Abstract
The cysteine proteases known as caspases play a central role in most apoptotic pathways. Here, we show that caspase inhibitors arrest the maturation of human erythroid progenitors at early stages of differentiation, before nucleus and chromatin condensation. Effector caspases such as caspase-3 are transiently activated through the mitochondrial pathway during erythroblast differentiation and cleave proteins involved in nucleus integrity (lamin B) and chromatin condensation (acinus) without inducing cell death and cleavage of GATA-1. These observations indicate a new function for caspases as key proteases in the process of erythroid differentiation.
Keywords: apoptosis, erythropoiesis, mitochondria, acinus, lamin B
Introduction
The production of red blood cells follows the sequential formation, from erythroid progenitors, of proerythroblasts—basophilic, polychromatophilic, and orthochromatic erythroblasts. Then, orthochromatic erythroblasts undergo terminal maturation leading to enucleation 1. The glycoprotein hormone erythropoietin (Epo) positively regulates erythropoiesis by preventing apoptosis and stimulating differentiation and proliferation of erythroid progenitors and erythroblasts 2. Epo protects cells from apoptosis by upregulating the expression of the antiapoptotic protein Bcl-xL 3 4. Conversely, Epo deprivation results in caspase activation and apoptosis 5. The transcription factor GATA-1 is required for Epo-mediated upregulation of Bcl-xL 6 and expression of erythroid genes involved in erythroid differentiation 7. In the absence of GATA-1, the differentiation process is arrested at the basophilic erythroblast stage, and cells die by apoptosis. A negative feedback loop was recently described in which the ligand of Fas (APO-1/CD95) expressed by mature erythroblasts activated Fas-mediated cell death of immature erythroblasts 8. Fas ligation results in the activation of caspase-8, then caspase-3, and the cleavage of GATA-1, the inactivation of which leads to maturation arrest at the basophilic erythroblast stage of differentiation 9. Altogether, these observations argued for a role of caspases in the negative regulation of erythropoiesis.
The morphology of erythroblasts changes dramatically during terminal erythroid differentiation. Little is known about the molecular mechanisms of these changes, which include nuclear and chromatin condensation, loss of organelles, and enucleation 10. Some of these morphological changes share similarities with features occurring during apoptosis as a consequence of caspase activation 11. Therefore, in this report we have investigated whether caspases could play a role during erythroid differentiation.
Materials and Methods
In Vitro Generation of Erythroid Cells.
Erythroid cells were generated from CD34+ progenitor cells in serum-free medium in the presence of Epo (2 mU/ml) + IL-3 (10 ng/ml) + stem cell factor (SCF; 50 ng/ml) (Epo) without or with TGF-β1 (2 ng/ml) (Epo + TGF-β1) as previously described 12.
Cell Proliferation, Differentiation, and Apoptosis Assays.
Throughout, the culture cell differentiation was assessed by morphological analysis of cells after cytocentrifugation and May-Grünwald-Giemsa coloration, and by analysis of glycophorin A and hemoglobin expression by flow cytometry and benzidine staining, respectively, as previously described 12. Cell proliferation was assessed by counting cells every day after trypan blue dye exclusion staining 12. Apoptosis was assessed by annexin V binding and propidium iodide (PI) staining as previously described 12.
Caspase Assays.
Caspase activities were evaluated by DEVD-AFC, IETD-AFC, LEHD-AFC, VDVAD-AFC, and ZEID-AFC cleavage in fluorogenic assay (Calbiochem) as previously described 13.
Mitochondrial Transmembrane Potential Study.
The cationic lipophilic fluorochrome 3,3′-dihexyloxacarbocyanine (DiOC6[3]; Sigma-Aldrich) was used to measure the transmembrane potential (ΔΨ) as described 14. ΔΨ disruption was assessed by cytometer analysis after cell labeling with DiOC6(3) and PI. The variation in ΔΨ was also investigated by microscopic fluorescence and cytometric analysis after cell labeling with the fluorescent carbocyanine dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1; Alexis). To assess nuclear integrity, 5 × 105 cells/ml were first incubated in the presence of Hoescht 33342 (Sigma-Aldrich) for 30 min (this first step was not performed for cytometric analysis) before being incubated with 10 μg/ml JC-1 for 30 min at 37°C in a humidified atmosphere of 5% CO2. Cells were then washed once with 1× PBS and resuspended in 250 μl of 1× PBS. Highly negative membrane potential in mitochondria produce JC-1 orange-red fluorescence. Loss of mitochondrial transmembrane potential results in green fluorescence and loss of the red fluorescence.
Reagents and Antibodies.
z-VAD-fmk and DEVD-cmk (Bachem) were resuspended in methanol and added at the indicated concentration. Anti-CD95 antibody (clone CH-11; Euromedex-Upstate Biotechnology) was added in the culture system at the time and concentration indicated (see Fig. 2 E).
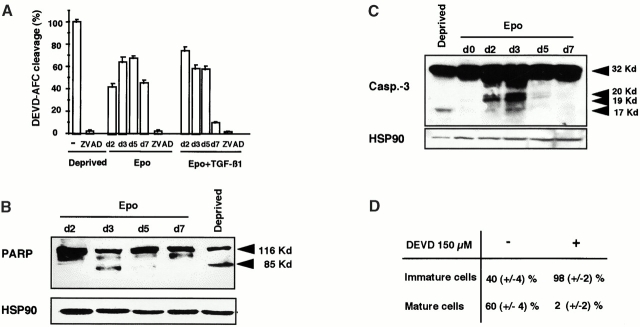
Figure 2.
Caspase-3, -2, and -9 are transiently activated, and PARP is cleaved during erythroid differentiation. DEVD-AFC peptide cleavage activity (caspase-3/-7) was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times (day 2, 3, 5, 7; d2, 3, 5, 7) in the presence of Epo or Epo + TGF-β1. Addition of 150 μM z-VAD-fmk (ZVAD) in all conditions inhibited caspases activation. Results are expressed as mean percentage ± SD (n = 3) of the activity measured in cells deprived for 12 h of growth factors (Deprived). (B and C) Immunoblots were performed on whole cell lysates from 0.5 × 106 CD36+ cells either deprived of growth factors for 12 h (Deprived) or cultured for indicated times in the presence of Epo to detect the 32-kD procaspase-3 and its p20, p19, and p17 cleavage products (B) or the native 116-kD PARP (PARP-p116) and its 85-kD cleavage product (PARP-p85) (C). Arrowheads indicate localization of specific products. HSP90 is shown as a loading control. (D) CD36 cells (2 × 105 cells/ml) were cultured in the presence of Epo with (+) or without (−) 150 μM DEVD-cmk (DEVD). Every 2 or 3 d, cells were diluted to 2 × 105 cells/ml, and cytokines and DEVD-cmk were added. Cell morphology was examined after May-Grünwald-Giemsa staining on day 8 of the culture. (E) The hydrolysis of IETD-AFC (caspase-8), LEHD-AFC (caspase-9), and VDVAD-AFC (caspase-2) peptide substrates was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times in the presence of Epo or Epo + TGF-β1. For caspase-3, -9, and -2 activation; results are expressed as percentage of the activity measured in 12-h growth factor–deprived cells (Deprived), and results for caspase-8 activity (A) are expressed as percentage of the activity measured in lysates of cells cultured with Epo + 500 ng/mL of an agonist anti-CD95 (Fas) mAb (Fas-L) for 12 h.
For detection of caspase-3 (BD PharMingen), poly(ADP-ribose) polymerase (PARP; Boehringer Mannheim), ICAD (inhibitor of caspase-activated DNase [CAD]) (DFF45; Euromedex-Upstate Biotechnology), lamin B (provided by J. Courvalin, Centre National de la Recherche Scientifique, Paris, France), acinus (provided by Y. Tsujimoto, Osaka University Medical School, Suita, Japan), HSP 90 (StressGen), and GATA-1 (provided by I. Dussanter, INSERM U363, Hopital Cochin, Paris, France), cells were lysed in Laemmli buffer or in 1% SDS, 10 mM Tris, pH 7.4, 1 mM vanadate, 1 mM PMSF, 1 mM dithiothreitol, and 10 μg/ml leupeptin, aprotinin, and pepstatin. Protein concentration was measured in the supernatant by the use of micro BCA protein assay (Pierce Chemical Co.). Proteins were analyzed by standard immunoblot procedure as described earlier 13.
Results and Discussion
To determine whether caspases could play a role in terminal erythroid differentiation, we used a two-step amplification culture system in which normal human CD34+ progenitor cells were amplified during 7 d in the presence of SCF, IL-3, and IL-6. Then, CD36+ erythroid progenitors were isolated and cultured in the presence of SCF, IL-3, and Epo. After 7 d of this second step of culture (day 7), all stages of erythroblast differentiation were observed (Fig. 1 A), though the percentage of enucleated erythrocytes remained limited to 1–5%. To increase the percentage of mature erythroblasts and erythrocytes, TGF-β1 (2.5 ng/ml) was added to the culture medium of CD36+ cells. While inhibiting cell proliferation, TGF-β1 accelerates and synchronizes erythroid differentiation with production at day 7 of 75 and 25% of mature polychromatic or orthochromatic erythroblasts and enucleated red cells, respectively 12.
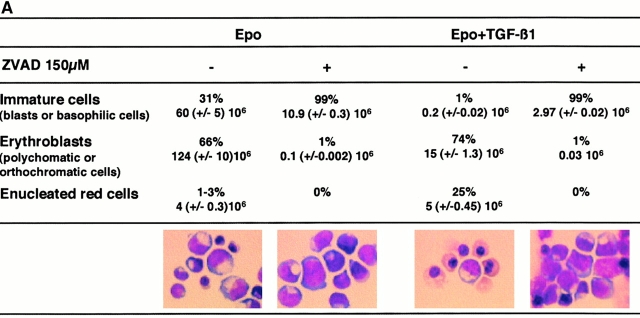
Figure 1.
The caspase inhibitor z-VAD-fmk arrests erythroid maturation at the basophilic stage. CD36 cells (2 × 105 cells/ml) were cultured for 8 d in the presence of Epo or Epo + TGF-β1 with (+) or without (−) 150 μM z-VAD-fmk (ZVAD). Every 2 or 3 d, cells were diluted to 2 × 105 cells/ml, and cytokines and z-VAD-fmk were added. (A) Cell morphology was examined after May-Grünwald-Giemsa staining on day 8 of the culture. Absolute numbers of each type of erythroblasts (immature cells, mature cells, and erythrocytes) in the presence or absence of z-VAD-fmk are indicated above percentages. (B) Percentage of cells containing hemoglobin was estimated after benzidine staining. All results are the mean of four independent experiments.
Addition to culture medium from 50–200 μM z-VAD-fmk, a permeant broad spectrum inhibitor of caspases 15, at the beginning (day 0) of CD36+ cell culture, induced a dose-dependent decrease of terminal erythroid differentiation. At 150 μM, z-VAD-fmk completely inhibited erythroid differentiation at the basophilic erythroblast stage, before nucleus and chromatin condensation (Fig. 1 A). This inhibitory effect was observed both in the absence and in the presence of TGF-β1 (Fig. 1 A). These observations suggested that caspase activation was required for erythroblast maturation after the basophilic stage of differentiation. To further explore this hypothesis, we added z-VAD-fmk to CD36+ cell culture medium at various times after culture initiation. When z-VAD-fmk was added at day 5 of culture in the absence of TGF-β1, terminal erythroid differentiation although reduced was not totally inhibited. Similarly, addition of z-VAD-fmk after 3 d of culture in the presence of TGF-β1, a time when most of the cells were at the end of basophilic stage of differentiation or already mature (polychromatophilic or orthochromatic erythroblasts), erythroid differentiation was no longer inhibited and enucleation occurred normally (data not shown). Addition of z-VAD-fmk to the culture medium of CD36+ cells also slightly reduced the number of hemoglobinized cells and their hemoglobin content, as determined by benzidine staining, in correlation with the blockade of maturation (Fig. 1 B). HPLC analysis of hemoglobin synthesis did not show any influence of z-VAD-fmk on the pattern of synthesized hemoglobin chains (not shown). Altogether, these results suggested that caspase activation might be required for earlier phases of erythroblast differentiation, while later events such as enucleation could be caspase independent.
Based on their structure and their ordering in cell death pathways, caspases have been classified into initiator and effector enzymes 16. Schematically, effector caspases, which include caspase-3, -6, and -7, cleave a variety of cellular substrates, while initiator caspases such as caspase-2, -8, and -9 control the activation of the former enzymes. To investigate which caspases were activated during normal erythroid differentiation, we studied the ability of cytosolic extracts from erythroid cells at various stages of differentiation to cleave fluorogenic peptide substrates 13. Cytosolic extracts of cells cultured in the presence of Epo cleaved the peptide substrate DEVD-AFC, suggesting activation of caspase-3 and/or caspase-7. This cleavage activity increased during the first 5 d of culture, reaching 75% of the activity measured in Epo-starved cells, then decreased the last 2 d of culture (Fig. 2 A). When the cells were cultured in the presence of Epo + TGF-β1, this DEVDase activity, although following the same kinetic profile with the same magnitude, occurred earlier, with a maximum at day 2. As expected, these activities were totally inhibited by addition of z-VAD-fmk (Fig. 2 A). Therefore, the kinetics of DEVDase activity were in accordance with the lack of effect of z-VAD-fmk on the later stages of erythroid maturation.
To determine whether DEVDase activity measured in cells undergoing erythroid maturation was related to caspase-3 activation, we performed immunoblot analyses. Procaspase-3 was observed to be cleaved to the active enzyme (p17 fragment; Fig. 2 B; reference 17). Notably, while this band was clearly individualized in the positive control from Epo-deprived erythroblasts, intermediate p20 and p19 bands were observed in erythroblasts cultured in the presence of Epo, suggesting that procaspase-3 proteolysis was less efficient during normal erythroid maturation than during starvation-induced apoptosis. Cleavage of the 116-kD PARP nuclear enzyme into a 85-kD fragment during erythroblast maturation further confirmed the activation of one or several effector caspases during the differentiation process 13 18 (Fig. 2 C). To emphasize the role of caspase-3, we cultured cells with 150 μM of DEVD-cmk, an inhibitor that is more specific for caspase-3 and related caspases than z-VAD-fmk. As expected, DEVD-cmk inhibited maturation with the same magnitude as z-VAD-fmk (Fig. 2 D).
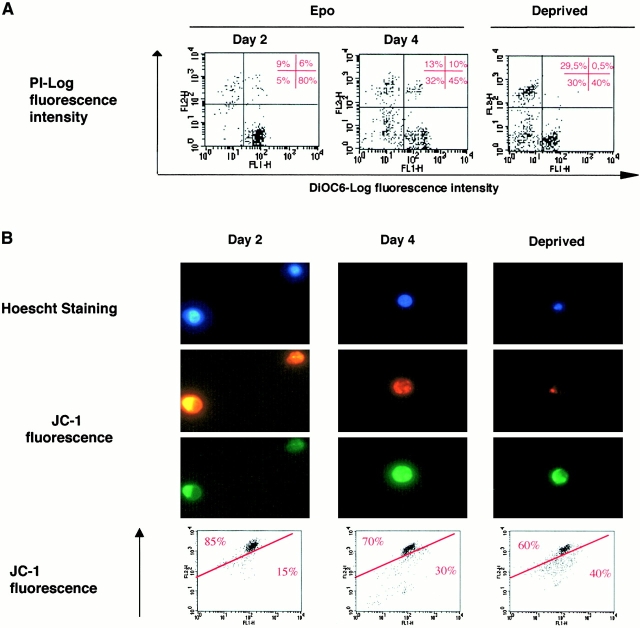
Two main pathways leading to caspase activation have been described 19. One involves death receptors such as Fas and results in the activation of the initiator caspase-8 that, in turn, activates effector caspases. This pathway is used for the negative regulation of erythropoiesis by Fas ligand (Fas-L) and leads to GATA-1 cleavage 9. The other pathway involves the disruption of the ΔΨ, resulting in opening of the permeability transition pore and the mitochondrial release of several procaspases, including procaspase-9 and -2 20 as well as apoptogenic factors 21. These phenomena result in activation of the caspase cascade through the initiator enzyme caspase-9. To determine whether one of these pathways was involved in caspase activation during normal erythropoiesis, we studied the ability of cell extracts to cleave in vitro the fluorogenic peptides IETD-AFC, VDVAD-AFC, and LEHD-AFC that mimic caspase-8, -2, and -9 targets, respectively. While a time-dependent cleavage of LEHD-AFC and VDVAD-AFC was clearly identified (Fig. 2 E), suggesting caspase-9 and -2 activation, no cleavage of IETD-AFC was detected, suggesting that neither caspase-8 nor a related caspase was involved in normal erythroid differentiation (Fig. 2 E). Involvement of the mitochondria in the pathway leading to caspase activation during erythroid differentiation was further confirmed by using the potential sensitive dyes DiOC6(3) and JC-1 that allow study of the mitochondrial transmembrane potential ΔΨ 14. While the cells cultured for 2 d in the presence of Epo incorporated DiOC6(3), as expected from viable cells, this incorporation decreased significantly at day 4, demonstrating a disruption of the ΔΨ during normal erythroid differentiation (Fig. 3 A). Similarly, at day 4, the JC-1 ratio orange-red over green fluorescence was significantly reduced (Fig. 3 B), further demonstrating that ΔΨ was disrupted during erythroid maturation. Nucleus counterstaining by Hoescht showed clearly that mitochondrial depolarization occurred in non apoptotic cells (Fig. 3 B).
Figure 3.
Mitochondrial transmembrane potential (ΔΨ) is disrupted during erythroid differentiation. For indicated times, ΔΨ disruption was assessed (A) by cytometer analysis after cell labeling with DiOC6(3) and PI. Cells with disruption of ΔΨ were DiOC6(3) negative and PI negative. A positive control of apoptosis was obtained by cytokines deprivation of CD36+ cells for 12 h (Deprived) (B) by fluorescence microscopy and cytometric analysis of cells labeled with the JC-1 fluorescent dye. Hoescht 33342 staining showed nucleus morphology.
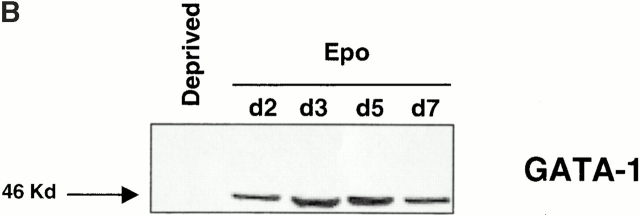
Reduction of the mitochondrial transmembrane potential and activation of the caspase cascade usually leads to externalization of phosphatidyl serine on the plasma membrane, DNA degradation, and apoptotic cell death 21. In our system of culture and in accordance with previous observations 22, <5–10% of erythroid cells exhibited features of apoptosis as assessed by annexin V binding/PI staining (Fig. 4 A), and internucleosomal DNA fragmentation could not be detected (data not shown). These two findings excluded the possibility that activation of caspases may favor erythroid maturation by inducing apoptosis in a subset of erythroid cells and strongly suggested that inhibition of red cell formation by z-VAD-fmk was not due to a selection of immature nonapoptotic cells. In addition, the disappearance of the transcriptional factor GATA-1 observed by immunoblotting in Epo-deprived erythroid cells undergoing caspase-dependent apoptosis was not identified during the normal maturation of erythroblasts (Fig. 4 B).
Figure 4.
Caspase activation does not induce either phosphatidylserine externalization or GATA-1 cleavage. (A) Cells cultured for 5 d in the presence of Epo or Epo + TGF-β1 with (+ZVAD) or without (−ZVAD) 150 μM z-VAD-fmk were labeled with annexin V and PI as described earlier. As negative and positive controls, cells were deprived of cytokines for 12 h (Deprived) with or without z-VAD-fmk, respectively. (B) Expression of the 46-kD erythroid transcription factor GATA-1 was studied by immunoblot in whole cell lysates from 0.5 × 106 CD36+ cells deprived of growth factor for 12 h (Deprived) or cultured for indicated times with Epo.
Other potential targets for caspases during erythroblastic maturation include critical proteins for nucleus integrity. Lamin B, which is highly expressed in differentiating erythroblasts 23 24, forms a fibrous meshwork on the nucleoplasmic surface of the nuclear membrane. In cells undergoing apoptosis, lamin B was shown to be cleaved by caspase-6 25, an effector caspase that is activated by caspase-3 in the proteolytic cascade 26. By using a peptide substrate (ZEID-AFC) that mimics the caspase-6 target site, we observed that caspase-6 is activated during erythroid maturation (Fig. 5 A). Accordingly, immunoblot analyzes showed that lamin B was cleaved during the differentiation process (Fig. 5 A). Inhibition of caspase activation by z-VAD-fmk, which blocked nuclear condensation, also inhibited lamin B cleavage, further supporting a correlation between caspase activation and morphological changes observed during erythroid maturation. Recently, the nuclear protein acinus was shown to play a role in chromatin condensation associated with apoptosis when cleaved by caspase-3 27. This protein is devoid of endonuclease activity and so differs from DFF40/CAD that possesses both activities (chromatin condensation and endonuclease activities) 28. DFF40 interacts and is specifically inhibited by DFF45/ICAD. During apoptosis, caspase-3 cleaves DFF45, thereby releasing DFF40 that digests DNA. Both acinus and DFF45/ICAD were expressed in our cultured cells and were cleaved in Epo-deprived cells undergoing apoptosis (Fig. 5 B). By immunoblotting, we observed that the expression of DFF45/ICAD remained unchanged during normal erythroid differentiation, while the native 96-kD acinus protein had almost disappeared at day 7 of CD36+ cell culture in the presence of Epo. The disappearance of the native acinus was related to the appearance of its active 23-kD fragment and that was prevented by z-VAD-fmk (Fig. 5 B). In contrast, no cleavage of DFF45 could be detected during erythroid differentiation (Fig. 5 B).
Figure 5.
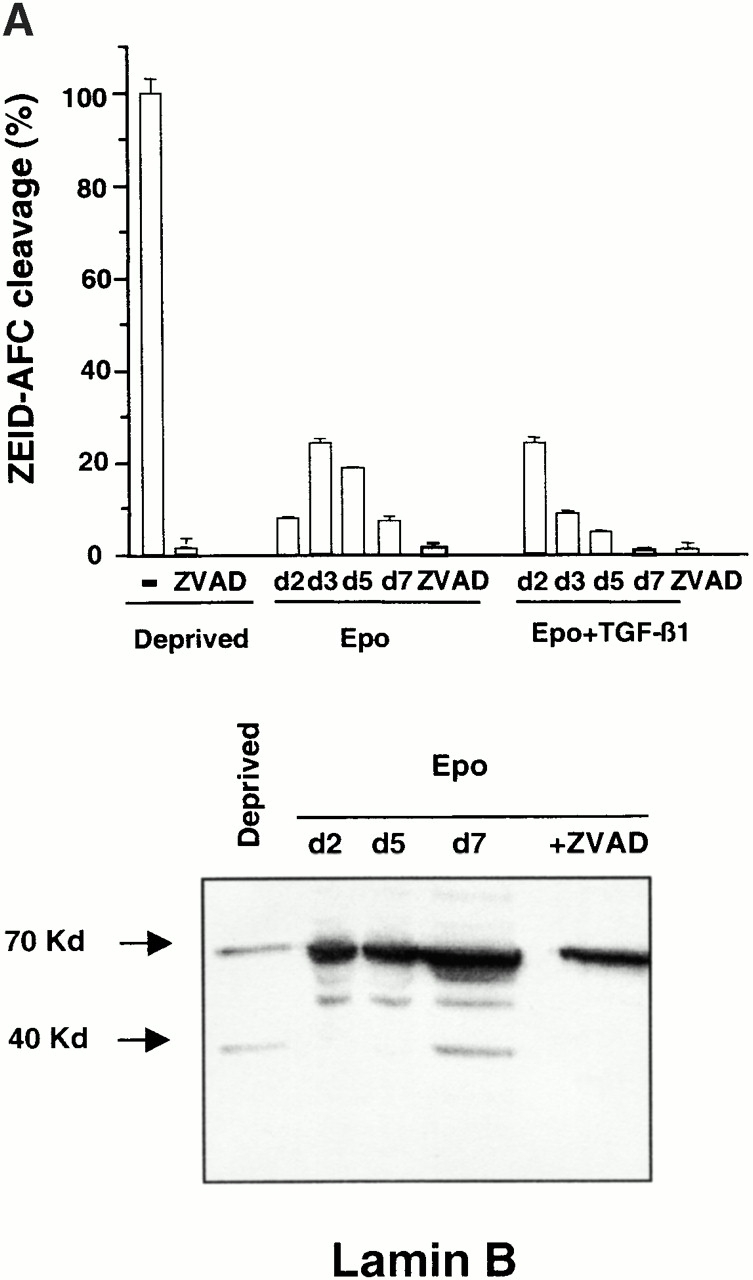
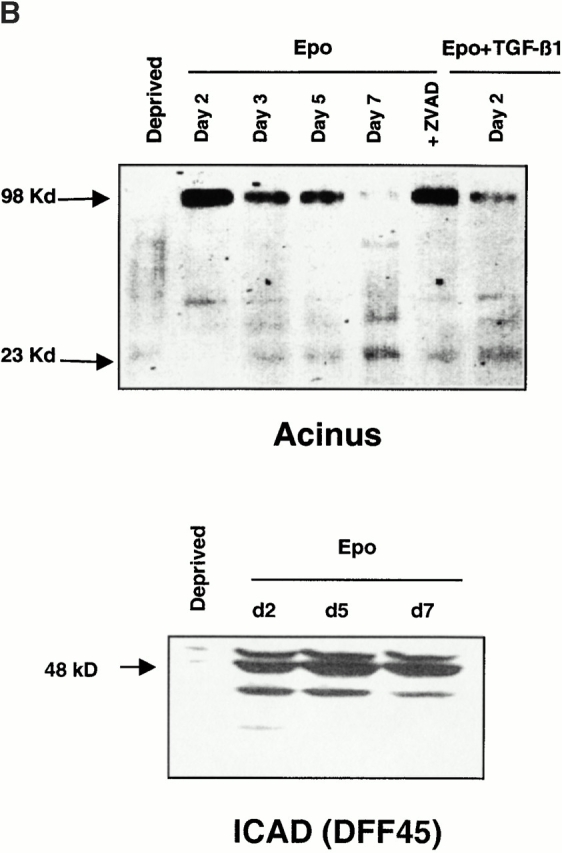
Cleavage of acinus and lamin B but not DFF45/ICAD during erythroid differentiation. (A) Top: hydrolysis of ZEID-AFC was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times in the presence of Epo or Epo + TGF-β1. Results are expressed as mean percentage ± SD (n = 3) of the activity measured in cells deprived for 12 h of growth factors (Deprived). Addition of z-VAD-fmk 150 μM (ZVAD) inhibited caspase-6 activation in all conditions of culture. Bottom: immunoblot analysis of the 70-kD lamin B and its 45-kD cleavage product in whole cell lysates from 0.5 × 106 CD36+ cells deprived of growth factor for 12 h (Deprived), cultured for indicated times with Epo, or cultured for 5 d with Epo + 150 μM z-VAD-fmk (+ZVAD). (B) Immunoblot analysis of the 98-kD protein acinus, its 23-kD cleavage product, and the 48-kD DFF45/ICAD protein in whole cell lysates from CD36+ cells cultured for indicated times with Epo or Epo + 150 μM z-VAD-fmk for 5 d (+ZVAD), or with Epo + TGF-β1 for 2 d. As positive control of apoptosis, cells were deprived with growth factors for 12 h (Deprived).
Taken together, our data demonstrate that normal erythroid differentiation requires the transient activation of several caspases. These enzymes have been involved in several physiological processes, including cell death, inflammation, and T cell proliferation 29. In erythroid cells, we show here that caspases are activated before the process of enucleation and cleave several proteins otherwise implicated in the apoptotic process such as acinus and lamin B. Cleavage of these proteins may account for the nuclear structural changes associated with the maturation of erythroblasts. These changes could also influence gene expression during the last steps of the differentiation and prepare the process of enucleation. How active caspases selectively cleave some target proteins such as acinus and lamin B without cleaving other targets such as GATA-1 or DFF45/ICAD remains currently unexplained. Caspases are only transiently activated during erythroid differentiation process, suggesting that regulatory mechanisms prevent further amplification of the proteolytic process that would lead to the cell demise. Upon Epo deprivation or Fas activation, the transient caspase activity is amplified via either the mitochondrial/caspase-9 or the caspase-8 pathway, respectively, and lead to apoptosis.
Therefore, in this report we have described a new function for caspases as enzymes critical for erythroid differentiation. Further exploration of the role of caspases in erythroid cells and other hematopoietic lineages would be of major interest for understanding the fate (differentiation versus apoptosis) of hematopoietic cells upon different physiological or pathological conditions.
Acknowledgments
We thank Yoshihide Tsujimoto, Jean Claude Courvalin, and Isabelle Dussanter for providing antibodies against acinus, lamin B, and GATA-1, respectively. We are indebted to Michel Dy, Yann Claesens, Frédéric Rieux-Laucat, Nega Beru, and William Vainchencker for helpful discussions and/or critical reading of the manuscript.
This work was supported by la Ligue Nationale contre le Cancer (LNC), l'Association de Recherche contre le Cancer (ARC), and la Fondation de France contre la Leucémie. Y. Zermati is a recipient of a Journées Biologiques Pasteur-Necker grant.
References
- Gregory C.J., Eaves A.C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978;51:527–537. [PubMed] [Google Scholar]
- Krantz S.B. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Socolovsky M., Fallon A.E., Wang S., Brugnara C., Lodish H.F. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- micea direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Motoyama N., Kimura T., Takahashi T., Watanabe T., Nakano T. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J. Exp. Med. 1999;189:1691–1968. doi: 10.1084/jem.189.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoli P.A., Bondurant M.C. Function of caspases in regulating apoptosis caused by erythropoietin deprivation in erythroid progenitors. J. Cell. Physiol. 1999;178:133–143. doi: 10.1002/(SICI)1097-4652(199902)178:2<133::AID-JCP2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Gregory T., Yu C., Ma A., Orkin S.H., Blobel G.A., Weiss M.J. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- Pevny L., Simon M.C., Robertson E., Klein W.H., Tsai S.F., D'Agati V., Orkin S.H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- De Maria R., Testa U., Luchetti L., Zeuner A., Stassi G., Pelosi E., Riccioni R., Felli N., Samoggia P., Peschle C. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood. 1999;93:796–803. [PubMed] [Google Scholar]
- De Maria R., Zeuner A., Eramo A., Domenichelli C., Bonci D., Grignani F., Srinivasula S.M., Alnemri E.S., Testa U., Peschle C. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Bessis M. Living Blood Cells and Their Ultrastructure 1973. Springer-Verlag, ; Berlin/Heidelberg/New York: pp. 85–140 [Google Scholar]
- Zamzami N., Kroemer G. Condensed matter in cell death. Nature. 1999;401:127–128. doi: 10.1038/43591. [DOI] [PubMed] [Google Scholar]
- Zermati Y., Fichelson S., Freyssinier J.M., Rouyer-Fessard P., Cramer E., Guichard J., Varet B., Hermine O. TGF-β1 inhibits erythropoiesis both by blocking proliferation and by accelerating differentiation of erythroid progenitors. Exp. Hematol. 2000;28:885–894. doi: 10.1016/s0301-472x(00)00488-4. [DOI] [PubMed] [Google Scholar]
- Bruey J.M., Ducasse C., Bonniaud P., Ravagnan L., Susin S.A., Diaz-Latoud C., Gurbuxani S., Arrigo A.P., Kroemer G., Solary E. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Castedo M., Macho A., Zamzami N., Hirsch T., Marchetti P., Uriel J., Kroemer G. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur. J. Immunol. 1995;25:3277–3284. doi: 10.1002/eji.1830251212. [DOI] [PubMed] [Google Scholar]
- Ekert P.G., Silke J., Vaux D.L. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W., Thornberry N.A. Caspaseskiller proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Han Z., Hendrickson E.A., Bremner T.A., Wyche J.H. A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J. Biol. Chem. 1997;272:13432–13436. doi: 10.1074/jbc.272.20.13432. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L.T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D.R., Poirier G.G., Salvesen G.S., Dixit V.M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A., Lazebnik Y. Caspasesenemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Susin S.A., Lorenzo H.K., Zamzami N., Marzo I., Brenner C., Larochette N., Prévost M.C., Alzari P.M., Kroemer G. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J. Exp. Med. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Dallaporta B., Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- Koury M.J., Bondurant M.C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Paddy M.R., Belmont A.S., Saumweber H., Agard D.A., Sedat J.W. Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell. 1990;62:89–106. doi: 10.1016/0092-8674(90)90243-8. [DOI] [PubMed] [Google Scholar]
- Morioka K., Tone S., Mukaida M., Takano-Ohmuro H. The apoptotic and nonapoptotic nature of the terminal differentiation of erythroid cells. Exp. Cell Res. 1998;240:206–217. doi: 10.1006/excr.1997.3927. [DOI] [PubMed] [Google Scholar]
- Buendia B., Santa-Maria A., Courvalin J.C. Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J. Cell Sci. 1999;112:1743–1753. doi: 10.1242/jcs.112.11.1743. [DOI] [PubMed] [Google Scholar]
- Slee E.A., Adrain C., Martin S.J. Serial killersordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1067–1074. doi: 10.1038/sj.cdd.4400601. [DOI] [PubMed] [Google Scholar]
- Sahara S., Aoto M., Eguchi Y., Imamoto N., Yoneda Y., Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401:168–173. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Elkon K.B. Caspasesmultifunctional proteases. J. Exp. Med. 1999;190:1725–1728. doi: 10.1084/jem.190.12.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]