Abstract
After ingestion by macrophages, Legionella pneumophila inhibits acidification and maturation of its phagosome. After a 6–10-h lag period, the bacteria replicate for 10–14 h until macrophage lysis releases dozens of progeny. To examine whether the growth phase of intracellular L. pneumophila determines the fate of its phagosome, interactions between the endosomal network and pathogen vacuoles were analyzed throughout the primary infection period. Surprisingly, as L. pneumophila replicated exponentially, a significant proportion of the vacuoles acquired lysosomal characteristics. By 18 h, 70% contained lysosomal-associated membrane protein 1 (LAMP-1) and 40% contained cathepsin D; 50% of the vacuoles could be labeled by endocytosis, and the pH of this population of vacuoles averaged 5.6. Moreover, L. pneumophila appeared to survive and replicate within lysosomal compartments: vacuoles harboring more than five bacteria also contained LAMP-1, inhibition of vacuole acidification and maturation by bafilomycin A1 inhibited bacterial replication, bacteria within endosomal vacuoles responded to a metabolic inducer by expressing a gfp reporter gene, and replicating bacteria obtained from macrophages, but not broth, were acid resistant. Understanding how L. pneumophila first evades and then exploits the endosomal pathway to replicate within macrophages may reveal the mechanisms governing phagosome maturation, a process also manipulated by Mycobacteria, Leishmania, and Coxiella.
Keywords: pathogenesis, autophagy, phagosomes, lysosomes, macrophages
Introduction
After inhalation, the gram-negative bacillus Legionella pneumophila can replicate within alveolar macrophages and cause severe pneumonia in immunocompromised people. The reservoir for L. pneumophila appears to be freshwater amebae, which also phagocytose but do not digest this opportunistic pathogen. Thus, L. pneumophila surmounts the formidable antimicrobial activities of professional phagocytes and establishes an intracellular niche that provides a ready supply of nutrients, protection from environmental stresses, and freedom from competition with other microbes.
Phagosomes harboring L. pneumophila have several unique features. After their internalization by coiling phagocytosis 1, the bacteria persist for at least 8 h in phagosomes that neither acidify nor fuse with lysosomes 2 3 4 5 6 7. Instead, by 4 h, endoplasmic reticulum envelopes the vacuole, a process that resembles autophagy 3 8. After a lag phase of 6–10 h, bacterial replication begins. By 24 h, the number of L. pneumophila has increased 50–100-fold, and lysis of phagocytes is evident 3 9 10. Several factors required by L. pneumophila to establish its protective vacuole have been discovered 11, but their modes of action remain elusive.
Broth cultures of L. pneumophila coordinately express several virulence traits in response to growth conditions 12. When amino acids are limiting, the second messenger ppGpp accumulates and triggers expression of traits likely to promote bacterial transmission to another phagocyte 13. Accordingly, postexponential phase (PE) L. pneumophila are competent to evade phagosome–lysosome fusion, whereas 90% of exponential phase (E) bacteria are destroyed rapidly in macrophage lysosomes. Whether L. pneumophila also downregulates competence to evade lysosomes during growth in macrophages has not been established, as few studies have analyzed phagosomes >8 h old.
The growth phase of an intracellular pathogen can affect phagosome maturation. Infectious Leishmania promastigotes evade phagosome–lysosome fusion 14, but, concomitant with their transformation into replicative amastigotes, the phagosomes merge with lysosomes, wherein the protozoa replicate 15 16 17. Similarly, the dormant small-cell variant form of Coxiella burnetii delays phagosome maturation 18; subsequently, its large-cell variant form replicates within phagolysosomes 19 20 21. Therefore, we tested the hypothesis that the growth phase of intracellular L. pneumophila determines the fate of its vacuole.
Materials and Methods
Bacteria Cultures.
L. pneumophila Lp02, a thymine auxotroph derived from Philadelphia strain 1 22, was cultured in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES; Sigma-Aldrich) buffered yeast extract broth containing 135 μg/ml ferric nitrate, 400 μg/ml l-cysteine, and 100 μg/ml thymidine (AYET). Macrophages were infected with L. pneumophila cultured to PE as assayed by absorbence at 600 nm of OD 3.3–4.0 and motility of >20% of the cells; E cultures were of OD 0.8–1.2 and nonmotile 12. Positive controls for phagosome–lysosome maturation assays were PE L. pneumophila incubated for 20 min at 80°C, which reduced viability 99.9%, and PE Escherichia coli strain DH5α cultured in Luria-Bertani broth and labeled with 5(6)-carboxy-fluorescein-N-hydroxysuccinamide ester (NHS-carboxyfluorescein; Roche) as described for pH assays. For some assays, labeled E. coli were fixed in 2.5% formaldehyde/PBS for 0.5 h and washed three times with PBS.
Macrophage Cultures.
Cultures of bone marrow–derived macrophages from A/J mice (The Jackson Laboratory) were prepared as described previously 8.
Microscopy.
2.0 × 105 macrophages per 12-mm glass coverslip were infected at 37°C for 1 h with L. pneumophila at a multiplicity of infection (MOI) of 0.5 in RPMI 1640 containing 10% heat-inactivated FCS (RPMI/FCS), washed three times in medium, then incubated for the period indicated in RPMI/FCS containing 100 μg/ml thymidine. To analyze the role of endosomal acidification, macrophages were infected for 1 h with L. pneumophila, washed three times in RPMI/FCS, then incubated for the times indicated in medium containing 25 nM bafilomycin A1 (BFA; Calbiochem). Cells were fixed for 0.5 h in periodate-lysine-paraformaldehyde containing 5% sucrose 8, washed once in PBS containing 5% sucrose, then permeabilized for 1 min with 1 mg Zwittergent (Roche) per ml PBS. To reduce nonspecific binding of antibodies, PBS containing 5% sucrose and 2% goat serum was used to preincubate cells and for antibody dilutions, as follows: rabbit anti–L. pneumophila (a gift from Dr. Ralph Isberg, Howard Hughes Medical Institute and Tufts University School of Medicine, Boston, MA), 1:1,000; rat anti–lysosomal-associated membrane protein 1 (LAMP-1) (1D4B; Developmental Hybridoma Bank), 1:100; rabbit anti–cathepsin D (a gift from Dr. Sadaki Yokota, Yamanashi Medical University, Yamanashi, Japan), 1:100; rabbit anti–procathepsin D 23, 1:20; and rat anti-BiP (a gift of David Bole, University of Michigan, Ann Arbor, MI), 1:200; all fluorescent secondary antibodies (Molecular Probes) were diluted 1:2,000. Cells were incubated with antibodies for 1 h at 37°C, then washed three times in PBS containing 5% sucrose. L. pneumophila were stained with either anti–L. pneumophila antibody or 0.1 μM of the nucleic acid dye 4′,6′-diamino-2-phenylindole (DAPI; Molecular Probes).
Samples were analyzed with a ZEISS Axioplan 2 epifluorescence microscope equipped with a 100× Plan-Neofluar objective, numerical aperture of 1.3, and filters 487901, 487910, and 487900. 50 vacuoles containing L. pneumophila were scored per coverslip, and no more than three vacuoles per cell were counted. Vacuoles were scored as positive for soluble markers when any fluorescence was detected within their lumens. For membrane markers, vacuoles were defined as positive only when a complete ring of fluorescence was observed at the vacuole periphery; this stringent criterion likely underestimates the fraction of LAMP-1–containing vacuoles. As >20 bacteria per vacuole could not be counted accurately, these were scored as >20. However, their size indicated that mature vacuoles held >50 bacteria.
Access to Endocytosed Material.
To label lysosomes fluorescently, macrophages were cultured for 0.5 h with 100 μg Texas red–ovalbumin (TR-OV) or Texas red–dextran (TR-DEX), 10 kD (Molecular Probes) per ml of RPMI/FCS, washed, incubated for 0.5 h in unlabeled medium, then infected with L. pneumophila at an MOI of 0.5. For pulse–chase experiments, infected macrophages were incubated with 250 μg/ml TR-OV for 15 min, unlabeled RPMI/FCS for an additional 5–120 min, then were fixed, permeabilized, stained with DAPI, and scored as described above.
pH Measurement.
To determine the pH of phagosomes <6 h old, bacteria were first surface labeled with a ratiometric fluorophore. Either L. pneumophila or E. coli were washed once in PBS, labeled with 1 mg NHS-carboxyfluorescein per ml of PBS, pH 8, for 0.5 h at 4°C, then washed three times in RPMI/FCS. Macrophages cultured on 25-mm coverslips were infected with L. pneumophila for 3–6 h at an MOI of 0.5 and with E. coli for 1–2 h at an MOI of 2. Surface labeling did not affect bacteria viability, as determined by colony formation, and only modestly affected L. pneumophila trafficking: 2 h after infection, an average of 20% labeled bacteria fused with lysosomes compared with 10% of control bacteria.
The pH of those pathogen vacuoles that were accessible to the endosomal pathway was also measured. Macrophages were infected with L. pneumophila at an MOI of 0.5 for 1 h, washed, then incubated for the period specified. 2 h before each end point, macrophages were incubated for 0.5 h with fluorescein dextran, 10 kD (Molecular Probes) at 200 μg/ml, then for 1.5 h in unlabeled RPMI/FCS. To test whether macrophage proton ATPase activity affected vacuolar pH, macrophages were treated with 1 μM BFA for 1 h before the end point. To label intracellular bacteria, DAPI was added to 0.3 μM for 30–45 min before the end point. DAPI did not affect vacuolar pH: the pH of phagosomes containing L. pneumophila surface labeled with fluorescein was similar to the pH of those harboring bacteria labeled with both fluorescein and DAPI.
Vacuolar pH was measured by the method of Tsang et al. 24 using Metamorph software (Universal Imaging Corp.). Infected macrophages were washed twice with 37°C Ringers buffer (155 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 2 mM NaH2PO4, 10 mM Hepes, and 10 mM glucose, pH 7.2), mounted into a 37°C coverslip holder, and visualized on a Nikon eclipse TE300 microscope with an attached Quantix Photometrics camera (Princeton Instruments). Vacuoles containing L. pneumophila were identified by DAPI fluorescence at 405-nm excitation (450-nm emission), then images of pathogen vacuoles that had acquired dextran were collected at 440- and 485-nm excitation (520-nm emission). The DAPI image was masked to define the bacterial pixel area, and the average gray area of the corresponding pixels in the 440- and 485-nm images was recorded. The pH of each vacuole was calculated as the ratio of 485-nm intensity (pH sensitive) to 440-nm intensity (pH insensitive). Calibration for individual fluorescent particles was conducted in situ for each experiment using cells treated with nigericin and isotonic potassium buffers of defined pH as described by Beauregard et al. 25. Young phagosomes were analyzed by masking the 440-nm image, then the ratios were calculated as described above. As a control, the lysosomes of uninfected macrophages were labeled with fluorescein dextran as described above, the 440-nm image was masked, then ratios for all of the vesicles in the field were calculated as described above.
Viability of Intracellular L. pneumophila.
Plasmid pMMB-GRN was constructed from which expression of green fluorescent protein (GFP) by L. pneumophila could be induced by isopropyl-thio-β-d-galactopyranoside (IPTG; Sigma). An SmaI–HindIII fragment from pGFPmut3 26 encoding GFP was ligated 3′ to the Ptac promoter of pMMB-Gent, which confers gentamicin resistance 13. To eliminate their inhibitory effect on L. pneumophila replication 27, mobA and mobB were deleted by digestion of pMMB-GRN with AgeI followed by intramolecular ligation. pMMB-GRN was transferred to L. pneumophila by electroporation and selection on CYET plates containing 10 μg/ml gentamicin. Analysis of broth cultures indicated GFP expression could be detected after an IPTG treatment of 4 h, or approximately two generation periods (data not shown).
To evaluate GFP expression, infected macrophages were incubated in 2 mM IPTG for 1.5 h, in TR-OV and IPTG for 0.5 h, then in unlabeled RPMI/FCS containing IPTG for 2 h. After fixation and DAPI staining, vacuoles were scored both for endosomal TR-OV and GFP fluorescence. Alternatively, macrophages were infected for 1 h, washed, incubated for an additional 13–17 h, treated with 2 mM IPTG for 4 h, then their LAMP-1 was stained as described above.
Intracellular Growth.
105 macrophages per well of 48-well tissue culture dishes were infected for 1 h with L. pneumophila at an MOI of 0.5, washed three times with RPMI/FCS, then incubated for the designated period in medium with or without 25 nM BFA (Calbiochem). To quantify CFU per well, culture supernatants were collected, monolayers were lysed by trituration in ice-cold PBS containing 0.05% saponin (PBS/saponin), then aliquots of four 10-fold dilutions of each pooled supernatent and lysate were cultured for 3–4 d on CYET agar.
A 24-h treatment with 50 or 500 nM BFA was not toxic to L. pneumophila, as judged by its failure to inhibit bacterial replication, motility, or virulence (data not shown). To ensure that the macrophages were viable and that their endocytic network was not irreversibly altered by BFA, phagocytes were treated with 25 nM BFA for 24 h before infection, incubated for 0.5 h in medium without drug, then allowed to endocytose 100 μg of TR-OV per ml of RPMI/FCS for 0.5 h. After macrophages phagocytosed L. pneumophila, the CFU per well and the accumulation of the endocytic probe in bacterial vacuoles were quantified as described above. Nearly 100% of the phagocytes endocytosed TR-OV, but a two- to fivefold decrease in L. pneumophila uptake was observed. However, intracellular L. pneumophila replication proceeded as in untreated macrophages, characterized by fusion with the endocytic network late in infection and a 10–20-fold increase in CFU over 24 h (data not shown).
To determine the density of bacteria in each vacuole (see Fig. 4), macrophages were infected for 1 h, then incubated with or without 25 mM BFA for the period indicated. After fixation, colocalization with LAMP-1 and the number of bacteria per vacuole were scored as described above.
Figure 4.
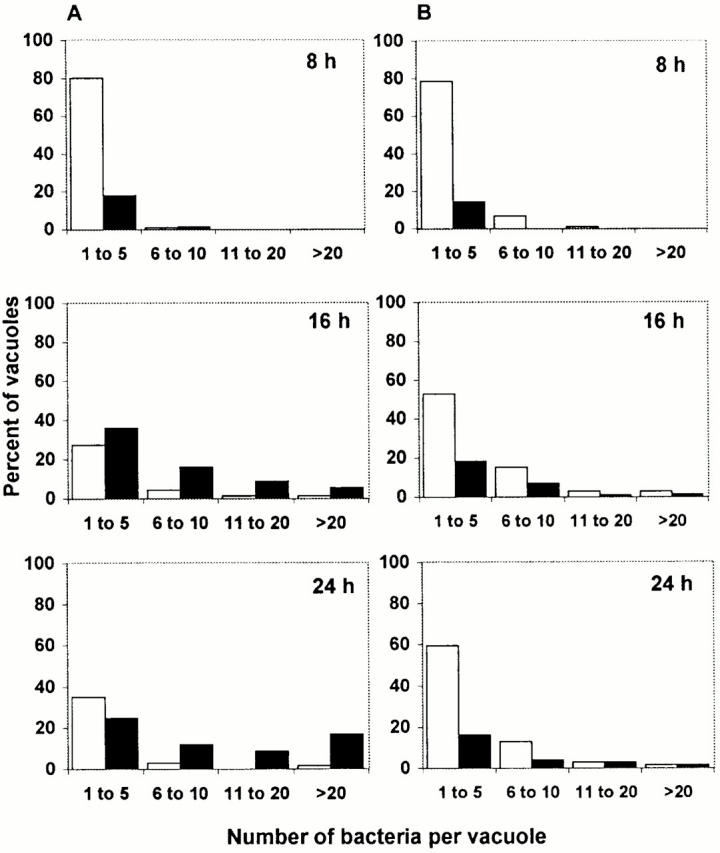
The bacterial density in vacuoles that contained LAMP-1 (black bars) or lacked LAMP-1 (white bars). (A) Macrophages infected for the period indicated were fixed and processed for microscopic localization of LAMP-1 and L. pneumophila. At the times indicated, vacuoles were scored for the presence of LAMP-1 and the number of bacteria. As it was not possible to count accurately >20 bacteria in one compartment, these were scored as >20. (B) After a 1-h infection, macrophages were treated with the proton ATPase inhibitor BFA, incubated for the times indicated, then processed as described above. In each of three independent experiments, 50 vacuoles were analyzed for each condition at the times indicated. The population of vacuoles analyzed for A was the same as that depicted in Fig. 1 B; the population of vacuoles analyzed for B was the same as that represented by Fig. 7.
Acid Tolerance.
1.5 × 106 macrophages per well of a 6-well tissue culture dish were infected for 1 h with L. pneumophila at an MOI of 0.5, washed three times with RPMI/FCS, then incubated for 2–17 h. Macrophages were lysed with 300 μl of PBS/saponin adjusted with HCl to either pH 6.9 or 5.0, then the lysate volume was brought to 1.5 ml with AYET of either pH 6.9 or 5.0. In parallel, E or PE L. pneumophila cultures in pH 6.9 AYE were diluted to 2 × 106 cells per ml of AYET of either pH 6.9 or 5.0 and containing 300 μl of either PBS/saponin or lysate prepared from 1.5 × 106 uninfected macrophages. After incubation at 37°C on a rotating wheel for 1–4 h, aliquots of four 10-fold dilutions of each sample were cultured for 3–4 d on CYET. Survival was calculated as (CFU/ml AYET, pH 5.0)/(CFU/ml AYET, pH 6.9) × 100.
Results
Mature L. pneumophila Vacuoles Interact with the Lysosomal Compartment.
To determine whether mature L. pneumophila vacuoles acquire characteristics of late endosomes and lysosomes, infected macrophages were analyzed by fluorescence microscopy after labeling with antibodies specific for the LAMP-1 or cathepsin D proteins, two hallmarks of the late stages of phagolysosome development 28 29 30, or soluble fluorescent endosomal tracers.
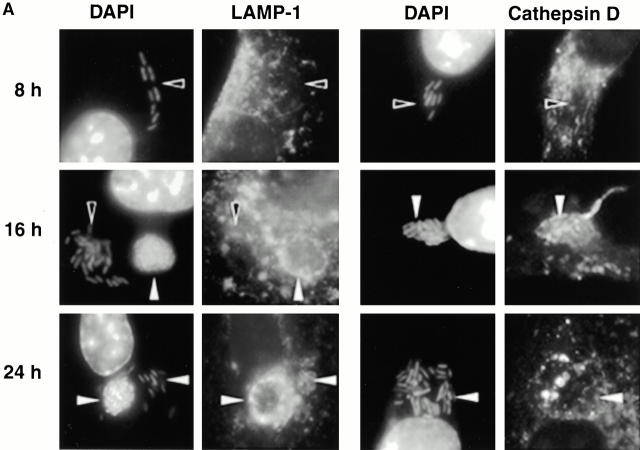
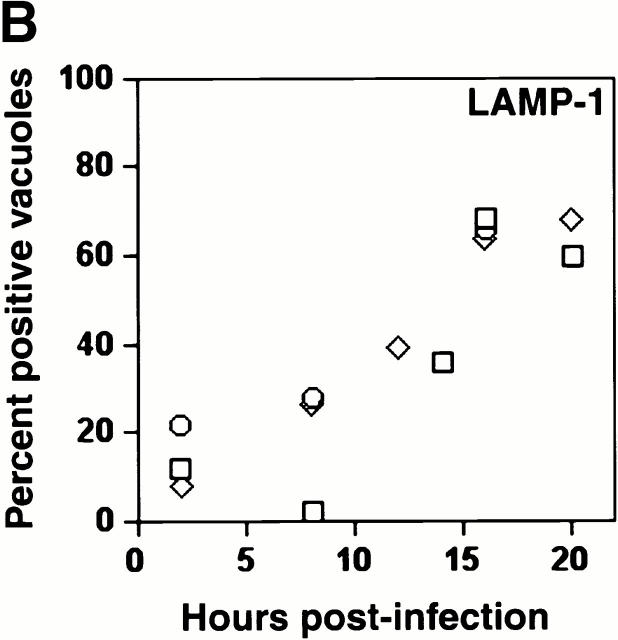
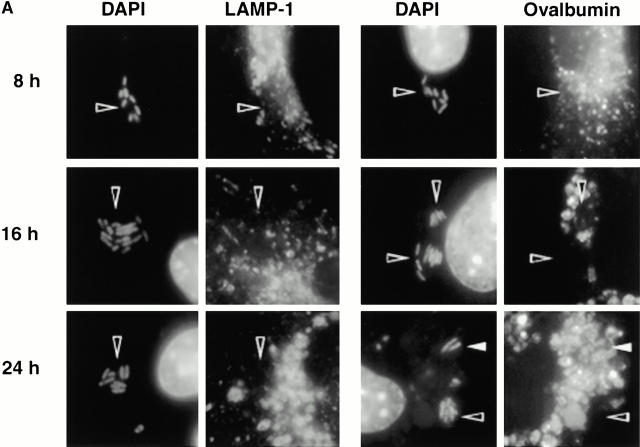
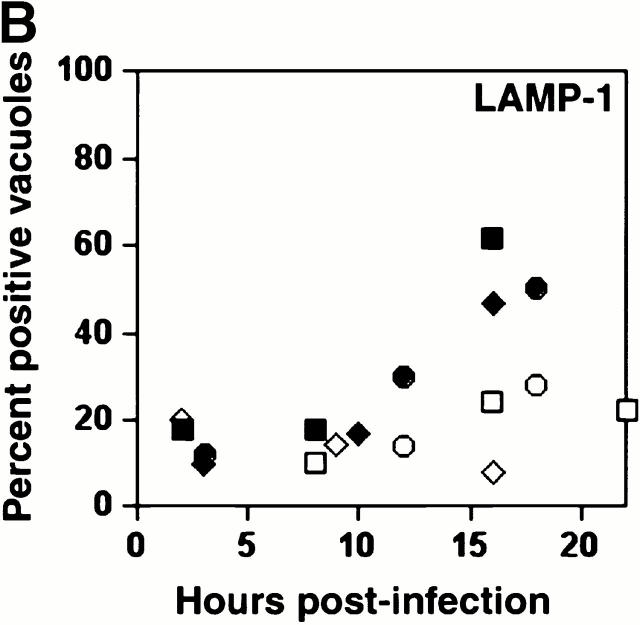
As expected, few early L. pneumophila vacuoles acquired the late endosomal and lysosomal protein LAMP-1 (6 7; Fig. 1a and Fig. b). However, by 16 h, 70% of the vacuoles contained this glycoprotein. LAMP-1–specific fluorescence was associated with the periphery of L. pneumophila vacuoles, suggesting either localization within the vacuolar membrane or juxtaposition of small vesicles to the vacuole membrane. In many cells, the lysosomes had coalesced around the bacterial vacuole (24 h; Fig. 1 A).
Figure 1.
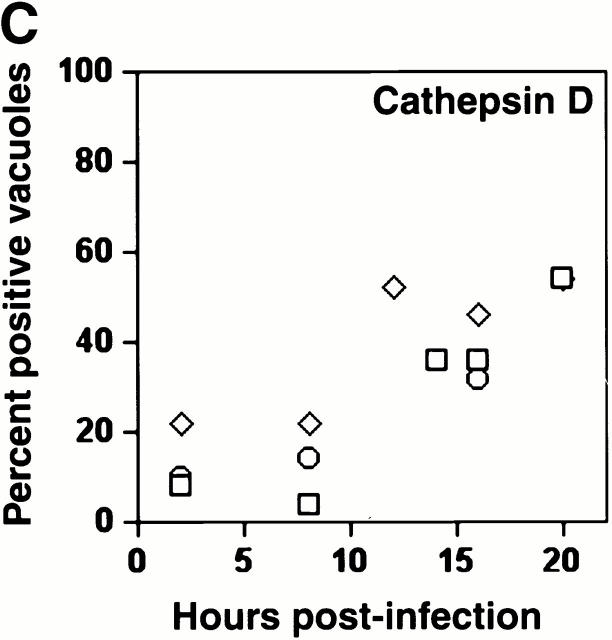
The acquisition of endosomal proteins by L. pneumophila vacuoles. Macrophages infected with L. pneumophila for the periods indicated were labeled with antibody specific for LAMP-1 or cathepsin D, then stained with DAPI. (A) At 8 h, L. pneumophila vacuoles rarely colocalized with LAMP-1 or cathepsin D (black arrowheads); after 16 h, many L. pneumophila vacuoles were ringed by LAMP-1 and contained cathepsin D (white arrowheads). The percentage of L. pneumophila vacuoles that contained either LAMP-1 (B) or cathepsin D (C) at the time indicated was scored in three separate experiments, each represented by a different symbol (circles, squares, or diamonds).
The lysosomal protease cathepsin D also accumulated in mature L. pneumophila vacuoles. Early in infection, <20% of the L. pneumophila phagosomes contained this enzyme, as reported previously 5; by 16 h, 40% or more of the vacuoles did so (Fig. 1a and Fig. c). As expected, this soluble enzyme appeared to be in the lumen of L. pneumophila vacuoles. In contrast, the proform of cathepsin D, which is delivered by the biosynthetic pathway to early endosomes 31 32, was not detected within mature L. pneumophila vacuoles (data not shown).
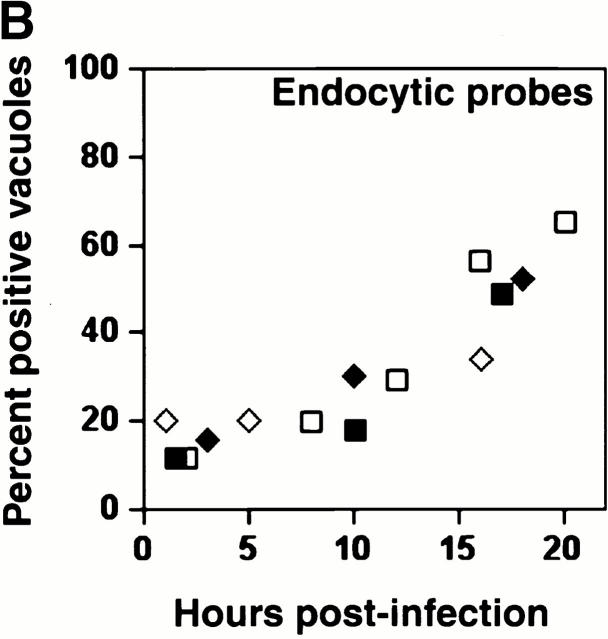
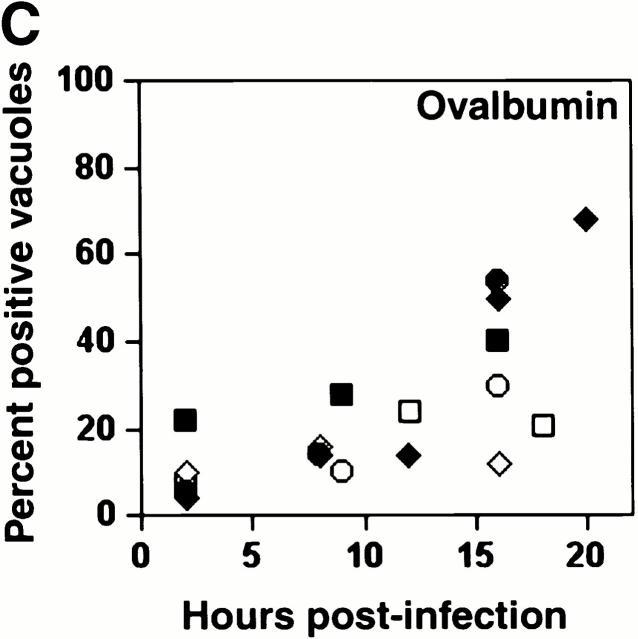
A similar result was obtained when macrophages whose lysosomes were prelabeled with the soluble fluorescent tracers TR-OV or TR-DEX were infected with L. pneumophila. Within 5 h of infection, <20% of the vacuoles contained the fluorescent probes; however, by 18–20 h, at least 50% had fused with lysosomes (Fig. 2).
Figure 2.
The acquisition of fluid-phase lysosomal markers by L. pneumophila vacuoles. Macrophages whose lysosomes were prelabeled by endocytosis of TR-OV or TR-DEX were infected with L. pneumophila for the period indicated, fixed, incubated with DAPI to label the bacteria, then analyzed microscopically. (A) At 8 h, bacterial vacuoles were isolated from the endocytic network (black arrowheads); by 16 h, the lumen of many vacuoles harboring L. pneumophila contained a fluorescent probe (white arrowheads). The 24-h panel reveals two separate vacuoles heavily laden with bacteria; the one at left had obviously fused (white arrowheads), whereas TR-OV accumulation by the vacuole at right was below the limit of detection (black arrowheads). (B) The percentage of 50 vacuoles that contained either TR-OV (filled symbols) or TR-DEX (open symbols) at the time indicated was scored by fluorescence microscopy in four separate experiments, each represented by a different symbol (diamonds or squares).
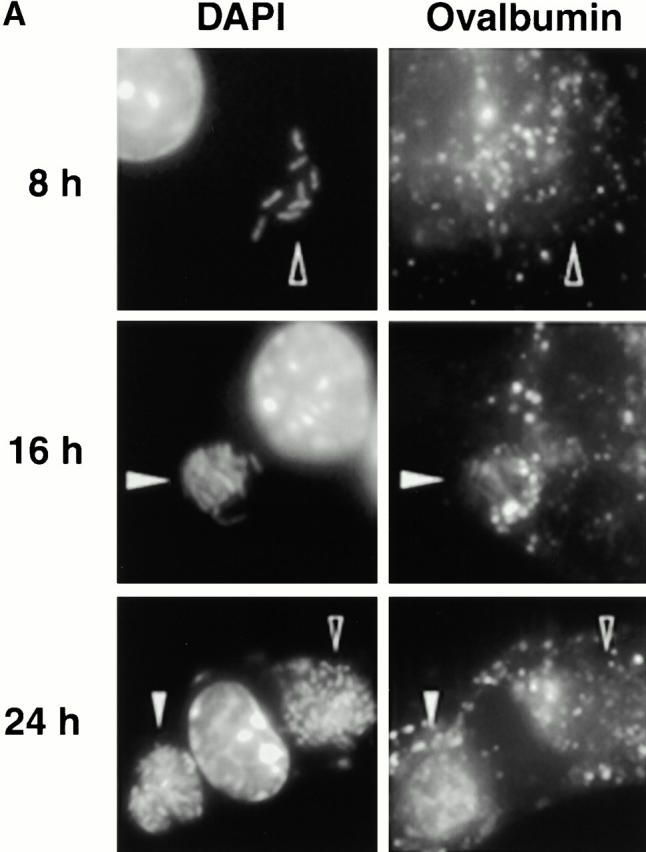
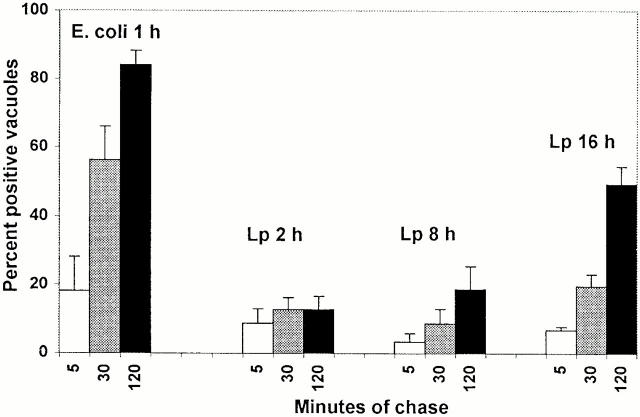
Next, the accessibility of L. pneumophila vacuoles to the endocytic pathway was assessed. Macrophages that had ingested either formaldehyde-fixed E. coli or live L. pneumophila were allowed to pinocytose TR-OV for 15 min, then the cells were incubated in fresh medium for an additional 5, 30, or 120 min. As expected, after the 120-min chase, ∼80% of phagosomes containing E. coli had accumulated fluid phase probe, whereas <20% of the nascent L. pneumophila vacuoles had done so (Fig. 3). In contrast, 50% of the mature pathogen vacuoles contained TR-OV.
Figure 3.
The accessibility of L. pneumophila vacuoles to endocytic probes. Macrophages incubated with either formaldehyde-fixed E. coli for 1 h or L. pneumophila (Lp) for the period indicated were incubated with TR-OV for 15 min, then in fresh medium for an additional 5, 30, or 120 min. After fixation and DAPI staining, blinded samples were scored for the percentage of vacuoles that had accumulated detectable TR-OV. Each bar represents the cumulative results from three separate experiments in which 50 vacuoles per condition were scored.
Although a significant proportion of mature replication vacuoles accumulated detectable quantities of LAMP-1, cathepsin D, and the endosomal tracers TR-OV and TR-DEX, not all of the vacuoles did so. However, analysis of mature replication vacuoles was hampered by two technical limitations. First, as vacuoles can be scored as positive only after accumulating visible quantities of marker, the earliest interactions with the endosomal pathway could not be detected. Second, by viewing live cultures by phase microscopy, it was evident that macrophages containing large replication vacuoles had rounded and many had detached from the coverslip. As this population of cells would be washed away during the immunofluorescence staining procedure, mature replication vacuoles likely are underrepresented in our data sets. Indeed, when macrophages were cultured on tissue culture-grade coverslips (no. 12-545-82; Fisher Scientific), 61% of mature replication vacuoles contained detectable TR-OV (50 vacuoles scored in each of three experiments). Taken together, the apparent absence of procathepsin D and the presence of LAMP-1, cathepsin D, and the endosomal tracers TR-OV and TR-DEX in a significant fraction of bacterial phagosomes indicated that as they age, L. pneumophila vacuoles merge with the lysosomal compartment.
Endosomal L. pneumophila Vacuoles Are Acidic.
To evaluate L. pneumophila phagosome maturation by an independent approach, we compared their pH to that of conventional phagolysosomes. Similar to the results obtained by Horwitz and Maxfield 2, phagosomes aged 3–6 h that contained live NHS-carboxyfluorescein–labeled L. pneumophila maintained a neutral pH (average pH 7.4), whereas vacuoles harboring similarly labeled E. coli or heat-killed L. pneumophila exhibited acidic pH values of 5.5 and 5.6, respectively (Table ). Next, the pH of those mature L. pneumophila vacuoles that had merged with the endosomal pathway was measured. For this purpose, infected macrophages were allowed to endocytose fluorescein-dextran, a process that labels nearly 50% of the mature L. pneumophila vacuoles (Fig. 2); the remaining bacterial vacuoles could not be evaluated. By 16–20 h after infection, the majority of fluorescein-dextran–containing L. pneumophila vacuoles were acidic, with an average pH of ∼5.6, a value comparable to that of lysosomal vesicles in uninfected macrophages (Table ).
Table 1.
pH of Young and Mature Vacuoles
| Condition | Age | n | pH | Error | |
|---|---|---|---|---|---|
| Phagosomes containing NHS-carboxyfluorescein–labeled particles | Live E. coli | 45–75 min | 17 | 5.6 | 0.32 |
| Live L. pneumophila | 3–6 h | 56 | 7.4 | 0.9 | |
| Heat-killed L. pneumophila | 2–4 h | 41 | 5.5 | 0.3 | |
| Vacuoles containing fluorescein dextran | L. pneumophila | 16–22 h | 64 | 5.6 | 0.8 |
| L. pneumophila + 1 μM BFA | 17–20 h | 22 | 7.3 | 0.8 | |
| Macrophage vesicles | 2 h | 300 | 5.5 | 0.3 | |
| Macrophage vesicles + 1 μM BFA | 2 h | 90 | 7.6 | 0.59 |
Lysosomes are acidified by the proton ATPase 33. To determine whether acidification of mature L. pneumophila vacuoles required the proton ATPase, infected macrophages were incubated with a specific inhibitor of its activity, BFA 34. After a 1-h treatment, mature L. pneumophila vacuoles were ∼pH 7.3; likewise, lysosomal vesicles in uninfected macrophages were pH 7.4 after BFA treatment (Table ). Therefore, macrophage proton ATPase activity was required to acidify mature L. pneumophila vacuoles.
L. pneumophila Survival and Replication within Endocytic, Acidic Vacuoles.
The observation that a significant proportion of mature L. pneumophila vacuoles acidify, accumulate endocytic probes, and contain LAMP-1 and cathepsin D strongly suggested that L. pneumophila are delivered to a lysosomal compartment during the intracellular replication period. Therefore, the viability and replication of L. pneumophila within lysosomal vacuoles was assessed by four methods.
First, at 8, 16, and 24 h after infection, the number of bacteria residing in each nonendosomal vacuole was compared with that in each endosomal vacuole, as judged by the presence of LAMP-1. The majority of vacuoles that harbored more than five bacteria were LAMP-1 positive, and the bacterial density within these endosomal vacuoles increased with time (Fig. 4 A). In contrast, vacuoles that lacked LAMP-1 typically contained less than five bacteria. A minority of vacuoles contained >20 bacteria yet lacked detectable LAMP-1, indicating that bacterial replication can precede accumulation of endosomal markers. We also observed that even 16 and 24 h after infection, approximately half of the bacterial vacuoles contained less than five bacteria. Although macrophages were infected with PE broth cultures, intracellular bacterial replication was not synchronous (Fig. 4); thus, this vacuole population likely represents both cells that never replicated during the primary infection and secondary infections by PE bacteria. Furthermore, because cells containing large replication vacuoles detach from the coverslips, the actual fraction of infected macrophages that contain vacuoles bearing one to five bacteria is likely to be smaller than the calculated value. Thus, microscopic analysis of individual phagosomes (Fig. 4 A) indicated that interaction with the endosomal network did not inhibit, and perhaps promoted, L. pneumophila replication.
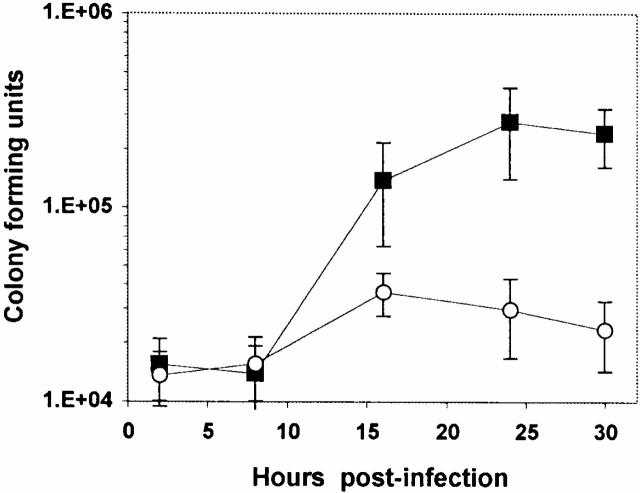
Next, the effect of neutralizing the macrophage endosomal pathway on L. pneumophila growth was examined. From 8 to 24 h of infection, the number of L. pneumophila in macrophage cultures increased ∼20-fold (Fig. 5). However, in macrophages whose endosomal network was neutralized with BFA, the yield of L. pneumophila increased only twofold. Enumeration of the bacteria in each vacuole confirmed and extended these results. In untreated macrophages 24 h after infection, 26% of L. pneumophila vacuoles harbored >11 bacteria (Fig. 4 A). In contrast, in BFA-treated macrophages, only 8% of the vacuoles contained >11 bacteria (Fig. 4 B). Notably, neutralization of the endosomal compartment did not block bacterial replication at the single cell stage. Apparently, an acidic endosomal network is not a prerequisite for initiation of bacterial replication, but macrophage proton ATPase activity did promote intracellular growth of L. pneumophila.
Figure 5.
The inhibition of L. pneumophila growth in macrophages by the proton ATPase inhibitor BFA. Infected macrophages were incubated with 25 nM BFA (open circles) or without drug (filled squares), and the yield of CFU was determined at the times indicated. Shown are the means and SE values calculated from four separate experiments, each performed in triplicate.
Concomitant with its inhibition of L. pneumophila replication, BFA also retarded the acquisition of late endosomal and lysosomal proteins by pathogen vacuoles. In untreated macrophages, 50% or more of mature L. pneumophila vacuoles contained LAMP-1 and accumulated TR-OV (Fig. 2), whereas after BFA treatment only ∼20% of the mature vacuoles did so (Fig. 6).
Figure 6.
The inhibition of L. pneumophila vacuole maturation by BFA. Macrophage lysosomes were labeled either with antibody specific for LAMP-1 or by endocytosis of TR-OV. 1 h after infection, 25 nM BFA was added to the culture medium for the duration of the experiment (open symbols) or was not (filled symbols). (A) In the presence of BFA, L. pneumophila vacuoles failed to merge with the endocytic network (black arrowheads), and each typically contained <15 bacteria. The percentage of 50 vacuoles that colocalized with either (B) LAMP-1 or (C) TR-OV was scored by fluorescence microscopy over the course of a primary infection in three separate experiments, each represented by a different symbol (circles, squares, or diamonds).
Although the interaction of the pathogen vacuole with the endocytic pathway appeared to be pH sensitive, its association with the endoplasmic reticulum was not. In control cultures, by 6 h after infection, 54% of the L. pneumophila vacuoles were enveloped by endoplasmic reticulum, as judged by immunofluorescence microscopic localization of BiP (n = 50 in each of three experiments [8]). Likewise, when infected macrophages were treated with BFA, 58% of pathogen vacuoles colocalized with BiP (n = 50 in each of three experiments). Furthermore, the yield of L. pneumophila CFU 24 h after infection was similar whether or not the cultures were treated with BFA during the first 6–8 h of infection (data not shown). Therefore, the pH-sensitive stage of the L. pneumophila life cycle occurs after formation of its autophagosome-like vacuole.
Results of several control experiments indicated that BFA did not affect bacterial or macrophage viability. First, growth of L. pneumophila in broth was not inhibited by even 500 nM BFA (data not shown). Second, BFA treatment did not permanently disrupt endosomal traffic. When macrophages were treated with BFA for 24 h before, rather than during, the infection with L. pneumophila, no difference in vacuole maturation or the yield of CFU was observed between pretreated and untreated cells (data not shown). Third, neutralization of the acidic compartments of cells, including phagosomes, with 25 μM chloroquine or 25 mM ammonium chloride 35 36 also inhibited L. pneumophila replication, although to a lesser extent than did BFA (data not shown). Therefore, L. pneumophila growth was maximal within macrophages whose endosomal compartment was acidic and competent to fuse with the bacterial vacuole.
Third, to assess the metabolic activity of L. pneumophila residing within endocytic vacuoles, macrophages were infected with a strain in which GFP expression could be induced by IPTG. After 16 h, a time when >60% of the replication vacuoles have merged with the endosomal pathway (Fig. 1), macrophages were incubated with IPTG and TR-OV for 4 h, then the capacity of bacteria within lysosomal vacuoles to express GFP was analyzed microscopically. Approximately 70% of the pathogen vacuoles harbored bacteria that expressed GFP, whether or not the vacuole had accumulated either LAMP-1 or TR-OV (Fig. 7 and Table ). Therefore, L. pneumophila appeared to survive within lysosomal vacuoles.
Figure 7.
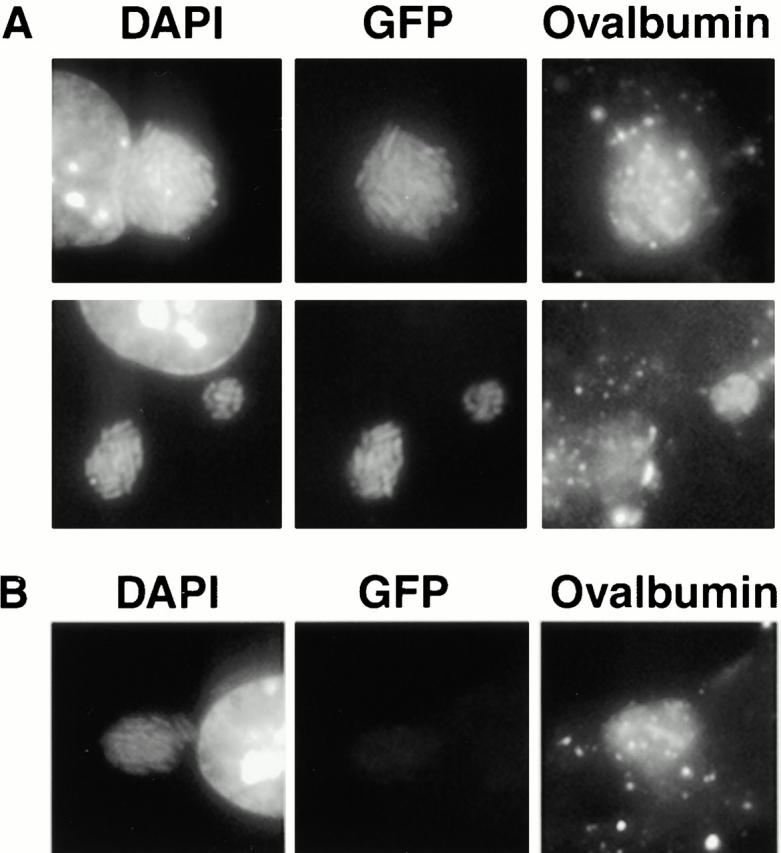
GFP expression by bacteria within endocytic vacuoles. Macrophages infected for 16 h with L. pneumophila carrying pMMB-GRN were incubated with IPTG to induce GFP expression and with TR-OV to label the lysosomes. Intracellular bacteria were identified by DAPI staining and scored for colocalization with the lysosomal probe and for expression of GFP. (A) The majority of lysosomal pathogen vacuoles harbored GFP-expressing L. pneumophila, (B) whereas a minority of the endosomal vacuoles contained bacteria that exhibited only DAPI fluorescence.
Table 2.
Comparison of GFP Expression by Bacteria within Endosomal Versus Nonendosomal Vacuoles
| Endosomalmarker | GFP− | GFP+ | Totalvacuoles | Percent GFP+ |
|---|---|---|---|---|
| n | n | n | % | |
| LAMP-1 negative | 28 | 66 | 94 | 70 |
| LAMP-1 positive | 46 | 111 | 157 | 71 |
| TR-OV negative | 75 | 187 | 262 | 71 |
| TR-OV positive | 55 | 118 | 173 | 68 |
16 h after infection with L. pneumophila carrying pMMB-GRN, macrophages were incubated for 4 h with IPTG and, where indicated, TR-OV, then colocalization of bacteria, GFP, and LAMP-1 or TR-OV was analyzed by fluorescence microscopy as described in Materials and Methods.
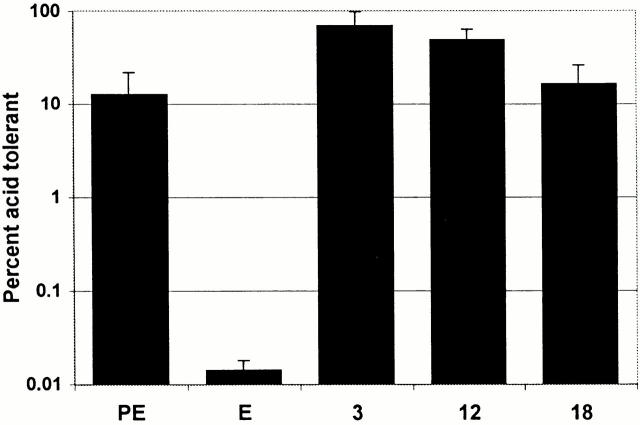
Finally, the prediction that L. pneumophila replicating in macrophages are acid resistant was tested. Macrophages infected with L. pneumophila for 3, 12, or 18 h were lysed, and the bacteria were transferred to broth of pH 6.9, which is standard, or pH 5.0, to mimic the lysosomal compartment (Table ). In parallel, E and PE broth cultures were incubated in AYET of pH 6.9 or 5.0. As is typical of many bacteria 37, PE L. pneumophila were acid resistant, whereas acid treatment reduced by 1,000-fold the CFU of E L. pneumophila (Fig. 8). In striking contrast, a similar treatment of replicating bacteria obtained from macrophages reduced the CFU less than fivefold. Macrophage lysates did not induce acid tolerance of L. pneumophila, as broth cultures of E L. pneumophila incubated with lysates prepared from uninfected macrophages were not protected from the pH 5.0 stress (data not shown). The prediction that L. pneumophila are replicating 12 and 18 h after infection (Fig. 1, Fig. 2, Fig. 4, and Fig. 5) was confirmed by the twofold increase in CFU that was observed after incubating parallel samples of macrophage lysates in AYET, pH 6.9 for 3 h, a period corresponding to less than two L. pneumophila doubling times (data not shown). Thus, consistent with our observation that a significant proportion of mature bacterial replication vacuoles are pH 5.6 (Table ), the phagosomal environment of macrophages appeared to induce L. pneumophila acid resistance.
Figure 8.
Acid tolerance of L. pneumophila isolated from macrophages. Macrophages infected with L. pneumophila for 3, 12, or 18 h were lysed, and the bacteria were transferred to broth of pH 5.0 or 6.9. In parallel, E or PE L. pneumophila from pH 6.9 broth were diluted into broth of pH 5.0 or 6.9. After 4 h, samples were plated on CYET to quantify CFU. Percentage survival was calculated as (CFU/ml of AYET, pH 5.0)/(CFU/ml of AYET, pH 6.9) × 100. Means and SE were calculated from four separate experiments.
Discussion
A parasite of amebae and macrophages, L. pneumophila escapes a rapid death in phagocytes by preventing phagosome–lysosome fusion. Classic experiments by Horwitz and colleagues determined that during the first 8 h of infection, L. pneumophila phagosomes fail to acidify or acquire lysosomal proteins 2 3 4. Nascent phagosomes also lack early and late endosomal proteins 5 6 7 38 39. Here, cell biological studies focused on the replication phase of the L. pneumophila intracellular life cycle revealed that the pathogen vacuole changes dramatically with time. Coincident with bacterial replication, a significant proportion of L. pneumophila vacuoles acidify, acquire lysosomal proteases, and accumulate material from the endocytic pathway. Moreover, residence within a lysosomal compartment appears to promote L. pneumophila growth: neutralization of the endosomal compartment inhibited vacuole maturation and bacterial replication.
Several previous observations are consistent with a phagolysosomal residence for replicating L. pneumophila. Bacteria obtained from E broth cultures are degraded in macrophage lysosomes, whereas PE L. pneumophila evade phagosome–lysosome fusion 12. L. pneumophila vacuoles aged >24 h frequently contain granular and membranous material 40 41 42. DotA, a protein that L. pneumophila requires during infection to arrest phagosome maturation 7 43, is dispensable during the intracellular replication period 44. Reagents that neutralize the endosomal compartment reportedly prevent L. pneumophila replication in macrophages, HeLa cells, or amebae 32 37 45 46. Finally, C. burnettii, an obligate intracellular pathogen closely related to L. pneumophila 47 48, replicates within acidic phagolysosomes of macrophages 19 20 21.
A 1-h treatment with BFA was sufficient to neutralize the macrophage endosomal compartment (Table ), yet it did not block replication of L. pneumophila at the single cell stage (Fig. 4 and Fig. 6). Therefore, whereas an acidic pH triggers development of dormant C. burnettii to its metabolically active variant 49, L. pneumophila differentiation from the PE virulent form to the replicative form 12 may be pH independent. However, robust growth of intracellular L. pneumophila did correlate with interaction between the pathogen vacuole and the endosomal compartment (Fig. 4 Fig. 5 Fig. 6).
Byrd and Horwitz postulated that experimental neutralization of the monocyte vacuolar pH inhibits dissociation of iron from endosomal transferrin, starving intracellular L. pneumophila of iron 35 50. In our experimental model, we were not able to demonstrate that BFA inhibits intracellular L. pneumophila replication by an iron-dependent mechanism. Addition to BFA-treated phagocytes of iron nitriloacetate to 100 μg/ml did not consistently restore L. pneumophila growth (data not shown), although interpretation of this result was complicated as, in the absence of BFA, this compound partially inhibited intracellular bacterial replication and was toxic to macrophages (data not shown). Moreover, L. pneumophila replicated efficiently for 24 h in broth lacking supplemental iron, in macrophages cultured in medium lacking serum, a source of transferrin, or in murine macrophages treated with the iron chelator deferoxamine at concentrations previously shown to inhibit bacterial growth in human monocytes (50; data not shown). Although ferric iron is essential for maximal L. pneumophila growth in broth, the concentration reportedly required varies from trace amounts to ∼20 μM 51 52 53 54 55. Finally, inhibition of proton ATPase activity with specific drugs, such as BFA and the concanamycins, has pleiotropic effects, including deregulation of trans-Golgi trafficking 56 57, inhibition of autophagy 58, and arrest of endosome maturation 59. Indeed, BFA inhibited not only intracellular L. pneumophila replication, but also fusion of its vacuole with the endocytic network (Fig. 6). Therefore, although it is clear that neutralizing the endosomal pH interferes with L. pneumophila replication, the mechanism of this inhibition warrants more detailed study.
Previously, Horwitz and Maxfield determined that L. pneumophila reside in neutral phagosomes by analyzing vacuoles that were <8 h old 2; our studies of phagosomes aged 3–6 h (Table ) corroborate their results. They also reported that 18-h phagosomes maintain a neutral pH, a conclusion contradictory to ours. However, their method to measure pH required that the bacteria retain fluorescent antibody opsonins bound before macrophage infection. As bacterial replication is expected to dilute significantly the surface opsonins, their 18-h pH values may represent vacuoles that contained nonreplicating L. pneumophila.
Our observation that macrophage vacuoles harboring replicating L. pneumophila are acidic presents a conundrum, as this pathogen grows optimally in broth of pH 6.3–7.2 53 60. However, consistent with a previous report 61, bacteria replicating intracellularly are acid resistant (Fig. 1, Fig. 2, Fig. 5, and Fig. 8). Presumably, during its several hours of isolation from the endocytic pathway, L. pneumophila responds to intracellular cues by inducing expression of factors that promote survival and perhaps replication within this hostile but nutrient-rich compartment. By analogy to other gram-negative bacilli, an early, moderate decline in its vacuolar pH may induce L. pneumophila acid resistance 62, or a variety of noxious conditions encountered during phagocytosis may induce cross-tolerance of L. pneumophila to other environmental stresses 31. In fact, L. pneumophila express heat shock proteins during growth in macrophages 10 61 63. Alternatively, acid resistance of L. pneumophila may be promoted by factors present in macrophages, but not AYET, such as high concentrations of amino acids that can be metabolized to produce basic compounds 64. Acid-resistant bacteria may exploit the lysosomal compartment for its ample supply of amino acids, the primary source of carbon and energy for L. pneumophila 65 66, and membrane, to accommodate numerous progeny.
Aspects of the L. pneumophila intracellular pathway resemble cellular autophagy. During this ubiquitous eukaryotic process, the endoplasmic reticulum engulfs cytoplasmic material and organelles which are then degraded as the autophagosome acquires LAMP-1, the proton ATPase, and lysosomal hydrolases 67. Likewise, by 4–6 h after formation, endoplasmic reticulum surrounds the L. pneumophila vacuole 8; by 12 h, the vacuole begins to acquire lysosomal characteristics. Furthermore, experimental conditions that increase macrophage autophagy also promote association of the endoplasmic reticulum with the pathogen vacuole and modestly stimulate bacterial growth 8. Similarly, vacuoles harboring either replicative Leishmania amastigotes or Brucella abortus acquire features of both the endoplasmic reticulum and the late endosomal compartments 68 69 70 71 72. Perhaps some vacuolar pathogens induce autophagy to obtain nutrients. Alternatively, autophagy may serve as a macrophage quality control mechanism that recognizes aborted phagosomes and delivers these organelles to the lysosomes.
An analogous correlation between microbial growth phase, autophagy, and vacuole maturation has been described for Leishmania. When infectious Leishmania promastigotes are ingested by macrophages, phagosome maturation is blocked by lipophosphoglycan on the parasites' surface 14. As promastigotes develop into the replicative amastigote form, lipophosphoglycan expression is downregulated, the vacuole associates with the endoplasmic reticulum and merges with the endosomal pathway, and the parasite replicates within an acidic phagolysosomal vacuole 68 73 74.
Because L. pneumophila is amenable to molecular genetics, it may serve as a tool for identifying the mechanisms by which pathogens, including Coxiella and Leishmania, tolerate or exploit the lysosomal environment. Understanding how L. pneumophila manipulates macrophage cellular processes to isolate its nascent phagosome from the endosomal pathway, form an autophagosomal vacuole, then survive and perhaps replicate, within an acidic, lysosomal compartment may elucidate the mechanisms that govern macrophage membrane traffic, as well as the virulence strategies of other intracellular pathogens.
Acknowledgments
We thank Brenda Byrne for technical assistance, Albert Tsang and Ken Christianson for video microscopy assistance, and Tom Zahrt, Joe Vogel, and Amrita Joshi for critically reviewing this manuscript. Much gratitude is given to Joel Swanson for the use of his video equipment and for thoughtful comments during the preparation of this manuscript.
These studies were supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, grant R29 A140694-01BM. S. Sturgill-Koszycki is a postdoctoral fellow in the University of Michigan Molecular Mechanisms of Microbial Pathogenesis Training Program, grant T32AI07528.
Footnotes
Abbreviations used in this paper: BFA, bafilomycin A1; E, exponential phase; GFP, green fluorescent protein; IPTG, isopropyl-thio-β-d-galactopyranoside; LAMP-1, lysosomal-associated membrane protein 1; MOI, multiplicity of infection; PE, postexponential phase; TR-DEX, Texas red–dextran; TR-OV, Texas red–ovalbumin.
References
- Horwitz M.A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanismengulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M.A., Maxfield F.R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J. Exp. Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D.L., Horwitz M.A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M.S., Isberg R.R. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect. Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.R., Berger K.H., Isberg R.R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- Swanson M.S., Isberg R.R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.A., Silverstein S.C. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Kwaik Y., Eisenstein B.I., Engleberg N.C. Phenotypic modulation by Legionella pneumophila upon infection in macrophages. Infect. Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.P., Isberg R.R. Cell biology of Legionella pneumophila . Curr. Opin. Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- Byrne B., Swanson M.S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer B.K., Swanson M.S. Coordination of Legionella pneumophila virulence with entry into stationary phase ppGpp. Mol. Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- Desjardins M., Descoteaux A. Inhibition of phagosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 1997;185:2061–2068. doi: 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine J.C., Prina E., Jouanne C., Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect. Immun. 1990;58:779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D.G., Xu S., Chakraborty P. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J. Cell Sci. 1992;103:1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- Turco S.J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Howe D., Mallavia L.P. Coxiella burnetti exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells. Infect. Immun. 2000;68:3815–3821. doi: 10.1128/iai.68.7.3815-3821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akporiaye E.T., Rowatt J.D., Aragon A.A., Baca O.G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii . Infect. Immun. 1983;40:1155–1162. doi: 10.1128/iai.40.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin M.A., Benoliel A.M., Bongrand P., Raoult D. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 1992;60:5013–5016. doi: 10.1128/iai.60.12.5013-5016.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen R.A., Scidmore M.A., Rockey D.D., Hackstadt T. Differential interaction with the endocytic and exocytic pathways distinguish parasitophorus vacuoles of Coxiella burnetti and Chlamydia trachomatis . Infect. Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K.H., Isberg R.R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila . Mol. Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Ullrich H.J., Beatty W.L., Russell D.G. Direct delivery of procathepsin D to phagosomesimplications for phagosome biogenesis and parasitism by Mycobacterium . Eur. J. Cell Biol. 1999;78:739–748. doi: 10.1016/S0171-9335(99)80042-9. [DOI] [PubMed] [Google Scholar]
- Tsang A., Oestergaard K., Myers J.T., Swanson J.A. Altered membrane trafficking in activated bone marrow-derived macrophages. J. Leukoc. Biol. 2000;68:487–494. [PubMed] [Google Scholar]
- Beauregard K.E., Lee K.-D., Collier R.J., Swanson J.A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes . J. Exp. Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack B.P., Valdivia R.H., Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Segal G., Shuman H.A. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol. Microbiol. 1998;30:197–208. doi: 10.1046/j.1365-2958.1998.01054.x. [DOI] [PubMed] [Google Scholar]
- Chen J.W., Cha Y., Yuksel K.U., Gracy R.W., August J.T. Isolation and sequencing of a cDNA clone encoding a lysosomal membrane protein mouse LAMP-1. Sequence similarity to proteins bearing onco-differentiation antigens. J. Biol. Chem. 1988;263:8754–8758. [PubMed] [Google Scholar]
- Peters C., von Figura K. Biogenesis of lysosomal membranes. FEBS Lett. 1994;346:108–114. doi: 10.1016/0014-5793(94)00499-4. [DOI] [PubMed] [Google Scholar]
- Oh Y.-K., Swanson J.A. Different fates of phagocytosed particles after delivery into macrophage lysosomes. J. Cell Biol. 1996;132:585–593. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diment S., Leech M.S., Stahl P.D. Cathepsin D is membrane-associated in macrophage endosomes. J. Biol. Chem. 1988;263:6901–6907. [PubMed] [Google Scholar]
- Delbruck R., Desel C., von Figura K., Hille-Rehfeld A. Proteolytic processing of cathepsin D in prelysosomal organelles. J. Cell Biol. 1994;64:7–14. [PubMed] [Google Scholar]
- Grinstein S., Nanda A., Lukacs G., Rotstein O. V-ATPases in phagocytic cells. J. Exp. Biol. 1992;172:179–192. doi: 10.1242/jeb.172.1.179. [DOI] [PubMed] [Google Scholar]
- Drose S., Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- Byrd T.F., Horwitz M.A. Chloroquine inhibits the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J. Clin. Invest. 1991;88:351–357. doi: 10.1172/JCI115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg L.G., Goldman W.E., Schlesinger P.H. Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 1993;177:1605–1611. doi: 10.1084/jem.177.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F.C., editor. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology Press; Washington, D.C.: 1996. pp. 1497–1512. [Google Scholar]
- Clemens D.L., Horwitz M.A. Membrane sorting during phagocytosisselective exclusion of major histocompatibility complex molecules but not complement receptor CR3 during conventional and coiling phagocytosis. J. Exp. Med. 1992;175:1317–1326. doi: 10.1084/jem.175.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D.L., Lee B.-Y., Horwitz M.A. Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect. Immun. 2000;68:2671–2684. doi: 10.1128/iai.68.5.2671-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H., Fields B.S., Shotts E.B., White E.H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoeba and human monocyte-like cells. Infect. Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T.W., Brown K., Friedman H. Differential morphologic and metabolic alterations in permissive versus nonpermissive murine macrophages infected with Legionella pneumophila . Infect. Immun. 1992;60:3231–3237. doi: 10.1128/iai.60.8.3231-3237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K.H., Merriam J.J., Isberg R.R. Altered intracellular targeting properties associated with mutations in the Legionella pnuemophila dotA gene. Mol. Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Coers J., Monahan C., Roy C.R. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1999;1:183–188. doi: 10.1038/15687. [DOI] [PubMed] [Google Scholar]
- Goldoni P., Pastoris M.C., Cattini L., Peluso C., Sinibaldi L., Orsi N. Effect of monensin on the invasiveness and multiplication of Legionella pneumophila . J. Med. Microbiol. 1995;42:269–275. doi: 10.1099/00222615-42-4-269. [DOI] [PubMed] [Google Scholar]
- Cattani L., Goldoni P., Pastoris M.C., Sinibaldi L., Orsi N. Bafilomycin A1 and intracellular replication of Legionella pneumophila . Antimicrob. Agents Chemother. 1997;41:212–214. doi: 10.1128/aac.41.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W.G., Dobson M.E., Samuel J.E., Dasch G.A., Mallavia L.P., Baca O., Mandelco L., Sechrest J.E., Weiss E., Woese C.R. Phylogenetic diversity of the Rickettsiae . J. Bacteriol. 1989;171:4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G., Shuman H.A. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii . Mol. Microbiol. 1999;33:667–672. doi: 10.1046/j.1365-2958.1999.01511.x. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Williams J.C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii . Proc. Natl. Acad. Sci. USA. 1981;78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T.F., Horwitz M.A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J. Clin. Invest. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W.J., Miller R.D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J. Clin. Microbiol. 1979;10:50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M.W., Pine L., Hutner S.H., George J.R., Harrell W.K. Metal requirements of Legionella pneumophila . J. Clin. Microbiol. 1981;13:688–695. doi: 10.1128/jcm.13.4.688-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J.D., Hedlund K.W., Gowda S. Chemically defined medium for Legionella pneumophila growth. J. Clin. Microbiol. 1981;13:115–119. doi: 10.1128/jcm.13.1.115-119.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James B.W., Mauchline W.S., Fitzgeorge R.B., Dennis P.J., Keevil C.W. Influence of iron-limited continuous culture on physiology and virulence of Legionella pneumophila . Infect. Immun. 1995;63:4224–4230. doi: 10.1128/iai.63.11.4224-4230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.D., O'Connell W.A., Cianciotto N.P. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect. Immun. 1996;64:629–636. doi: 10.1128/iai.64.2.629-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Weert A.W.M., Geuze H.J., Stoorvogel W. Heterogeneous behavior of cells with respect to induction of retrograde transport from the trans-Golgi network to the Golgi upon inhibition of the vacuolar proton pump. Eur. J. Cell Biol. 1997;74:417–423. [PubMed] [Google Scholar]
- Reaves B., Banting G. Vacuolar ATPase inactivation blocks recycling to the trans-Golgi network from plasma membrane. FEBS Lett. 1994;345:61–66. doi: 10.1016/0014-5793(94)00437-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Clague M.J., Urbe S., Aniento F., Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Feeley J.C., Gorman G.W., Weaver R.E., Mackel D.C., Smith H.W. Primary isolation media for Legionnaires disease bacterium. J. Clin. Microbiol. 1978;8:320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Kwaik Y., Gao L.-Y., Harb O.S., Stone B.J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol. Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- Foster J.W. When protons attackmicrobial strategies of acid adaptation. Curr. Opin. Microbiol. 1999;2:170–174. doi: 10.1016/S1369-5274(99)80030-7. [DOI] [PubMed] [Google Scholar]
- Susa M., Hacker J., Marre R. De novo synthesis of Legionella pneumophila antigens during intracellular growth in phagocytic cells. Infect. Immun. 1996;64:1679–1684. doi: 10.1128/iai.64.5.1679-1684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E.R. Influence of pH on bacterial gene expression. Mol. Microbiol. 1993;8:5–14. doi: 10.1111/j.1365-2958.1993.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Pine L., George J.R., Reeves M.W., Harrell W.K. Development of a chemically defined liquid medium for growth of Legionella pneumophila . J. Clin. Microbiol. 1979;9:615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauchline W.S., Araujo R., Wait R., Dowsett A.B., Dennis P.J., Keevil C.W. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen tension. J. Gen. Microbiol. 1992;138:2371–2380. doi: 10.1099/00221287-138-11-2371. [DOI] [PubMed] [Google Scholar]
- Dunn W.A. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Schaible U.E., Schlesinger P.H., Steinberg T.H., Mangel W.F., Kobayashi T., Russell D.G. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 1999;112:681–693. doi: 10.1242/jcs.112.5.681. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J., Meresse S., Parton R.G., Van der Goot G., Sola-Landa A., Lopez-Goni I., Moreno E., Gorvel J.P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detilleux P.G., Deyoe B.L., Cheville N.F. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells Am. J. Vet. Res. 52 1990. 1658 1664a [PubMed] [Google Scholar]
- Detilleux P.G., Deyoe B.L., Cheville N.F. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro Infect. Immun. 58 1990. 2320 2328b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte F., Liautard J.P., Kohler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M.J., Blackwell J.M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovanii: characterization of the promastigote and amastigote glycolipids. J. Biol. Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- Turco S.J., Sacks D.L. Expression of a stage-specific lipophosphoglycan in Leishmania major amastigotes. Mol. Biochem. Parasitol. 1991;45:91–100. doi: 10.1016/0166-6851(91)90030-a. [DOI] [PubMed] [Google Scholar]