Abstract
Antibodies that bind to antigens expressed on the merozoite form of the malaria parasite can inhibit parasite growth by preventing merozoite invasion of red blood cells. Inhibitory antibodies are found in the sera of malaria-immune individuals, however, the specificity of those that are important to this process is not known. In this paper, we have used allelic replacement to construct a Plasmodium falciparum parasite line that expresses the complete COOH-terminal fragment of merozoite surface protein (MSP)-119 from the divergent rodent malaria P. chabaudi. By comparing this transfected line with parental parasites that differ only in MSP-119, we show that antibodies specific for this domain are a major component of the inhibitory response in P. falciparum–immune humans and P. chabaudi–immune mice. In some individual human sera, MSP-119 antibodies dominated the inhibitory activity. The finding that antibodies to a small region of a single protein play a major role in this process has important implications for malaria immunity and is strongly supportive of further understanding and development of MSP-119–based vaccines.
Keywords: Plasmodium, merozoite, invasion, human sera, malaria
Introduction
Infection by the protozoan parasite Plasmodium falciparum results in several hundred million clinical cases of malaria each year of which approximately two million are fatal. The development of a malaria vaccine is now a major global initiative. Progress toward this goal requires an understanding of the mechanisms that underpin both naturally acquired and vaccine-induced immunity. Antibodies that inhibit the growth of bloodstage P. falciparum parasites in vitro are found in the sera of some, but not all, individuals living in malaria endemic regions 1 2 3 4. Inhibitory antibodies are likely to contribute to the clinical immunity observed in highly exposed individuals but their overall significance to protection remains unclear 5 6.
Inhibitory antibodies function by preventing invasion of RBCs by the extracellular merozoite form of the parasite. A number of merozoite antigens have been shown to be targets of invasion inhibitory antibodies including some that localize to the merozoite surface, parasitophorous vacuole, and apical organelles. One target of inhibitory antibodies is the membrane-associated 19-kD COOH-terminal fragment of merozoite surface protein (MSP)-119, a molecule that is now a leading malaria vaccine candidate 7 8. MSP-119 is composed almost entirely of two cysteine-rich epidermal growth factor (EGF)-like domains that form a tightly packed, disc-like structure 9 10. The function of MSP-119 is unknown, however, allelic replacement experiments have shown that the function of most of the two EGF domains is conserved across distantly related Plasmodium species 11.
The MSP-119 EGF domains form reduction-sensitive epitopes that are recognized by invasion-inhibitory monoclonal and polyclonal antibodies 11 12 13 14 15. MSP-119–specific inhibitory antibodies are also present in the sera of individuals naturally exposed to P. falciparum 16. These antibodies recognize epitopes formed by the double EGF domain and by the second EGF domain alone 16. The mechanism of inhibition by MSP-119 antibodies is not fully understood, however, those that prevent the secondary processing of a precursor molecule and hence the formation of MSP-119 also effectively inhibit merozoite invasion of RBCs 17.
Here, by constructing a transfected P. falciparum line that expresses an antigenically distinct MSP-119 domain from the distantly related species P. chabaudi, we show that MSP-119–specific antibodies comprise a large component of the total invasion–inhibitory response of sera from many P. falciparum–immune adults from Papua New Guinea. Also, we show that considerable amounts of MSP-119–inhibitory antibodies are elicited in mice experimentally infected with a rodent malaria parasite P. chabaudi. This is the first time that the relative contribution of specific antibodies to the invasion-inhibitory activity of immune sera has been examined.
Materials and Methods
Plasmids.
Construction of the plasmids pPfM3′ and pPcM3′ has been described previously 11. The plasmid pPcMEGF was constructed by the insertion of a 1,200-bp XhoI fragment into the unique XhoI site of a plasmid pHC2 18. This target fragment comprises a 900-bp internal region of the P. falciparum MSP-1 gene fused in frame to the MSP-119 region of P. chabaudi. The fragment was generated by PCR amplification from P. falciparum (D10) and P. chabaudi (adami DS) genomic DNA (gDNA) using the oligonucleotide pairs Pf#1 5′-atttctcgag-aatccgaagataatgacg-3′, PfEGF-R 5′-GAAACATC- CAGCATTTTCTGGAAGTTTGTTCCTATGCATTGGTG- TTGTGAAATG-3′, and PcEGF-F 5′-CCAGAAAATGC- TGGATGTTTCAGATATGATGATGGTAAAGAAGAATG- G-3′, Pc3′ 5′-TCACTCGAGTTAAAATAAATTAAATACA- ATTAATGTG-3′. The resulting amplicons were sewn together via PCR for insertion into pHC2. The XhoI sites are shown in bold.
Parasite Culture and Transfection Procedures.
P. falciparum line D10 was cultivated and synchronized as per standard procedures 19 20. Ring-stage parasites (∼5% parasitemia) were transfected with 50–100 μg of CsCl-purified plasmid DNA as described previously 21 22 but using the electroporation conditions as described by Fidock and Wellems 23. After transfection and initial selection using 0.1 μM pyrimethamine for ∼4 wk, parasites were subjected to repeated cycles of 1 μM pyrimethamine for 3 wk proceeded by removal of the drug for 3–4 wk. gDNA was extracted from mixed trophozoite/schizont stage parasites as described previously 24, and Southern blot analysis was carried out using standard procedures 25.
Western Blot Analysis.
Parasite proteins were obtained from extracted enriched schizont or merozoite preparations and separated using 7.5 and 12% SDS-PAGE nonreducing gels, respectively, and transferred to PVDF membranes (Amersham Pharmacia Biotech). Membranes were probed with either mouse ascitic fluid containing 4H9/19, a monoclonal antibody specific for P. falciparum MSP-119 14, diluted 1:80,000 or rabbit αPcM19 polyclonal antibodies diluted 1:2,500 that are specific for P. chabaudi MSP-119 11. Horseradish peroxidase–conjugated rabbit anti–mouse (Dako) or sheep anti–rabbit (Silenus) Igs were used for detection, and bands were visualized by enhanced chemiluminescence (NEN Life Science Products).
Indirect Immunofluorescence.
For indirect immunofluorescence assay (IFA), D10-PfM3′ and D10-PcMEGF schizont-stage parasites were incubated with a mixture of 4H9/19 ascitic fluid and αPcM19 sera diluted 1:4,000 and 1:1,000, respectively. After incubation in the presence of a mixture of FITC-conjugated sheep anti–mouse and rhodamine-conjugated goat anti–rabbit Igs (Dako), both diluted 1:150, parasites were visualized by fluorescence microscopy. The same fields were photographed using filters to detect the FITC or rhodamine fluorochromes.
Sera.
The Papua New Guinean sera used were collected in the Madang Province from adults living in and around Madang town in 1980–82 (denoted PNG-M sera) and from adults currently living on Bagabag Island (denoted PNG-B sera). Both locations have high prevalence rates of P. falciparum (over all rates of 25.7 and 24%, respectively, with the highest rates observed in 1–9 yr-old children in both communities) that are indicative of intense transmission 26. Transmission in these localities is perennial with similar rates in the wet and dry seasons.
To generate P. chabaudi immune mouse sera (Pc immune), six 7-wk-old C57BL/6 male mice were injected intraperitoneally with 5 × 103 P. chabaudi (adami DS)–infected RBCs and rechallenged at 3 wk with the same dose. At weeks 7 and 21, mice were administered a higher challenge of 104 P. chabaudi–infected RBCs before serum collection at week 24.
MSP-119 Glutathione S Transferase Fusion Proteins.
The DNA sequence corresponding to the MSP-119 fragment lacking the glycosylphosphatidylinositol anchor sequence (amino acids Asn 1631–Ser 1723, according to reference 27) was amplified from P. falciparum D10 or HB3 gDNA (which contains the MAD20 or K1 MSP-119 alleles, respectively; reference 27) using the oligonucleotides: PfM19f 5′-cgcggatccaacatttcacaacaccaatgcg-3′ and PfM19r 5′-ggaagatcttaactgcagaaaataccatcgaaaag-3′. The corresponding sequence was amplified from P. chabaudi (adami DS) gDNA using the oligonucleotides: PcM19f 5′-CGCGGATCCGGTATAGGTTCTAATCATGTATG-3′ and PcM19r 5′-GGAAGATCTTAGCTACAGAATACACCATCATAAT-3′. The resulting PCR products were ligated into the BamHI site of pGEX-4T-1, expressed as glutathione S transferase (GST) fusion proteins in Escherichia coli 28 and purified using glutathine-sepharose as described by the manufacturer (Amersham Pharmacia Biotech). GST alone was produced using the pGEX-4T-1 plasmid.
ELISA.
Antibodies reacting with recombinant P. falciparum MSP-119 and P. chaubaudi MSP-119 were detected by ELISA. Microtitre plates (Dynex) were coated overnight at 4°C with 0.5 μg/ml recombinant protein diluted in carbonate buffer (0.015 M Na2CO3, 0.035 M NaHCO3, pH 9.6). Plates were washed three times with PBS containing 0.05% Tween 20 (PBST), blocked for 2 h at 37°C with PBST containing 10 mg/ml BSA, and washed again. Sera diluted in PBST containing 5 mg/ml BSA was then added to the plates (50 μl), which were then incubated further for 1 h at 37°C. After washing with PBST, horseradish peroxidase–conjugated sheep anti–human IgG (1:4,000; Silenus), rabbit anti–mouse IgG (1:5,000), or sheep anti–rabbit IgG (1:2,500; Dako) were added to the plates, incubated for 1 h at 37°C and, after washing with PBST, developed with H2O2 and 3,3′,5,5′-tetramethylbenzidine dihydrochloride for 10 min at room temperature. The reaction was stopped by the addition of 20 μl 2.5 M H2SO4 and plates were read at 450 nm. All human and mouse sera were tested in duplicate at three dilutions (1:1,000, 1:3,200, and 1:10,000) against GST-PfM19, GST-PcM19, and GST alone. The mean optical density (OD) value derived for GST alone was subtracted from the mean OD obtained for each GST fusion protein. Values at a 1:3,200 dilution were considered most likely to be on the slope of a titration curve, hence, these values are represented here.
Inhibition of Invasion Assays.
Ring-stage parasites were synchronized by sorbitol lysis twice at 4-h intervals and then allowed to mature through to trophozoite/schizont stages. The purified parasites were adjusted to 4% hematocrit with 0.5–2% infected RBCs and aliquots of 50 μl placed into the wells of a 96-well tray. An equal volume of serum, prediluted 1:10 in culture media, was added (resultant hematocrit of 2%) and cultures incubated for ∼26 h to allow for schizont rupture and merozoite invasion. Note that when two parasite lines were being compared the same batch of prediluted serum was added to each line. For the microscopy analysis, smears were made of the duplicate wells, stained with Giemsa, and the number of ring-stage parasites per 500 RBCs were determined for each well. The mean parasitemia from duplicate wells was calculated and this was expressed as a percentage of the mean parasitemia observed in parallel cultures of each parasite line in the presence of pooled human nonimmune sera (HNIS). For the [3H]hypoxanthine uptake assay, media was removed from triplicate wells at ∼24 h after cultivation and replaced with hypoxanthine-free media supplemented with [3H]hypoxanthine (10 μCi/ml). A further 24 h later, cultures of mature parasites were frozen and thawed to lyse infected RBCs. Samples were transferred to glass fiber filters via a cell harvester and quantitated using a scintillation counter. Statistical analysis to address whether the mean invasion rates of these sera were the same between two lines was performed using a two-sample Student's t test assuming unequal variances.
Cocultivation Assays.
For cocultivation, D10-PfM3′ and D10- PcMEGF ring-stage parasites were doubly synchronized as described above and then cultured together at an equal ratio in the presence of pooled sera. Sera was pooled on the basis of either appearing to contain significant proportions of anti–MSP-119 inhibitory antibodies (pool 2) or having a less inhibitory effect between the two parasite lines (pool 1). The pools included the following sera: (PNG-B pool 1) 8, 247, 322, and 962; (PNG-B pool 2) 413, 604, 614, and 954; (Pc-immune pool 1) 2 and 4; and (Pc-immune pool 2) 1, 3, 5, and 6. Additional controls included a HNIS pool and αPcM19 rabbit serum. Parasites were smeared at the trophozoite/schizont stage (i.e., every 2 d beginning at day 1) and assessed by indirect IFA using a mixture of 4H9/19 and αPcM19 as described above. For each of the triplicate slides, FITC (D10-PfM3′) and rhodamine (D10-PcMEGF) were observed and counted in 16 individual fields that each contained at least 10 mature (pigmented) parasites. In total, between 400 and 1,400 mature stage parasites were counted for each cocultivation sample.
Results
Replacement of Complete EGF Domains of P. falciparum MSP-119 with those from P. chabaudi.
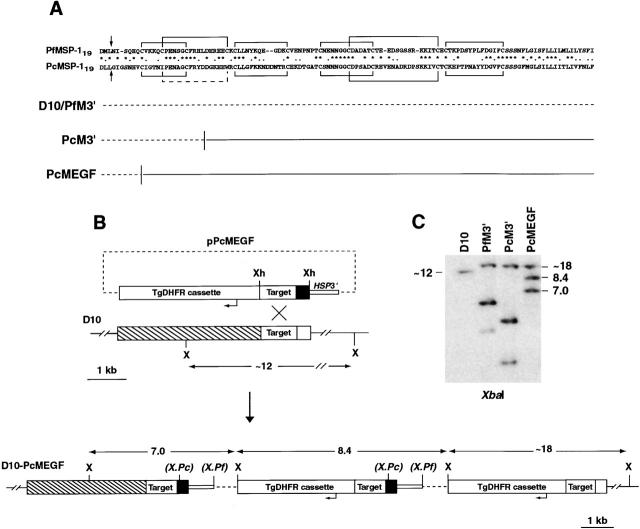
The aim of this study was to generate a P. falciparum line that possesses an antigenically distinct MSP-119 domain and to investigate whether this line differs from parental parasites in its susceptibility to inhibition by sera from malaria-immune individuals. We have described previously the construction of a parasite line D10-PcM3′ which expresses an MSP-1 chimera in place of the endogenous molecule 11. This chimera incorporates ∼3/4 of the two EGF-like domains that comprise MSP-119 from the divergent rodent malaria P. chabaudi (Fig. 1 A). We also constructed a control transfectant D10-PfM3′ which expresses endogenous MSP-1 (Fig. 1 A; reference 11). These transfected lines displayed no observable phenotypic differences to parental D10 parasites revealing that the function of most of MSP-119 is conserved across divergent Plasmodium species. Here we describe transfection of a plasmid, pPcMEGF, designed to replace the entire EGF domains from MSP-119 with those from P. chabaudi (Fig. 1). Upon transfection and drug cycling, pPcMEGF was shown to have integrated into the MSP-1 gene. The transfected population, D10-PcMEGF, was cloned and two randomly selected clones (D10-PcMEGF.1 and D10-PcMEGF.2) were analyzed further. Southern blot analysis showed the plasmid had integrated into the target site through the expected recombination event replacing the entire endogenous P. falciparum MSP-119 EGF domains with those from P. chabaudi. This line could be distinguished from both D10-PfM3′ and D10-PcM3′ by restriction endonuclease digestion with XbaI (Fig. 1B and Fig. C).
Figure 1.
Generation of a transfected P. falciparum line containing the complete MSP-119 EGF domains from P. chabaudi in place of the endogenous molecule. (A) Alignment of MSP-119 sequences from P. falciparum (MAD20 allele; GenBank/EMBL/DDBJ accession no. M19143) and P. chabaudi (adami DS line; GenBank/EMBL/DDBJ accession no. AF149303). The arrows indicate the sites of secondary cleavage, asterisks denote identical residues, and dots highlight conserved residues. The disulfide bonds expected for EGF-like domains are shown (black lines). Note the absent disulfide bond in P. chabaudi (dashed line). The nature of the gene fusions in the various MSP-1 hybrid lines is represented underneath the alignment with the dashed line representing endogenous P. falciparum sequence and the solid line P. chabaudi sequence. (B) The plasmid pPcMEGF was constructed by ligating a DNA fragment containing P. falciparum MSP-1 sequence (Target) fused to sequence encoding MSP-119 from P. chabaudi MSP-1 (dark shading) into the XhoI site of pHC2. The predicted structure of the MSP-1 loci following integration of pPcMEGF and the location of the XbaI (X) sites used to map these loci are shown. The location of XbaI sites unique to D10-PcM3′ and D10-PfM3′ are bracketed and are represented as X.Pc and X.Pf, respectively. All sizes are to scale with the exception of the plasmid backbone (dashed line). (C) Southern blot analysis of gDNA restricted with XbaI showing that pPcMEGF had integrated into MSP-1 as predicted and that the resultant line (D10-PcMEGF) differs from the previously established lines D10-PfM3′ and D10-PcM3′ 11. The 0.9-kb PfMSP-1 fragment (Target) was used to probe the blot.
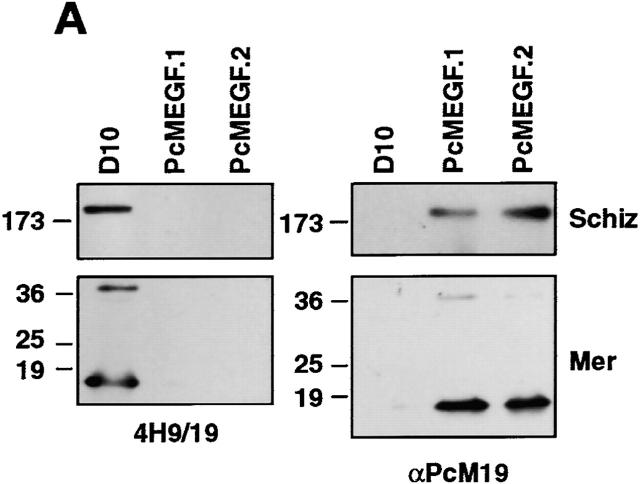
To determine if the chimeric MSP-1 was expressed in D10-PcMEGF parasites, extracts of mature schizonts and free merozoites were examined by immunoblot analysis (Fig. 2 A). Bands corresponding to endogenous full-length MSP-1 (∼200 kD) and MSP-119 (∼18 kD) were detected in parental D10 schizonts and merozoites, respectively, using the P. falciparum–specific antibody 4H9/19 14. No reactive bands were observed in extracts from the two D10-PcMEGF clones. Conversely, when replicate immunoblots were probed with a rabbit antiserum specific for P. chabaudi MSP-119 (αPcM19; reference 11), species corresponding to both forms of MSP-1 were observed in the D10-PcMEGF extracts but not in parental D10 (Fig. 2 A). The larger band (∼40 kD) in the merozoite samples is consistent with the presence of the primary MSP-1 processing product, MSP-142. The localization of the MSP-1 chimera was assessed by an IFA (Fig. 2 B). D10-PfM3′ and D10-PcMEGF parasites were incubated with a mixture of mouse 4H9/19 and rabbit αPcM19 antibodies followed by FITC-labeled anti–mouse (to detect endogenous MSP-1) and rhodamine-labeled anti–rabbit (to detect the MSP-1 chimera) IgG. “Grape-like” fluorescence was observed in both lines indicative of merozoite surface labeling. D10-PcMEGF parasites showed only rhodamine fluorescence supporting the absence of endogenous MSP-119 expression in this line. Fluorescence was also observed in ring-stage parasites indicating that the P. chabaudi MSP-119 domain is carried into the newly invaded RBCs in D10-PcMEGF parasites as has been described for P. falciparum MSP-119 (data not shown; references 11 and 12).
Figure 2.
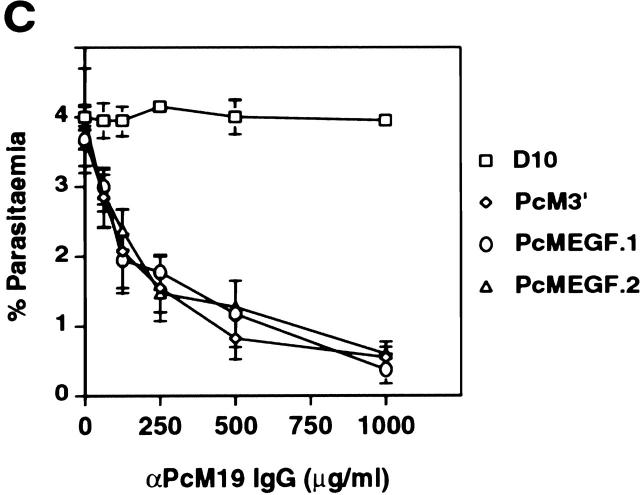
Transfected D10-PcMEGF parasites express a functional MSP-1 chimera. (A) Western blot analysis of parasite proteins from extracted enriched schizont (Schiz) or merozoite (Mer) preparations of parental D10 and the D10-PcMEGF clones (PcMEGF.1 and PcMEGF.2). Proteins were separated by SDS-PAGE under nonreducing conditions, transferred to PVDF membranes, and probed with either 4H9/19 or αPcM19 antibodies as indicated. The position of molecular weight standards are shown to the left and are in kD. (B) Localization of MSP-1 expressed in the transfected lines by indirect IFA. D10-PfM3′ (PfM3′) and D10-PcMEGF.1 (PcMEGF.1) schizont-stage parasites were incubated with a mixture of 4H9/19 and αPcM19 antibodies. After incubation in the presence of a mixture of FITC-conjugated anti–mouse and rhodamine-conjugated anti–rabbit Igs, parasites were visualized by microscopy. Original magnification: 1,000×. The same fields were photographed under fluorescence conditions to detect the FITC or rhodamine fluorochromes. (C) In vitro inhibition assays of D10, D10-PcM3′, and D10-PcMEGF clones (PcMEGF.1 and PcMEGF.2) with different concentrations of αPcM19 antibodies (IgG). Error bars represent SDs.
To ensure that the chimeric MSP-1 was functional, an in vitro inhibition of invasion assay was carried out (Fig. 2 C). Mature stage parasites from parental D10, D10-PcM3′, and two clones from D10-PcMEGF were incubated in the presence of αPcM19 IgG. These antibodies specifically inhibited RBC invasion of D10-PcMEGF and D10-PcM3′ parasites in a dose-dependent manner but had no effect on parental D10. These results are consistent with the correct expression, processing, localization and functioning of the expected hybrid MSP-1 molecule in D10-PcMEGF parasites. This also reveals that the complete EGF domains of MSP-119 are functionally conserved across distantly related Plasmodium species.
Invasion-inhibition of Transfected P. falciparum Parasites by Immune Sera Reveals an Important Role for MSP-119–specific Antibodies.
The availability of parasite lines that are identical except for the presence of antigenically distinct MSP-119 domains provided a unique opportunity to address the relative importance of MSP-119 antibodies to invasion-inhibition by immune sera. Sets of human sera were obtained from adults in two malaria endemic areas in Papua New Guinea that have intense transmission rates of P. falciparum (PNG-M and PNG-B). The majority, if not all, of these individuals are likely to be clinically immune to P. falciparum malaria. Sera from six C57BL/6 mice that had been repeatedly infected with P. chabaudi were also generated (Pc-immune sera). To determine the presence and the specificity of MSP-119 antibodies, each human and mouse serum was tested in ELISA against recombinant forms of P. falciparum MSP-119 (GST-PfM19) and P. chabaudi MSP-119 (GST-PcM19). All PNG-B sera (47/47) and most PNG-M sera (27/33) reacted against GST-PfM19 while only five human sera (all from PNG-B) showed detectable cross-reactivity with GST-PcM19 (Table ). The OD450 values against GST-PcM19 of these five cross-reactive PNG-B sera ranged from 0.277 to 0.900 (mean = 0.451). The remaining 42 PNG-B serum samples had OD450 values against GST-PcM19 below 0.113.
Table 1.
Reactivity in ELISA of Sera from Malaria-infected Humans and Mice Against Recombinant P. falciparum and P. chabaudi MSP-119
| Mean OD450nm | |||||
|---|---|---|---|---|---|
| Sera | n | GST-PfM19 | GST-PcM19 | GST-PfM19+ | GST-PcM19+ |
| PNG-B | 47 | 0.846 ± 0.200 | 0.072 ± 0.158 | 47 (100%) | 5 (11%) |
| PNG-M | 33 | 0.481 ± 0.374 | 0.022 ± 0.032 | 27 (82%) | 0(0%) |
| Pc immune | 6 | 0.016 ± 0.012 | 0.613 ± 0.197 | 0(0%) | 6(100%) |
The six mouse sera showed no reactivity to GST-PfM19 but each showed strong reactivity with GST-PcM19. These results reveal that MSP-119 antibodies were generated in response to infection with either P. falciparum or P. chabaudi and that these were mostly highly specific for the homologous MSP-119 domain. The P. falciparum MSP-119 sequence of GST-PfM19 represented the “MAD20” allele; however, each serum was also tested in parallel with a GST-MSP-119 fusion protein comprising “KI/Wellcome” allelic sequence 27 29. The OD readings against this fusion protein were very similar to those obtained for the “MAD20” allele across all PNG-B and PNG-M sera (R2 = 0.914). This cross-reactivity between alleles is consistent with the findings of others 30.
Preliminary experiments in our laboratory had indicated that D10-PcMEGF were relatively resistant to inhibition by human sera from malaria-immune individuals. To explore this more thoroughly, all PNG-M, PNG-B, and Pc-immune mouse sera were assessed for their ability to inhibit invasion of D10 and D10-PcMEGF merozoites in a microscopy-based invasion inhibition assay. All sera were tested in the one assay with the same parasite preparations (assay 1; Fig. 3 A). PNG-B sera were relatively effective at inhibiting invasion of parental D10 parasites with a mean invasion of 26.7%. PNG-M sera were generally less inhibitory of D10 parasites (43.1%). The difference between PNG-B and PNG-M sera, both in invasion-inhibition and total MSP-119 antibodies (Table ), may simply reflect a loss of potency of PNG-M sera over relatively long-term cryopreservation period (∼20 yr) although this was not explored further.
Figure 3.
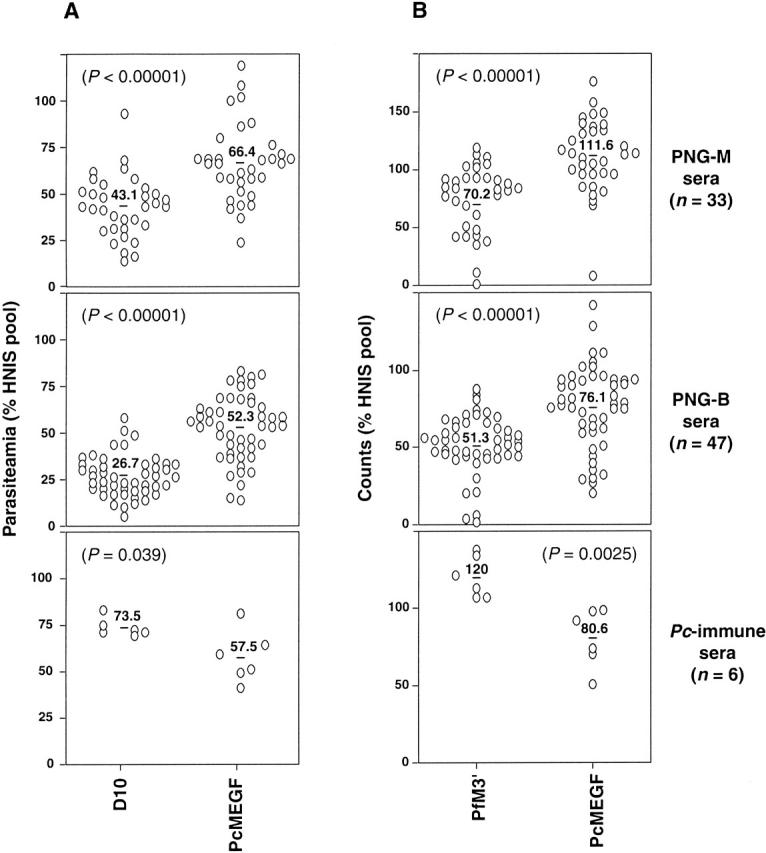
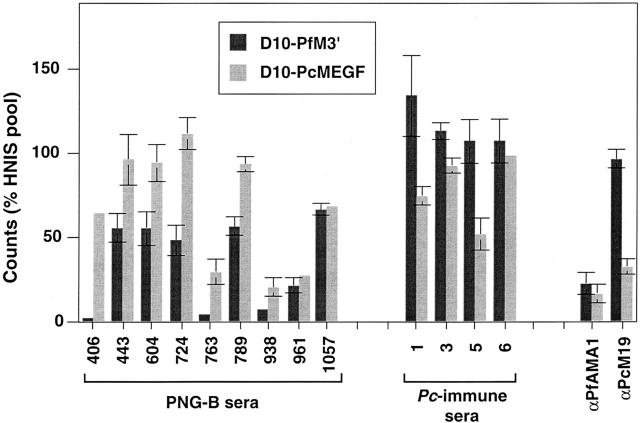
Invasion inhibition of transfected P. falciparum parasites expressing divergent MSP-119 domains by sera from clinically immune individuals reveals an important role for MSP-119–specific antibodies. (A) Assay 1, microscopy. Microscopy-based invasion inhibition assay involving the detection of ring-stage D10 and D10-PcMEGF (PcMEGF) parasites after cultivation in the presence of each individual serum. (B) Assay 2, hypoxanthine uptake. Alternative invasion-inhibition assay comparing D10-PfM3′ (PfM3′) and D10-PcMEGF parasites using [3H]hypoxanthine uptake as a measure of parasite growth. Invasion is represented as either parasitemia (A) or counts (B) and is expressed as a percentage of the invasion observed in parasites cultured in negative control sera (HNIS). The means of samples within a serum set against each parasite line are indicated. P values from a Student's t test comparing the means in each panel are shown.
Strikingly, we found that both PNG-B and PNG-M sera were generally much less effective at inhibiting the invasion of D10-PcMEGF merozoites (Fig. 3 A). Here, mean invasion rates of 52.3 and 66.4% were obtained for PNG-B and PNG-M, respectively, which in both cases was ∼25% higher than that obtained for D10. In contrast, the Pc-immune sera were more effective at inhibiting D10-PcMEGF (mean invasion rate 57.5%) than parental D10 (mean invasion 73.5%). In each case, the difference in the mean invasion rate was either significant or highly significant (Fig. 3 A).
In an attempt to independently confirm these results, the inhibitory potential of these sera was tested by a different assay that utilizes [3H]hypoxanthine uptake as a measure of parasite growth (assay 2; Fig. 3 B). In this assay, D10-PfM3′ was used as the parental control instead of D10 and again all sera were tested together in the one assay. The results were similar to those obtained in assay 1 with D10-PfM3′ parasites more susceptible than D10-PcMEGF to inhibition by PNG-M and PNG-B sera. Again, as in Fig. 3 A, D10-PcMEGF parasites were more susceptible than D10-PfM3′ to inhibition by Pc-immune sera. In each case, the difference in the mean invasion rates was highly significant. These results are consistent with a major role for MSP-119 antibodies in invasion/growth inhibition by malaria immune sera.
Fig. 4 shows inhibition results (from assay 2) that are representative of the data obtained for individual sera. Although some individual human sera did not appear to contain high levels of P. falciparum MSP-119–specific inhibitory antibodies (e.g., 938, 961, and 1,057), a major proportion of the invasion-inhibitory component of other samples was directed against MSP-119 (eg, 406, 604, 724). Most human samples (59/80) showed some level of P. falciparum MSP-119–specific inhibitory antibodies in either assay 1 or 2. All Pc-immune sera had detectable levels of P. chabaudi MSP-119–specific inhibitory antibodies in either assay 1 or 2. Results for the two control sera used in assay 2 are also shown (Fig. 4). The first was a polyclonal rabbit anti–P. falciparum AMA-1 IgG 31 used at a concentration of 250 μg/ml and the second was αPcM19 purified IgG used at a concentration of 750 μg/ml. Both lines were equally susceptible to inhibition by αAMA-1 IgG, whereas only D10-PcMEGF was inhibited with αPcM19.
Figure 4.
Invasion-inhibition assay with representative individual sera from PNG-B and Pc-immune serum sets against D10-PfM3′ and D10-PcMEGF parasite lines. Samples were selected from assay 2 and represent typical examples of the inhibitory activities observed. The results obtained for the control sera in assay 2, anti–P. falciparum AMA-1 (αPfAMA1), and αPcM19 IgG are shown. Error bars represent the range observed in duplicate samples.
We also examined if there was any relationship between the amounts of MSP-119–specific invasion-inhibitory antibody and total MSP-119 IgG. MSP-119 invasion-inhibitory antibody in each human serum was calculated from microscopy-based assay by subtracting the percentage of invasion for D10-PfM3′ from the value obtained with D10-PcMEGF. These values showed no correlation with the OD450 readings obtained for each serum against GST-PfM19 antigen (R2 = 0.013 and 0.0003 for PNG-M and PNG-B sera, respectively). However, it should be noted that four of the six PNG sera that were negative for GST-PfM19 antibodies (at a 1:3,200 dilution) in ELISA (Table ) also showed no detectable levels of MSP-119–specific invasion-inhibitory antibodies. The amount of P. chabaudi MSP-119–specific inhibitory antibody present in individual Pc-immune sera also showed no relationship to total P. chabaudi MSP-119–specific IgG (R2 = 0.0048).
Cocultivation Assays in the Presence of Sera from Immune Individuals Support a Major Role for MSP-119 Antibodies in Growth Inhibition.
As an alternative means of addressing the specificity of the inhibitory antibodies in immune sera for MSP-119, D10-PfM3′ and D10-PcMEGF parasites were cocultivated at an equal ratio in the presence of pooled sera. Several individual sera were pooled on the basis of the amount of anti–MSP-119–inhibitory antibody determined by the inhibition assays described above. Those with lower levels of MSP-119–specific inhibitory antibody comprised pool 1 while those with more apparent MSP-119–inhibitory antibody comprised pool 2. Parasites were detected by indirect IFA using a mixture of 4H9/19 and αPcM19 to detect D10-PfM3′ and D10-PcMEGF, respectively. The insert for Fig. 5 shows a typical field after incubation with pooled HNIS showing similar numbers of D10-PfM3′ (green) and D10-PcMEGF (red) parasites and illustrates the ease with which the two different lines were visualized in the mixed culture.
Figure 5.
Cocultivation of D10-PfM3′ and D10-PcMEGF parasites in the presence of immune sera confirms an important role for MSP-119 antibodies in invasion inhibition. Ring-stage D10-PfM3′ and D10-PcMEGF parasites were combined at an equal ratio and cultured in the presence of the pooled sera indicated at right. Smears from days 1 and 5 were analyzed by double-labeling IFA. Mature stage (pigmented) green and red parasites were counted in 16 fields each containing at least 10 parasites. The same fields were observed by fluorescence microscopy using filters to detect the FITC or rhodamine fluorochromes. Results are expressed as a ratio of D10-PfM3′ to D10-PcMEGF. A representative field of parasites (at day 3) cultured in the presence of HNIS pool is shown (inset).
Red and green fluorescent parasites were counted after 1 and 5 d of cocultivation in the presence of the different pooled sera. After 1 d of culture, where parasites were expected to have matured but not reinvaded fresh RBCs, no change in parasite ratio was observed with any sera. Cocultivation in the presence of HNIS for 5 d also had no effect on the ratio of the two parasite lines confirming that D10-PfM3′ and D10-PcMEGF have very similar growth rates (Fig. 5). However, incubation of the parasite mix with αPcM19 or Pc-immune sera had a dramatic effect on parasite ratio with the number of D10-PfM3′ parasites in 3–4-fold excess of D10-PcMEGF. In contrast, incubation with PNG-B–pooled sera had the opposite effect. It is important to note that the human and mouse sera pooled on the basis of possessing the most MSP-119–inhibitory antibody in the aforementioned assay (Fig. 3) also exhibited the greatest growth inhibition here.
Discussion
These data reveal a major role for MSP-119–specific antibodies in mediating the invasion-inhibitory effect of sera from P. falciparum–immune adult humans and from P. chabaudi–immune mice. This study was made possible by the use of allelic replacement to derive a P. falciparum parasite line, D10-PcMEGF, that expresses the antigenically distinct MSP-119 domain from P. chabaudi which allowed comparisons of this line with control P. falciparum lines that are identical to D10-PcMEGF, except for their MSP-119 domains. Our central conclusion was the consistent finding of three different approaches. First, we used a standard microscopy-based invasion-inhibition assay to show that D10-PcMEGF parasites were generally far less susceptible to inhibition by P. falciparum–immune human sera than the parent D10 line. Conversely, in this same assay, it was also evident that D10-PcMEGF was considerable more susceptible to inhibition by P. chabaudi–immune mouse sera than D10 parasites.
Secondly, we obtained very similar results using an independent approach that involves [3H]hypoxanthine uptake as a measure of parasite growth. The percentage invasion values in the [3H]hypoxanthine uptake assays were generally higher than those observed in the microscopy-based invasion assays and sometimes values of >100% were observed. This most likely reflects the fact that this assay is a measure of parasite growth (rather than invasion) and that parasite metabolism can differ in the presence of certain sera, perhaps because of variations in exogenous folate levels 32. However, this does not influence the finding that D10-PcMEGF and D10-PfM3′ parasites showed a dramatically altered susceptibility to the same malaria-immune sera in this assay.
Finally, we showed that malaria-immune sera exerted strong selective effects on cocultivated P. falciparum parasite lines that differed only in their MSP-119 domains. Growth of the mixed D10-PcMEGF and D10-PfM3′ parasite lines in the presence of P. falciparum–immune human sera enriched for D10-PcMEGF. The opposite effect was observed with sera from P. chabaudi–immune mice. Taken together, all three approaches strongly indicated that MSP-119–specific antibodies play a major invasion inhibitory role in malaria-immune sera.
The significant contribution of just one antibody specificity to the inhibitory effect of immune sera is surprising given that antibodies directed against a wide range of merozoite antigens are known to inhibit RBC invasion. However, our results are consistent with epidemiological evidence linking the presence of MSP-119–specific antibodies to protection from clinical malaria 33 34 35. Together with a considerable body of evidence highlighting the protective efficacy of MSP-119–specific antibodies, our data supports a significant role for MSP-119–specific invasion inhibitory antibodies in immunity to malaria in highly exposed individuals. An examination of sera from longitudinal studies, which involves testing individuals with a known clinical outcome, using the assays described here would directly address the importance of inhibitory MSP-119 antibodies to protection from natural infection. Such a study could also be performed in mice experimentally infected with P. chabaudi where clinical signs are carefully monitored.
Although our results are supportive of the development of MSP-119–based vaccines, as with our previous study 11, they also confirm that this domain is not tightly constrained by function and can accommodate considerable antigenic diversity. Considering also that the data in this paper suggests that MSP-119 is under considerable selection pressure in the field, it remains somewhat of a mystery as to why MSP-119 is relatively conserved in P. falciparum isolates. It is possible that single point mutations that lead to antibody escape result in structural instability of MSP-119 such that only certain amino acid changes are accommodated or even that compensatory mutations elsewhere in the molecule are required for the integrity of the domain to be maintained. Such a circumstance would make it relatively difficult to select for escape mutants and would explain why more extensive antigenic diversity in P. falciparum MSP-119 has not emerged since the relatively recent evolutionary “bottleneck” that has been proposed for P. falciparum 36. Allelic replacement of MSP-119 domains provides a unique approach to map inhibitory epitopes in polyclonal immune sera and to test if individual point mutations in these epitopes leads to viable parasites that are less susceptible to inhibitory antibodies. With respect to this, it is worth noting that the susceptibility of P. falciparum lines expressing partial (D10-PcM3′) and complete (D10-PcMEGF) P. chabaudi EGF domains were equally susceptible to anti–P. chabaudi MSP-119 rabbit antisera (αPcM19; Fig. 2 C). This indicates that the inhibitory epitopes recognized by αPcM19 are not in close proximity to the secondary processing site.
MSP-119–specific antibodies in human immune sera include a mix of inhibitory and noninhibitory antibodies as well as “blocking” antibodies that interfere with the inhibitory effect of MSP-119 antibodies 17 37. Hence, measurement of total MSP-119 IgG is unlikely to be an accurate correlate of immunity. Consistent with this, we found no correlation between total MSP-119 IgG and amount of inhibitory MSP-119 antibodies present in the individual sera tested in this study. The availability of a suitable in vitro correlate of protection assay is particularly important to assess the efficacy of MSP-119–based vaccines; however, the mechanism of immunity induced by these vaccines is not completely understood. In vaccinated Aotus monkeys, the presence of P. falciparum MSP-1 processing–inhibitory antibodies has not proven to be a good predictor of immunity 38 39. This has suggested that other mechanisms of immunity may contribute to protection from MSP-119 vaccines in this system, including the possibility that some MSP-119 antibodies may interfere with an event other than the secondary processing of MSP-1. In the rodent P. yoelii, challenge system immunity induced by MSP-119–based vaccines is clearly mediated by antibodies although it is not known how these antibodies exert their protective effect 40 41 42. The ability to quantitate MSP-119–specific inhibitory antibodies in serum using the parasite lines and approaches described here may prove valuable for the prediction of vaccine efficacy in P. falciparum or P. chabaudi MSP-119 vaccine trials. Such an assay should be especially useful in determining if MSP-119–inhibitory antibodies are elicited by combination vaccines that include MSP-119.
Acknowledgments
We thank Terry McElwain and Graham Brown for helpful advice and input into this study, Robin Anders for the provision of P. falciparum AMA-1 antiserum, Allan Saul and Laura Martin for monoclonal antibody 4H9/19, and the Australian Red Cross Blood Service for the provision of human blood and serum. The use of Papua New Guinean sera is approved by the Medical Research Advisory Council of PNG (MRAC No. 01.05).
This work was supported by the National Health and Medical Research Council of Australia. R.A. O'Donnell is the recipient of an Australia Postgraduate Research Award and B.S. Crabb is a Howard Hughes Medical Institute International Research Scholar.
Footnotes
Abbreviations used in this paper: EGF, epidermal growth factor; GST, glutathione S transferase; HNIS, human nonimmune sera; IFA, immunofluorescence assay; MSP, merozoite surface protein; OD, optical density.
References
- Marsh K., Otoo L., Hayes R.J., Carson D.C., Greenwood B.M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Brown G.V., Anders R.F., Mitchell G.F., Heywood P.F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro . Nature. 1982;297:591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- Brown G.V., Anders R.F., Knowles G. Differential effect of immunoglobulin on the in vitro growth of several isolates of Plasmodium falciparum . Infect. Immun. 1983;39:1228–1235. doi: 10.1128/iai.39.3.1228-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H., Attanath P., Sabchareon A., Chongsuphajaisiddhi T., Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K., Stevenson M.M. Acquired immunity to asexual blood stages. In: Sherman I.W., editor. Malaria, Parasite Biology, Pathogenesis and Protection. ASM Press; Washington, DC: 1998. pp. 467–493. [Google Scholar]
- McGregor I.A., Wilson R.M.J. Specific immunity acquired in man. In: Wernsdorfer W.H., McGregor I.A., editors. Malaria, Principles and Practices of Malariology. Churchill Livingston, Inc.; New York: 1988. pp. 559–619. [Google Scholar]
- Diggs C.L., Ballou W.R., Miller L.H. The major merozoite surface protein as a malaria vaccine target. Parasitol. Today. 1993;9:300–302. doi: 10.1016/0169-4758(93)90130-8. [DOI] [PubMed] [Google Scholar]
- Good M.F., Kaslow D.C., Miller L.H. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 1998;16:57–87. doi: 10.1146/annurev.immunol.16.1.57. [DOI] [PubMed] [Google Scholar]
- Morgan W., Birdsall B., Frenkiel T., Gradwell M., Burghaus P., Syed S., Uthaipibull C., Holder A., Feeney J. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J. Mol. Biol. 1999;289:113–122. doi: 10.1006/jmbi.1999.2753. [DOI] [PubMed] [Google Scholar]
- Chitarra V., Holm I., Bentley G.A., Petres S., Longacre S. The crystal structure of C-terminal merozoite surface protein 1 at 1.8A resolution, a highly protective malaria vaccine candidate. Mol. Cell. 1999;3:457–464. doi: 10.1016/s1097-2765(00)80473-6. [DOI] [PubMed] [Google Scholar]
- O'Donnell R.A., Saul A., Cowman A.F., Crabb B.S. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 2000;6:91–95. doi: 10.1038/71595. [DOI] [PubMed] [Google Scholar]
- Blackman M.J., Heidrich H.-G., Donachie S., McBride J.S., Holder A.A. A single fragment of a malaria merozoite surface protein remains on the parasite during red blood cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappel J.A., Holder A.A. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol. Biochem. Parasitol. 1993;60:303–311. doi: 10.1016/0166-6851(93)90141-j. [DOI] [PubMed] [Google Scholar]
- Cooper J.A., Cooper L.T., Saul A.J. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol. Biochem. Parasitol. 1992;51:301–312. doi: 10.1016/0166-6851(92)90080-4. [DOI] [PubMed] [Google Scholar]
- Chang S.P., Gibson H.L., Lee Ng C.T., Barr P.J., Hui G.S. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J. Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- Egan A., Burghaus P., Druilhe P., Holder A., Riley E. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- Blackman M.J., Scott Finnigan T.J., Shai S., Holder A.A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Healer J., Caruana S.R., Hodder A.N., Anders R.F., Crabb B.S., Cowman A.F. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 2000;38:706–718. doi: 10.1046/j.1365-2958.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Crabb B.S., Cowman A.F. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum . Proc. Natl. Acad. Sci. USA. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B.S., Triglia T., Waterkeyn J.G., Cowman A.F. Stable transgene expression in Plasmodium falciparum . Mol. Biochem. Parasitol. 1997;90:131–144. doi: 10.1016/s0166-6851(97)00143-6. [DOI] [PubMed] [Google Scholar]
- Fidock D.A., Wellems T.E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R.L., Bianco A.E., Culvenor J.G., Crewther P.E., Brown G.V., Anders R.F., Kemp D.J. A cDNA clone expressing a rhoptry protein of Plasmodium falciparum . Mol. Biochem. Parasitol. 1987;25:73–81. doi: 10.1016/0166-6851(87)90020-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 1989. [Google Scholar]
- Cattani J.A., Tulloch J.L., Vrbova H., Jolley D., Gibson F.D., Moir J.S., Heywood P.F., Alpers M.P., Stevenson A., Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am. J. Trop. Med. Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- Miller L.H., Roberts T., Shahabuddin M., McCutchan T.F. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol. Biochem. Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- Smith D.B., Johnson K.S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J.G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum . J. Mol. Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Egan A.F., Chappel J.A., Burghaus P.A., Morris J.S., McBride J.S., Holder A.A., Kaslow D.C., Riley E.M. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum . Infect. Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder A.N., Crewther P.E., Anders R.F. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Sims P.F., Hyde J.E. A modified in vitro sulfadoxine susceptibility assay for Plasmodium falciparum suitable for investigating Fansidar resistance. Parasitology. 1997;115:223–330. doi: 10.1017/s0031182097001431. [DOI] [PubMed] [Google Scholar]
- Egan A.F., Morris J., Barnish G., Allen S., Greenwood B.M., Kaslow D.C., Holder A.A., Riley E.M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- Conway D.J., Cavanagh D.R., Tanabe K., Roper C., Mikes Z.S., Sakihama N., Bojang K.A., Oduola A.M., Kremsner P.G., Arnot D.E. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 2000;6:689–692. doi: 10.1038/76272. [DOI] [PubMed] [Google Scholar]
- Branch O.H., Udhayakumar V., Hightower A.W., Oloo A.J., Hawley W.A., Nahlen B.L., Bloland P.B., Kaslow D.C., Lal A.A. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infantsassociations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 1998;58:211–219. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- Rich S.M., Ayala F.J. Population structure and recent evolution of Plasmodium falciparum . Proc. Natl. Acad. Sci. USA. 2000;97:6994–7001. doi: 10.1073/pnas.97.13.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino J.A., Holder A.A., McBride J.S., Blackman M.J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan A.F., Blackman M.J., Kaslow D.C. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive, -exposed, and/or -rechallenged Aotus vociferans monkeys. Infect. Immun. 2000;68:1418–1427. doi: 10.1128/iai.68.3.1418-1427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Collins W., Egan A., Yadava A., Garraud O., Blackman M.J., Guevara Patino J.A., Diggs C., Kaslow D.C. Immunogenicity and efficacy in aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 2000;68:2215–2223. doi: 10.1128/iai.68.4.2215-2223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirunpetcharat C., Tian J.H., Kaslow D.C., van Rooijen N., Kumar S., Berzofsky J.A., Miller L.H., Good M.F. Complete protective immunity induced in mice by immunization with the 19-kiloDalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiaecorrelation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- Daly T.M., Long C.A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- Ling I.T., Ogun S.A., Holder A.A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]