Abstract
T cell–specific adapter (TSAd) protein is an Src homology 2 (SH2) domain–containing adapter molecule implicated in T cell receptor for antigen (TCR)-mediated interleukin 2 (IL-2) secretion in T cells. Here, we demonstrate that a substantial fraction of TSAd is found in the T cell nucleus. Nuclear import of TSAd is an active process that depends on TSAd SH2 domain recognition of a phosphotyrosine-containing ligand. Importantly, we show that TSAd can act as a potent transcriptional activator in T cells. Furthermore, the TSAd SH2 domain appears to be essential for this transcription-activating function independent of its role in nuclear import. Biochemical analyses suggest that a single TSAd SH2 domain ligand of 95–100 kD may be involved in these processes. Consistent with a role as a transcription activator, cotransfection of TSAd with an IL-2 promoter–reporter gene construct results in a considerable upregulation of IL-2 promoter activity. Further, we show that this augmentation requires a functional TSAd SH2 domain. However, TSAd does not appear to modulate the activity of the major recognized IL-2 gene transcription factors, nuclear factor κB (NF-κB), nuclear factor of activated T cells (NFAT), or activator protein 1 (AP-1). These findings point to the function of TSAd as a novel transcription-regulatory protein in T cells and illustrate the importance of the TSAd SH2 domain in this role.
Keywords: transcription, T lymphocyte, interleukin 2, signal transduction, SH2 domain
Introduction
Adapter proteins play important roles in mammalian cell signaling through the formation of intracellular signaling complexes with catalytically active molecules 1. In human T cells, one such adapter protein is the T cell–specific adapter (TSAd) protein, whose expression is induced by TCR cross-linking 2 3. TSAd comprises of an NH2-terminal region of unknown function, a centrally located Src homology 2 (SH2) domain with the potential to bind phosphotyrosine-containing protein ligands, and a COOH- terminal region which contains both a proline-rich stretch and tyrosine residues which, if phosphorylated, could function as docking sites for other signaling proteins.
The murine ortholog of TSAd is known as LCK-associated adapter protein (LAD) or RLK/ITK-binding protein (RIBP), based on its ability to interact with these respective Src and Tec family kinases, at least in yeast hybrid systems 4 5. Like TSAd, LAD/RIBP is restricted in expression to T cells and is induced upon TCR engagement. The function of TSAd and LAD/RIBP in T cells is unknown. Constitutive expression of TSAd in human Jurkat T leukemic cell line stable transfectants was previously shown to result in inhibition of TCR plus phorbol ester–induced activation of the promoter for the T cell autocrine growth factor, IL-2. In addition, TSAd was shown to inhibit the tyrosine kinase activity of LCK when both were transfected into a fibroblast cell line 3. These findings, therefore, suggested that TSAd might function as a negative feedback regulator of T cell activation. However, in stark contrast to this, T cells from LAD/RIBP knockout mice were demonstrated to produce considerably reduced quantities of IL-2 and IFN-γ upon TCR challenge, compared with control T cells 5. This finding indicates that TSAd/LAD/RIBP in fact performs a net positive role in T cell signal transduction, in induction of Th1-type T cell cytokines.
In these studies we show that TSAd has a predominant nuclear localization in T cells and can function as a potent activator of gene transcription in in vivo transactivation assays. Both TSAd nuclear import and transcription-activating function appear to be mediated by TSAd SH2 domain recognition of a phosphotyrosine-containing ligand. We confirm that, under certain conditions in T cell transient transfection assays, TSAd can indeed activate gene transcription from an IL-2 promoter–reporter gene construct and that this activation requires an intact TSAd SH2 domain. These findings illustrate the function of TSAd as a potential transcription-regulatory protein in T cells.
Materials and Methods
TSAd DNA Constructs.
DNA corresponding to the TSAd full-length coding region or SH2 domain only was generated by PCR and inserted into the multiple cloning sites of pEF-FLAG 6, pSG424 7, or pGEX3X (Amersham Pharmacia Biotech). Resulting constructs encode for NH2-terminal FLAG, GAL4 DNA-binding domain (dbd) (1–147), and glutathione S-transferase (GST)-tagged TSAd proteins, respectively. Arginine to lysine 120 (R120K) mutations of the TSAd SH2 domain were made with the use of a QuickChange site-directed mutagenesis kit (Stratagene).
Intracellular Staining.
Jurkat Tag C15 human T leukemia cells 8, COS-7 African Green Monkey kidney fibroblast–like cells (American Type Culture Collection), and 293T human kidney epithelial cells were transfected with pEF-FLAG or pSG424 constructs by electroporation (250 V, 960 μF, 0.4-cm gap cuvettes). After 24 h, cells were cytospun, and fixed and permeabilized in 3.75% formaldehyde/0.1% Triton X-100, and stained with anti-FLAG M2 (Sigma-Aldrich) or anti-GAL4 (Santa Cruz Biotechnology, Inc.) antibodies followed by goat anti–mouse Ig (GAM)-Alexa 488 or Texas red-X (Molecular Probes). To detect endogenous TSAd, regular Jurkat cells (American Type Culture Collection) were prestimulated with PMA (1 ng/ml; Sigma-Aldrich) plus ionomycin (1 μM; Sigma-Aldrich) for 24 h. Fixed and permeabilized cells were then stained with an affinity-purified monospecific anti-TSAd antibody 3 followed by goat anti–rabbit Ig (GAR), rabbit anti–goat Ig, and GAR-Alexa 488 (Molecular Probes). In all experiments, cells were counter-stained with Hoechst 33342 (Sigma-Aldrich) or TO-PRO-3 (Molecular Probes) to reveal nuclei. Cells were viewed on a Nikon Eclipse E600 or ZEISS LSM510 laser scanning confocal microscope.
Reporter Assays.
For GAL4 transcription transactivation assays, EL-4 murine thymoma cells (American Type Culture Collection) and regular Jurkat cells (American Type Culture Collection) were transfected with pSG424 constructs, the pG4M2 GAL4 operator-luciferase reporter 9 and control pRL-TK renilla-luciferase reporter (Promega) by lipofection using lipofect AMINE-2000 (Life Technologies) and X-tremeGENE Q2 transfection reagent (Roche), respectively. After 6 h, EL-4 cells were stimulated with the 145-2C11 mAb (CD3 component of the TCR complex, 1 μg/ml; BD PharMingen) or PMA (50 ng/ml) and Jurkat cells with PMA (1 ng/ml) plus ionomycin (1 μM) for a further 24 h. Luciferase activity in cell lysates was measured with the use of a Dual-Luciferase Reporter Assay System (Promega).
For other promoter assays, Jurkat cells were transfected with pEF-FLAG constructs, together with a −326 to +46 IL-2 promoter-luciferase reporter 10, a nuclear factor κB (NF-κB)-luciferase reporter (Stratagene), or nuclear factor of activated T cells (NFAT)/activator protein 1 (AP-1)-luciferase reporter 11 and control pRL-TK by lipofection as above. After 24 h, cells were stimulated for a further 12–14 h with different combinations of OKT3 mAb (CD3, 1 μg/ml; American Type Culture Collection) plus CD28.2 mAb (CD28, 1 μg/ml; BD PharMingen), PMA (1 ng/ml), and ionomycin (1 μM). Luciferase activity was measured as above.
GST Pulldown Assays.
GST fusion proteins were produced in protease-negative BL21(DE3)pLysS(Ion,ompT−) bacteria (Novagen) and purified on glutathione-agarose beads. Jurkat cells were preactivated with PMA (1 ng/ml) plus ionomycin (1 μM) for 48 h followed by pervanadate treatment (0.5 mM) for 20 min. Aliquots of beads, coated with 400 ng of protein, were rotated for 2 h in a 1-ml volume with NP-40 lysates or with cytoplasmic or nuclear lysates of Jurkat 12 prepared from ∼4 × 107 cells. Beads were washed extensively, and proteins were eluted in reducing SDS sample buffer and run on 10% polyacrylamide gels. Tyrosine phosphorylated proteins were detected by Western blotting using the RC20 antiphosphotyrosine antibody (Transduction Laboratories) as described 13. The quality of nuclear and cytoplasmic lysates was determined by stripping and reprobing of blots with antiinhibitor κ (IκB-α) and anti-poly ADP-ribose polymerase (PARP) antibodies (Santa Cruz Biotechnology, Inc.).
Results and Discussion
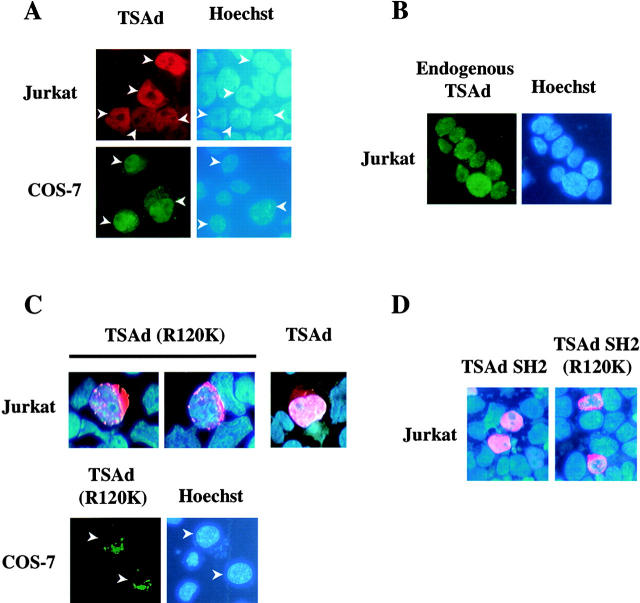
The subcellular distribution of TSAd has not previously been examined in detail. One recent study, which looked at the expression of retroviral-transduced LAD/RIBP in the murine D10 T hybridoma, suggested a predominant cytoplasmic localization for this molecule. However, LAD/RIBP was clearly also expressed in the nucleus of this cell line 14. During the course of our own experiments, designed to examine the function of TSAd in T cell signal transduction, we examined the subcellular localization of transfected FLAG-tagged TSAd in the human Jurkat T leukemia cell line. Surprisingly, and in contrast to the previously reported results, we found that although TSAd was present in the cytoplasm, the majority of transfected TSAd was in fact nuclear localized (Fig. 1 A). Likewise, in transfected COS-7 fibroblast-like cells and 293T epithelial cells (where nuclear and cytoplasmic compartments can be more easily discerned), TSAd was found predominantly in the nucleus (Fig. 1 A and 2). Confocal microscopic studies of 293T transfectants confirmed that TSAd did not just simply overlie nuclei but was present throughout the nuclear body (excluding nucleoli; Fig. 2). The same results were also obtained using green fluorescent protein–tagged TSAd and LAD/RIBP and in live as well as fixed cells (not shown). Consistently, nuclear localization was observed in >90% of transfected cells. In a minority of cells, TSAd was found mostly in the cytoplasm. However, this type of distribution was generally seen in cells that express very high levels of transfected protein. Most importantly, with the use of an anti-TSAd antibody we determined that endogenous TSAd, expressed in Jurkat cells activated with PMA plus ionomycin (Fig. 1 B) or CD3 plus CD28 mAb (not shown), was predominantly nuclear in all cells.
Figure 1.
Subcellular distribution of TSAd. Jurkat T leukemia cells or COS-7 cells were transiently transfected with plasmids encoding FLAG-tagged wild-type TSAd (A), a TSAd (R120K) mutant (C), or the TSAd SH2 domain only with or without the R120K mutation (D). Alternatively, Jurkat cells were not transfected but were stimulated for 24 h with PMA and ionomycin to induce endogenous TSAd expression (B). The location of TSAd was determined by immunostaining using an anti-FLAG antibody (A, C, and D) or an affinity-purified monospecific anti-TSAd antibody (B; shown in red or green). The position of nuclei was determined by Hoescht staining (blue color). In C, the subcellular distribution of wild-type TSAd in Jurkat is shown for comparison with TSAd (R120K) using the same imaging method.
Figure 2.
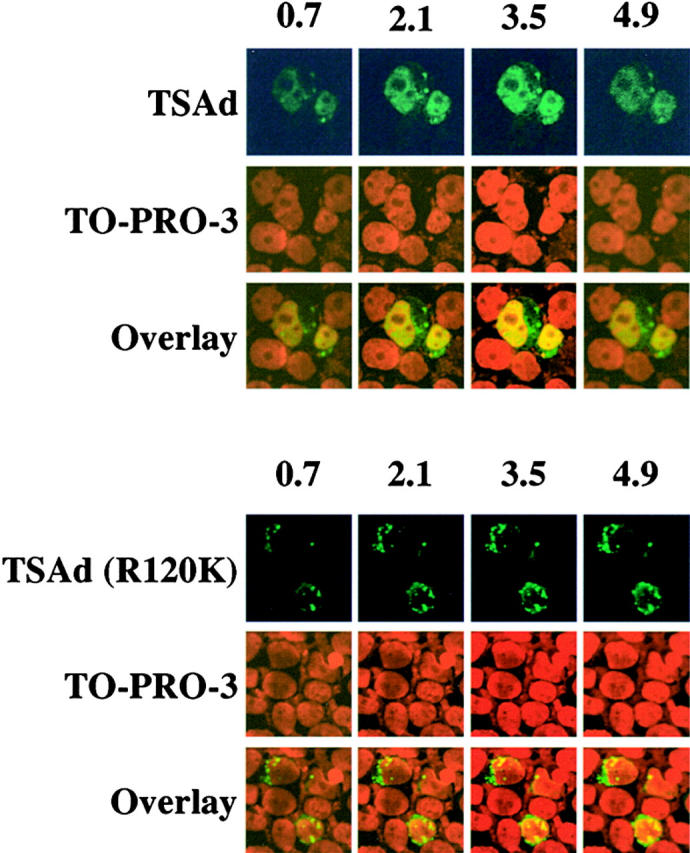
Confocal microscopic analysis of TSAd nuclear expression. Expression of TSAd in 293T cells, transiently transfected with FLAG-TSAd (top) or FLAG-TSAd (R120K; bottom), was examined by immunostaining for the FLAG epitope (green) and confocal microscopy. Nuclei were revealed with the use of TO-PRO-3 (red). Serial sections, in 0.7-μm increments, from the top to bottom of samples, were viewed. Shown are select sections for each sample. The section number (in μm) is indicated at the top of columns.
We next investigated the molecular mechanism of TSAd nuclear import. As TSAd does not contain any obvious nuclear localization sequence (NLS; reference 15), we instead asked if the TSAd SH2 domain was involved. To determine if a functional SH2 domain is required for TSAD nuclear import, we examined the subcellular distribution of a TSAd mutant in which arginine residue 120 of the SH2 domain was mutated to a lysine (R120K). Arginine residue 120, which is conserved in all SH2 domains, is predicted to contact the phosphate group of phosphotyrosine in SH2 domain protein ligands 16. Substitution of a lysine at this position would be expected to prevent ligand recognition. In contrast to wild-type TSAd, TSAd (R120K) was restricted to the cytoplasm of Jurkat, COS-7, and 293T cells (Fig. 1 C and 2; TSAd was confined to the cytoplasm in essentially all transfected cells). This finding, therefore, indicates that a functional SH2 domain is necessary for nuclear localization of TSAd.
A requirement for the TSAd SH2 domain in nuclear import of TSAd could be explained on the basis that either: (a) nuclear import of TSAd is directly mediated by a tyrosine phosphorylated ligand of the SH2 domain, or (b) that a functional SH2 domain is necessary to allow for any posttranslational modifications of the TSAd amino and/or carboxyl regions which constitute the import signal (either because they expose a cryptic NLS or lead to the release of TSAd from the constraints of an otherwise cytoplasmic retention protein). To distinguish between these possibilities, we examined the subcellular distribution of the isolated TSAd SH2 domain that was deleted of flanking amino and carboxyl sequences. As shown in Fig. 1 D, the isolated TSAd SH2 domain was essentially completely nuclear in Jurkat. In contrast, an isolated SH2 domain with an R120K mutation was excluded from the nucleus and found only in cytoplasm. These results argue strongly in favor of the notion that a tyrosine-phosphorylated ligand of the TSAd SH2 domain directly mediates import of TSAd into the nucleus. Given these results, therefore, it is conceivable that a lesser extent of nuclear localization of LAD/RIBP in the D10 T hybridoma may reflect a lower level expression of this ligand and/or its degree of tyrosine phosphorylation in this cell line. Moreover, in overexpression systems, the availability of the ligand may be limiting such that above a certain level of expression, TSAd/LAD/RIBP accumulates in the cytoplasm. Indeed, this would be consistent with our observation of a correlation between expression level of transfected protein and cytoplasmic localization.
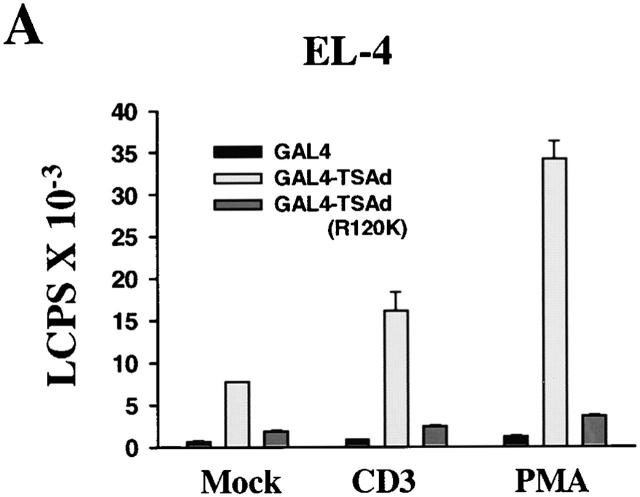
As TSAd was found in the nucleus, we next asked if TSAd possessed transcription-activating ability. To examine this, we produced a GAL4(dbd)–TSAd hybrid that was transfected into EL-4 thymoma cells or Jurkat cells along with a GAL4 operator-luciferase reporter. As shown in Fig. 3a and Fig. b, GAL4(dbd)–TSAd behaved as a potent transcription activator compared with GAL4(dbd) alone. In EL-4 cells, this transcription-activating ability was seen even in the absence of cellular stimulation, although it was accentuated if, after transfection, cells were stimulated with CD3 mAb or PMA. In Jurkat cells, constitutive TSAd transcriptional activity was less apparent. However, after stimulation of cells with agents such as PMA plus ionomycin, TSAd transcriptional activity was readily observed. Interestingly, in both T cell lines, the TSAd (R120K) mutant displayed much reduced transcriptional activity in this assay. This, despite the fact that the GAL4(dbd)–TSAd (R120K) fusion protein was found to be strongly nuclear in transfected cells (Fig. 3 C, compare with Fig. 1 and Fig. 2), presumably because of the presence of a strong NLS contained within the GAL4(dbd) component. These findings, therefore, reveal, (a) an additional role for the TSAd SH2 domain in transcription activation distinct from its role in nuclear import, and (b) that TSAd transcription activation is indirect and mediated by a TSAd SH2 domain ligand.
Figure 3.
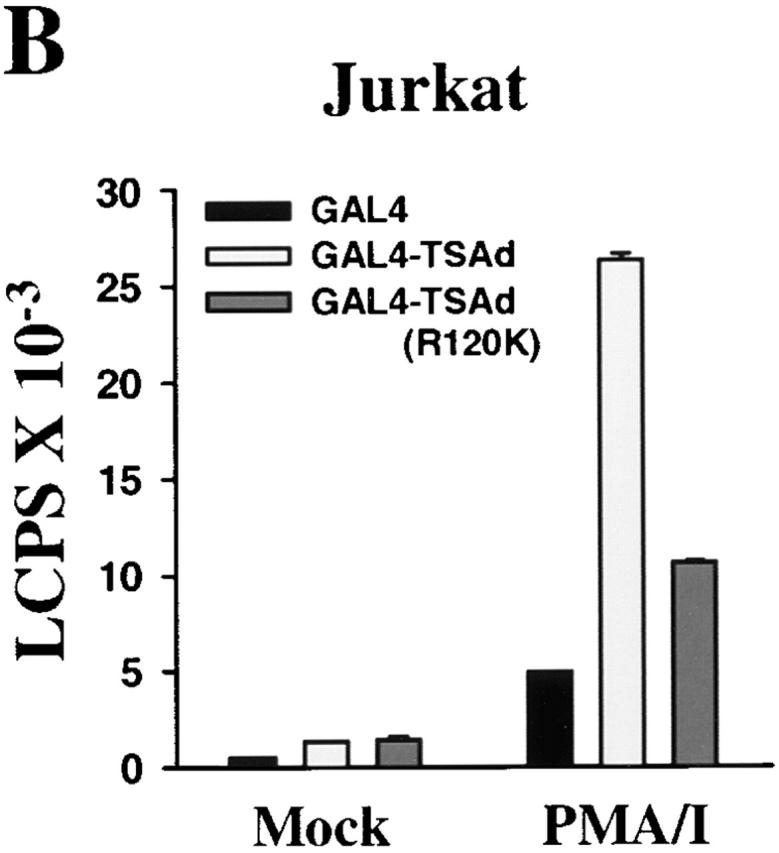
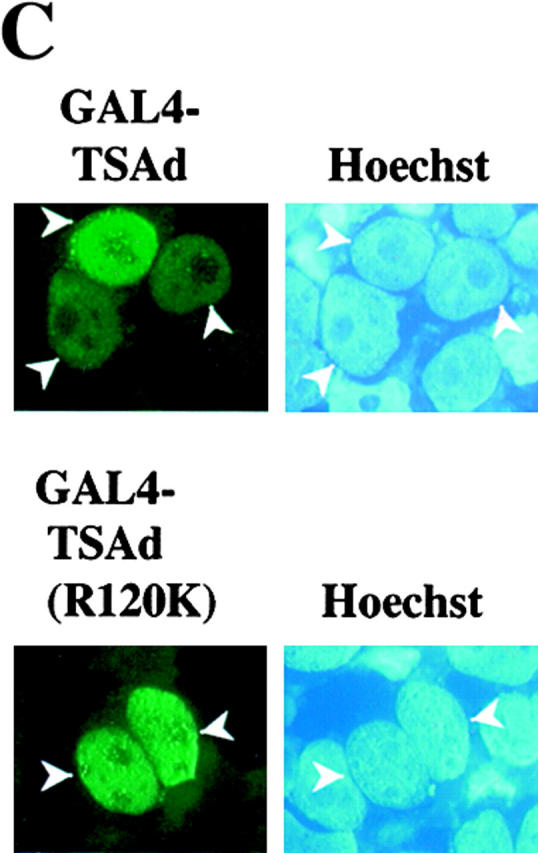
TSAd transcription activation. EL-4 thymoma cells (A) and Jurkat cells (B) were transiently transfected with a GAL4 operator-luciferase reporter and a control renilla-luciferase reporter together with plasmids encoding the GAL4 (dbd) alone or GAL4(dbd)–TSAd or –TSAd (R120K) fusion proteins. After 6 h, cells were activated or not (Mock) with CD3 mAb, PMA, or PMA plus ionomycin for a further 24 h at which point cells were lysed and luciferase activity in cell lysates determined. Results are expressed as luminescence counts per second (LCPS). Shown are the means plus 1 SE of triplicate determinations. Data have been normalized to renilla-luciferase counts obtained from mock stimulated samples. The same results have been obtained in at least 20 repeat experiments for EL-4 and 5 repeat experiments for Jurkat. In C, 293T cells were transiently transfected with plasmids encoding for GAL4(dbd)–TSAd or GAL4(dbd)–TSAd (R120K). The subcellular distribution of fusion proteins was determined by immunostaining using an anti-GAL4(dbd) mAb (green). The position of nuclei was shown by Hoechst staining (blue).
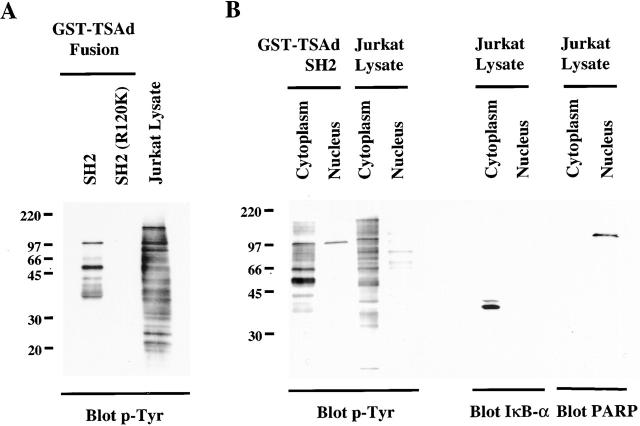
So as to characterize ligands of the TSAd SH2 domain that may be involved in nuclear import and/or transcription activation, we performed GST pulldown assays with the use of a GST–TSAd SH2 domain fusion protein. Fig. 4 A shows that the TSAd SH2 domain bound several tyrosine phosphorylated proteins present in NP-40 lysates of pervanadate-stimulated Jurkat cells. Each of these proteins represent phosphotyrosine-dependent ligands of the SH2 domain, as none were detected in pulldowns using a GST–TSAd SH2 (R120K) fusion protein. The most prominent tyrosine-phosphorylated species were ∼57 and 95–100 kD. Other species were also identified but less consistently. Specifically, Src family kinases (including LCK) and Tec family kinases (including ITK and the human ortholog of RLK known as TXK) were not identified as binding partners of the TSAd SH2 domain in these experiments (not shown).
Figure 4.
TSAd SH2 domain ligands. Glutathione agarose beads coated with GST–TSAd SH2 domain or –TSAd SH2 (R120K) fusion proteins were incubated with NP-40 lysates (A) or cytoplasmic and nuclear lysates (B) of PMA plus pervanadate-stimulated Jurkat cells (cytoplasmic and nuclear lysates were derived from the same number of cells). After extensive bead washing, fusion protein–bound tyrosine-phosphorylated proteins were eluted and detected by Western blotting using an anti-phosphotyrosine antibody (p-Tyr). In panel A, that equivalent quantities of fusion proteins were used in experiments was confirmed by Coomassie blue staining of replicate SDS-page gels (not shown). In B, blots were stripped and reprobed with anti-IκB-α and PARP antibodies (right). IκB-α was detected only in cytoplasmic lysates and PARP only in nuclear lysates indicating relative purity of the respective fractions.
One feature that might be predicted of a TSAd SH2 domain nuclear import and transcription-activating ligand is, at the least, a part presence in the nucleus. To examine this, therefore, we performed the same GST pulldown experiments only using Jurkat lysates that had been fractionated into cytoplasmic and nuclear components. As shown in Fig. 4 B, the 57-kD ligand was detected only in the cytoplasm. In contrast, the 95–100-kD ligand was found in both the cytoplasm and nucleus in approximately equal proportion. Within the nucleus, the 95–100-kD species was the only ligand of the TSAd SH2 domain that was identified. Immunoblotting of nuclear and cytoplasmic lysates for IκB-α (restricted to the cytoplasm) and PARP (restricted to the nucleus) confirmed lack of contamination of lysates by material from the opposing fraction. These results, therefore, are consistent with the notion that the 95–100-kD species alone is involved in both TSAd nuclear import and transcription activation.
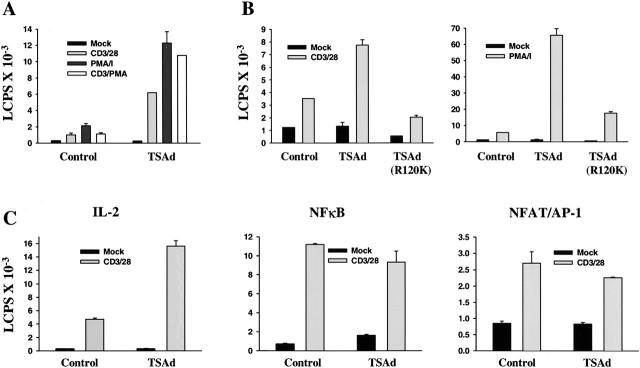
Given the results from transcription assays, as well as data generated from LAD/RIBP knockout mice, we reexamined if TSAd could activate and/or enhance transcription from an IL-2 promoter–reporter construct in Jurkat cells. A previous study that had examined this issue used stable Jurkat transfectants that expressed very high levels of TSAd 3. To address this, therefore, we examined the influence of transiently transfected TSAd upon IL-2 promoter activity and for this purpose used regular Jurkat cells (rather than TagC15 Jurkat cells) that express lower, more physiologic levels of transfected protein. Indeed, as shown in Fig. 5 A, transient transfection of TSAd resulted in a dramatic enhancement of IL-2 promoter activity in Jurkat cells that was induced in response to different stimuli including CD3 plus CD28 mAb, PMA plus ionomycin, and CD3 mAb plus PMA. Generally, this influence was more pronounced at earlier (9–14 h) rather than later (>24 h) time points of activation (not shown); an effect that can presumably be accounted for on the basis that, during the course of the assay, Jurkat would be induced to express progressively increasing quantities of their own endogenous TSAd 3. Furthermore, in the same assays, we repeatedly observed that a TSAd (R120K) mutant was unable to augment induced IL-2 promoter activity (Fig. 5 B). This result is consistent with the findings that a functional SH2 domain is necessary for TSAd nuclear import and transcription activation.
Figure 5.
Influence of TSAd on IL-2 promoter activity. Jurkat cells were transiently transfected with an IL-2 promoter-luciferase reporter construct (A–C) or with NF-κB or NFAT/AP-1-luciferase reporter constructs (C only), a control renilla-luciferase reporter, and constructs encoding the FLAG epitope alone (Control) or FLAG-tagged TSAd (A–C) or FLAG-TSAd (R120K) (B only). After 24 h, cells were activated or not (Mock) with the indicated stimuli for 12 h before determination of luciferase activity. Throughout, data have been normalized to renilla-luciferase counts obtained from mock-stimulated samples and represent the means plus 1 SE of triplicate determinations. In C, the shown data are from the same experiment. The same data shown in A, B, and C have been obtained in five, three, and four independent repeat experiments, respectively.
The major recognized transcription factors involved in control of IL-2 expression in T cells are NF-κB, NFAT, and AP-1 17. Therefore, we asked if the influence of TSAd on IL-2 promoter activity was mediated through any of these transcription factors. To examine this, TSAd was cotransfected into Jurkat cells with an NF-κB reporter (comprising of five tandem repeats of an NF-κB binding site upstream of the luciferase gene) or a composite NFAT/AP-1 reporter (comprising of three tandem repeats of an NFAT/AP-1 binding site derived from the IL-2 promoter, also upstream of the luciferase gene). Fig. 5 C shows that these NF-κB and NFAT/AP-1–based promoters were activated in response to CD3 plus CD28 mAb stimulation of transfected Jurkat. However, cotransfection of TSAd failed to augment this induced activity. In contrast, in the same assays, TSAd did augment IL-2 promoter activity induced by the same stimulus. Thus, these findings suggest that TSAd may regulate IL-2 expression through communication with a distinct element of the IL-2 promoter.
In conclusion, we have identified TSAd as a potential transcription control protein in T cells. Aside from IL-2, a full description of which genes TSAd regulates awaits. Whether or not TSAd directly contacts DNA has also to be determined. As with transcription activation, contact with DNA could be indirect. If so, then this raises the possibility that TSAd might function as a transcription adapter protein that mediates the formation of transcription complexes from component DNA binding and transcription-activating parts.
Acknowledgments
We thank Drs. Gary Koretzky (University of Pennsylvania), C. Wilson Xu (Memorial Sloan-Kettering Cancer Center), Jeff Northrop (Affymax Research Institute), and David J. McKean (Mayo Foundation) for the provision of pEF-FLAG, reporter plasmids, and Jurkat TagC15 cells. Dr. Anne Spurkland (The National Hospital, Oslo, Norway) is thanked for providing the anti-TSAd antibody.
This work was supported by Public Health Service Grant no. R01 AI44926 from the National Institutes of Health to P.D. King.
References
- Clements J.L, Boerth N.J., Lee J.R., Koretzky G.A. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Ann. Rev. Immunol. 1999;17:89–108. doi: 10.1146/annurev.immunol.17.1.89. [DOI] [PubMed] [Google Scholar]
- Spurkland A., Brinchmann J.E., Markussen G., Pedeutou F., Munthe E., Lea T., Vartdal F., Aasheim H.-C. Molecular cloning of a T cell-specific adapter protein (TSAd) containing an Src homology (SH) 2 domain and putative SH3 and phosphotyrosine binding sites. J. Biol. Chem. 1998;273:4539–4546. doi: 10.1074/jbc.273.8.4539. [DOI] [PubMed] [Google Scholar]
- Sundvold V., Torgersen K.M., Post N.H., Marti F., King P.D., Rottingen J.A., Spurkland A., Lea T. T cell-specific adapter protein inhibits T cell activation by modulating Lck activity. J. Immunol. 2000;165:2927–2931. doi: 10.4049/jimmunol.165.6.2927. [DOI] [PubMed] [Google Scholar]
- Choi Y.B., Kim C.K., Yun Y. Lad, an adapter protein interacting with the SH2 domain of p56lck, is required for T cell activation. J. Immunol. 1999;163:5242–5249. [PubMed] [Google Scholar]
- Rajagopal K., Sommers C.L., Decker D.C., Mitchell E.O., Korthauer U., Sperling A.I., Kozak C.A., Love P.E., Bluestone J.A. RIBP, a novel Rlk/Itk-binding adaptor protein that regulates T cell activation. J. Exp. Med. 1999;90:1657–1668. doi: 10.1084/jem.190.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motto D.G., Ross S.E., Wu J., Hendriks-Taylor L.R., Koretzky G.A. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor–mediated interleukin 2 production. J. Exp. Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989;17:7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J.P., Ullman K.S., Crabtree G.R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J. Biol. Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- Sadowski I., Bell B., Broad P., Hollis M. GAL4 fusion vectors for expression in yeast or mammalian cells. Gene. 1992;118:137–141. doi: 10.1016/0378-1119(92)90261-m. [DOI] [PubMed] [Google Scholar]
- Fraser J.D., Irving B.A., Crabtree G.R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Hedin K.E., Bell M.P., Kalli K.R., Huntoon C.J., Sharp B.M., McKean D.J. δ-Opioid receptors expressed by Jurkat T cells enhance IL-2 secretion by increasing AP-1 complexes and activity of the NF-AT/AP-1-binding promoter element. J. Immunol. 1997;159:5431–5440. [PubMed] [Google Scholar]
- Singh S., Saunders G.F. A method for obtaining reporter gene activity and nuclear extracts simultaneously from transiently transfected cells. Anal. Biochem. 1998;265:185–187. doi: 10.1006/abio.1998.2882. [DOI] [PubMed] [Google Scholar]
- King P.D., Sadra A., Teng J.M.C., Liu X.-R., Han A., Selvakumar A., August A., Dupont B. Analysis of CD28 cytoplasmic tail residues as regulators and substrates for the protein tyrosine kinases, EMT and LCK. J. Immunol. 1997;158:580–590. [PubMed] [Google Scholar]
- Sun S., Kesavan K., Schaefer B., Garrington T.P., Ware M., Lassingal Johnson N., Gelfand E.W., Johnson G.L. Mek kinase 2 associates with the adapter protein Lad/RIBP, and regulates the Mek5-BMK1/ERK5 pathway. J. Biol. Chem. 2001;276:5093–5100. doi: 10.1074/jbc.M003719200. [DOI] [PubMed] [Google Scholar]
- Gorlich D., Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Cowburn D. Modular peptide recognition domains in eukaryotic signaling. Annu. Rev. Biophys. Biomol. Struct. 1997;26:259–288. doi: 10.1146/annurev.biophys.26.1.259. [DOI] [PubMed] [Google Scholar]
- Jain J., Loh C., Rao A. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]