Abstract
Dendritic cells (DCs), unique antigen-presenting cells (APCs) with potent T cell stimulatory capacity, direct the activation and differentiation of T cells by providing costimulatory signals. As such, they are critical regulators of both natural and vaccine-induced immune responses. A new B7 family member, B7-DC, whose expression is highly restricted to DCs, was identified among a library of genes differentially expressed between DCs and activated macrophages. B7-DC fails to bind the B7.1/2 receptors CD28 and cytotoxic T lymphocyte–associated antigen (CTLA)-4, but does bind PD-1, a receptor for B7-H1/PD-L1. B7-DC costimulates T cell proliferation more efficiently than B7.1 and induces a distinct pattern of lymphokine secretion. In particular, B7-DC strongly costimulates interferon γ but not interleukin (IL)-4 or IL-10 production from isolated naive T cells. These properties of B7-DC may account for some of the unique activity of DCs, such as their ability to initiate potent T helper cell type 1 responses.
Keywords: dendritic cells, T cell activation, B7, interferon γ, costimulatory molecule
Introduction
The qualitative and quantitative nature of an immune response depends on the type of APC that processes and presents antigen to T cells. Critical determinants of T cell activation include the density of peptide-MHC ligand available for TCR engagement as well as the provision of soluble and membrane-bound costimulatory signals. Among the different types of APCs, dendritic cells (DCs) are the central initiator of antigen-specific T cell responses. Early DCs have specialized antigen uptake and processing machinery, whereas mature DCs efficiently present antigen to and activate T cells. Functionally, mature DCs are >100 times more potent than macrophages in activating naive T cell in vitro 1 2.
T cell–dependent immune responses initiated by DCs depend on their expression of specific costimulatory signals. Among the most important costimulatory signals are those delivered by the B7 family, which is currently composed of four members with demonstrated immunologic activity. Two of the members, B7.1 and B7.2, bind both CD28 and CTL-associated antigen (CTLA)-4 on T cells. CD28 delivers a positive costimulatory signal 3 4 5, enhancing the production of certain lymphokines such as IL-2 via transcriptional 6 and posttranscriptional mechanisms 7. CTLA-4, which is expressed a few days after T cell activation, delivers a counterregulatory negative signal that antagonizes CD28 signals and thereby limits the magnitude and duration of T cell activation 8 9. B7.1 and B7.2 are expressed by all bone marrow–derived APCs including B cells, macrophages, and DCs, although at different ratios and with different kinetics. Despite many reports suggesting different functions of B7.1 and B7.2 in Th1/Th2 differentiation, antibody production, and CTL generation 10 11 12 13, their distinct immunologic roles remain to be clarified 14 15 16.
Two additional B7 family members, B7-H1/PD-L1 and B7h/B7RP-1, have been identified recently 17 18 19 20. B7-H1/PD-L1 and B7h/B7RP-1 bind the PD-1 and inducible costimulator (ICOS) receptors, respectively, both of which are induced upon T cell activation 18 21. Expression of all the currently known B7 family members is broadly distributed on multiple hematopoietic and nonhematopoietic tissues, raising interesting questions as to their relative role in immune response regulation. In vivo experiments in transgenic and knockout mice have confirmed a role for B7h/B7RP-1 in effector responses, particularly involving antibody production 21 22 23 24. Although expression of the currently described B7 family molecules is clearly important in mediating T cell activation, their ubiquitous expression on multiple APC types suggests that other molecules contribute to the extraordinary stimulatory capacity of DCs.
In an effort to understand more about genes involved in DC function, we screened a subtractive cDNA library between DCs and activated macrophages. We have isolated a new member of the B7 family that appears to be expressed primarily in DCs in mouse and human. Our initial functional studies indicate that B7-DC is more active than B7-1 in stimulating IFN-γ production by T cells.
Materials and Methods
Cell Preparation and Culture.
6–12-wk-old female BALB/c mice were purchased from National Cancer Institute and used for DC and macrophage preparation. Bone marrow–derived DCs were cultured in RPMI 1640 (GIBCO BRL) supplemented with 5% FCS (Hyclone), penicillin/streptomycin (JRH Biosciences), gentamycin (Sigma-Aldrich), nonessential amino acids (aa; RH Biosciences), l-glutamate (JRH Biosciences), sodium pyruvate (Sigma-Aldrich), 2-mercaptoethanol (Sigma-Aldrich), and 1,000 U/ml recombinant murine GM-CSF (Immunex), as described previously 25. Day 8 bone marrow–derived DCs were stained with monoclonal antibodies by conventional methods. Monoclonal antibody against MHC class II, 14-4-4s, was purified from hybridoma supernatant. Dr. W. Baldwin (Johns Hopkins University) supplied CTLA-4-Ig fusion molecule. Antibodies for MHC class I (28-14-8), F4/80 (C1.A3-1), B7.1 (1G10), B7.2 (GL1), Fcγ RII/III (2.4G2), and Mac-1 (M1/70) were purchased from BD PharMingen. For tester cDNA preparation, day 8 cells were purified by cell sorter using 14-4-4s and CTLA-4-Ig at the Johns Hopkins University Oncology Center. The purity of MHC class IIhi and B7hi population after sorting was 93–98%. Bone marrow–derived macrophages were cultured with RPMI 1640 supplemented with 10% FCS, penicillin/streptomycin, nonessential aa, sodium pyruvate, l-glutamine, 2-mercaptoethanol, and 250 U/ml recombinant murine M-CSF, and they were treated with 500 U/ml IFN-γ (BD PharMingen) and 5 μg/ml LPS (Sigma-Aldrich), as described previously 26. After stimulation, cell surface expression of MHC class II and B7 were confirmed using flow cytometric analysis on day 10 of culture. Macrophage cell lines WEHI-3, RAW264.7, J774.A.1, and PU5-1.8 were provided by Dr. J. Farber (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). They were cultured with American Type Culture Collection recommended medium.
cDNA Subtractive Hybridization.
Total RNA from sorted DCs and activated macrophages was extracted with TRIzol (GIBCO BRL). Messenger RNA was purified by Oligotex mRNA purification kit (QIAGEN). We used the PCR-based SMART cDNA synthesis system (CLONTECH Laboratories, Inc.) to amplify cDNA followed by the PCR-based subtraction system PCR Select (CLONTECH Laboratories, Inc.). Subtraction was performed following the manufacturer's protocol. Plasmid dot blot was then performed to confirm that the cDNA cloned is DC specific. Alkaline-denatured miniprep DNAs were spotted on Hybond N+ membrane (Amersham Pharmacia Biotech) and hybridized with SMART cDNA probe derived from sorted DCs or activated macrophages. These cDNA probes were 32P-labeled using random primer labeling method (Prime-It II; Stratagene). Hybridizations and washing were done as described previously 27.
cDNA Library Construction and Screening: Cloning of B7-DC.
Bone marrow–derived DCs were harvested on day 8 without sorting. About 20 to 40% of these cells expressed high MHC class II and B7. Total RNA extraction followed by poly A RNA purification was done as described above. For oligo dT primed DC library construction, we used Lambda ZAP Express cDNA synthesis system (Stratagene). The PCR DNA fragment of B7-DC was probed and used for screening. Membrane transfer, denaturation, and renaturation were performed using Stratagene's protocol. Positive clones were isolated and second screening was performed. After second screening, plasmids were excised by in vivo excision and tested by dot blotting and sequencing. The BLAST program was used to do homology search of the nucleotide sequence against GenBank (National Center for Biotechnology Information) for similarity to previously reported genes. The full-length B7-DC cDNA clone was pulled out from the DC cDNA library. 5′ rapid amplification of cDNA ends (RACE) was performed using SMART RACE cDNA amplification kit (CLONTECH Laboratories, Inc.). 5′-RACE products were cloned into pCR2.1 vector and sequenced. Two more full-length B7-DC clones were obtained by reverse transcription (RT)-PCR and their sequences were compared to avoid sequence error.
For cloning of the human B7-DC, human DCs were obtained from normal peripheral blood mononuclear cells by culture in GM-CSF plus IL-4 as described previously 28. A BLAST search identified an overlapping expressed sequence tag (EST) clone, under GenBank/EMBL/DDBJ accession no. AK001879, with homology to mouse B7-DC. 5′ RACE was performed as described above. We sequenced a 5′-RACE PCR fragment and designed a primer corresponding to 5′-UTR of human B7-DC. The following primers in the 5′-UTR and 3′-UTR of B7-DC was used to amplify full-length human B7-DC: 5′-GGAGCTACTGCATGTTGATTGTTTTG-3′ and 5′-TGCAAACTGAGGCACTGAAAAGTC-3′. The full-length cDNA sequences of the human and murine B7-DC cDNAs are available from GenBank/EMBL/DDBJ under accession nos. AF329193 and AF142780.
Bacterial Artificial Chromosome (129SVJ) Library Screening/Genomic Cloning and Mapping.
Bacterial artificial chromosome (BAC) library screening followed manufacturer's protocol (Genome Systems, Inc.). Primers used: 5′-TTGTTGTCTCCTTCTGTCTCCCAAC-3′ and 5′-ACAGTTGCTCCTTGTATCAGGTTC-3′.
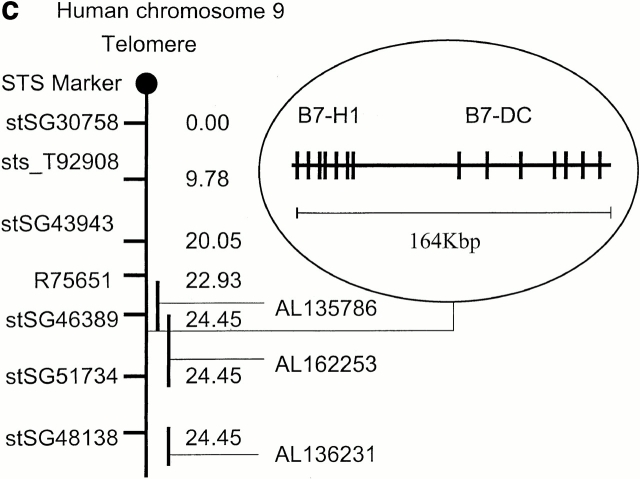
BAC library screening yielded three positive clones. Chromosome location mapping was done by fluorescence in situ hybridization (Genome Systems, Inc.). A total of 80 metaphase cells were analyzed, with 79 exhibiting specific labeling. The human B7-DC mapping was done using available bioinformatic tools, National Center for Biotechnology Information's BLAST program, and the International RH Mapping Consortium. The hB7-DC sequence was searched in GenBank/EMBL/DDBJ and was found to map to two BAC clones RP11-574F11 (AL162253) and Rp11-635N21 (AL354744) localizing on chromosome 9.
Hybridization Analysis.
RNA extraction and SMART cDNA synthesis for cell lines, sorted DCs, and activated macrophages were performed as described above. SMART PCR cDNAs were purified by PCR purification kit (QIAGEN). 0.5 μg/lane purified DNAs were run on a 1% agarose gel and transferred on a Nytran nylon membrane (Schleicher & Schuell). To make radioactive probes, subtracted library-derived plasmid DNAs were amplified as templates. DNA was amplified by PCR using primer sets just adjacent to the cloning site of plasmid DNA and used purified PCR DNA of each of the clones for hybridization probes. The nucleotide sequences of these primers are as follows: 5′-GTAACGGCCGCCAGTGTGCTG-3′ and 5′-CGCCAGTGTGATGGATATCTGCA-3′. Hybridization analysis of total RNA of human DCs and control placenta was also performed. The probes used and RNA preparation was described above. Radiolabeling of probes, hybridization, washing, and autoradiography were done as described above.
CD28-Ig, CTLA-4-Ig, and PD-1-Ig Binding Assay.
293T cells were transfected with B7.1-pCAGGS or B7-DC-pCAGGS using LipofectAMINE 2000 (GIBCO BRL). After 24 h, cells were resuspended in standard FACS® buffer and spun at 1,000 rpm for 5 min at 4°C, stained, and analyzed on a FACScan™ (Becton Dickinson). Recombinant CD28-Ig, CTLA-4-Ig, and PD-L1-Ig chimera were used at 2 μg/ml. Goat F(ab′)2 anti–human IgG-PE was used at 1:20 dilution (Southern Biotechnology Associates, Inc.).
B7-DC-Ig Dimer Synthesis.
The B7-DC-Ig construct was made by fusing the sequence encoding the NH2-terminal aa of B7-DC without the transmembrane domain in-frame to the sequence encoding the COOH-terminal aa of the human IgG1 Fc in the pIg-Tail Plus vector (R&D Systems). COS-7 cells were transiently transfected with pIg/B7-DC using LipofectAMINE 2000 (GIBCO BRL) or GINE JAMMER (Stratagene). The B7-DC-Ig fusion protein was purified from the serum-free supernatants using the saturated ammonium sulfate precipitation. SDS-PAGE and silver staining demonstrated a purity >90%.
T Cell Proliferation and Cytokine Assays.
For costimulation assays with anti-CD3, 96-well flat-bottommed plates (Immulon4; Dynex) were precoated with anti-CD3 antibodies (2C11; BD PharMingen) and B7.1-Ig (R&D Systems), B7-DC-Ig, or isotype control (Sigma-Aldrich) at 100 ng/ml were diluted in 1× PBS (GIBCO BRL), pH 7.4, for 2 h at 37°C. The plates were then washed three times with 1× PBS and blocked with RPMI 1640 supplemented with 10% FCS for one-half hour before adding T cells. T cells from spleens and lymph nodes were purified using dynabeads M-280 (Dynex) with the indirect method using an antibody cocktail composed of anti-IEd and B220/CD45RO or CD8α (BD PharMingen). For proliferation and cytokine secretion assays, cells were plated at 2 × 105 cells/well.
For costimulation assays using the RENCA system to present hemagglutinin (HA) antigen, RENCA MHC class II expression was induced with IFN-γ (75 U/ml) for 72 h. They were then irradiated (13,200 rad) and plated at 2 × 104 cells/well (96-well flat-bottomed plates). HA110–120 peptide was then added at 2.5 μg/well and various concentrations of the Ig-fusion molecules were added. Transgenic I-Ed plus HA-specific T cells (gift of H. von Boehmer, Harvard University, Cambridge, MA) were isolated as described above and plated at 4 × 105 cells/well.
For analysis by cytokine ELISA, cultures were set up as described above and supernatants were harvested at the indicated times. IL-2, IL-10, IFN-γ (Endogen), and IL-4 (R&D Systems) concentrations were determined using commercially available ELISA kits.
Results
Identification and Characterization of B7-DC.
B7-DC was isolated from a subtracted library between DCs and activated macrophages. The two populations used for cDNA subtraction were bone marrow–derived GM-CSF–cultured DCs as the “tester” population and IFN-γ plus LPS-activated adherent bone marrow–derived M-CSF macrophages as the “driver” population. Day 8 MHC class IIhiB7hi “mature” DCs were sorted to >93% purity as the source of tester cDNA. DCs were characterized by flow cytometry as having ∼50-fold higher MHC class II levels than macrophages. Both populations expressed B7.1 and B7.2, although B7.2 levels were significantly higher in the DCs. F4/80 and CD16 were expressed at higher levels on the macrophage population. Functional comparison of the two populations demonstrated that the DC population was ∼100-fold more potent than the macrophage population in stimulating an allo-MLR (data not shown).
After RNA extraction from both populations, we used a PCR-based cDNA synthesis system followed by the PCR-based subtraction procedure, PCR Select. One of the differentially expressed clones encoded a novel Ig supergene family member, which we name B7-DC. The murine B7-DC cDNA is ∼1.7 kb in length encoding a 247-aa precursor protein with a 23-aa NH2-terminal signal peptide and a predicted molecular weight of ∼25 kD (Fig. 1 a). The putative leader sequence and transmembrane domain were identified using the SOSUI program 29. Two charged aa are found within the 23-aa transmembrane domain of mB7-DC, suggesting a possible binding partner. At the aa level, murine B7-DC is 70% identical to the human B7-DC, indicating that they are orthologues (Fig. 1 a). The hB7-DC differs slightly from the murine B7-DC in that it has a longer cytoplasmic tail.
Figure 1.
Aa sequence of B7-DC and comparison with other B7 family members. (a) Aa sequence comparison of murine B7-DC to human B7-DC. The mB7-DC putative leader and transmembrane domain are underlined. The alignment was done using Clustawl-Gonnet Pam250 matrix. The red lettering shows identical aa and the blue lettering shows conservative substitutions. The asterisks indicate cysteine residues that may be important in the formation of disulfide bonds inside the IgV or IgC domains. (b) Aa sequence comparison of mB7-DC to mB7-H1/ PD-L1. (c) Both hB7-DC and B7-H1/PD-L1 are localized on human chromosome 9p24 and map to within 150 kb of each other on BAC clone RPCI-11.2. hB7-DC is centromeric to B7-H1.
Through a homology search, we found that B7-DC has significant homology to B7-H1/PD-L1 (34% identity, 48% similarity; Fig. 1 b), to a lesser extent butyrophilin (30% identity, 45% similarity), and <20% identity to B7.1 and B7.2. Even though no immunologic function has yet been demonstrated for butyrophilin, phylogenetic studies indicate that it is likely related to the B7 family through exon shuffling 30 31. They each possess the canonical IgV-IgC structure and a transmembrane domain. In contrast to the other B7 family members, murine B7-DC has an extremely short cytoplasmic tail (4 aa).
To determine the genomic structure of mB7-DC, we isolated a genomic clone by screening a pooled BAC library using probes from the 5′ and 3′ UTRs. Chromosome location mapping using the BAC clones showed that mB7-DC is located between the regions of chromosome 19C2 and 19C3, which corresponds to a region of human chromosome 9, where hB7-H1/PD-L1 has been mapped (data not shown). hB7-DC has been found to be located on two chromosome 9 BAC clones. In addition, both hB7-DC and hB7-H1/PD-L1 were found to be located on a single chromosome 9 BAC clone with an insertion size of ∼164 kb (Fig. 1 c). The genomic proximity of B7-DC and B7-H1/PD-L1 is reminiscent of the B7.1/B7.2 pair, which map to within 1 megabase of each other.
B7-DC Is Selectively Expressed in DCs.
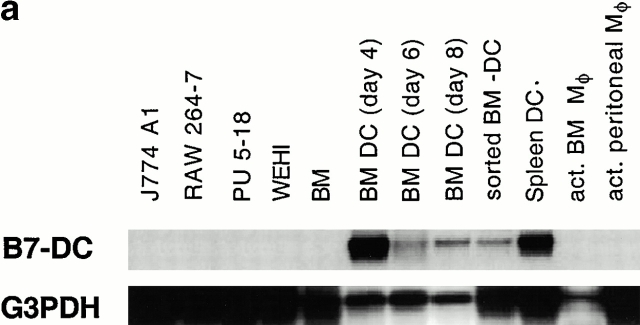
To determine the expression pattern of B7-DC, virtual Northern blot was performed using RNA extracted from multiple macrophage cell lines, macrophage cultures, and DCs derived from both bone marrow and spleen. Although strong hybridization was detected using a B7-DC probe in immature (day 4 and 6) and mature (day 8 and sorted MHC IIhiB7hi) bone marrow–derived DCs and splenic DCs, no signal was detected in any of four macrophage cell lines, activated bone marrow macrophages, or peritoneal macrophages (Fig. 2 a). We were also able to detect strong expression of hB7-DC in human DCs grown from peripheral blood mononuclear cells with GM-CSF plus IL-4 (Fig. 2 b).
Figure 2.

B7-DC is differentially expressed between DCs and macrophages. (a) Distribution of mB7-DC mRNA in murine bone marrow DCs, splenic DCs, macrophages (Mφ), and macrophage lines was assessed by hybridization analysis using 0.5 μg/lane purified cDNA generated by SMART cDNA synthesis and run on a 1% agarose gel. G3PDH is used as control. J774A1, RAW 264.7, Pu5-1.8, and WEHI are macrophage cell lines. act., activated; BM, bone marrow. (b) hB7-DC is expressed in human DCs. Lane 1 shows human placenta and lane 2 shows human DCs cultured with GM-CSF plus IL-4. Oligos from the 5′ and 3′ UTR of human B7-DC were used to make PCR DNA probe for virtual Northern analysis of total RNA of human DCs. β-actin was used as control to ensure the quality of mRNA.
B7-DC Does Not Bind to CD28 or CTLA-4 but Does Bind to PD-1.
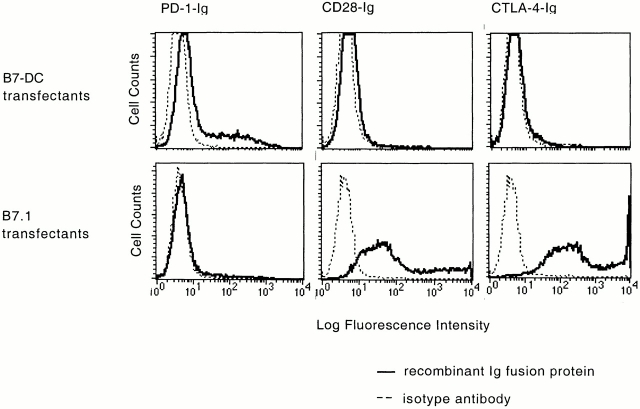
Although B7-DC has structural and sequence homology to the B7 family, it does not contain the putative CD28/CTLA-4 binding sequence, SQDXXXELY or XXXYXXRT 32. To directly assess binding, we analyzed the ability of dimeric CD28-Ig, CTLA-4-Ig to stain 293T cells transfected with either B7-DC or B7.1. Whereas strong binding was observed with B7.1 transfectants, there was no binding to B7-DC transfectants (Fig. 3). Based on the homology and genomic proximity between B7-DC and B7-H1/PD-L1, we also tested PD-1 as a candidate binding partner for B7-DC. Indeed, PD-1-Ig bound to B7-DC transfectants but not to B7.1 transfectants. The binding of PD-1-Ig to B7-DC transfectants is lower than the binding of CTLA-4-Ig and CD28-Ig to B7.1 transfectants, although it is totally specific. Further confirmation of the binding of PD-1 to B7-DC was obtained from positive staining of stable B7-DC-GFP transfectants with PD-1-Ig (data not shown). These results suggest that PD-1 is indeed a receptor for B7-DC.
Figure 3.
B7-DC binds PD-1 but not CD28 or CTLA-4. 293T cells were transiently transfected with pCAGGS-B7.1 or pCAGGS-B7-DC. Transfectants were stained with PD-1-Ig, CD28-Ig, and CTLA-4-Ig fusion molecules followed by PE-labeled secondary antibody. Staining of pCAGGS (empty vector) transfectants was negative (data not shown).
B7-DC Functions As a Costimulatory Molecule for T Cells.
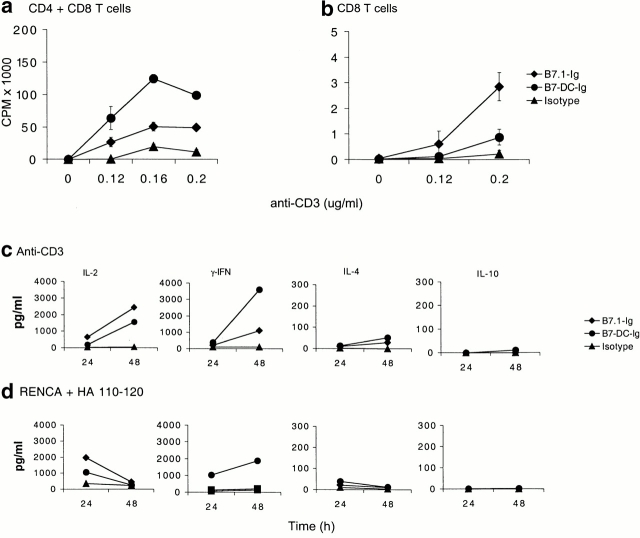
To determine whether B7-DC possesses costimulatory activity, we produced a soluble B7-DC-Ig fusion protein which could be added to T cell stimulation assays. We first measured the proliferative response to increasing amounts of plate-bound anti-CD3 in the presence of B7-DC-Ig, B7.1-Ig, or an isotype control. Fig. 4 a shows that, in the presence of suboptimal amounts of anti-CD3, B7-DC costimulates a T cell proliferative response to a greater level than B7.1. Furthermore, B7-DC costimulates proliferative responses among CD4 cells to a much greater extent than among CD8 cells (Fig. 4 b). B7-DC fails to stimulate T cells in the absence of a TCR-dependent stimulus, indicating that it provides a true costimulatory signal.
Figure 4.
Costimulation of T cells by B7-DC. (a) Purified T cells (CD4 plus CD8) were cultured in the presence of increasing concentrations of precoated antibody against CD3 (monoclonal antibody 2C11) and a constant amount of immobilized B7.1-Ig (♦), B7-DC-Ig (•), or isotype control (▴). The concentration of the Ig-fusion molecules used was 0.1 μg/ml. Data depict one representative experiment of three. (b) Purified CD8 T cells were cultured in the presence of increasing concentrations of precoated antibody against CD3 and a constant amount of immobilized B7.1-Ig (♦), B7-DC-Ig (•), or isotype control (▴) as in panel a. Data depict one representative experiment of two. Cells were incubated for 72 h and labeled with [3H]thymidine. (c) For analysis of cytokine secretion, purified T cells were cultured in the presence of precoated anti-CD3 (0.12 μg/ml) and 0.1 μg/ml of immobilized B7.1-Ig (♦), B7-DC-Ig (•), or isotype control (▴) as in panel a. Supernatants were collected after 24 and 48 h culture and assayed for the indicated lymphokines using ELISA. Data depict one representative experiment of three. (d) IFN-γ–treated RENCA cells expressing MHC class II were loaded with 12.5 μg/ml HA110–120 peptide and incubated with purified HA plus I-Ed–specific transgenic T cells together with 2 μg/ml of soluble B7.1-Ig (♦), B7-DC-Ig (•), or isotype control (▴). Data depict one representative experiment of two. Supernatants were collected after 24 and 48 h culture and assayed for the indicated lymphokines using ELISA.
Patterns of Lymphokine Production Costimulated by B7-DC.
The best characterized T cell costimulatory activity of B7 family molecules relates to lymphokine production, one of the most important mediators of T cell function. We therefore analyzed production of a panel of lymphokines by T cells stimulated with anti-CD3 or MHC-peptide costimulated with B7-DC-Ig, B7.1-Ig, or an isotype control. For experiments in which signal 1 is MHC-peptide, RENCA cells (which do not express any endogenous B7.1, B7.2, or B7-DC by RT-PCR analysis) were treated with IFN-γ to induce MHC class II expression and loaded with the I-Ed–restricted HA 110–120 peptide (FERFEIFPKE; reference 33). Purified splenic T cells from an I-Ed plus HA 110–120-specific TCR transgenic mouse line were added and the proliferative response was measured in the presence of either B7-DC-Ig, B7.1-Ig, or an isotype control.
Fig. 4c and Fig. d, demonstrate that patterns of lymphokine costimulation are fairly consistent whether anti-CD3 or MHC-peptide complex is used as “signal 1”. Significantly, although both costimulate similar levels of IL-2, B7-DC costimulates much greater levels of IFN-γ production than B7.1. In contrast, B7-DC fails to costimulate IL-4 or IL-10 production. These findings implicate B7-DC as a DC signal to drive Th1 responses.
Discussion
We have characterized here a new B7 family member whose expression is highly restricted to DCs and which has potent costimulatory properties for T cells. In particular, B7-DC strongly costimulates IFN-γ production much more strongly than B7.1 while not costimulating IL-4 or IL-10 production. These in vitro findings suggest that, in addition to IL-12, B7-DC may be an important DC mediator of Th1 responses. The human orthologue of B7-DC is also expressed in monocyte-derived DCs, though confirmation of biological equivalence awaits functional studies with human T cells. Mature monocyte-derived DCs in humans and CD8α+ DCs in mice induce very strong Th1 responses, whereas immature human DCs induce IL-10 production and differentiation of regulatory T cells that inhibit Th1 effector responses 34 35 36. The role of plasmacytoid DCs in humans and murine CD8α− DCs in mediating Th2 responses remains unresolved 37 38. It will be important to directly correlate B7-DC expression in these different DC subtypes with their ability to initiate Th1 effector responses.
The restricted expression of B7-DC contrasts with the previously described B7 family members, suggesting that it participates in different immune responses than the defined B7.1/2 pathways. Although a weak B7-DC signal was detected by RT-PCR in activated macrophages, preliminary real-time RT-PCR analysis indicates that B7-DC mRNA expression in DCs is >15-fold higher than in activated macrophages (data not shown). Antibody staining likewise detects very low levels of B7-DC on the surface of activated macrophages but it is unclear whether such low levels could contribute significantly to T cell activation by macrophages.
Although B7-DC fails to bind CD28 or CTLA-4, it does bind PD-1, a receptor for B7-H1/PD-L1 18 39 40. The strong homology between B7-DC and B7-H1/PD-L1 (greater than that between B7.1 and B7.2), the close physical linkage between B7-DC and hB7-H1/PD-L1, and their binding to a common receptor suggests that they are related by a relatively recent duplication event. This is highly analogous to B7.1 and B7.2, which both map to within 1 megabase on mouse chromosome 16 and human chromosome 3 41. It will be important to discern the relative biologic roles of B7-DC versus B7-H1/PD-L1 as mediated by PD-1 and other putative receptor(s). PD-1 is expressed subsequent to T cell activation and appears to inhibit T cell activation and induce apoptosis under conditions of T cell stimulation with high concentrations of anti-CD3. PD-1 knockout mice develop an autoimmune syndrome 18 characterized by clinical manifestations of hypertrophic cardiomyopathy. In contrast, Dong et al. 17 found that B7-H1/PD-L1 costimulated T cell proliferation and cytokine release at lower concentrations of anti-CD3. Thus, by analogy to the situation with CD28 and CTLA-4, PD-L1 may be a counterreceptor for an as yet unidentified activating receptor. Despite sharing PD-1 as a binding partner, B7-DC and B7-H1/PD-L1 appear to demonstrate differences in lymphokine costimulation patterns. For example, B7-H1/PD-L1 has been reported to costimulate IL-10 production by T cells whereas B7-DC does not. The molecular basis for these differences awaits the elucidation of binding affinities as well as the complete receptor complement for these two costimulatory molecules.
Acknowledgments
We thank Dan Meuller, Leiping Chen, Katie Wartenby, Frederique Rattis, and members of our Immunology Program for helpful suggestions. We thank James Hildreth for advice on B7-DC-Ig fusion molecule production.
This work was supported by National Institutes of Health grants, a Burroughs Wellcome Award, and gifts from Dorothy Needle and Bill and Betty Topercer. D. Pardoll is a Janney Foundation Scholar.
Footnotes
S.-Y. Tseng, M. Otsuji, and K. Gorski contributed equally to this work.
Abbreviations used in this paper: aa, amino acid(s); BAC, bacterial artificial chromosome; CTLA, CTL-associated antigen; DC, dendritic cell; HA, hemagglutinin; RACE, rapid amplification of cDNA ends; RT, reverse transcription.
References
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Thompson C.B., Lindsten T., Ledbetter J.A., Kunkel S.L., Young H.A., Emerson S.G., Leiden J.M., June C.H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F.A., McArthur J.G., Gross J.A., Raulet D.H., Allison J.P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Brady W., Grosmaire L., Aruffo A., Damle N.K., Ledbetter J.A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J.D., Irving B.A., Crabtree G.R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Lindstein T., June C.H., Ledbetter J.A., Stella G., Thompson C.B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Brady W., Urnes M., Grosmaire L.S., Damle N.K., Ledbetter J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P., Marengere L.E., Mittrucker H.W., Mak T.W. CTLA-4, a negative regulator of T-lymphocyte activation. Immunol. Rev. 1996;153:183–207. doi: 10.1111/j.1600-065x.1996.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Kuchroo V.K., Das M.P., Brown J.A., Ranger A.M., Zamvil S.S., Sobel R.A., Weiner H.L., Nabavi N., Glimcher L.H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathwaysapplication to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Hathcock K.S., Laszlo G., Pucillo C., Linsley P., Hodes R.J. Comparative analysis of B7-1 and B7-2 costimulatory ligandsexpression and function. J. Exp. Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L., O'Fallon S., Somoza C., Phillips J.H., Linsley P.S., Okumura K., Ito D., Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- Van Parijs L., Sethna M.P., Schweitzer A.N., Borriello F., Sharpe A.H., Abbas A.K. Functional consequences of dysregulated B7-1 (CD80) and B7-2 (CD86) expression in B or T lymphocytes of transgenic mice. J. Immunol. 1997;159:5336–5344. [PubMed] [Google Scholar]
- Abbas A.K., Sharpe A.H. T-cell stimulationan abundance of B7s. Nat. Med. 1999;5:1345–1346. doi: 10.1038/70905. [DOI] [PubMed] [Google Scholar]
- Borriello F., Sethna M.P., Boyd S.D., Schweitzer A.N., Tivol E.A., Jacoby D., Strom T.B., Simpson E.M., Freeman G.J., Sharpe A.H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- Sharpe A.H. Analysis of lymphocyte costimulation in vivo using transgenic and ‘knockout’ mice. Curr. Opin. Immunol. 1995;7:389–395. doi: 10.1016/0952-7915(95)80115-4. [DOI] [PubMed] [Google Scholar]
- Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow M.M., Wallin J.J., Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhu G., Chapoval A.I., Dong H., Tamada K., Ni J., Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- Yoshinaga S.K., Whoriskey J.S., Khare S.D., Sarmiento U., Guo J., Horan T., Shih G., Zhang M., Coccia M.A., Kohno T. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- McAdam A.J., Greenwald R.J., Levin M.A., Chernova T., Malenkovich N., Ling V., Freeman G.J., Sharpe A.H. ICOS is critical for CD-40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- Dong C., Juedes A.E., Temann U., Shresta S., Allison J.P., Ruddle N.H., Flavell R. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Tafuri A., Shahinian A., Bladt F., Yoshinaga S.K., Jordana M., Wakeham A., Boucher L., Bouchard D., Chan V.S.F., Duncan G. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Inaba K., Swiggard W.J., Steinman R., Romani N., Schuler G. Isolation of Dendritic Cells 1998. John Wiley & Sons, Inc; New York: pp. 421 pp [Google Scholar]
- Fortier A.H., Falk L.A. Isolation of Murine Macrophages 1994. John Wiley & Sons, Inc; New York: pp. 216 pp [Google Scholar]
- Orimoto K., Tsuchiya H., Sakurai J., Nishizawa M., Hino O. Identification of cDNAs induced by the tumor suppressor Tsc2 gene using a conditional expression system in Tsc2 mutant (Eker) rat renal carcinoma cells. Biochem. Biophys. Res. Commun. 1998;247:728–733. doi: 10.1006/bbrc.1998.8853. [DOI] [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kampgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P.O., Steinman R.M., Schuler G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirolawa T., Boon-Chieng S., Mitaku S. SOSUIclassification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Peach R., Gladstone P., Bajorath J. Extending the B7 (CD80) gene family. Protein Sci. 1994;3:1341–1343. doi: 10.1002/pro.5560030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J., Ribouchon M., Depetris D., Mattei M., Offer C., Tazi-Ahnini R., Pontarotti P. Cloning, structural analysis, and mapping of the B30 and B7 multigenic families to the major histocompatibility complex (MHC) and other chromosomal regions. Immunogenetics. 1997;46:383–395. doi: 10.1007/s002510050292. [DOI] [PubMed] [Google Scholar]
- Fargeas C.A., Truneh A., Reddy M., Hurle M., Sweet R., Sekaly R.P. Identification of residues in the V domain of CD80 (B7-1) implicated in functional interactions with CD28 and CTLA4. J. Exp. Med. 1995;182:667–675. doi: 10.1084/jem.182.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirberg J., Baron A., Jakob S., Rolink A., Karjalainen K., von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex–restricted receptor. J. Exp. Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C., Bhadwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsk E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo . Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P., Hilkens C.M., Wierenga E.A., Kapsenberg M.L. T-cell priming by type-1 and type-2 polarized dendritic cellsthe concept of a third signal. Immunol. Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Kapsenberg M.L., Kalinski P. The concept of type 1 and type 2 antigen-presenting cells. Immunol. Lett. 1999;69:5–6. doi: 10.1016/s0165-2478(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T., Taniwaki M., Ishida Y., Kawaichi M., Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1) Genomics. 1994;23:704–706. doi: 10.1006/geno.1994.1562. [DOI] [PubMed] [Google Scholar]
- Reeves R.H., Patch D., Sharpe A.H., Borriello F., Freeman G.J., Edelhoff S., Disteche C. The costimulatory genes Cd80 and Cd86 are linked on mouse chromosome 16 and human chromosome 3. Mamm. Genome. 1997;8:581–582. doi: 10.1007/s003359900508. [DOI] [PubMed] [Google Scholar]