Abstract
The T helper (Th) cell pool is composed of specialized cells with heterogeneous effector functions. Apart from Th1 and 2 cells, CXCR5+ T cells have been suggested to be another type of effector T cell specialized for B cell help. We show here that CXCR5+ T cells are heterogeneous, and we identify subsets of CXCR5+ CD4 T cells that differ in function and microenvironmental localization in secondary lymphoid tissues. CD57+CXCR5 T cells, hereafter termed germinal center Th (GC-Th) cells, are localized only in GCs, lack CCR7, and are highly responsive to the follicular chemokine B lymphocyte chemoattractant but not to the T cell zone EBI1-ligand chemokine. Importantly, GC-Th cells are much more efficient than CD57−CXCR5+ T cells or CXCR5− T cells in inducing antibody production from B cells. Consistent with their function, GC-Th cells produce elevated levels of interleukin 10 upon stimulation which, with other cytokines and costimulatory molecules, may help confer their B cell helper activity. Our results demonstrate that CXCR5+ T cells are functionally heterogeneous and that the GC-Th cells, a small subset of CXCR5+ T cells, are the key helpers for B cell differentiation and antibody production in lymphoid tissues.
Keywords: germinal center, chemokine receptor, CXCR5, B cell help, T helper cells
Introduction
CD4 Th cells are composed of naive T cells and functionally heterogeneous memory T cell subsets at various differentiation stages 1 2 3. Naive CD4 T cells originate in the thymus and are developmentally programmed to home to secondary lymphoid tissues 4. After activation by APCs, vigorous proliferation and maturation of CD4 T cells occur in the secondary lymphoid tissues, generating antigen-specific memory T cells. In contrast to naive cells, some memory cells home well to nonlymphoid tissues. However, many memory T cells can also home to secondary lymphoid organs, but the functional significance of these different homing subsets is only now being elucidated.
Lymphocyte homing from blood to organs or tissues and movement from one microenvironment to another are controlled at multiple levels 4 5. These include initial interactions of lymphocyte adhesion molecules and their receptors on endothelial cells for rolling, chemokine-induced cell arrest through integrin activation, diapedesis, and chemotaxis to specific microenvironments within tissues, and adhesive interactions with stromal cells and extracellular matrix components. Chemokines expressed in specific microenvironments and conditions play important roles in the regulation of leukocyte trafficking for homeostasis and during progression of immune response 6 7 8 9 10. Secondary lymphoid tissue chemokine (SLC), also called CCL21, Exodus2, 6Ckine, or TCA-4, expressed on high endothelial venules, and its receptor CCR7, participate in recruiting T cells to secondary lymphoid tissues 11 12 13. Along with another ligand of CCR7, EBI1-ligand chemokine (ELC, also called CCL19/CKβ-11/MIP-3β), SLC may also help define boundaries of T and B cell zones in secondary lymphoid tissues 13 14 15 16 17 18. Both CCR7-deficient mice and plt mice, which apparently lack functional lymphoid organs SLC and ELC, display abnormal localization of lymphocytes in secondary lymphoid tissues and impaired immune responses, further implicating the T cell zone chemokines and their receptor CCR7 in lymphocyte homing to the lymphoid organs and immunity 19 20.
Circulating B cells also home to secondary lymphoid tissues in search of trapped antigens. Unlike T cells, chemokines that trigger B cell arrest on high endothelial venules and diapedesis into the secondary lymphoid organs remain to be characterized 11. After entry into lymphoid tissues, homing B cells localize in follicles that are separated from, but adjacent to, the T cell zones. After stimulation, antigen-specific T and B cells initially interact at the edges of the follicles via their surface receptors and the cytokines they secrete 21 22. Some B cells differentiate at this stage into short-lived antibody-forming cells, but other activated B cells and antigen-specific T cells migrate into the follicles where they form germinal centers (GCs). In GCs, B cells undergo rapid clonal expansion, somatic hypermutation, isotype switching, and clonal selection 23. Surface receptors on B cells such as CD40, OX40L, and cytokine receptors play important roles in receiving the signals from T cells required for the GC reaction 24. The chemokine receptor CXCR5 is expressed on B cells and required for development of follicles in some secondary lymphoid tissues as well as for B cell localization in the follicles of spleens and Peyer's patches 25. The CXCR5 ligand B lymphocyte chemoattractant (BLC) is expressed by stromal cells (most likely follicular dendritic cells) in B cell follicles and is one of the strongest B cell chemoattractants 26 27 28. BLC is required for B cell migration to follicles in lymph nodes and spleen 29. Interestingly, CXCR5 is also expressed on a subset of circulating human memory CD4 T cells 30 and is upregulated on some antigen-responding mouse CD4 T cells after immunization 31. Thus, expression of CXCR5 provides a possible mechanism for T cells to enter the B cell follicles and may be an important marker for a specialized subset of CD4 T cells for helping B cells.
In line with this, it has been recently reported that CXCR5 defines Th cells with B cell helper function 32 33. In contrast with these reports, we demonstrate that CXCR5 alone does not define B cell helpers in either lymphoid tissue or circulation. Instead, CXCR5+ T cells are actually heterogeneous and in tonsils only a small subset of CXCR5+ T cells, specifically found in GCs, effectively stimulates B cell antibody production. Unlike the GC CXCR5+ T cells, circulating CXCR5+ T cells are resting and thus need to be activated to help B cells. T cell receptor stimulation can induce B cell helper activity in blood CXCR5+ T cells but also in CXCR5− T cells. Therefore, CXCR5 expression by itself does not imply specialization for B cell helper activity.
Materials and Methods
Antibody and Cytokines.
Antibodies to the following antigens were obtained from BD PharMingen unless indicated otherwise: CD3 (UCHT1); CD4 (RPA-T4); CD19 (HIB19); CD27 (M-T271); CD28 (CD28.2); CD45RA (HI100); CD45RO (UCHL1); CD57 (NK-1); CD69 (CH/4; Caltag); CD134 (ACT35); IL-2 (MQ1-17H12); IL-4 (3010.211; Becton Dickinson); IL-5 (JES1-39D10); IL-10 (JES3-19F1); IL-12 (C11.5); IL-13 (JES10-5A2); IFN-γ (4S.B3); TNF-α (mAb11); TNF-β (359.81.11); CLA (HECA 452; E.C. Butcher's laboratory); CD62L (DREG-200; E.C. Butcher's laboratory); α4β7 (ACT-1; LeukoSite); CCR7 (7H12-12-2; LeukoSite); CXCR5 (51505.111; R&D Systems); and CD154 (24 25 26 27 28 29 30 31; Ansell Corp.). Cytokines, IL-2, IL-4, and IL-12 were purchased from BD PharMingen. The following chemokines were from PeproTech, Inc., unless indicated otherwise: SLC; stromal cell–derived factor (SDF)-1; MIP-3β; TARC; IP-10 (R&D Systems); and BLC (B cell–attracting chemokine [BCA]-1; I. Clark-Lewis' laboratory).
Cell Isolation.
PBMCs from human peripheral blood (Stanford University Blood Center, Stanford, CA) were prepared by density gradient centrifuge on histopaque 1077 (Sigma-Aldrich). Tonsil CD4 T cells were isolated from human tonsils obtained from young patients (3–10 yr) undergoing tonsillectomy to relieve obstruction of respiratory passages and improve drainage of the middle ear. Untouched CD4+ T cells (purity >97%) were isolated by depleting non-CD4+ T cells using a magnetic bead depletion method (Miltenyi Biotec). After staining of the isolated CD4 T cells with appropriate antibodies, CD57+/− and/or CXCR5+/− naive and memory cells were sorted using FACSVantage™ SE (Becton Dickinson). Sorted CD4 T cell subsets were on average 99% pure. B cells were isolated from the low density PBMCs or tonsils using CD19 beads (Dynal, Inc.) and detached from the beads according to the manufacturer's protocol. Isolated CD19+ peripheral blood B cells were stained with anti-CD19 and anti–IgD antibodies and further sorted by FACSVantage™ to isolate naive CD19+IgD+ and memory CD19+ IgD− B cells (purity >99%).
Immunohistochemistry.
Frozen sections of tonsils were stained using directly labeled antibodies and/or three-step streptavidin-biotin staining for CXCR5. In brief, acetone-fixed sections were stained with the primary monoclonal antibody to CXCR5 (R&D Systems), followed by incubation in biotinylated goat anti–mouse IgG (Vector Laboratories). After incubation with streptavidin-PE and directly labeled antibodies to CD57 (FITC labeled; BD PharMingen), CD4 (APC; Caltag), and/or IgD-PE (BD PharMingen) in 10% mouse serum, the sections were observed under a confocal microscope (MRC 1024ES; Bio-Rad Laboratories).
Chemotaxis Assay.
Isolated blood or tonsil CD4+ T cells (106 cells per well) were used as input cells for chemotaxis assay. Transwells (5-μm pore size; Corning, Inc.) were used to measure chemotactic responses of various T cell subsets. Cells were allowed to migrate for 3 h. Input cells and cells migrated to the lower chambers were stained with antibodies to CXCR5, CD57, and CD4 and counted using FACSCalibur™ (Becton Dickinson) as described previously 16. 15-μm beads (Polysciences, Inc.) were used as counting standards, and data were presented as the percentage of migration of each cell subset in the input cells.
Cytokine Expression (Intracellular Staining and ELISA).
The sorted cells (CD45RA+CXCR5−, CD45RA−CXCR5−, and CD45RA− CXCR5+ cells) were activated for 4.5 h at 37°C with 50 ng/ml PMA and 1 μg/ml ionomycin in RPMI 1640 supplemented with penicillin/streptomycin, 10% fetal bovine serum, and 1 μg/ml brefeldin A (Sigma-Aldrich). For experiments in Fig. 3 A and 5 B, isolated CD4 T cells were prestained with antibodies (anti-CXCR5, anti-CCR7, anti–CD57-FITC, and/or anti–CD45RA- Tricolor) and activated with PMA and ionomycin in the presence of 10 μg/ml monensin (Sigma-Aldrich). This prestaining method yielded similar results to FACS® sorting followed by activation. Activated cells were fixed and permeabilized using cytofix/cytoperm solution (BD PharMingen) and stained with phycoerythrin-conjugated isotype control antibodies or monoclonal antibodies to IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IFN-γ, TNF-α, and TNF-β. In preliminary experiments, these antibodies were tested on in vitro–generated polarized Th1 and 2 CD4 T cells 34. Data were analyzed on FACSCalibur™ using CELLQuest™ (Becton Dickinson). Supernatants of activated tonsil T cells (105 cells per well in 96-well plates) by 50 ng/ml PMA and 1 μg/ml ionomycin for 24 h were assayed for IL-4, IL-10, and IFN-γ using ELISA kits (Quantikine; R&D Systems) according to the manufacturer's protocols.
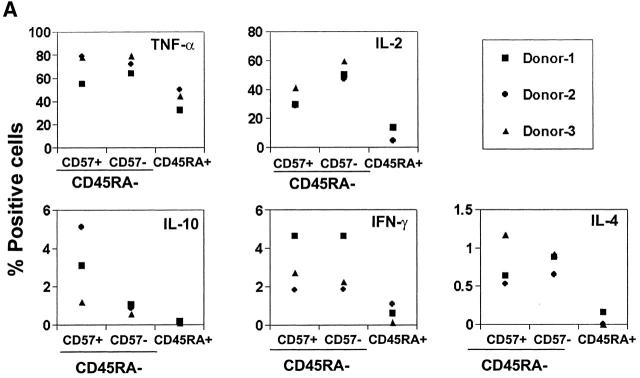
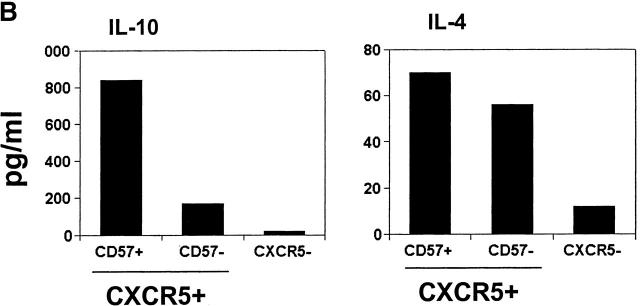
Figure 3.
Effector machinery of CD57+/−CD45RA+/− or CD57+/− CXCR5+/− CD4 T cells. (A) Intracellular cytokine analyses of TNF-α, IL-2, IL-10, IFN-γ, and IL-4. (B) ELISA of IL-10 and IL-4. Isolated T cells were activated with 50 ng/ml PMA and 1 μg/ml ionomycin for 4 h for intracellular cytokine analyses, or 24 h for ELISA of IL-10 and IL-4. Representatives of at least four independent experiments are shown.
T–B Cell Coculture and ELISA of Antibody Production.
Sorted tonsil CD57+CXCR5+, CD57−CXCR5+, and CXCR5− T cells (105) were cultured for 11–13 d in 96-well plates with an equal number of isolated B cells from the same tonsil. For blood T cells, various numbers of sorted T cells (103, 5 × 103, 104, 2.5 × 104, 5 × 104, and 105) were cultured for 14 d in anti-CD3–coated round-bottomed 96-well plates containing B cells from the same donors (2 × 104), 2 μg/ml anti-CD28, and 10% fetal bovine serum in RPMI 1640. 96-well ELISA plates (Corning, Inc.) were coated with 100 μl of anti–human IgG, IgM, or IgA goat polyclonal antibodies (Kirkegaard & Perry Lab) at 10 μg/ml in PBS overnight at 4°C. The plates were extensively washed with PBS and then blocked at 37°C for at least 0.5 h with blocking buffer (1% BSA, 0.05% Tween 20, 1× PBS). Culture supernatants and standard antibodies were diluted and added to the coated wells. After 2 h, plates were washed with PBS before incubation for an additional 2 h with peroxidase-conjugated goat polyclonal antibodies to human IgG, IgM, or IgA (final 1 μg/ml in PBS; Kirkegaard & Perry Lab). After extensive washing with PBS, plates were developed with developing solution (O-phenylenediamine dihydrochloride; Sigma-Aldrich) and read with an ELISA reader at OD 490.
Results
A Subset of CXCR5+ CD4 T Cells Expresses CD57 and Is Specifically Localized in the GCs of Tonsils.
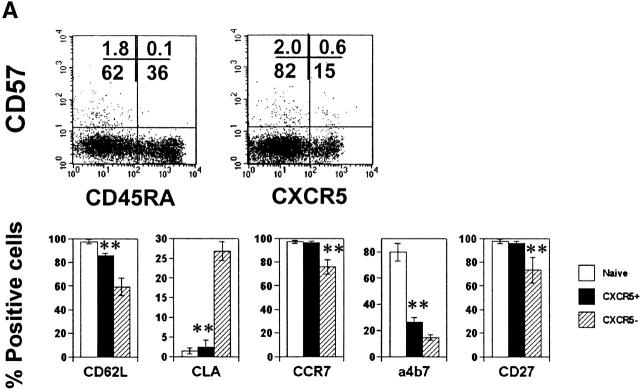
As in blood, both naive CD45RO− (or CD45RA+) and memory type CD45RO+ (CD45RA−) T cells are found in tonsils. While most naive T cells in tonsils do not express CXCR5, ∼90% of tonsil memory type CD4 T cells express CXCR5 (Fig. 1 A), a frequency much higher than peripheral blood CD4 T cells (∼20% of memory T cells in blood are CXCR5+). CD57 is an antigen associated with GCs 35 36 and expressed on a subset of CD4 T cells that are positive for CD69 and CD45RO 37. Expression of CD57 subdivides tonsil CXCR5+ CD4 T cells into two populations (Fig. 1 A). CD57 expression is detected only on 15–25% of tonsil CXCR5+ CD4 T cells but is not seen on CXCR5− or naive CD4 T cells. We examined localization of these CXCR5+ CD4 T cell subsets in tonsil by immunohistochemistry and confocal microscopy. CD57+ CXCR5+ T cells are abundant in GCs but not in primary follicles of IgD+ B cells or in the interfollicular/T cell area (Fig. 1B and Fig. C). In contrast, as reported previously by Forster et al. 30, CXCR5+ CD4 T cells are found not only in B cell follicles but, in great numbers, in the interfollicular/T cell area (Fig. 1 C). Their differential localization within lymphoid tissues suggests that the two CXCR5+ T cell populations have distinct functions.
Figure 1.
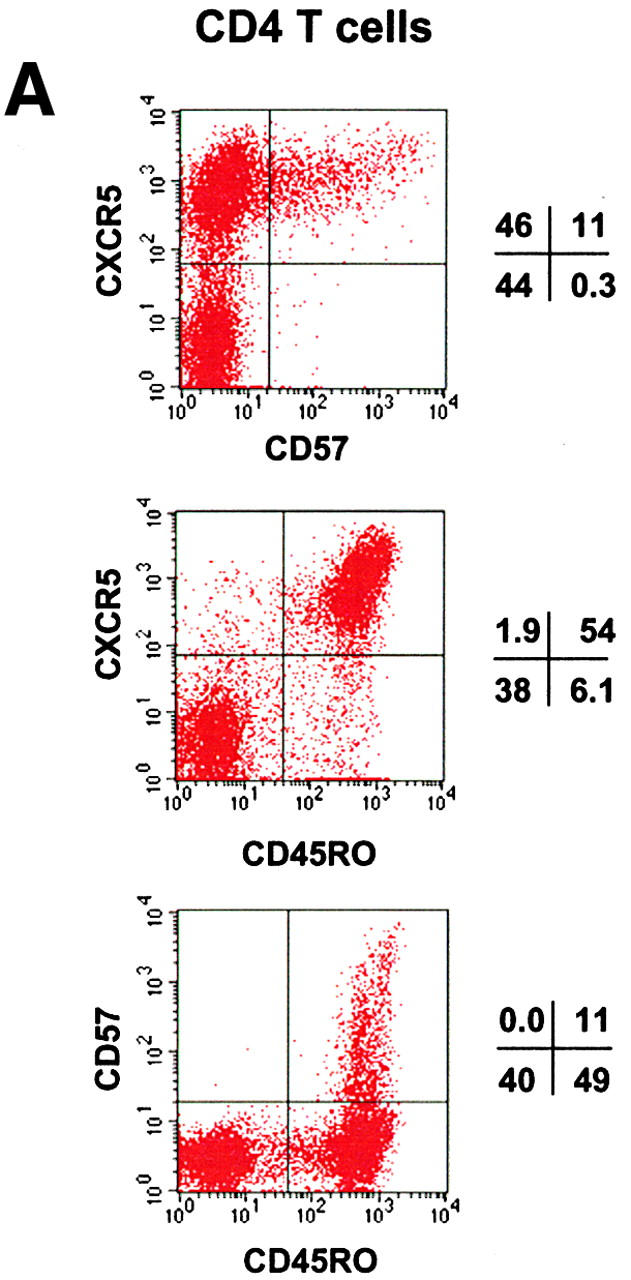
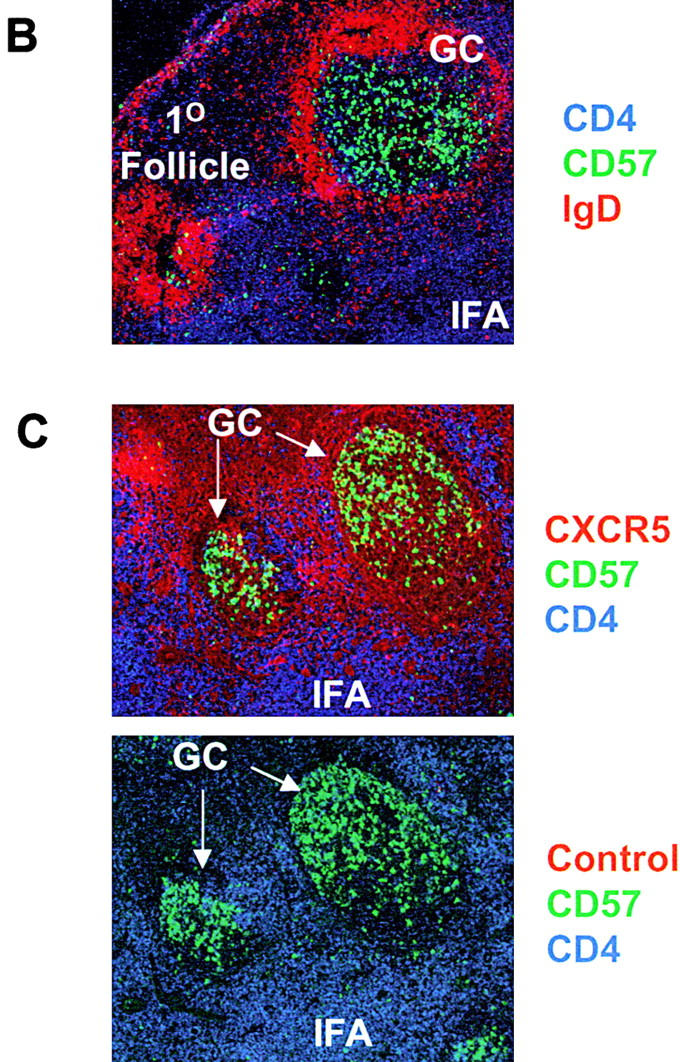
Identification of CD57+ CXCR5+ T cells and their localization in GCs. (A) Flow analyses of CD57 and CXCR5 expression on tonsil CD4 T cells. (B) Specific localization of CD57+ CD4 T cells in GCs. (C) Localization of CD57+ CXCR5+ CD4 T cells in GCs. Antibodies to CD57 (green), CD4 (blue), and IgD (red; B) or CXCR5 (red; C) were used for in situ immunohistochemistry.
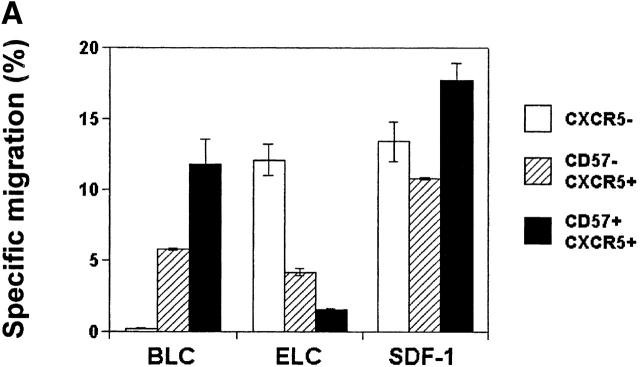
CD57+CXCR5+ T Cells Are Highly Responsive to BCA-1/BLC but Not Responsive to ELC.
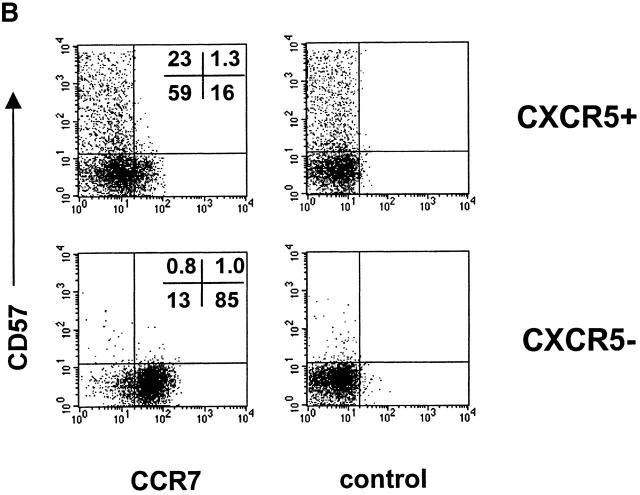
In an effort to understand the differential localization of tonsil CD4 T cell subsets, we examined the chemotactic response of tonsil CD4 T cell subsets to the follicular chemokine BCA-1/BLC and to a T cell area chemokine ELC. A general chemoattractant SDF-1 was also included in this assay. CD57+CXCR5+ T cells demonstrate a greater chemotactic response to BLC than CD57−CXCR5+ T cells, and unlike the CD57− CXCR5+ subset, do not significantly respond to the T cell area chemokine ELC (Fig. 2 A). Reduced response to ELC is thought to be important to allow T cells to enter the GCs 38. Both CD57+ and CD57−CXCR5+ T cells are highly responsive to SDF-1. CXCR5− CD4 T cells do not respond to BLC but respond vigorously to ELC and SDF-1. Consistent with their chemotactic responses, the ELC receptor CCR7 is not expressed on CD57+CXCR5+ T cells but on a subset of CD57−CXCR5+ T cells and on most CXCR5− T cells (Fig. 2 B). Thus, their chemotactic responses and chemokine receptor expression pattern correlate with the specific localization of CD57+CXCR5+ T cells in GCs.
Figure 2.
Chemotactic responses (A) and chemokine receptor expression (B) by CD57+/2CXCR5+/− CD4 T cells. Optimal concentrations of 5 μg/ml BLC, 1 μg/ml ELC, and 100 ng/ml SDF-1 were used for chemotaxis experiments. Freshly isolated tonsil cells were used for chemotaxis and flow analyses of tonsil CD4 T cell subsets. Representatives of three independent experiments are shown.
Effector Machinery of CD57+CXCR5+T Cells.
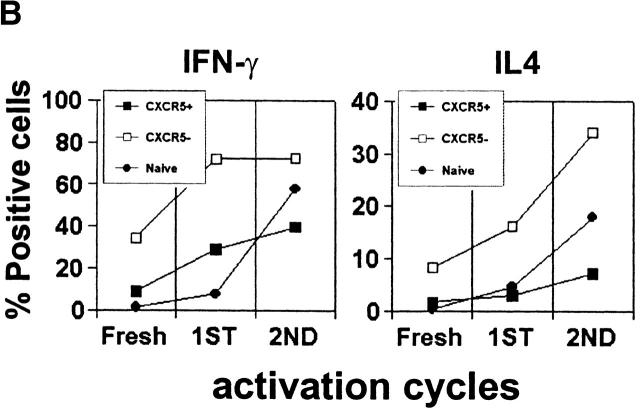
Next, we examined cytokine production by different T cell subsets from tonsils. Similar frequencies of TNF-α, IL-4, and IFN-γ producers are found in CD57+ and CD57− memory CD4 T cells (Fig. 3 A). However, one notable difference between these T cell populations is that the CD57+ T cells are more efficient in production of IL-10 as evidenced by intracellular staining (Fig. 3 A) and ELISA (Fig. 3 B). On the other hand, naive or CXCR5− T cells did not or very poorly produce IL-10, IL-4, and IFN-γ, and contained lower frequencies of IL-2 and TNF-α producers than either memory or CXCR5+ subset. OX40 and CD40L are inducible T cell antigens of the TNF receptor–TNF ligand family that play important roles in B cell help and antibody switching 39 40. Therefore, we examined expression of these molecules by the tonsil T cell subsets before and after brief (1–3 h) stimulation with PMA and ionomycin. While the two CXCR5+ T cell populations are much more efficient than CXCR5− T cells in upregulation of surface OX40 and CD40L after stimulation, no notable difference between the CD57+ and CD57−CXCR5+ T cells was found (data not shown).
Only the CD57+ Subset of Tonsillar CXCR5+ T Cells Efficiently Helps B Cells Produce Antibodies.
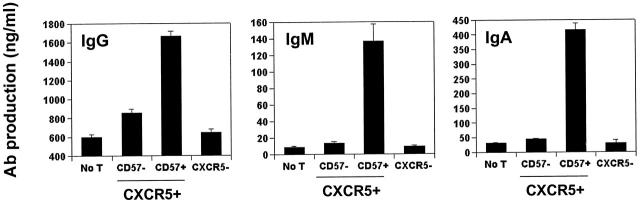
To determine the efficiency of CD57- and CXCR5-defined T cell subsets in helping B cells produce antibody, sorted tonsil CD57+/− CXCR5+/− CD4 T cells were cultured with CD19+ B cells from the same tonsils followed by ELISAs of secreted IgG, IgA, and IgM. Strikingly, the CD57+CXCR5+ T cells very efficiently induced B cell production of all three isotypes examined, while the CD57−CXCR5+ T cells, which account for the majority (∼80%) of tonsil CXCR5+ T cells, only weakly induced antibody production (Fig. 4). Indeed, they were little or no more efficient than CXCR5− T cells in providing B cell help.
Figure 4.
Spontaneous B cell helper activity of CD57+/− CXCR5+/− CD4 T cells. Sorted CD57+CXCR5+, CD57− CXCR5+, and CXCR5− CD4 T cells were cocultured with B cells from the same tonsil for 11–13 d in the absence of any stimulatory agents followed by analyses of secreted IgG, IgA, and IgM. Representatives of at least three independent experiments are shown.
Phenotype of Blood CXCR5+ T Cells.
In blood only ∼20% of memory T cells are CXCR5+. Most blood CXCR5+ T cells are CD57−CD27+CD45RAlow/− (Fig. 5 A). The majority of them are CD62L+, CCR7+, and CLA−. Additionally, 20–25% of CXCR5+ T cells are α4β7+ cells. This pattern of adhesion and chemokine receptor expression suggests that the blood CXCR5+ T cells home to peripheral and mucosal lymphoid tissues. In contrast to the CXCR5− memory T cells, fewer CXCR5+ T cells from blood produce the Th1/2 effector cytokines IL-4 and IFN-γ, even after repeated stimulation with anti-CD3 and anti-CD28 (Fig. 5 B). Expression of other cytokines such as IL-5, IL-10, IL-12, and TNF-β was not readily detected in the circulating CXCR5+ T cell population (data not shown).
Figure 5.
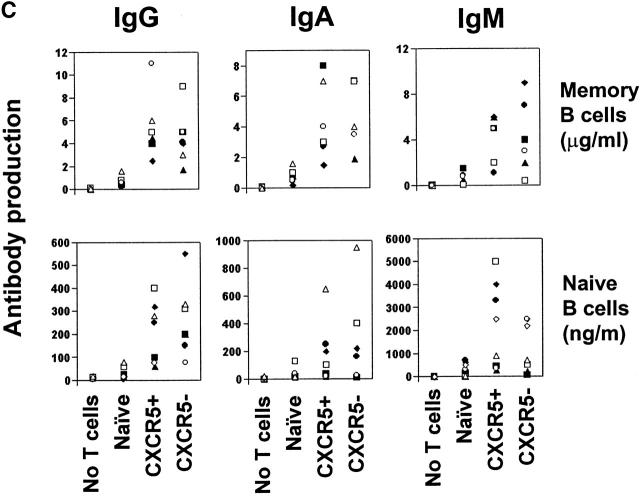
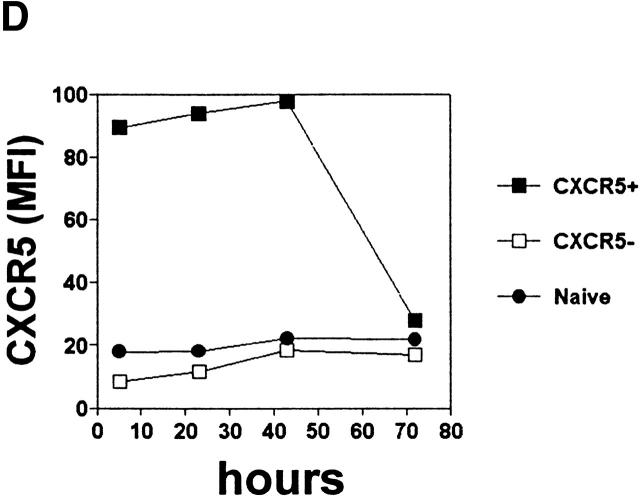
Phenotype and effector function of circulating CXCR5+ T cells. (A) Surface phenotype of circulating naive or CXCR5+/− memory T cells. **Significant differences between CXCR5+ and CXCR5− memory cells. (B) IL-4/IFN-γ production capabilities of CXCR5+/− T cells during repeated T cell receptor activation (each cycle is composed of 4-d activation with anti-CD3 and anti-CD28 followed by 3-d resting in the presence of IL-2). (C) B cell help activity of circulating CXCR5+ T cells after T cell receptor activation. Naive (IgD+) or memory (IgD−) B cells (2 × 104) from peripheral blood were cultured for 14 d in the presence or absence of various numbers (103, 5 × 103, 104, 2.5 × 104, 5 × 104, and 105) of autologous T cells (CXCR5−CD45RA+, CXCR5+CD45RA−, or CXCR5− CD45RA−). Concentrations of IgG, IgA, and IgM in the culture supernatants were measured by ELISA. T cell numbers required for peak levels of antibody production varied among donors or experiments. One peak value with the best antibody production in each T cell group is shown. (D) Loss of CXCR5 expression during T cell receptor activation with anti-CD3 and anti-CD28. Error bars indicate SD of results from at least five different experiments (A). Representatives of three independent experiments are shown (B and D), and results from seven different donors (C) are shown.
CXCR5 Is Not a Specific Maker for B Cell Helpers in Blood.
Although blood CXCR5+ cells may be able to home to B cell follicles in lymphoid tissues, it is known that, unlike the tonsil GC CXCR5+ T cell studied above, these resting blood T cells (which neither express surface costimulatory molecules nor secrete effector cytokines) cannot constitutively support B cell antibody production. However, B helper activity can be induced in blood T cells by appropriate stimulation 41 42. To determine if CXCR5 expression correlates with enhanced potential for B cell help, sorted peripheral blood CXCR5+ and CXCR5− memory and naive CD4 T cells were cultured in the presence of anti-CD3 and anti-CD28 with isolated peripheral blood B cells. Secreted IgG, IgA, and IgM in culture media were quantified by ELISA. Cultures of B cells alone or with stimulated naive T cells did not produce significant levels of antibodies (Fig. 5 C). In contrast, both circulating CXCR5+ and CXCR5− memory T cells induced B cell production of IgG, IgA, and IgM. Thus, CXCR5 expression neither predicts constitutive B helper capacity nor indicates a special potential to provide B cell help. Interestingly, when activated with anti-CD3 and anti-CD28, CXCR5+ T cells lose surface CXCR5 expression and become CXCR5− T cells within 3 d (Fig. 5 D). Thus, the circulating CXCR5− cells with B cell helping function could be originated from CXCR5+ T cells in vivo.
Discussion
CD57+CXCR5+ T Cells Are the B Cell Helper T Cells within the Tonsil CXCR5+ T Cell Population.
Thus far, immunologists have believed that effector CD4 T cells exist mainly in two forms: Th1 or Th2 cells. Indeed, this Th1/2 paradigm provides convincing explanations for pathological progression of many polarized inflammatory diseases. Also it has been believed that Th cells for B cell differentiation are contained within these Th1/2 populations. However, almost all circulating Th1/2 cells lack expression of CXCR5, a molecule thought to be necessary (but not sufficient) for homing to B cell areas of lymphoid tissues. Recent reports indeed suggest that lymphoid tissue homing CXCR5+ T cells are a new third type of T follicular helper effector cell specialized for B cell help 32 33 43. However, the fact that these CXCR5+ T cells are extraordinarily abundant in tonsils, constituting the majority of total memory T cells, and are found not only in follicles but also in interfollicular areas/T cell zones, prompted us to examine this hypothesis further. Our results demonstrate that CXCR5+ T cells are functionally heterogeneous and that the majority of CXCR5+ T cells are neither capable nor clearly specialized for providing efficient B cell help. We identify the efficient B cell helping effector T cells, CD57+CXCR5 T cells, hereafter termed GC T helper (GC-Th) cells, as a small subset of CXCR5+ T cells that are localized in GCs and display the surface phenotype: CD4+CD45RO+CD57+CXCR5+ CCR7−.
GC-Th cells account for only 15–25% of total CXCR5+ T cells in tonsils and are specifically localized in GCs, while other CXCR5+ T cells are found scattered in B cell follicles and interfollicular areas. These CD57+ CD4 T cells are also restricted to the GCs in other lymphoid tissues such as the spleen and lymph nodes 35. This strategic localization of GC-Th cells in lymphoid tissues is relevant to their functions in GCs, where B cells undergo activation and differentiation to become functional memory and antibody-producing plasma B cells. Additionally, the localization of GC-Th cells correlates very well with their chemotactic phenotype: GC-Th cells express CXCR5, but not CCR7, and chemotax well to BLC but poorly to T cell zone–chemokine ELC. One the other hand, as a population, CD57−CXCR5+ T cells respond equally well to ELC and BLC, consistent with their presence in interfollicular and follicular areas of lymphoid tissue. Tonsil CXCR5− T cells respond only to ELC but not to BLC, suggesting their exclusion from B cell areas.
Among the CD4 T cell populations in tonsils, only GC-Th cells demonstrate efficient constitutive B cell helper activity, supporting B cell production of IgG, IgA, and IgM. Other CD4 T cell types, CD57−CXCR5+ T cells and CXCR5− T cells, induce only low or no secretion of IgG, IgA, and IgM from B cells. Thus, our study reveals a further specialization of CXCR5+ T cells. The efficient B cell help activity of GC-Th cells undoubtedly reflects a unique effector machinery. GC-Th cells can produce cytokines IL-10, IL-2, IL-4, IFN-γ, and TNF-α, and surface costimulatory molecules OX40 and CD40L. Consistent with these findings, it has been reported previously that mRNA expression of IL-4, IL-10, TNF-α, and IFN-γ was detected by reverse transcription PCR in CD57+ CD4 T cells 44. IL-10, a cytokine important for antibody class switching and B cell differentiation into plasma cells 24, is particularly more efficiently provided by GC-Th cells than other T cells. In this regard, it is interesting that inducible costimulator is highly expressed on tonsillar T cells in the apical light zone of GCs, (where CD57+ GC-Th cells are localized), and activation of inducible costimulator on T cells superinduces the synthesis of IL-10 45.
It is not known how blood CXCR5+ T cells are related to their lymphoid tissue counterparts. From the fact that naive T cells quickly become CD57−CXCR5+ T cells in response to dendritic cell stimulation within 4–5 d (data not shown), we propose that nonB cell–helping lymphoid CD57−CXCR5+ T cells may be the earliest CXCR5+ T cells freshly generated from naive T cells in response to antigen and dendritic cells. Further activation of these CD57−CXCR5+ T cells may lead to more differentiated GC-Th cells with efficient B cell helping activity. Another possibility is that GC-Th and CD57−CXCR5+ T cells are interchangeable populations in which CD57 is upregulated when cells receive GC-specific signal(s), and they either apoptose or revert to a CD57−CXCR5+ phenotype after the signal or antigen stimulation is cleared. Blood CXCR5+ T cells have a similar phenotype to lymphoid CD57−CXCR5+ T cells in that they are CD57− and CCR7+ and have dramatically reduced capacities to produce IL-4 and/or IFN-γ. Thus, it is likely that blood CXCR5+ T cells may be the recirculating counterparts of lymphoid CD57−CXCR5+ T cells. Few GC-Th cells (CXCR5+CD57bright) are found in circulation, suggesting that GC-Th cells stay in GCs for B cell differentiation and, like GC B cells, do not recirculate.
In contrast to recent publications, we find that CXCR5 expression alone is not a specific indicator of Th cells for B cell development. CXCR5+ but CD57− tonsil T cells help B cells poorly. CXCR5+ and CXCR5− subsets of blood memory CD4 T cells also do not provide help, unless they are activated, and CXCR5+ and CXCR5− T cells display a similar potential for B helper activity after T cell receptor stimulation. Tonsil CD57−CXCR5+ cells and circulating CXCR5+ T cells, which are uniquely deficient in effector cytokine production compared with circulating CXCR5− T cells, may represent a precursor pool for the CD57+ GC-Th cells. Interestingly, CXCR5+ T cells can quickly lose CXCR5 expression upon T cell receptor activation. Thus, CXCR5 is not a stable marker for T cells with B helper activity.
In conclusion, based upon recent reports and our findings in this paper, we hypothesize that naive CD4 T cells upon antigen stimulation can differentiate into either CXCR5+ or CXCR5− CD4 T cells. CXCR5+ CD4 T cells can further differentiate to CD57+CXCR5+ CD4 T cells and localize in GCs to help B cell differentiation, while CXCR5− CD4 T cells take the Th1/2 memory/effector pathway to become CD4 cells efficient in effector cytokine production. CXCR5+ CD4 T cells emigrate and recirculate through the blood and lymphoid organs and are relatively deficient in cells targeted to extralymphoid sites of tissue inflammation, which reside primarily in the CXCR5− population.
Acknowledgments
We thank June Twelves and Evelyn Resurreccion for their technical assistance, and Drs. Anna Messner and Kay Chang (Stanford University Medical Center) for tonsil specimens.
This work is supported by grants AI47822, GM37734, and AI37832 from the National Institutes of Health, an award from the Department of Veterans Affairs (to E.C. Butcher), and the FACS® core facility of the Stanford Digestive Disease Center. C.H. Kim is a Leukemia and Lymphoma Society Fellow. D.J. Campbell is the recipient of a fellowship from the Arthritis Foundation.
Footnotes
Abbreviations used in this paper: BCA, B cell–attracting chemokine; BLC, B lymphocyte chemoattractant; ELC, EBI1-ligand chemokine; GC, germinal center; SDF, stromal cell–derived factor; SLC, secondary lymphoid tissue chemokine.
References
- Metz D.P., Bottomly K. Function and regulation of memory CD4 T cells. Immunol. Res. 1999;19:127–141. doi: 10.1007/BF02786482. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Carter L.L., Swain S.L. From naive to memory. Development and regulation of CD4+ T cell responses. Immunol. Res. 1998;18:1–13. doi: 10.1007/BF02786509. [DOI] [PubMed] [Google Scholar]
- Butcher E.C., Williams M., Youngman K., Rott L., Briskin M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. Molecules controlling lymphocyte migration to the gut. Gut. 1999;45:148–153. doi: 10.1136/gut.45.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Butcher E.C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Broxmeyer H.E. Chemokinessignal lamps for trafficking of T and B cells for development and effector function. J. Leukoc. Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokinesa new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Warnock R.A., Campbell J.J., Dorf M.E., Matsuzawa A., McEvoy L.M., Butcher E.C. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J.V., Rot A., Luo Y., Narasimhaswamy M., Nakano H., Gunn M.D., Matsuzawa A., Quackenbush E.J., Dorf M.E., von Andrian U.H. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function–associated antigen 1–mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 2000;191:61–76. doi: 10.1084/jem.191.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann K., Tam C., Cyster J.G., Rosen S.D., Williams L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Bowman E.P., Murphy K., Youngman K.R., Siani M.A., Thompson D.A., Wu L., Zlotnik A., Butcher E.C. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3β receptor CCR7. J. Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromas R., Kim C.H., Klemsz M., Krathwohl M., Fife K., Cooper S., Schnizlein-Bick C., Broxmeyer H.E. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J. Immunol. 1997;159:2554–2558. [PubMed] [Google Scholar]
- Kim C.H., Pelus L.M., White J.R., Applebaum E., Johanson K., Broxmeyer H.E. CK β-11/macrophage inflammatory protein-3 β/EBI1-ligand chemokine is an efficacious chemoattractant for T and B cells. J. Immunol. 1998;160:2418–2424. [PubMed] [Google Scholar]
- Ngo V.N., Tang H.L., Cyster J.G. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J. Exp. Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Lu Z., Luo Y., Quackenbush E.J., Berman M.A., Collins-Racie L.A., Mi S., Reilly C., Lo D., Jacobs K.A., Dorf M.E. Identification of a new mouse β-chemokine, thymus-derived chemotactic agent 4, with activity on T lymphocytes and mesangial cells. J. Immunol. 1997;159:5671–5679. [PubMed] [Google Scholar]
- Forster R., Schubel A., Breitfeld D., Kremmer E., Renner-Muller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Nakano H., Mori S., Yonekawa H., Nariuchi H., Matsuzawa A., Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–2895. [PubMed] [Google Scholar]
- Garside P., Ingulli E., Merica R.R., Johnson J.G., Noelle R.J., Jenkins M.K. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Tarlinton D. Germinal centersform and function. Curr. Opin. Immunol. 1988;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Banchereau J. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin. Immunol. 1997;9:235–240. doi: 10.1006/smim.1997.0080. [DOI] [PubMed] [Google Scholar]
- Forster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Ngo V.N., Ansel K.M., Ekland E.H., Cyster J.G., Williams L.T. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Legler D.F., Loetscher M., Roos R.S., Clark-Lewis I., Baggiolini M., Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E.P., Campbell J.J., Soler D., Dong Z., Manlongat N., Picarella D., Hardy R.R., Butcher E.C. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J. Exp. Med. 2000;191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Forster R., Sedgwick J.D., Browning J.L., Lipp M., Cyster J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Forster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein–coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Ansel K.M., McHeyzer-Williams L.J., Ngo V.N., McHeyzer-Williams M.G., Cyster J.G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P., Willimann K., Lang A.B., Lipp M., Loetscher P., Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornasse T., Larenas P.V., Davis K.A., deVries J.E., Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J. Exp. Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A.W., James K., Micklem H.S. The distribution and possible significance of cells identified in human lymphoid tissue by the monoclonal antibody HNK-1. Clin. Exp. Immunol. 1983;51:439–447. [PMC free article] [PubMed] [Google Scholar]
- Velardi A., Tilden A.B., Millo R., Grossi C.E. Isolation and characterization of Leu 71 germinal-center cells with the T helper-cell phenotype and granular lymphocyte morphology. J. Clin. Immunol. 1986;6:205–215. doi: 10.1007/BF00918700. [DOI] [PubMed] [Google Scholar]
- Bowen M.B., Butch A.W., Parvin C.A., Levine A., Nahm M.H. Germinal center T cells are distinct helper-inducer T cells. Hum. Immunol. 1991;31:67–75. doi: 10.1016/0198-8859(91)90050-j. [DOI] [PubMed] [Google Scholar]
- Randolph D.A., Huang G., Carruthers C.J., Bromley L.E., Chaplin D.D. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. 1999;286:2159–2162. doi: 10.1126/science.286.5447.2159. [DOI] [PubMed] [Google Scholar]
- Stuber E., Neurath M., Calderhead D., Fell H.P., Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Calderhead D.M., Buhlmann J.E., van den Eertwegh A.J., Claassen E., Noelle R.J., Fell H.P. Cloning of mouse Ox40a T cell activation marker that may mediate T-B cell interactions. J. Immunol. 1993;151:5261–5271. [PubMed] [Google Scholar]
- Gmelig-Meyling F., UytdeHaag A.G., Ballieux R.E. Human B-cell activation in vitro. T cell-dependent pokeweed mitogen-induced differentiation of blood B lymphocytes. Cell. Immunol. 1977;33:156–169. doi: 10.1016/0008-8749(77)90143-5. [DOI] [PubMed] [Google Scholar]
- Hirohata S., Jelinek D.F., Lipsky P.E. T cell-dependent activation of B cell proliferation and differentiation by immobilized monoclonal antibodies to CD3. J. Immunol. 1988;140:3736–3744. [PubMed] [Google Scholar]
- Mackay C.R. Follicular homing T helper (Th) cells and the Th1/Th2 paradigm. J. Exp. Med. 2000;192:F31–F34. doi: 10.1084/jem.192.11.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butch A.W., Chung G.H., Hoffmann J.W., Nahm M.H. Cytokine expression by germinal center cells. J. Immunol. 1993;150:39–47. [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]