Abstract
Immune responses induced during the early stages of chronic viral infections are thought to influence disease outcome. Using HIV as a model, we examined virus-specific cytotoxic T lymphocytes (CTLs), T helper cells, and viral genetic diversity in relation to duration of infection and subsequent response to antiviral therapy. Individuals with acute HIV-1 infection treated before seroconversion had weaker CTL responses directed at fewer epitopes than persons who were treated after seroconversion. However, treatment-induced control of viremia was associated with the development of strong T helper cell responses in both groups. After 1 yr of antiviral treatment initiated in acute or early infection, all epitope-specific CTL responses persisted despite undetectable viral loads. The breadth and magnitude of CTL responses remained significantly less in treated acute infection than in treated chronic infection, but viral diversity was also significantly less with immediate therapy. We conclude that early treatment of acute HIV infection leads to a more narrowly directed CTL response, stronger T helper cell responses, and a less diverse virus population. Given the need for T helper cells to maintain effective CTL responses and the ability of virus diversification to accommodate immune escape, we hypothesize that early therapy of primary infection may be beneficial despite induction of less robust CTL responses. These data also provide rationale for therapeutic immunization aimed at broadening CTL responses in treated primary HIV infection.
Keywords: cytotoxic T lymphocytes, T helper cell responses, viral evolution, cytotoxic T lymphocyte epitopes, human leukocyte antigen
Introduction
The outcome of many viral infections is containment rather than the eradication of infection, suggesting a central role of the immune system in viral control. Increasing data indicate the importance of CTLs in HIV-1 infection (for reviews, see references 1 2 3). During untreated primary infection, HIV-1–specific CTL activity is associated with the initial decrease of viremia 4 5 6 and in vitro studies have shown potent inhibition of viral replication by CD8+ T cells, mediated by both lytic and nonlytic mechanisms 7. Studies in individuals with long-term nonprogressing HIV-1 infection, in whom persistent low viral loads have been associated with strong CTL responses 8 9 10 11, and observations of declining CTL responses in persons with disease progression 11 12 13, further support the potential important role of the cellular immune response. In addition, recent animal model data have shown that CD8+ cell depletion is associated with an increase in viral load 14 15 16. The inverse correlation between HIV-1–specific CTLs and viral load has also been demonstrated by flow cytometry in untreated persons with chronic HIV-1 infection using MHC class I tetramers 10.
Despite the strong antiviral effect of CTLs, most HIV-1–infected individuals have poorly controlled viremia and progress to AIDS without effective antiretroviral therapy. Several studies, predominantly in the murine lymphocytic choriomeningitis virus model, indicate that maintenance of effective CTLs requires the presence of virus-specific T helper cells 17 18 19. Recent studies in HIV-1 infection, showing an inverse association between virus-specific CD4+ cell responses and viral load 20 and an association between strong HIV-1–specific CTL and T helper cell responses 21 suggest that virus-specific T helper cells are also required for maintaining an effective CTL response in HIV-1 infection (for reviews, see references 1 and 22). As T helper cell function is impaired early after HIV-1 infection 20 23 24, loss of T helper cell support may be an important reason for subsequent insufficient virus control by CTLs. Additional factors contributing to the failure of the immune system to control HIV-1 infection observed in the majority of infected individuals may be the generation of viral escape mutants 25 26 27 28 29 and the development of high viral diversity over time 30 31 32 33.
Several studies indicate that early antiviral therapy results in the generation of strong HIV-1–specific T helper cell responses 20 23 34. However, effects of early therapy on CTL responses, which are normally closely linked to T helper cell responses in HIV infection 21, have not been well defined. In addition, there is a paucity of data relating overall viral diversity to immune responses in acute or chronic viral infections. Here, we present a detailed analysis of the HIV-1 epitope-specific cellular immune responses in subjects that were treated with highly active antiretroviral therapy (HAART) during acute HIV-1 infection, before seroconversion, or within 180 d of HIV-1 infection. In addition, as viral diversification has been linked to disease outcome in a chronic human viral infection 32 35 36, we examine the diversity of the HIV-1–env C2-V5 region in these subjects compared with those in individuals treated during chronic HIV-1 infection.
Materials and Methods
Study Subjects.
A cohort of subjects with acute HIV-1 infection has been established at the Massachusetts General Hospital and at the San Francisco General Hospital, the first 30 of whom are reported here. These subjects were divided in two groups: those individuals who were diagnosed and treated with HAART before or at the time of HIV-1 seroconversion (referred to as “preseroconversion acute HIV”, n = 19) and those individuals treated with HAART after HIV-1 seroconversion but within 180 d of primary HIV-1 infection (“postseroconversion primary HIV”, n = 11; reference 37).
Acute HIV-1 infection in the preseroconversion group (group 1) was defined by symptomatic disease, recent high risk exposure, positive plasma HIV-1 RNA and either a negative HIV-1 ELISA or a negative/indeterminate HIV-1 Western blot. 16 subjects in this cohort had a negative HIV-1 ELISA at diagnosis of infection and 3 (AC08, AC09, and AC26) had a weakly positive ELISA but indeterminate Western blot. 18 were Caucasian and one was Hispanic (18 male and 1 female), and mode of exposure to HIV-1 was sexual in all. Upon diagnosis of acute HIV-1 infection, all subjects were treated with HAART, including two nucleoside analogues and either a protease inhibitor (n = 14) or a nonnucleoside reverse transcriptase (RT) inhibitor (n = 5). Individuals in the postseroconversion primary HIV-1 group (group 2) were enrolled and treated with HAART within 180 d of HIV-1 infection 37. All individuals had fully seroconverted when HAART, including two nucleoside analogues and either a protease inhibitor or a nonnucleoside RT inhibitor, was started. Recent seroconversion was determined by either documentation of a negative HIV Ab test within the prior 180 d or a history consistent with recent infection supported by laboratory evidence consisting of a nonreactive less-sensitive enzyme immunoassay (EIA) Ab test 37. 9 individuals were Caucasian and 2 were Hispanic (all male) and mode of exposure to HIV-1 was sexual in 10 subjects and by intravenous drug use in 1 subject. All individuals were adherent to their treatment during the study period except individual AC22, who was not fully adherent to the antiretroviral regiment.
For comparison, another group of 10 individuals who were treated during chronic HIV-1 infection (“chronic HIV” group; group 3) was studied. For these subjects, no pretreatment samples were available, but only a single time point after at least 7 mo (median 20 mo, range 7–38 mo) of effective antiretroviral treatment resulting in an undetectable viral load. These individuals were infected for at least 12 mo (median 2 yr, range 1–10 yr) before HAART, including two nucleoside analogues and either a protease inhibitor or a nonnucleoside RT inhibitor, was started. Seven individuals were Caucasian and three were of African descent (eight male and two female) and mode of exposure to HIV-1 was sexual in all subjects studied.
This study was approved by the Massachusetts General Hospital and University of California at San Francisco Institutional Review Boards and all individuals gave informed consent for participation in the study.
HLA Typing.
HLA typing was performed at the Massachusetts General Hospital Tissue Typing Laboratory and at the Department of Human Genetics, Roche Molecular Systems (Alameda, CA) using sequence-specific primer (SSP)-PCR 38.
Viral Load Monitoring.
Plasma viral loads were measured using either the Roche Amplicor Monitor assay (detection limit of 400 HIV-1 RNA copies/ml plasma) or the Roche Ultradirect assay (detection limit of 50 HIV-1 RNA copies/ml plasma), according to the manufacturer's specifications.
Cell Lines and Media.
EBV-transformed B lymphoblastoid cell lines (B-LCLs) were established and maintained in R20 medium (RPMI 1640; Sigma-Aldrich) supplemented with 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 10 mM Hepes, and 20% heat-inactivated FCS; Sigma-Aldrich) as described 39. For culture of CTL clones, medium containing 10% FCS (R10) supplemented with 50 U/ml rIL-2 (provided by Dr. M. Gately, Hoffmann-La Roche, Nutley, NJ) was used.
Generation of CTL Clones.
CTL clones were isolated by limiting dilution and characterized and maintained as described 40 41 using the CD3-specific mAb 12F6 as stimulus for T cell proliferation. Developing clones were screened for HIV-1–specific CTL activity by 51Cr release assay 39 against autologous B cell lines pulsed with peptides recognized in the enzyme-linked immunospot (Elispot) assays. Fine mapping of the novel CD8+ T cell responses identified in the Elispot assay was achieved in a 51Cr release assay using truncations of the recognized 15–20-mer peptide as described 40.
Synthetic HIV-1 Peptides.
Peptides corresponding to described optimal HIV-1 CTL epitopes 42 were synthesized on an automated peptide synthesizer (model 432A; Applied Biosystems). In addition, a panel of 259 overlapping peptides, 15–20 amino acids in length and overlapping by 10 to 11 amino acids, spanning the entire p17 Gag, p24 Gag, gp41 Env, gp120 Env, RT, and Nef B-clade SF2 sequence, were used. These peptides were provided in part by the National Institute for Biological Standards and Control Centralized Facility for AIDS Reagents, supported by European Union Program EVA and the UK Medical Research Council.
Elispot Assay.
Fresh or frozen PBMCs were plated in 96-well polyvinylidene difluoride–backed plates (MAIP S45; Millipore) that had been coated previously with 100 μl of an anti–IFN-γ mAb 1-D1k (0.5 μg/ml; Mabtech) overnight at 4°C. Peptides were added directly to the wells at a final concentration of 10−5 M. Cells were added to the wells at 25,000–200,000 cells/well. The plates were incubated at 37°C, 5% CO2 overnight (14–16 h) and then processed as described 43 44. IFN-γ–producing cells were counted by direct visualization and are expressed as spot-forming cells (SFCs) per 106 cells. The number of specific IFN-γ–secreting T cells was calculated by subtracting the negative control value from the established SFC count. The negative controls were always <30 SFCs/106 input cells (median 5, range 0–25 SFCs/106 input cells). The positive control consisted of incubation of 100,000 PBMCs with phytohemagglutinin (PHA). CD8+ T cell dependence of all responses to synthetic peptides was confirmed by loss of IFN-γ production after CD8+ T cell depletion using magnetic beads (MACS; Miltenyi Biotech), according to the manufacturer's protocol.
HIV-1–specific T Helper Cell Assays.
Lymphocyte proliferation assays were performed with baculovirus-derived HIV-1 Gag protein 20. PBMCs were incubated with Gag protein (5 μg/ml) for 6 d and then pulsed with [3H]thymidine at 1.0 μCi/well for 6 h, as described 20. For the purposes of data interpretation, a stimulation index (SI) of 5 or greater was considered significant.
Heteroduplex Mobility Assay for the Evaluation of HIV-1 Genetic Heterogeneity.
Assessing quasispecies complexity requires the number of amplifiable templates in a specimen to be determined. This is especially important in individuals with low viral load where limited sampling of unique viral templates may lead to inaccurate low estimates of viral diversity 45. DNA was extracted from PBMCs from 37 of the 40 individuals (insufficient samples were available for 3 persons) using Isoquick DNA extraction kit (Orca Research). The proviral DNA encoding the C2-V5 region of env was amplified by a nested PCR amplification strategy using primer pairs ED31-BH2 in the first round and DR7-DR8 in the second round. Primer sequences for ED31, DR7, and DR8 have been described previously 31 45; primer BH2 is 7759-5′-CCTTGGTGGGTGCTACTCCTAATGGTTCA, where the number corresponds to the position in the HXB2 genome (sequence data available from GenBank/EMBL/DDBJ under accession no. K03455) of the 5′ nucleotide. The first and second round amplification conditions were 3 cycles of 95°C for 1 min, 55°C for 1 min, 72°C for 1 min, followed by 32 cycles of 94°C for 15 s, 55°C for 45 s, 72°C for 1 min, and a final extension at 72°C for 5 min. Three serial fivefold dilutions of PBMC DNA were amplified in triplicate by nested PCR and the results were used to estimate HIV-1 proviral copy number using the program Quality 46. Based on the presence of sufficient numbers of viral templates in PBMCs, seven patients in each group were selected for evaluation of viral heterogeneity by heteroduplex mobility assay (HMA). The first round PCR products were pooled from an average of 65 copies of viral template in each patient and amplified using second round primers. PBMC samples yielding <20 copies of proviral templates were not used in analysis. The 700-bp products from the second round were subjected to HMA 47 on a 5% polyacrylamide gel electrophoresed at 200 V for 3 h. The gels were stained with ethidium bromide and photographed on a UV transilluminator (GelDoc system; Bio-Rad Laboratories). Pixel intensity and the relative position of bands were quantitated using NIH Image v1.62 (http://rsb.info.nih.gov/nih-image). Estimates of viral diversity were made based on the intensity and relative migration of heteroduplexes. In brief, pixel intensities were multiplied by the relative migration at that position and summed over the entire lane to represent the average pairwise DNA distance.
Statistical Analysis.
Statistical analysis and graphical presentation was done using SigmaPlot 5.0 (SPSS Inc.). Results are given as mean ± SE or median with range. Statistical analysis of significance (P values) were based on a two-tailed t test.
Online Supplemental Data Section.
CTL epitopes and frequency of recognition at 0–12 mo of HAART in the individuals studied are shown in online supplemental Tables S1–S3. The differences in recognition of these CTL epitopes in the different groups studied is shown in online supplemental Tables S4 and S5. Online supplemental material is available at http://www.jem/org/cgi/content/full/193/2/169/DC1.
Results
Plasma Viral RNA Levels in the Subjects Studied.
The 30 subjects with primary HIV-1 infection were divided into two groups, according to the time between HIV-1 infection and initiation of antiretroviral treatment. Median HIV-1 RNA plasma load before initiation of therapy in the 19 individuals of the preseroconversion acute HIV-1 group was 7 × 106 copies/ml (range 0.25 × 106–95.5 × 106 copies/ml) and median CD4+ T cell counts were 395 cells/mm3 (range 42–1,023 cells/mm3). In the postseroconversion primary HIV group 37, median HIV-1 RNA plasma load before initiation of treatment was 105 copies/ml (range 500–8.3 × 105 copies/ml) and median CD4+ T cell counts were 544 cells/mm3 (range 314–981 cells/mm3).
Analysis of HIV-1–specific CTL Responses in Acute and Early HIV Infection.
To maximally detect CTL responses to potentially novel epitopes, PBMCs from all subjects were screened with 259 individual overlapping 15–20-mer peptides spanning the protein sequences of HIV-1 p17, p24, RT, gp41, gp120, and Nef, using an Elispot assay. In addition, a median of 19 (range 2–33) described optimal HIV-1 CTL epitope peptides per individual were tested for recognition, depending on the individual's HLA type 42. CD8+ T cell dependence of responses to overlapping peptides was confirmed by CD4+ and CD8+ cell depletion studies (data not shown). For each response to an overlapping peptide, the optimal CTL epitope contained within the 15–20 mer was characterized and used for the subsequent quantification of CTL responses. This approach, using overlapping peptides as well as described optimal CTL epitopes for the corresponding HLA class I type, is exemplified for one patient in Fig. 1A and Fig. B. Furthermore, peptide-specific CTL lines or clones were generated in order to confirm that the peptide-specific responses measured by Elispot assay were reflective of cytotoxic activity in a standard 51Cr release assay (Fig. 1C, Fig. D, and Fig. E).
Figure 1.
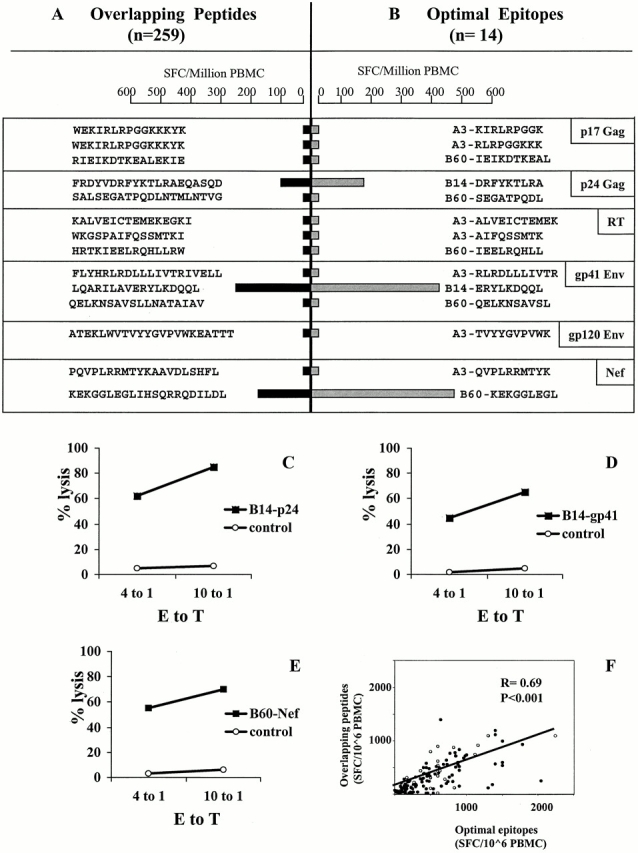
HIV-1–specific CTL responses determined by screening PBMCs in an Elispot assay using overlapping peptides (15–20 mer) spanning HIV-1 p17 (Gag), p24 (Gag), RT, gp41 (Env), gp120 (Env), and Nef sequences (A, black bars) and reported or subsequently defined optimal epitopes (B, gray bars). CTL magnitudes are expressed as SFCs/106 PBMCs. B shows all the previously reported and newly defined optimal CTL epitopes described for the HLA-type of the individual (AC05: HLA-A3/-, B14/60, Cw3/8). These epitopes are contained in the corresponding overlapping peptides in A. In C–E, the cytolytic activity of CTL clones isolated for the three epitopes recognized in this individual in p24 (Gag), gp41 (Env), and Nef is shown. Lytic activity is given as percentage of specific lysis at two different E/T ratios for autologous target cells either pulsed or not pulsed with the corresponding peptide. In F, the correlation between CTL responses to optimal HIV-1 CTL epitopes (9 to 10 mer) and corresponding overlapping peptides (15–20 mer) including the sequence of the optimal CTL epitope is shown. Each optimal epitope/overlapping peptide pair is represented by a single dot and frequencies are given as SFCs/106 PBMCs.
The data in Fig. 1A and Fig. B show CTL responses to the longer peptides and the subsequently defined optimal epitopes for subject AC01. For each of the three epitope-specific responses defined, the level of reactivity was greater than the response to the larger peptide containing the optimal epitope. To more comprehensively address this observation, we compared the magnitude of HIV-1–specific CTL responses against overlapping peptides (15–20 mer) directly to responses against the corresponding optimal HIV-1 CTL epitopes (8–10 mer) for all epitopes defined in all 30 subjects. A total of 62 different epitope-specific responses were detected, including 6 novel epitopes first described here (see below). All responses that were detectable with the optimal epitopes were also detected using the overlapping peptides containing the corresponding optimal sequence. Furthermore, the magnitude of responses to overlapping peptides correlated significantly (R = 0.7, P < 0.001) to the responses to the corresponding optimal epitopes (Fig. 1 F). However, responses were significantly higher when the optimal CTL epitope was used (median 440 SFCs/106 PBMCs, range 0–2,240) compared with the larger peptides (median 240 SFCs/106 PBMCs, range 0–1,400; P < 0.0001). None of the individuals recognized all epitopes tested for their HLA class I type. Taken together, these results show optimal peptides are more sensitive for detecting responses, but that the use of only previously described epitopes would lead to underestimation of CTL responses in HIV-1 infection, both in acute and early infection.
Longitudinal Changes of HIV-1–specific CTL Responses under HAART.
HIV-1–specific CTL responses in the individuals treated before seroconversion (group 1, preseroconversion acute HIV) and in the individuals treated within 180 d of HIV-1 infection (group 2, postseroconversion primary HIV) were analyzed longitudinally during the first year of treatment with HAART. Overall CTL frequencies, represented by the total reactivity against all reported and newly defined optimal HIV-1 peptides, are shown in Fig. 2A and Fig. B. The individual data from which these figures are derived are in online supplemental Tables S1 and S2. Pretreatment HIV-1–specific CTL responses were of lower magnitude in the individuals treated preseroconversion, compared with the individuals treated postseroconversion (210 ± 115 SFCs/106 PBMCs vs. 778 ± 1,021 SFCs/106 PBMCs, P < 0.05). Furthermore, fewer HIV-1 CTL epitopes were targeted at baseline in the preseroconversion group than in those identified postseroconversion (0.5 vs. 2.5 targeted, P = 0.06). In both groups, the magnitude of HIV-1–specific CTL responses increased significantly after initiating antiretroviral treatment from baseline to 2 mo after starting HAART (Fig. 2A and Fig. B). CTL frequencies subsequently declined over the following 10 mo of treatment. The significant differences between pre- and postseroconversion group in the magnitude of CTL responses observed at baseline were lost after initiation of antiretroviral treatment. At 12 mo of HAART, the mean CTL responses were comparable in both groups (518 ± 129 SFCs/106 PBMCs in the preseroconversion group and 600 ± 203 SFCs/106 PBMCs in the postseroconversion group; P = 0.73).
Figure 2.
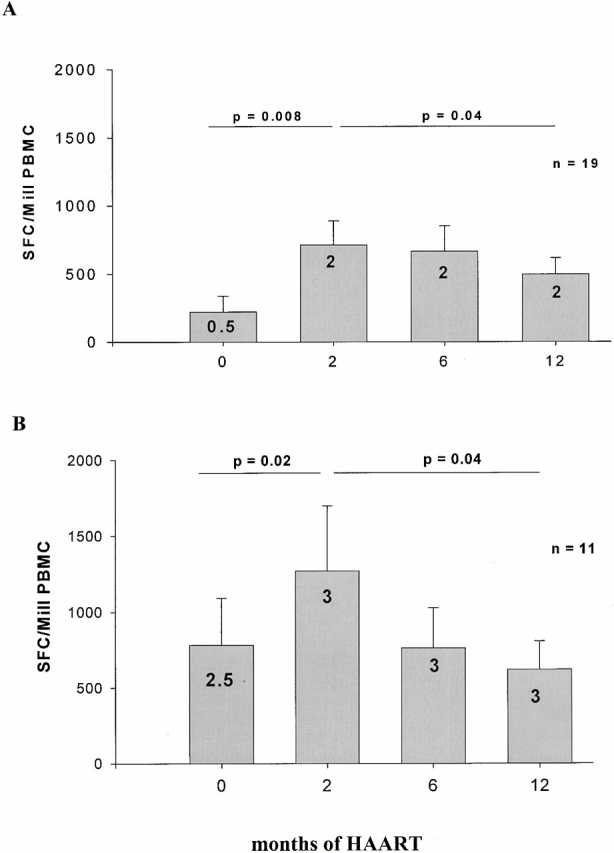
(A) Longitudinal CTL frequencies in persons with acute HIV-1 infection. Mean total CTL frequencies directed against optimal CTL epitopes and standard error for the 19 subjects treated before HIV-1 seroconversion (group 1, preseroconversion acute HIV) during acute HIV-1 infection before treatment (0 mo) and after 2, 6, and 12 mo of treatment with HAART are shown as SFCs/106 (Mill) PBMCs. The median number of optimal HIV-1 CTL epitopes recognized is indicated by the number within the bar. Significance of differences between mean total CTL frequencies were calculated by two-tailed paired t test and only P values < 0.05 are shown. (B) Longitudinal CTL frequencies in persons with early HIV-1 infection. Mean total CTL frequencies directed against optimal CTL epitopes and standard error for the 11 subjects treated after HIV-1 seroconversion, but within 180 d of infection (group 2, postseroconversion primary HIV) before treatment (0 mo) and after 2, 6, and 12 mo of treatment with HAART are shown as SFCs/106 PBMCs. The median number of optimal HIV-1 CTL epitopes recognized is indicated by the figure within the bar. Significance of differences between mean total CTL frequencies were calculated by two-tailed paired t test and only P values < 0.05 are shown.
In parallel to the increase in CTL magnitude in the preseroconversion acute HIV-1 group after the initiation of antiretroviral treatment, the number of recognized optimal HIV-1 CTL epitopes increased from a median of 0.5 epitopes per person recognized at baseline (range 0–6) to a median of 2 epitopes per person at 2 mo (range 0-7, P = 0.005). Overall, the total number of CTL epitopes targeted more than doubled from 20 epitopes pretreatment to 44 epitopes at 2 mo, and no established responses were lost (see online supplemental Tables S1 and S2 for individual responses). No further broadening of CTL responses was observed after the 2-mo time point in these persistently treated individuals. In the individuals treated after seroconversion, but within 180 d of infection, this increase in the number of CTL epitopes was less pronounced, augmenting from a median of 2.5 CTL epitopes (total 23 epitopes, range 0–5 per person) to a median of 3 epitopes (total 35 epitopes, range 0–7 per person, P = 0.08) in the 11 subjects studied (Fig. 2 B). Again, none of the established CTL responses against HIV-1–specific epitopes was lost during the observation period of 12 mo (online supplemental Tables S1 and S2).
Broadening of Virus-specific CTL Responses with Prolonged Exposure to HIV-1.
To compare the HIV-1–specific CTL responses in subjects treated during early infection to CTL responses in individuals treated during chronic infection, the breadth and magnitude of CTL responses were analyzed using the same comprehensive approach in 10 chronically infected individuals, who were effectively treated for at least 7 mo with HAART (group 3, online supplemental Table S3). The mean HIV-1–specific CTL response at this time point in subjects treated during chronic infection (2458 ± 521 SFCs/106 PBMCs) was significantly higher than those seen at 6 or 12 mo of treatment in group 1 (P = 0.01 and 0.0002) or group 2 (P = 0.07 and 0.02), respectively. Furthermore, CTLs in group 3 targeted a significantly greater number of HIV-1–specific CTL epitopes than the group 1 individuals receiving immediate treatment of acute infection (median 5.5 epitopes, range 0–13 vs. median 2 epitopes, range 0–7; P = 0.03; Fig. 3 A). The median breadth of CTL responses in the group 2 individuals treated within 180 d of infection was three epitopes (range 0–7) and was not significantly different compared with individuals treated during acute or chronic infection (P = 0.25 and 0.1, respectively). Taken together, these data are consistent with a broadening of virus-specific CTL responses with prolonged exposure to presumably more diversified HIV-1 antigens.
Figure 3.
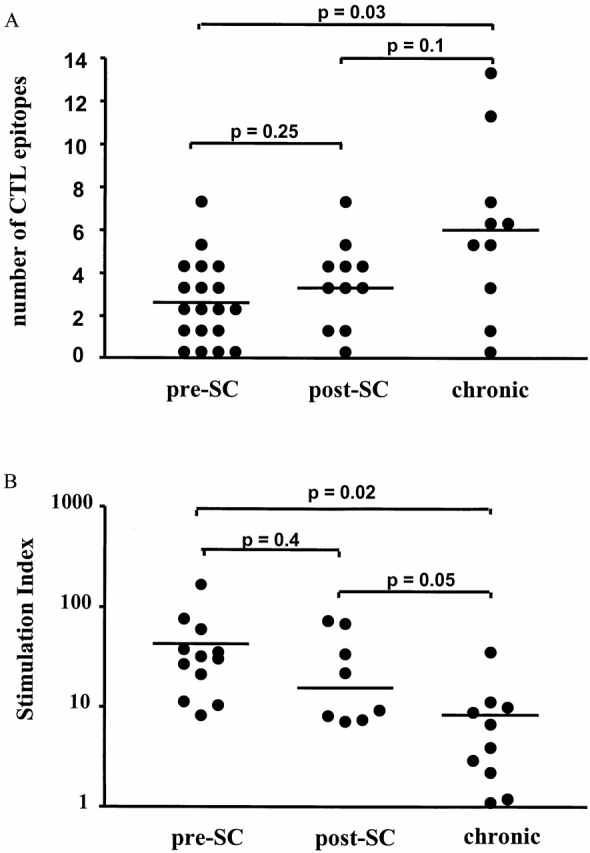
(A) Breadth of CTL responses in HIV infection: the number of optimal HIV-1–specific CTL epitopes recognized in each individual studied after 1 yr of treatment with HAART is shown as a single dot. Breadth of CTL responses was compared between the persons with treated acute HIV-1 infection (preseroconversion [pre-SC]; n = 19), the persons treated postseroconversion (post-SC), but within 180 d of infection (n = 11), and the persons first treated in the chronic phase of infection (chronic; n = 10). A horizontal line indicates median number of recognized CTL epitopes for each group, and significance of differences between the groups was calculated by two-tailed t test. (B) T helper cell responses in HIV-1 infection: HIV-1 Gag-specific T helper cell responses were assessed among persons with treated acute HIV-1 infection (preseroconversion; n = 19) after 1 yr on therapy. These were compared with HIV-1 Gag-specific T helper cell responses in persons treated postseroconversion, but within 180 d of infection (n = 11) and persons first treated in the chronic phase of infection (n = 10). A horizontal line indicates the mean SI for each group and significance of differences between the groups was calculated by two-tailed t test.
Lack of HIV-1–specific T Helper Cells despite Persistent CTL Responses in Chronic HIV-1 Infection.
Several studies have shown that HIV-1–specific T helper cell responses play an important role in the effectiveness and maintenance of HIV-1–specific CTL responses (for reviews, see references 1 and 22). To address the influence of the time of initiation of antiretroviral therapy on HIV-1–specific T helper cell responses, Gag-specific T helper cell responses were quantified in a standard lymphoproliferation assay after 12 mo of treatment in the two primary infection groups and the chronically infected group (Fig. 3 B). Samples of 12/19 individuals from the preseroconversion acute HIV group, 8/11 samples from the postseroconversion primary HIV group, and 10/10 samples from the group of individuals with chronic HIV-1 infection were available for analysis (Fig. 3 B). The T helper cell responses of most individuals treated before seroconversion and two individuals treated postseroconversion (AC10 and AC14) have been described in part elsewhere 48.
Gag-specific T helper cell responses were undetectable or of low magnitude in the individuals treated during chronic HIV-1 infection (SI: 8 ± 3; Fig. 3 B). In contrast, significantly stronger T helper cell responses to Gag were generated by the individuals treated during acute HIV-1 infection (SI: 43 ± 13, P = 0.02) or individuals treated within 180 d of HIV-1 infection (SI: 29 ± 10, P = 0.05). The differences in Gag-specific T helper cell responses between the two groups of primary infected subjects did not reach statistical significance (P = 0.4). The lack of detectable responses in chronically infected subjects is in line with the previously reported lack of detectable HIV-1–specific T helper cell responses in persons with chronic progressive HIV-1 infection using this assay 20 23 24. Overall, these data confirm previous reports demonstrating the generation of strong HIV-1–specific T helper cell responses in individuals treated during acute HIV-1 infection 20, and show that this immune response can be achieved in both acute and early infection.
Lack of Viral Diversification in Individuals Treated during Acute HIV-1 Infection.
The data on the cellular immune responses directed against HIV-1 described above show that early initiation of antiretroviral treatment, before HIV-1 seroconversion, leads to the generation of strong Gag-specific T helper cell responses, which otherwise are typically absent or low in infected persons. In contrast to T helper cells, HIV-1–specific CTL responses are weaker and more narrowly directed in the early treated subjects compared with persons treated in chronic infection. The effect of early or late treatment of HIV-1 infection on the diversity of the virus was therefore addressed, using the HMA focused on the env C2-V5 region 47.
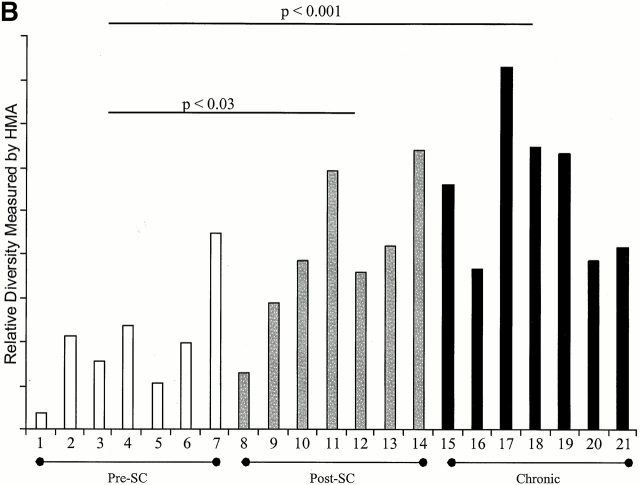
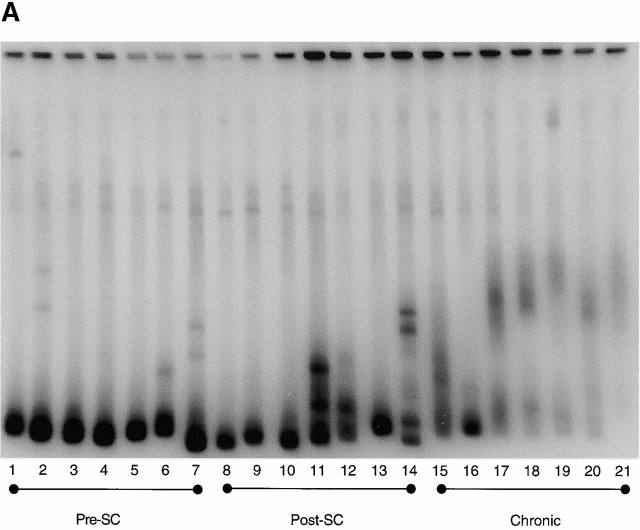
After effective treatment with HAART for 6–12 mo, samples of 37 from the 40 individuals were analyzed, and in 21 individuals (7 from each group) sufficient amounts of proviral DNA were isolated to perform heteroduplex analysis. HIV-1 envelope genes had very low levels of diversity in six of the seven individuals treated before HIV-1 seroconversion, all of whom were highly adherent to medication regimens (relative diversity [RD] = 2.7 ± 0.3; Fig. 4). The seventh individual in this group (AC22) had a higher level diversity (RD = 3.4), associated with poor adherence to his antiretroviral drug regimen, and several viral rebounds during the first year of treatment. However, HIV-1–specific CTL responses did not increase in magnitude or breadth during or after the period of poor adherence in this single individual (online supplemental Table S1). In contrast, the individuals treated within 180 d of HIV-1 infection or during chronic infection showed significantly higher diversity in the env gene of the virus (RD = 3.3 ± 0.5, P < 0.03 and 3.7 ± 0.5, P < 0.001, respectively; Fig. 4). Differences in viral diversity between the two latter groups did not reach statistical significance (P = 0.14). Taken together these data indicate that the generation of genetic variants of HIV-1 may be reduced by the very early initiation of antiretroviral treatment with effective suppression of viral replication.
Figure 4.
Viral diversification using the HMA focused on the env C2-V5 region: diversification of the HIV-1 env C2-V5 region is shown for persons with treated acute HIV-1 infection (preseroconversion [pre-SC]), persons treated postseroconversion, but within 180 d of infection (post-SC) and persons first treated in the chronic phase of infection (chronic) in A. B shows the RD measured by HMA for the seven individuals of each group, for whom sufficient amounts of proviral DNA was isolated to perform analysis (1, AC16; 2, AC01; 3, AC03, 4, AC04, 5, AC09; 6, AC13; 7, AC22; 8, AC14; 9, AC10; 10, AC25; 11, AC21; 12, AC29; 13, OP286; 14, OP314; 15, 6001; 16, 6002; 17, 6003; 18, 6006; 19, 6007; 20, 6009; and 21, 6010).
The Breadth of HIV-1–specific CTL Responses Is Inversely Correlated to Viral Load during Acute Infection.
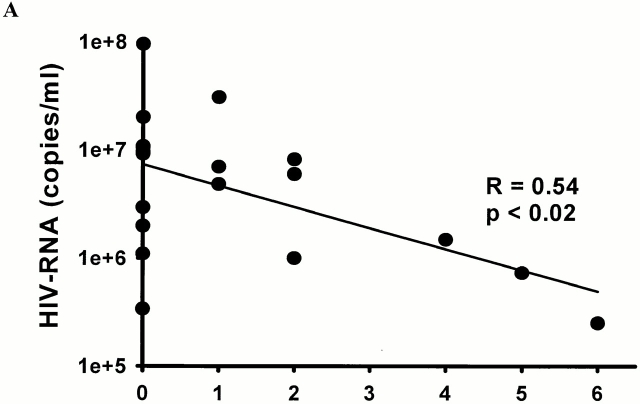
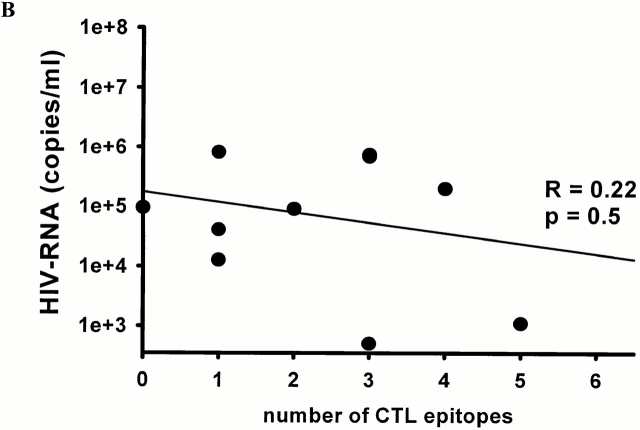
The temporal association of the initial decline in viremia during acute HIV-1 infection and the first development of virus-specific CTL responses has been described in several studies 4 5. To investigate whether the breadth of CTL responses was associated with early viral load, the epitope-specific CTL responses in individuals with primary HIV-1 infection were compared with viral load before initiation of antiretroviral treatment. The breadth of the CTL responses was inversely associated with the viral load at presentation in the subjects treated before HIV-1 seroconversion (R = 0.54, P < 0.02; Fig. 5 A). In contrast, no significant correlation between viral load and breadth of CTL responses at the time of presentation was observed in the subjects who were treated after seroconversion, within the first 180 d of infection (R = 0.22, P = 0.5; Fig. 5 B). Furthermore, we observed no correlation between CTL magnitude and plasma HIV-1 RNA levels, CD4+ T cell counts or HIV-1–specific T helper cell responses at 12 mo of treatment in either group. These data indicate a negative correlation between breadth of CTL responses and viral load in the earliest phase of HIV-1 infection during the initial reduction of HIV-1 viremia.
Figure 5.
Correlation between baseline HIV-1 RNA load (copies per ml plasma) before the initiation of treatment and the number of optimal HIV-1 CTL epitopes recognized for persons with treated acute HIV-1 infection (A, n = 19) and persons treated postseroconversion, but within 180 d of infection (B, n = 11).
Qualitative Differences in HIV-1–specific CTL Responses during Acute and Chronic Infection.
After assessing quantitative differences in CTL responses to HIV-1 depending on the time between infection and initiation of antiretroviral treatment, differences in the CTL-recognition of specific regions of HIV-1 were addressed. Table shows the percentage of individuals with CTL responses directed against different regions of HIV-1. Independent of the time point of initiation of HAART, HIV-1–Gag was preferentially targeted by HIV-1–specific CTLs, followed by HIV-1–Env and HIV-1–Nef. HIV-1–RT was recognized by CTLs in only 17–20% of individuals with acute or early infection, but was as frequently recognized as HIV-1–Env (40%) in the individuals treated during chronic infection.
Table 1.
Percentage of Individuals with CTL Responses against Specific HIV-1 Proteins
| p17 | p24 | Gag | RT | gp41 | gp120 | Env | Nef | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre-SC | Baseline | 22 | 11 | 33 | 17 | 17 | 11 | 28 | 17 |
| 12 mo | 33 | 33 | 61 | 16 | 28 | 17 | 39 | 56 | |
| Post-SC | Baseline | 40 | 50 | 70 | 0 | 40 | 20 | 50 | 40 |
| 12 mo | 50 | 60 | 80 | 20 | 40 | 30 | 50 | 40 | |
| Chronic | 12 mo | 50 | 70 | 80 | 40 | 20 | 30 | 40 | 50 |
Overall percentages for the entire protein are indicated in bold. Pre-SC, preseroconversion; post-SC, postseroconversion.
Discussion
Accumulating data indicate that the antiviral immune activity generated in acute infection may be critical for determining the ultimate progression of disease 49 50 51. A better understanding of the dynamic interactions between HIV-1 and the human immune system during acute infection is therefore crucial for defining the role of host defense mechanisms in controlling viremia. To date the analysis of HIV-1–specific immune responses has been dominated by studies in chronic infection. In this study, epitope-specific CTL responses against HIV-1 were prospectively analyzed in individuals identified during acute and early HIV-1 infection and compared after treatment to responses in treated chronically infected individuals. Furthermore, HIV-1–specific T helper cell responses and the diversity of the HIV-1–env gene were also compared between these different cohorts after treatment with HAART.
An increase of HIV-1–specific CTL responses was observed with longer exposure to viral antigen. In individuals treated during acute HIV-1 infection before seroconversion virus–specific CTL responses were weaker and more narrowly directed against fewer epitopes than in individuals treated during chronic HIV-1 infection. These data indicate that the very early initiation of effective antiretroviral treatment before seroconversion, when HIV-1–specific CTL responses were still developing, may have inhibited a further broadening of CTL responses. This finding is further supported by preliminary longitudinal data on HIV-1–specific CTL responses in individuals who were not treated during acute infection. These individuals experienced an increase in breadth and magnitude of CTL responses over the first 6 mo of follow up (Altfeld, M., unpublished data). Our findings of weak CTL responses in acute HIV-1 infection are consistent with more limited cross-sectional data of Dalod et al., in which the breadth of responses was less extensively characterized 52. Using overlapping peptides spanning p17 (Gag), p24 (Gag), RT, gp41 (Env), gp120 (Env), and Nef in this study along with an extensive set of optimal epitopes, detailed longitudinal assessment of both breadth and magnitude of CTL responses during acute HIV-1 infection could be defined. This approach allowed the identification of 6 novel CTL epitopes, representing almost 10% of the total of 62 different CTL epitopes that were recognized by the 40 individuals studied. Responses to these novel CTL epitopes contributed importantly to the breadth of CTL responses in 6 of the 30 individuals with acute and early HIV-1 infection, representing 13–100% (median 47%) of the recognized CTL epitopes in these subjects. These data indicate that CTL responses in acute and early HIV-1 infection would have been underestimated with the use of reported optimal epitopes alone.
The comprehensive analysis of CTL epitopes also allowed us to identify differences in epitopes targeted in early and later stages of HIV infection, and the effects of HAART on these responses (summarized in online supplemental Tables S4 and S5). Only 1 of the 10 HLA-A2–positive subjects with acute infection recognized the p17 epitope SLYNTVATL (10%), in contrast to 2/6 individuals (33%) treated within 180 d of infection and 2/4 individuals (50%) treated during chronic infection (summarized in online supplemental Tables S4 and S5). The low frequency of recognition of this epitope by individuals expressing HLA-A2 during acute HIV-1 infection is in striking contrast to the higher frequency of recognition seen in the limited number of chronically infected individuals included in this study, as well as to data derived from published studies in chronically HIV-1–infected subjects, in whom SLYNTVATL is targeted in ∼70% 10 53 54 55. The response to this epitope may only develop after a longer period of exposure to the antigen, which has been shortened in this study by the early initiation of HAART 56. A similar pattern of increased recognition of CTL epitopes in later treated individuals was observed for the other HLA-A2–restricted epitopes, the HLA-B44–restricted epitope in gp120, and the HLA-B8–restricted epitopes in p17 and RT (online supplemental Tables S4 and S5). In contrast, the HLA-A3–restricted epitopes as well as the HLA-B8–restricted CTL epitopes in p24 and Nef were frequently recognized in individuals with acute HIV-1 infection (online supplemental Tables S4 and S5). The differences in frequency of recognition of specific CTL epitopes in acute compared with chronic HIV-1 infection indicate that certain epitopes are more immunogenic and induce CTL responses more readily and earlier after viral infection. These findings therefore have important implications for prophylactic and therapeutic vaccine design and testing and also for immunotherapy of treated primary infection.
These longitudinal studies also allowed for analysis of the effects of HAART on CTL activity in acute and early HIV-1 infection. We observed an increase in the number of subjects with detectable HIV-1–specific CTL responses after initiation of HAART that was also associated with an increase in magnitude and breadth of preexisting CTL responses. The increase was more pronounced in individuals treated before seroconversion, in whom CTL magnitude more than doubled after initiation of HAART. The initial increase in CTL frequencies after initiation of HAART may be due to different factors. The drop in viremia after effective antiretroviral treatment may lead to redistribution of T cells from the lymphoid tissue to the peripheral circulation 57 58 allowing for the detection of higher HIV-1–specific CTL responses in peripheral blood. Furthermore, it required several weeks of effective treatment for viral loads to decline below levels of detection, potentially providing an antigenic stimulus for the development of new CTL responses during this period. In contrast, once viral load was suppressed below the limit of detection (<50 copies HIV-1 RNA per ml plasma), virus-specific CTL responses started to decline in both groups with primary infection. This study did not allow for the analysis of virus-specific CTL responses in chronically infected individuals after initiation of HAART. However, a decline of CTL responses has been described in chronically infected patients after initiation of antiretroviral therapy 59 60, suggesting that the decline in HIV-1–specific CTL responses is a general phenomenon, independent of the time antiretroviral treatment is initiated. Further longitudinal comparative studies will be required to determine if the rate of decline is similar in these groups.
CD8+ T cell activity against HIV-1 in early infection has been reported previously in several studies, indicating that CTLs are important in the initial drop in viremia. The more detailed studies presented here, which include an assessment of the specific epitopes targeted over time, show that during acute HIV-1 infection, before HIV-1 seroconversion, broader HIV-1–specific CTL responses were associated with lower viral loads, suggesting that these responses are functionally relevant. In contrast, this inverse correlation between viral load and breadth of CTL responses was lost in individuals treated later during infection. Several factors may be contributing to this observation. It has been described that HIV-1 escapes from CTL-mediated immune pressure early after primary infection by the generation of escape variants 25 26 27 28 29. The general variability of HIV-1 can be addressed by analyzing the relative diversity of HIV-1–Env by the HMA 47. Using this assay, it was shown that the viral population is typically homogenous in individuals treated before HIV-1 seroconversion, but significantly greater diversity occurs in individuals treated after HIV-1 seroconversion, even within the first 180 d after infection.
In conclusion, HIV-1–specific CTL responses are relatively modest and narrowly directed in individuals with acute HIV-1 infection treated before HIV-1 seroconversion, but early treatment allows for the generation of strong HIV-1–specific T helper cell responses and the conservation of a very homogeneous virus population. In these individuals, an inverse correlation between breadth of CTL responses and viral load was observed. In contrast, virus-specific T cell help is lost and the virus population is highly diversified in individuals treated later in HIV-1 infection. In these individuals, no inverse correlation between CTL and viral load was observed even with overall stronger CTL responses, suggesting a complex interaction between viral diversity, virus-specific T helper cells and CTLs in HIV-1 infection. These findings provide new insights in the dynamic changes during acute HIV-1 infection and suggest that patients treated early versus those treated later in infection suffer from distinct deficiencies. Individuals treated in early infection may benefit from strategies aimed at enhancing virus-specific CTL responses, whereas individuals treated later in infection may benefit from strategies aimed at enhancing HIV-1–specific T helper cell functions as well as broadening CTL responses to combat increased viral diversity.
Acknowledgments
We thank Barbara Wilkes, Hong Zhao, and Alicja Trocha for expert technical assistance.
The Doris Duke Charitable Foundation, the National Institutes of Health (R37 AI28568, RO1 AI30914, R01 AI44656, R01 AI40873, U01 AI41535, and U01 AI41531), the Deutscher Akademischer Austauschdienst, the Deutsche Forschungsgemeinschaft, the Lloyd Foundation, and several private donors supported this research. B.D. Walker is the recipient of a Doris Duke Distinguished Clinical Scientist Award, and P.J.R. Goulder is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
Footnotes
Abbreviations used in this paper: Elispot, enzyme-linked immunospot; HAART, highly active antiretroviral therapy; HMA, heteroduplex mobility assay; RD, relative diversity; RT, reverse transcriptase; SFC, spot-forming cell; SI, stimulation index.
The online version of this article contains supplemental material.
R. Shankarappa and J.S. Mukherjee contributed equally to this work.
References
- Altfeld M., Rosenberg E.S. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 2000;12:375–380. doi: 10.1016/s0952-7915(00)00103-5. [DOI] [PubMed] [Google Scholar]
- Brander C., Walker B.D. T lymphocyte responses in HIV-1 infectionimplications for vaccine development. Curr. Opin. Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- Goulder P.J.R. Anti-HIV cellular immunityrecent advances towards vaccine design. AIDS. 1999;13:S121–S136. [PubMed] [Google Scholar]
- Borrow P., Lewicki H., Hahn B.H., Shaw G.M., Oldstone M.B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R.A., Safrit J.T., Cao Y., Andrews C.A., McLeod G., Borkowsky W., Farthing C., Ho D.D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Demarest J.F., Soudeyns H., Graziosi C., Denis F., Adelsberger J.W., Borrow P., Saag M.S., Shaw G.M., Sekaly R.P. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- Yang O.O., Kalams S.A., Trocha A., Cao H., Luster A., Johnson R.P., Walker B.D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cellsevidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer T., Harrer E., Kalams S.A., Elbeik T., Staprans S.I., Feinberg M.B., Cao Y., Ho D.D., Yilma T., Caliendo A.M. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retrovir. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- Harrer T., Harrer E., Kalams S.A., Barbosa P., Trocha A., Johnson R.P., Elbeik T., Feinberg M.B., Buchbinder S.P., Walker B.D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J. Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- Ogg G.S., Jin X., Bonhoeffer S., Dunbar P.R., Nowak M.A., Monard S., Segal J.P., Cao Y., Rowland-Jones S.L., Cerundolo V. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Klein M.R., van Baalen C.A., Holwerda A.M., Kerkhof Garde S.R., Bende R.J., Keet I.P., Eeftinck-Schattenkerk J.K., Osterhaus A.D., Schuitemaker H., Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infectiona longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C., Huang X.L., Fan Z.F., Ding M., Beltz L., Logar A., Panicali D., Mazzara G., Liebmann J., Cottrill M. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael A., Jin X., Sissons P., Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)–specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infectiondifferential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Bauer D.E., Tuttleton S.E., Lewin S., Gettie A., Blanchard J., Irwin C.E., Safrit J.T., Mittler J., Weinberger L. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J.E., Kuroda M.J., Santra S., Sasseville V.G., Simon M.A., Lifton M.A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B.J. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Castro B.A., Nepomuceno M., Lerche N.W., Eichberg J.W., Levy J.A. Persistent infection of baboons and rhesus monkeys with different strains of HIV-2. Virology. 1991;184:219–226. doi: 10.1016/0042-6822(91)90838-3. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Concepcion R.J., Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay M., Moskophidis D., Rahemtulla A., Hengartner H., Mak T.W., Zinkernagel R.M. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrath M.G., Yokoyama M., Dockter J., Oldstone M.B., Whitton J.L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Billingsley J.M., Caliendo A.M., Boswell S.L., Sax P.E., Kalams S.A., Walker B.D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- Kalams S.A., Buchbinder S.P., Rosenberg E.S., Billingsley J.M., Colbert D.S., Jones N.G., Shea A.K., Trocha A.K., Walker B.D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams S.A., Walker B.D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A., Price D.A., Easterbrook P.J., O'Callaghan C.A., Kelleher A.D., Whelan J.A., Sontag G., Sewell A.K., Phillips R.E. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musey L.K., Krieger J.N., Hughes J.P., Schacker T.W., Corey L., McElrath M.J. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J. Infect. Dis. 1999;180:278–284. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- Goulder P.J., Phillips R.E., Colbert R.A., McAdam S., Ogg G., Nowak M.A., Giangrande P., Luzzi G., Morgan B., Edwards A. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- Price D.A., Goulder P.J., Klenerman P., Sewell A.K., Easterbrook P.J., Troop M., Bangham C.R., Phillips R.E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Lewicki H., Wei X., Horwitz M.S., Peffer N., Meyers H., Nelson J.A., Gairin J.E., Hahn B.H., Oldstone M.B., Shaw G.M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Evans D.T., O'Connor D.H., Jing P., Dzuris J.L., Sidney J., da Silva J., Allen T.M., Horton H., Venham J.E., Rudersdorf R.A. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- Goulder P.J., Walker B.D. The great escape—AIDS viruses and immune control. Nat. Med. 1999;5:1233–1235. doi: 10.1038/15184. [DOI] [PubMed] [Google Scholar]
- Delwart E.L., Sheppard H.W., Walker B.D., Goudsmit J., Mullins J.I. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J. Virol. 1994;68:6672–6683. doi: 10.1128/jvi.68.10.6672-6683.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E.L., Pan H., Sheppard H.W., Wolpert D., Neumann A.U., Korber B., Mullins J.I. Slower evolution of human immunodeficiency virus type 1 quasispecies during progression to AIDS. J. Virol. 1997;71:7498–7508. doi: 10.1128/jvi.71.10.7498-7508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashov V.V., Kuiken C.L., Goudsmit J. Intrahost human immunodeficiency virus type 1 evolution is related to length of the immunocompetent period. J. Virol. 1995;69:6911–6916. doi: 10.1128/jvi.69.11.6911-6916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M.A., Anderson R.M., Boerlijst M.C., Bonhoeffer S., May R.M., McMichael A.J. HIV-1 evolution and disease progression. Science. 1996;274:1008–1011. doi: 10.1126/science.274.5289.1008. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J., Rosenberg E., Lieberman J., Jessen H., Lopalco L., Siliciano R., Walker B., Lori F. Control of HIV despite the discontinuation of antiretroviral therapy. N. Engl. J. Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- Shankarappa R., Margolick J.B., Gange S.J., Rodrigo A.G., Upchurch D., Farzadegan H., Gupta P., Rinaldo C.R., Learn G.H., He X. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P., Shimoda A., Coiana A., Diaz G., Peddis G., Melpolder J.C., Strazzera A., Chien D.Y., Munoz S.J., Balestrieri A. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- Janssen R.S., Satten G.A., Stramer S.L., Rawal B.D., O'Brien T.R., Weiblen B.J., Hecht F.M., Jack N., Cleghorn F.R., Kahn J.O. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA (J. Am. Med. Assoc.). 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- Bunce M., Fanning G.C., Welsh K.I. Comprehensive, serologically equivalent DNA typing for HLA-B by PCR using sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;45:81–90. doi: 10.1111/j.1399-0039.1995.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Walker B.D., Chakrabarti S., Moss B., Paradis T.J., Flynn T., Durno A.G., Blumberg R.S., Kaplan J.C., Hirsch M.S., Schooley R.T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker B.D., Flexner C., Birch-Limberger K., Fisher L., Paradis T.J., Aldovini A., Young R., Moss B., Schooley R.T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.P., Trocha A., Yang L., Mazzara G.P., Panicali D.L., Buchanan T.M., Walker B.D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J. Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- Brander C., Goulder P.J.R. Recent advances in HIV-1 CTL epitope characterization. In: Korber B.T.M., Brander C., Walker B.D., Koup R.A., Moore J., Haynes B., Meyer G., editors. HIV Molecular Database. Los Alamos National Laboratory; Los Alamos, NM: 1999. [Google Scholar]
- Altfeld M.A., Trocha A., Eldridge R.L., Rosenberg E.S., Phillips M.N., Addo M.M., Sekaly R.P., Kalams S.A., Burchett S.A., McIntosh K. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopesrapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 2000;74:8541–8549. doi: 10.1128/jvi.74.18.8541-8549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder P.J., Brander C., Annamalai K., Mngqundaniso N., Govender U., Tang Y., He S., Hartman K.E., O'Callaghan C.A., Ogg G.S. Differential narrow focusing of immunodominant human immunodeficiency virus gag-specific cytotoxic T-lymphocyte responses in infected african and caucasoid adults and children. J. Virol. 2000;74:5679–5690. doi: 10.1128/jvi.74.12.5679-5690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.L., Rodrigo A.G., Shankarappa R., Learn G.H., Hsu L., Davidov O., Zhao L.P., Mullins J.I. HIV quasispecies and resampling. Science. 1996;273:415–416. doi: 10.1126/science.273.5274.415. [DOI] [PubMed] [Google Scholar]
- Rodrigo A.G., Goracke P.C., Rowhanian K., Mullins J.I. Quantitation of target molecules from polymerase chain reaction-based limiting dilution assays. AIDS Res. Hum. Retrovir. 1997;13:737–742. doi: 10.1089/aid.1997.13.737. [DOI] [PubMed] [Google Scholar]
- Delwart E.L., Shpaer E.G., Louwagie J., McCutchan F.E., Grez M., Rubsamen-Waigmann H., Mullins J.I. Genetic relationships determined by a DNA heteroduplex mobility assayanalysis of HIV-1 env genes. Science. 1993;262:1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Rosenberg E.S., Altfeld M., Poon S.H., Phillips M.N., Wilkes B.M., Eldridge R.L., Robbins G.K., D'Aquila R.T., Goulder P.J., Walker B.D. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- Watson A., McClure J., Ranchalis J., Scheibel M., Schmidt A., Kennedy B., Morton W.R., Haigwood N.L., Hu S.L. Early postinfection antiviral treatment reduces viral load and prevents CD4+ cell decline in HIV type 2-infected macaques. AIDS Res. Hum. Retrovir. 1997;13:1375–1381. doi: 10.1089/aid.1997.13.1375. [DOI] [PubMed] [Google Scholar]
- Cooper S., Erickson A.L., Adams E.J., Kansopon J., Weiner A.J., Chien D.Y., Houghton M., Parham P., Walker C.M. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- Lechner F., Wong D.K., Dunbar P.R., Chapman R., Chung R.T., Dohrenwend P., Robbins G., Phillips R., Klenerman P., Walker B.D. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M., Dupuis M., Deschemin J.C., Goujard C., Deveau C., Meyer L., Ngo N., Rouzioux C., Guillet J.G., Delfraissy J.F. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J. Clin. Invest. 1999;104:1431–1439. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder P.J., Sewell A.K., Lalloo D.G., Price D.A., Whelan J.A., Evans J., Taylor G.P., Luzzi G., Giangrande P., Phillips R.E., McMichael A.J. Patterns of immunodominance in HIV-1–specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J. Exp. Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander C., Hartman K.E., Trocha A.K., Jones N.G., Johnson R.P., Korber B., Wentworth P., Buchbinder S.P., Wolinsky S., Walker B.D., Kalams S.A. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J. Clin. Invest. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C.M., Lawrence J., Schapiro J.M., Altman J.D., Winters M.A., Crompton M., Loi M., Kundu S.K., Davis M.M., Merigan T.C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J. Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- Goulder, P.J.R., M.A. Altfeld, E.S. Rosenberg, T. Nguyen, Y. Tang, R.L. Eldridge, M.M. Addo, S. He, J.S. Mukherjee, M.N. Phillips, et al. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. 193:181–193. [DOI] [PMC free article] [PubMed]
- Pakker N.G., Notermans D.W., de Boer R.J., Roos M.T., de Wolf F., Hill A., Leonard J.M., Danner S.A., Miedema F., Schellekens P.T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infectiona composite of redistribution and proliferation. Nat. Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- Bucy R.P., Hockett R.D., Derdeyn C.A., Saag M.S., Squires K., Sillers M., Mitsuyasu R.T., Kilby J.M. Initial increase in blood CD4+ lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J. Clin. Invest. 1999;103:1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg G.S., Jin X., Bonhoeffer S., Moss P., Nowak M.A., Monard S., Segal J.P., Cao Y., Rowland-Jones S.L., Hurley A. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams S.A., Goulder P.J., Shea A.K., Jones N.G., Trocha A.K., Ogg G.S., Walker B.D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J. Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]