Abstract
We have previously shown that antimicrobial peptides like defensins have the capacity to mobilize leukocytes in host defense. LL-37 is the cleaved antimicrobial 37-residue, COOH-terminal peptide of hCAP18 (human cationic antimicrobial protein with a molecular size of 18 kD), the only identified member in humans of a family of proteins called cathelicidins. LL-37/hCAP18 is produced by neutrophils and various epithelial cells. Here we report that LL-37 is chemotactic for, and can induce Ca2+ mobilization in, human monocytes and formyl peptide receptor–like 1 (FPRL1)-transfected human embryonic kidney 293 cells. LL-37–induced Ca2+ mobilization in monocytes can also be cross-desensitized by an FPRL1-specific agonist. Furthermore, LL-37 is also chemotactic for human neutrophils and T lymphocytes that are known to express FPRL1. Our results suggest that, in addition to its microbicidal activity, LL-37 may contribute to innate and adaptive immunity by recruiting neutrophils, monocytes, and T cells to sites of microbial invasion by interacting with FPRL1.
Keywords: cathelicidin, hCAP18, chemotaxis, phagocyte, lymphocyte
Introduction
A variety of small (<100 amino acids) antimicrobial peptides, produced by cells of insects, plants, and animals, act as endogenous antibiotics. In humans, over a dozen of these antimicrobial peptides have thus far been identified and include several salivary histatins, lactoferricin, six α-defensins, two β-defensins, and an 18-kD human cationic antimicrobial protein, hCAP18 1. hCAP18, a protein possessing 140 amino acid residues (references 2–4), belongs to a family of proteins called cathelicidins, which usually consist of a highly conserved preproregion of ∼128–143 residues, including a putative ∼29–30-residue signal peptide and a ∼99–114-residue cathelin-like domain and a COOH-terminal antimicrobial domain ranging in length from 12 to >100 amino acid residues 5. The COOH-terminal domain of hCAP18 also has the capacity to bind and neutralize bacterial LPS 2. Cleavage of hCAP18 was initially predicted 2 3 and later confirmed 6 to occur between Ala103 and Leu104, giving rise to LL-37, a 37-residue mature antimicrobial peptide with two leucine residues on its NH2 terminus. LL-37/hCAP18 is present in neutrophil granules 3 and is produced by bone marrow and testis 4, inflamed skin keratinocytes 7, lung epithelia 8, and squamous epithelia of human mouth, tongue, esophagus, cervix, and vagina 9. Both purified and chemically synthesized LL-37 peptides exhibit potent and comparable antimicrobial activities in vitro 6 10.
Although antimicrobial peptides have generally been considered to contribute to host innate antimicrobial defense 1, some of them may also contribute to adaptive immunity against microbial infection in higher vertebrates. Human neutrophil α-defensin is chemotactic for human peripheral blood CD4/CD45RA and CD8 T cells, as well as immature dendritic cells 11 12. When administered simultaneously with protein antigens to mice, human α-defensins can promote antigen-specific immune responses 13. Similarly, human epithelial cell derived β-defensins have the capacity to induce the migration of human peripheral blood CD4/CD45RO T cells and immature dendritic cells by interacting with one of the chemokine receptors, CCR6 14. However, it is not known whether antimicrobial peptides other than defensins (e.g., LL-37) have similar activities.
In the course of characterizing neutrophil granule–derived monocyte chemotactic activities, we previously established that the major HPLC peak of activity was attributable to cathepsin G 15. Another broader peak with significant chemotactic activity contained a complex mixture of proteins. By the use of more recently developed sensitive mass spectroscopic methods, we have identified most of the proteins present in that peak, one of them being LL-37 (Wooters, J., E. Nickbarg, and O. Chertov, unpublished observation). PR-39, the COOH-terminal antimicrobial peptide of one member of the porcine cathelicidins, has been reported to have the capacity to chemoattract porcine neutrophils 16. Therefore, we sought to determine whether LL-37 could chemoattract human leukocytes. The results indicate that LL-37 utilizes formyl peptide receptor–like 1 (FPRL1) as a receptor to activate human neutrophils, monocytes, and T cells.
Materials and Methods
Media and Reagents.
DME and RPMI 1640 were purchased from Biowhittaker. Synthetic fMLP (N-formyl-Met-Leu-Phe) and human AB serum were purchased from Sigma-Aldrich. FBS was purchased from Hyclone. Su peptide, a shorter version (lacking the NH2-terminal five amino acids) of T21/DP107 corresponding to amino acid 563–595 of HIV envelope protein gp41 17, was synthesized and purified by Macromolecular Resources. Su peptide is a specific agonist for FPRL1 receptor (Wang, J.M., unpublished observation). Su peptide was 99% pure as verified by mass spectrometry. LL-37 was synthesized by solid phase Fmoc (9-fluorenylmethyloxycarbonyl) chemistry. The first 11 amino acids were each coupled once, and the remaining amino acids were doubly coupled. After cleavage from the resin, the peptide was dissolved in buffer A and purified by reverse phase chromatography on a Dynamax C18 HPLC column using a gradient of 5–30% B in A buffer in 10 min, followed by 30–100% B in 90 min at a flow rate of 45 ml/min (buffer A: 0.1% TFA in water; buffer B: 0.1% TFA in acetonitrile). The fractions were characterized by analytical HPLC, mass spectrometry, and capillary electrophoresis, combined, and lyophilized. The LL-37 peptide thus prepared was 99.9% pure. Both LL-37 and Su peptide solutions were free of endotoxin.
Cells.
Human PBMCs were isolated from leukopacks (courtesy of the Transfusion Medicine Department, National Institutes of Health [NIH] Clinic Center, Bethesda, MD) by routine Ficoll–Hypaque density gradient centrifugation. Monocytes were purified from human PBMCs with the use of a MACS™ CD14 monocyte isolation kit (Miltenyi Biotec) according to the manufacturer's recommendation 12. Neutrophils were purified from the same leukopacks by 3% dextran sedimentation. T cells were purified from PBMCs by the use of Human CD4 T Cell Enrichment Columns (R&D Systems) 12. The purity of neutrophils, monocytes, and T cells by FACScan™ (Becton Dickinson) analysis was >95%. Rat basophilic leukemia cells stably transfected with epitope-tagged FPR (ETFR cells) were provided by Drs. H. Ali and R. Snyderman (Duke University, Durham, NC) and maintained in the presence of 0.8 mg/ml of geneticin in DME supplemented with 10% FBS. Human embryonic kidney (HEK)293 cells stably transfected with FPRL1 (hereafter designated FPRL1/293 cells) were a gift of Drs. P.M. Murphy and J.-L. Gao (National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD) and were maintained in the presence of 2 mg/ml of geneticin in DME supplemented with 10% FBS.
Chemotaxis Assay.
Migration of neutrophils, monocytes, T cells, and HEK293 or transfectant cells in response to chemotactic factors was assessed using a 48-well microchemotaxis chamber technique as previously described 17 18. In brief, chemotactic factors diluted in chemotaxis medium (CM; RPMI 1640 containing 1% BSA) were placed in the wells of the lower compartment of the chamber (Neuro Probe), and cells suspended in CM were added to the upper compartment. The lower and upper compartments were separated by a polycarbonate membrane (Osmonics). After incubation at 37°C in humidified air with 5% CO2 for a period of time (60 min for neutrophils, 90 min for monocytes, 180 min for T cells, and 300 min for HEK293, ETFR, and FPRL1/293 cells), the membranes were removed, scraped, stained, and counted. The results are presented as number of cells per high power field (no./HPF) or as chemotactic index (C.I.).
Ca2+ Mobilization Assay.
Monocytes or FPRL1/293 cells (107 cells/ml in RPMI 1640 containing 10% FBS) were loaded by incubating with 5 μM Fura-2 (Molecular Probes) at 24°C for 30 min in the dark. Subsequently, the loaded cells were washed with and resuspended (106 cells/ml) in saline buffer (138 mM NaCl, 6 mM KCl, 1 mM CaCl2, 10 mM Hepes, 5 mM glucose, and 1% BSA, pH 7.4). Each 2 ml of loaded cell suspension was then transferred into a quartz cuvette, which was placed in a luminescence spectrometer LS50 B (PerkinElmer). Ca2+ mobilization of the cells in response to chemotactic factors was measured by recording the ratio of fluorescence emitted at 510 nm after sequential excitation at 340 and 380 nm.
Statistical Analysis.
Unless otherwise specified, all experiments were performed at least three times, and the results show representative experiments. The significance of the difference between test and control groups was analyzed with a Student's t test.
Results
We first tested whether LL-37 could induce the in vitro migration of human peripheral blood monocytes, a critical correlate of monocyte accumulation at sites of inflammation or injury. LL-37 induced monocyte migration in a dose-dependent manner with an optimal concentration at 10−5 M (Fig. 1 A). In addition, LL-37 also dose-dependently induced Ca2+ flux in monocytes (Fig. 1 B). A checkerboard analysis revealed that, when equal concentrations of LL-37 were present in both the lower and the upper wells, only a slight increase in cell migration was observed (Table ). Thus, the migration of monocytes induced by LL-37 was based predominantly on chemotaxis rather than chemokinesis. Interestingly, after monocytes were differentiated into immature dendritic cells, the immature dendritic cells could not respond to LL-37 in terms of either chemotaxis or Ca2+ mobilization, presumably due to the loss of functional expression of the receptor for LL-37 (data not shown).
Figure 1.
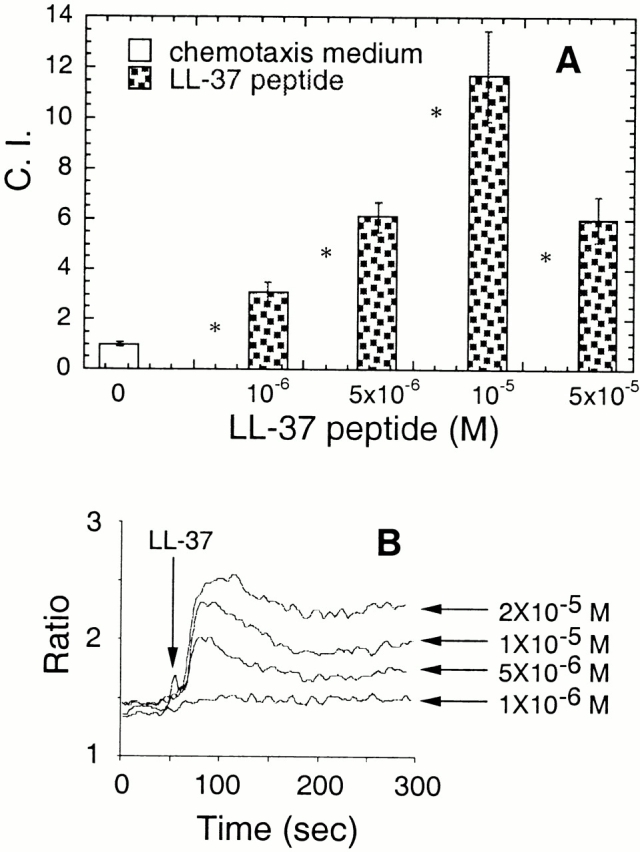
Induction by LL-37 of migration of (A) and Ca2+ flux in (B) human monocytes. (A) The migration of monocytes (106 cells/ml) was assessed by chemotaxis assay using 5-μm uncoated membranes. Spontaneous cell migration (without LL-37) was ∼30–50 cells per HPF. The average C.I. (mean ± SD) of triplicate wells is shown. *P < 0.05 when compared with chemotaxis medium alone (open bar). (B) Arrow indicates the time point at which LL-37 was applied to the cells.
Table 1.
Checkerboard Analysis of LL-37–induced Monocyte Migration
| LL-37 in lower wells (μM) | LL-37 in upper wells (μM) | ||
|---|---|---|---|
| 0 | 1 | 10 | |
| 0 | 40 ± 5 | 45 ± 6 | 44 ± 7 |
| 1 | 135 ± 15‡ | 50 ± 8 | 40 ± 6 |
| 10 | 495 ± 29‡ | 265 ± 5‡ | 95 ± 2* |
Human monocytes were used at 106 per ml. LL-37 peptide at the specified concentrations was added to the lower wells of the chemotaxis chamber, and monocytes in the absence or presence of the specified concentration of LL-37 peptide were added to the upper wells of the chemotaxis chamber. The average (mean ± SD) of migrated monocytes of triplicate wells is shown as no./HPF. *P < 0.05 and ‡ P < 0.01 as compared with spontaneous monocyte migration (in bold type).
To characterize the nature of the receptor for LL-37, we examined the effect of pertussis toxin (PTX), a reagent known to selectively block Gi protein–coupled signaling, on LL-37–induced monocyte chemotaxis. Treatment of monocytes with PTX (200 ng/ml) at 37°C for 30 min almost completely inhibited subsequent cell migration in response to LL-37, indicating that a Gi protein–coupled receptor might be involved in LL-37–induced monocyte activation (Fig. 2 A).
Figure 2.
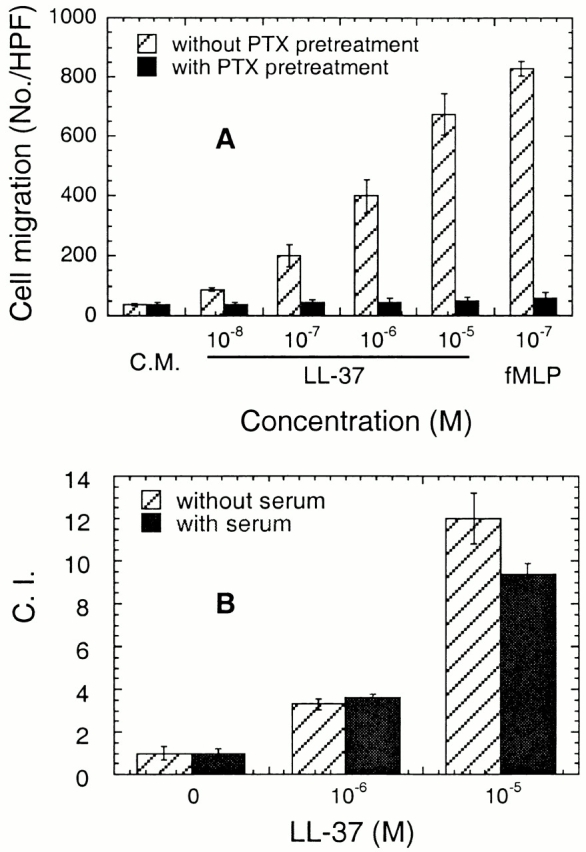
Effect of PTX (A) and serum (B) on LL-37–induced chemotaxis of monocytes. (A) Monocytes were incubated with (closed bar) or without (hatched bar) PTX at a final concentration of 200 ng/ml for 30 min at 37°C before performing chemotaxis assay. To show that the spontaneous cell migration (C.M.) was not affected by PTX pretreatment, the results are presented as no./HPF. (B) Chemotaxis assay was performed in the absence (hatched bar) or presence (closed bar) of 10% human AB serum, which can completely block the antimicrobial activity of LL-37 at 10−5 M.
The only previously known effect of LL-37 on mammalian cells is its capacity to damage them at concentrations above 5 × 10−5 M. This cytocidal effect of LL-37 can be blocked completely by the presence of human serum and partially by porcine serum 10. To investigate the relationship of the chemotactic effect of LL-37 to its cytocidal effect, the influence of 10% human AB serum on LL-37–induced chemotaxis was studied. LL-37–induced chemotaxis of monocytes was not significantly inhibited by the presence of 10% human AB serum (Fig. 2 B), suggesting that the chemotactic effect of LL-37 was independent of its cytocidal effect.
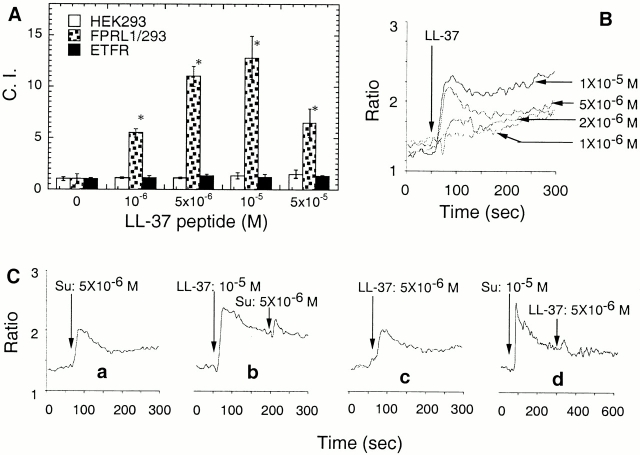
Next, we proceeded to identify the receptor LL-37 utilizes to mediate its chemotactic effect on monocytes. LL-37 must use a chemotactic receptor expressed by monocytes, but not by monocyte-derived immature dendritic cells. Of the 22 human chemotactic receptors (including 17 chemokine receptors and 5 classical chemoattractant receptors) identified so far 19 20, 9 of them, namely FPR, FPRL1, platelet activating factor receptor (PAFR), C5aR, CXCR4, CCR1, CCR2, CCR5, and CCR8, have been demonstrated to be expressed by freshly isolated peripheral blood monocytes at mRNA, protein, and functional levels 19 20 21. 7 out of the 9 (FPR, PAFR, C5aR, CXCR4, CCR1, CCR5, and CCR8) have also been shown to be functionally expressed by monocyte-derived immature dendritic cells 21 22. Examination of HEK293 cells stably transfected to express CCR2 indicated that CCR2 was not a receptor for LL-37 (data not shown). Fortuitously, we recently determined that FPRL1 was downregulated in the process of differentiation of monocytes into immature dendritic cells (Yang, D., Q. Chen, J.M. Wang, and J.J. Oppenheim, unpublished observation). We therefore investigated whether FPRL1 serves as a receptor for LL-37. As shown by Fig. 3 A, FPRL1/293 cells, HEK293 cells transfected to stably express FPRL1, migrated in response to LL-37 in a dose-dependent manner, whereas the parental HEK293 cells did not respond, suggesting that FPRL1 is a receptor for LL-37. The specificity of LL-37 interaction with FPRL1 is further supported by the fact that cells expressing FPR (ETFR cells), which share the highest homology with FPRL1, also did not respond to LL-37 (Fig. 3 A). As expected, LL-37 also induced Ca2+ flux in a dose-dependent manner in FPRL1/293 cells (Fig. 3 B) but not in ETFR cells (data not shown).
Figure 3.
LL-37 uses FPRL1 as its receptor. (A) Selective induction of FPRL1/293 cell chemotaxis by LL-37. The migration of parental HEK293 (open bars), FPRL1/293 (dotted bars), or ETFR (closed bars) cells was assessed by chemotaxis assay with the use of collagen-coated 10-μm membranes. Cells were used at a concentration of 106 cells/ml. The average C.I. (mean ± SD) of triplicate wells is shown. Spontaneous cell migration (without LL-37) was ∼10–20 cells per HPF. *P < 0.05 when compared with chemotaxis medium alone. (B) LL-37–induced Ca2+ flux in FPRL1/293 cells. Arrow indicates the time point at which LL-37 was applied to the cells. (C) Cross-desensitization of LL-37–induced Ca2+ flux in monocytes by FPRL1-specific agonistic ligand. Arrows indicate the time points at which LL-37 and Su peptides were applied to the cells.
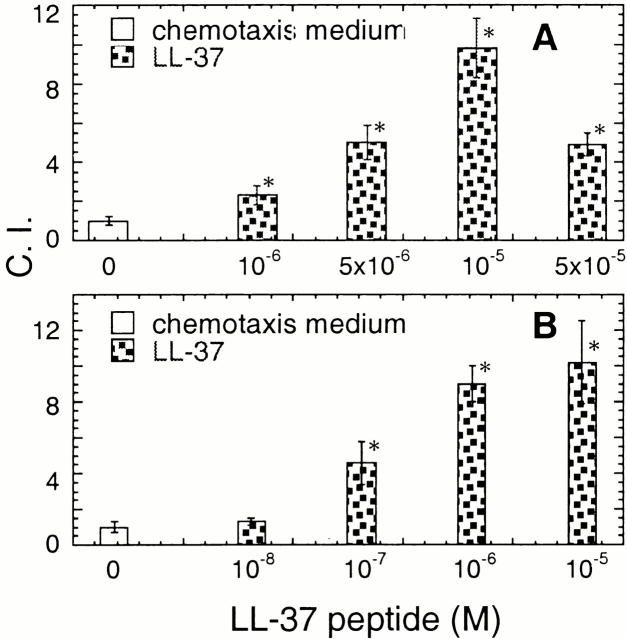
To ascertain that LL-37 also utilizes FPRL1 as a receptor to mediate its effect on monocytes, we examined whether LL-37–induced Ca2+ flux in monocytes could be cross-desensitized by Su peptide, an FPRL1-specific agonist. As shown in Fig. 3 C, both Su peptide and LL-37 at 5 × 10−6 M induced Ca2+ flux to a similar extent in freshly isolated human peripheral blood monocytes (a and c, respectively). Furthermore, the Ca2+ flux induced by Su peptide at 5 × 10−6 M was almost completely desensitized by LL-37 at 10−5 M (Fig. 3 C, b) and vice versa (Fig. 3 C, d). As cross-desensitization of Ca2+ flux is often due to two agonists acting on the same receptor, we conclude that LL-37 utilizes FPRL1 as a functional receptor. We further investigated whether LL-37 could chemoattract freshly isolated human peripheral blood neutrophils and T lymphocytes that are known to express functional FPRL1 17 19. As expected, LL-37 induced dose-dependent migration of highly purified human neutrophils (Fig. 4 A) and CD4 T cells (Fig. 4 B).
Figure 4.
Chemotaxis of human neutrophils (A) and CD4 T lymphocytes (B) in response to LL-37. The cell migration was assessed by chemotaxis assay with the use of uncoated (A) or fibronectin-coated (B) 5-μm membranes. The results are presented as the average C.I. (mean ± SD) of triplicate wells. *P < 0.05 when compared with spontaneous cell migration (chemotactic medium alone). Neutrophils and CD4 T cells were used at a concentration of 106 cells/ml and 5 × 106 cells/ml, respectively. Spontaneous neutrophil and T cell migration (without LL-37) was ∼30–50 and ∼30–40 cells per HPF, respectively.
Discussion
In this study, we have demonstrated that LL-37 is chemotactic for freshly isolated human peripheral blood neutrophils, monocytes, and T cells and determined that LL-37 utilizes FPRL1 as a receptor to mediate its chemotactic and Ca2+-mobilizing effects. So far, LL-37/hCAP18 has been considered to contribute to human innate defense against microbial infection, based primarily on its selective expression by neutrophils and epithelial cells facing the external environment, its in vitro antimicrobial activity, and its capacity to bind and neutralize the biological effect of bacterial endotoxin 1 5. As leukocytes participate in both innate and adaptive immunity, the fact that LL-37 can chemoattract human leukocytes may provide one additional mechanism by which LL-37 contributes to host defense against microbial invasion, by participating in the recruitment of leukocytes to sites of infection. This mechanism is potentially important in vivo, because the chemotactic activity of LL-37, unlike its antimicrobial action 10, is not significantly inhibited by the presence of 10% human AB serum (Fig. 2 B).
The optimal concentration for LL-37 to chemoattract human leukocytes was 10−5 M (Fig. 1 A and Fig. 4). Most chemotactic responses are usually based on “high-affinity ligand–receptor interaction” 19 20. It is likely that LL-37–FPRL1 interaction represents a low-affinity ligand–receptor interaction. Previously identified low-affinity ligand–receptor interactions include multiple CXC chemokines (growth-regulated oncogene, neutrophil-activating peptide 2, and epithelial cell–derived neutrophil-activating peptide 78) for CXCR1 23 and macrophage inflammatory protein 1β and monocyte chemoattractant protein 1 for CCR1 24. Low-affinity ligand–receptor interactions also contribute to the recruitment of leukocytes to the focus of inflammation 25. Multiple leukocyte chemotactic factors, including LL-37, are likely to be generated at sites of microbial invasion and presumably form a complex of concentration gradients. As leukocytes traffic from the lower to the higher levels of the chemotactic gradients, their high-affinity chemotactic receptors are likely to become deactivated through desensitization 25. Subsequent low-affinity ligand–receptor interactions, such as LL-37–FPRL1 interaction, can thus direct leukocytes closer to inflammatory foci. LL-37 expression can increase at both mRNA and protein levels by >10–50-fold in response to inflammatory stimuli 7 9. The concentration of LL-37 in the fluid from xenografts prepared with normal human airway epithelia is 2 μg/ml, equivalent to 4 × 10−7 M 26. Assuming that airway inflammation could also induce a 50-fold increase in LL-37 expression, the LL-37 at the site of airway inflammation would reach 2 × 10−5 M. Thus, LL-37 can potentially reach its optimal chemotactic concentration (10−5 M) at local inflammatory sites.
In addition to LL-37, other known ligands for FPRL1 include bacterial formyl peptides 19, an ectodomain of HIV gp41 termed T21 17, a hexapeptide (Trp-Lys-Tyr-Met-Val-d-Met-NH2) termed W peptide 27, serum amyloid A 28, and even lipoxin A4, a lipid derivative of arachidonate metabolism 29. Of these, serum amyloid A, lipoxin A4, and LL-37 are endogenously generated and increase dramatically during inflammation 7 9 28 29. Activation of the chemotactic receptor FPRL1 by these endogenous ligands, including LL-37, results in a G protein–mediated signaling cascade leading to not only chemotaxis of leukocytes but also increased adhesion, enhanced phagocytosis, release of oxygen intermediates, and augmented bacterial killing 19 20, thereby promoting antimicrobial immunity. As LL-37 can also bind and neutralize bacterial LPS 2, it may, on the other hand, limit the adverse effects of inflammation by downregulating LPS-induced inflammatory responses.
Defensins and cathelicidins represent two major types of endogenous antimicrobial antibiotic peptides in higher mammals, and their contribution to innate host defense against microbial invasion is well established 1 5. Based on the chemotactic activities of α- and β-defensins 11 12 14 and the capacity of α-defensins to promote antigen-specific immune responses 13, we proposed that defensins also contribute to adaptive antimicrobial immunity by alarming the adaptive immune system 12 14. The results showing that LL-37, the mature form of human cathelicidin, is also chemotactic for human leukocytes predicts that cathelicidins will also have the capacity to augment innate and adaptive host defenses. Thus, mobilization and amplification of immune responses may be a common feature for endogenous antimicrobial peptides in higher vertebrates. The identification of FPRL1 as the receptor that LL-37 utilizes to interact with human leukocytes should facilitate more detailed investigation of the mechanism of cathelicidins' roles in adaptive immunity.
Acknowledgments
The authors wish to thank N. Dunlop for technical assistance, Drs. H. Ali and R. Snyderman (Duke University) for providing ETFR cells, and Drs. P.M. Murphy and J.-L. Gao (National Institute of Allergy and Infectious Disease, NIH) for sharing FPRL1/293 cells. The support of the laboratory manager Ms. C. Fogle and the secretarial assistance of Ms. C. Nolan is greatly appreciated.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under contract number 01-CO-56000.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- Lehrer R.I., Ganz T. Antimicrobial peptides in mammalian and insect host defense. Curr. Opin. Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- Larrick J.W., Hirata M., Balint R.F., Lee J., Zhong J., Wright S.C. Human CAP18a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowland J.B., Johnsen A.H., Borregaad N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- Agerberth B., Gunne H., Odeberg J., Kogner P., Boman H.G., Gudmundsson G.H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M., Gennaro R., Romeo D. Cathelicidinsa novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- Gudmundsson G.H., Agerberth B., Odeberg J., Bergman T., Olsson B., Salcedo R. The human gene FALL-39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- Frohm M., Agerberth B., Ahangari G., Stahle-Backdahl M., Liden S., Wigzell H., Gudmundsson G.H. The expression of the gene coding for the antimicrobial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- Bals R., Wang X., Zasloff M., Wilson J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad anti-microbial activity at the airway surface. Proc. Natl. Acad. Sci. USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M.F., Sandstedt B., Sorensen O., Weber G., Borregaad N., Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J., Gudmundsson G.H., Rottenberg M.E., Berndt K.D., Agerberth B. Conformation-dependent antibacterial activity of naturally occurring human peptide LL-37. J. Biol. Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- Chertov O., Michiel D.F., Xu L., Wang J.M., Tani K., Murphy W.J., Longo D.L., Taub D.D., Oppenheim J.J. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- Yang D., Chen Q., Chertov O., Oppenheim J.J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- Lillard J.W., Jr., Boyaka P.N., Chertov O., Oppenheim J.J., McGhee J.R. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Chertov O., Bykovskaia S.N., Chen Q., Buffo M.J., Shogan J., Anderson M., Schroder J.M., Wang J.M., Howard O.M.Z. β-Defensinslinking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Chertov O., Ueda H., Xu L.L., Tani K., Murphy W.J., Wang J.M., Howard O.M.Z., Sayers T.J., Oppenheim J.J. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J. Exp. Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.J., Ross C.R., Blecha F. Chemoattractant properties of PR-39, a neutrophil antibacterial peptide. J. Leukoc. Biol. 1997;61:624–629. doi: 10.1002/jlb.61.5.624. [DOI] [PubMed] [Google Scholar]
- Su S.B., Gao J.-L., Gong W.-H., Dunlop N.M., Murphy P.M., Oppenheim J.J., Wang J.M. T21/DP107, a synthetic leucine zipper-like domain of the HIV-1 envelope gp41, attracts and activates human phagocytes by using G-protein-coupled formyl peptide receptors. J. Immunol. 1999;162:5924–5930. [PubMed] [Google Scholar]
- Yang D., Howard O.M.Z., Chen Q., Oppenheim J.J. Cutting edgeimmature dendritic cells generated from monocytes in the presence of TGF-β1 express functional C-C chemokine receptor 6. J. Immunol. 1999;163:1737–1741. [PubMed] [Google Scholar]
- Murphy P.M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- Zlotnick A., Morales J., Hedrick J.A. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- Sozzani S., Allavena P., Vecchi A., Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J. Leukoc. Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- Yang D., Chen Q., Stoll S., Chen X., Howard O.M.Z., Oppenheim J.J. Differential regulation of responsiveness to fMLP and C5a upon dendritic cell maturationcorrelation with receptor expression. J. Immunol. 2000;165:2694–2702. doi: 10.4049/jimmunol.165.5.2694. [DOI] [PubMed] [Google Scholar]
- Ahuja S.K., Murphy P.M. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- Neote K., DiGregorio D., Mak J.Y., Horuk R., Schall T.J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- Foxman E.F., Campbell J.J., Butcher E.C. Multi-step navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Weiner D.J., Meegalla R.L., Wilson J.M. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J. Clin. Invest. 1999;103:1113–1117. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y., Gong W.-H., Li B., Dunlop N.M., Shen W., Su S.B., Ye R.D., Wang J.M. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J. Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- Su S.B., Gong W., Gao J.-L., Shen W., Murphy P.M., Oppenheim J.J., Wang J.M. A seven-transmembrane, G protein–coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S., Maddox J.F., Peres H.D., Serhan C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]