Abstract
The thymus in mice lacking both the receptor tyrosine kinase c-kit and the common cytokine receptor γ chain (γc) is alymphoid because these receptors provide essential signals at the earliest stages of thymocyte development. The signals transduced by these receptors potentially regulate proliferation, survival, or differentiation, but the contribution of each receptor to distinct intracellular signaling cascades is only poorly defined. Here, we have examined whether enforced expression of Bcl-2 can rescue thymocyte development in c-kit and γc single or double mutant mice. A bcl-2 transgene (Eμ-bcl-2-25; expressed in the T cell lineage) was introduced into (a) c-kit and γc wild-type (c-kit+γc +bcl+), (b) c-kit–deficient (c-kit−γc +bcl+), (c) γc-deficient (c-kit+γc −bcl+), or (d) c-kit and γc double-deficient mice (c-kit−γc −bcl+). The bcl-2 transgene was functionally active in wild-type and c-kit or γc single mutants, as it promoted survival of ex vivo isolated thymocytes, including pro-T cells. In vivo, however, transgenic Bcl-2 did not release T cell precursors from their phenotypic block and failed to increase progenitor or total thymocyte cellularity in c-kit or γc single or double mutants. These data argue strongly against a role for Bcl-2 as a key mediator in signaling pathways linked to cytokine and growth factor receptors driving early thymocyte development.
Keywords: growth factors, c-kit, common cytokine receptor γ chain, bcl-2, T cell development
Introduction
Prothymocytes colonizing the thymus are very rare cells. This small pool of thymic immigrants undergoes in the order of >100 fold (unpublished data) expansion before rearrangement of the TCR β chain. This expansion is mediated by two key growth/cytokine receptors expressed on prothymocytes, the tyrosine kinase c-kit and common cytokine receptor γ chain (γc)–IL-7Rα chain complexes. The ligands binding to these receptors, stem cell factor (SCF) and IL-7, are provided by the epithelial thymic stroma (for reviews, see references 1 2 3). In mutants lacking c-kit and γc together, but not in single c-kit– or γc-deficient mice, the thymus is alymphoid, and TCR-β, -γ, and -δ rearrangements are essentially undetectable 4 5. Thus, c-kit and γc provide essential, synergistic signals in pro-T cells (for a review, see reference 2). Synergy between growth factor and cytokine signaling is a common theme in many hematopoietic cells, including stem cells (see, for example, reference 6). Due to the complete lack of thymocytes, c-kit and γc double mutant mice may provide a useful model in which individual signaling pathways can be selectively rescued in vivo. At present, the downstream signaling pathways of each receptor are, at least in primary thymocytes, only poorly defined.
Expression of the prototypic antiapoptotic protein Bcl-2 is developmentally regulated in thymocytes. Bcl-2 expression is high in immature CD3−CD4−CD8− (triple negative [TN]) and mature CD3+CD4+CD8− or CD3+CD4− CD8+ (single positive [SP]), and is downmodulated in intermediate CD3lowCD4+CD8+ (double positive [DP]) thymocytes 7 8. Bcl-2 expression is reduced in thymocytes from mice lacking IL-7Rα 9, γc 8, or IL-7 10, and is upregulated after exposure of thymocytes from IL-7–deficient mice to IL-7 in vitro 1 10. Hence, signaling via IL-7R/γc can upregulate Bcl-2 expression in thymocytes. Thymocyte development in bcl-2–deficient mice is normal until early adult life (∼4 wk of age) followed by a strongly increased sensitivity to apoptotic stimuli affecting, among other cell types, all stages of thymocytes and peripheral lymphocytes 11 12 13. Similarly, fetal liver–derived T cell development is more Bcl-2 independent than bone marrow–derived T cell development 13. Collectively, Bcl-2 can act as an antiapoptotic factor in thymocytes, in particular during adult life. As early T cell development is cytokine/growth factor dependent, it is of interest whether growth factor withdrawal–induced thymocyte death can be prevented by Bcl-2 expression. There are conflicting data on the role of Bcl-2 for the rescue of T cell development is the absence of IL-7Rα/γc signaling. Transgene-driven overexpression of Bcl-2 was reported to rescue T cell development in IL-7Rα chain 9 14 or γc 8 15 mutant mice (for a review, see reference 1). In contrast, other groups failed to observe a Bcl-2–mediated rescue of γc-deficient thymocytes (16 17; for a review, see reference 2).
SCF stimulation can provide antiapoptotic signals in hematopoietic cells. This survival signal may be relayed via activation of phosphatidylinositol 3 (PI-3) kinase and Akt kinase (for a review, see reference 18), but c-kit–induced survival may also involve Bcl-2 expression 19. Given that c-kit−γc − mice are completely devoid of thymocytes, we reasoned that these mutants would be a very sensitive model to reveal potential, even minor, effects of Bcl-2 downstream of c-kit and γc. In this paper, we show that the bcl-2 transgene was functionally active, and efficiently promoted survival of thymocytes, including the earliest T cell progenitors ex vivo. In vivo, however, transgenic bcl-2 expression failed to release T cell progenitors, both in terms of phenotype and cell numbers, from their developmental block in all mutants tested. As our data, using postnatal day 5 old mutant mice, did not confirm other reports analyzing adult γc-deficient bcl-2 transgenic mice 1 8 15, we extended our studies to large numbers of adult γc-deficient mice with or without the bcl-2 transgene. Notably, thymus cell numbers in adult γc mutants without the bcl-2 transgene showed considerable heterogeneity (ranging over two logs), an observation pointing at a possible role for modifier genes in the genetic background of the γc mutants. In line with our analysis of neonatal γc mutants, enforced expression of Bcl-2 also failed to rescue T cell development in adult γc-deficient mice. Our study, therefore, challenges the current view that enforced expression of Bcl-2 can rescue defects in thymocyte development caused by lack of γc, and suggests that gene products other than Bcl-2 might modulate signaling pathways linked to cytokine (IL-7) and growth factor (SCF) receptors in early thymocyte development in vivo.
Materials and Methods
Mice.
WB-kitW/+ 20 (SLC), γc − 21, and Eμ-bcl-2-25 22 (The Jackson Laboratory) were maintained under specific pathogen free-conditions. C-kit and γc single and double mutant mice were bred as described 4, and crossed to Eμ-bcl-2-25 mice. Experiments involving c-kit mutant mice were performed on postnatal day 5 old mice, as white spotting locus (W/W) mice die within 10 d after birth 23. W mutant mice were phenotyped by white skin color. Experiments on γc mutants were done either on postnatal day 5, or on adult 6–12-wk-old mice. γc mutant mice were genotyped by PCR on genomic DNA as described 4. Eμ-bcl-2-25 mice 22 were genotyped by PCR on genomic DNA using oligonucleotides amplifying the human bcl-2 gene (5′ oligo: 5′-GGTCATGTGTGTGGAGAGCGTCA-3′) linked to the SV40 polyA site (3′ oligo: 5′-GTTTCAGGTTCAGGGGGAGGT-3′) yielding a diagnostic DNA fragment of ∼1 kb. In addition, in some experiments, thymocytes were typed by Western blotting using anti–human Bcl-2–specific antibodies (Santa Cruz Biotechnology, Inc.).
Monoclonal Antibodies and Flow Cytometry.
The following monoclonal antibodies were used for flow cytometric analyses: Anti-“lineage” antibodies were 2C11 (CD3ε), H129.19 (CD4), 53-6.7 (CD8α) (both from GIBCO BRL), M1/70.15 (Mac-1; Caltag), Gr-1 (granulocytes), TER119 (red blood cells), DX-5 (NK lineage), B220 (B cells; all PE-labeled), biotinylated ACK-4 (c-kit), FITC-labeled 3C7 (CD25), Cy-Chrome–labeled Pgp-1 (CD44; all from BD PharMingen, unless otherwise indicated); streptavidin-allophycocyanin (APC) (Molecular Probes) was used as second step reagent. For FACS® analysis, thymocytes were stained with monoclonal antibodies at 5–10 μg/ml in PBS/5% FCS for 15–30 min on ice and washed once in PBS/5% FCS. Cell sorting and FACS® analysis were done on FACStar™ and FACSCalibur™, respectively (Becton Dickinson). Fluorescence data are displayed as dot plots using CELLQuest™ software (Becton Dickinson).
Ex Vivo Survival Assay.
Total or cell sorter purified thymocyte subpopulations were placed in normal cell culture medium (DMEM plus 10% FCS plus 1 mM pyruvate plus 2 mM glutamine plus 2 × 10−5 M β-mercaptoethanol) and cultured without additional growth factors. At time points indicated in Fig. 1, absolute numbers (or percentage of the starting population) of viable cells were determined by cell counting and trypan blue exclusion in a Neubauer counting chamber.
Figure 1.
The bcl-2 transgene (Eμ-bcl-2-25) is functional in T cell progenitors in c-kit and γc mutant thymocytes. Nontransgenic (open symbols) or Eμ-bcl-2-25 transgenic (filled symbols) total thymocytes from 5-d-old c-kit+γc + (A) or c-kit+γc − mice (B), or c-kit−γc + mice (C), or cell sorter–purified progenitor subsets (CD3−CD4−CD8− [TN]) c-kit+CD25-CD44+ (TN 1) (D), or c-kit+CD25+CD44+ (TN 2) (E) from 5-d-old c-kit+γc + mice were compared for their survival capacity ex vivo. Each symbol represents an individual mouse for each genotype. Thymocytes were placed in normal cell culture medium in vitro, and, at indicated times after initiation of the culture, numbers of viable cells were determined. The Eμ-bcl-2-25 transgene is functional in all of these growth factor receptor mutants, and, importantly, promotes thymocyte survival at those stages, in which mutations in c-kit or γc impair thymocyte development (TN 1 and TN 2). Due to the complete absence of thymocytes in the combined absence of c-kit and γc, the corresponding analysis could not be performed on c-kit−γc − mice.
Results and Discussion
The Eμ-bcl-2-25 Transgene Promotes Survival of c-kit and γc Mutant Thymocytes Ex Vivo.
To analyze whether enforced expression of the bcl-2 transgene could promote survival of thymocyte populations in c-kit or γc mutant mice, the Eμ-bcl-2-25 mouse line was crossed to c-kit and γc single and double mutants. In the Eμ-bcl-2-25 mouse line, transgene expression is T lineage specific 22 with an early onset of transgene expression at the CD3−CD4−CD8− stage of development 24. Functional transgenic Bcl-2 expression can be monitored by extended survival times of primary thymocytes isolated ex vivo 24. To test whether the Eμ-bcl-2-25 transgene was functional in these growth factor receptor mutants, total thymocytes from each strain were cultured in normal cell culture medium, and, at various times after initiation of the culture, numbers of viable cells were determined (Fig. 1A–C). The results from γc and c-kit wild-type mice demonstrated, as expected, that cell death of transgenic thymocytes was delayed by about 2 d when compared with nontransgenic cells cultured in parallel (Fig. 1 A). Bcl-2 also mediated in vitro survival of thymocytes from γc (Fig. 1 B) or c-kit (Fig. 1 C) single mutant mice. Due to the complete absence of thymocytes (see below), the corresponding analysis could not be performed in c-kit−γc − mice.
C-kit and γc play essential roles at the most immature stages of T cell development defined by the phenotypes CD25−CD44+ (TN 1) and CD25+CD44+ (TN 2) 4. In a previous analysis of the Eμ-bcl-2-25 transgenic mice line, functional expression of the bcl-2 transgene was shown for TN 1 cells 24 but c-kit expression was not used to identify pro-T cell within TN 1. The TN 1 population contains c-kit+ and c-kit− cells, and the pro-T cell activity resides only in the c-kit+ fraction 25. Therefore, we analyzed bcl-2 transgene function in c-kit+ TN 1 as well as in c-kit+ TN 2 cells by comparison of the ex vivo survival potential of cell sorter–purified progenitors from wild-type and Eμ-bcl-2-25 transgenic mice. The results demonstrate that this bcl-2 transgene clearly promoted survival of both c-kit+ TN 1 (Fig. 1 D) and c-kit+ TN 2 (Fig. 1 E) cells in vitro. Thus, the Eμ-bcl-2-25 transgene is functional in all of these growth factor receptor mutants, and, importantly, promotes thymocyte survival at those stages, in which thymocyte development is impaired due to lack of c-kit and γc.
These data confirm the findings of O'Reilly and colleagues 24 on the function of this bcl-2 transgene in TN 2 cells, and extend functional bcl-2 expression to the c-kit+ (pro-T cell fraction) within the TN 1 subset. Kondo et al. 15 reported that the Eμ-bcl-2-25 transgene was not expressed in the earliest thymic precursors defined as CD3−CD25−c-kit+ (which would include c-kit+TN 1 cells). However, our experiments clearly show that this is not the case, as both c-kit+ TN 1 and c-kit+ TN 2 cells from Eμ-bcl-2-25 were markedly protected from growth factor withdrawal–induced apoptosis. Collectively, the Eμ-bcl-2-25 transgene is functional at exactly those stages of development which are affected by lack of SCF and IL-7.
Enforced Expression of Bcl-2 Does Not Normalize Thymus Cellularity in Postnatal c-kit and γc Single and Double Mutant Mice In Vivo.
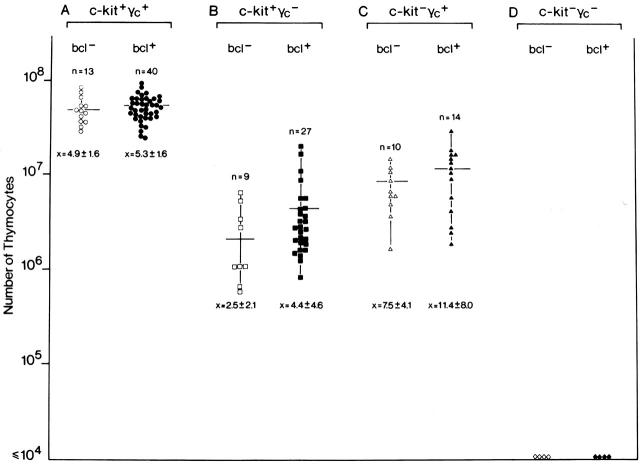
Given that the bcl-2 transgene was functional in the relevant progenitor subsets, we next analyzed the effect of the transgene on thymus cellularity of various growth factor receptor mutants. Mice of eight different genotypes were generated and analyzed: (a) wild-type mice with (c-kit+γc +bcl+) or (b) without (c-kit+γc +bcl−) the transgene, (c) mice deficient for c-kit with (c-kit−γc +bcl+), or (d) without (c-kit−γc +bcl−) the transgene, (e) mice deficient for γc with (c-kit+γc −bcl+), or (f) without (c-kit+γc −bcl−) the transgene, and, finally, mice deficient for both c-kit and γc with (g) (c-kit−γc −bcl+) or (h) without (c-kit−γc −bcl−) the transgene. In Fig. 2, thymocyte numbers are plotted for all individual mice of the indicated genotypes. In total, 121 mice were analyzed (numbers of mice for each group are indicated in Fig. 2). As null mutations in c-kit (c-kitW/W) are lethal within 10 d after birth, these analyses were done on day 5.
Figure 2.
Failure of transgenic Bcl-2 to normalize thymocyte cellularity in c-kit and γc single and double mutant mice in vivo. To asses the effect of transgenic Bcl-2 expression on thymus cellularity in growth factor receptor mutants, mice of eight different genotypes were generated: (A) wild-type mice without (c-kit+γc +bcl−; ○) or with (c-kit+γc +bcl+; •) the transgene; (B) mice deficient for γc without (c-kit+γc −bcl−; □) or with (c-kit+γc −bcl+; ▪) the transgene; (C) mice deficient for c-kit without (c-kit−γc +bcl−; ▵) or with (c-kit−γc +bcl+; ▴) the transgene; (D) mice deficient for both c-kit and γc without (c-kit−γc −bcl−; ⋄) or with (c-kit−γc −bcl+; ♦) the transgene. All mice were analyzed on postnatal day 5 because c-kitW/W mice die by postnatal day 10. Numbers of mice per group are indicated above each group; below, the mean cell number ± 1 SD are shown. No significant differences were observed between bcl-2 transgenic and nontransgenic mice (Wilcoxon rank-sum test: A: transgenic vs. nontransgenic, rank-sum normal statistic with correction Z = −0.8997, P value = 0.3683; B: transgenic vs. nontransgenic, rank-sum normal statistic with correction Z = −1.645, P value = 0.1; C: transgenic vs. nontransgenic, rank-sum normal statistic with correction Z = −1.0839, P value = 0.2784, alternative hypothesis: true μ is not equal to 0; in D, no statistical comparison was done because thymocytes were undetectable in both groups of mice). Thymocytes were undetectable in c-kit−γc − mice regardless of the bcl-2 transgene.
Thymocyte numbers are reduced ∼15-fold in γc single and ∼6-fold in c-kit single mutants when compared with wild-type mice 4. As shown in Fig. 2, and as reported previously 22, the bcl-2 transgene did not increase the total cell number in wild-type mice (Fig. 2 A). Notably, there was no significant (for the statistical evaluation, see the legend to Fig. 2) effect of the bcl-2 transgene on thymocyte cellularity in γc single (Fig. 2 B) and c-kit single (Fig. 2 C) mutants. Thus, enforced expression of bcl-2 does not rescue overall thymic cellularity caused by lack of γc or c-kit. The thymus anlage in c-kit−γc − mice, lacking lymphoid cells, is composed of disorganized thymic epithelium 26 and thymic dendritic cells, the latter cell type developing independently of these growth factor receptors and any detectable pro-T cell compartment 27. Given that thymocytes are undetectable in the double mutant thymus, this “null base line mutant” should be very sensitive to uncover even marginal effects of the bcl-2 transgene, i.e., even a partial Bcl-2–mediated rescue should be evident by the reappearance of thymocytes. However, even in bcl-2 transgenic c-kit−γc − mice, thymocytes failed to appear.
In the Adult Thymus, the Developmental Block Caused by γc Deficiency Is Not Rescued by Transgenic Bcl-2.
Given that c-kit null mutations are lethal by postnatal day 10, all experiments involving c-kit single and double mutants, including the relevant control mice (Fig. 2), were performed on 5-d-old mice. While these experiments were underway, other investigators reported that bcl-2 transgenes, when bred onto γc-deficient mice, rescued T lymphopoiesis, but not B or NK cell development 15. Similarly, bcl-2 transgenes were also reported to rescue thymocytes cellularity in IL-7Rα–deficient mice 9 14. The discrepancy between lack of rescue of the γc deficiency phenotype, in our hands, and rescue by bcl-2 transgenes, reported by others, prompted us to analyze whether a Bcl-2 effect was only evident in adult mice, but not in postnatal mice. Therefore, the impact of the Eμ-bcl-2-25 transgene on thymocyte cellularity in adult γc-deficient mice was analyzed. In Fig. 3, thymus cellularity is compared between γc − and γc −bcl+ mice. When thymus cell numbers were analyzed in large numbers (n = 25) of γc − mice, we surprisingly observed a wide range of cell numbers spreading over more than two logs. This data set suggests that γc − mice fall into three groups: (a) mice with a relatively mild phenotype (>107 thymocytes), (b) mice with an intermediate phenotype (between 106 and 107 thymocytes), and (c) mice with the most severe phenotype (between 105 and 106 thymocytes). Of note, a similar distribution was found in γc −bcl+ (n = 34) mice. As these mice, but also mice examined by Kondo et al. 15, were on mixed genetic backgrounds (here WB, C57Bl/6, Balb/c, and 129/ola), it is possible that the penetrance of the γc mutation is subject to modifier genes, reminiscent of phenotypic heterogeneity in IL-7R mutants 28. Nevertheless, comparing γc − versus γc −bcl+ mice, both range and mean values were overlapping and not significantly different. Thus, in our hands, a functionally active bcl-2 transgene does not rescue thymus cellularity in adult γc-deficient mice.
Figure 3.
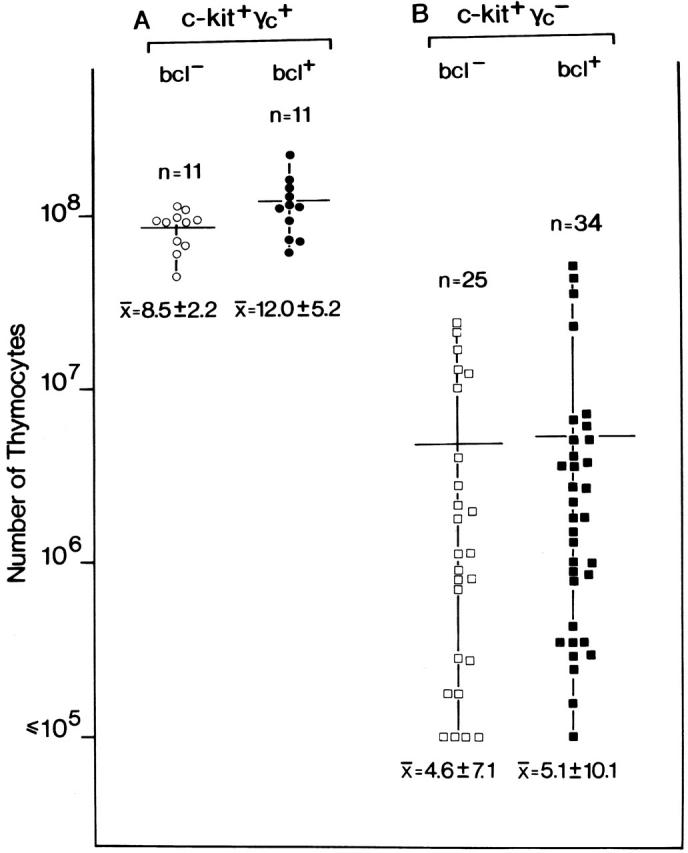
Failure of transgenic bcl-2 to rescue thymus cellularity in adult γc-deficient mice in vivo. To analyze the effect of transgenic bcl-2 expression on thymus cellularity in adult mice, animals of four different genotypes were generated: (A) wild-type mice without (c-kit+γc +bcl−; ○) or with (c-kit+γc +bcl+; •) the transgene, and (B) mice deficient for γc without (c-kit+γc −bcl−; □) or with (c-kit+γc −bcl+; ▪) the transgene. Null mutations in c-kit (W/W) are lethal by postnatal day 10 precluding an analysis of adult c-kit–deficient mice. Numbers of mice per group are indicated above each group; below, the mean thymocyte cell number ± 1 SD are shown. Although the range of cell numbers per thymus was very large (over two logs), no differences were observed between bcl-2 transgenic and nontransgenic mice.
Failure of Transgenic bcl-2 to Release γc-deficient Postnatal or Adult Pre-T Cells from Their Phenotypic Block at the CD25+CD44+/− Stage.
A genetic rescue of a developmental block in the thymus should be evident by increased cell numbers, and by a release from the phenotypic developmental block. For instance, introduction of a TCR β chain into SCID or recombination activating gene (RAG) mutant mice releases thymocytes from their block in differentiation at the TN 3 stage, and promotes the development of TN 4 cells and large numbers of CD4+CD8+ thymocytes (for a review, see reference 29). In mice lacking γc or IL-7Rα or IL-7, TN thymocyte development is severely affected. The transition from TN 2 (CD44+CD25+) to TN 3 (CD44−CD25+) stages is (incompletely) blocked causing increased percentages of TN 2 and decreased percentages of TN 3 cells 30 31. As a result of this block, TN 4 (CD44−CD25−) cells are reduced >10-fold in γc/IL-7Rα/IL-7 mutants (for a review, see reference 2). To test the possibility that such “phenotypic release” took place without affecting overall cell numbers (Fig. 2 and Fig. 3), lineage marker (CD3, CD4, CD8, B220, Ter119, Mac 1, Gr-1, DX5)-negative thymocytes were analyzed by flow cytometry for expression of CD44 versus CD25 (Fig. 4). This analysis included postnatal 5-d-old (Fig. 4A–C) and adult (Fig. 4D–F) mice which had a wild-type (Fig. 4A and Fig. D), γc-deficient (Fig. 4B and Fig. E), or γc-deficient bcl-2 transgenic (Fig. 4C and Fig. F) genotype. In agreement with previous reports 30 31, data shown in Fig. 4 confirmed that the transition from TN 2 to TN 3 was inhibited, and that TN 4 cells were almost absent in postnatal (γc +: 16% TN 2, 63% TN 3, and 14% TN 4, versus γc −: 42% TN 2, 36% TN 3, and 1% TN 4), and in adult (γc +: 11% TN 2, 63% TN 3, and 21% TN 4, versus γc −: 40% TN 2, 57% TN 3, and 1% TN 4) γc − mice. A release from the “γc block” should be detectable by decreased percentages of TN 2, increased percentages of TN 3, and, most visibly, by rescue of the lacking TN 4 compartment. Notably, the pattern of TN subsets was unaltered comparing γc − and γc −bcl+ mice. This holds true for both young (Fig. 4, compare B versus C) and adult (Fig. 4, compare E versus F) mice.
Figure 4.
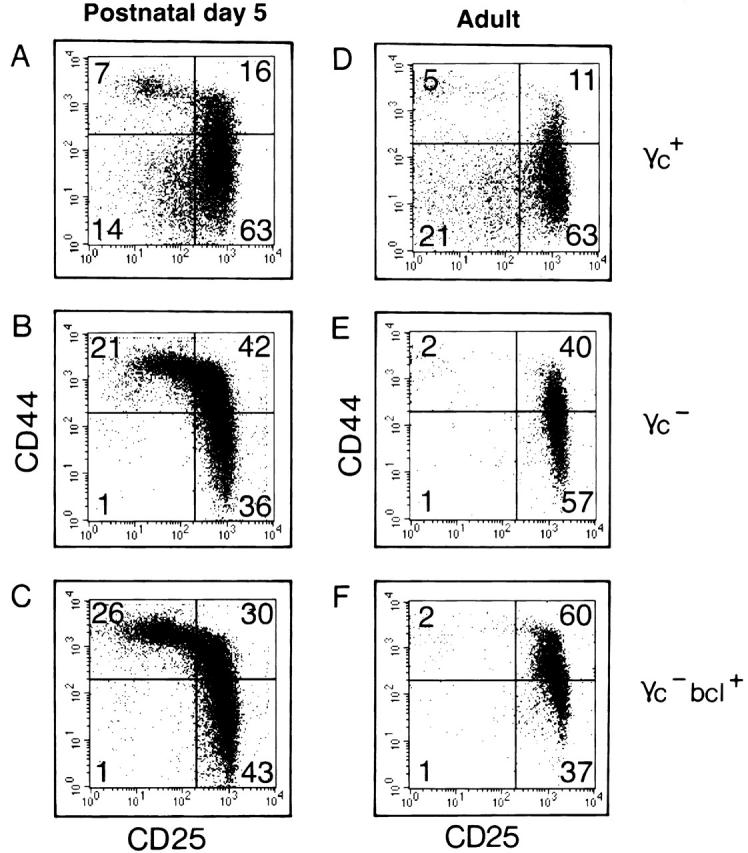
Failure of transgenic bcl-2 to release postnatal or adult pre-T cells from their γc dependence at the CD44+/−CD25+ stage. Lineage marker (CD3, CD4, CD8, B220, Ter119, Mac 1 Gr-1, DX5) negative thymocytes from postnatal day 5 (A–C) or adult (D–F) γc wild-type (A and D), γc − (B and E), or γc −bcl+ (C and F) mice were analyzed by flow cytometry for expression of CD44 vs. CD25. Thymocytes develop from TN 1 (CD44+CD25−) via TN 2 (CD44+CD25+) and TN 3 (CD44−CD25+) to TN 4 (CD44−CD25−). In γc − mice, thymocyte development is incompletely blocked at the transition from TN 2 to TN 3 causing a strong reduction in TN 4 cells (14% [postnatal] or 21% [adult] TN 4 in γc wild-type vs. 1% [for both postnatal or adult] TN 4 in γc −). Enforced expression of Bcl-2 does not release postnatal or adult thymic precursors from the developmental block caused by lack of γc (compare B vs. C, and E vs. F).
In addition to the overall reduction in thymocyte cellularity, cell numbers of lineage marker–negative thymocytes are also reduced in γc − mice (for a review, see reference 2). To analyze lin− cell numbers in these mutant mice, CD4−CD8− (DN) thymocytes were prepared (by αCD4 and αCD8 antibody binding and C′ lysis), and the resulting populations were stained with the lineage-mix antibodies (see above). In postnatal γc + mice, the DN population contained 43% lin− cells (which split into TN 1 to 4 subsets as shown in Fig. 4). In contrast, in postnatal γc − mice, the DN population contained only 27% lin− cells, demonstrating an overall reduction in thymic lin− cellularity of ∼30-fold (15-fold reduction in overall thymocyte cell numbers and 2-fold reduction of lin− cells). The proportion of thymic lin− cells among DN thymocytes was strongly reduced in adult mice compared with young mice (young γc + mice: 43% lin− cells; adult γc + mice: 7.8% lin− cells). Of note, the proportion of thymic lin− cells among DN thymocytes was not increased by Bcl-2 (postnatal mice: γc + = 43%; γc − = 27%; γc −bcl+ 25% lin− cells; adult mice: γc + = 7.8%; γc − = 4.4%; γc −bcl+ 3.1% lin− cells). Collectively, enforced expression of Bcl-2 failed to rescue the phenotypic block in γc − mice, and did not increase the lin− thymic progenitor pool. This finding is consistent with the observed inability of Bcl-2 to rescue overall thymus cell numbers.
In two previous reports suggesting that transgenic bcl-2 can rescue T cell development in γc − mice 8 15, the phenotype of TN subsets, according to CD44 versus CD25 staining on lineage-negative thymocytes, had not been investigated. Kondo et al. analyzed progenitor populations for expression of c-kit versus CD25 gated on CD3− cells, an analysis which may have obscured phenotype and distribution within TN subsets because many other nonprogenitor cells types such as NK cell or myeloid cells are included in the CD3− cells. Clearly, failure of a Bcl-2–mediated rescue of the strongest γc − phenotype, i.e., restoration of TN 4 cells, cannot be detected by this type of analysis. Phenotypic and quantitative analysis of the TN 1 to 4 subsets in γc − mice now demonstrates that enforced expression of Bcl-2 does not rescue the γc- mutant phenotype.
Growth Factor– and Cytokine Receptor–mediated Signals Are Essential at the First Stages of Thymocyte Development: A Role for Bcl-2?
Thymocyte development can be separated into three major stages: (a) growth factor– and cytokine-driven expansion of rare colonizing T cell progenitors; (b) selection of thymocytes for further development on the basis of productive TCR β chain/pre-TCR complexes (β-selection); and (c) α/β TCR–based repertoire selection. In this first expansion, c-kit and γc act in synergy, i.e., each receptor mutant alone is permissive for T cell development but mice lacking both receptors lack all thymocytes. The molecular mechanisms underlying this signaling synergy in thymocytes are unknown. Clearly, one or both of these receptors may induce an intracellular antiapoptotic signal.
In this paper, we have analyzed bcl-2 transgenic young c-kit and γc single and double mutants, as well as adult γc mutants. Enforced expression of Bcl-2 (a) failed to increase thymocyte cell numbers, and (b) TN 2/TN 3 cells were not released from their block in differentiation. To account for possible variability within each group of mice, large numbers of animals were analyzed, both for young and adult mice. Consistent with our finding that Bcl-2 expression cannot overcome defects in c-kit− thymocytes, Domen and Weissman showed that development of mast cells, a c-kit–dependent lineage, was not rescued by expression of transgenic bcl-2 in viable c-kit mutants (W41/W41) 6. In stem cells, Bcl-2 has been suggested to synergize with c-kit signaling in preventing cell death but here, as in early thymocytes (this study), Bcl-2 does not act downstream of c-kit 6.
The effect of bcl-2 transgenes on the thymus in γc-deficient mice, has been studied by us (this paper) and others 8 15, and the results are clearly conflicting. Kondo et al., crossed the very same bcl-2 transgenic line (Eμ-bcl-2-25) into γc-deficient mice, and reported partial rescue of thymocyte cell numbers (6.8 × 106 [γc −] vs. 2.8 × 107 [γc − Eμ-bcl-2-25]). Although these authors observed almost no effect of the bcl-2 transgene on the precursor pool (defined as CD3− c-kit+), the increase in cell numbers was somewhat stronger in more mature thymocytes (defined as CD69+ cells). We cannot reproduce the findings by Kondo et al. on overall cellularity, and have no evidence that bcl-2 expression can release γc-deficient thymocytes from their phenotypic block (Fig. 4). This discrepancy is not easily explained. One caveat in this type of genetic rescue experiment by crossing mouse lines is based on the fact that γc deficiency appears to be sensitive to modifier genes, and mice used in our study, but also mice used by others, were on mixed genetic backgrounds (only three backcrosses to B6 15, or two backcrosses to B6 or Balb/c 8). As we observed a massive heterogeneity of cell numbers ranging over two logs which became evident only after analysis of large numbers of mice (Fig. 3), such heterogeneity might account for the observed discrepancies. A detailed analysis of the developmental block in γc-mutants with “mild” versus “intermediate” versus “severe” reduction in thymus cell numbers could give insights into the nature of any modifier gene(s). Enforced Bcl-2 expression may “tilt” death versus differentiation choices constantly towards survival along various intra- and extrathymic T cell differentiation stages, causing “nonspecific” accumulation of thymocytes. Nevertheless, we conclude that a Bcl-2–mediated survival signal is insufficient to substitute for the stage-specific signals provided by c-kit and γc in prothymocyte development.
Acknowledgments
We thank Drs. H.J. Fehling, U. Grawunder (Ulm), J.P. DiSanto (Paris), and H. Spits (Amsterdam) for discussions, and Dr. Louise Ryan (Dana-Farber Cancer Institute, Boston, MA) for statistical evaluations.
The Basel Institute for Immunology was founded and supported by F. Hoffmann-La Roche (Basel, Switzerland). H.R. Rodewald was supported by the Deutsche Forschungsgemeinschaft (SFB-497-B5), and by Landesforschungschwerpunkt Baden-Württemberg.
References
- Akashi K., Kondo M., Weissman I.L. Role of interleukin-7 in T-cell development from hematopoietic stem cells. Immunol. Rev. 1998;165:13–28. doi: 10.1111/j.1600-065x.1998.tb01226.x. [DOI] [PubMed] [Google Scholar]
- DiSanto J.P., Rodewald H.-R. In vivo roles of receptor tyrosine kinases and cytokine receptors in thymocyte development. Curr. Opin. Immunol. 1998;10:196–207. doi: 10.1016/s0952-7915(98)80249-5. [DOI] [PubMed] [Google Scholar]
- Haks M.C., Oosterwegel M.A., Blom B., Spits H.M., Kruisbeek A.M. Cell-fate decisions in early T cell developmentregulation by cytokine receptors and the pre-TCR. Semin. Immunol. 1999;11:23–37. doi: 10.1006/smim.1998.0153. [DOI] [PubMed] [Google Scholar]
- Rodewald H.-R., Ogawa M., Haller C., Waskow W., DiSanto J.P. Pro-thymocyte expansion by c-kit and the common cytokine receptor γ chain is essential for repertoire formation. Immunity. 1997;6:265–272. doi: 10.1016/s1074-7613(00)80329-5. [DOI] [PubMed] [Google Scholar]
- Rodewald H.-R., Haller C. Antigen receptor junctional diversity in growth factor receptor deficient mice. Dev. Comp. Immunol. 1998;22:351–365. doi: 10.1016/s0145-305x(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Domen J., Weissman I.L. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J. Exp. Med. 2000;192:1707–1718. doi: 10.1084/jem.192.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N.C., Anderson G., Williams G.T., Owen J.J., Jenkinson E.J. Developmental regulation of bcl-2 expression in the thymus. Immunology. 1994;81:115–119. [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Leonard W.J. Role of Bcl-2 in alpha beta T cell development in mice deficient in the common cytokine receptor gamma-chainthe requirement for Bcl-2 differs depending on the TCR/MHC affinity. J. Immunol. 1999;162:782–790. [PubMed] [Google Scholar]
- Akashi K., Kondo M., von Freeden-Jeffry U., Murray R., Weissman I.L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry, U., N. Solvason, M. Howard, and R. Murray. 1997. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 147–154. [DOI] [PubMed]
- Veis D.J., Sorenson C.M., Shutter J.R., Korsmeyer S.J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Negishi I., Kuida K., Sawa H., Loh D.Y. Targeted disruption of Bcl-2 alpha beta in miceoccurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl. Acad. Sci. USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y., Nakayama K., Tomita T., Isoda M., Loh D.Y., Nakauchi H. Role of bcl-2 in the development of lymphoid cells from the hematopoietic stem cell. Blood. 1997;89:853–862. [PubMed] [Google Scholar]
- Maraskovsky E., O'Reilly L.A., Teepe M., Corcoran L.M., Peschon J.J., Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- Kondo M., Akashi K., Domen J., Sugamura K., Weissman I.L. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- Blom B., Spits H., Krimpenfort P. The role of the common gamma chain of the IL-2, IL-4, IL-7 and IL-15 receptors in development of lymphocytes. Constitutive expression of bcl-2 does not rescue the developmental defects in gamma common deficient mice. In: Smit Sibinga C.T., Das P.C., Löwenberg B., editors. Cytokines and Growth Factors in Blood Transfusion. B. Kluwer Academic Publishers; Dordrecht, Netherlands: 1997. pp. 3–11. [Google Scholar]
- Jacobs H., Krimpenfort P., Haks M., Allen J., Blom B., Demolliere C., Kruisbeek A., Spits H., Berns A. PIM1 reconstitutes thymus cellularity in interleukin 7- and common gamma chain-mutant mice and permits thymocyte maturation in Rag- but not CD3gamma-deficient mice. J. Exp. Med. 1999;190:1059–1068. doi: 10.1084/jem.190.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Carson W.E., Haldar S., Baiocchi R.A., Croce C.M., Caligiuri M.A. The c-kit ligand suppresses apoptosis of human natural killer cells through the upregulation of bcl-2. Proc. Natl. Acad. Sci. USA. 1994;91:7553–7557. doi: 10.1073/pnas.91.16.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. Hereditary anemias of the mousea review for geneticists. Adv. Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- DiSanto J.P., Müller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A.W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Russell E.S., Lawson F.A. Selection and inbreeding for longevity of a lethal type. J. Hered. 1959;50:19–25. [Google Scholar]
- O'Reilly L., Harris A., Strasser A. bcl-2 transgene expression promotes survival and reduces proliferation of CD3−CD4−CD8− T cell progenitors. Int. Immunol. 1997;9:1291–1301. doi: 10.1093/intimm/9.9.1291. [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Zlotnik A., Suda T. Phenotypic and functional characterisation of c-kit expression during intrathymic T cell development. J. Immunol. 1992;149:2281–2285. [PubMed] [Google Scholar]
- Rodewald H.-R., Fehling H.J. Molecular and cellular events in early thymocyte development. Adv. Immunol. 1998;69:1–112. doi: 10.1016/s0065-2776(08)60606-9. [DOI] [PubMed] [Google Scholar]
- Rodewald H.R., Brocker T., Haller C. Developmental dissociation of thymic dendritic cell and thymocyte lineages revealed in growth factor receptor mutant mice. Proc. Natl. Acad. Sci. USA. 1999;96:15068–15073. doi: 10.1073/pnas.96.26.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Siegler S.F., Williams D.E., Ware C.B. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P., von Boehmer H. Development and selection of T cellsfacts and puzzles. Adv. Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Moore T.A., von-Freeden-Jeffry U., Murray R., Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7−/− mice. J. Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- He Y.W., Nakajima H., Leonhard W.J., Adkins B., Malek T.R. The common γ-chain of cytokine receptors regulates intrathymic T cell development at multiple stages. J. Immunol. 1997;158:2592–2599. [PubMed] [Google Scholar]