Abstract
The physiologic role of L-selectin shedding is unknown. Here, we investigate the effect of L-selectin shedding on firm adhesion and transmigration. In a tumor necrosis factor α–induced model of inflammation, inhibition of L-selectin shedding significantly increased firm adhesion and transmigration by a lymphocyte function–associated antigen (LFA)-1 and intercellular adhesion molecule (ICAM)-1–dependent mechanism. We examined the quality of leukocyte rolling and L-selectin–mediated signaling. Blockade of L-selectin shedding significantly reduced the “jerkiness” of leukocyte rolling, defined as the variability of velocity over time. A low level of jerkiness was also observed in the rolling of microbeads conjugated with L-selectin, a model system lacking the mechanism for L-selectin shedding. Inhibition of L-selectin shedding potentiated activation of LFA-1 and Mac-1 induced by L-selectin cross-linking as shown by activation epitope expression and binding of ICAM-1–conjugated beads. We conclude that inhibition of L-selectin shedding increases leukocyte adhesion and transmigration by (a) increasing leukocyte exposure to the inflamed endothelium by decreasing jerkiness and (b) promoting leukocyte activation by outside-in signaling. These observations help to resolve the apparent discrepancy between the minor contribution of L-selectin to rolling and the significant leukocyte recruitment defect in L-selectin knockout mice.
Keywords: inflammation, signaling, trafficking, rolling velocity, ICAM-1
Introduction
Leukocyte rolling, the initial step in the recruitment of leukocytes to sites of acute inflammation, is followed by leukocyte activation, firm adhesion, and transmigration into the interstitial tissue 1. L-selectin contributes to physiologic leukocyte rolling 2 and is rapidly shed from the surface of leukocytes upon activation 3. TNF-α converting enzyme is involved in PMA-induced shedding of L-selectin in murine fetal thymocytes 4; however, the L-selectin sheddase on granulocytes has not yet been identified. Recently, we identified shedding of L-selectin as an influential regulator of leukocyte rolling velocity 5, consistent with previous in vitro data 6.
Several lines of evidence show that L-selectin–ligand interactions lead to activation of leukocytes 7 8 9 10. Cross-linking of L-selectin initiates signaling through the Ras pathway via src-like tyrosine kinases and the small G protein Rac 11 12 and also causes L-selectin shedding 13. Although L-selectin shedding modulates physiologic leukocyte rolling velocity, it remains unknown whether L-selectin shedding influences other functions such as L-selectin–dependent signaling or leukocyte recruitment.
Chemokines such as IL-8, chemoattractants (N-formylmethionine leucylphenylalanine, platelet activating factor [PAF]) 3, and some nonsteroidal antiinflammatory drugs 14 are known to induce L-selectin shedding. Tissue inhibitor of metalloproteinase 3 15, morphine 16, and hydroxamic acid derivatives (Ro31-9790 17, KD-IX-73-4 18) inhibit shedding of L-selectin, presumably by inhibition of the shedding protease. Glucocorticoids, such as dexamethasone, reduce PAF-induced shedding of L-selectin 19.
In vitro, L-selectin and immobilized intercellular adhesion molecule (ICAM)-1 synergize for the transition of rolling lymphocytes to firm adhesion in the presence of chemokines 20. This is corroborated by in vivo data showing a synergistic role of L-selectin and ICAM-1 in leukocyte rolling 21. Ligation and cross-linking of L-selectin result in β2-integrin–dependent adhesion 7 9 10; however, the role of L-selectin shedding in this process has not been explored.
This study aims to elucidate the role of L-selectin shedding in leukocyte recruitment in vivo. To address potential mechanisms, we investigate the change in the quality of leukocyte rolling with blockade of L-selectin shedding by measuring the “jerkiness” of rolling, defined as the variability of velocity over time. We present evidence for two complementary mechanisms that could explain the impact of L-selectin shedding on leukocyte activation and recruitment: 1 increased leukocyte exposure to the endothelium caused by decreased rolling velocity, increased rolling flux, and decreased jerkiness; and 2 enhanced L-selectin–mediated signaling after blockade of L-selectin shedding, resulting in upregulation of β2-integrin expression and function.
Materials and Methods
Reagents.
KD-IX-73-4 (INH-3850-PI) and the related hydroxamic acid–based compound SI (PCS-3999-PI) were purchased from Peptides International and were dissolved in 10 mg/ml DMSO and diluted with saline to 1 μg:1 μl. 150 μg of KD-IX-73-4 was injected into the jugular vein of mice for an estimated initial serum concentration of 50 μg/ml.
mAbs.
mAb CBRM1/5 22 was a gift of Dr. T.A. Springer (Harvard Medical School, Boston, MA). mAb RB40.34 (rat IgG1, 30 μg/mouse) blocks murine P-selectin and was purified from hybridoma supernatant (a gift of Dr. D. Vestweber, University of Münster, Münster, Germany) 23. mAb 9A9 (rat IgG1, 30 μg/mouse) blocks murine E-selectin 24 (a gift of Dr. B. Wolitzky, MitoKor, San Diego, CA). The L-selectin mAb MEL-14 (rat IgG2a, 30 μg/mouse) was purified from hybridoma supernatant (American Type Culture Collection). TNF-α (0.5 μg/mouse) was obtained from Genzyme. Purified anti–mouse CD11a (M17/4/01840D) and CD18 (GAME-46/28040D) were purchased from BD PharMingen.
Animals.
8–10-wk-old male mice, 22–24 g, including wild-type (wt) C57BL/6 (Hilltop) and mice deficient in E-selectin (E−/−; reference 25) or L-selectin (L−/−; reference 26), E- and P-selectin (EP−/−; reference 25), E-, P-, and L-selectin (EPL−/−; reference 26a), and E-, P-selectin, and ICAM-1 (EPI−/−; reference 27) were used. Gene-targeted mice were backcrossed into a C57BL/6 background for at least seven generations.
Anesthesia and Surgery.
Mice were anesthetized with ketamine hydrochloride (100 mg/kg, Ketalar; Parke-Davis) after pretreatment with xylazine (10 mg/kg intraperitoneally; Phoenix Scientific, Inc.) and atropine sulfate (0.2 mg/kg intraperitoneally; Elkins-Sinn) and kept at 37°C with a water mattress. Some mice were pretreated for 3 or 6 h with an intrascrotal injection of 0.5 μg murine TNF-α (Genzyme) in 0.3 ml of saline.
Conjugated Microspheres.
Carboxylated fluorescent (18338) and nonfluorescent (23526) 2-μm microspheres (Polysciences, Inc.) were covalently conjugated to protein G (P-4689; Sigma-Aldrich) using a carbodiimide coupling kit (19539; Polysciences, Inc.). 20 μl of these microspheres was incubated with 5 μg of murine Fc-L-selectin constructs or Fc-CD4 28 (both gifts from Dr. S.R. Watson, NeXstar Pharmaceuticals, Boulder, CO) for 15 min at 37°C. Some L-selectin–conjugated beads were incubated with mAb MEL-14 for 30 min at 37°C. Recombinant human ICAM-1 (R&D Systems) was coated to the beads at 0.1 mg/ml and 4°C overnight. All microspheres were washed in PBS with 10% FBS before use in vivo.
Intravital Microscopy.
Microscopic observations were made on an axioscope (ZEISS) with a saline immersion objective (SW 40/0.75 numerical aperture) with modifications for stroboscopic epifluorescence microscopy (60/s, Strobex 236; Chadwick Helmuth). Venules were observed 90–120 s for velocities of rolling cells and numbers of adherent leukocytes, defined as cells remaining stationary for 30 s or longer. Images were obtained with a CCD camera (VE-1000CD; Dage-MTI) and recorded on S-VHS. Distances and vessel dimensions were measured using a digital-image processing system. Each rolling leukocyte passing a line perpendicular to the vessel wall was followed for 0.5–1 s. Leukocyte rolling velocities were calculated by dividing the traveled distance by the tracking time. The movements of individual rolling leukocytes were monitored by freeze-frame advancing. The variability of the rolling velocity (acceleration/deceleration) was calculated at 0.1 s intervals to quantify the jerkiness of leukocyte rolling. Mean jerkiness values (Jm), as shown in Table , were calculated as below, Δx being the displacement of a cell, Δt the time interval, and n the number of measurements:
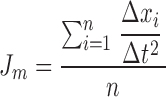 |
Table 1.
Rolling Velocity and Jerkiness under Different Conditions
| Genotype | Condition | Rollingvelocity | Jerkiness (Jm) | |
|---|---|---|---|---|
| TNF-α | KD-IX-73-4 | |||
| μm/s | μm/s2 | |||
| Wt | − | − | 58 ± 5 | 453 ± 48 |
| − | + | 42 ± 6 | 163 ± 17 | |
| + | − | 4.5 ± 0.3 | 115 ± 12 | |
| + | + | 4.1 ± 0.3 | 131 ± 11 | |
| (Microspheres) | + | − | 103 ± 5 | 135 ± 9 |
| EP−/− | + | − | 72 ± 8 | 518 ± 77 |
| + | + | 30 ± 2 | 232 ± 35 | |
| E−/− | + | − | 24 ± 1 | 176 ± 16 |
| + | + | 18 ± 1 | 117 ± 9 | |
| L−/− | − | − | 37 ±17 | 274 ± 60 |
| − | + | 38 ± 15 | 297 ± 90 | |
| EPL−/− | + | − | 13 ± 1 | 207 ± 27 |
| + | + | 14 ± 1 | 204 ± 16 | |
Data are mean ± SEM.
Centerline blood velocities were measured by a dual photo-diode connected to a cross-correlation program (Circusoft Instrumentation). Mean blood flow velocities were calculated by multiplying centerline velocities with 0.625 29. Wall shear rate (γw) equals 2.12 × 8Vb/d, where Vb is the mean blood flow velocity, d, the vessel diameter, and 2.12, a median empirical factor 30. The critical velocity (Vcrit) equals Vb × ε × (2 − ε), where ε is the ratio of cell to vessel diameter 31.
Flow Cytometry.
Mouse peripheral blood was stained with PE-conjugated anti–L-selectin mAb MEL-14 (01265B; BD PharMingen), anti-CD11b mAb M1/70 (01715B; BD PharMingen), or isotype control (rat IgG2 aκ, 11025A; BD PharMingen), 2 μl/ml at 37°C for 15 min. After lysis of the erythrocytes (0.15 M NH4Cl, 0.01 M NaHCO3, and 0.001 M disodium EDTA), cells were washed with phosphate buffered saline containing 10% FBS and fixed in 1% paraformaldehyde/PBS, and 104 cells/sample were analyzed by flow cytometry on a FACScan™ (Becton Dickinson). The results reported are for granulocytes, gated by their characteristic forward and side scatter. For analysis of L-selectin (CD62L) and Mac-1 (CD11b) expression on human polymorphonuclear granulocytes (PMNs), PE-conjugated mAbs (DREG56, 32235X; ICRF44, 30455X; BD PharMingen) were used in whole blood, followed by RBC lysis. For unlabeled primary mAbs, a FITC-labeled secondary mAb (goat anti–mouse [GAM] or goat anti–rat [GAR]) was used. For quantitative analysis of L-selectin chimeras on the surface of the microbeads, the flow cytometer was calibrated using the Quantum Simply Cellular kit (FCSC 815; Fishers) in combination with the provided software (QuickCal) for regression analysis.
Cross-Linking.
F(ab′)2 fragments of mAb DREG200 were prepared by digestion with ficin (ImmunoPure IgG1 Fab and F(ab′)2 kit 44880; Pierce Chemical Co.). F(ab′)2 fragments of mAb MEL-14 were prepared by digestion with immobilized pepsin (ImmunoPure F[ab′]2 kit 44888; Pierce Chemical Co.). Digests were purified from undigested mAbs and Fc fragments by passage over protein A Sepharose columns. Neutrophils were incubated with a saturating concentration of the F(ab′)2 fragments for 15 min at 37°C. Cells were washed with PBS to remove unbound F(ab′)2 fragments and subsequently incubated with secondary GAM F(ab′)2 fragments (115-006-006; Jackson ImmunoResearch Laboratories) for cross-linking of DREG200 F(ab′)2 fragments or with secondary GAR F(ab′)2 fragments (Mouse ads., CLCC40100; Cedarlane Laboratory Ltd.) for cross-linking of MEL-14 F(ab′)2 fragments at 37°C for 30 min. Fucoidin was used at a concentration of 20 μg/ml (F-5631; Sigma-Aldrich). In some experiments, cross-linking of the PE-conjugated mAb DREG56 was achieved by addition of secondary GAM F(ab′)2 fragments.
Statistics.
Rolling velocity, jerkiness, and number of adherent leukocytes from the same vessels before and after treatment were compared using two-tailed paired Student's t test for differences in mean, since the source populations from which these data were randomly drawn passed the tests for normal distribution. All statistical tests were performed using SigmaStat software. Statistical significance was set at P < 0.05 or P < 0.01 as indicated.
Results
L-Selectin Shedding Regulates Inflammatory Leukocyte Recruitment.
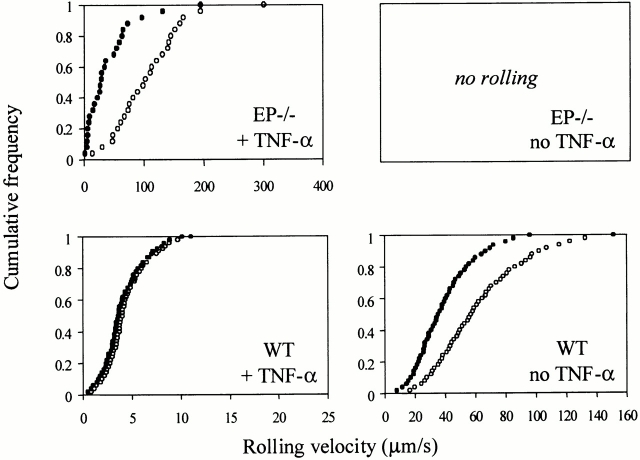
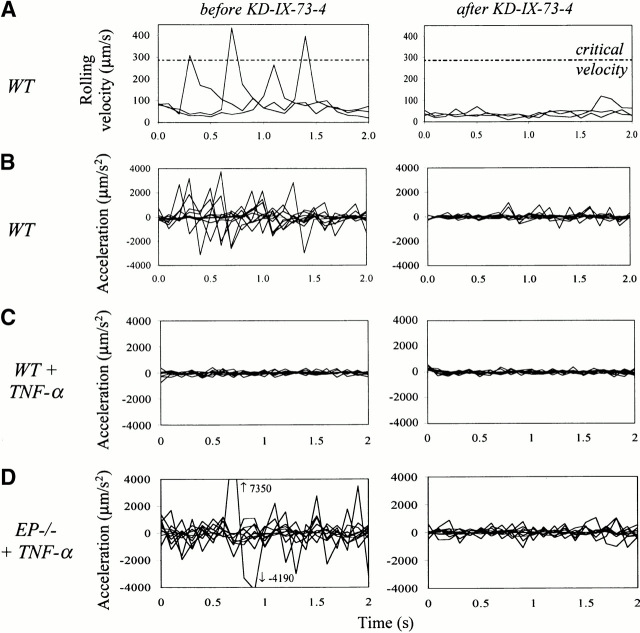
To explore the role of L-selectin shedding in leukocyte recruitment, we chose a well characterized model of inflammation induced by TNF-α in wt 2 and EP−/− mice 32. In TNF-α–treated EP−/− mice, where leukocyte rolling is predominantly mediated by L-selectin and its endothelial ligands, blockade of L-selectin shedding with KD-IX-73-4 caused a 63% reduction in rolling velocity (Fig. 1). To explore this change in rolling, we examined jerkiness, measured as acceleration/deceleration of rolling cells at 0.1 s intervals. Jerkiness was dramatically reduced in both untreated wt mice (Fig. 2 B) and TNF-α–treated EP−/− mice (Fig. 2 D) after blocking shedding. The velocity profiles (Fig. 2 A) underlying the jerkiness calculations show that physiologically rolling cells can temporarily travel above the critical velocity. This does not occur after blockade of shedding (Fig. 2 A) or when E-selectin is expressed (data not shown). However, in L−/− and EPL−/− mice jerkiness remained unchanged after application of KD-IX-73-4, showing that the effect of KD-IX-73-4 is specifically mediated by L-selectin (Table ). To address why blockade of L-selectin shedding does not affect leukocyte rolling velocity when E-selectin is expressed 5, we measured the jerkiness in 3-h TNF-α–treated wt and E−/− mice. Jerkiness in TNF-α–treated wt mice (Table ) was unaffected by the blockade of L-selectin shedding (Fig. 2 C); however, in E−/− mice jerkiness was significantly reduced after blockade of L-selectin shedding (Table ). When L-selectin was blocked with mAb MEL-14 in TNF-α–treated E−/− mice, jerkiness was reduced by 19.7% (30 cells from seven venules in two mice; P < 0.05). These data suggest that L-selectin shedding regulates jerkiness of rolling and that jerkiness of rolling on E-selectin is so low that inhibition of L-selectin shedding is of no further consequence to the jerkiness or to the rolling velocity. Blood pressure, centerline velocities, diameters, shear rates of the observed venules, and the systemic leukocyte counts were unaffected by the application of KD-IX-73-4 (Table , some data not shown).
Figure 1.
Leukocyte rolling velocities and L-selectin expression. Cumulative frequency of velocities of rolling leukocytes in wt and EP−/− mice with or without TNF-α treatment. wt data are shown for clarity and comparison from (reference 5). Significant reduction in rolling velocity in EP−/− with TNF-α treatment. ○, before the blockade of L-selectin shedding; •, 1–10 min after the blockade of L-selectin shedding. P < 0.01.
Figure 2.
Impact of L-selectin shedding on jerkiness of rolling. (A) Velocity profiles of three representative rolling leukocytes measured at 0.1 s intervals in untreated wt mice before (left) and after (right) the blockade of L-selectin shedding. Dotted line, critical rolling velocity. (B) Jerkiness (variability of velocity) of 10 rolling leukocytes over 0.1-s intervals before (left) and after (right) the blockade of L-selectin shedding in wt mice. (C) Jerkiness of 10 rolling leukocytes over 0.1 s intervals in TNF-α–treated wt mice before (left) and after (right) blockade of L-selectin shedding with KD-IX-73-4. (D) Jerkiness of 10 rolling leukocytes before (left) and after (right) the blockade of L-selectin shedding in TNF-α–treated EP−/− mice. Arrows, values of the data points outside of the panel.
Table 2.
Hemodynamic Parameters before and after Injection of KD-IX-73-4
| Centerline velocity | Diameter | Wall shear rate γ | ||||
|---|---|---|---|---|---|---|
| Genotype | Before | After | Before | After | Before | After |
| mm/s | μm | s−1 | ||||
| Wt | 2.3 ± 0.3 | 2.4 ± 0.4 | 37 ± 2 | 36 ± 1 | 661 ± 42 | 705 ± 92 |
| EP−/− | 2.7 ± 0.3 | 2.8 ± 0.3 | 43 ± 8 | 45 ± 9 | 837 ± 121 | 891 ± 206 |
| EPI−/− | 2.2 ± 0.4 | 2.3 ± 0.3 | 32 ± 3 | 31 ± 3 | 815 ± 217 | 869 ± 198 |
| EPL−/− | 2.6 ± 0.1 | 2.7 ± 0.3 | 23 ± 1 | 23 ± 2 | 1,251 ± 61 | 1,244 ± 80 |
Data are mean ± SEM.
To model the interaction of leukocytes after the blockade of L-selectin shedding, we constructed L-selectin–conjugated microspheres that lacked the machinery needed to shed L-selectin. The copy number of L-selectin chimera was quantified at ∼5.8 × 104/bead by comparison with the Ab binding capacity of calibrated beads in flow cytometry (Fig. 3 C). However, even though the copy number of L-selectin on these microspheres is in the same order of magnitude as on leukocytes, its relation to physiologic site densities on leukocyte microvilli is unclear. These microspheres rolled at a low jerkiness on TNF-α–treated cremaster microvenules of wt mice (Fig. 3A and Fig. B), similar to KD-IX-73-4–treated leukocytes (Fig. 2 C), although at a higher velocity (Table ). Incubation of the microspheres with function blocking mAb MEL-14 significantly reduced the average interaction of the microspheres with the endothelium from 23 ± 5 to 4 ± 1%. As a control, when chimeric CD4 was conjugated instead of L-selectin, microspheres showed 2 ± 1% interaction with the endothelium.
Figure 3.
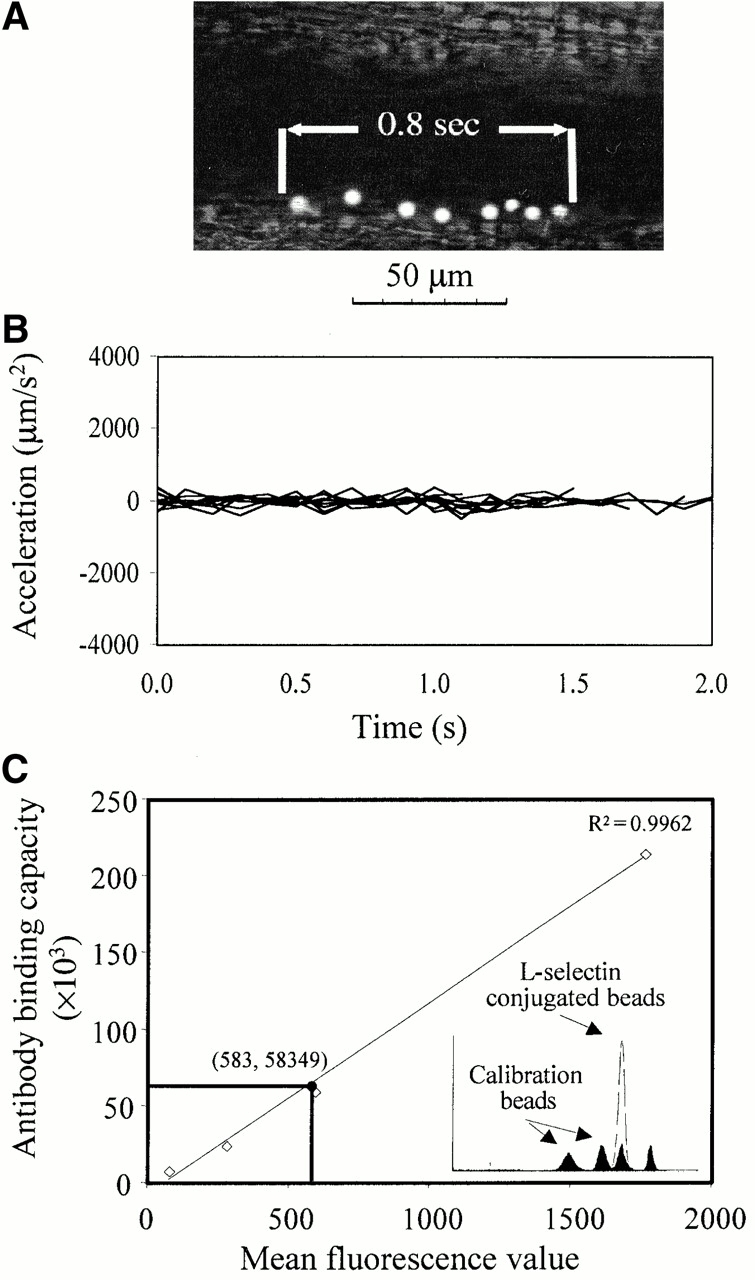
(A) Composite of eight superimposed video frames of a representative rolling L-selectin–conjugated microsphere in vivo. Time between the first and the last frame, 0.8 s. (B) Jerkiness of 10 L-selectin–conjugated microspheres on cytokine-stimulated endothelium of wt mice over 0.1 s intervals. (C) Number of L-selectin molecules on microspheres. Mean fluorescence values of calibration beads (open squares) with known mAb binding capacity obtained in flow cytometry (filled histograms). Fluorescence value of PE-DREG56 mAb binding to L-selectin–conjugated nonfluorescent beads (open histogram). Copy number of the PE-DREG56 bound to L-selectin–conjugated microspheres based on the regression line (filled circle).
Inhibition of L-Selectin Shedding Promotes Leukocyte Adhesion.
We next investigated the impact of L-selectin shedding on leukocyte adhesion by comparing leukocyte accumulation over 10 min after application of KD-IX-73-4 or vehicle in TNF-α–treated wt and EP−/− mice. In TNF-α–treated wt mice, firm adhesion significantly increased twofold after inhibition of shedding (P < 0.05; Fig. 4 A). In EP−/− mice, firm adhesion increased 2.7-fold after inhibition of shedding (P < 0.05; Fig. 4 A). Concomitant with increased adhesion, inhibition of L-selectin shedding increased the number of transmigrating cells, recognized by their characteristic change of shape during their transendothelial passage (Fig. 4 B). As a control, application of KD-IX-73-4 in TNF-α–treated L−/− and EPL−/− mice did not change firm adhesion or transmigration (Fig. 4), or decrease rolling velocity or jerkiness (Table , and data not shown).
Figure 4.
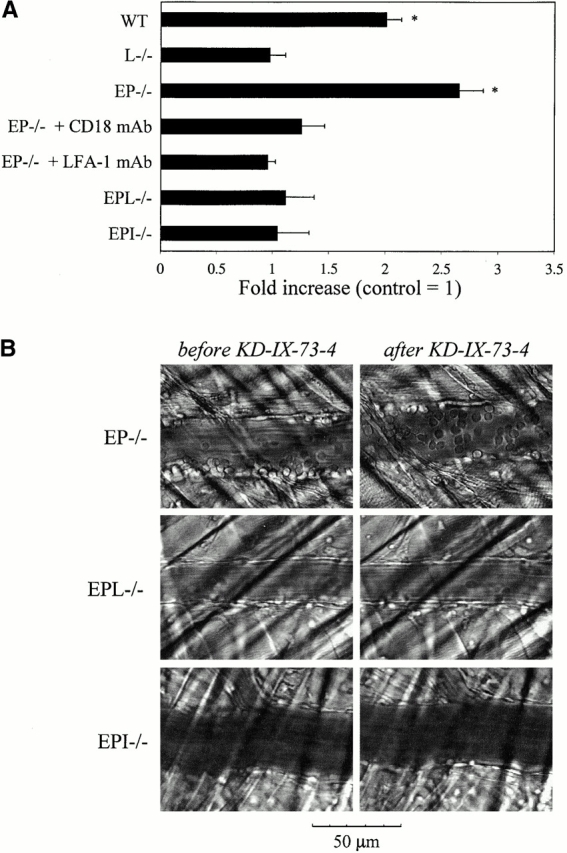
L-selectin–mediated arrest on cytokine-stimulated endo-thelium. (A) Bars represent mean (± SEM) increase of adherent leukocytes within 8–10 min after blockade of L-selectin shedding with KD-IX-73-4 in various mouse genotypes, compared with adhesion in the same venule without KD-IX-73-4. Studied number of venules/mice for leukocyte adhesion: wt, 17/7; EP−/−, 8/4; the remaining groups, 5/2 or higher. For CD18 and LFA-1 blockade, mAbs systemically injected before the blockade of L-selectin shedding in EP−/− mice. Control, onefold increase of adhesion after vehicle in wt or EP−/− mice within the same time frame. *Significantly different from all other groups. P < 0.05. (B) Representative video micrographs showing the increase of firmly adhering and transmigrating leukocytes before and 8–10 min after blockade of L-selectin shedding in three different gene-targeted mice.
Role of LFA-1 and ICAM-1.
LFA-1 and Mac-1 interact with ICAM-1, mediating firm leukocyte adhesion 33 34. We therefore tested whether the increase in leukocyte recruitment after blockade of L-selectin shedding is ICAM-1 dependent. In TNF-α–treated EPI−/− mice, inhibition of L-selectin shedding did not increase firm adhesion or transmigration, suggesting ICAM-1 is necessary for L-selectin–mediated leukocyte recruitment (Fig. 4). Functional blockade of CD18 with mAb GAME-46 or of LFA-1 with mAb M17/4 also prevented the elevated recruitment after blockade of L-selectin shedding (Fig. 4 A). These data show that increased leukocyte adhesion after blockade of L-selectin shedding requires LFA-1 and ICAM-1.
L-Selectin Shedding Regulates Leukocyte Activation.
Since cross-linking L-selectin downregulates its expression 13, activates the leukocyte, and upregulates β2-integrin expression and avidity 7 8 9, we hypothesized that L-selectin–mediated activation may be self-limiting by L-selectin's rapid cleavage upon ligation. We reasoned that cross-linking together with blockade of L-selectin shedding would result in a higher leukocyte activation compared with cross-linking alone. To test this hypothesis, we cross-linked L-selectin with and without prior incubation with KD-IX-73-4 and measured L-selectin, Mac-1 expression, the expression of a Mac-1 activation epitope recognized by mAb CBRM1/5 22, and the binding of these leukocytes to ICAM-1–coated microspheres. To show that cross-linking–mediated shedding of L-selectin can be blocked with KD-IX-73-4, we added secondary F(ab′)2 fragments to human neutrophils labeled with PE-DREG56 mAb with and without KD-IX-73-4, and measured L-selectin expression (Fig. 5 A). Incubating leukocytes with KD-IX-73-4 alone did not increase Mac-1 expression (Fig. 5 B). Cross-linking L-selectin on human PMNs with F(ab′)2 fragments of mAb DREG200 increased Mac-1 expression (Fig. 5 C). L-selectin cross-linking after blockade of shedding caused a larger increase in Mac-1 expression than cross-linking alone. Incubating leukocytes with fucoidin, a polyvalent L-selectin ligand that cross-links L-selectin 35, increased Mac-1 expression, which was further elevated after blockade of shedding (Fig. 5 D). To understand whether the increased expression of Mac-1 is accompanied by conformational activation, we used mAb CBRM1/5, which binds to the active conformation of the I-domain 22. Human neutrophils bound more CBRM1/5 when L-selectin was cross-linked by fucoidin and shedding was blocked, compared with incubation with fucoidin alone (Fig. 5 E).
Figure 5.
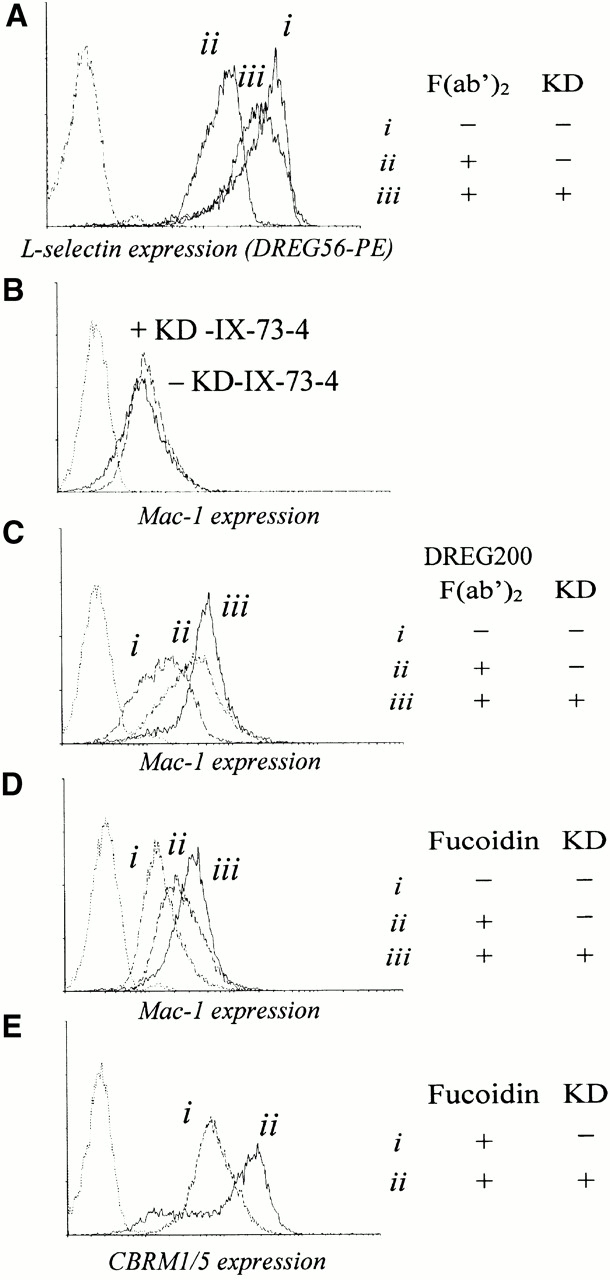
Regulatory function of shedding on L-selectin–mediated PMN activation. (A) L-selectin expression on human neutrophils with or without cross-linking of PE-DREG56 with secondary F(ab′)2 fragments and with or without incubation with KD-IX-73-4 (50 μg/ml at 37°C for 20 min). (B–D) Mac-1 expression on human neutrophils. (B) With or without incubation with KD-IX-73-4 (50 μg/ml at 37°C for 20 min); (C) after cross-linking with DREG200 F(ab′)2 fragments and secondary F(ab′)2 fragments with or without incubation with KD-IX-73-4; and (D) L-selectin ligation with fucoidin with or without incubation with KD-IX-73-4. (E) Expression of a Mac-1 activation epitope, detected by CBRM1/5 after L-selectin ligation with fucoidin with or without incubation with KD-IX-73-4. Dotted line, unstained PMNs. Data shown is representative of three independent experiments. KD, KD-IX-73-4.
To measure the impact of L-selectin cross-linking on adhesiveness of neutrophils with and without shedding, we quantified neutrophil binding to ICAM-1–coated beads (Fig. 6). The number of neutrophils with no bound ICAM-1 beads decreased systematically from 30% for resting neutrophils, to 24% with L-selectin cross-linked, and to 13% when L-selectin was cross-linked and shedding blocked. Conversely, the number of neutrophils with four or more bound ICAM-1 beads increased from 11 to 22 to 36%, respectively. KD-IX-73-4 alone did not affect ICAM-1 bead binding (30% for no beads, 15% for four beads, and data not shown).
Figure 6.
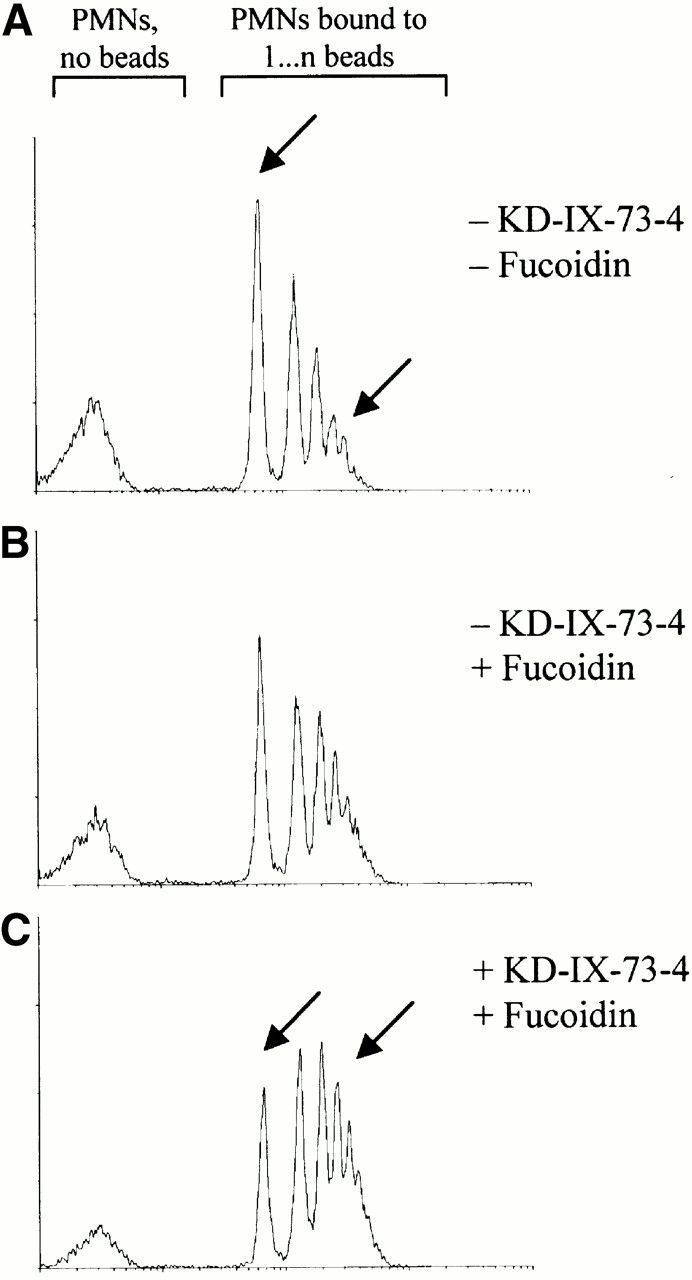
Regulatory function of shedding on PMN adhesive function. Binding of ICAM-1–coated microspheres to human PMNs. (A) No treatment. (B) L-selectin ligation with fucoidin. (C) L-selectin ligation with fucoidin and blockade of L-selectin shedding. Peaks in each panel from left to right, PMNs bound to 0, 1, … n beads, respectively. Data shown is representative of three independent experiments.
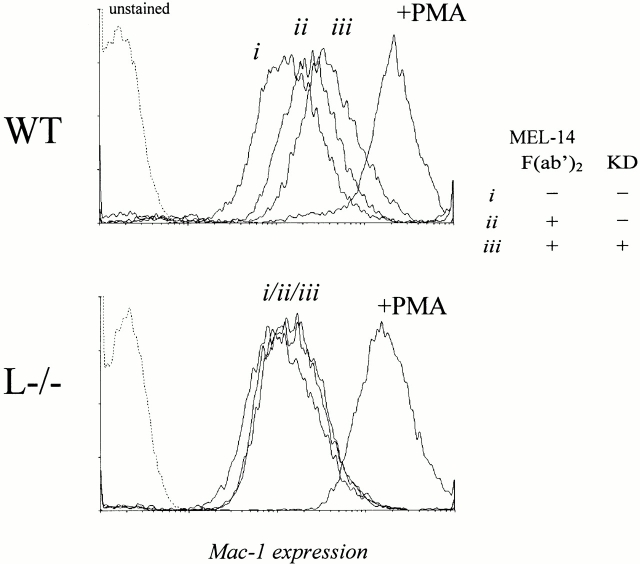
To elucidate whether the enhanced leukocyte activation after blockade of shedding is specific for L-selectin, we generated MEL-14 F(ab′)2 fragments against murine L-selectin. Cross-linking of L-selectin on murine wt neutrophils with MEL-14 F(ab′)2 and secondary GAR F(ab′)2 fragments, with or without blockade of shedding, revealed results completely corresponding to those seen in human neutrophils (Fig. 7). In contrast, incubation of neutrophils from L−/− mice with MEL-14 F(ab′)2 and secondary GAR F(ab′)2 fragments did not change the expression of Mac-1 (Fig. 7). The Mac-1 expression also remained unchanged if, in addition to the F(ab′)2 fragments, the shedding inhibitor KD-IX-73-4 was added to the neutrophils (Fig. 7).
Figure 7.
Regulatory function of shedding on L-selectin–mediated leukocyte activation. Mac-1 expression on neutrophils, obtained from wt and L−/− mice. (i)Without treatment; (ii) L-selectin cross-linked with MEL-14 F(ab′)2 fragments plus secondary GAR F(ab′)2 fragments; and (iii) L-selectin cross-linked with MEL-14 F(ab′)2 fragments plus secondary GAR F(ab′)2 fragments and blockade of L-selectin shedding. Dotted line, unstained PMNs. Data shown is representative of three independent experiments.
Discussion
L-selectin shedding has a surprisingly large impact on the stages of leukocyte recruitment subsequent to rolling. Our data suggest two mechanisms linking L-selectin shedding with leukocyte recruitment.
Increased Leukocyte Exposure to the Endothelium When L-Selectin Shedding Is Blocked.
The velocity of rolling leukocytes decreased significantly with the blockade of L-selectin shedding. Decreased velocity increases transit time of rolling leukocytes on cytokine-stimulated endothelium and can increase the chance of activation of the leukocyte by endothelial mediators and the likelihood for a successful arrest 36. Shedding of L-selectin appears to be a regulatory parameter that impacts the transition from rolling to firm adhesion.
Conventionally, rolling has been understood as movement below the critical velocity 31. Based on our data, a physiologically rolling leukocyte can temporarily travel faster than the critical velocity. The process of a rolling leukocyte accelerating above the critical rolling velocity, traveling transiently at a hydrodynamic velocity, and decelerating below critical velocity represents a “microjump.” Blocking shedding decreases the jerkiness of rolling and diminishes microjumps. Jerkiness reflects discontinuous and nonuniform contact between the rolling leukocyte and the endothelium. Rolling at a lower jerkiness means fewer microjumps and longer contact time between the rolling leukocyte and the endothelium. This allows the rolling leukocyte to ‘scan’ the surface of the endothelial layer more closely and sample endothelial signaling molecules, such as chemokines. Thus, inhibition of L-selectin shedding increases the exposure of a rolling leukocyte to the inflamed endothelium by (a) increasing the transit time and (b) elevating the continuous contact time with the endothelium by diminishing the microjumps.
Enhanced Leukocyte Activation After Blockade of L-Selectin Shedding.
L-selectin's dual function in leukocyte capture/rolling 1 and as a signaling molecule 7 8 9 11 is well established. Cross-linking of L-selectin activates leukocytes 7 8 and causes its own downregulation 13. We have shown that L-selectin is shed during rolling in vivo 5, agreeing with previous in vitro findings 6. However, the physiological consequences of L-selectin signaling and shedding were previously unknown. Our present data suggest that blockade of L-selectin shedding also enhances signaling to the rolling leukocyte, as shown by upregulated Mac-1 expression, increased binding to ICAM-1, and increased expression of activated Mac-1. We provide direct in vivo evidence for the importance of this mechanism by showing that blockade of L-selectin shedding enhances leukocyte adhesion in cytokine-treated wt and EP−/− mice. Furthermore, this adhesion is ICAM-1 and LFA-1 dependent. Inhibition of L-selectin shedding without L-selectin cross-linking does not activate the leukocyte. Since blocking L-selectin shedding in combination with ligand interaction or cross-linking activates the cell more than ligand interaction or cross-linking alone, we propose that engagement of L-selectin during rolling results in cell signals that enhance β2-integrin adhesive responses, which are further increased, if shedding is blocked.
Based on the current data, both increased exposure to the endothelium and enhanced signaling represent equally attractive models, contributing to an unknown extent in regulation of leukocyte recruitment. However, the fact that in TNF-α–treated wt mice, blockade of L-selectin shedding increases firm adhesion without any apparent change to the rolling velocity or jerkiness, compared with EP−/− mice, where an increase in firm adhesion coincides with a reduction in rolling velocity and jerkiness, suggests that enhanced signaling through L-selectin after blockade of shedding is critical. However, neither enhanced signaling alone nor increased endothelial contact time alone appears to be sufficient for leukocyte arrest in vivo. This is supported by our finding that blockade of L-selectin shedding in untreated wt mice causes a reduction of rolling velocity but does not induce arrest 5. In addition to the increased activation of the rolling leukocyte by blockade of L-selectin shedding, activation of the endothelium, i.e., endothelial presentation of chemokines induced by TNF-α treatment, appears to be necessary for arrest 37 38.
Recruitment Deficit in L−/− Mice.
Although originally identified as a homing receptor for lymphocyte recruitment to peripheral lymph nodes, L-selectin also plays a vital role in leukocyte recruitment to peripheral sites of inflammation, as shown by the significant deficit in PMN recruitment of L−/− mice in many models 26 39 40. However, this important role of L-selectin is not explained by the relatively minor contribution of L-selectin to leukocyte rolling 2 32. Our findings provide a mechanism that may explain the requirement for L-selectin in leukocyte recruitment.
Role of CD11a/CD18 and ICAM-1 in L-Selectin–induced Firm Adhesion.
CD18-dependent arrest of neutrophils on ICAM-1 in shear flow is induced by L-selectin ligation 9. Our data indicate that ICAM-1 and LFA-1 are necessary for the transition of L-selectin–mediated rolling to firm adhesion on cytokine-activated endothelium in vivo, since the increase of firm adhesion through inhibition of L-selectin shedding only occurs if ICAM-1 is expressed.
Rolling of L-Selectin–conjugated Microspheres.
Rolling of these microspheres clearly indicates that, although shedding of L-selectin is necessary for rolling at physiologic velocities, it is not required for rolling in general. The extracellular domain of L-selectin is sufficient to support L-selectin–mediated rolling on its endothelial ligands at a comparable low jerkiness as the rolling of leukocytes after blockade of L-selectin shedding. The bead data also confirm previous findings that L-selectin does not have to be expressed on microvilli to support rolling 41.
Physiologic Relevance of Shedding.
Since blockade of shedding caused enhanced activation of leukocytes with higher levels of recruitment in both wt and EP−/− mice but not in L−/− and EPL−/− mice, shedding of L-selectin appears to be a physiologic mechanism to limit neutrophil recruitment during inflammation. L-selectin ligation activates the leukocyte, but L-selectin–mediated rolling alone is not sufficient to elevate firm adhesion and transmigration. Shedding during L-selectin ligation may be responsible for keeping the activation status of a rolling leukocyte below the level necessary for successful arrest. By blocking shedding, we disable this mechanism, resulting in increased activation of the surface cross-linked cell in vitro or an increased firm adhesion in vivo. This is consistent with the finding that leukemia cells with lower expressions of L-selectin show impaired transendothelial migration in vitro 42. Some of the antiinflammatory effects of nonsteroidals 14 may also be related to enhanced L-selectin shedding.
Previously we showed that reduction of rolling velocity does not occur with blockade of L-selectin shedding when E-selectin is expressed 5. Here we show that rolling on E-selectin expressing endothelium reveals the lowest level of jerkiness among all examined leukocyte rolling conditions, which did not further decrease after blockade of L-selectin shedding. The lack of microjumps when E-selectin is expressed may explain why the velocity does not change upon blockade of shedding. However, if only P-selectin is expressed, as in cytokine-treated E−/− mice, rolling leukocytes perform some microjumps, which can be reduced by blockade of L-selectin shedding. These L-selectin–independent, occasional microjumps, for instance during P-selectin–mediated rolling or in EPL−/− mice, may be attributable to local variations of hydrodynamic conditions or nonuniform expression patterns of endothelial adhesion molecules 43.
In conclusion, our data suggest that L-selectin plays at least two roles in the recruitment of leukocytes to peripheral sites of inflammation. Continuous L-selectin shedding regulates the quality of leukocyte rolling by affecting the jerkiness of rolling and, consequently, the close leukocyte endothelial contact time and thus the accessibility of endothelial activation signals including chemokines. In addition, L-selectin–mediated signaling appears to activate rolling leukocytes and to promote their arrest if other costimulatory signals such as endothelially presented chemokines are expressed. Further studies will be necessary to elucidate whether a rolling leukocyte is able to integrate signals from chemokine receptor– and adhesion molecule–ligand interactions to initiate arrest. These mechanisms probably act in concert to promote leukocyte adhesion at sites of inflammation.
Acknowledgments
We would like to thank John C. Chappel, Jennifer Bryant, and William Ross for their technical assistance and Tim Olson for generation of F(ab′)2 fragments. L−/− mice were from a colony based on breeders provided by Dr. T.F. Tedder, Duke University, Durham, NC. E−/−, EP−/−, EPL−/−, and EPI−/− mice were from colonies based on breeders provided by Drs. D.C. Bullard, R.G. Collins, and A.L. Beaudet, Baylor College of Medicine, Houston, TX. We thank Drs. D. Vestweber, University of Münster, Münster, Germany for mAb RB40, B. Wolitzky, Roche, Inc. for mAb 9A9, and S.R. Watson for L-selectin IgG. We appreciate the gift of the mAb CBRM1/5 from Dr. T.A. Springer.
This work was supported by National Institutes of Health grants HL54136 and 64381 to K. Ley.
Footnotes
Abbreviations used in this paper: GAM, goat anti–mouse; GAR, goat anti–rat; ICAM, intercellular adhesion molecule; wt, wild-type.
References
- Butcher E.C. Leukocyte-endothelial cell recognitionthree (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Ley K., Bullard D.C., Arbones M.L., Bosse R., Vestweber D., Tedder T.F., Beaudet A.L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T.K., Jutila M.A., Berg E.L., Butcher E.C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Slack J.L., Reddy P., Stocking K.L., Sunnarborg S.W., Lee D.C., Russell W.E., Castner B.J., Johnson R.S., Fitzner J.N. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A., Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J. Exp. Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcheck B., Kahn J., Fisher J.M., Wang B.B., Fisk R.S., Payan D.G., Feehan C., Betageri R., Darlak K., Spatola A.F., Kishimoto T.K. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- Simon S.I., Burns A.R., Taylor A.D., Gopalan P.K., Lynam E.B., Sklar L.A., Smith C.W. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) β2-integrin. J. Immunol. 1995;155:1502–1514. [PubMed] [Google Scholar]
- Crockett-Torabi E., Sulenbarger B., Smith C.W., Fantone J.C. Activation of human neutrophils through L-selectin and Mac-1 molecules. J. Immunol. 1995;154:2291–2302. [PubMed] [Google Scholar]
- Gopalan P.K., Smith C.W., Lu H., Berg E.L., McIntire L.V., Simon S.I. Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin. J. Immunol. 1997;158:367–375. [PubMed] [Google Scholar]
- Steeber D.A., Engel P., Miller A.S., Sheetz M.P., Tedder T.F. Ligation of L-selectin through conserved regions within the lectin domain activates signal transduction pathways and integrin function in human, mouse, and rat leukocytes. J. Immunol. 1997;159:952–963. [PubMed] [Google Scholar]
- Brenner B., Gulbins E., Schlottmann K., Koppenhoefer U., Busch G.L., Walzog B., Steinhausen M. L-selectin activates the Ras pathway via the tyrosine kinase p56lck. Proc. Natl. Acad. Sci. USA. 1996;93:15376–15381. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Weinmann S., Grassme H., Lang F., Linderkamp O., Gulbins E. L-selectin activates JNK via src-like tyrosine kinases and the small G-protein Rac. Immunology. 1997;92:214–219. doi: 10.1046/j.1365-2567.1997.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecanda A., Walcheck B., Bishop D.K., Jutila M.A. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur. J. Immunol. 1992;22:1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez F., Gonzalez-Alvaro I., Campanero M.R., Mollinedo F., del Pozo M.A. Prevention of in vitro neutrophil-endothelial attachment through shedding of L-selectin by nonsteroidal antiinflammatory drugs. J. Clin. Invest. 1995;95:56–1765. doi: 10.1172/JCI117853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G., Murphy G., Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L-selectin from leukocytes. J. Biol. Chem. 1999;274:2810–2815. doi: 10.1074/jbc.274.5.2810. [DOI] [PubMed] [Google Scholar]
- Wang T.L., Chang H., Hung C.R., Tseng Y.Z. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am. J. Cardiol. 1997;80:1532–1535. doi: 10.1016/s0002-9149(97)00788-1. [DOI] [PubMed] [Google Scholar]
- Preece G., Murphy G., Ager A. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J. Biol. Chem. 1996;271:11634–11640. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- Feehan C., Darlak K., Kahn J., Walcheck B., Spatola A.F., Kishimoto T.K. Shedding of the lymphocyte L-selectin adhesion molecule is inhibited by a hydroxamic acid-based protease inhibitor. Identification with an L-selectin-alkaline phosphatase reporter. J. Biol. Chem. 1996;271:7019–7024. doi: 10.1074/jbc.271.12.7019. [DOI] [PubMed] [Google Scholar]
- Filep J.G., Delalandre A., Payette Y., Foldes-Filep E. Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils. Circulation. 1997;96:295–301. doi: 10.1161/01.cir.96.1.295. [DOI] [PubMed] [Google Scholar]
- Tangemann K., Gunn M.D., Giblin P., Rosen S.D. A high endothelial cell-derived chemokine induces rapid, efficient, and subset-selective arrest of rolling T lymphocytes on a reconstituted endothelial substrate. J. Immunol. 1998;161:6330–6337. [PubMed] [Google Scholar]
- Steeber D.A., Campbell M.A., Basit A., Ley K., Tedder T.F. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc. Natl. Acad. Sci. USA. 1998;95:7562–7567. doi: 10.1073/pnas.95.13.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M.S., Springer T.A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse R., Vestweber D. Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur. J. Immunol. 1994;24:3019–3024. doi: 10.1002/eji.1830241215. [DOI] [PubMed] [Google Scholar]
- Norton C.R., Rumberger J.M., Burns D.K., Wolitzky B.A. Characterization of murine E-selectin expression in vitro using novel anti-mouse E-selectin monoclonal antibodies. Biochem. Biophys. Res. Commun. 1993;195:250–258. doi: 10.1006/bbrc.1993.2037. [DOI] [PubMed] [Google Scholar]
- Bullard D.C., Kunkel E.J., Kubo H., Hicks M.J., Lorenzo I., Doyle N.A., Doerschuk C.M., Ley K., Beaudet A.L. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J. Exp. Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbones M.L., Ord D.C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D.J., Tedder T.F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Collins, R.G., U. Jung, D.C. Bullard, J. Hicks, K. Ley, and A.L. Beaudet. 1999. Viable phenotype but impaired leukocyte rolling and peritoneal emigration in triple selectin (E, L, and P) null mice. Keystone Symposia: Inflammatory Paradigms and the Vasculature, Santa Fe, NM. C4:39.
- Mizgerd J.P., Quinlan W.M., LeBlanc B.W., Kutkoski G.J., Bullard D.C., Beaudet A.L., Doerschuk C.M. Combinatorial requirements for adhesion molecules in mediating neutrophil emigration during bacterial peritonitis in mice. J. Leukoc. Biol. 1998;64:291–297. doi: 10.1002/jlb.64.3.291. [DOI] [PubMed] [Google Scholar]
- Watson S.R., Imai Y., Fennie C., Geoffroy J.S., Rosen S.D., Lasky L.A. A homing receptor–IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J. Cell Biol. 1990;110:2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky H.H., Zweifach B.W. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc. Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- Reneman R.S., Woldhuis B., oude Egbrink M.G.A., Slaaf D.W., Tangelder G.J. Concentration and velocity profiles of blood cells in the microcirculation. In: Hwang N.H.C., Turitto V.T., Yen M.R.T., editors. Advances in Cardiovascular Engineering. Plenum Publishing Corp; New York: 1992. pp. 25–40. [Google Scholar]
- Ley K., Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ. Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- Jung U., Ramos C.L., Bullard D.C., Ley K. Gene-targeted mice reveal importance of L-selectin-dependent rolling for neutrophil adhesion. Am. J. Physiol. 1998;43:H1785–H1791. doi: 10.1152/ajpheart.1998.274.5.H1785. [DOI] [PubMed] [Google Scholar]
- Marlin S.D., Springer T.A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Diamond M.S., Staunton D.E., de Fougerolles A.R., Stacker S.A., Garcia-Aguilar J., Hibbs M.L., Springer T.A. ICAM-1 (CD54)a counter-receptor for Mac-1 (CD11b/CD18) J. Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin P.A., Hwang S.T., Katsumoto T.R., Rosen S.D. Ligation of L-selectin on T lymphocytes activates β1-integrins and promotes adhesion to fibronectin. J. Immunol. 1997;159:3498–3507. [PubMed] [Google Scholar]
- Jung U., Norman K.E., Scharffetter-Kochanek K., Beaudet A.L., Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainger G.E., Fisher A.C., Nash G.B. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am. J. Physiol. 1997;272:H114–H122. doi: 10.1152/ajpheart.1997.272.1.H114. [DOI] [PubMed] [Google Scholar]
- Gerszten R.E., Garcia-Zepeda E.A., Lim Y.C., Yoshida M., Ding H.A., Gimbrone M.A., Jr., Luster A.D., Luscinskas F.W., Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- Tedder T.F., Steeber D.A., Pizcueta P. L-selectin–deficient mice have impaired leukocyte recruitment into inflammatory sites. J. Exp. Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.L., Hale L.P., Steeber D.A., Tedder T.F. L-selectin is involved in lymphocyte migration to sites of inflammation in the skindelayed rejection of allografts in L-selectin-deficient mice. J. Immunol. 1997;158:5191–5199. [PubMed] [Google Scholar]
- Stein J.V., Cheng G., Stockton B.M., Fors B.P., Butcher E.C., von Andrian U.H. L-selectin–mediated leukocyte adhesion in vivomicrovillous distribution determines tethering efficiency, but not rolling velocity. J. Exp. Med. 1999;189:37–50. doi: 10.1084/jem.189.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.R., Gu B.J., Dao L.P., Bradley C.J., Mulligan S.P., Wiley J.S. Transendothelial migration of lymphocytes in chronic lymphocytic leukaemia is impaired and involved down-regulation of both L-selectin and CD23. Br. J. Haematol. 1999;105:181–189. [PubMed] [Google Scholar]
- Damiano E.R., Westheider J., Tozeren A., Ley K. Variation in the velocity, deformation, and adhesion energy density of leukocytes rolling within venules. Circ. Res. 1996;79:1122–1130. doi: 10.1161/01.res.79.6.1122. [DOI] [PubMed] [Google Scholar]