Abstract
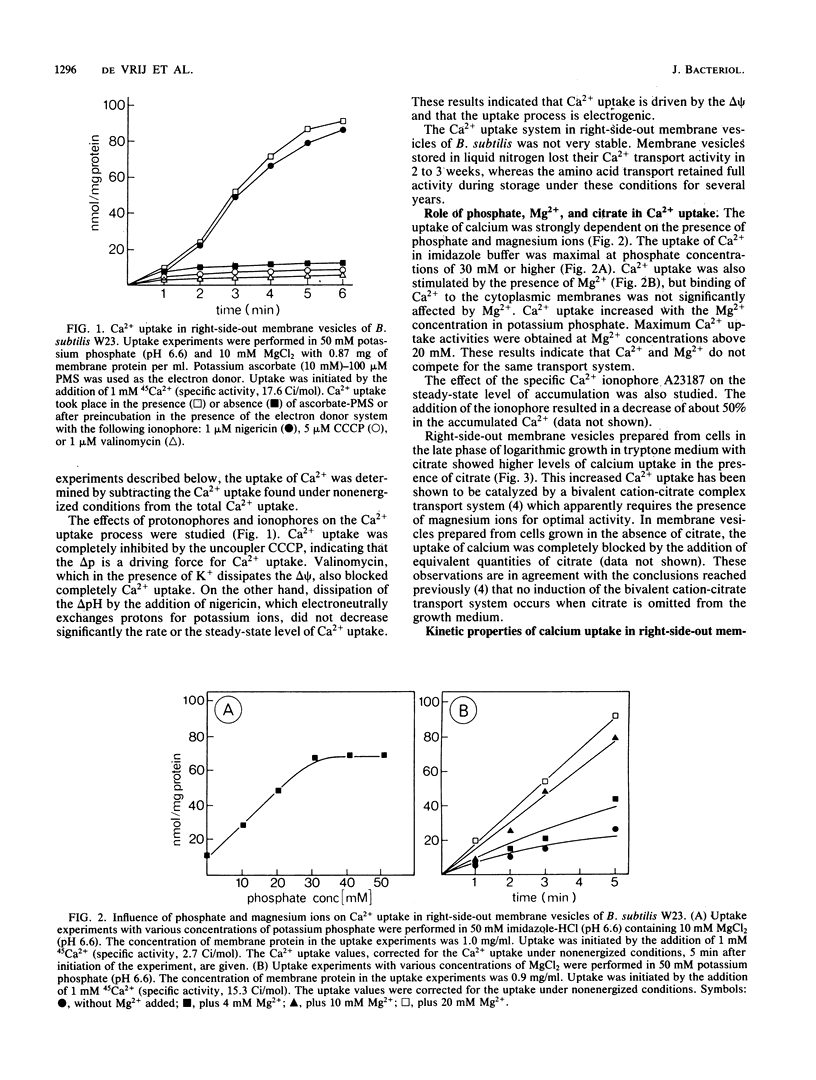
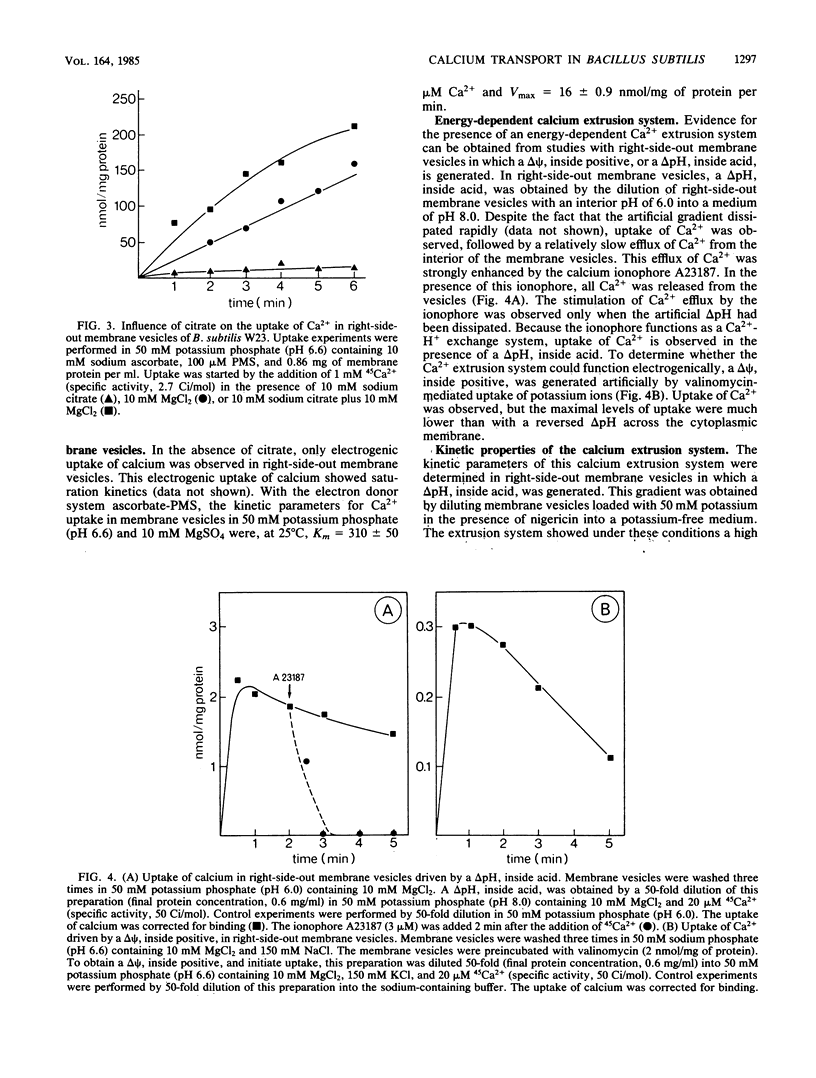
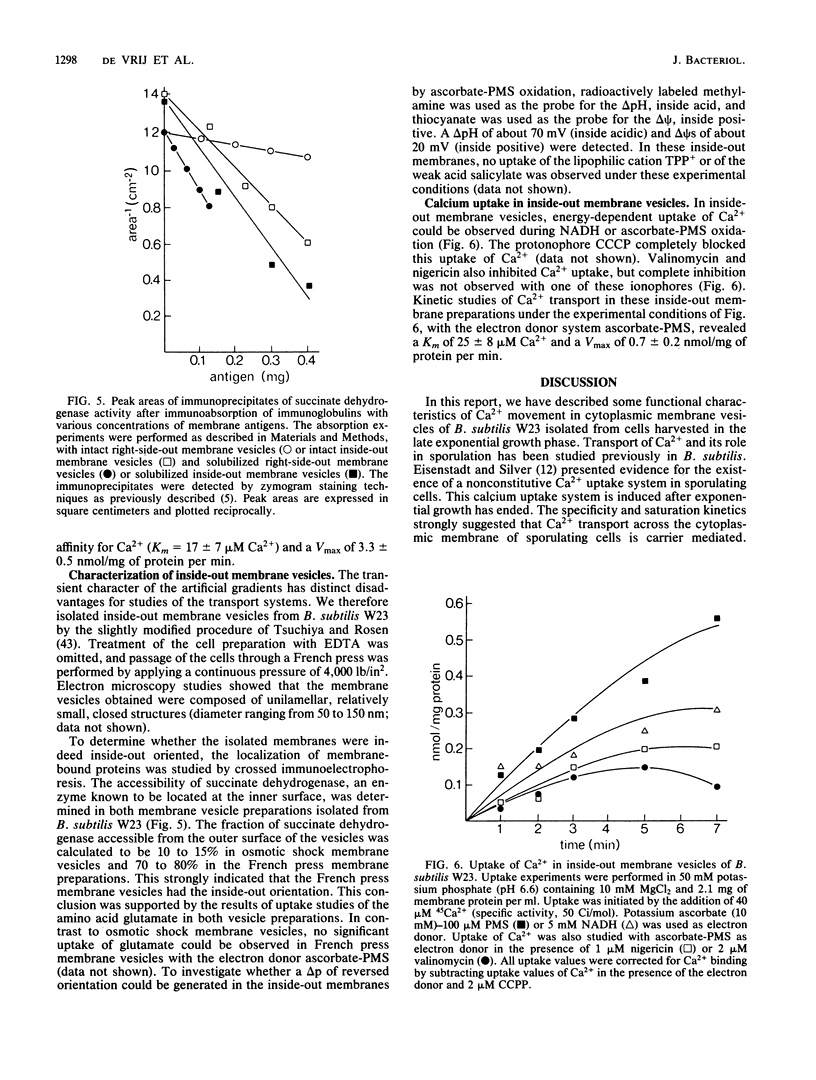
Right-side-out membrane vesicles of Bacillus subtilis W23 grown on tryptone-citrate medium accumulated Ca2+ under aerobic conditions in the presence of a suitable electron donor. Ca2+ uptake was an electrogenic process which was completely inhibited by carbonyl cyanide m-chlorophenylhydrazone or valinomycin and not by nigericin. This electrogenic uptake of calcium was strongly dependent on the presence of phosphate and magnesium ions. The system had a low affinity for Ca2+. The kinetic constants in membrane vesicles were Km = 310 microM Ca2+ and Vmax = 16 nmol/mg of protein per min. B. subtilis also possesses a Ca2+ extrusion system. Right-side-out-oriented membrane vesicles accumulated Ca2+ upon the artificial imposition of a pH-gradient, inside acid. This system had a high affinity for Ca2+; Km = 17 microM Ca2+ and Vmax = 3.3 nmol/mg of protein per min. Also, a membrane potential, inside positive, drove Ca2+ transport via this Ca2+ extrusion system. Evidence for a Ca2+ extrusion system was also supplied by studies of inside-out-oriented membrane vesicles in which Ca2+ uptake was energized by respiratory chain-linked oxidation of NADH or ascorbate-phenazine methosulfate. Both components of the proton motive force, the pH gradient and the membrane potential, drove Ca2+ transport via the Ca2+ extrusion system, indicating a proton-calcium antiport system with a H+ to Ca2+ stoichiometry larger than 2. The kinetic parameters of this Ca2+ extrusion system in inside-out-oriented membranes were Km = 25 microM and Vmax = 0.7 nmol/mg of protein per min.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Zlotnick G. W., Rosen B. P. Calcium efflux from Escherichia coli. Evidence for two systems. J Biol Chem. 1984 May 25;259(10):6142–6146. [PubMed] [Google Scholar]
- Ando A., Yabuki M., Kusaka I. Na+-driven Ca2+ transport in alkalophilic Bacillus. Biochim Biophys Acta. 1981 Jan 8;640(1):179–184. doi: 10.1016/0005-2736(81)90543-5. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. New histochemical techniques for the demonstration of tissue oxidase (cytochrome oxidase). J Histochem Cytochem. 1959 Mar;7(2):112–122. doi: 10.1177/7.2.112. [DOI] [PubMed] [Google Scholar]
- Bergsma J., Konings W. N. The properties of citrate transport in membrane vesicles from Bacillus subtilis. Eur J Biochem. 1983 Jul 15;134(1):151–156. doi: 10.1111/j.1432-1033.1983.tb07545.x. [DOI] [PubMed] [Google Scholar]
- Bergsma J., Strijker R., Alkema J. Y., Seijen H. G., Konings W. N. NADH dehydrogenase and NADH oxidation in membrane vesicle from Bacillus subtilis. Eur J Biochem. 1981 Dec;120(3):599–606. doi: 10.1111/j.1432-1033.1981.tb05742.x. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Op den Kamp J. A., van Deenen L. L. The distribution of lipids in the protoplast membranes of Bacillus subtilis. A study with phospholipase C and trinitrobenzenesulphonic acid. Eur J Biochem. 1977 Nov 1;80(2):381–391. doi: 10.1111/j.1432-1033.1977.tb11893.x. [DOI] [PubMed] [Google Scholar]
- Bisschop A., Doddema H., Konings W. N. Dicarboxylic acid transport in membrane vesicles from Bacillus subtilis. J Bacteriol. 1975 Nov;124(2):613–622. doi: 10.1128/jb.124.2.613-622.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H. Metabolic patterns in photosynthetic bacteria. Bacteriol Rev. 1951 Dec;15(4):183–210. doi: 10.1128/br.15.4.183-210.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. E., Bronner F. Bacterial calcium transport: energy-dependent calcium uptake by membrane vesicles from Bacillus megaterium. J Bacteriol. 1974 Sep;119(3):840–843. doi: 10.1128/jb.119.3.840-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A., Larsen B. Biosynthesis of alginate. II. Polymannuronic acid C-5-epimerase from Azotobacter vinelandii (Lipman). Carbohydr Res. 1971 Apr;17(2):297–308. doi: 10.1016/s0008-6215(00)82537-9. [DOI] [PubMed] [Google Scholar]
- Hellingwerf K. J., van Hoorn P. A polyvinylchloride-membrane based anion selective electrode for continuous registration of delta pH (interior alkaline) with salicylate as the indicator probe. J Biochem Biophys Methods. 1985 Aug;11(2-3):83–93. doi: 10.1016/0165-022x(85)90044-2. [DOI] [PubMed] [Google Scholar]
- Inesi G. Active transport of calcium ion in sarcoplasmic membranes. Annu Rev Biophys Bioeng. 1972;1:191–210. doi: 10.1146/annurev.bb.01.060172.001203. [DOI] [PubMed] [Google Scholar]
- Jasper P., Silver S. Divalent cation transport systems of Rhodopseudomonas capsulata. J Bacteriol. 1978 Mar;133(3):1323–1328. doi: 10.1128/jb.133.3.1323-1328.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Van Brunt J., Harold F. M. ATP-linked calcium transport in cells and membrane vesicles of Streptococcus faecalis. J Biol Chem. 1978 Apr 10;253(7):2085–2092. [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K. Induction of pleomorphy and calcium ion deficiency in Lactobacillus bifidus. J Bacteriol. 1970 Apr;102(1):217–220. doi: 10.1128/jb.102.1.217-220.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N. Active transport of solutes in bacterial membrane vesicles. Adv Microb Physiol. 1977;15:175–251. doi: 10.1016/s0065-2911(08)60317-3. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Daatselaar M. C.C. Transport of L-glutamate and L-aspartate by membrane vesicles of Bacillus subtilis W 23. FEBS Lett. 1972 Aug 15;24(3):260–264. doi: 10.1016/0014-5793(72)80368-5. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Konings W. N., Robillard G. T. Physical mechanism for regulation of proton solute symport in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5480–5484. doi: 10.1073/pnas.79.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Matin A., Konings W. N. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a pseudomonas species. Eur J Biochem. 1973 Apr 2;34(1):58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Silver S., Toth K., Scribner H. Facilitated transport of calcium by cells and subcellular membranes of Bacillus subtilis and Escherichia coli. J Bacteriol. 1975 Jun;122(3):880–885. doi: 10.1128/jb.122.3.880-885.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975 Oct 10;250(19):7687–7692. [PubMed] [Google Scholar]
- Zimniak P., Barnes E. M., Jr Characterization of a calcium/proton antiporter and an electrogenic calcium transporter in membrane vesicles from Azotobacter vinelandii. J Biol Chem. 1980 Nov 10;255(21):10140–10143. [PubMed] [Google Scholar]



