ramanathan S., de Kozak Y., Saoudi A., Goureau O., Van der Meide P.H., Druet P., Bellon B. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J. Immunol. 1996;157:2209–2215. [PubMed] [Google Scholar]
- Mencacci A., Del Sero G., Cenci E., d'Ostiani C.F., Bacci A., Montagnoli C., Kopf M., Romani L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans . J. Exp. Med. 1998;1873:307–317. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F.P., Sadick M.D., Holaday B.J., Coffman R.L., Locksley R.M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E., Belkaid Y., Jakob T., Sacks D.L., Udey M.C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin–derived dendritic cellsimplications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F., Magram J., Ferrante J., Launois P., Di Padova K., Behin R., Gately M.K., Louis J.A., Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- Gorak P.M., Engwerda C.R., Kaye P.M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Li J., Hunter C.A., Farrell J.P. Anti-TGF-beta treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J. Immunol. 1999;162:974–979. [PubMed] [Google Scholar]
- Hondowicz B.D., Scharton-Kersten T.M., Jones D.E., Scott P. Leishmania major-infected C3H mice treated with anti-IL-12 mAb develop but do not maintain a Th2 response. J. Immunol. 1997;159:5024–5031. [PubMed] [Google Scholar]
- Zimmermann S., Egeter O., Hausmann S., Lipford G.B., Rocken M., Wagner H., Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]