Abstract
T cells secreting interleukin (IL)-4 and IL-5 (T helper cell type 2 [Th2] cells) play a detrimental role in a variety of diseases, but specific methods of regulating their activity remain elusive. T1/ST2 is a surface ligand of the IL-1 receptor family, expressed on Th2- but not on interferon (IFN)-γ–producing Th1 cells. Prior exposure of BALB/c mice to the attachment (G) or fusion (F) protein of respiratory syncytial virus (RSV) increases illness severity during intranasal RSV challenge, due to Th2-driven lung eosinophilia and exuberant Th1-driven pulmonary infiltration, respectively. We used these polar models of viral illness to study the recruitment of T1/ST2 cells to the lung and to test the effects of anti-T1/ST2 treatment in vivo. T1/ST2 was present on a subset of CD4+ cells from mice with eosinophilic lung disease. Monoclonal anti-T1/ST2 treatment reduced lung inflammation and the severity of illness in mice with Th2 (but not Th1) immunopathology. These results show that inhibition of T1/ST2 has a specific effect on virally induced Th2 responses and suggests that therapy targeted at this receptor might be of value in treating Th2-driven illness.
Keywords: bronchiolitis, viral; immunity, mucosal; immunity, cellular; pulmonary infection; eosinophil
Introduction
In several common diseases, some CD4+ T cells cause enhanced pathology, while others are protective. Th1 cells produce IFN-γ, activate macrophages, and are associated with inflammatory disorders, whereas Th2 cells secrete predominantly IL-4 and IL-5 and are pivotal in asthma and atopy 1 2 3. Selective depletion of Th1 or Th2 cells would provide a novel therapeutic strategy but requires the identification of reliable markers and targets against which therapy could be directed.
To date, the Th1 phenotype is associated with chemokine receptor 1, 3, and 5 (references 4 and 5) and IL-18 receptor expression 6. The Th2 phenotype, on the other hand, preferentially upregulates the transcription factors c-maf 7 and GATA-3 8, chemokine receptors 3 and 4 (references 4, 9, and 10), and the orphan receptor T1/ST2 (references 11 12 13 14 15). Resting murine Th2 cells constitutively express the multidrug resistance protein transmembrane pump, whereas expression on Th1 and Th2 cells is equal after antigenic stimulation 16 17. Recently, a novel leukocyte chemoattractant receptor expressed on activated human Th2 but not Th1 cells has been reported (designated CRTH2, chemoattractant receptor-homologous molecule expressed on Th2 cells). Allergen-induced proliferation of peripheral blood mononuclear cells is significantly reduced by antibody-mediated depletion of CRTH2+ cells 18.
T1/ST2 is an IL-1 receptor family member originally identified in murine fibroblasts 19 20. Alternate 3′ processing generates a long and short mRNA. The short mRNA encodes a secreted glycoprotein that is constitutively expressed in embryonic tissues and mammary tumors and is inducible in fibroblasts. The long mRNA encodes a membrane-spanning protein expressed only in the lung and hematopoietic tissue 21 22 23. Studies of T1/ST2 knockout mice provide conflicting evidence regarding its functional significance 24 25 26. Anti-T1/ST2 treatment of immunocompetent mice partially inhibits Th2 differentiation and allergic airway inflammation 13, but the effects of anti-T1/ST2 treatment have not been evaluated in virus-infected animals.
Respiratory syncytial virus (RSV) is a common cold virus that causes bronchiolitis in infants, killing up to one million children per year worldwide 27. Children hospitalized with bronchiolitis often suffer recurrent wheezing in later childhood and are frequently diagnosed as asthmatic 28. There is strong evidence that viral bronchiolitis is a T cell–mediated immunopathological condition 29 30. In the BALB/c mouse, primary intranasal infection with RSV causes mild self-limiting illness characterized by transient T cell infiltration. However, prior sensitization to the attachment protein (G; expressed by recombinant vaccinia virus) results in extensive lung eosinophilia and enhanced weight loss after RSV challenge. CD4+ T cells secreting IL-4 and IL-5 are necessary and sufficient for this response 31. This phenomenon is reminiscent of human vaccine trials using formalin-inactivated RSV that caused disease enhancement and peripheral and lung eosinophilia and lead to some deaths in RSV-infected vaccinees 29. After sensitization with the fusion (F) protein, an exuberant T cell infiltrate causes enhanced weight loss, but eosinophil recruitment is not seen 32. A major advantage of the BALB/c model is that immune responses can be manipulated in parallel groups of mice undergoing Th1- or Th2-mediated pathology to the same pathogen.
We now report that CD4+ T cells expressing T1/ST2 occur exclusively during the Th2-driven eosinophilic RSV disease and are reduced in number by treatment with IL-12 (which antagonizes the development of eosinophilia). Furthermore, treatment of mice with anti-T1/ST2 antibody decreases inflammatory cell recruitment and weight loss only in mice with eosinophilic immunopathology. These results indicate that T1/ST2+ cells are specifically associated with RSV-induced eosinophilia in this model and that blockade of this cell surface receptor may be of therapeutic use in Th2-driven illnesses.
Materials and Methods
Generation of mAb 3E10.
The generation and characterization of rat anti-T1/ST2 mAb (clone 3E10) is described elsewhere 15. In brief, a DNA sequence containing the extracellular domain of T1/ST2 was amplified by PCR and cloned into a vector containing the CD5 signal sequence and the human IgG1 constant region. COS cells were transiently transfected by using the LipofectAMINE™ (GIBCO BRL) protocol and cultured in Ultra Low IgG Fetal Bovine Serum™ (GIBCO BRL) for 1 wk. Recombinant protein was purified from culture supernatants using a protein A column. Lou/M rats were immunized with 0.5 mg of purified recombinant T1/ST2 subcutaneously, followed by intraperitoneal boosting twice at 2-wk intervals. Sera were analyzed for reactivity to the fusion protein by ELISA 10 d after the final boost. Animals with serum antibody to T1/ST2 were boosted again 4 wk later and killed after a further 3 d. Splenocytes were fused with SP/2 myeloma cells, and resulting clones were screened for selective binding to murine Th2 clones. The hybridoma 3E10 produced a blocking rat IgG1 anti-T1/ST2 mAb. Serum from unimmunized Lou/M rats was used as negative control.
Mice and Virus Stocks.
8–10-wk-old female BALB/c mice were purchased from Harlan Olac Ltd. and kept in pathogen-free conditions. RSV and recombinant vaccinia virus expressing the attachment protein (Gvac) or the fusion protein (Fvac) of RSV or control β-galactosidase (β gal-vac) were grown in HEp-2 cells and assayed for infectivity as previously described 33. All stocks were mycoplasma free as assessed by DNA hybridization (Gen-Probe Inc.).
Mouse Infection and Treatment.
Anesthetized mice were scarified on the rump on day 0 with 3 × 106 pfu Gvac, Fvac, or β gal-vac in a final volume of 10 μl (four or five mice per group). On day 14, mice were challenged intranasally with 3 × 106 pfu of human RSV (A2 strain). Some mice were injected intravenously with 100 μg of anti-T1/ST2 (rat IgG) or isotype-matched control antibody each day, starting 1 d before RSV challenge. In addition, some mice were treated with recombinant IL-12 (300 ng in PBS) daily from day −2 to day +2 relative to scarification as described previously 34. The appearance of illness was scored by a blinded observer, and the weights of mice were monitored daily after RSV challenge. Illness was scored using the following scale: 0 = healthy; 1 = barely ruffled fur; 2 = ruffled fur but active; 3 = ruffled fur and inactive; 4 = ruffled fur, inactive, and hunched; and 5 = dead. Mice were killed on day 21 (7 d after intranasal RSV challenge) by injection of 3 mg of pentobarbitone and exanguinated via the femoral vessels.
Lung Virus Titer.
Clearance of RSV was assessed in lung homogenates on days 2 and 4 after virus challenge. Lungs were removed from four mice per group and homogenized. After centrifugation at 4,000 rpm for 4 min, the supernatant was titrated in doubling dilutions on HEp-2 cell monolayers in 96-well flat-bottomed plates. 24 h later, monolayers were washed and incubated with peroxidase-conjugated goat anti-RSV antibody (Biogenesis). Infected cells were detected using 3-amino-9-ethylcarbazole (AEC), and infectious units were enumerated by light microscopy.
Cell Recovery.
After sacrifice, bronchoalveolar lavage (BAL) fluid, lung tissue, and serum were harvested as previously described 35. In brief, the lungs of each mouse were inflated six times with 1 ml of 12 mM lidocaine in Eagle's MEM and placed undiluted on ice in sterile tubes. 100 μl of BAL fluid from each mouse was cytocentrifuged onto glass slides, and eosinophils were enumerated using hematoxylin and eosin staining based on cell morphology and presence of red granules. The remainder of the BAL fluid was centrifuged, and the supernatant was removed and stored at −70°C in 200-μl aliquots for analysis of cytokines by ELISA. The pelleted cells were resuspended in RPMI containing 10% FCS, 2 mM/ml l-glutamine, 50 U/ml penicillin, and 50 mg/ml streptomycin (R10F). Viable cells were counted by trypan blue exclusion
Flow Cytometric Analysis.
T1/ST2 antibody (2 μg) or control antibody was added to 106 pelleted cells on ice for 30 min; cells were then washed and treated with goat anti–rat FITC (Sigma-Aldrich). After blocking with rat serum, Quantum Red™–conjugated CD8 or B220 and PE-conjugated CD4 or DX5 (anti-NK cell antibody) (Sigma-Aldrich) were added for 30 min. After washing again, cells were fixed for 20 min at room temperature with 2% formaldehyde. Samples were analyzed on a Beckman Coulter EPICS Elite™ flow cytometer collecting data on at least 40,000 lymphocytes. Eosinophils were enumerated as granulocytes by flow cytometry using their distinctive forward and side scatter properties.
Cytokine ELISAs.
IL-4, IL-5, IFN-γ, and TNF-α in BAL fluid were quantified using OptEIA™ kits from PharMingen. In brief, microtiter plates were coated with 100 μl of capture antibody diluted in the recommended buffer overnight at 4°C. After five washes with PBS containing 0.5% Tween-20, plates were blocked with 200 μl of PBS containing 10% FCS and left for 1 h at room temperature. Samples and standards (diluted in PBS plus 10% FCS) were then incubated for a further 2 h at room temperature. After five washes, bound cytokine was detected using biotinylated antibodies premixed with avidin–horseradish peroxidase followed by tetramethylbenzidine and hydrogen peroxidase. Optical densities were read at 450 nm. The mean optical density of wells containing no cytokine was subtracted from the results obtained for samples and standards. The concentration of cytokine in each sample was calculated from a standard curve using linear regression analysis. The standard curve range for IL-4 lies between 7.8 and 500 pg/ml, for IL-5 between 15.6 and 1,000 pg/ml, for TNF between 15.6 and 1,000 pg/ml, and for IFN-γ between 31.3 and 2,000 pg/ml.
RSV-specific Antibody ELISA.
Serum antibody was assessed by ELISA as described previously 36. ELISA antigen was prepared by infecting HEp-2 cells with RSV strain A2 at 1 pfu/cell. When significant cytopathic effect was observed the infected cells were harvested, centrifuged at 400 g, resuspended in 3 ml of distilled water, and then subjected to 2 min of sonication (Ultrawave Ltd.). Uninfected HEp-2 cells were similarly treated and used as control antigen. 50-μl aliquots were stored at −20°C until required. Microtiter plates were coated overnight with 100 μl of a 1:200 dilution of either sonicated RSV or HEp-2 control antigen. After blocking with 2% normal rabbit serum for 2 h, dilutions of test samples (diluted in PBS containing 1% HEp-2 lysate) were added for a further hour at room temperature. Bound antibody was detected using peroxidase-conjugated rabbit anti–mouse Ig and O-phenylene-diamine as a substrate. The reaction was stopped with 50 μl of 2.5 M sulfuric acid. Optical densities were read at 490 nm. The amount of RSV-specific antibody was determined by subtracting the optical density obtained by incubating serum on HEp-2–coated plates from the same sample incubated on RSV-coated plates.
Statistical Analysis.
One-way ANOVA (analysis of variance) assuming unequal variance was used and significance assumed at P < 0.05.
Results
To simplify the description in Results and Discussion, we will refer to Gvac-primed, RSV-challenged mice as G/RSV, Fvac-primed, RSV-challenged mice as F/RSV, and control β gal-vac–primed, RSV-challenged mice as β gal/RSV.
T1/ST2+ Cells Are Present in the BAL during Eosinophilic Illness.
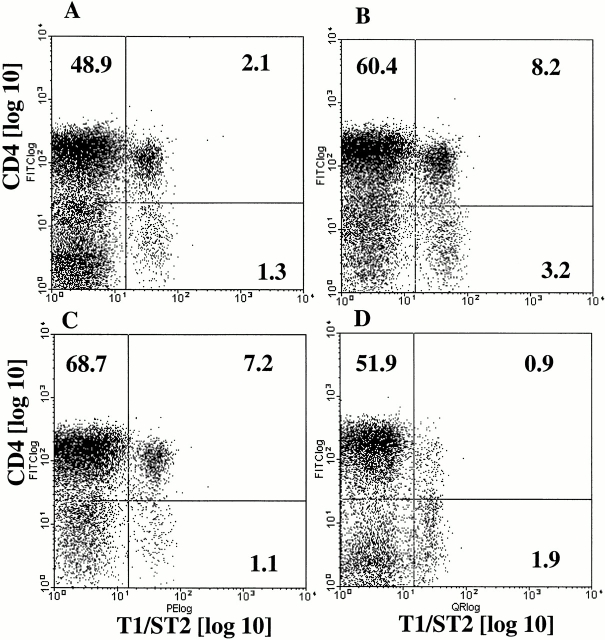
No T1/ST2+ cells were observed in the lungs of uninfected mice (data not shown). T1/ST2+ cells were apparent 3 d after RSV challenge in G/RSV (which showed lung eosinophilia) but not F/RSV mice (which did not have lung eosinophilia). T1/ST2+ cells were CD4+ (4.4% of CD4+ T cells at day 3; Fig. 1 A) and did not express CD8, B220 (CD45R), or DX5 (data not shown). The number of T1/ST2+ cells in the lungs peaked at day 5 after RSV challenge in G/RSV mice (13.3% of CD4+ T cells; Fig. 1 B) and were mostly CD45RB low (>90%), indicating recent activation. A small population of T1/ST2+ cells were CD4− but did not express any of the other lymphoid markers described above. It is possible that these cells have downregulated CD4 expression after antigen activation. These data show that T1/ST2+ cells are present only in the lungs of mice undergoing eosinophilic inflammatory responses.
Figure 1.
T1/ST2+ cells are present in the lung during viral induced eosinophilia. Mice were scarified with Gvac and challenged intranasally with RSV 14 d later. T1/ST2+ cells were identified in BAL samples by indirect immunofluorescence. Cells were then blocked with rat Ig and stained with quantum red conjugated antibodies to CD4. Samples were analyzed on a Beckman Coulter EPICS Elite™ flow cytometer collecting data on at least 40,000 cells. A and B show T1/ST2+ cells in G/RSV mice at days 3 and 5 after intranasal RSV challenge. C and D show G/RSV mice treated with PBS (C) or IL-12 (D) at 4 d after intranasal RSV challenge. Representative plots for five mice per group are shown.
Anti-T1/ST2 Treatment Prevents Enhanced Illness in G/RSV but Not in F/RSV Mice.
During a primary RSV infection, BALB/c mice experience mild weight loss after 5–6 d, coinciding with the peak of the inflammatory infiltrate. In G/RSV or F/RSV mice, the inflammatory infiltrate is greater and weight loss is enhanced and accelerated. However, anti-T1/ST2 treatment only prevented weight loss in G/RSV (eosinophilic) animals and had no effect on F/RSV (noneosinophilic) mice (Fig. 2). Similarly, there was no effect of anti-T1/ST2 treatment in β gal/RSV mice undergoing a primary RSV infection (data not shown).
Figure 2.
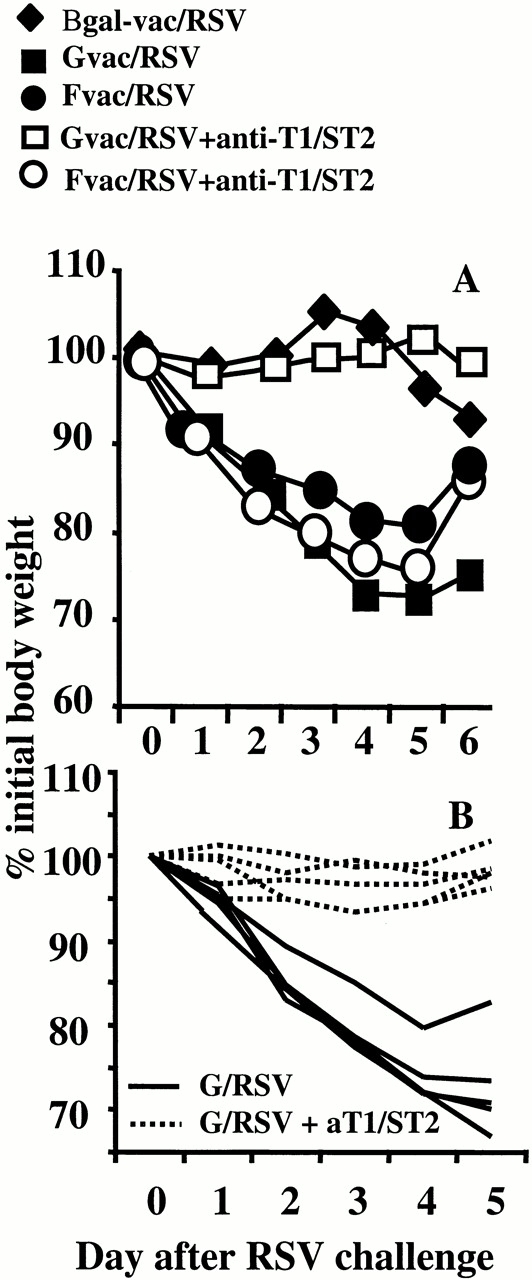
Anti-T1/ST2 treatment prevents RSV-induced weight loss during immune responses dominated by Th2-driven eosinophilia. Mice were primed with Gvac, Fvac, or β gal-vac and challenged with RSV intranasally 14 d later. Half of each group was given anti-T1/ST2 antibody daily from the day before intranasal RSV challenge (open symbols), whereas the other half was given isotype-matched irrelevant antibody (closed symbols). The percent weight loss or gain on subsequent days was calculated from the original weight of each individual mouse. The mean and standard deviation of five individual mice are shown (A). The data for β gal/RSV mice treated with anti-T1/ST2 is not shown for clarity, but weight loss was not significantly different from control treated β gal/RSV mice. In B, a separate experiment with just G/RSV-infected mice is shown. Each line represents an individual mouse treated with anti-T1/ST2 (dotted line) or irrelevant isotype-matched control (solid line).
Anti-T1/ST2 Treatment Reduces Inflammatory Cell Influx and BAL Cytokine Levels in G/RSV but Not in F/RSV Mice.
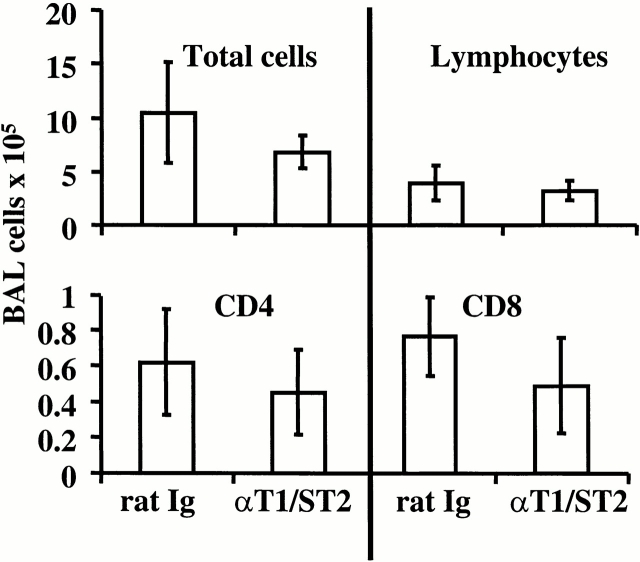
Anti-T1/ST2+ treatment significantly reduced total BAL cell recovery from G/RSV mice, affecting eosinophils, lymphocytes, CD4+ T cells, and CD8+ T cells (Fig. 3). The reduction of eosinophils was particularly prominent, as both the proportion and total number present in the sample were reduced by anti-T1/ST2 treatment. A corresponding reduction in BAL fluid TNF, IL-5, and IFN-γ (but not IL-4) was also observed (Fig. 4). Anti-T1/ST2 treatment had no significant effect on cell recruitment or phenotype in F/RSV mice (P > 0.05 for all comparisons; Fig. 5). Furthermore, no T1/ST2-expressing cells were found in the BAL of anti-T1S/T2–treated mice (data not shown).
Figure 3.
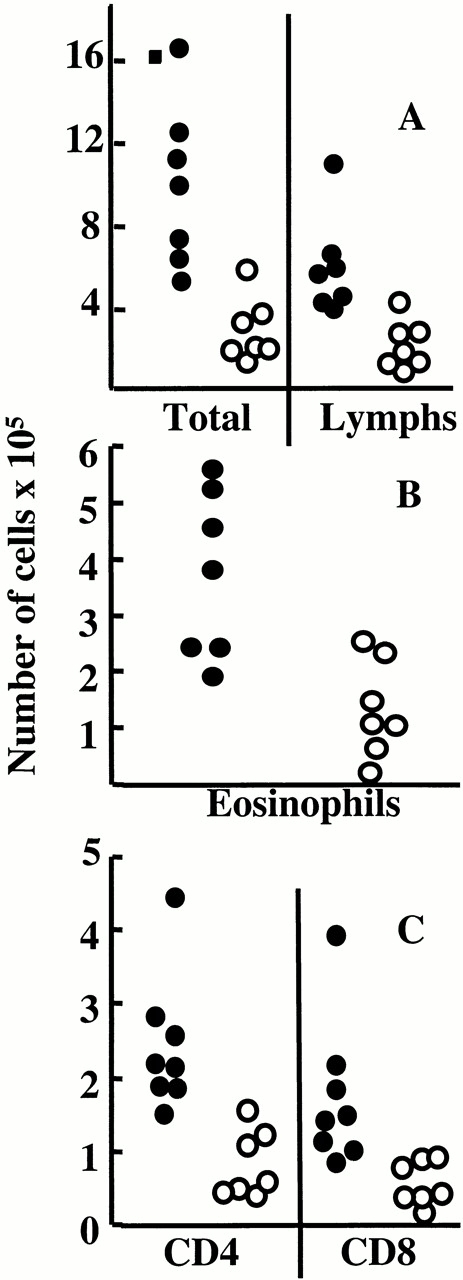
Anti-T1/ST2 treatment reduces inflammation in the lung. G/RSV-infected mice were treated with rat Ig (closed symbols) or anti-T1/ST2 (open symbols), and cells from the airways were removed by BAL. Total viable cells were determined by trypan blue exclusion. Total lymphocytes were calculated by multiplying this number by the percent lymphocytes identified in forward and side scatter plots by flow cytometry (the position of lymphocytes being determined by expression of T and B cell markers). In the BAL, both total cell numbers (P = 0.02) and lymphocyte numbers (P = 0.03) were decreased in the anti-T1/ST2–treated group. (A). The percent of BAL eosinophils (B) was determined by differential counting in hematoxylin and eosin–stained cytocentrifuge preparations (identified by nuclear morphology and presence of eosinophilic granules). The data represents this percentage multiplied by the total viable cell count and is lower in the anti-T1/ST2 treated group (P = 0.005). The number of CD4+ and CD8+ T cells (C) is calculated by multiplying the percent of each subset (by flow cytometry) by the number of lymphocytes present. Both CD4+ (P = 0.02) and CD8+ T cells (P = 0.03) are fewer in anti-T1/ST2–treated mice. Each point represents an individual mouse from one of two independent experiments.
Figure 4.
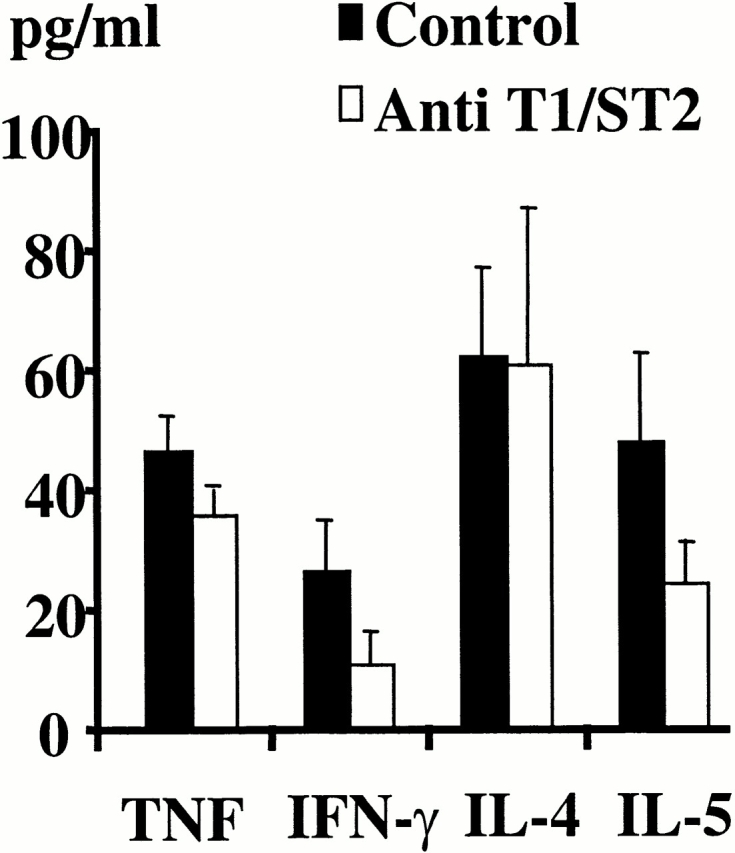
Anti-T1/ST2 treatment reduces cytokine production in the lung. G/RSV-infected mice were treated with rat Ig control (solid bars) or anti-T1/ST2 (open bars). Lung lavage was performed 7 d after RSV challenge, and the cytokines in the BAL supernatant were assayed by ELISA. The results represent the mean and standard deviation of four mice per group and are representative of two independent experiments. Anti-T1/ST2 treatment decreases TNF (P = 0.04), IFN-γ (P = 0.029), and IL-5 (P = 0.04), but not IL-4.
Figure 5.
Anti-T1/ST2 treatment has no effect on the influx of cells into the lungs of mice primed with the fusion protein. Mice were scarified with Fvac and intranasally challenged with RSV 14 d later. Mice were treated with rat Ig or anti-T1/ST2 as shown. The number of total cells, lymphocytes, and CD4+ and CD8+ T cells was determined as described in Fig. 3. The results represent the mean and standard deviation of groups of five mice. Similar results were obtained in a separate experiment containing four mice per group.
Anti-T1/ST2 Treatment Reduces RSV-specific Antibody and Viral Clearance in G/RSV but Not F/RSV Mice.
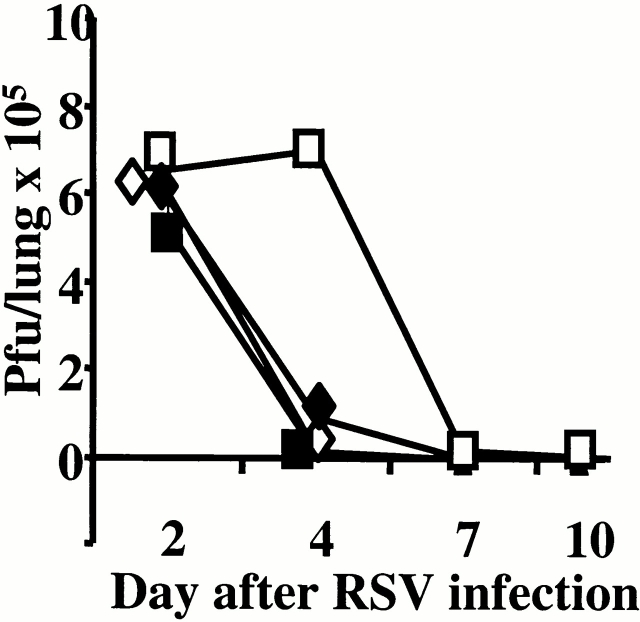
Prior scarification with Gvac or Fvac induces enhanced virus-specific antibody production after RSV challenge compared with mice infected with RSV alone. Anti-T1/ST2 treatment reduced this boost in antibody levels in G/RSV but not F/RSV mice (Table ). The reduction in inflammatory infiltrate and antibody titer was reflected by the delayed clearance of RSV from the lung in anti-T1/ST2–treated animals. On day 2 after RSV challenge, there was no difference in the titer of RSV recovered from the lungs of treated and untreated mice. However, G/RSV-infected mice treated with anti-T1/ST2 had reduced viral clearance at day 4 compared with untreated mice (P > 0.01), but treatment did not affect RSV clearance in F/RSV animals (P > 0.05) (Fig. 6). No virus was recovered from any group at day 7 or 10 after RSV infection.
Table 1.
Depletion of T1/ST2 Reduces Antibody Titers in G/RSV-infected Mice
| Mouse group | Endpoint antibody titer |
|---|---|
| F/RSV control | 1/3,218 ± 1/1,121 |
| F/RSV + anti-T1/ST2 | 1/3,413 ± 1/916 |
| G/RSV control | 1/1,638 ± 1/458 |
| G/RSV + anti-T1/ST2 | 1/107 ± 1/37 |
Mice were scarified with Gvac or Fvac and intranasally infected with whole RSV after 14 d. Mice were treated with anti-T1/ST2 or control Ig as indicated. Serum was removed 7 d after the intranasal RSV challenge and tested in an RSV-specific ELISA.
Figure 6.
Anti-T1/ST2 treatment slows the elimination of RSV from the lungs in eosinophilic mice. Mice were scarified with Fvac (diamonds) or Gvac (squares) and challenged with RSV 14 d later. At the time points shown, lungs were removed from rat Ig–treated (closed symbols) or anti-T1/ST2–treated (open symbols) mice. Homogenized lung was assessed for the presence of RSV by plaque assay on HEp-2 cells. The results represent the mean from four individual mice.
G/RSV Mice Treated with Recombinant IL-12 Have Reduced Numbers of T1/ST2+ Cells.
We have previously shown that IL-12 treatment reduces pulmonary eosinophilia but also enhances the weight loss and illness in animals infected with G/RSV 34. We therefore tested the effect of IL-12 on the recruitment of T1/ST2+ cells to the lung. Mice were scarified with Gvac on day 0 and treated intraperitoneally with IL-12 or PBS daily from day −2 to day +2. In this experiment, 10.5% of CD4+ BAL T cells expressed T1/ST2 on day 4 of RSV infection (Fig. 1 C); IL-12 treatment reduced this figure to <1% (Fig. 1 D). Similar to previous reports 34, IL-12 treatment enhanced cellular recruitment to the lung in the G/RSV group, which was not significantly affected by anti-T1/ST2 treatment (Fig. 7 A). IL-12 treatment caused a reduction in pulmonary eosinophilia (P = 0.005), which was unaffected by the additional depletion of T1/ST2+ cells (Fig. 7 B; P = 0.07). Therefore, the proportion of recovered T1/ST2+ cells from eosinophilic mice is reduced by IL-12 treatment.
Figure 7.
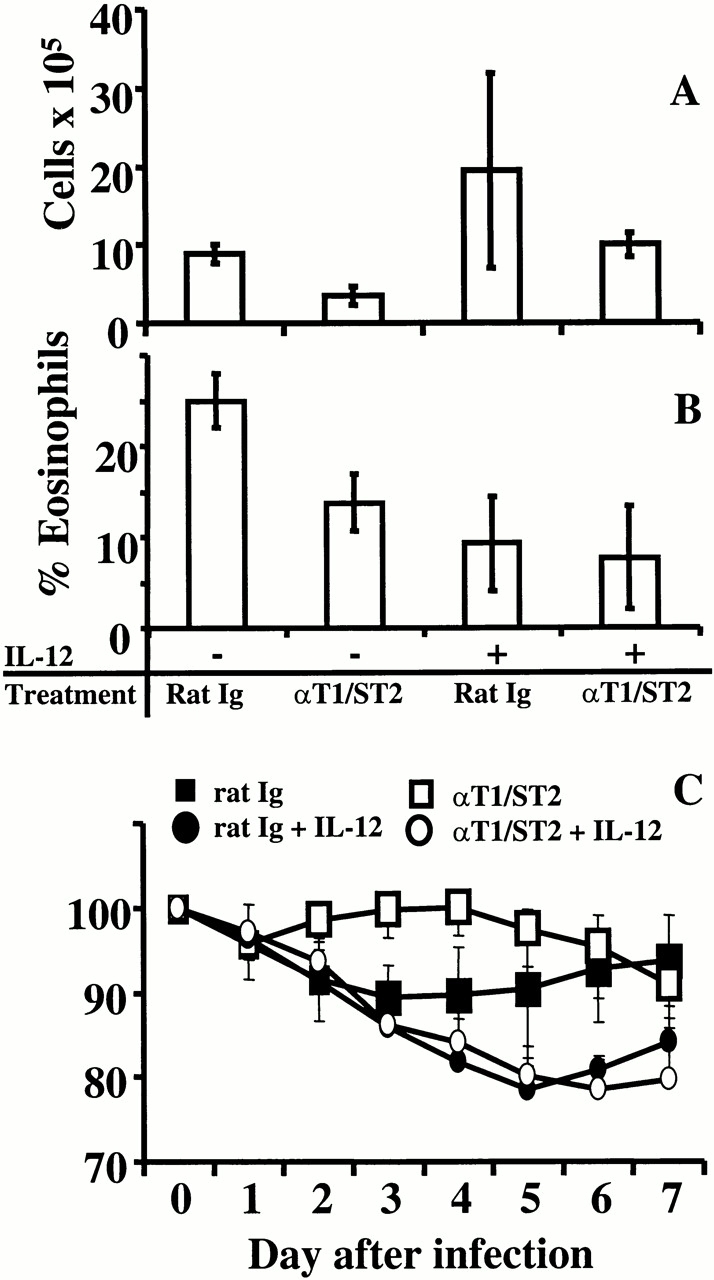
Anti-T1/ST2 treatment does not alter illness in IL-12–treated mice. Mice were scarified with Gvac on day 0 and treated with PBS (−) or IL-12 (+) from days −2 to +2. RSV was administered intranasally on day 14, and lung lavage was performed after a further 7 d. Total viable cells (A) were determined by trypan blue exclusion, and the percent eosinophilia (B) was determined by histological analysis of hematoxylin and eosin–stained cytocentrifuge preparations. Anti-T1/ST2 treatment decreased the percentage of eosinophils (P = 0.005), as did IL-12 treatment alone, but IL-12 treatment in conjunction with anti-T1/ST2 did not further decrease this percentage. Weight loss (C) was monitored throughout RSV challenge and represents the percentage of initial starting weight. Open symbols represent mice treated with anti-T1/ST2 antibody; closed symbols represent mice treated with rat Ig. Circles represent IL-12–treated mice; PBS control mice are represented by squares. The mean and standard deviation of five individual mice is shown.
As shown in Fig. 2 and Fig. 7 C, anti-T1/ST2 treatment prevented weight loss in G/RSV-infected mice. IL-12 treatment of G-primed mice enhanced weight loss during RSV challenge, as previously described 34. However, blocking of anti-T1/ST2 had no effect on IL-12–enhanced weight loss in G/RSV mice (Fig. 7 C). Therefore, although anti-T1/ST2 treatment prevented weight loss in G/RSV mice, it had no effect when the immune response to the G protein is altered by IL-12 and skewed towards a Th1 phenotype or dominated by CD8+ T cells.
Discussion
Our results show that anti-T1/ST2 treatment reduces the severity of Th2-mediated eosinophilic immunopathology in the BALB/c mouse model of RSV infection. Treatment decreased weight loss, local recruitment of inflammatory cells (including eosinophils), and lung lavage levels of IL-5, TNF, and IFN-γ in RSV-infected mice presensitized with the attachment protein G. However, no benefit of anti-T1/ST2 treatment was found in the noneosinophilic immunopathology seen in F/RSV mice or in G/RSV mice previously treated with IL-12.
Previous studies show that T1/ST2 is preferentially expressed on T cells that predominantly produce IL-4, IL-5, or IL-10 but not IFN-γ or IL-2 15. Expression is found in Th2-induced granulomatous lung disease of Schistosoma mansoni infection 14, and in vitro studies demonstrate upregulation of T1/ST2 under Th2-polarizing conditions 11. Signaling through T1/ST2 after adoptive transfer of OVA-treated dendritic cells is essential for sensitization to inhaled OVA and development of Th2-dependent airway eosinophilia 37. In this study, T1/ST2 expression was only observed in G protein–sensitized (eosinophilic) mice and not in those previously sensitized with the fusion protein or those experiencing a primary RSV infection (both noneosinophilic). These results, together with the observation that IL-12 treatment reduces T1/ST2 expression in G/RSV-infected mice, support the Th2-restricted nature of this molecule.
The ability of anti-T1/ST2 antibody treatment of G/RSV-infected mice to abrogate T1/ST2 expression on BAL cells does not clarify whether T1/ST2+ cells are depleted or the receptor merely masked by the antibody. The effect on Th2-mediated responses was striking, however. Anti-T1/ST2 treatment was accompanied by a reduction in IL-5, TNF, and IFN-γ but not IL-4 levels in the lung lavage of G/RSV-infected animals. The reduction in pulmonary eosinophilia may be explained by reduced IL-5 and is similar to that reported in T1/ST2 knockout mice infected with S. mansoni eggs 24. The reduction in both Th1 and Th2 cytokines is intriguing considering that reduced IL-4 and IL-5 but increased IFN-γ is observed when OVA-specific Th2 cells are incubated with a T1/ST2 Ig fusion protein in vitro 11. When OVA-specific cells are adoptively transferred, however, T1/ST2 depletion reduces Th2 cytokine levels in the lung lavage but does not significantly alter IFN-γ. Mediastinal lymph node cells from T1/ST2 knockout mice produce less IL-4 and IL-5 and increased IFN-γ in response to S. mansoni eggs 38. The discrepancies between our results and those of others are likely to reflect the nature of the antigen (i.e., replicating versus nonreplicating) and the infected cell type. Our results also suggest that removal of T1/ST2-expressing cells does not necessarily enhance Th1 responses. The G protein only induces CD4+ T cells and, in the absence of CD8+ T cells, these assume a Th2 cytokine profile 36. As anti-T1/ST2 treatment was performed during the RSV challenge (not during recombinant vaccinia priming), the induction of a replacement Th1 population would not be anticipated. The lack of an effect on IL-4 in lavage fluid of anti-T1/ST2–treated, G/RSV-infected mice may indicate IL-4 production by an alternative cell type. CD4+NK1.1+ cells secrete IL-4 but are T1/ST2− 15 and would therefore not be inhibited in our experimental design.
The reduced weight loss in the G/RSV group treated with anti-T1/ST2 may be linked to lower TNF levels. TNF has multiple actions and plays a critical role in inflammation. TNF also plays an important role in recruitment of cells to normal and inflamed tissues 39 40 and may induce early neutrophil and eosinophil recruitment 41. When released in large quantities, it enters the bloodstream and is associated with weight loss and cachexia. TNF production is seen predominantly, but not exclusively, in T1/ST2−CD4+ T cells 14, and the reduced levels of this cytokine may be due in part to the decreased total inflammatory cell recruitment seen with T1/ST2 depletion in this study. A reduction in S. mansoni–induced primary granuloma volume in T1/ST2 knockout mice 24 is also consistent with the reduced inflammatory infiltrate seen in our study. No previous reports show a decrease in TNF by T1/ST2 inhibition. T1/ST2 depletion did not inhibit illness in G/RSV-infected mice treated with IL-12 or those previously sensitized with the F protein. As IL-12 treatment reduces T1/ST2+ cells and such cells are scarce in F protein–sensitized mice, anti-T1/ST2 treatment does not alter total cell recruitment to the lung and weight loss remains unaltered.
An enhanced T cell–dependent antibody response occurs in G/RSV-infected mice and promotes rapid viral clearance compared with mice experiencing a primary infection (β gal/RSV). Considering the reduced T cell recruitment to the lung, it is not surprising that anti-T1/ST2 treatment decreases RSV-specific antibody production and delays viral clearance. To our knowledge, there are no previous reports of delayed clearance of a pathogen due to anti-T1/ST2 treatment. As illness in this model does not depend on uncontrolled viral replication but rather on the extent of cell recruitment, the beneficial effects of T1/ST2 inhibition are not offset by the delayed viral clearance. A reduction in antibody has previously been observed in OVA-challenged mice after T1/ST2 depletion 11 but not in T1/ST2 knockout animals infected with S. mansoni 24.
Studies of T1/ST2-knockout mice have produced conflicting results. One group reported that T1/ST2-deficient animals fail to show decreased Th2 cytokine or Ig production in response to Nippostrongylus braziliensis infection or allergen-induced airway inflammation 25. An independently derived T1/ST2 knockout model, however, shows that basal immunological functions are not affected but that Th2 cytokine responses are impaired in a fashion similar to that seen in depletion studies. The findings in the latter study suggest that this molecule plays an important role in the early events of Th2 development, as primary but not secondary granuloma formation was of decreased magnitude 24. In another study, both a T1/ST2-deficient mouse strain and a transgenic mouse strain expressing high levels of an extracellular T1/ST2 domain–Fc fusion protein (acting as T1/ST2 ligand scavenger) cleared N. brasiliensis infection as efficiently as control mice. Lung lymphocytes, however, did produce less IL-5, and these mice had a slightly diminished recruitment of eosinophils into the lung 26. As T1/ST2 is not only expressed on CD4+ T cells but also on mast cells and fibroblasts 42, the knockout mice may not only reflect T helper cell phenotype modulation but also harbor other developmental changes.
Inhibition of specific Th cell phenotypes is a promising approach to the treatment of various inflammatory conditions. Th2 inhibition may be beneficial in both prevention and management of allergic inflammation, but to date treatments have not been able to reliably counter Th2-driven illness in the clinical setting. T1/ST2 neutralization may offer a new therapeutic opportunity, but more information is needed to define its role. In these studies, we have been able to demonstrate a beneficial role for anti-T1/ST2 antibody treatment in selective Th2-directed immunopathology in G-primed mice during RSV infection. Our results offer the prospect that selective anti-T1/ST2 treatment will be beneficial in Th2-driven conditions.
Acknowledgments
We thank Professor Brigitte Askonas for critical comments and Professor G. Wertz for supplying vaccinia virus stocks.
This work was supported by the Wellcome Trust (003296), the Royal Society (RSRG21219), the Medical Research Council UK (B0000077), and the National Asthma Campaign (00/007).
Footnotes
Abbreviations used in this paper: BAL, bronchoalveolar lavage; RSV, respiratory syncytial virus.
Gerhard Walzl and Tracy Hussell's present address is Centre for Molecular Microbiology and Infection, Department of Biochemistry, Imperial College of Science, Technology and Medicine, London SW7 2AZ, UK.
References
- Sher A., Coffman R.L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Sad S. The expanding universe of T-cell subsetsTh1, Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D'Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P., Uguccioni M., Bordoli L., Baggiolini M., Moser B., Chizzolini C., Dayer J.M. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- Xu D., Chan W.L., Leung B.P., Hunter D., Schulz K., Carter R.W., McInnes I.B., Robinson J.H., Liew F.Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J. Exp. Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I.C., Hodge M.R., Rooney J.W., Glimcher L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Zheng W., Flavell R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Mackay C.R., Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Mackay C.R., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Coyle A.J., Lloyd C., Tian J., Nguyen T., Erikkson C., Wang L., Ottoson P., Persson P., Delaney T., Lehar S. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2–mediated lung mucosal immune responses. J. Exp. Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf P., Schopf L.R., Chung C.L., Xu D., Liew F.Y., Sypek J.P., Muller I. Expression of Th2 cytokines and the stable Th2 marker ST2L in the absence of IL-4 during Leishmania major infection. Eur. J. Immunol. 1999;29:3621–3628. doi: 10.1002/(SICI)1521-4141(199911)29:11<3621::AID-IMMU3621>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Xu D., Chan W.L., Leung B.P., Huang F., Wheeler R., Piedrafita D., Robinson J.H., Liew F.Y. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohning M., Grogan J.L., Coyle A.J., Yazdanbakhsh M., Meisel C., Gutierrez R.J., Radbruch A., Kamradt T. T1/ST2 expression is enhanced on CD4+ T cells from schistosome egg-induced granulomasanalysis of Th cell cytokine coexpression ex vivo. J. Immunol. 1999;162:3882–3889. [PubMed] [Google Scholar]
- Lohning M., Stroehmann A., Coyle A.J., Grogan J.L., Lin S., Gutierrez R.J., Levinson D., Radbruch A., Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff M., Prechtl S., Sommer F., Roellinghoff M., Schmitt E., Gradehandt G., Rohwer P., Stride B.D., Cole S.P., Deeley R.G. A multidrug-resistance protein (MRP)-like transmembrane pump is highly expressed by resting murine T helper (Th) 2, but not Th1 cells, and is induced to equal expression levels in Th1 and Th2 cells after antigenic stimulation in vivo. J. Clin. Invest. 1998;101:703–710. doi: 10.1172/JCI824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtl S., Roellinghoff M., Scheper R., Cole S.P., Deeley R.G., Lohoff M. The multidrug resistance protein 1a functionally important activation marker for murine Th1 cells. J. Immunol. 2000;164:754–761. doi: 10.4049/jimmunol.164.2.754. [DOI] [PubMed] [Google Scholar]
- Nagata K., Tanaka K., Ogawa K., Kemmotsu K., Imai T., Yoshie O., Abe H., Tada K., Nakamura M., Sugamura K. Selective expression of a novel surface molecule by human Th2 cells in vivo. J. Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- Rossler U., Andres A.C., Reichmann E., Schmahl W., Werenskiold A.K. T1, an immunoglobulin superfamily member, is expressed in H-ras-dependent epithelial tumours of mammary cells. Oncogene. 1993;8:609–617. [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Werenskiold A.K. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA. 1989;86:5708–5712. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa K., Takagi T., Tsukamoto T., Tetsuka T., Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- Rossler U., Thomassen E., Hultner L., Baier S., Danescu J., Werenskiold A.K. Secreted and membrane-bound isoforms of T1, an orphan receptor related to IL-1-binding proteins, are differently expressed in vivo. Dev. Biol. 1995;168:86–97. doi: 10.1006/dbio.1995.1063. [DOI] [PubMed] [Google Scholar]
- Bergers G., Reikerstorfer A., Braselmann S., Graninger P., Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M.J., Fallon P.G., Matthews D.J., Jolin H.E., McKenzie A.N.J. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 2000;191:1069–1075. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Kashiwamura S., Kuribayashi K., Kodama T., Tsujimura T., Nakanishi K., Matsuyama T., Takeda K., Akira S. The absence of IL-1R–related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 1999;190:1541–1547. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn K.A., McCoy K.D., Maloy K.J., Stark G., Frohli E., Rulicke T., Klemenz R. T1-deficient and T1-Fc-transgenic mice develop a normal protective Th2-type immune response following infection with Nippostrongylus brasiliensis . Eur. J. Immunol. 2000;30:1929–1938. doi: 10.1002/1521-4141(200007)30:7<1929::AID-IMMU1929>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Simoes E.A. Respiratory syncytial virus and subsequent lower respiratory tract infections in developing countriesa new twist to an old virus. J. Pediatr. 1999;135:657–661. doi: 10.1016/s0022-3476(99)70079-x. [DOI] [PubMed] [Google Scholar]
- Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitisa prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- McIntosh K., Fishaut J.M. Immunopathologic mechanisms in lower respiratory tract disease of infants due to respiratory syncytial virus. Prog. Med. Virol. 1980;26:94–118. [PubMed] [Google Scholar]
- Chanock R.M., Parrott R.H., Connors M., Collins P.L., Murphy B.R. Serious respiratory tract disease caused by respiratory syncytial virusprospects for improved therapy and effective immunization. Pediatrics. 1992;90:137–143. [PubMed] [Google Scholar]
- Alwan W.H., Kozlowska W.J., Openshaw P.J.M. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan W.H., Openshaw P.J.M. Distinct patterns of T and B cell immunity to respiratory syncytial virus induced by individual proteins. Vaccine. 1993;11:431–437. doi: 10.1016/0264-410x(93)90284-5. [DOI] [PubMed] [Google Scholar]
- Bangham C.R.M., Cannon M.J., Karzon D.T., Askonas B.A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J. Virol. 1985;56:55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T., Khan U., Openshaw P.J.M. IL-12 treatment attenuates Th2 and B cell responses but does not improve vaccine-enhanced lung illness. J. Immunol. 1997;159:328–334. [PubMed] [Google Scholar]
- Hussell T., Spender L.C., Georgiou A., O'Garra A., Openshaw P.J.M. Th1 and Th2 cytokine induction in pulmonary T-cells during infection with respiratory syncytial virus. J. Gen. Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- Hussell T., Baldwin C.J., O'Garra A., Openshaw P.J.M. CD8+ T-cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N., De V.M., Coyle A.J., Gutierrez R.J., Thielemans K., Pauwels R.A. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esolen L.M., Ward B.J., Moench T.R., Griffin D.E. Infection of monocytes during measles. J. Infect. Dis. 1993;168:47–52. doi: 10.1093/infdis/168.1.47. [DOI] [PubMed] [Google Scholar]
- Sean R.D., Korner H., Strickland D.H., Lemckert F.A., Pollard J.D., Sedgwick J.D. Challenging cytokine redundancyinflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J. Exp. Med. 1998;187:1517–1528. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J.D., Riminton D.S., Cyster J.G., Korner H. Tumor necrosis factora master-regulator of leukocyte movement. Immunol. Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- Lukacs N.W., Strieter R.M., Chensue S.W., Widmer M., Kunkel S.L. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J. Immunol. 1995;154:5411–5417. [PubMed] [Google Scholar]
- Gachter T., Werenskiold A.K., Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J. Biol. Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]