Abstract
Two hematopoietic-specific adapters, src homology 2 domain–containing leukocyte phosphoprotein of 76 kD (SLP-76) and linker for activation of T cells (LAT), are critical for T cell development and T cell receptor (TCR) signaling. Several studies have suggested that SLP-76 and LAT function coordinately to promote downstream signaling. In support of this hypothesis, we find that a fraction of SLP-76 localizes to glycolipid-enriched membrane microdomains (GEMs) after TCR stimulation. This recruitment of SLP-76 requires amino acids 224–244. The functional consequences of targeting SLP-76 to GEMs for TCR signaling are demonstrated using a LAT/SLP-76 chimeric protein. Expression of this construct reconstitutes TCR-inducted phospholipase Cγ1 phosphorylation, extracellular signal–regulated kinase activation, and nuclear factor of activated T cells (NFAT) promoter activity in LAT-deficient Jurkat T cells (J.CaM2). Mutation of the chimeric construct precluding its recruitment to GEMs diminishes but does not eliminate its ability to support TCR signaling. Expression of a chimera that lacks SLP-76 amino acids 224–244 restores NFAT promoter activity, suggesting that if localized, SLP-76 does not require an association with Gads to promote T cell activation. In contrast, mutation of the protein tyrosine kinase phosphorylation sites of SLP-76 in the context of the LAT/SLP-76 chimera abolishes reconstitution of TCR function. Collectively, these experiments show that optimal TCR signaling relies on the compartmentalization of SLP-76 and that one critical function of LAT is to bring SLP-76 and its associated proteins to the membrane.
Keywords: adapter proteins, signal transduction, T cell activation, lipid rafts, protein tyrosine kinase
Introduction
One of the earliest detectable biochemical events after TCR engagement is the tyrosine phosphorylation of several proteins by protein tyrosine kinases (PTKs) 1 2 3. PTK activation is required for the subsequent initiation of intracellular signaling leading to new gene expression and other features of T cell activation 4 5 6 7. Although much is known about both the proximal phosphorylation events as well as the downstream signaling pathways required for T cell activation, the mechanisms by which these steps are integrated after TCR ligation remain less clear. Several studies have shown that the early phosphorylation events are required for the creation of multimolecular protein complexes that coordinate the various signals important for successful T cell activation (for review see references 8 9 10). These complexes are nucleated by adapter molecules, proteins that contain modular domains responsible for mediating interactions with other molecules within the cell. Recently, two such adapter proteins, src homology (SH)2 domain–containing leukocyte phosphoprotein of 76 kD (SLP-76) and linker for activation of T cells (LAT), have been shown to be indispensable for T cell development and activation 11 12 13 14 15 16.
SLP-76 is a cytosolic protein expressed in T cells, macrophages, NK cells, and platelets 17 18. It consists of an NH2-terminal acidic region that includes several tyrosines that are phosphorylated after TCR engagement 19 20. These phosphotyrosines bind to SH2 domains of key signaling molecules, including Vav 21 22 23 24, an exchange factor for the Rac GTPase, and inducible T cell kinase (ITK), a Tec family PTK 25 26 27. The central region of SLP-76 is rich in proline residues, enabling SLP-76 to associate constitutively with Gads (also known as GrpL, Grf40, or Mona) via the two Gads SH3 domains 28 29 30 31. The COOH-terminal region of SLP-76 contains an SH2 domain that binds to another hematopoietic specific adapter molecule (SLP-76–associated phosphoprotein of 130 kD [SLAP-130], also known as Fyb) after TCR engagement and tyrosine phosphorylation of SLAP-130 32 33.
LAT is expressed in the same tissues as SLP-76; however, in contrast to SLP-76, it is a transmembrane protein 34. Due to posttranslational fatty acid modifications, LAT is targeted to glycolipid-enriched membrane microdomains (GEMs, also known as lipid rafts), compartments known to be critical for concentrating TCR-stimulated signaling molecules 35 36. After TCR engagement, GEM-localized LAT is phosphorylated on multiple tyrosine residues, enabling it to bind SH2 domains of numerous signaling molecules, including phospholipase C (PLC)γ1, the 85-kD subunit of phosphatidylinositol-3 kinase, and Grb2 34. Mutation of these tyrosine residues produces a dominant negative effect on TCR signaling 34. Interestingly, LAT also inducibly binds to Gads, allowing for the creation of a LAT–Gads–SLP-76 trimolecular complex 28 29 30 34.
Several lines of evidence have demonstrated that both SLP-76 and LAT play critical roles in T cell function. Overexpression of SLP-76 in transformed T cell lines results in increased efficiency of TCR signaling 37. Mutant variants of Jurkat T cells, deficient in either SLP-76 13 or LAT 14 15, fail to signal effectively via their TCRs. Although activation of src and syk family PTKs is retained in Jurkat cells lacking SLP-76 or LAT, phosphorylation of key substrates (such as PLCγ1) is absent, preventing translation of the most proximal TCR-mediated signaling events into cellular activation 13 14 15. The most compelling evidence for the importance of both SLP-76 and LAT in T cell biology has come from the analysis of mice made deficient in these proteins by targeted gene disruption 11 12 16. Both SLP-76 and LAT null mice have no peripheral T cells, as development is arrested during early thymopoiesis, presumably due to a failure of the pre-TCR to signal effectively.
Similar signaling defects in SLP-76– and LAT-deficient Jurkat cells and mice, coupled with the observation that the two adapters can associate indirectly via Gads, suggested the possibility that these two proteins function coordinately to regulate TCR-mediated events. In this report, we describe experiments performed to test the hypothesis that a major function of LAT is to recruit SLP-76 to the plasma membrane. First, we demonstrate the inducible translocation of SLP-76 to GEMs. This targeting requires amino acids 224–244, which were shown previously to encompass the Gads binding domain of SLP-76. Then, using LAT-deficient Jurkat T cells and various chimeric molecules combining modules of SLP-76 and LAT, we show that all of the tyrosines of LAT are dispensable if SLP-76 is covalently attached to LAT sequences that target the chimeric molecule to GEMs. We show further that if SLP-76 is tethered to LAT, the Gads binding site of SLP-76 becomes unnecessary for the support of TCR signaling, but SLP-76 tyrosines (Y113, 128, and 145) remain essential. Additionally, although necessary for optimal TCR function, GEM localization of the LAT/SLP-76 chimera is not absolutely essential. Collectively, our experiments support the conclusion that in the Jurkat model system, an important role for LAT is to recruit SLP-76 and its associated molecules to the membrane, where signaling molecules are concentrated.
Materials and Methods
Cells and Cell Culture.
Mutant variants of Jurkat deficient in LAT expression (J.CaM2; reference 14) and SLP-76 expression (J14-v-29; reference 13) were provided by A. Weiss (University of California, San Francisco, CA). E6-1 Jurkat T cells, J.CaM2, and J14-v-29 cells were maintained in RPMI 1640 media with 10% FCS, penicillin (1,000 U/ml), streptomycin (1,000 U/ml), and glutamine (20 mM) in a 5% CO2 humidified atmosphere at 37°C as described previously 38.
Antibodies and Reagents.
The following antibodies were used: clonotypic Jurkat anti-TCR mAb C305 (gift from A. Weiss) 38, anti-LAT polyclonal antisera (gift from L.A. Samelson, National Cancer Institute, Bethesda, MD), anti-Gads polyclonal antisera (gift from J. McGlade, Hospital for Sick Children, Toronto, Ontario), anti-FLAG mAb M2 (International Biotechnologies, Inc.), antiphospho extracellular signal–regulated kinase (ERK; New England BioLabs, Inc.), anti-ERK1/2 (Zymed Laboratories), antiphosphotyrosine Ab (4G10), anti-Myc mAb and anti-PLCγ1 mixed mAb (Upstate Biotechnology), and horseradish peroxidase–conjugated goat anti–mouse IgG (Bio-Rad Laboratories). Luciferin was purchased from Sigma-Aldrich. Horseradish peroxidase–conjugated cholera toxin B subunit was purchased from Calbiochem-Novabiochem.
cDNA Constructs.
The cDNA for pEF/Myc/LAT (Myc-LAT) was a gift from L.A. Samelson. Flag-SLP-76 (pEF/Flag/SLP-76, wild type) and the Flag-tagged SLP-76 mutants pEF/Flag/SLP-76 Δ224–244 (Δ224–244), pEF/Flag/SLP-76 Y113/128/145F (Y3F), and pEF/Flag/SLP-7 R448K (R448K) were cloned as described previously 39. The pEF/LAT/SLP-76 chimeric construct was generated using PCR to generate a cDNA encoding amino acids 1–35 of the human LAT cDNA (sense primer, GCAGCGTCGACCCCTGCAGATGGAGGAG; antisense primer, GCGTAGGATCCTGGCAGTCTGTGGCAGTG) for ligation into pCRScript easy (Stratagene). The LAT partial cDNA was then subcloned into pEF/Flag/SLP-76 at the SalI and BamHI sites, replacing Flag and in frame with the SLP-76 coding sequence. The LAT/SLP-76 chimeric constructs with mutations in the SLP-76 coding sequence were generated by replacing the wild-type SLP-76 cDNA with the previously described SLP-76 mutants at the BamHI and XbaI sites. The LAT/SLP-76 chimera with point mutations in C26 and C29 was generated by PCR of pEF/Myc/LAT using the antisense primer CGGATCCTGGCAGTCTGTGGCTGTGCACACTCAGTGC (underline represents point mutations changing cysteine to serine) and the original sense primer. The subsequent PCR product was subcloned in frame with the wild-type SLP-76 sequence as described previously.
pEF/HLA-A2, the expression vector containing the HLA-A2 cDNA, was a gift of B. Shraven (University of Heidelberg, Heidelberg, Germany). pIL-2 nuclear factor of activated T cells (NFAT)-luciferase (NFAT-luc) was a gift from G. Crabtree (Stanford University, Palo Alto, CA). pCMV/β-galactosidase (β-gal) was a gift from G. MacGregor (Emory University, Atlanta, GA).
Transfections.
Cells were washed in PBS and suspended in cytomix (120 mM KCl; 0.15 mM CaCl2; 10 mM K2HPO4/KH2PO4; 25 mM Hepes, pH 7.6; 2 mM EGTA; 5 mM MgCl2; pH adjusted with KOH) at a concentration of 2 × 107 cells per 400 μl of cytomix per cuvette 40. Cells were electroporated at 250 V, 960 μF using a Gene Pulser (Bio-Rad Laboratories). The cells were placed at 37°C, 5% CO2 for 24 h, followed by functional analysis.
Isolation of GEM Fractions Using Equilibrium Density Gradients.
For each GEM preparation, five sets of cells were transfected with 40 μg of plasmid and were then combined. 24 h after transfection, 5 × 107 live cells were either left unstimulated or stimulated via the TCR (1:1,000 C305), followed by lysis at 4°C for 20 min in 1 ml of MES-buffered saline (25 mM MES, pH 6.5; 150 mM NaCl) containing 1% Triton X-100, 50 mg/ml aprotinin, 10 mg/ml leupeptin, 50 mg/ml pepstatin A, 1 mM phenylmethylsulfonyl fluoride, 400 mM sodium vanadate, 10 mM sodium fluoride, and 10 mM sodium pyrophosphate 36 41. The lysates were then mixed with 1 ml 80% sucrose in MES-buffered saline and transferred to ultracentrifuge tubes. The samples were overlaid with 2 ml of 30% sucrose in MES-buffered saline, followed by 1 ml 5% sucrose in MES-buffered saline. The Triton-insoluble fractions were separated from the cell lysates by ultracentrifugation for 18 h at 45,000 rpm in a Beckman SW55Ti rotor at 4°C (no break). 400-μl fractions were removed sequentially starting from the top of the gradient.
To assess the presence of particular proteins within the cytosol versus GEMs, 25 μl of each fraction was subjected to SDS-PAGE (10% for SLP-76, Gads, and LAT; 15% for ganglioside GM1), followed by transfer to nitrocellulose for immunoblot analysis using anti–SLP-76, anti-LAT, or anti-Gads polyclonal antisera or probed for GM1 with horseradish peroxidase–conjugated cholera toxin B subunit with detection via ECL (Amersham Pharmacia Biotech).
Luciferase Assays.
Cells were transfected with 25 μg of NFAT-luc construct, 5 μg of pCMV/β-gal, and 40 μg of the expression vectors. The total amount of plasmid DNA was equilibrated to 100 μg with the vector control pEF/HLA-A2. After 24 h, 5 × 10 5 live cells were stimulated in triplicate for 16 h with media, immobilized anti-TCR mAb C305 (ascites 1:1,000), or 50 ng/ml phorbol ester (PMA) plus 1 μM ionomycin (for maximal response). Additionally, triplicate samples of 5 × 10 5 unstimulated cells were assayed for β-gal activity using the Galacto-Light Plus Reporter Gene Assay System (Tropix Inc.). Luciferase activity was determined as described previously 39. Luciferase light units were normalized to β-gal activity present in each transfectant to standardize for transfection efficiency.
For examination of the expression levels of the transfected molecules, 106 transfected cells were lysed in NP-40 lysis buffer (50 mM Tris buffer, pH 7.4; 1% NP-40; 150 mM NaCl) including protease inhibitors (50 μg/ml aprotinin, 10 μg/ml leupeptin, 50 μg/ml pepstatin A, and 1 mM PMSF). The cell lysates were subjected to SDS-PAGE (10%), followed by transfer to nitrocellulose for immunoblot analysis using either anti–SLP-76 polyclonal antisera or anti-Myc mAb.
Immunoprecipitations.
Transfected Jurkat T cells were left unstimulated or stimulated with anti-TCR mAb (C305 ascites, 1:1,000) for 5 min and lysed in NP-40 lysis buffer including protease inhibitors (50 μg/ml aprotinin, 10 μg/ml leupeptin, 50 μg/ml pepstatin A, and 1 mM PMSF) and protein phosphatase inhibitors (400 μM sodium vanadate, 10 μM sodium fluoride, and 10 μM sodium pyrophosphate) 39. In experiments involving the detection of phosphorylated PLCγ1, cells stimulated with pervanadate (PV) were used to assess TCR-independent phosphorylation as a positive control. For immunoprecipitations, antibodies (2 μg per immunoprecipitation for anti-PLCγ1) were conjugated to GammaBind Plus Sepharose (Amersham Pharmacia Biotech) for 2 h at 4°C. Lysates were subjected to precipitation with the indicated Ab-conjugated Sepharose beads for 2 h at 4°C. The immune complexes were washed three times with NP-40 lysis buffer with 500 mM NaCl, subjected to SDS-PAGE (10% polyacrylamide gels), and transferred to nitrocellulose for immunoblot analysis using either 4G10 or anti-PLCγ1 Ab.
Measurement of ERK Activation.
Transfected cells were left unstimulated or were stimulated for 5 min with either C305 or PMA (50 ng/ml). Then, 107 cells were lysed in Triton lysis buffer (1% Triton X-100; 50 mM Hepes, pH 7.6; 150 mM NaCl; 1 mM PMSF; 1 μM aprotinin; 1 mM sodium vanadate; 50 mM NaFl; 0.5 mM EGTA). Lysates from 2 × 107 cell equivalents were subjected to reducing SDS-PAGE (12%) for visualization of ERK activation by immunoblot analysis using an antiphospho ERK Ab. For standardization of gel loading, the nitrocellulose membrane was reprobed for ERK levels by immunoblot analysis.
Results
SLP-76 Is Recruited to GEMs after TCR Ligation via Prolines within the Central Domain of SLP-76.
We and others have found that SLP-76 associates inducibly with LAT after TCR engagement 28 29 30 34. As LAT is a GEM resident protein, we reasoned that TCR ligation should therefore stimulate translocation of SLP-76 to GEMs. Jurkat T cells were left unstimulated or stimulated via their TCR and then lysed in a Triton X-100–based buffer. Lysates were subjected to sucrose density gradient ultracentrifugation to separate detergent-resistant GEMs from the Triton-soluble fraction. As described previously 41 42 43, purity of the GEM preparation was determined by examining fractions for the presence of the ganglioside GM1 (Fig. 1 A). As shown, this marker of GEMs is present only in fractions 2 and 3. Similarly (and confirming previous work of others [35, 36]), LAT is also found predominantly in GEMs (Fig. 1 B). In contrast, in unstimulated cells, neither SLP-76 nor Gads is present in the GEM fractions (Fig. 1C and Fig. D). After TCR engagement with the clonotypic anti-TCR C305, a pool of SLP-76 is detected in the GEMs. As expected, Gads is also inducibly recruited to GEMs, supporting the notion that the LAT–Gads–SLP-76 complex is GEM localized. We have also observed inducible recruitment of SLP-76 to GEMs by confocal microscopy and colocalization of SLP-76 with FITC-conjugated cholera toxin after TCR engagement (data not shown).
Figure 1.

Recruitment of SLP-76 and Gads to GEMs after TCR ligation. Jurkat T cells were either left unstimulated (US) or stimulated with C305 for 5 min, followed by lysis in MES lysis buffer plus protease and phosphatase inhibitors. Lysates were subjected to sucrose gradient ultracentrifugation for GEM purification. Sequential fractions were removed starting from the top of the gradient and are indicated as fraction number. The gradient fractions were separated by SDS-PAGE, followed by detection of GM1 using horseradish peroxidase–conjugated cholera toxin B subunit (A) or immunoblot analysis using anti-LAT (B), anti–SLP-76 (C), or anti-Gads (D).
To further test the possibility that it is Gads that bridges SLP-76 with LAT within GEMs, we transfected Jurkat T cells with cDNAs encoding either FLAG-tagged wild-type SLP-76 or similarly tagged SLP-76 variants with mutations in each of the known SLP-76 protein interaction domains (Fig. 2 A). Cells were left unstimulated or stimulated via the TCR and then lysed and fractionated by sucrose density gradient centrifugation. As shown in Fig. 2 B, wild type and each of the mutant variants are present in the cytosol in unstimulated cells. After TCR engagement, wild-type SLP-76 translocates to GEMs. Additionally, SLP-76 molecules with alterations in either the tyrosine phosphorylation sites (Y3F) or the SH2 domain (R448K) also translocate to GEMs after TCR stimulation. In contrast, the SLP-76 mutant that cannot bind to Gads (Δ224–244) fails to appear in GEMs after TCR ligation. (Fig. 2, SLP-76 blot).
Figure 2.
SLP-76 amino acids 224–244 are required for recruitment of SLP-76 to GEMs. (A) Schematic of the constructs encoding wild-type SLP-76 (WT), the SLP-76 mutant with tyrosines 113, 128, and 145 altered to phenylalanine (Y3F), the SLP-76 mutant incapable of binding Gads (Δ224–244), and the SLP-76 mutant with a nonfunctional SH2 domain (R448K). (B) Jurkat T cells were transfected with the various constructs and then left unstimulated or stimulated with C305, followed by lysis and preparation of GEM fractions as described for Fig. 1. A Triton-soluble fraction (fraction 11) and a GEM fraction (fraction 3) were separated by SDS-PAGE, followed by immunoblot analysis using anti-Flag (top) or anti–SLP-76 (bottom). Note that in the anti–SLP-76 blot, the band in the unstimulated Δ224–244 lane is broader than all other SLP-76 bands. This is due to immunoreactivity of both the endogenous wild-type SLP-76 and the Δ224–244 mutant. Also, note that in the stimulated lanes, only the slower migrating species (wild-type SLP-76) appears.
Targeting SLP-76 to GEMs Replaces the Need for LAT in Jurkat T Cells.
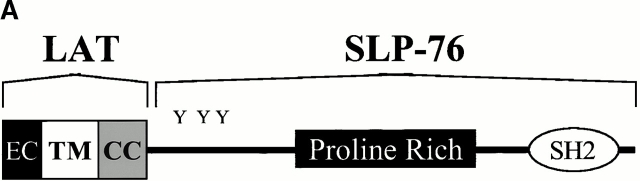
Others have shown that the tyrosines within the cytoplasmic domain of LAT are critical for TCR signaling function, presumably because they recruit effector proteins to a multimolecular signaling complex 34. We addressed the importance of SLP-76 as one of these effectors by asking if LAT could function without its tyrosine residues if SLP-76 was constitutively targeted to GEMs. This was accomplished by expressing a chimeric protein including regions of both LAT and SLP-76. As illustrated in Fig. 3 A, the NH2 terminus of the chimera consists of the extracellular and transmembrane domains and a limited portion of the cytoplasmic tail of LAT, including the two cysteine residues (C26 and C29) previously shown to be necessary and sufficient for GEM localization of LAT 35 36. COOH-terminal to the cysteines, the chimera consists of full length, wild-type SLP-76. Note that the chimera contains none of the LAT tyrosine residues shown to be phosphorylated after TCR engagement.
Figure 3.
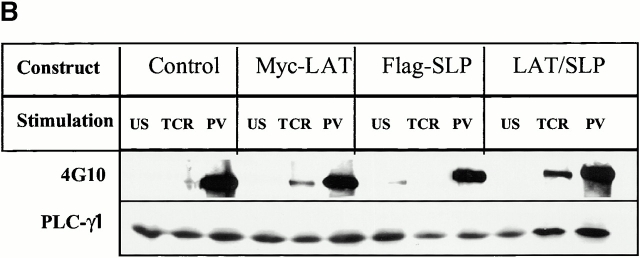
Targeting of SLP-76 to GEMs reconstitutes proximal TCR signaling in LAT-deficient T cells. (A) Schematic of the LAT/SLP-76 chimeric construct used to target SLP-76 to GEMs. The NH2 terminus contains LAT amino acids 1–35, including the LAT extracellular domain (EC), transmembrane domain (TM), and amino acids surrounding cysteines 26 and 29 (CC). (B) J.CaM2 cells were transfected with HLA-A2 (control), Myc-LAT, Flag–SLP-76, or the LAT/SLP-76 chimera and then left unstimulated (US), stimulated with C305 (TCR), or stimulated with PV for 5 min. Lysates were subjected to immunoprecipitation with anti-PLCγ1. Immune complexes were analyzed by SDS-PAGE and immunoblot analysis with 4G10 (top) or anti-PLCγ1 (bottom). (C) J.CaM2 cells were transfected with HLA-A2 (control), Myc-LAT, Flag–SLP-76, or the LAT/SLP-76 chimera and then left unstimulated (US), stimulated with C305 (TCR), or stimulated with PMA for 5 min. Lysates were subjected to SDS-PAGE, followed by immunoblot analysis with antiphospho-ERK (top) and with anti-ERK to ensure equal loading of lanes (bottom).
We made use of J.CaM2, a LAT-deficient variant of the Jurkat T cell line, to study the function of the LAT/SLP-76 chimera. Cells were transfected with a vector control (encoding the A2 allele of MHC class I), or cDNA encoding SLP-76, wild-type LAT, or the LAT/SLP-76 chimera. Transfectants were left unstimulated or stimulated via the TCR. Cellular lysates were subjected to immunoprecipitation with Ab directed against PLCγ1. Immune complexes were then analyzed for the presence of phosphotyrosine (Fig. 3 B, top) and quantitation of PLCγ1 present (Fig. 3 B, bottom). As reported by several groups and shown in Fig. 3 B, stimulation of the TCR on the LAT-deficient mutant fails to induce tyrosine phosphorylation of PLCγ1. The TCR-induced PTKs and substrate, however, are intact, as PV stimulates PLCγ1 phosphorylation in control transfected J.CaM2 cells. Confirming published studies 14 35, TCR-induced PLCγ1 phosphorylation is restored by transfection of cells with wild-type LAT. As expected, overexpressed SLP-76 does not rescue PLCγ1 phosphorylation, even though SLP-76 levels in the transfected cells are substantially higher than endogenous. Surprisingly, expression of the LAT/SLP-76 chimera also rescues TCR-stimulated PLCγ1 tyrosine phosphorylation. In every experiment, the rescue with the chimera was substantially higher than when wild-type LAT is overexpressed (data not shown). The chimeric molecule is also consistently more efficient at supporting this means of TCR function than is the combination of transfected wild-type LAT plus transfected wild-type SLP-76 (data not shown).
Another TCR-stimulated signaling pathway known to be regulated by both SLP-76 and LAT is activation of ERK. As shown in Fig. 3 C, stimulation of the TCR on J.CaM2 fails to activate ERK as assessed by ERK phosphorylation, although these cells demonstrate an ERK response after stimulation with the phorbol ester, PMA. The TCR signaling defect is reversed by transfection of cDNA encoding LAT but not SLP-76. Again, expression of the LAT/SLP-76 chimera restores TCR-induced ERK activation more efficiently than does expression of wild-type LAT.
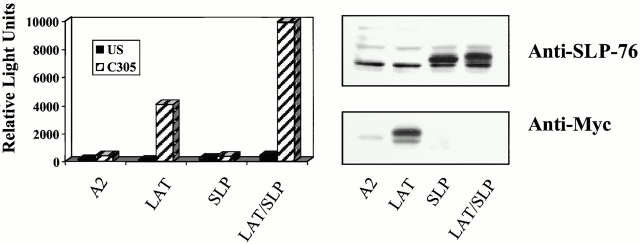
NFAT is stimulated downstream of the PLCγ1 and RAS/ERK pathways 8. Therefore, we asked if expression of the LAT/SLP-76 chimera in LAT-deficient cells could support TCR-stimulated NFAT activation. Cells were transfected with the various LAT and SLP-76 constructs along with a reporter construct including triplicated NFAT binding sites (derived from the IL-2 promoter) upstream of the luciferase gene. As shown in Fig. 4, the signaling defect in J.CaM2 leading to NFAT activation is reversed more efficiently with the LAT/SLP-76 chimera than with wild-type LAT. Collectively, these experiments demonstrate that SLP-76 is a critical molecule recruited by LAT to support TCR signal transduction in Jurkat T cells.
Figure 4.
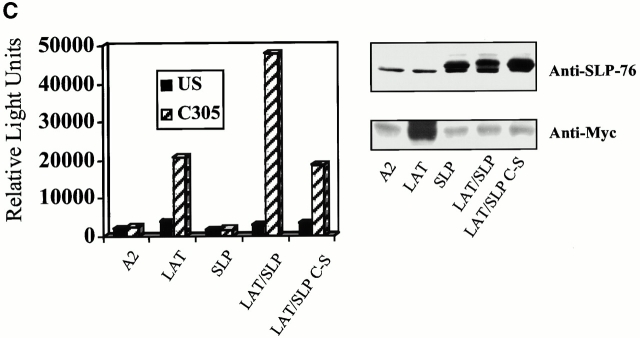
Targeting of SLP-76 to GEMs supports reconstitution of NFAT promoter activity in LAT-deficient T cells. J.CaM2 cells were transfected with NFAT-luc and pCMV/β-gal plus the indicated constructs. 24 h after transfection, cells were left unstimulated (US), stimulated with C305, or stimulated with PMA plus ionomycin for 16 h. Samples were assayed for luciferase activity (normalized to β-gal; left panel). PMA plus ionomycin responses were similar (∼100,000 relative light units) for each sample. This experiment is representative of 10 independent transfections. Expression of the transfected proteins for this experiment was determined by examining whole cell lysates by immunoblot analysis with anti–SLP-76 (to detect transfected SLP-76 or the chimera) or anti-Myc (to detect transfected LAT) antibodies (right panel).
As SLP-76 is covalently attached to LAT in the chimeric molecule and hence does not need to be recruited after TCR engagement, we anticipated that transfection of the chimera would be more efficient than wild-type LAT at restoring TCR signaling in J.CaM2. The experiment shown in Fig. 5 supports this notion, showing that at each of four concentrations of transfected cDNA, TCR-induced NFAT activation is more efficient in cells reconstituted with LAT/SLP-76 when compared with cells receiving wild-type LAT. Additionally, it appears that the ability of LAT to increase efficiency of TCR signaling plateaus as the amount of LAT cDNA is transfected. This is not seen when the LAT/SLP-76 chimera is expressed (at least at the concentrations we have studied). This difference may be due to limiting amounts of SLP-76 present in J.CaM2 cells transfected with wild-type LAT. In support of this possibility, we have found that cotransfection of SLP-76 with LAT into J.CaM2 is more efficient at rescuing TCR signaling than transfection of LAT alone (Fig. 5 B).
Figure 5.
The LAT/SLP-76 chimera is more efficient at restoring TCR signaling in J.CaM2 than wild-type LAT. (A) J.CaM2 cells were transfected with NFAT-luc and pCMV/β-gal plus the indicated constructs. 40 μg of plasmid was used for both the A2 and SLP-76 controls. Varying amounts of plasmid (from 5 to 40 μg, as shown) encoding LAT or the LAT/SLP-76 chimera were used. 24 h after transfection, cells were left unstimulated (US), stimulated with C305, or stimulated with PMA plus ionomycin for 16 h. Samples were assayed for luciferase (normalized to β-gal activity; left panel). PMA plus ionomycin responses were similar (∼120,000 relative light units) for each sample. This experiment is representative of four independent transfections. Expression of the transfected proteins for this experiment was determined by examining whole cell lysates by immunoblot analysis with anti–SLP-76 (to detect transfected SLP-76 or the chimera) or anti-Myc (to detect transfected LAT) antibodies (right panel). (B) J.CaM2 cells were transfected with NFAT-luc and pCMV/β-gal plus the indicated constructs. 24 h after transfection, cells were left unstimulated (US), stimulated with C305, or stimulated with PMA plus ionomycin for 16 h. Samples were assayed for luciferase (normalized to β-gal activity; left panel). PMA plus ionomycin responses were similar (∼120,000 relative light units) for each sample. This experiment is representative of three independent transfections. Note the doublet in the SLP-76 blot examining expression of the LAT/SLP-76 chimera (panel A, right). The slower migrating species represents the chimera, while the faster migrating band is endogenous wild-type SLP-76.
GEM Localization of the LAT/SLP-76 Chimera Is Required for Optimal Rescue of the J.CaM2 Signaling Defect.
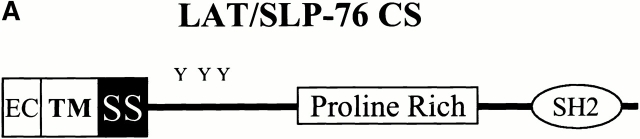
Next, we addressed whether GEM localization of SLP-76 is absolutely required to reconstitute TCR function in J.CaM2. For these experiments, we prepared a mutant LAT/SLP-76 chimera altering the two LAT cysteines (C26 and C29) responsible for GEM targeting to serine residues (LAT/SLP-76 CS) (Fig. 6 A). The mutant chimera was transfected first into J14-v-29, an SLP-76–deficient variant of Jurkat 13. We chose this cell as a host because it lacks endogenous SLP-76, making detection of the chimera unequivocal. As shown in Fig. 6 B, whereas the LAT/SLP-76 chimera with intact C26 and C29 is found exclusively in the GEMs (fraction 3), the LAT/SLP-76 CS mutant is found in the cytosol (fraction 11).
Figure 6.
Tethering of SLP-76 to the membrane is sufficient to reconstitute signaling in LAT-deficient T cells. (A) Schematic of the LAT/SLP-76 chimera with point mutations altering cysteines 26 and 29 to serine. (B) J.14-v-29 cells were transfected with LAT/SLP-76 wild type, LAT/SLP-76 CS, or FLAG–SLP-76 and then lysed for GEM purification. The Triton-soluble fractions (fraction 11) and GEM fractions (fraction 3) were separated by SDS-PAGE, followed by immunoblot analysis using anti-SLP-76. (C) J.CaM2 cells were transfected with NFAT-luc, pCMV/β-gal, and the indicated constructs. 24 h after transfection, cells were left unstimulated (US), stimulated with C305, or stimulated with PMA plus ionomycin for 16 h. The samples were assayed for luciferase activity, which is normalized to the β-gal activity (left panel). PMA plus ionomycin responses were similar (∼250,000 relative light units) for each condition. This experiment is representative of four independent transfections. Expression of the transfected proteins in the experiment shown was determined by immunoblot analysis of whole cell lysates with anti–SLP-76 (to detect transfected SLP-76 or the chimera) or anti-Myc (to detect transfected LAT) antibodies (right panel).
J.CaM2 cells were then transfected with the NFAT reporter construct plus control cDNA (A2), wild-type SLP-76, wild-type LAT, the original LAT/SLP-76 chimera, or the LAT/SLP-76 CS mutant. Cells were left unstimulated or stimulated via the TCR and analyzed for NFAT activation. As shown in Fig. 6 C, expression of LAT/SLP-76 CS reconstitutes TCR-induced NFAT activation, although always with less efficiency than the chimera that localizes to GEMs. These data indicate that when expressed at the membrane, SLP-76 can support TCR signaling. However, our results also suggest that for SLP-76 to function optimally, it is necessary for SLP-76 to be targeted to microdomains in the membrane.
The SLP-76 Gads Binding Site and SH2 Domain, but Not Its Tyrosine Phosphorylation Sites, Are Dispensable if SLP-76 Is Tethered to LAT.
The experiments using the LAT/SLP-76 chimeric proteins suggest that a major function of LAT is to serve as a scaffold for the recruitment of SLP-76 to a TCR-stimulated signaling complex. The availability of the LAT-deficient J.CaM2 cells provides an excellent reagent for us to address the structural features of SLP-76 within the context of the chimera required for the reconstitution of TCR signaling. To approach this issue, we generated a series of LAT/SLP-76 chimeras with mutations in each of the three functional SLP-76 domains. As shown in Fig. 7 A, all of the constructs contain the LAT extracellular, transmembrane, and GEM localization sequences. The LAT/SLP-76 Y3F mutant has three point mutations in the SLP-76 component preventing its tyrosine phosphorylation. The LAT/SLP-76 Δ224–244 mutant has a deletion in the Gads binding site, and the LAT/SLP-76 R448K mutant has a point mutation in the SH2 domain preventing this protein from binding SLAP-130/Fyb. Each of the cDNAs for the chimeras was transfected into J.CaM2 cells for analysis of TCR function.
Figure 7.
Structure/function analysis of SLP-76 domains required to support TCR-induced NFAT activity in LAT-deficient cells. (A) Schematic of the LAT/SLP-76 chimeric constructs used in this experiment. Y3F contains three point mutations altering tyrosines 113, 128, and 145 to phenylalanine, abrogating tyrosine phosphorylation of the chimera; Δ224–244 includes a 20–amino acid deletion eliminating the Gads binding site; and R448K contains a point mutation in arginine 448, eliminating function of the SLP-76 SH2 domain. (B) J.CaM2 cells were transfected with NFAT-luc, pCMV/β-gal, and the indicated constructs. 24 h after transfection, cells were left unstimulated (US), stimulated with C305, or stimulated with PMA plus ionomycin for 16 h. The samples were assayed for luciferase activity, which is normalized to the β-gal activity (left panel). The PMA plus ionomycin response was similar (∼200,000 relative light units) for each condition. This experiment is representative of five independent transfections. Expression of the transfected proteins in the experiment shown was determined by immunoblot analysis of whole cell lysates with anti–SLP-76 (to detect transfected SLP-76 or the chimera) or anti-Myc (to detect transfected LAT) antibodies (right panel).
Fig. 7 B shows the results of representative NFAT assays for cells expressing each of the chimeric molecules or various control constructs. As can be appreciated from this experiment, mutation of either the Gads binding site or the SH2 domain of SLP-76 decreases the ability of the chimera to support TCR signaling. However, TCR function is largely preserved even though expression of these constructs compared with LAT or SLP-76 is relatively equal (Fig. 7 B, right panel). In contrast, mutation of the SLP-76 tyrosine phosphorylation sites completely abrogates the ability of the chimera to function. This is true also if the readout of activation is inducible phosphorylation of PLCγ1 or activation of ERK (Fig. 8 A and Fig. 8 B).
Figure 8.
Structure/function analysis of SLP-76 domains required to support TCR-induced PLCγ1 and ERK activity in LAT-deficient cells. (A) J.CaM2 cells were transfected with the indicated constructs. 24 h later, cells were left unstimulated (US) or were stimulated with C305 (TCR) or PV for 5 min and then lysed and subjected to immunoprecipitation using anti-PLCγ1. The immune complexes were analyzed for phosphotyrosine contain (4G10, top) and amount of PLCγ1 (bottom). (B) Transfected J.CaM2 cells were stimulated and then analyzed for phospho-ERK (top) and with anti-ERK to ensure equal loading of lanes (bottom) as described for Fig. 3.
Thus, if tethered to LAT, the SLP-76 Gads binding site is no longer critical, presumably because SLP-76 is already localized to the TCR-stimulated signaling complex. Similarly, mutation of the SH2 domain of SLP-76 does not prevent the chimera from restoring the ability of the TCR to signal, suggesting that SLP-76 SH2 binder(s) are not critical for the positive effects of SLP-76 on T cell activation in this model system. In contrast, however, mutation of the SLP-76 tyrosine phosphorylation sites completely abolishes the ability of the chimera to function. We interpret these findings to suggest that for TCR signaling to progress, SLP-76 must coordinate the assembly of a complex including molecules that associate with the SLP-76 NH2-terminal domain. Thus far, several such proteins have been identified, including Vav, Nck, and ITK, all of which have been shown previously to play important roles in T cell function.
Discussion
It is becoming increasingly appreciated that effective signal transduction requires not only the activation of critical effector molecules but also their concentration into particular subdomains within the cell. Much recent attention has focused on GEMs or lipid rafts, membrane subdomains characterized by detergent insolubility 44 45. In T cells, it has been established that the integrity of GEMs and the localization of specific molecules to these microdomains is necessary for TCR-initiated signals to be translated into cellular activation 46. Some of these proteins are GEM resident (due to their posttranslational modification, e.g., LAT), whereas others are recruited into GEMs via induced associations with other molecules 35 46 47 48 49.
In the experiments described in this report, we focused on the relationship of LAT and SLP-76 and the importance of their colocalization into GEMs for TCR signaling. We found that, similar to other critical modulators of the T cell response, SLP-76 inducibly translocates to GEMs after TCR ligation. We show additionally that this translocation requires amino acids within the proline-rich region of SLP-76, supporting the notion that GEM localization of SLP-76 is indirect. The recruitment of SLP-76 to GEMs likely occurs via an interaction with Gads, an adapter whose binding to SLP-76 requires the sequence mutated in the SLP-76 Δ224–244 construct. To test the importance of the LAT–SLP-76 interaction, we created a LAT/SLP-76 chimera that constitutively places SLP-76 within the GEMs and found that expression of this protein in a LAT-deficient cell restores the ability of the TCR to signal. In fact, this rescue is more efficient than reconstituting the mutant cell with wild-type LAT. Although the chimera possesses no LAT tyrosines, expression of the chimera reconstitutes multiple TCR-inducible biochemical events leading to transcriptional activation of an NFAT reporter construct. As SLP-76 has no intrinsic effector activity, we speculated that the LAT/SLP-76 chimera must function by allowing SLP-76 to recruit other molecules to a larger signaling complex. This appears to be the case, as the signal transduction rescue is completely abrogated if the chimeric construct is mutated so the SLP-76 component can no longer be phosphorylated and therefore is incapable of recruiting other phosphotyrosine binding proteins.
Although we began these experiments with the hypothesis that the LAT/SLP-76 interaction would be critical to support some aspects of TCR signaling, we speculated that expression of the chimera on J.CaM2 would provide only a partial rescue of TCR function. In addition to the residue responsible for Gads binding, LAT possesses multiple tyrosine residues capable of interacting with proteins known to be important for TCR signal transduction 34. In particular, LAT binds PLCγ1 independent of its interaction with Gads (and hence SLP-76). Therefore, we predicted that the LAT/SLP-76 chimera would not support TCR-induced PLCγ1 function in the absence of endogenous LAT. Our surprising finding that the chimera very efficiently supports TCR-stimulated PLCγ1 phosphorylation (and presumably function, based on the NFAT results) suggests that in addition to being recruited by LAT, PLCγ1 may enter a TCR-initiated signaling complex via other avenues. In this regard, it is important to note that in addition to possessing SH2 domains, PLCγ1 contains other modules able to interact with other proteins. In fact, a recent report demonstrated that the SH2 domains of PLCγ1 are not critical for its recruitment after engagement of the platelet-derived growth factor receptor 50. An alternative means by which PLCγ1 may be activated after TCR engagement may involve its association with other proteins by means of the PLCγ1 SH3 domain. Among the potential binders is SLP-76, as SLP-76 contains a region rich in proline residues. Experiments are currently underway to determine if there is a direct recruitment of PLCγ1 to the LAT/SLP-76 chimera.
Additional experiments will also be required to determine which protein(s) must bind to the NH2 terminus of SLP-76 to maintain the integrity of the TCR signaling pathway. Several molecules are known to bind to tyrosines located within this region of SLP-76, and each is being evaluated as a candidate. These include Vav, an exchange factor for Rho family GTPases 51; Nck, an adapter protein 52; and ITK, a Tec family PTK 53. We are particularly interested in the possibility that a SLP-76–ITK interaction is required for function of the chimera, as Tec family PTKs are known to regulate PLCγ isoforms 54.
As expected, mutation of the Gads binding site in the context of the LAT/SLP-76 chimera does not prevent the rescue of TCR function. This is presumably because once tethered to LAT, SLP-76 no longer needs to bind Gads. Interestingly, however, in every experiment where this was tested, the LAT/SLP-76 Δ224–244 chimera was less efficient than the chimera containing wild-type SLP-76. Thus, it appears that optimal signaling may be facilitated by recruitment of Gads or other protein(s) whose association with SLP-76 requires integrity of the region between amino acids 224 and 244. Similarly, mutation of the SLP-76 SH2 domain decreases, but does not abolish, the ability of the chimera to reconstitute TCR function. The only identified binder to the SLP-76 SH2 is SLAP-130/Fyb, a protein whose function remains unclear 55 56 57 58. Our data suggest that it is not critical for SLAP-130/Fyb to bind the targeted SLP-76 chimera for the support of TCR function; however, optimal activity may rely on this intermolecular interaction.
It should be noted also that TCR signaling in J.CaM2 cells is still restored even if the LAT/SLP-76 chimera is not targeted directly to GEMs. In every experiment, this rescue is substantially decreased compared with studies where there are similar levels of expression of the LAT/SLP-76 chimera targeted to GEMs. The ability of the nontargeted chimera to support TCR signaling is comparable to the reconstitution of activity seen when wild-type LAT is reintroduced. These results indicate that merely bringing SLP-76 to the membrane is sufficient to replace the need for LAT in the support of TCR signaling; however, this process is considerably more efficient if SLP-76 is targeted to GEMs.
One potential explanation for why SLP-76 can function to replace LAT even when SLP-76 is not GEM localized constitutively is that the LAT/SLP-76 CS chimera may be brought to GEMs through other intermolecular interactions. This is unlikely, however, as the compartmentalization of SLP-76 to lipid rafts appears to rely on its Gads binding domain alone (Fig. 2). A second possibility is that when expressed at high enough levels at the plasma membrane, SLP-76 may function even when not in GEMs, although GEM localization is critical at lower concentrations of SLP-76. A third potential explanation is that GEM localization is necessary for LAT phosphorylation (as demonstrated by Zhang et. al. [15]) and hence SLP-76 recruitment. However, if SLP-76 is tethered to LAT, the requirement for GEM localization is obviated. Placing the LAT/SLP-76 chimera within GEMs may still increase the efficiency of TCR signaling but is not absolutely required for TCR function.
One limitation of these studies is that by tethering SLP-76 to LAT, we eliminate the ability of the interaction of these proteins to be regulated. Thus, while our results indicate that fixing SLP-76 to LAT eliminates the need for other LAT tyrosines, it is possible that under more physiological conditions, LAT-mediated interactions with other proteins play additional key roles in TCR signaling. It should be emphasized also that these experiments were performed in variants of the Jurkat T cell leukemic line. Although conclusions from studies in Jurkat have often been borne out by complementary experiments using freshly isolated human T cells or murine models, there is the potential that results using Jurkat may not recapitulate precisely the normal biology of T cell activation. We therefore are planning experiments to examine the effect of expression of the LAT/SLP-76 chimera in LAT-deficient mice. Additionally, to test most rigorously the importance of the LAT–SLP-76 interaction in the regulation of TCR signaling, it will be necessary to develop a system lacking both endogenous SLP-76 and LAT. To this end, we are establishing a line of SLP-76/LAT double-knockout mice. These mice will provide an excellent model system to test the importance of targeting SLP-76 (both in its wild-type and mutant forms) to the membrane in general, and GEMs in particular, for the support of TCR function in vivo.
Acknowledgments
The authors are grateful to B. Hostager (University of Iowa) for technical assistance in initiating the GEM localization studies. The authors are grateful to Drs. E. Peterson and X. Zhang for providing review of this manuscript and helpful discussions of the data.
This work was supported in part by grants from the National Institutes of Health, GM53256 to G. Koretzky and National Research Service Award 5033700 to N. Boerth.
Footnotes
Abbreviations used in this paper: ERK, extracellular signal–regulated kinase; GEMs, glycolipid-enriched membrane microdomains; ITK, inducible T cell kinase; LAT, linker for activation of T cells, NFAT, nuclear factor of activated T cells; PLC, phospholipase C; PTK, protein tyrosine kinase; PV, pervanadate; SLP-76, src homology 2 domain–containing leukocyte phosphoprotein of 76 kD.
References
- June C.H., Fletcher M.C., Ledbetter J.A., Samelson L.E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J. Immunol. 1990;144:1591–1599. [PubMed] [Google Scholar]
- Chan A.C., Iwashima M., Turck C.W., Weiss A. ZAP-70a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Williams B.L., Schreiber K.L., Zhang W., Wange R.L., Samelson L.E., Leibson P.J., Abraham R.T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptorreconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D.M., Newton M.E., Kadlecek T., Weiss A. Stimulation of the phosphatidylinositol pathway can induce T-cell activation. Nature. 1990;348:66–69. doi: 10.1038/348066a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Koretzky G., Schatzman R.C., Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc. Natl. Acad. Sci. USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnitz L., Sutor S.L., Torigoe T., Reed J.C., Bell M.P., McKean D.J., Leibson P.J., Abraham R.T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol. Cell. Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Willebrand M., Baier G., Couture C., Burn P., Mustelin T. Activation of phosphatidylinositol-3-kinase in Jurkat T cells depends on the presence of the p56lck tyrosine kinase. Eur. J. Immunol. 1994;24:234–238. doi: 10.1002/eji.1830240137. [DOI] [PubMed] [Google Scholar]
- Wange R.L., Samelson L.E. Complex complexessignaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- Pawson T., Scott J.D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Myung P.S., Boerthe N.J., Koretzky G.A. Adapter proteins in lymphocyte antigen-receptor signaling. Curr. Opin. Immunol. 2000;12:256–266. doi: 10.1016/s0952-7915(00)00085-6. [DOI] [PubMed] [Google Scholar]
- Clements J.L., Yang B., Ross-Barta S.E., Eliason S.L., Hrstka R.F., Williamson R.A., Koretzky G.A. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- Pivniouk V., Tsitsikov E., Swinton P., Rathbun G., Alt F.W., Geha R.S. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- Yablonski D., Kuhne M.R., Kadlecek T., Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- Finco T.S., Kadlecek T., Zhang W., Samelson L.E., Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- Zhang W., Irvin B.J., Trible R.P., Abraham R.T., Samelson L.E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int. Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sommers C.L., Burshtyn D.N., Stebbins C.C., DeJarnette J.B., Trible R.P., Grinberg A., Tsay H.C., Jacobs H.M., Kessler C.M. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- Jackman J.K., Motto D.G., Sun Q., Tanemoto M., Turck C.W., Peltz G.A., Koretzky G.A., Findell P.R. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J. Biol. Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- Clements J.L., Ross-Barta S.E., Tygrett L.T., Waldschmidt T.J., Koretzky G.A. SLP-76 expression is restricted to hemopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J. Immunol. 1998;161:3880–3889. [PubMed] [Google Scholar]
- Fang N., Motto D.G., Ross S.E., Koretzky G.A. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J. Immunol. 1996;157:3769–3773. [PubMed] [Google Scholar]
- Wardenburg J.B., Fu C., Jackman J.K., Flotow H., Wilkinson S.E., Williams D.H., Johnson R., Kong G., Chan A.C., Findell P.R. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J. Biol. Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- Wu J., Motto D.G., Koretzky G.A., Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- Tuosto L., Michel F., Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J. Exp. Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M., da Silva A.J., Findell P.R., Rudd C.E. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity. 1997;6:155–164. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- Onodera H., Motto D.G., Koretzky G.A., Rothstein D.M. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J. Biol. Chem. 1996;271:22225–22230. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- Su Y.W., Zhang Y., Schweikert J., Koretzky G.A., Reth M., Wienands J. Interaction of SLP adaptors with the SH2 domain of tec family kinases. Eur. J. Immunol. 1999;29:3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bunnell S.C., Diehn M., Yaffe M.B., Findell P.R., Cantley L.C., Berg L.J. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- Schneider H., Guerette B., Guntermann C., Rudd C.E. Resting lymphocyte kinase (Rlk/Txk) targets lymphoid adaptor SLP-76 in the cooperative activation of interleukin-2 transcription in T-cells. J. Biol. Chem. 2000;271:3835–3840. doi: 10.1074/jbc.275.6.3835. [DOI] [PubMed] [Google Scholar]
- Liu S.K., Fang N., Koretzky G.A., McGlade C.J. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- Asada H., Ishii N., Sasaki Y., Endo K., Kasai H., Tanaka N., Takeshita T., Tsuchiya S., Konno T., Sugamura K. Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 1999;189:1383–1390. doi: 10.1084/jem.189.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C.L., Ewings M.K., Chaudhary P.M., Solow S.A., Yun T.J., Marshall A.J., Hood L., Clark E.A. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J. Exp. Med. 1999;189:1243–1253. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourette R.P., Arnaud S., Myles G.M., Blanchet J.P., Rohrschneider L.R., Mouchiroud G. Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:7273–7281. doi: 10.1093/emboj/17.24.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci M.A., Hendricks-Taylor L.R., Motto D.G., Paskind M., Kamens J., Turck C.W., Koretzky G.A. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- da Silva A.J., Li Z., de Vera C., Canto E., Findell P., Rudd C.E. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc. Natl. Acad. Sci. USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Lin J., Weiss A., Finco T.S. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J. Biol. Chem. 1999;274:28861–28864. doi: 10.1074/jbc.274.41.28861. [DOI] [PubMed] [Google Scholar]
- Motto D.G., Ross S.E., Wu J., Hendricks-Taylor L.R., Koretzky G.A. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor-mediated interleukin 2 production. J. Exp. Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Stobo J. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci M.A., Motto D.G., Ross S.E., Fang N., Koretzky G.A. Three domains of SLP-76 are required for its optimal function in a T cell line. J. Immunol. 1997;159:1639–1647. [PubMed] [Google Scholar]
- van den Hoff M.J., Moorman A.F., Lamers W.H. Electroporation in ‘intracellular’ buffer increases cell survival. Nucleic Acids Res. 1992;20:2902. doi: 10.1093/nar/20.11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi A., Saitoh S., Noda S., Yasuda K., Hayashi F., Ogata M., Hamaoka T. Translocation of tyrosine-phosphorylated TCRzeta chain to glycolipid-enriched membrane domains upon T cell activation. Int. Immunol. 1999;11:1395–1401. doi: 10.1093/intimm/11.9.1395. [DOI] [PubMed] [Google Scholar]
- Fra A.M., Williamson E., Simons K., Parton R.G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Schnitzer J.E., McIntosh D.P., Dvorak A.M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Brown R.E. Sphingolipid organization in biomembraneswhat physical studies of model membranes reveal. J. Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Kabouridis P.S., Magee A.I., Ley S.C. S-acylation of LCK protein tyrosine kinase is essential for its signaling function in T lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montixi C., Langlet C., Bernard A.M., Thimonier J., Dubois C., Wurbel M.A., Chauvin J.P., Pierres M., He H.T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes P.W., Ley S.C., Magee A.I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin B., Sekiya F., Rhee S.G. Differential roles of the Src homology 2 domains of phospholipase C-gamma1 (PLC-gamma1) in platelet-derived growth factor-induced activation of PLC-gamma1 in intact cells. J. Biol. Chem. 2000;275:6411–6416. doi: 10.1074/jbc.275.9.6411. [DOI] [PubMed] [Google Scholar]
- Crespo P., Schuebel K.E., Ostrom A.A., Gutkind J.S., Bustelo X.R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J., Pappu R., Bu J.Y., Mayer B., Chernoff J., Straus D., Chan A.C. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- Schaeffer E.M., Schwartzberg P.L. Tec family kinases in lymphocyte signaling and function. Curr. Opin. Immunol. 2000;12:282–288. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- Liu K.Q., Bunnell S.C., Gurniak C.B., Berg L.J. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L., Raab M., Rudd C.E. Cutting edgeSLP-76 cooperativity with FYB/FYN-T in the up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J. Immunol. 1999;163:5753–5757. [PubMed] [Google Scholar]
- Raab M., Kang H., da Silva A., Zhu X., Rudd C.E. FYN-T-FYB-SLP-76 interactions define a T-cell receptor zeta/CD3-mediated tyrosine phosphorylation pathway that up-regulates interleukin 2 transcription in T-cells. J. Biol. Chem. 1999;274:21170–21179. doi: 10.1074/jbc.274.30.21170. [DOI] [PubMed] [Google Scholar]
- Veale M., Raab M., Li Z., da Silva A.J., Kraeft S.K., Weremowicz S., Morton C.C., Rudd C.E. Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production. J. Biol. Chem. 1999;274:28427–28435. doi: 10.1074/jbc.274.40.28427. [DOI] [PubMed] [Google Scholar]
- Boerth N.J., Judd B.A., Koretzky G.A. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J. Biol. Chem. 2000;275:5143–5152. doi: 10.1074/jbc.275.7.5143. [DOI] [PubMed] [Google Scholar]