Abstract
B cells recruited into splenic antibody responses grow exponentially, either in extrafollicular foci as plasmablasts, or in follicles where they form germinal centers. Both responses yield plasma cells. Although many splenic plasma cells survive <3 d, some live much longer. This study shows that early plasma cell death relates to a finite capacity of the spleen to sustain plasma cells rather than a life span endowed by the cell's origin or the quality of antibody it produces. Antibody responses were compared where the peak numbers of plasma cells in spleen sections varied between 100 and 5,000 cells/mm2. In each response, plasmablast clones divided some five times, with the peak numbers of plasma cells produced relating directly to the number of B cells recruited into the response. The spleen seems to have the capacity to sustain between 20 and 100 plasma cells/mm2. When this number is exceeded, there is a loss of excess cells. Immunoglobulin variable region gene sequencing, and 5-bromo-2′-deoxyuridine pulse–chase studies indicate that long-lived splenic plasma cells are a mixture of cells derived from the extrafollicular and germinal center responses and cells derived from virgin and memory B cells. Only a proportion has switched immunoglobulin class.
Keywords: plasma cell survival, plasmablast growth, hypermutation, immunoglobulin class switch, spleen
Introduction
B cells activated in the outer T zone in the spleen migrate either to extrafollicular sites where they grow as plasmablasts or to follicles where they form germinal centers 1 2 3 4. The growth of plasmablasts in mouse spleen typically occurs in extrafollicular foci, located at the junction of the red pulp with the T zone 2 3 4. In mice with transgenic B cells, when exceptional numbers of antigen-specific B cells are recruited into the extrafollicular response, plasmablast growth can occur throughout the red pulp 5. Plasmablasts growing in the extrafollicular response differentiate locally into plasma cells, and many of these are lost from the spleen within 2–3 d 6 7—many by apoptosis in situ 7. Some antigen-specific plasma cells are found in the spleen for several weeks after immunization 4 6. The origin, fate, and life span of these cells are considered in this report.
In this study, information about the origin of persistent red pulp plasma cells is provided by analysis of their Ig V region genes. Plasmablasts growing in extrafollicular foci do not appear to acquire the mutations in their Ig V region genes that arise in B cells in germinal centers 3. Individual immunohistologically identified antigen-specific plasma cells have been picked from the red pulp of tissue sections at different times after immunization. The Ig V regions of these have been amplified and sequenced, using the principles established by Küppers et al. in studies of single cells from human germinal centers 8. It is shown that a mixture of mutated and nonmutated splenic plasma cells avoid an early death.
Plasma cells in the bone marrow play a dominant role in sustaining systemic antibody production, and many of these plasma cells are derived from B cells activated in the spleen 9 10 11 12. Ig V region analysis of bone marrow plasma cells in responses to hapten protein indicates that most, but not all, have mutations in their Ig V region genes 11 12. Those with mutations are likely to come from B cells that have undergone hypermutation and selection in germinal centers 13. It is not clear if some or all of the nonmutated bone marrow plasma cells come from extrafollicular antibody responses. Some may be nonmutated B cells emerging from germinal centers. Despite the dominant role of the bone marrow in systemic antibody production, recent studies have identified a contribution by long-lived splenic plasma cells to the continued production of virus-neutralizing antibody 14 and antiovalbumin antibody 15.
Further information about the origin of long-lived plasma cells in the spleen has been obtained using 5-bromo-2′-deoxyuridine (BrdU) pulse–chase experiments. BrdU pulses were given during antibody responses either during extrafollicular plasmablast growth, which ends on the fourth day after immunization, or in the second or third week of the response when B cell proliferation only continues in germinal centers 2 4. This approach suggests that the long-lived plasma cells in the spleen are a mixture of cells produced in follicles and extrafollicular foci; some are derived from activation of virgin B cell and some from memory cells.
Materials and Methods
Mice.
Specific pathogen-free female B10A and C57BL/6 mice (Harlan) were maintained in isolators within the Animal Unit of The Birmingham University. Both strains are homozygous for the IgHb allotype. Quasimonoclonal (QM) mice were provided by Marilia Cascalho and Matthias Wabl (University of California at San Francisco, San Francisco, CA 16) and were bred and maintained in our animal unit.
Antigens and Immunization Procedures.
Mice were immunized when aged 6–10 wk. The hapten (3-nitro-4-hydroxy-phenyl) acetyl (NP) was conjugated as its succinimide ester (Cambridge Research Biochemicals) to chicken gamma globulin (CGG; Sigma-Aldrich) to give a substitution ratio of 18 NP per CGG as described previously 17. Alum precipitates were freshly prepared from stock solutions of CGG as described elsewhere 18. Primary immunization was intraperitoneal using 50 μg alum-precipitated CGG plus 5 × 108 chemically killed Bordetella pertussis (Evans Medical). Secondary immunization (5 wk after priming) used 50 μg soluble NP-CGG in 200 μl sterile PBS, injected intraperitoneally. Cells in S phase were identified by the uptake of BrdU given as a 2-mg intraperitoneal injection 2 h before tissues were taken for histology. In longer pulses, a 2-mg intraperitoneal injection of BrdU was followed by administering BrdU at 1 mg/ml in the drinking water.
Tissue Preparation for Immunohistology.
Mice were killed by CO2 asphyxiation; after taking blood, the spleens were removed and immediately snap frozen by repeated dipping in liquid N2. 5-μm cryostat sections were prepared and stained as described previously 4. The following antibodies were used in addition to those described in reference 4: rat anti–mouse CD3 (17A2; a gift from H. Acha-Orbea, University of Lausanne, Lausanne, Switzerland), rat anti–mouse syndecan-1 (281-2; BD PharMingen), and a cocktail of rat anti–mouse mAbs specific for all four IgG subclasses, IgG1 (LO-MG1-2), IgG2a (LO-MG2A-3), IgG2b (LO-MG2b-2), and IgG3 (LO-MG3-7) (Serotec Ltd.). Tissue sections were triple-stained to show the expression of: (a) CD3, IgD, and BrdU; (b) NP- or CGG-binding antibody, IgD, and BrdU; (c) NP- or CGG-binding antibody, syndecan-1 19, and BrdU; and (d) NP- or CGG-binding antibody, IgG, and BrdU. The first named molecule in each of these triple stains was revealed in blue by immunoalkaline phosphatase, the second molecule as brown precipitate by immunoperoxidase. After this, the nuclei of cells that had incorporated BrdU were identified in red, again using immunoalkaline phosphatase, after the sections had been treated with acid to expose the BrdU-associated epitopes on DNA.
Quantification of NP- and CGG-binding B Cells in Tissue Sections.
The T zones, follicles, and red pulp were located using triple staining for CD3, IgD, and BrdU. NP-binding cells and CGG-binding cells were quantified on two adjacent sections, one stained to show NP-binding cells, the other to show CGG-binding cells. Together with the antigen-specific cells, the sections were stained to show IgD expression and BrdU incorporation. This allowed the location of antigen-specific B cells and plasma cells, in either the T zone or red pulp, to be identified. The size of splenic compartments and the number of cells per square millimeter of each compartment were determined using a point counting technique 20. The data are expressed as the numbers of antigen-specific cells per square millimeter of total section area, or per square millimeter of splenic compartment.
Isolation of Individual Antigen-specific Antibody-containing Cells.
Adjacent sections were stained for NP binding with IgD and NP binding with syndecan-1. Cells were identified by bright field microscopy. NP-specific plasmablasts and plasma cells can be readily identified as cells staining strongly for cytoplasmic Ig and syndecan-1 expression. Staining for IgD expression on adjacent sections allows the demarcation of the splenic compartments. Individual NP-binding plasma cells in the red pulp were selected at random. Cells were removed from the stained sections using a micromanipulator-controlled glass capillary with a fine tip, being careful not to take material from surrounding antigen-specific cells.
Amplification of V Region Genes from Single Plasma Cells.
The digestion and DNA amplification protocols were adapted from Jacob et al. 13 with modifications. Material from each cell recovered by microdissection was put into a 0.2-ml well of a 96-well PCR plate containing 25 μl of 0.2× PBS with 10 μg proteinase K (Boehringer). The plate was incubated at 56°C for 2 h followed by heat inactivation of the proteinase (10 min, 96°C). The lysates were then subjected to 2 sets of 40 cycles of PCR with nested primers as described 13. The first round PCR was carried out in a 50-μl volume containing 25 μl of the digestion product, 1× Pfu buffer (Stratagene), 0.01% gelatin type B from bovine skin (Sigma-Aldrich), 0.4 mM dNTP (Promega), 0.4 μM of each primer, and 1.25 U recombinant Pfu polymerase (Stratagene). No mineral oil was added; the plates were sealed with adhesive sheets (Hybaid). The reaction was run in a Touch Down thermal cycler (Hybaid). The second nested PCR used 2 μl of the reaction mixture from the first PCR.
DNA Sequencing.
25 μl of secondary PCR products was subjected to agarose gel electrophoresis. The DNA was extracted from the agarose using a commercial extraction kit (QIAquick Gel Extraction kit; QIAGEN). The extracted DNA was sequenced in both directions using the dye terminator cycle sequencing technique (ABI PRISM ready reaction kit; PerkinElmer) using the same primers as for the secondary PCR. Fluorescent-labeled cycle sequencing product was ethanol precipitated and read by Alta Biosciences on an ABI 377 machine (PerkinElmer).
Frequency of Pfu-induced Artefactual Mutations.
The frequency of polymerase-induced mutations was determined by sequencing the rearranged Ig V region gene from single B1-8 cells which contains an unmutated V186.2 V region segment linked to DFL16.1 and JH2 (21; a gift of G. Kelsoe, Duke University, Durham, NC). Each cell was recovered from a cytospin preparation; the digestion and amplification protocols were identical to those used on splenic sections. No mutation was observed in 6,000 bp sequenced. The measured error rate of the Pfu has been reported as 1–2 × 10−6 misincorporations/bp/cycle 22. This low error frequency is sufficient to allow the assumption that virtually all recovered mutations represent in vivo events, a conclusion supported by the analysis of sequences from 9 NP-specific plasma cells taken at day 7 of a primary response to NP-CGG and 16 plasma cells taken from day 6 of a primary response to NP-Ficoll. All of these had nonmutated V segments; CDR3 junctional diversity showed them to be from separate clones 23.
Results and Discussion
The Peak Number of Antibody-producing Cells Generated during a Splenic Extrafollicular Antibody Response Is Directly Related to the Number of B Cells Recruited into the Response, but the Life Span of Plasma Cells Reflects a Finite Capacity of the Spleen to Sustain Their Survival.
The peak numbers of plasma cells produced in the spleen in different extrafollicular antibody responses vary greatly. To investigate the factors that account for this variability, a range of immune responses was analyzed in which the number of antigen-specific B cells available varied. B cell recruitment into each response, the peak numbers of antibody-producing cells generated, and the proportion of these cells that survived for >1 wk were determined. The analysis identifies a direct relationship between the number of B cells recruited into an antibody response and the peak numbers of plasma cells produced. It also indicates that the capacity of the spleen to maintain plasma cells for >1 wk is finite and unrelated to the peak number of antibody-producing cells generated.
Plasmablast Growth and Plasma Cell Survival When Low Numbers of B Cells Are Recruited into the Extrafollicular Response.
The primary responses to intraperitoneal immunization with alum-precipitated NP-CGG plus killed B. pertussis were used to exemplify the situation in which relatively low numbers of antigen-specific B cells are available 3 4. In this response, the median peak number of antibody-producing cells generated was 112 cells/mm2 of red pulp (Fig. 1). Of these, a median of 88% (range 81–98%) was NP specific and 12% (range 2–19%) CGG specific. This presumably reflects the relative frequency of virgin B cells of these two specificities that were recruited into the response. The peak numbers of NP-specific plasma cells and CGG-specific plasma cells were both reached on the tenth day after immunization. After this, there was no significant fall in the number of antigen-specific plasma cells in the spleen through day 18. The number of B cells recruited into this response was too low to determine accurately, and its determination is complicated by the likely continued recruitment of B cells into the response for some days.
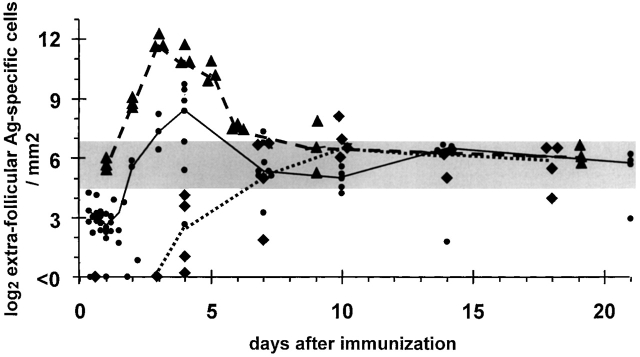
Figure 1.
Evidence that plasma cells go through a fixed number of cell cycles irrespective of the number of B cells recruited into extrafollicular antibody responses, but that the spleen has a finite capacity to sustain plasma cells produced. The horizontal gray bar indicates the range of sustainable plasma cell numbers. Three examples are given of plasmablast growth, differentiation, and survival where the starting number of B cells recruited into the extrafollicular response varies. The number of cells recruited into the response is identified by the number of antigen-specific B cells found in the outer T zone 12–24 h after immunization. These then go into exponential growth for 3 d. Diamonds represent values obtained from individual B10A mice during a primary response to NP-CGG (reference 4); the dotted line is drawn through median values. The delay in the onset of plasmablast growth in this response reflects the time taken for help from primed T cells to become available. Filled circles show values for a response of CGG-primed B10A mice to NP-CGG (reference 4); the solid line is drawn through median values for this response. Triangles represent values for red pulp NP-specific plasma cells obtained during the response of QM mice to NP-Ficoll (reference 5). Some 60% of the B cells these mice have transgenic receptors specific for NP; the dashed line is drawn through median values of this response.
Plasmablast Growth and Plasma Cell Survival When Intermediate Numbers of B Cells Are Recruited into the Extrafollicular Response.
Study of the recruitment of intermediate numbers of B cells was carried out in the response to soluble intravenous NP-CGG in mice primed 5 wk before with alum-precipitated CGG plus killed B. pertussis 4. A median of eight CGG-specific B cells/mm2 of spleen accumulated in the outer T zone by 12 h after immunization, and this number did not increase significantly by 24 h, reflecting the short period when B cells are recruited into this response (Fig. 1). Most of the cells were in S phase of the cell cycle at 24 h, as assessed by the uptake of BrdU given 2 h before the spleen was taken. Fewer than 1 NP-specific B cell/mm2 was seen in the T zone at this stage. After this, the numbers of antigen-specific cells in the extrafollicular response increased exponentially to peak at 96 h, when a median of 413 CGG-specific antibody-containing cells/mm2 was present. These were mainly found in extrafollicular foci located at the junction of the T zone and red pulp (Fig. 2, a and b). By 7 d after immunization, the numbers of plasma cells had fallen into the range of 10–170 cells/mm2 and were sustained at this level through 21 d. This range of plasma cell frequency is similar to that sustained into the third week of the primary response to NP-CGG (Fig. 1). By 21 d, the plasma cells were no longer concentrated in extrafollicular foci, but were scattered throughout the red pulp (Fig. 2 c). At the peak of the response, medians of 80% (range 63–87%) of the antigen-specific plasma cells were CGG specific and 20% (range 13–37%) were NP specific (Fig. 2 b and Fig. 3). This is the reverse of the CGG-specific to NP-specific cell ratio seen in the primary response to NP-CGG, indicating that almost all of the CGG-specific cells recruited into the response in carrier-primed mice were memory B cells.
Figure 2.
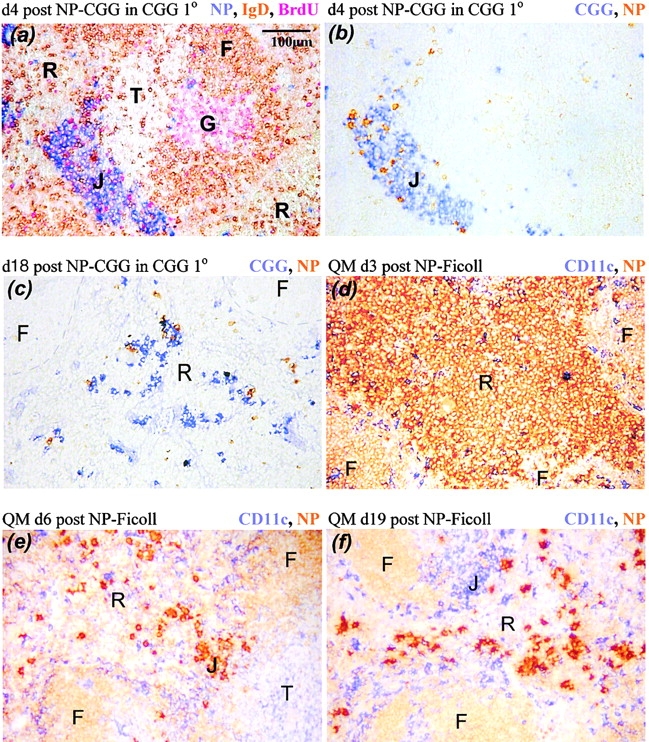
The distribution of plasma cells during the responses to NP-CGG in CGG-primed normal mice (a–c) and to NP-Ficoll in QM mice in which some 60% of the B cells have high affinity receptors for NP (d–f). The color that identifies molecules in each section is shown at the top right of each panel. (a and b) Sections showing extrafollicular foci (J) at the junction of the red pulp (R) and T zone (T) 4 d after NP-CGG challenge. G, germinal center; F, follicular mantle. Most of the plasma cells in the focus are CGG specific (b). (c and f) The typical dispersed pattern of plasma cells in the red pulp late in antibody responses, showing (f) a junction zone with CD11chigh dendritic cells now largely free of NP-specific plasma cells. (d) NP-specific plasmablasts filling the red pulp of QM mice 3 d after immunization with NP-Ficoll. (e) A QM mouse 3 d later in the response when most of the NP-specific antibody-forming cells have died, with a cluster of residual plasma cells in a junction zone (J).
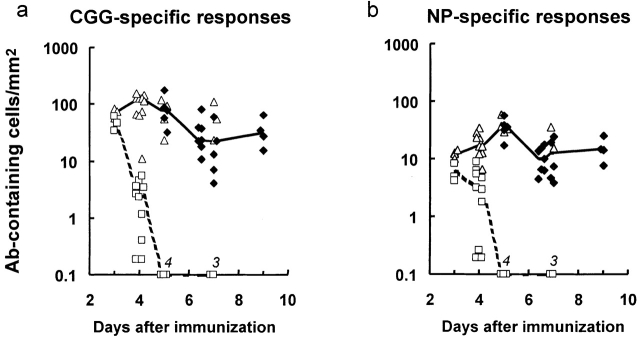
Figure 3.
The peak number, proliferation, and decline of red pulp antigen-specific antibody-containing cells in mice primed with CGG and reimmunized with NP-CGG. (a) The total number of CGG-binding plasmablasts and plasma cells/mm2 of red pulp in individual mice (♦ and ▵, respectively); medians are joined by a solid line. The number of CGG-binding cells that had taken up BrdU in the 2 h before the spleen was taken are shown (□); medians are joined by a dashed line (the BrdU labeling data were obtained from the mice whose total CGG-specific antibody-containing cell counts are shown [▵]). (b) Equivalent results for NP-binding plasmablasts and plasma cells in the same mice studied in panel a. Numbers on x-axis in italics indicate observations with values below the scale.
Detailed analysis of the transition from plasmablasts, which are in cell cycle and label with BrdU, to plasma cells, which are not proliferating, is shown in Fig. 3. This second series of experiments also shows the early loss of plasma cells. After immunization of CGG-primed mice with NP-CGG, CGG-specific cell numbers peak on day 4, when most of the cells have come out of cell cycle. Plasma cell numbers fall between days 5 and 6.5 and thereafter remain constant through day 10. The kinetics of the NP-specific cells is similar, the only difference being a delay of up to 24 h in the time peak numbers are reached and plasmablasts come out of cell cycle. The proportions of NP-specific cells and CGG-specific cells lost between days 5 and 6.5 are roughly equal (Fig. 3), indicating that a similar proportion of the plasma cells generated from memory B cells as well as those generated from virgin B cells dies early.
Plasmablast Growth and Plasma Cell Survival When Large Numbers of B Cells Are Recruited into the Extrafollicular Response.
High level recruitment of NP-specific cells into the extrafollicular response was achieved in QM mice immunized with NP-Ficoll; some 60% of QM B cells have high affinity for NP 14.
The frequency of responding B cells is far greater than in the response to NP-CGG in CGG-primed mice. Nevertheless, the extent of extrafollicular growth per cell recruited into the two responses is similar; the cells in both go through five cell cycles (Fig. 1). The plasmablasts in the QM response to NP-Ficoll completely fill the red pulp of the spleen after 3 d (Fig. 2 d), when the peak number of antigen-specific cells is reached. There is massive loss of plasma cells in this response until a plateau is reached by 6 d. The number of residual plasma cells is in the same range as that seen in the later stages of the other two responses where peak numbers of plasmablasts produced are, respectively, some one and two orders of magnitude lower (Fig. 1). Some of the residual plasma cells after 6 d in the QM response were distributed in clusters in the junction zone, but others were scattered in the red pulp (Fig. 2 e). As in the other responses, by the third week after immunization the residual plasma cells were mainly in the red pulp (Fig. 2 f).
Despite great differences in the number of B cells recruited into the three responses studied, the extent of plasmablast growth appears similar in all three responses. This result makes it unlikely that the growth of plasmablasts is controlled by stromal capacity to sustain growth. The results are more consistent with the growth being regulated within the cell, i.e., the trigger that induces B cells to proliferate and differentiate into plasmablasts sets a program for a finite number of cell divisions after which the cells come out of cell cycle and differentiate into plasma cells.
Persistent Plasma Cells in Splenic Antibody Responses Are Associated with Prolonged Survival Rather Than a Low Rate of Renewal of Short-lived Plasma Cells.
BrdU pulse–chase experiments were carried out to probe whether splenic plasma cells found late in antibody responses originate from plasmablast growth outside follicles or from germinal centers. The approach takes advantage of differences in the times when follicular and extrafollicular plasma cell precursors proliferate. Extrafollicular growth induced by NP-CGG in carrier-primed mice stops on the fourth day after immunization (Fig. 3). The main proliferative burst of plasmablasts in QM mice immunized with NP-Ficoll stops at the same stage of the response. Thus, BrdU given in drinking water between 72 and 96 h after the challenge would be expected to label most plasma cells formed in extrafollicular foci. Cells in germinal centers continue to proliferate after this time and rapidly dilute any BrdU taken up; this falls to levels undetectable by immunohistology within three cell divisions 24.
Two experiments were carried out in CGG-primed mice after challenge with NP-CGG: (a) with BrdU given from 60 to 96 h after immunization when the proportions of labeled antigen-specific cells on days 5 and 18 were compared (○, Fig. 4, a–d); and (b) with BrdU from 72 to 96 h and analysis at days 5, 6.5, and 9 (•, Fig. 4, a–d). There is a major loss of plasma cells between days 5 and 6.5, with relatively little further loss in the following 12 d. Many plasma cells labeled by the BrdU pulse survived the early period of apoptosis. It seems likely that most of the BrdU-labeled plasma cells found on day 18 were derived from plasmablasts growing in extrafollicular foci during the BrdU pulse, although it is also possible that some of these were early emigrants from germinal centers. It may be significant that the number of CGG-specific plasma cells fell more than the number of NP-specific cells and that the proportion of BrdU-labeled CGG-specific plasma cells remained higher. These findings might relate to the dominance of NP-specific germinal centers in the response of CGG-primed mice to NP-CGG 4, i.e., a high proportion of the plasma cells coming from germinal centers in this response are likely to be NP specific.
Figure 4.
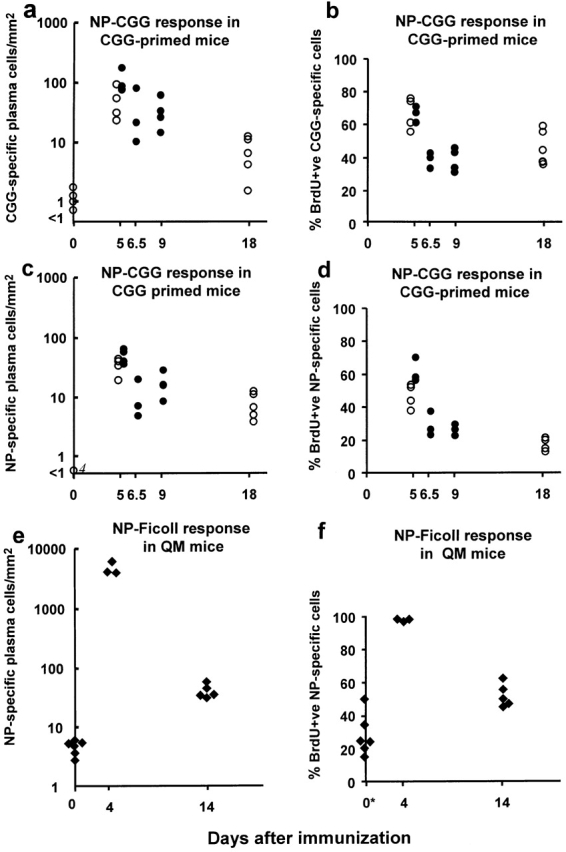
The number of antibody-containing cells and the proportion labeled by a BrdU pulse given from 60 to 96 h (○) or from 72 to 96 h (•) after CGG-primed mice had been immunized with NP-CGG, or for the first 4 d of the response of QM mice to NP-Ficoll (♦). (a) The total number of CGG-binding cells in the red pulp at intervals after immunization. (b) The percentage of the CGG-binding cells that were BrdU labeled. (c and d) The equivalent results obtained in the same mice for NP-binding red pulp cells. (e and f) The results for QM mice immunized with NP-Ficoll. *Unimmunized QM mice were given BrdU 14–10 d before the spleen was taken to assess the equivalent turnover of the significant background number of NP-specific plasma cells in the spleens of these mice. Each point shows data from one mouse. Number of cells is expressed as the number/mm2 of red pulp assessed in tissue sections.
Further BrdU pulse–chase experiments were carried out in QM mice in the response to NP-Ficoll. In these studies, BrdU was given in the drinking water during the first 4 d after giving NP-Ficoll, and the number and proportion of labeled NP-specific plasma cells were assessed at the end of the 4-d pulse and 10 d later (Fig. 4e and Fig. f). As in the NP-CGG response, a substantial proportion of the plasma cells in the red pulp 14 d after immunization was labeled by the BrdU given in the first 4 d of the response.
In complete contrast to the control of plasmablast growth, the persistence of splenic plasma cells follows a pattern consistent with the spleen having a finite capacity to support plasma cell survival. Those plasma cells that survive >3 d are located mainly in the junction zones between the T zone and red pulp. At this stage, the surviving cells show a close association with the CD11chigh dendritic cells that are located in the junction zones 24. After a few days, the plasma cells become more dispersed in the red pulp, leaving the junction zone with its CD11chigh dendritic cells relatively free of plasma cells (Fig. 2 f).
Splenic Plasma Cells Derived from Follicles Are Not Simply Migrants on Their Way from Follicles to the Bone Marrow.
To label plasma cells emerging from germinal centers selectively, further BrdU pulse labeling experiments were performed in which BrdU was given in the drinking water between days 14 and 18. This is already 10 d after the end of extrafollicular growth, but the mainly NP-specific germinal centers are still active at this time. After this late pulse, 5% of CGG-specific plasma cells were labeled on day 18 and, surprisingly, the median number of labeled plasma cells at that time and 10 d later was similar (0.85 vs. 0.66 cells/mm2; Table ). As in the previous experiments, there were fewer NP-specific plasma cells on day 18, but ∼15% of these were labeled with BrdU. A higher proportion (80%) of these cells had been lost 10 d later (1.23 vs. 0.28 cells/mm2). These data may reflect slow replacement of splenic plasma cells late in antibody responses from the predominantly NP-specific germinal centers present. Only a small proportion of the splenic plasma cells at 18 d appears to be migrants on the way to the bone marrow.
Table 1.
A Proportion of Red Pulp Plasma Cells Is Produced in Germinal Centers and Persists in the Red Pulp for >10 d
| Mouse no. | CGG-specific cells/mm2 | BrdU+ CGG-specific cells/mm2 | NP-specific cells/mm2 | BrdU+ NP-specific cells/mm2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Day 18 | Day 28 | Day 18 | Day 28 | Day 18 | Day 28 | Day 18 | Day 28 | Day 18 | Day 28 |
| 1 | 5 | 16.6 | 6.7 | 0.75 | 0.66 | 7.9 | 4.3 | 1.32 | 0.18 |
| 2 | 6 | 0.6 | 7.0 | 0.12 | 0.53 | 9.5 | 5.9 | 1.52 | 0.41 |
| 3 | 7 | 20.2 | 12.8 | 0.95 | 1.01 | 8.5 | 4.0 | 1.33 | 0.28 |
| 4 | 8 | 14.4 | 14.8 | 0.95 | 0.87 | 7.2 | 3.6 | 1.14 | 0.15 |
| 9 | 10.6 | 0.44 | 5.4 | 0.51 | |||||
| Median | 15.5 | 10.6 | 0.85 | 0.66 | 8.2 | 4.3 | 1.23 | 0.28 | |
Mice primed with CGG were reimmunized with NP-CGG and given a BrdU pulse 14–18 d later; at this time, B cell growth in the spleen is confined to germinal centers. The total number and number of BrdU+ antigen-specific plasma cells in the red pulp were determined on day 18 and day 28 after the NP-CGG boost.
A Small Proportion of NP-specific Red Pulp Plasma Cells Generated in the Response to NP-CGG in Carrier-primed Mice Has Mutations in the Ig V Region Genes.
The experiments in the previous section indicate that a substantial minority of plasma cells found in the red pulp is long-lived. Some of these appear to arise from B cells growing in extrafollicular foci, whereas others are later emigrants from follicles. To investigate this further, the sequences of IgH V region genes from single NP-specific red pulp plasma cells were determined at 5 and 18 d after CGG-primed mice had been challenged with NP-CGG. The genomic DNA was amplified using primers designed to bind to members of the V186.2 and V3 V-segment families. Sequences were obtained from 33 individual NP-specific plasma cells picked from day 5 tissue sections. Seven of these contained V region mutations; five had five or more mutations in their V region (Table , and Fig. 5 a). Among the 33 sequences derived on day 5, sequences 5b-25 and 5b-26 (Fig. 5 a) were identical and mutated. The V region sequences, 5b-9 and 5b-10, obtained from two further cells taken from the same day 5 spleen section (Fig. 5 a) were identical, but had no mutations. Germinal centers reach peak size on the fourth day after immunization in this response, and memory cells are clearly identifiable in the marginal zones from day 4 onwards 2 4; it seems likely that the NP-specific plasma cells with mutated V region sequences found in the red pulp at 5 d are early emigrants from germinal centers.
Table 2.
V Segment Use and the Frequency of Framework versus CDR Mutations in NP-specific Red Pulp Plasma Cells from Mice during the Response to NP-CGG in Carrier-primed Mice
| 5 d after NP-CGG | 18 d after NP-CGG | |
|---|---|---|
| Total no. of sequences | 33 | 53 |
| No. of sequences with mutations | 7 | 12 |
| No. of sequences with V186.2 | 12 | 14 |
| No. of V186.2 mutated | 3 | 5 |
| No. of V186.2 codon 33 Trp→Leu | 0 | 1 |
| Silent framework mutations | 7 | 13 |
| Replacement framework mutations | 6 | 10 |
| Silent CDR1 and 2 mutations | 2 | 5 |
| Replacement CDR1 and 2 mutations | 16 | 22 |
Figure 5.
Individual Ig V region DNA sequences obtained from (a) 33 and (b) 53 single NP-binding red pulp plasma cells from sections of spleen taken (a) 5 and (b) 18 d after CGG-primed mice had been reimmunized with NP-CGG. The sequences are assigned to individual V segment families, and any mutations from the germline sequence are indicated; only those codons that differ from the reference sequences are shown. The sequences are set out in two broad Ig V segment families, V3 and V186.2. Lowercase letters indicate silent mutations, uppercase characters replacement mutations; dashes indicate identity with the reference sequence. FR, framework region of the V segment. The sequence data summarized in this figure are available from EMBL/GenBank/DDBJ under accession nos. AJ240347, AJ240348, AJ240349, AJ240350, AJ240351, AJ240352, AJ240353, AJ240354, AJ240355, AJ240356, AJ240357, AJ240358, AJ240359, AJ240360, AJ240361, AJ240362, AJ240363, AJ240364, AJ240365, AJ240366, AJ240367, AJ240368, AJ240369, AJ240370, AJ240371, AJ240372, AJ240373, AJ240374, AJ240375, AJ240376, AJ240377, AJ240378, AJ240379 (a) and AJ240293, AJ240294, AJ240295, AJ240296, AJ240297, AJ240298, AJ240299, AJ240300, AJ240301, AJ240302, AJ240303, AJ240304, AJ240305, AJ240306, AJ240307, AJ240308, AJ240309, AJ240310, AJ240311, AJ240312, AJ240313, AJ240314, AJ240315, AJ240316, AJ240317, AJ240318, AJ240319, AJ240320, AJ240321, AJ240322, AJ240323, AJ240324, AJ240325, AJ240326, AJ240327, AJ240328, AJ240329, AJ240330, AJ240331, AJ240332, AJ240333, AJ240334, AJ240335, AJ240336, AJ240337, AJ240338, AJ240339, AJ240340, AJ240341, AJ240342, AJ240343, AJ240344, AJ240345, AJ240346 (b). Sequences V303 and V304 are likely to be new germline V segment genes, as they have been found after immunization with a TI-2 antigen by others (EMBL/GenBank/DDBJ accession no. AF 045500), and we have found them repeatedly in responses to NP-Ficoll.
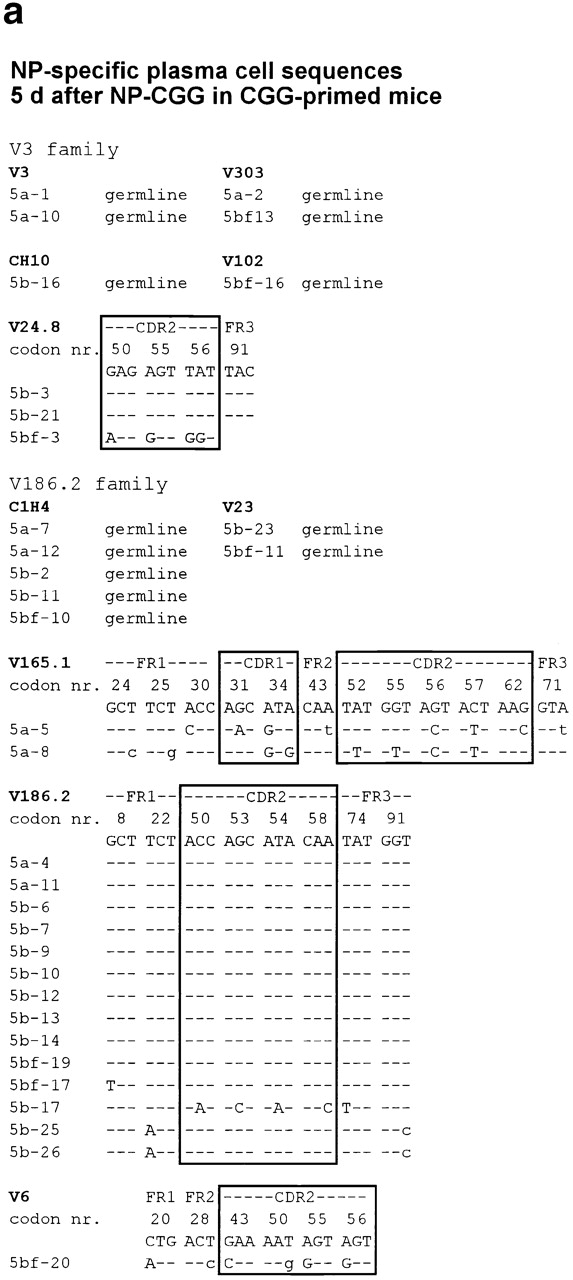
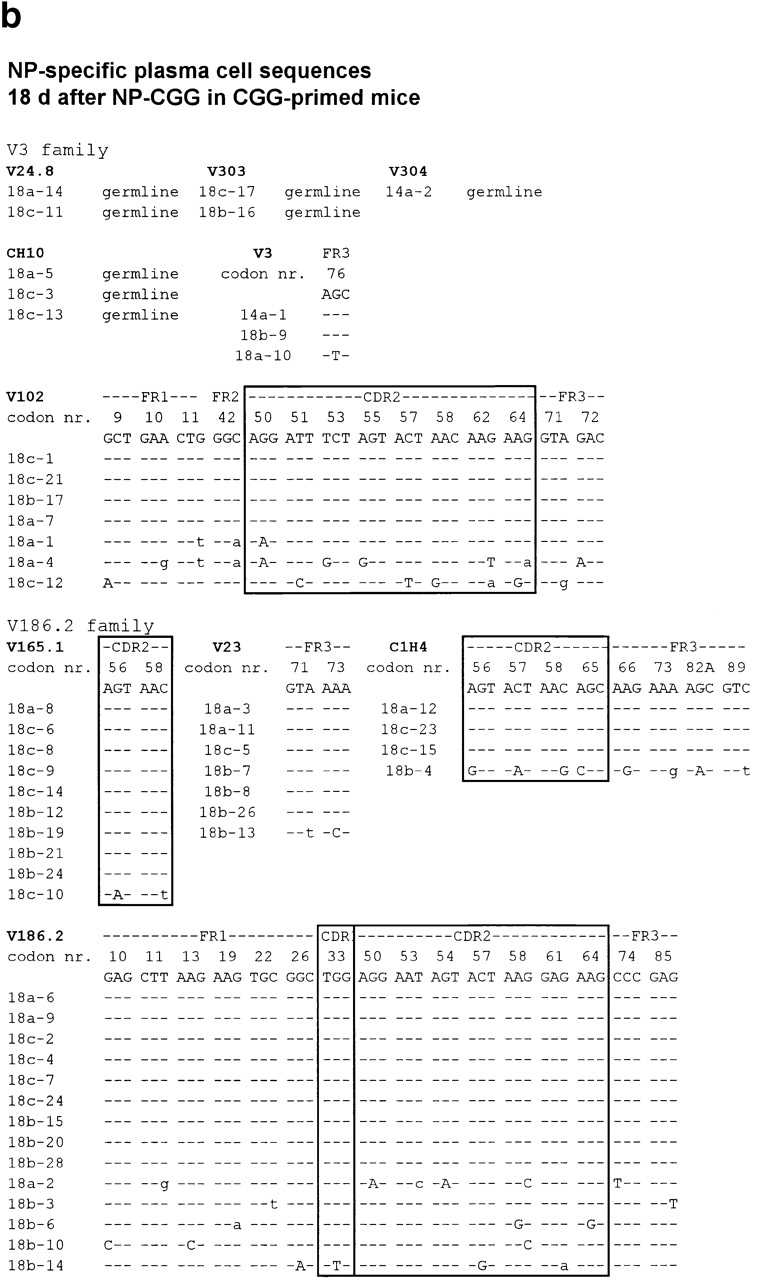
A further 53 sequences from NP-specific red pulp plasma cells were obtained at day 18. 12 of these had V region gene mutations (Table , and Fig. 5 b). All of the 53 sequences derived from NP-specific plasma cells on day 18 were from different clones, as judged by CDR3 diversity. Both the day 5 and day 18 sequences show evidence of antigen-driven selection; the numbers of silent and replacement mutations in framework sequences were comparable, whereas replacements were markedly greater than silent mutations in CDR1 and 2 (Table ).
The canonical V186.2 V segment associated with NP-specific cells in the response to NP-CGG in IgHb mice was used by 38% of the NP-specific plasma cells picked from day 5 sections and 26% of those taken at day 18. Only 1 of these V186.2-containing regions had the classical position 33 mutation from tryptophan to leucine, which increases the affinity of anti-NP antibodies using this V segment ∼10-fold 25. The broad range of anti-NP sequences seen in these mice, which were carrier-primed before immunizing with NP-CGG, is a feature of carrier priming; it is quite unlike the more restricted range of V regions seen in a primary response to NP-CGG 2 11 12. This effect of carrier priming has been analyzed in detail and will be reported elsewhere.
These sequencing studies reinforce the conclusions drawn from the BrdU pulse–chase studies that long-lived plasma cells in the spleen are derived partially from germinal centers, but that many are derived from plasmablast growth in extrafollicular foci, as the majority lacks Ig V region gene mutations.
Ig Class Switching Does Not Appear to Influence Which Splenic Plasma Cells Become Long-lived.
Sections from the pulse–chase experiment described above, in which BrdU was given during extrafollicular plasmablast growth, were triple-stained to identify the NP-specific plasma cells. These indicate: (a) whether these cells had taken up BrdU during the fourth day after immunization, and (b) whether they had switched to produce IgG. The results are shown in Table . They show no obvious influence of switch recombination on the life span of splenic plasma cells.
Table 3.
Lack of Correlation between the Class of Antibody Produced by Red Pulp Plasma Cells and Their Life Span
| Spleen code/ day assessed | CGG-specific IgG+ cells | CGG-specific IgG− cells | NP-specific IgG+ cells | NP-specific IgG− cells | ||||
|---|---|---|---|---|---|---|---|---|
| n/mm2 | % BrdU+ | n/mm2 | % BrdU+ | n/mm2 | % BrdU+ | n/mm2 | % BrdU+ | |
| A/day 5 | 134 | 57 | 40 | 30 | 20 | 50 | 30 | 23 |
| B/day 5 | 79 | 63 | 9 | 30 | 15 | 31 | 17 | 29 |
| C/day 5 | 69 | 56 | 10 | 23 | 11 | 55 | 45 | 40 |
| D/day 5 | 72 | 63 | 10 | 17 | 8 | 32 | 27 | 77 |
| Median/day 5 | 76 | 63 | 10 | 27 | 13 | 32 | 29 | 35 |
| E/day 6.5 | 20 | 38 | 2 | 20 | 4 | 31 | 3 | 13 |
| F/day 6.5 | 71 | 50 | 10 | 33 | 12 | 6 | 6 | 37 |
| G/day 6.5 | 9 | 38 | 2 | 21 | 2 | 25 | 2 | 17 |
| Median/day 6.5 | 20 | 38 | 2 | 21 | 4 | 25 | 3 | 17 |
| H/day 9 | 51 | 62 | 12 | 37 | 7 | 10 | 7 | 20 |
| I/day 9 | 29 | 45 | 4 | 23 | 17 | 22 | 8 | 19 |
| J/day 9 | 13 | 43 | 3 | 6 | 5 | 9 | 3 | 16 |
| K/day 9 | 23 | 28 | 4 | 23 | 6 | 27 | 8 | 23 |
| Median/day 9 | 26 | 44 | 4 | 23 | 7 | 16 | 8 | 20 |
The mice shown in this table were primed with CGG and boosted with NP-CGG. They were given a BrdU pulse between 72 and 96 h after immunization; the number of antigen-specific plasma cells and the proportions of these that had taken up BrdU during the pulse were assessed on the day after immunization indicated in the leftmost column.
Concluding Discussion.
Why are so many splenic plasma cells formed in extrafollicular foci lost within 3 d? The findings of this study argue against preordained early death among a subset of plasma cells; rather, it appears that a random proportion of all plasma cells dies. It has been suggested that CD11chigh dendritic cells found in extrafollicular foci of plasmablast growth play a role in plasmablast differentiation and their subsequent survival 5. When plasmablasts outgrow the number of these dendritic cells, surviving plasma cells are closely associated with the dendritic cells. Conversely, experimental conditions that expand the dendritic cell stroma have been found to be associated with survival of an increased number of plasma cells. This association with dendritic cells ceases to be as strong later in antibody responses when the plasma cells have mostly left extrafollicular foci and are scattered throughout the red pulp. This move of the plasma cells clears extrafollicular foci for further antibody responses. The signals or nutrients that sustain plasma cell survival in foci and the broader red pulp are still unknown.
The data obtained in this study are consistent with the following conclusions: (a) red pulp plasmablasts go through some five cycles of exponential growth over 3 d before coming out of cell cycle as plasma cells, or entering apoptosis; (b) mouse spleen can support the continued survival of a finite number of antibody-secreting cells (in the mice investigated, this is ∼20–100 cells/mm2 of red pulp; (c) when plasmablasts and plasma cell numbers exceed this capacity, the excess cells die after 2–3 d; and (d) after a few days, plasma cells leave the site of extrafollicular growth and become more scattered in the red pulp.
Extrafollicular plasmablast growth provides the first antibody produced after exposure to antigen; B cells differentiate into plasmablasts ∼1 d after cognate interaction with T cells 4 19, or activation by a T cell–independent antigen 5. This early production can be critical in the control of virus spread or neutralization of bacterial toxins. Antibody-producing cells do not leave germinal centers for a further 2–3 d 11 12. The later response in germinal centers can improve the quality of the antibody 11 12, although in certain infections there are sufficient high affinity virgin B cells to produce efficient neutralizing antibody in the extrafollicular response 26.
The scenario in which plasmablasts growth overshoots the capacity of stroma to sustain long-term plasma cell survival fulfills the requirement for early protective antibody production. At the same time, a lower level of sustained splenic antibody production protects where there is no follicular response—as for example in responses to some bacterial capsular polysaccharides.
Acknowledgments
The authors would like to acknowledge Garnet Kelsoe for his great patience and generosity in helping with our Ig V region gene mutation studies.
This work was supported by a programme grant from the British Medical Research Council. C. García de Vinuesa is supported by a Medical Research Council Clinical Research Fellowship, and D. Sze was supported by a United Birmingham Hospitals Trust grant.
Footnotes
Abbreviations used in this paper: BrdU, 5-bromo-2′-deoxyuridine; CGG, chicken gamma globulin; NP, (4-hydroxy-3-nitrophenyl) acetyl; QM, quasimonoclonal.
References
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Zhang J., Lane P.J.L., Chan E.Y.-T., MacLennan I.C.M. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. II. A common clonal origin for periarteriolar lymphoid sheath–associated foci and germinal centers. J. Exp. Med. 1992;176:679–688. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toellner K.-M., Gulbranson-Judge A., Taylor D.R., Sze D.M.-Y., MacLennan I.C.M. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García de Vinuesa C., Gulbranson-Judge A., Khan M., O'Leary P., Cascalho M., Wabl M., Klaus G.G.B., Owen M.J., MacLennan I.C.M. Dendritic cells associated with plasmablast survival. Eur. J. Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ho F., Lortan J.E., Khan M., MacLennan I.C.M. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- Smith K.G., Hewitson T.D., Nossal G.J.V., Tarlinton D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- Küppers R., Zhao M., Hansmann M.L., Rajewsky K. Tracing B-cell development in human germinal-centers by molecular analysis of single cells picked from histological sections. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner R., Hijmans W., Haaijman J.J. The bone marrowthe major source of immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- Tew J.G., DiLosa R.M., Burton G.F., Kosco M.H., Kupp L.I., Masuda A., Szakal A.K. Germinal centers and antibody production in bone marrow. Immunol. Rev. 1992;126:99–112. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Smith K.G., Light A., Nossal G.J., Tarlinton D.M. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Dutta P.R., Cerasoli D.M., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Slifka M.K., Antia R., Whitmire J.K., Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Manz R.A., Lohning M., Cassese G., Thiel A., Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- Cascalho M., Ma A., Lee S., Masat L., Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- Nossal G.J.V., Karvelas M. Soluble antigen abrogates the appearance of anti-protein IgG1-forming cell precursors during primary immunization. Proc. Natl. Acad. Sci. USA. 1990;87:1615–1619. doi: 10.1073/pnas.87.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M.W. Preparation of immunogens. In: Williams C.A., Chase M.W., editors. Methods in Immunology and Immunochemistry. Academic Press; New York: 1967. pp. 197–209. [Google Scholar]
- Luther S.A., Gulbranson-Judge A., Acha-Orbea H., MacLennan I.C.M. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J. Exp. Med. 1997;185:551–562. doi: 10.1084/jem.185.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible E.R. Principle and methods for the morphometric study of the lung and other organs. Lab. Invest. 1963;12:131–135. [PubMed] [Google Scholar]
- Kelsoe G., Reth M., Rajewsky K. Control of idiotope expression by monoclonal anti-idiotope and idiotope-bearing antibody. Eur. J. Immunol. 1981;11:418–423. doi: 10.1002/eji.1830110513. [DOI] [PubMed] [Google Scholar]
- Lundberg K.S., Shoemaker D.D., Adams M.W., Short J.M., Sorge J.A., Mathur E.J. High fidelity amplification using a thermostable DNA polymerase isolated from Pyroccocus furiosus . Gene. 1991;108:1–6. doi: 10.1016/0378-1119(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Sze D.M.-Y. The selection and selective use of the B cell repertoire. Ph.D. thesis 1998. University of Birmingham; Birmingham, UK: pp. 233 [Google Scholar]
- Wynford-Thomas D., Williams E.D. Use of bromodeoxyuridine for cell kinetic studies in intact animals. Cell Tissue Kinet. 1986;19:179–182. doi: 10.1111/j.1365-2184.1986.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Allen D., Cumano A., Dildrop R., Kocks C., Rajewsky K., Rajewsky N., Roes J., Sablitzky F., Siekevitz M. Timing, genetic requirements and functional consequences of somatic hypermutation during B cell development. Immunol. Rev. 1987;96:5–22. doi: 10.1111/j.1600-065x.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Kalinke U., Bucher E.M., Ernst B., Oxenius A., Roost H.P., Geley S., Kofler R., Zinkernagel R.M., Hengartner H. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus. Immunity. 1996;5:639–652. doi: 10.1016/s1074-7613(00)80277-0. [DOI] [PubMed] [Google Scholar]