The diverse antigen receptor repertoires of B and T lymphocytes are generated by somatic rearrangement of Ig and TCR V, D, and J gene segments during lymphocyte development. A basic mechanistic understanding of V(D)J recombination reaction is now at hand. The process is initiated by recombinase proteins recombination activating gene (RAG)-1 and RAG-2, which bind to recombination signal sequences (RSSs) that flank Ig and TCR gene segments, recruit a pair of RSSs into a synaptic complex, and generate double strand breaks (DSBs) between the RSSs and coding segments. The reaction is then completed with the additional participation of DSB repair proteins, which help to process and rejoin the coding and signal end molecules to generate coding joints and signal joints, respectively.
V(D)J recombination at the various Ig and TCR loci is highly regulated during lymphocyte development, with lineage- and developmental stage–specific rearrangement events distinguishing individual loci, and even individual gene segments within loci. It has long been appreciated that this developmental regulation cannot be accounted for by the expression of either RAG or DNA repair proteins. It has been noted that transcription of unrearranged gene segments (germline transcription) parallels their developmental activation for V(D)J recombination. Yancopoulos and Alt interpreted this transcriptional activity to reflect a permissive chromatin structure, and on this basis initially proposed that a permissive chromatin structure determines the suitability of particular gene segments as targets for the V(D)J recombinase 1. Thus, developmental control of V(D)J recombination would be exerted at the level of gene segment accessibility within chromatin.
Accessibility control has been a tremendously useful concept that has influenced research in this area for quite some time 2. However, gaining a molecular understanding of accessibility has been difficult. The accessibility hypothesis received perhaps its strongest confirmation when Stanhope-Baker and Schlissel showed that chromosomal RSSs could be cleaved by introducing exogenous RAG proteins into isolated nuclei in vitro 3. The ability of particular RSSs to serve as substrates for RAG was determined by the developmental stage of the cells from which the nuclei were isolated, an indication of developmentally regulated changes in RSS accessibility. In addition, a host of studies have established that transcriptional enhancers and promoters play critical roles in establishing the efficiency, lineage specificity, and developmental stage specificity of V(D)J recombination events 2. However, the molecular mechanisms by which promoter and enhancer function translate to accessibility for V(D)J recombination have remained elusive.
Chromatin exists in a highly compacted structure in the eukaryotic nucleus, and it has long been appreciated that this structure poses a barrier to gene expression. The basic unit of chromatin structure is the nucleosome, which is composed of 146 bp of DNA wound around an octamer of core histones 4. Although nucleosomes are further organized into higher order structures in chromatin, even the mononucleosome is known to present a barrier to transcription factors. On the basis of recent studies it is clear that mononucleosomes present a barrier to RAG proteins as well 5 6. How is this barrier overcome?
A posttranslational modification of histones, the acetylation of lysine residues in their amino terminal tails, has received much attention as a regulator of chromatin structure and gene expression 7 8. This modification reduces the interaction between histones and nucleosomal DNA and also reduces contacts between nucleosomes. Chromatin regions that are transcriptionally active or poised for activation typically contain hyperacetylated histones. Histone hyperacetylation can be targeted to promoters and enhancers by transcriptional coactivators with histone acetyltransferase (HAT) activity, can promote increased transcription factor binding to nucleosomal DNA, and can be a critical step in transcriptional activation. Recent studies have therefore addressed the role of histone acetylation in accessibility for V(D)J recombination.
McMurry and Krangel mapped the acetylation status of histones in the context of both a transgenic V(D)J recombination reporter substrate and the endogenous TCR-α/δ locus, and showed that the TCR δ and α enhancers, previously implicated as developmental regulators of V(D)J recombination, function as long range developmental regulators of histone acetylation 9. Moreover, those regions of the loci that were accessible for V(D)J recombination were shown to contain hyperacetylated histones, indicating a tight linkage between accessibility and acetylation status. The TCR-β enhancer has similarly been shown to control V(D)J recombination and acetylation within a defined segment of the TCR-β locus, and incubation of Eβ−/− thymocytes with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) was found to partially rescue V(D)J recombination in this region 10. TSA has been used to activate V(D)J recombination in other recent studies as well 11 12 13. However, TSA treatment has the potential for broader effects, and none of the above studies evaluated the extent to which locus acetylation was actually increased by TSA treatment or the step at which V(D)J recombination was rescued.
In this issue, Agata et al. analyze the developmental regulation of Vγ gene segment rearrangement at the TCR-γ locus 14. Rearrangement of Vγ3 to Jγ1 occurs in fetal but not adult thymocytes due to a change in the developmental potential of stem cells that seed the thymus 15. By contrast, rearrangement of nearby Vγ2 to Jγ1 occurs at low levels in the fetus but predominates in the adult. The authors show that the absence of Vγ3 rearrangement in adult thymocytes is associated with reduced Vγ3 acetylation. They further show that although adult bone marrow cells fail to generate Vγ3+ cells in fetal thymus organ culture, they can do so if cultured in the presence of TSA. The appearance of Vγ3+ cells is shown to be associated with increases in Vγ3 acetylation, germline transcription, rearrangement, and DSB formation. Thus, TSA appears to promote V(D)J recombination by promoting RAG-mediated cleavage at the Vγ3 RSS, consistent with an effect on Vγ3 accessibility. This strengthens the notion that histone acetylation is a critical regulator of accessibility for V(D)J recombination in vivo.
As the case for histone acetylation builds, it may be asked whether hyperacetylated chromatin is truly sufficient for accessibility to RAG proteins. In addition to the covalent modification of histones by HATs and HDACs, chromatin structure can also be modified by the activity of several ATP-dependent chromatin remodeling complexes 16 17. These remodeling complexes can create a modified nucleosome conformation with altered histone–DNA interactions, and can promote nucleosome displacement, although their precise mechanism of action is still unclear. HATs and ATP-dependent remodeling complexes cooperate to remodel nucleosomes and to assemble preinitiation complexes at eukaryotic promoters. Do they function similarly in providing accessibility to RAG proteins?
Short DNA fragments containing RSSs have been assembled into mononucleosomes and have been found to serve as poor substrates for RAG-mediated cleavage in vitro 5 6. However, recent experiments have shown that in the presence of the nonhistone chromosomal protein high mobility group 1 (HMG1), RAG-mediated cleavage can be substantially increased if nucleosomes are assembled from hyperacetylated histones, or if assembled nucleosomes are incubated with the switch/sucrose nonfermenting (SWI/SNF) chromatin remodeling complex 18. Together, the effects of acetylation and SWI/SNF increase RSS cleavage to levels approaching that of naked DNA. Hence, these two classes of remodeling events appear to cooperate to provide accessibility for V(D)J recombination. This said, it should be noted that another study failed to detect the effect of acetylation on cleavage of nucleosomal RSSs, and the reason for the discrepancy is not yet clear 6. Several additional issues should be considered in evaluating the results of the in vitro experiments. First, it must be remembered that multiple levels of chromatin structure have already been eliminated by the simple nature of the mononucleosomal substrates used in vitro. It will be important to examine the accessibility issue in vitro using more complex chromatinized templates, and under conditions in which cleavage requires synapsis of two RSSs as is the case in vivo. Second, the in vitro experiments are conducted using truncated versions of RAG proteins. Although the core RAG proteins used appear sufficient for basic enzymatic activity, the missing portions could have unappreciated functions that impact access to and cleavage of chromatin-embedded RSSs. Thus we should expect future in vitro studies of accessibility to focus on the use of more complex and more physiological components. As well, we might expect future efforts in vivo to focus on ways to more specifically recruit and manipulate remodeling activities at endogenous loci and in chromatinized V(D)J recombination reporter substrates.
Although the correlation between germline transcription and V(D)J recombination provided the impetus for the accessibility hypothesis, the precise relationships between germline transcription and V(D)J recombination, on the one hand, and germline transcription and accessibility, on the other hand, have remained ambiguous. Does transcription play a causal role in modulating chromatin structure and thereby provide accessibility for V(D)J recombination? Alternatively, are transcription and V(D)J recombination independent consequences of an accessible chromatin structure? As histone acetylation appears to be intimately associated with chromatin remodeling, transcription, and V(D)J recombination, it may be useful to consider the ways in which cis-acting elements establish hyperacetylated regions of chromatin, and the potential implications for the functioning of TCR and Ig loci.
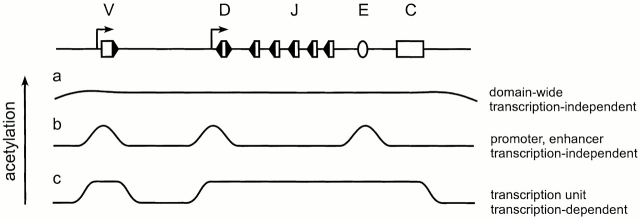
First, acetylation and remodeling may depend on changes in subnuclear localization (Fig. 1 a). Active and inactive regions of chromosomes are segregated into distinct regions of interphase nuclei 19 20. Foci of inactive, centromeric heterochromatin are thought to establish a repressive environment, and specific genes may be recruited into this environment in association with silencing, or may be excluded from this environment in association with activation 21. Studies of the human β-globin locus have linked movement away from centromeric heterochromatin to domain-wide increases in locus accessibility and histone acetylation, even in the absence of transcription 22. Such changes in localization are under the control of specific cis-acting elements, but the associated domain-wide changes in acetylation may occur nonspecifically due to changes in the concentrations of various factors between repressive and permissive environments. This provides a mechanism whereby accessibility and acetylation may be modulated in the absence of transcription.
Figure 1.
Possible mechanisms by which histone acetylation may be regulated at a prototypical TCR/Ig locus. (a) Low-level, domain-wide acetylation associated with locus repositioning in the nucleus; (b) localized acetylation targeted to enhancers and promoters by the assembly of transcriptional activators and coactivators; (c) acetylation extending across transcription units as a consequence of transcriptional elongation. Arrows denote transcriptional promoters, open rectangles denote gene segments (V, D, J, and C), an open oval denotes a transcriptional enhancer (E), and filled triangles denote RSSs.
Second, acetylation and remodeling may depend on sequence-specific targeting to promoters and enhancers (Fig. 1 b). Sequence-specific transcription factors can recruit coactivator HATs and chromatin remodeling complexes to promoters and enhancers and can result in localized hyperacetylation and remodeling that typically extends no farther than a few nucleosomes 23 24. This remodeling is associated with the assembly of a preinitiation complex and with transcriptional activation, but precedes and can be segregated from transcriptional activation per se 24 25.
Finally, acetylation and remodeling may depend on transcriptional elongation (Fig. 1 c). HATs have been found to be components of elongating RNA Pol II complexes 26, and promoter distal chromatin disruption can be a consequence of transcriptional elongation 27. Consistent with this, intergenic transcripts that extend across large regions of the human β globin locus have been implicated in chromatin remodeling over a region corresponding to the entire transcription unit 28. Whether acetylation is restricted to the promoter or extended across the transcription unit might depend on the nature of the HATs and other factors that are recruited to a particular promoter. Note that the three mechanisms outlined here could operate sequentially and contribute additively to remodeling at a given locus.
With these considerations in mind we can return to accessibility control at TCR and Ig loci. Enhancers have been shown, by themselves, to modulate histone acetylation and chromatin structure 29 30. However, their effects on accessibility and histone acetylation have been documented over only relatively short distances. Rather, the ability of enhancers to remodel chromatin and provide accessibility to RAG over many kilobases seems to depend on activation of a promoter 31 32 33. Thus, although locus repositioning is likely to play a critical role in locus activation (Fig. 1 a), remodeling events related to promoter activation (Fig. 1b and Fig. c) seem to be a key additional requirement for accessibility to RAG. RSSs located in the immediate vicinity of a promoter might be made accessible to RAG due to local effects of recruited HATs and chromatin remodeling complexes (Fig. 1 b), without any mechanistic requirement for transcription per se. However, RSSs located at a distance from a promoter (for example, J segment RSSs in Fig. 1) may depend critically on transcription-coupled remodeling (Fig. 1 c). Hence, when it comes to germline transcription and V(D)J recombination, we may be able to have it both ways. Germline transcription and V(D)J recombination may be independent consequences of accessibility at some RSSs, whereas accessibility and V(D)J recombination may be consequences of germline transcription at others. It should be kept in mind that the various pathways to acetylation are likely to involve the activities of distinct HATs that modify different lysines in histones H3 or H4 22 30. It is not known whether accessibility for RAG reflects a generic chromatin accessibility or might require a specific pattern of acetylation. With continued progress, these and other aspects of chromatin control of V(D)J recombination should become more readily accessible in the near future.
Acknowledgments
I would like to thank Drs. Carolyn Doyle, Barry Sleckman, Yuan Zhuang, and Rajkamal Tripathi for reviewing the manuscript.
Work in the author's laboratory is supported by a grant from the National Institutes of Health (GM41052).
References
- Yancopoulos G., Alt F. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Sleckman B.P., Gorman J.R., Alt F.W. Accessibility control of antigen receptor variable region gene assemblyrole of cis-acting elements. Annu. Rev. Immunol. 1996;14:459–481. doi: 10.1146/annurev.immunol.14.1.459. [DOI] [PubMed] [Google Scholar]
- Stanhope-Baker P., Hudson K.M., Shaffer A.L., Constantinescu A., Schlissel M.S. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;85:887–897. doi: 10.1016/s0092-8674(00)81272-6. [DOI] [PubMed] [Google Scholar]
- Luger K., Mader A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Kwon J., Imbalzano A.N., Matthews A., Oettinger M.A. Accessibility of nucleosomal DNA to V(D)J cleavage is modulated by RSS positioning and HMG1. Mol. Cell. 1998;2:829–839. doi: 10.1016/s1097-2765(00)80297-x. [DOI] [PubMed] [Google Scholar]
- Golding A., Chandler S., Ballestar E., Wolffe A.P., Schlissel M.S. Nucleosome structure completely inhibits in vitro cleavage by the V(D)J recombinase. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:3712–3723. doi: 10.1093/emboj/18.13.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- Brown C.E., Lechner T., Howe L., Workman J.L. The many HATs of transcriptional coactivators. TIBS (Trends Biochem. Sci.). 2000;25:15–19. doi: 10.1016/s0968-0004(99)01516-9. [DOI] [PubMed] [Google Scholar]
- McMurry M.T., Krangel M.S. A role for histone acetylation in the developmental regulation of V(D)J recombination. Science. 2000;287:495–498. doi: 10.1126/science.287.5452.495. [DOI] [PubMed] [Google Scholar]
- Mathieu N., Hempel W.M., Spicuglia S., Verthuy C., Ferrier P. Chromatin remodeling by the T cell receptor (TCR)-β gene enhancer during early T cell developmentimplications for the control of TCR-β locus recombination. J. Exp. Med. 2000;192:625–636. doi: 10.1084/jem.192.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durum S.K., Candeias S., Nakajima H., Leonard W.J., Baird A.M., Berg L.J., Muegge K. Interleukin 7 receptor control of T cell receptor γ gene rearrangementrole of receptor-associated chains and locus accessibility. J. Exp. Med. 1998;188:2233–2241. doi: 10.1084/jem.188.12.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S.R., Baltimore D. Chromatin remodeling directly activates V(D)J recombination. Proc. Natl. Acad. Sci. USA. 1999;96:10788–10793. doi: 10.1073/pnas.96.19.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBlane F., Boyes J. Stimulation of V(D)J recombination by histone acetylation. Curr. Biol. 2000;10:483–486. doi: 10.1016/s0960-9822(00)00449-8. [DOI] [PubMed] [Google Scholar]
- Agata Y., Katakai T., Ye S.-K., Sugai M., Gonda H., Honjo T., Ikuta K., Shimizu A. Histone acetylation determines the developmentally regulated accessibility for T cell receptor γ gene recombination. J. Exp. Med. 2001;193:873–879. doi: 10.1084/jem.193.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y.-H., Weissman I.L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Kingston R.E., Narlikar G.J. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 1999;13:2339–2352. doi: 10.1101/gad.13.18.2339. [DOI] [PubMed] [Google Scholar]
- Vignali M., Hassan A.H., Neely K.E., Workman J.L. ATP-dependent chromatin-remodeling complexes. Mol. Cell. Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J., Morshead K.B., Guyon J.R., Kingston R.E., Oettinger M.A. Histone acetylation and hSWI/SNF remodeling act in concert to stimulate V(D)J cleavage of nucleosomal DNA. Mol. Cell. 2000;6:1037–1048. doi: 10.1016/s1097-2765(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Lamond A.I., Earnshaw W.C. Structure and function in the nucleus. Science. 1998;280:547–553. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- Cockell M., Gasser S.M. Nuclear compartments and gene regulation. Curr. Opin. Genet. Dev. 1999;9:199–205. doi: 10.1016/S0959-437X(99)80030-6. [DOI] [PubMed] [Google Scholar]
- Brown K.E., Baxter J., Graf D., Merkenschlager M., Fisher A.G. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol. Cell. 1999;3:207–217. doi: 10.1016/s1097-2765(00)80311-1. [DOI] [PubMed] [Google Scholar]
- Schubeler D., Francastel C., Cimbora D.M., Reik A., Martin D.I.K., Groudine M. Nuclear localization and histone acetylationa pathway for chromatin opening and transcriptional activation of the human β-globin locus. Genes Dev. 2000;14:940–950. [PMC free article] [PubMed] [Google Scholar]
- Parekh B.S., Maniatis T. Virus infection leads to localized hyperacetylation of histones H3 and H4 at the IFN-β promoter. Mol. Cell. 1999;3:125–129. doi: 10.1016/s1097-2765(00)80181-1. [DOI] [PubMed] [Google Scholar]
- Kuo M.-H., vom Baur E., Struhl K., Allis C.D. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell. 2000;6:1309–1320. doi: 10.1016/s1097-2765(00)00129-5. [DOI] [PubMed] [Google Scholar]
- Agalioti T., Lomvardas S., Parekh B., Yie J., Maniatis T., Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell. 2000;103:667–678. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Travers A. Chromatin modification by DNA tracking. Proc. Natl. Acad. Sci. USA. 1999;96:13634–13637. doi: 10.1073/pnas.96.24.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Kingston R.E. Disruption of downstream chromatin directed by a transcriptional activator. Genes Dev. 1997;11:3116–3121. doi: 10.1101/gad.11.23.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribnau J., Diderich K., Pruzina S., Calzolari R., Fraser P. Intergenic transcription and developmental remodeling of chromatin subdomains in the human β-globin locus. Mol. Cell. 2000;5:377–386. doi: 10.1016/s1097-2765(00)80432-3. [DOI] [PubMed] [Google Scholar]
- Jenuwein T., Forrester W.C., Fernandez-Herrero L.A., Laible G., Dull M., Grosschedl R. Extension of chromatin accessibility by nuclear matrix attachment regions. Nature. 1997;385:269–272. doi: 10.1038/385269a0. [DOI] [PubMed] [Google Scholar]
- Fernandez L.A., Winkler M., Grosschedl R. Matrix attachment region-dependent function of the immunoglobulin μ enhancer involves histone acetylation at a distance without changes in enhancer occupancy. Mol. Cell. Biol. 2001;21:196–208. doi: 10.1128/MCB.21.1.196-208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villey I., Caillol D., Selz F., Ferrier P., de Villartay J.-P. Defect in rearrangement of the most 5′ TCR-Jα following targeted deletion of T early α (TEA)implications for TCR α locus accessibility. Immunity. 1996;5:331–342. doi: 10.1016/s1074-7613(00)80259-9. [DOI] [PubMed] [Google Scholar]
- Whitehurst C.E., Chattopadhyay S., Chen J. Control of V(D)J recombinational accessibility of the Dβ1 gene segment at the TCRβ locus by a germline promoter. Immunity. 1999;10:313–322. doi: 10.1016/s1074-7613(00)80031-x. [DOI] [PubMed] [Google Scholar]
- Sikes M.L., Suarez C.C., Oltz E.M. Regulation of V(D)J recombination by transcriptional promoters. Mol. Cell. Biol. 1999;19:2773–2781. doi: 10.1128/mcb.19.4.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]