Abstract
Natural killer T (NKT) cells are a highly conserved subset of T cells that have been shown to play a critical role in suppressing T helper cell type 1–mediated autoimmune diseases and graft versus host disease in an interleukin (IL)-4–dependent manner. Thus, it is important to understand how the development of IL-4– versus interferon (IFN)-γ–producing NKT cells is regulated. Here, we show that NKT cells from adult blood and those from cord blood undergo massive expansion in cell numbers (500–70,000-fold) during a 4-wk culture with IL-2, IL-7, phytohemagglutinin, anti-CD3, and anti-CD28 mAbs. Unlike adult NKT cells that preferentially produce both IL-4 and IFN-γ, neonatal NKT cells preferentially produce IL-4 after polyclonal activation. Addition of type 2 dendritic cells (DC2) enhances the development of neonatal NKT cells into IL-4+IFN-γ− NKT2 cells, whereas addition of type 1 dendritic cells (DC1) induces polarization towards IL-4−IFN-γ+ NKT1 cells. Adult NKT cells display limited plasticity for polarization induced by DC1 or DC2. Thus, newly generated NKT cells may possess the potent ability to develop into IL-4+IFN-γ− NKT2 cells in response to appropriate stimuli and may thereafter acquire the tendency to produce both IL-4 and IFN-γ.
Keywords: cord blood, interleukin 4, interferon γ, autoimmune diseases, graft versus host disease
Introduction
NKT cells are a conserved subset of T cells that express a highly limited TCR repertoire, composed of an invariant TCR α chain (Vα24-JαQ in humans) and a biased set of TCR β chains (predominantly Vβ11 in humans) 1. These TCRs recognize a nonpolymorphic, MHC class I–like molecule, CD1d, which presents glycolipids 2. NKT cells have a conspicuous ability to rapidly produce large amounts of IL-4 and IFN-γ upon TCR triggering 1, suggesting a regulatory function at the early stage of immune responses.
Development of various autoimmune diseases in mice 3 4 and humans 5 6 was found to be associated with a decrease in the number of NKT cells. NKT cells have been shown to prevent diabetes in nonobese diabetic mice 7 and graft versus host disease (GVHD) after bone marrow transplantation 8 in an IL-4–dependent manner. These findings indicate that NKT cells are an important regulatory cell type that prevents a variety of deleterious immune responses. Thus, it is important to understand how the development of IL-4– versus IFN-γ–producing NKT cells is regulated. Furthermore, obtaining a large number of IL-4–producing NKT cells may be instrumental in controlling certain autoimmune diseases and GVHD. To this end, two questions need to be addressed. First, because the frequency of NKT cells in human blood is extremely low 9 10 11, how can we expand NKT cell numbers? Second, because most NKT cells produce both IL-4 and IFN-γ, how can we promote NKT cells to produce only IL-4 but not IFN-γ?
In humans, small numbers of NKT cells exist in umbilical cord blood and adult blood 9 10 11. Recent studies have shown that not only NKT cells in adult blood (adult NKT cells) but also those in cord blood (neonatal NKT cells) have an activated/memory phenotype 10 11, suggesting that human NKT cells are stimulated even before birth, probably by endogenous ligands. However, in contrast to adult NKT cells, neonatal NKT cells do not produce IL-4 or IFN-γ upon primary stimulation 11. This suggests that neonatal NKT cells are functionally immature at birth and need to be primed to become fully functional. Thus, the nature of initial stimuli may strongly influence the direction of neonatal NKT cell differentiation, i.e., toward IL-4−IFN-γ+ NKT1 versus IL-4+IFN-γ− NKT2 cells.
Recent studies have shown that distinct types of dendritic cells (DCs) are capable of directing Th differentiation toward a Th1 or Th2 type 12. In humans, monocyte-derived DCs (designated DC1) produce a large amount of IL-12 after stimulation with maturation-inducing factors such as CD40 ligand (L) and induce Th1 differentiation of naive CD4+ T cells 13. In contrast, type 2 dendritic cells (DC2), which develop from CD4+CD3−CD11c− plasmacytoid cells in the presence of IL-3 and CD40L 13 or TNF-α 14, do not produce a significant amount of IL-12 and preferentially induce Th2 differentiation. Recent studies have shown that CD1d-expressing DCs stimulate mouse 2 and human 15 NKT cells. Thus, the type of DCs may also influence NKT cell differentiation into NKT1 or NKT2 cells.
Here, we studied how to obtain a large number of IL-4–producing NKT2 cells in vitro. To this end, we cultured NKT cells from cord blood or adult blood with anti-CD3 and anti-CD28 mAbs, DC1, or DC2 in the presence of IL-2, IL-7, and PHA. We show that neonatal NKT cells undergo massive proliferation and strong polarization toward NKT2 cells during 4-wk culture with IL-2, IL-7, PHA, anti-CD3, and anti-CD28 mAbs. Addition of DC2 enhances the differentiation of neonatal NKT cells into NKT2 cells.
Materials and Methods
Isolation of NKT Cells from Cord Blood or Adult Blood.
Cord blood and buffy coat from healthy donors were purchased from Advanced Bioscience Technologies and Stanford Blood Center, respectively. B cells and monocytes were depleted from mononuclear cells using anti-CD19 and anti-CD14 mAbs and magnetic beads coated with goat anti–mouse IgG (Dynabeads M-450). The cells were frozen until needed. They were stained with FITC-conjugated anti-Vα24 (C15) and PE-conjugated anti-Vβ11 mAbs (C21; Immunotech). Vα24+Vβ11− conventional T cells and Vα24+Vβ11+ NKT cells were purified by cell sorting.
Generation of DC1 and DC2.
Monocytes and CD4+ CD11c−lin− DC2 precursors were isolated from human peripheral blood as described previously 16. The cells were cultured in RPMI 1640 (BioWhittaker) supplemented with 10% FCS (BioWhittaker), 2 mM l-glutamine, 10 mM Hepes, 1 mM sodium pyruvate, 55 μM 2-mercaptoethanol, penicillin G, and streptomycin (all from Life Technologies). Monocytes were cultured in the presence of 50 ng/ml GM-CSF (Schering-Plough) and 200 U/ml IL-4 (Schering-Plough) for 6 d. The resulting immature DCs were washed and cultured for 2 d with human CD40L–transfected L cells 16 (irradiated at 5,500 rads), to obtain mature DC1. CD4+CD11c−lin− DC2 precursors were cultured with 10 ng/ml IL-3 (R&D Systems) and irradiated CD40L-transfected L cells for 6 d to obtain mature DC2.
Sequencing of TCR Vα24 mRNA.
cDNAs of TCR-α transcripts were amplified by PCR. The primers were Vα24 sense, 5′-GATATACAGCAACTCTGGATGCA-3′ and Cα antisense, 5′-TGAAGTCCATAGACCTCATGTC-3′. PCR products were subcloned using a TA Cloning® Kit (Invitrogen), and the nucleotide sequences were determined by automated sequencing using an Applied Biosystems 373 sequencer.
In Vitro Expansion of NKT Cells and Coculture with DCs.
Vα24+Vβ11+ NKT cells were stimulated with allogeneic DC1, allogeneic DC2, or plate-bound anti-CD3 mAb SPV-T3b (10 μg/ml; reference 16) and 1 μg/ml soluble anti-CD28 mAb L293.1 (Becton Dickinson) in Yssel's medium (Irvine Scientific) containing 10% FCS, 100 U/ml IL-2 (Schering-Plough), 50 ng/ml IL-7 (R&D Systems), and 0.5 μg/ml PHA (Wellcome Diagnostics). NKT cells from cord blood were cultured in Terasaki plates (300–1,500 T cells per well depending on available cell numbers), and those from adult blood were cultured in 96-well round-bottomed culture plates (1,500–16,000 T cells per well). DC1 and DC2 were irradiated (3,000 rads) and cultured with NKT cells at a 1:2 DCs/T cells. The proliferating cells were supplemented with medium containing IL-2 and IL-7 but not PHA. After 14 d of stimulation, the cells were restimulated for another 14 d under the same condition as the first stimulation. The resulting cells were used for cytokine assays. The purity of Vα24+Vβ11+ cells was >95% at the time of restimulation for cytokine assays.
Analysis of Cytokine Production by ELISA or Flow Cytometry.
106 NKT cells per milliliter were restimulated with plate-bound anti-CD3 and 1 μg/ml soluble anti-CD28 mAbs for 24 h. IFN-γ and IL-4 in supernatants were measured with a Quantikine ELISA kit (R&D Systems). Intracellular cytokines produced by NKT cells were analyzed by flow cytometry as described 13.
Results
Both Adult and Neonatal NKT Cells Undergo Massive Expansion in Cultures with IL-2, IL-7, PHA, Anti-CD3, and Anti-CD28 mAbs.
NKT cells were isolated from cord blood and adult blood by cell sorting, based on the double expression of Vα24 and Vβ11 17. The frequency of Vα24+Vβ11+ NKT cells in cord blood was 0.031 ± 0.023% (SD) of total lymphocytes (range 0.001–0.083%, n = 11), which tended to be lower than the frequency in adult blood (0.094 ± 0.129% of total lymphocytes, range 0.007–0.540%, n = 16). Previous studies showed that IL-2 and IL-7 are important for NKT cell proliferation 18 19. Therefore, 1,500–16,000 adult NKT cells or 300–1,500 neonatal NKT cells were cultured with IL-2, IL-7, PHA, anti-CD3, and anti-CD28 mAbs. After 2 wk of culture, between 0.08 and 6.2 × 106 NKT cells were generated, representing a 30–7,600-fold expansion (Table ). After two more weeks of culture in the same condition, 1.2–27 × 106 NKT cells were obtained, representing a total 500–70,000-fold expansion. As compared with the expansion during the first 2 wk (30–7,600-fold), the expansion during the second 2 wk was modest (2–16-fold), possibly due to clonal exhaustion caused by continuous stimulation. These results indicate that although only small numbers of NKT cells are isolated from adult and cord blood, NKT cells can be efficiently expanded in cultures.
Table 1.
Adult and Neonatal NKT Cell Expansion after Cultures with Anti-CD3 and Anti-CD28 mAbs
| Day 0 | Day 14 | Day 28 | ||||
|---|---|---|---|---|---|---|
| Cell number | Cell number | Fold increase | Cell number | Fold increase | ||
| Adult NKT | Exp. 1 | 16 | 2,200 | 140 | 7,700 | 480 |
| Exp. 2 | 16 | 500 | 31 | NA | NA | |
| Exp. 3 | 1.5 | 78 | 52 | 1,210 | 810 | |
| Neonatal NKT | Exp. 1 | 1.5 | 6,200 | 4,100 | NA | NA |
| Exp. 2 | 0.4 | 3,030 | 7,600 | 27,000 | 67,500 | |
| Exp. 3 | 0.3 | 920 | 3,100 | 1,910 | 6,400 | |
Vα24+Vβ11+ NKT cells were purified by cell sorting and expanded with plate-bound anti-CD3 and soluble anti-CD28 mAbs in the presence of PHA, IL-2, and IL-7 for two rounds (14 d each). Cell numbers were counted on days 0, 14, and 28, and are shown at ×10−3. Fold increase: compared to the starting cell numbers.
Exp., experiment.
NA, not available.
More than 95% of cultured NKT cells expressed Vα24+Vβ11+ cells after the 4 wk of culture by FACS® analyses (data not shown). In addition, sequence analyses of TCR α chains of these cultured cells showed the invariant Vα24-JαQ TCR without N sequences, which is identical to that reported for NKT cells (reference 20 and data not shown). In contrast, cDNA sequences of TCR α chains of Vα24+Vβ11− T cells were polyclonal at the V–J junctions and the J regions (data not shown). These data indicate that the expanded cells after 4 wk of culture are NKT cells but not contaminating cells.
Neonatal but Not Adult NKT Cells Preferentially Become IL-4+IFN-γ− NKT2 Cells after Polyclonal Stimulation.
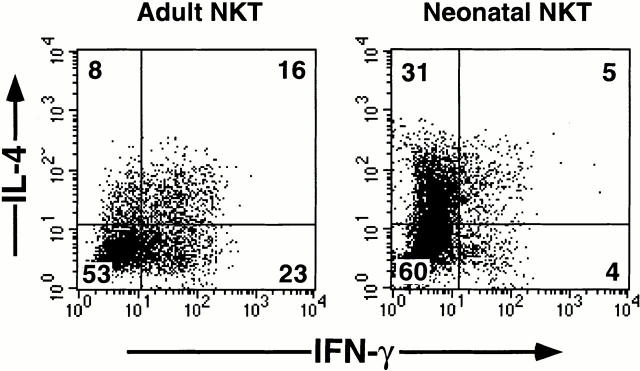
To determine the potential of cytokine production by the expanded NKT cells, the cells were restimulated with anti-CD3 and anti-CD28 mAbs for either 5 (intracellular cytokine staining) or 24 h (cytokine analyses by ELISA). Adult NKT cells produced considerable amounts of both IL-4 (268–885 pg/ml) and IFN-γ (2,914–3,366 pg/ml; Table ). Intracellular cytokine staining showed that the adult NKT cells mainly differentiated into IL-4−IFN-γ+ NKT1 cells (23%) and IL-4+IFN-γ+ NKT0 (16%; Fig. 1). Only 8% of the cells differentiated into IL-4+IFN-γ2 NTT2 cells. This confirms the previous finding that NKT cells produce both IL-4 and IFN-γ upon activation through TCRs 1.
Table 2.
IFN-γ and IL-4 Production by NKT Cells Stimulated with Anti-CD3 and Anti-CD28 mAbs
| IFN-γ | IL-4 | IL-4/IFN-γratio | ||
|---|---|---|---|---|
| pg/ml | pg/ml | |||
| Adult blood NKT | Exp. 1 | 3,321 | 484 | 0.15 |
| Exp. 2 | 3,366 | 885 | 0.26 | |
| Exp. 3 | 2,914 | 268 | 0.09 | |
| Cord blood NKT | Exp. 1 | 1,671 | 1,069 | 0.64 |
| Exp. 2 | 1,889 | 5,559 | 2.94 | |
| Exp. 3 | 650 | 548 | 0.84 |
Vα24β11+ NKT cells were purified by cell sorting, expanded with plate-bound anti-CD3 and soluble anti-CD28 mAbs in the presence of PHA, IL-2, and IL-7 for two rounds (14 d each), and restimulated with immobilized anti-CD3 and soluble anti-CD28 mAbs at ×106 cells per milliliter for 24 h. IFN-γ and IL-4 in the supernatants were measured by ELISA.
Exp., experiment.
Figure 1.
Intracellular staining for IL-4 and IFN-γ in NKT cells from adult or cord blood stimulated with anti-CD3 and anti-CD28 mAbs. Vα24+Vβ11+ NKT cells were purified by cell sorting, expanded with anti-CD3 and anti-CD28 mAbs in the presence of PHA, IL-2, and IL-7 for two rounds (14 d each), and restimulated with immobilized anti-CD3 mAb and soluble anti-CD28 mAb at 106 cells per milliliter ml for 5 h. Brefeldin A was added at 2.5 h for intracellular cytokine staining. Percentages in each quadrant are indicated on the plots. The data shown are from one experiment representative of three experiments.
Interestingly, neonatal NKT cells produced two to six times more IL-4 (548–5,559 pg/ml) but two to four times less IFN-γ (650–1,889 pg/ml) than adult NKT cells (Table ). These differences are reflected in higher IL-4/IFN-γ ratios from neonatal NKT cells than those from adult NKT cells (Table ). Intracellular cytokine staining showed that the predominant population of neonatal NKT cells became IL-4+IFN-γ− NKT2 cells (31%; Fig. 1). Only 5 and 4% of the NKT cells differentiated into IL-4+IFN-γ+ NKT0 and IL-4−IFN-γ+ NKT1 cells, respectively. These data indicate that neonatal NKT cells and adult NKT cells display strikingly different patterns of cytokine production; neonatal and adult NKT cells preferentially develop into IL-4+IFN-γ− NKT2 cells and IL-4+IFN-γ1 NKT0 cells, respectively, in response to TCR engagement.
DC2 Promote Neonatal NKT Cells To Become IL-4+IFN-γ− NKT2 Cells.
Because neonatal NKT cells preferentially develop into IL-4+IFN-γ− NKT2 cells after polyclonal stimulation, we asked whether addition of Th2-inducing DCs could further promote neonatal NKT cell development into the NKT2 type. Our previous study showed that while CD40L-activated monocyte-derived DC1 preferentially induce Th1 differentiation, CD40L-activated CD11c− plasmacytoid cell–derived DC2 preferentially induce Th2 differentiation of naive CD4+ T cells 13. Thus, DC2 were added to the NKT cell cultures together with PHA, IL-2, and IL-7 at the beginning of the culture and at the end of 2 wk of culture. After a total of 4 wk of culture, NKT cells were restimulated with anti-CD3 and anti-CD28 mAbs for either 5 (for intracellular cytokine staining) or 24 h (for cytokine analyses by ELISA). In parallel, the effect of DC1 on neonatal NKT cell differentiation was analyzed.
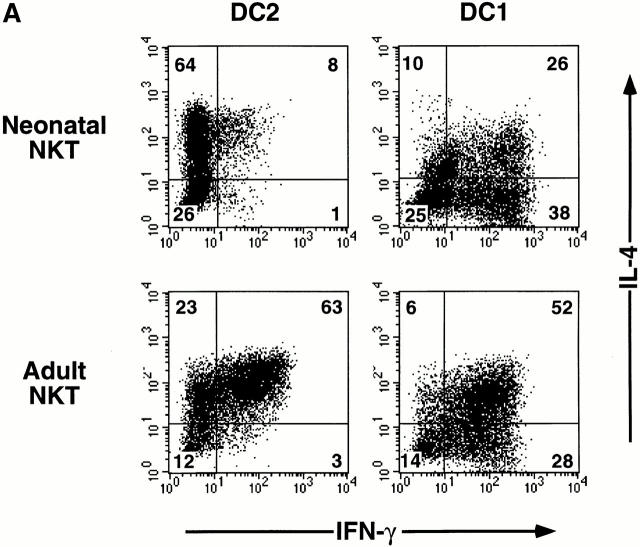
Addition of DC2 resulted in a twofold increase in the percentage of IL-4+IFN-γ2 NKT2 cells (64%; Fig. 2 A) compared with the polyclonal stimulation with anti-CD3 and anti-CD28 mAbs (31%; Fig. 1). Only 8 and 1% of the NKT cells cultured with DC2 became IL-4+IFN-γ+ NKT0 cells and IL-4−IFN-γ+ NKT1 cells, respectively (Fig. 2 A). In contrast, addition of DC1 preferentially induced neonatal NKT cells to become IL-4−IFN-γ+ NKT1 cells (38%), and to a lesser extent IL-4+IFN-γ+ NKT0 cells (26%) and IL-4+IFN-γ− NKT2 cells (10%; Fig. 2 A). Neonatal NKT cells cultured with DC2 produced more IL-4 (4,900–16,200 pg/ml) and similar amounts of IFN-γ (230–1,450 pg/ml; Fig. 2 B) compared with those stimulated with anti-CD3 and anti-CD28 mAbs (548–5,559 pg/ml IL-4 and 650–1,889 pg/ml IFN-γ; Table ). Neonatal NKT cells cultured with DC1 produced much more IFN-γ (97,000–222,000 pg/ml) and less IL-4 (1,400–6,900 pg/ml) than those cultured with DC2 (230–1,450 pg/ml IFN-γ and 4,900–16,200 pg/ml IL-4; Fig. 2 B). These results suggest that DC2 further promote development of neonatal NKT cells into IL-4+IFN-γ− NKT2 cells. In addition, a large fraction of neonatal NKT cells can be polarized into IL-4−IFN-γ+ NKT1 cells by DC1.
Figure 2.

IL-4 and IFN-γ production by NKT cells from cord blood or adult blood cultured with DC1 or DC2. Vα24+Vβ11+ NKT cells were purified by cell sorting, expanded with DC1 or DC2 in the presence of PHA, IL-2, and IL-7 for two rounds (14 d each), and restimulated as in Fig. 1. (A) Intracellular staining for IL-4 and IFN-γ. Percentages in each quadrant are indicated on the plots. The data shown are from one experiment representative of three experiments. (B) ELISA. Data from three experiments are shown by scattergram.
Adult NKT Cells Have Limited Plasticity for Polarization Induced by DC1 or DC2.
Next, we examined whether the addition of DC2 would induce a large number of IL-4+IFN-γ− NKT2 cells from adult NKT cells, which produce both IL-4 and IFN-γ after polyclonal TCR stimulation (Table , and Fig. 1). Although DC2 induced an increase in the percentage of IL-4+IFN-γ− NKT2 cells (23%; Fig. 2 A) compared with polyclonal TCR stimulation (8%; Fig. 1), the predominant population of adult NKT cells cultured with DC2 became IL-4+IFN-γ+ NKT0 cells (63%; Fig. 2 A). Accordingly, adult NKT cells cultured with DC2 produced large amounts of IL-4 (2,400–10,700 pg/ml) as well as IFN-γ (5,000–46,000 pg/ml; Fig. 2 B). Similarly, adult NKT cells cultured with DC1 preferentially developed into IL-4+IFN-γ+ NKT0 cells (52%) and to a lesser extent into IL-4−IFN-γ+ NKT1 cells (28%; Fig. 2 A). Accordingly, adult NKT cells cultured with DC1 produced a large amount of IFN-γ (48,000–124,000 pg/ml) and IL-4 (2,600–10,100 pg/ml; Fig. 2 B). These results indicate that the predominant population of adult NKT cells has an NKT0 phenotype and poor plasticity for polarization to NKT1 or NKT2 cells.
Discussion
Accumulating findings indicate that IL-4–producing NKT cells are likely to play an important role in controlling various Th1-mediated pathological conditions such as type 1 diabetes 7 and GVHD 8. Thus, it is important to understand how the development of IL-4– versus IFN-γ–producing NKT cells is regulated. Furthermore, expanding the population of IL-4–producing NKT cells may serve to reduce the activity of these diseases. Here we demonstrated that the rare human NKT cells isolated from adult or cord blood have great potential to expand in cultures with IL-2, IL-7, PHA, anti-CD3, and anti-CD28 mAbs. Although adult NKT cells displayed an IL-4+IFN-γ+ NKT0 phenotype, a large population of neonatal NKT cells became IL-4+IFN-γ− NKT2 cells in this culture condition. Moreover, neonatal NKT cells could be further polarized into IL-4+IFN-γ− NKT2 cells in cultures with DC2, whereas adult NKT cells showed poor plasticity for polarization. These results suggest that newly generated and functionally immature NKT cells, such as the ones in cord blood rather than the already primed mature NKT cells, such as the those in adult blood, may have greater potential to develop into IL-4–producing NKT2 cells and thus to inhibit Th1-mediated pathological conditions.
It has been shown that adult but not neonatal NKT cells have oligoclonally expanded and have obtained primary effector functions 11. In addition, mouse NKT cell numbers increase after birth 1. Here we showed that adult blood tends to contain more NKT cells than cord blood. These findings suggest that NKT cells may continue to be stimulated by self- and/or environmental antigens presented on CD1d, leading to chronic activation and expansion after birth. This, together with our finding that adult NKT cells produce more IFN-γ and display less plasticity for polarization than neonatal NKT cells, suggests that NKT cells may progressively acquire the bias to produce IFN-γ by chronic stimulation and may thus trigger the onset of autoimmune diabetes in predisposed individuals at certain ages 6.
It remains to be determined whether neonatal NKT cells have an intrinsic tendency to differentiate into IL-4+IFN-γ2 NKT2 cells or if the culture conditions used in this study selectively expanded the cells that had acquired the ability to produce this pattern of cytokines. Nevertheless, the clear differences in cytokine profiles between neonatal and adult NKT cells cultured in comparable conditions indicate greater flexibility of neonatal NKT cells in changing their cytokine profiles as a population level. This property of neonatal NKT cells may be related to their functional immaturity, as demonstrated by the inability to produce IL-4 or IFN-γ upon primary stimulation 11, and is reminiscent of a property of conventional naive CD4+ T cells, which need to be primed to acquire the ability to produce Th1 or Th2 cytokines.
The invariant Vα24+ TCR on human NKT cells specifically recognizes CD1d 21. CD40L-activated DC1 expressed a low level of CD1d as reported 15, and anti-CD1d mAb diminished IFN-γ production by NKT cells cultured with DC1 (data not shown). However, CD40L-activated DC2 did not express a detectable level of CD1d, and anti-CD1d mAb did not diminish IFN-γ production by NKT cells cultured with DC2 (data not shown). Thus, DC1 stimulate NKT cells through CD1d, whereas DC2 promote NKT2 cell differentiation in a TCR-independent manner, as DCs modulate functions of B cells 22 and NK cells 23 independently of the interaction between MHC class molecules and TCRs. In addition, compared with culture with anti-CD3 and anti-CD28 mAbs, culture with DC1 or DC2 overall increased the absolute level of cytokines from NKT cells, except for IFN-γ from neonatal NKT cells cultured with DC2. This enhancement of cytokine production is probably due to combined effects of various costimulatory molecules and cytokines derived from DCs.
In summary, this study demonstrates the remarkable capacity of cord blood NKT cells to preferentially develop into IL-4+IFN-γ− NKT2 cells. Furthermore, the cytokine-producing capacity of NKT cells can be modulated by the distinct subsets of DCs, DC1 and DC2. Developing efficient methods to isolate and expand NKT cells, particularly the IL-4+IFN-γ− NKT2 population, may offer the possibility of using them in cell therapies for autoimmune diseases and GVHD.
Acknowledgments
We thank J. Cupp for flow cytometry, R. de Waal Malefyt for many helpful suggestions, and D. Wylie for critical review of the manuscript.
DNAX Research Institute is supported by the Schering-Plough Corporation.
Footnotes
N. Kadowaki's present address is Department of Hematology and Oncology, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawara-cho, Sakyo-ku, Kyoto 606-8507, Japan.
References
- Bendelac A., Rivera M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Takeda K., Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1–positive cellsevidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J. Exp. Med. 1993;177:155–164. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieza M.A., Itoh T., Cui J.Q., Makino Y., Kawano T., Tsuchida K., Koike T., Shirai T., Yagita H., Matsuzawa A. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- Sumida T., Sakamoto A., Murata H., Makino Y., Takahashi H., Yoshida S., Nishioka K., Iwamoto I., Taniguchi M. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J. Exp. Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.B., Kent S.C., Patton K.T., Orban T., Jackson R.A., Exley M., Porcelli S., Schatz D.A., Atkinson M.A., Balk S.P. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- Hammond K.J.L., Poulton L.D., Palmisano L.J., Silveira P.A., Godfrey D.I., Baxter A.G. α/β-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D., Lewis D., Dejbakhsh-Jones S., Lan F., Garcia-Ojeda M., Sibley R., Strober S. Bone marrow NK1.1− and NK1.1+ T cells reciprocally regulate acute graft versus host disease. J. Exp. Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Tanaka Y., Shimizu E., Kaneko Y., Kamata N., Sato H., Osada H., Sekiya S., Nakayama T., Taniguchi M. A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int. Immunol. 1999;11:881–887. doi: 10.1093/intimm/11.6.881. [DOI] [PubMed] [Google Scholar]
- van Der Vliet H.J., Nishi N., de Gruijl T.D., von Blomberg B.M., van den Eertwegh A.J., Pinedo H.M., Giaccone G., Scheper R.J. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95:2440–2442. [PubMed] [Google Scholar]
- D'Andrea A., Goux D., De Lalla C., Koezuka Y., Montagna D., Moretta A., Dellabona P., Casorati G., Abrignani S. Neonatal invariant Vα24+ NKT lymphocytes are activated memory cells. Eur. J. Immunol. 2000;30:1544–1550. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Kadowaki N., Rissoan M.C., Soumelis V. T cell activation and polarization by DC1 and DC2. Curr. Top. Microbiol. Immunol. 2000;251:149–159. doi: 10.1007/978-3-642-57276-0_19. [DOI] [PubMed] [Google Scholar]
- Rissoan M.-C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Arpinati M., Green C.L., Heimfeld S., Heuser J.E., Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- Spada F.M., Borriello F., Sugita M., Watts G.F., Koezuka Y., Porcelli S.A. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 2000;30:3468–3477. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kadowaki N., Antonenko S., Lau J.Y., Liu Y.J. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C., Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J. Immunol. 1997;159:5862–5870. [PubMed] [Google Scholar]
- Chen H., Paul W.E. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J. Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- Vicari A., de Moraes M.D.C., Gombert J.M., Dy M., Penit C., Papiernik M., Herbelin A. Interleukin 7 induces preferential expansion of Vβ 8.2+CD4-8− and Vβ 8.2+CD4+8− murine thymocytes positively selected by class I molecules. J. Exp. Med. 1994;180:653–661. doi: 10.1084/jem.180.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S., Yockey C.E., Brenner M.B., Balk S.P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8− α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley M., Garcia J., Balk S.P., Porcelli S. Requirements for CD1d recognition by human invariant Vα24+CD4−CD8− T cells. J. Exp. Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Vanbervliet B., Fayette J., Massacrier C., Van Kooten C., Briere F., Banchereau J., Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N.C., Lozier A., Flament C., Ricciardi-Castagnoli P., Bellet D., Suter M., Perricaudet M., Tursz T., Maraskovsky E., Zitvogel L. Dendritic cells directly trigger NK cell functionscross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]