Abstract
Active suppression by T regulatory (Tr) cells plays an important role in the downregulation of T cell responses to foreign and self-antigens. Mouse CD4+ Tr cells that express CD25 possess remarkable suppressive activity in vitro and in autoimmune disease models in vivo. Thus far, the existence of a similar subset of CD25+CD4+ Tr cells in humans has not been reported. Here we show that human CD25+CD4+ Tr cells isolated from peripheral blood failed to proliferate and displayed reduced expression of CD40 ligand (CD40L), in response to T cell receptor–mediated polyclonal activation, but strongly upregulated cytotoxic T lymphocyte–associated antigen (CTLA)-4. Human CD25+CD4+ Tr cells also did not proliferate in response to allogeneic antigen-presenting cells, but they produced interleukin (IL)-10, transforming growth factor (TGF)-β, low levels of interferon (IFN)-γ, and no IL-4 or IL-2. Importantly, CD25+CD4+ Tr cells strongly inhibited the proliferative responses of both naive and memory CD4+ T cells to alloantigens, but neither IL-10, TGF-β, nor CTLA-4 seemed to be directly required for their suppressive effects. CD25+CD4+ Tr cells could be expanded in vitro in the presence of IL-2 and allogeneic feeder cells and maintained their suppressive capacities. These findings that CD25+CD4+ Tr cells with immunosuppressive effects can be isolated from peripheral blood and expanded in vitro without loss of function represent a major advance towards the therapeutic use of these cells in T cell–mediated diseases.
Keywords: suppressor, T lymphocytes, immune tolerance, CTLA-4, IL-2 receptor α chain, human
Introduction
T regulatory (Tr) cells have a key role in the maintenance of immune tolerance to both self- and harmless foreign antigens. Many subsets of Tr cells have been described and recently much progress has been made in understanding their ontogeny, function, and mechanisms of action (for a review, see reference 1). Some Tr cells do not produce cytokines and suppress T cell responses via a mechanism that requires direct cell–cell contact 2 3. Other subsets of Tr cells produce immunoregulatory cytokines, such as IL-10 and TGF-β, and exert their suppressive functions at least in part via the effects of these cytokines 4 5 6 7 8.
CD4+ Tr cells that constitutively express the IL-2 receptor (IL-2R) α chain (CD25) have been identified in the mouse (for a review, see references 2 and 3). These CD25+CD4+ Tr cells show a remarkable suppressive capacity both in vitro and in vivo. Transfer of these Tr cells reduces the pathology of experimentally induced autoimmune diseases such as thyroiditis, gastritis, insulin-dependent diabetes mellitus, and colitis 9 10 11 12, whereas depletion of CD25+CD4+ Tr cells results in the development of systemic autoimmune diseases 11 13 14.
Murine CD25+CD4+ Tr cells are anergic when stimulated in vitro with anti-CD3 mAbs, but proliferate upon addition of exogenous IL-2 15 16. After TCR-mediated stimulation, CD25+CD4+ Tr cells suppress the activation and proliferation of other CD4+ and CD8+ T cells in an antigen-nonspecific manner 16 17 via a mechanism that requires cell–cell contact and, in most systems, is independent of production of immunosuppressive cytokines 15 16. Murine CD25+CD4+ Tr cells constitutively express cytotoxic T lymphocyte–associated antigen (CTLA)-4 9, a negative regulator of T cell activation, and expression of this molecule may be required for the ability of these cells to suppress immune responses in vivo 10 18. In addition, CD25+CD4+ Tr cells may act by downregulating the expression of CD80 and CD86 on APCs 19, although some reports suggest that APCs are not required for the suppressive activity and indicate that direct T cell–T cell interaction is involved 17.
Considering the potent suppressive capacities of murine CD25+CD4+ Tr cells, it was critical to determine whether a similar subset of Tr cells is also present in humans. Results shown here demonstrate that human CD25+CD4+ Tr cells inhibit the proliferative responses of both naive and memory T cells. In addition, human CD25+CD4+ Tr cells can be expanded in vitro and maintain not only their cell surface phenotype but also their potent regulatory functions.
Materials and Methods
Purification of CD25+CD4+ Tr Cells.
Human peripheral blood was obtained from healthy donors in accordance with local ethical committee approval. PBMCs were prepared by centrifugation over Ficoll-Hypaque gradients (Nycomed Amersham), and CD4+ T cells were purified by positive or negative selection (by depletion of CD8, CD11b, CD16, CD19, CD36, and CD56 positive cells) with the CD4+ MultiSort kit or the Untouched CD4+ T cell isolation kit, respectively (Miltenyi Biotec). After isolation of CD4+ T cells, CD25+ cells were stained with PE-coupled anti-CD25 mAbs and purified after the addition of anti-PE–coupled magnetic beads (Miltenyi Biotec). Alternatively, CD4+ T cells were purified with magnetic beads directly coupled to anti-CD25 (Miltenyi Biotec) to facilitate FACS® analysis. Results obtained with CD25+CD4+ Tr cells isolated by negative or positive selection and directly or indirectly coupled CD25 mAbs were identical. Starting with 2 × 108 PBMCs, typically 2–3 × 106 CD25+CD4+ Tr cells were isolated with a purity ranging from 90–95%. CD25−CD4+ T cells were also collected with a purity ranging from 70–90%. For purification of CD25+ cells after in vitro activation of CD25− cells, CD25−CD4+ T cells were activated for 48 h with 10 μg/ml immobilized anti-CD3 and 1 μg/ml soluble anti-CD28 Abs, and CD25+ T cells were purified as described previously.
In Vitro Expansion of T Cell Lines.
CD25+CD4+ or CD25− CD4+ T cells were isolated as described previously. T cells (2 × 105 cells per milliliter) were stimulated with 1 μg/ml anti-CD3 (OKT3, Orthoclone) in the presence of an allogeneic feeder cell mixture consisting of 106 PBMCs per milliliter (irradiated 6,000 rads), 105 JY cells per milliliter (irradiated 10,000 rads), an EBV-LCL that expresses high levels of histocompatibility leukocyte antigen (HLA), costimulatory molecules, and cytokines as described previously 20 21. All cultures were performed in X-VIVO-15 medium (BioWhittaker) supplemented with 10% FCS (Mascia Brunelli), 1% pooled human serum, 100 U/ml penicillin/streptomycin (Bristol-Myers Squibb), and 2 mM glutamine (GIBCO BRL) (hereafter referred to as complete medium). 3 d after activation, 40 U/ml recombinant interleukin (rIL)-2 (Chiron Corp.) was added. Cells were split as necessary and fresh medium with IL-2 was added. T cell lines were restimulated every 14 d. All experiments on expanded cells were performed at least 10 d after activation.
Proliferation and Suppression of T Cells.
To analyze proliferation in response to polyclonal activation, 96-well round-bottomed plates (Costar) were coated overnight at 4°C with anti-CD3 mAbs (10 μg/ml) in 0.1 M Tris, pH 9.5, and washed three times with PBS. T cells were plated at an initial density of 5 × 105 cells per milliliter (100,000 cells per well) in a final volume of 200 μl of complete medium in the absence or presence of 1 μg/ml soluble anti-CD28 mAbs (BD PharMingen), 10 μg/ml soluble secondary rabbit anti–mouse Abs (Sigma Aldrich), and/or 100 U/ml IL-2.
To test antigen-specific T cell proliferation, freshly isolated CD25+CD4+ Tr or CD25−CD4+ T cells (2.5 × 105 cells per milliliter) were stimulated with irradiated (6,000 rads) allogeneic PBMCs (2.5 × 105 cells per milliliter) that had been depleted of CD3+ cells by negative selection (Dynal). For suppression, increasing numbers (up to 2.5 × 105 cells per milliliter) of freshly isolated autologous CD25+CD4+ Tr cells were added. Cells were cocultured in a final volume of 200 μl of complete medium in 96-well round-bottomed plates. 10 μg/ml control IgG (BD PharMingen), 10 μg/ml neutralizing anti–IL-10R (3F9; BD PharMingen), and/or 10 μg/ml or 50 μg/ml anti–TGF-β1,2,3 (R&D Systems), or 10 μg/ml F(ab′)2 anti–CTLA-4 (Ancell) mAbs were added as indicated. For suppression of memory T cells, cells from expanded CD25−CD4+ T cell lines were cultured with allogeneic APCs (from a donor different from that used in the allogeneic feeder cell mixture), and increasing numbers of expanded autologous CD25+CD4+ Tr cells were added as described previously. For control experiments, CD25+CD4+ T cells purified from in vitro–activated CD25−CD4+ T cells were added in increasing numbers to freshly isolated autologous CD25−CD4+ T cells and allogeneic APCs.
After the indicated time, wells were pulsed for 16 h with 1 μCi per well [3H]thymidine (Amersham Pharmacia Biotech). Cells were harvested and counted in a scintillation counter.
ELISAs.
For detection of IL-10, IL-4, IL-2, IFN-γ, and TGF-β1, capture ELISAs were performed on supernatants of cells (106 T cells per milliliter) that had been stimulated with 10 μg/ml immobilized anti-CD3 mAbs with or without 1 μg/ml anti-CD28 and 100 U/ml IL-2, or irradiated CD3-depleted allogeneic PBMCs (106 cells per milliliter) for 24 (for IL-2) or 72 h. ELISAs were performed according to the manufacturer's instructions. All capture and detection mAbs were purchased from BD PharMingen. The limits of detection were as follows: 19 pg/ml IL-2; 9.4 pg/ml IL-4; 15.6 pg/ml IL-10; 62.5 pg/ml IFN-γ; and 62.5 pg/ml TGF-β1.
FACS® Analysis.
Anti-CD4, -CD25, -HLA-DR, -CD45RO, -CD62L, -CD69, and -CD40 ligand (CD40L) mAbs were purchased from Becton Dickinson and were directly coupled to FITC or PE. Expression of IL-2Rβ (CD122) and IL-2Rγ (CD132) was determined by staining with the relevant biotinylated mAbs (BD PharMingen) and streptavidin-coupled Tricolor (Caltag). Cells that were resting, or that had been activated with 10 μg/ml immobilized anti-CD3, or 10 ng/ml PMA (Sigma Aldrich) and calcium ionophore (500 ng/ml A23187; Sigma Aldrich), were incubated with the indicated mAbs for 20 min at 4°C in PBS, 2% FCS, washed once, and analyzed with a FACScan™ flow cytometer using CELLQuest™ software (Becton Dickinson). Expression of CTLA-4 was determined by intracytoplasmic staining with biotinylated anti–CTLA-4 (BD PharMingen) followed by streptavidin-coupled PE (Caltag). Resting or activated cells were fixed with 2% formaldehyde, and membranes were permeabilized by incubation in saponin buffer (PBS, 2% BSA, and 0.5% saponin; Sigma Aldrich) for 10 min. Staining and washing were performed in saponin buffer, and cells were washed once in PBS, 2% BSA before analysis.
Statistical Analysis.
All analysis for statistically significant differences were performed with the Student's paired t test. P values <0.05 were considered significant. Results are expressed as means ± SEM.
Results and Discussion
Isolation and Cell Surface Phenotype of Human CD25+ CD4+ Tr Cells.
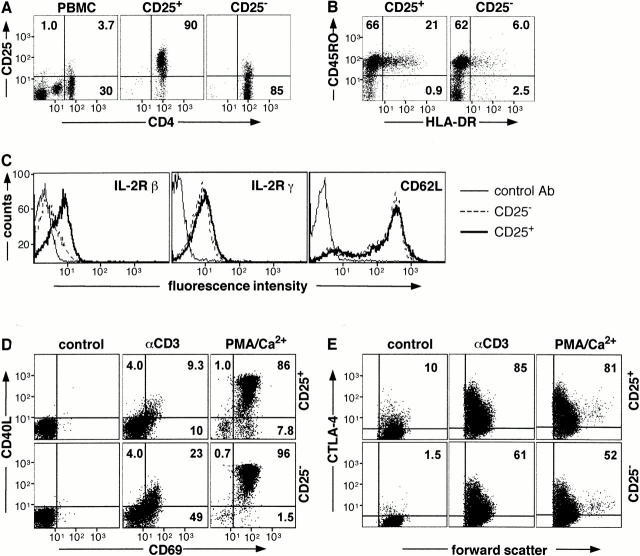
CD25+CD4+ Tr cells are present in human PBMCs. On average they represent 3.0% (range 1.6–4.4%, n = 6) of the total PBMCs and 12.8% (range 9.8–18.1%, n = 6) of CD4+ T cells. These cells could be readily isolated with purities >90% (Fig. 1 A). The majority (82 ± 5.1%) of freshly isolated CD25+CD4+ Tr cells also expressed CD45RO and the percentage of CD25+CD4+ Tr cells expressing HLA-DR was significantly higher than the CD25−CD4+ population (17.3 ± 4.9% vs. 6.4 ± 2.9%, n = 6; Fig. 1 B). In addition, human CD25+CD4+ Tr cells expressed higher levels of the IL-2Rβ in comparison to CD25−CD4+ T cells (61.9 ± 2.6% vs. 33.1. ± 2.5%, n = 5; Fig. 1 C) and a subset of freshly isolated CD25+CD4+ Tr cells expressed CTLA-4 (8.4 ± 1.6%, n = 6; Fig. 1 E). In contrast, expression of the IL-2Rγ and CD62L was equivalent on both populations of T cells. CD25+CD4+ Tr cells and CD25−CD4+ T cells were CD3+TCR-α/β+ (data not shown). Thus, human CD25+CD4+ Tr cells express markers that are characteristic of memory T cells and low constitutive levels of CTLA-4, similar to murine CD25+CD4+ Tr cells 9 10 15 17 18.
Figure 1.
Isolation and cell surface phenotype of human CD25+CD4+ Tr cells. CD4+ T cells were isolated from PBMCs and separated into CD25+ and CD25− fractions. Purity (A) and expression of CD45RO, HLA-DR (B), IL-2Rβ, IL-2Rγ, and CD62L (C) was determined by FACS®. CD25+CD4+ and CD25−CD4+ T cells were cultured either in medium alone or activated with immobilized anti-CD3 mAbs or PMA and calcium ionophore for 6 (D) or 24 h (E). Cells were analyzed for surface expression of CD40L and CD69 (D) and for intracytoplasmic expression of CTLA-4 (E). Results are representative of six independent experiments.
After TCR-mediated stimulation, human CD25+CD4+ Tr cells expressed lower levels of activation markers in comparison to CD25−CD4+ T cells. The proportion of CD40L+ cells was 17.3 ± 2.3% in CD25+CD4+ Tr cells versus 28.4 ± 1.8% in the CD25−CD4+ T cell subset (P ≤ 0.005), whereas 30.9 ± 7.0% of CD25+CD4+ Tr cells versus 54 ± 9.1% of CD25−CD4+ T cells expressed CD69 (P ≤ 0.006; Fig. 1 D). Time-course experiments demonstrated that the reduced levels of CD40L and CD69 on CD25+CD4+ Tr cells were not due to altered kinetics of expression (data not shown). After activation with PMA and calcium ionophore there was no statistically significant difference between expression of CD69 or CD40L on CD25+CD4+ or CD25−CD4+ T cells, although in general fewer CD25+CD4+ Tr cells expressed CD40L.
After activation with anti-CD3 mAbs or PMA and calcium ionophore, the percentage of CD25+CD4+ Tr cells expressing CTLA-4 was higher than CD25−CD4+ T cells. In addition, CTLA-4 expression levels were approximately threefold higher on CD25+CD4+ Tr cells (Fig. 1 E). Collectively, these data demonstrate that upon TCR activation human CD25+CD4+ Tr cells have a surface molecule expression profile that is unique and distinct from other CD4+ T cell subsets.
Proliferation and Cytokine Production by Human CD25+ CD4+ Tr Cells.
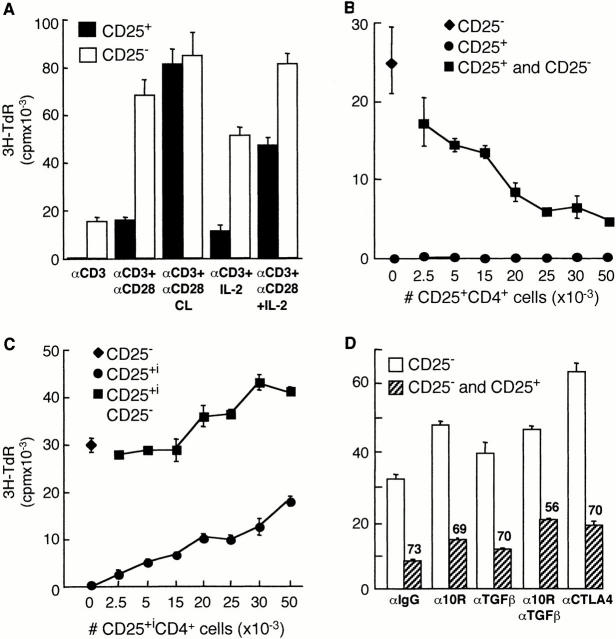
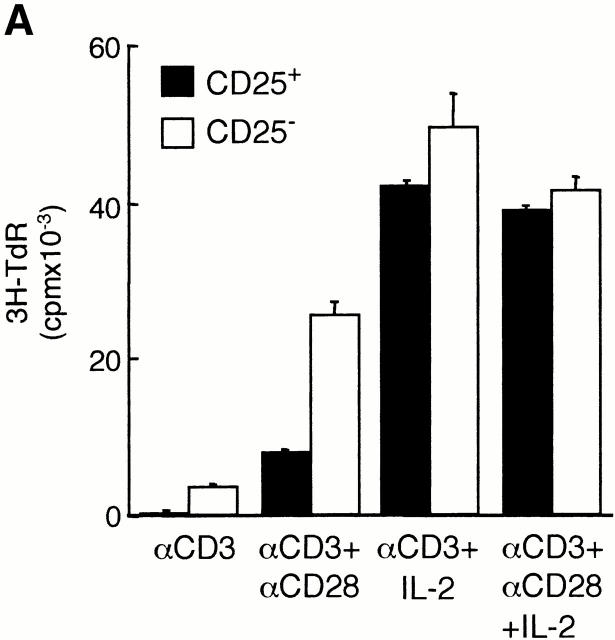
Freshly isolated CD25+CD4+ Tr cells did not proliferate in response to immobilized anti-CD3 mAbs, and the addition of soluble anti-CD28 mAbs resulted in a modest and variable increase in proliferation. In contrast, cross-linked anti-CD28 mAbs completely reversed the unresponsiveness of CD25+CD4+ Tr cells to TCR activation. Addition of exogenous IL-2 partially restored the proliferation of CD25+CD4+ Tr cells in response to anti-CD3 mAbs, and proliferation was further enhanced by soluble anti-CD28 mAbs (Fig. 2 A). These results indicate that human CD25+CD4+ Tr cells have a specific defect in their ability to proliferate after TCR-mediated activation 15 16.
Figure 2.
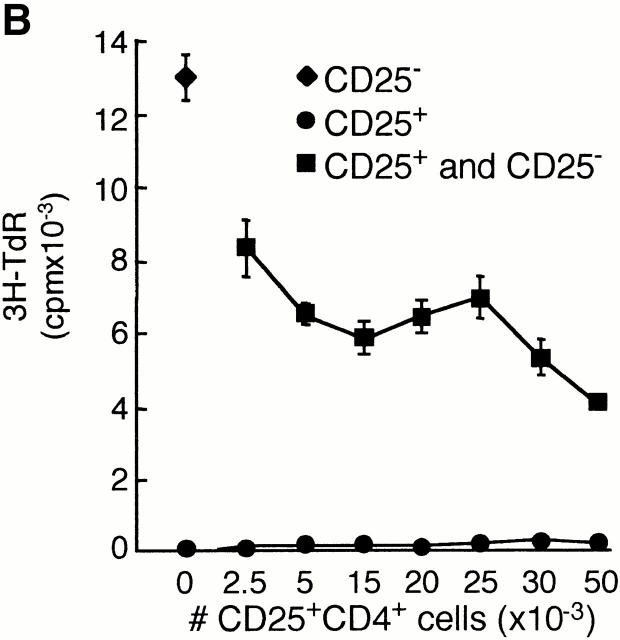
CD25+CD4+ Tr cells are anergic and suppress proliferation to alloantigens. Purified CD25+CD4+ Tr cells (100,000 cells per well) were tested for their ability to proliferate in response to immobilized anti-CD3 mAbs (αCD3; 10 μg/ml) in the absence or presence of soluble anti-CD28 mAbs (αCD28; 1 μg/ml), secondary rabbit anti–mouse Abs (αCD28 CL; 10 μg/ml), and/or 100 U/ml IL-2. After 72 h of culture, [3H]thymidine was added for an additional 16 h (A). CD25−CD4+ T cells (50,000 cells per well) were tested for their ability to proliferate in response to allogeneic APCs in the absence or presence of increasing numbers of autologous CD25+CD4+ Tr cells (B). CD25−CD4+ T cells were activated to induce CD25 expression. After 48 h, T cells that became CD25+ (CD25+i) were purified and tested for their ability to suppress proliferation of CD25− T cells in response to alloantigens (C). CD25−CD4+ T cells were activated by alloantigens with or without CD25+CD4+ Tr cells (1:1 ratio) in the presence of the indicated mAbs (10 μg/ml). Numbers represent the percent reduction in proliferation compared with culture in the absence of CD25+CD4+ Tr cells (D). For B–D, after 96 h, [3H]thymidine was added for an additional 16 h. Results are representative of six independent experiments for A, nine for B, and three for C and D.
Human CD25+CD4+ Tr cells were analyzed for their capacity to produce cytokines. After stimulation with immobilized anti-CD3 mAbs, with or without soluble anti-CD28 mAbs, no detectable levels of IL-2, IL-10, IL-4, TGF-β, or IFN-γ could be measured (data not shown). In contrast, when stimulated with anti-CD3 and soluble anti-CD28 mAbs in the presence of exogenous IL-2, CD25+CD4+ Tr cells produced significant levels of IL-4, IL-10, IFN-γ, and TGF-β (Table ). Under these stimulation conditions, CD25+CD4+ Tr cells had a cytokine profile that was comparable to CD25−CD4+ T cells. In contrast, differences in cytokine production were observed after activation with allogeneic APCs. Both CD25+CD4+ and CD25−CD4+ T cells produced IL-10, TGF-β, and IFN-γ, but no IL-4. However, the striking difference between the CD25+CD4+ and CD25−CD4+ T cell populations was that the CD25+ cells failed to secrete IL-2, indicating that these cells have a specific defect in production of IL-2. Interestingly, CD25+CD4+ Tr cells consistently produced less IFN-γ upon alloantigen stimulation than did CD25−CD4+ T cells.
Table 1.
Cytokine Production by CD25+CD4+ Tr Cells
| Stimuli | Cells | IL-2 | IL-4 | IL-10 | IFN-γ | TGF-β |
|---|---|---|---|---|---|---|
| αCD3/28 + IL-2 | CD25+ | ND | 153 | 1148 | 5723 | 1322 |
| αCD3/28 + IL-2 | CD25− | ND | 94 | 840 | 9773 | 1225 |
| allogeneic APCs | CD25+ | <19 | <9 | 298 | 527 | 509 |
| allogeneic APCs | CD25− | 99.5 | <9 | 251 | 5744 | 637 |
Purified cells were stimulated as indicated and supernatants were collected after 24 (for IL-2) or 72 h. The amount of cytokine was determined by ELISA. Data represent the average values (pg/ml) of pooled data from four independent experiments. Cytokine production by allogeneic APCs alone has been subtracted.
Human CD25+CD4+ Tr Cells Suppress the Proliferative Responses of Naive CD25−CD4+ T Cells to Alloantigens.
We investigated the regulatory properties of CD25+CD4+ Tr cells by testing their ability to suppress the proliferative responses of naive CD25−CD4+ T cells to alloantigens. CD25−CD4+ T cells were stimulated with allogeneic APCs and increasing numbers of autologous CD25+CD4+ Tr cells were added. Addition of as few as 2,500 CD25+CD4+ Tr cells to 50,000 CD25−CD4+ T cells resulted in a reduced proliferation of CD25−CD4+ T cells. At a ratio of 1:1, CD25+CD4+ Tr cells inhibited the proliferation of naive CD25−CD4+ T cells by an average of 75.0 ± 2.9% (n = 9; Fig. 2 B). The CD25+CD4+ Tr cells themselves failed to proliferate in response to alloantigens. Furthermore, CD25+CD4+ Tr cells suppressed the proliferation of CD25−CD4+ T cells in response to PHA in the presence of APCs (by an average of 81.2 ± 5.0%, n = 6; data not shown) and to immobilized anti-CD3 alone (by an average of 79.4 ± 14.6%, n = 3; data not shown). These latter data indicate that CD25+CD4+ Tr cells have a direct suppressive effect on T cells that is independent of APCs, similar to murine CD25+CD4+ Tr cells 17. In addition, CD25+CD4+ Tr cells suppressed production of IFN-γ and IL-2 by CD25−CD4+ T cells activated with anti-CD3 mAbs or allogeneic APCs (data not shown).
To demonstrate that this suppressive capacity was an intrinsic property of T cells constitutively expressing CD25 in vivo, we tested whether CD25−CD4+ T cells which expressed CD25 after activation in vitro showed regulatory effects. To this aim, CD25−CD4+ T cells were activated with anti-CD3 and anti-CD28 mAbs, and after 48 h the CD25+ T cells were isolated and tested for their ability to suppress freshly isolated autologous CD25− T cells. As shown in Fig. 2 C, T cells that became CD25+ after in vitro activation proliferated in response to alloantigens and enhanced, rather than suppressed, the response of CD25− CD4+ T cells. These data indicate that inhibition of T cell proliferation is a unique property of cells which constitutively express CD25 in vivo.
Several studies show that some subsets of Tr cells, such as type 1 Tr (Tr1) and Th3 cells, secrete IL-10 and/or TGF-β and suppress immune responses via production of these cytokines 4 5 6 7 8. Since CD25+CD4+ Tr cells produced both IL-10 and TGF-β upon stimulation with allogeneic APCs (Table ), the role of these cytokines on inhibition of allogeneic responses by human CD25+CD4+ Tr cells was investigated. As shown in Fig. 2 D, addition of neutralizing anti–IL-10R or anti–TGF-β mAbs had no significant effect on the ability of CD25+CD4+ Tr cells to suppress the proliferation of CD25−CD4+ T cells in response to alloantigens. In three independent experiments, an average of 67.2 ± 6.0% suppression was observed in the presence of 10 μg/ml of control IgG, 63.8 ± 5.8% with 10 μg/ml of anti–IL-10R, and 69.0 ± 3.4% with 10 μg/ml of anti–TGF-β mAbs. Similar results were obtained with a fivefold higher concentration of anti–TGF-β mAbs (data not shown). Addition of both anti–IL-10R and anti–TGF-β mAbs resulted in a slight, but not statistically significant, reversal of suppression mediated by CD25+CD4+ Tr cells (from 67.2 ± 6.0% to 52.4 ± 5.7%, P ≤ 0.06).
F(ab′)2 fragments from Abs which specifically block the ability of CTLA-4 to bind to CD80/86, without affecting signals via CD28, have been shown previously to inhibit the production of TGF-β by Tr1 cells 22. Addition of the same blocking anti–CTLA-4 mAbs had no significant effect on the suppressive activity of CD25+CD4+ Tr cells. These data suggest that despite the fact that CD25+CD4+ Tr cells express high levels of CTLA-4 (Fig. 1 E), this molecule is not required for their suppressive activity.
Human CD25+CD4+ Tr Cells Can Be Expanded In Vitro.
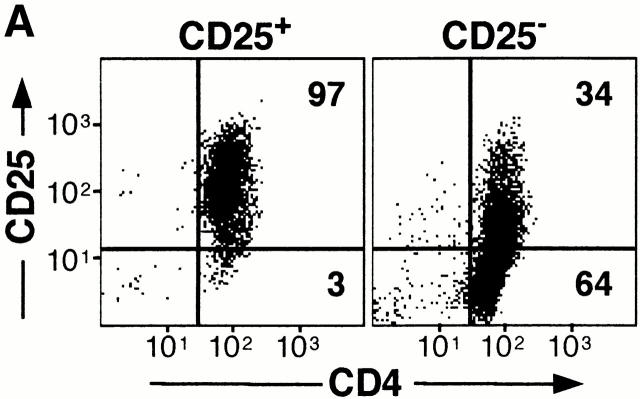
We have shown previously that human Tr1 cells which have been cloned and expanded in vitro maintain their regulatory activity 6. Using a protocol similar to that described for Tr1 cells, we determined whether CD25+CD4+ Tr cells could be expanded. CD25+CD4+ Tr cells did not proliferate in response to anti-CD3 alone (Fig. 2 A), but when activated with anti-CD3 mAbs in the presence of an allogeneic feeder cell mixture and exogenous IL-2, an expansion which was similar to CD25−CD4+ T cells was obtained (20–30-fold increase at day 14). In vitro–expanded human CD25+CD4+ Tr cells remained positive for CD25 even after culture for >1 mo (Fig. 3 A). In contrast, all CD25−CD4+ T cells expressed CD25 after activation, but the expression gradually decreased with time. Persistent expression of CD25 has also been observed in murine CD25+CD4+ Tr cells activated in vivo 23.
Figure 3.
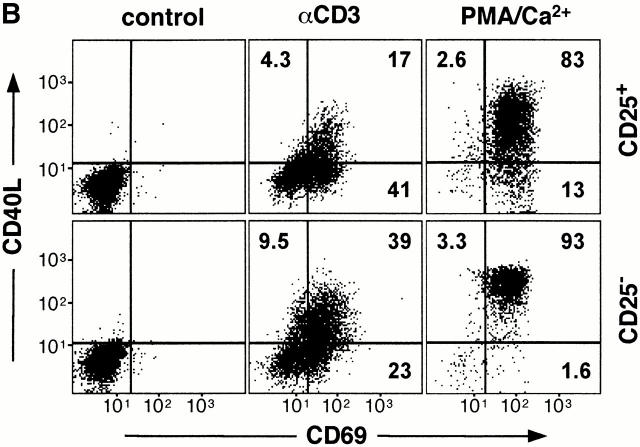
Expansion and cell surface phenotype of CD25+CD4+ Tr cells. CD25+ and CD25−CD4+ T cells were purified and activated with anti-CD3 mAbs, allogeneic feeder cell mixture, and exogenous IL-2. Cells were split as necessary and after 2 wk were analyzed by FACS® for expression of CD25 and CD4 (A). In parallel, cells were analyzed for cell surface expression of CD40L and CD69 after activation for 6 h with immobilized anti-CD3 mAbs or PMA and calcium ionophore (B). Constitutive levels of CTLA-4 expression was determined by intracytoplasmic staining (C). Results are representative of three independent experiments.
Similar to freshly isolated CD25+CD4+ Tr cells, the proportion of in vitro–expanded CD25+CD4+ Tr cells expressing CD40L after activation with anti-CD3 mAbs was consistently lower in comparison to CD25−CD4+ T cells (12.7 ± 3.3% vs. 36.9 ± 8.3%, P ≤ 0.01). However, in contrast to freshly isolated cells, cultured CD25+CD4+ Tr cells expressed normal levels of CD69 after polyclonal TCR-mediated activation (Fig. 3 B). Thus, reduced upregulation of CD40L is also a characteristic of expanded CD25+CD4+ Tr cells. As expected, cultured CD25+ CD4+ and CD25−CD4+ T cells expressed similar levels of both CD40L and CD69 after activation with PMA and calcium ionophore. In vitro–expansion of CD25+CD4+ Tr cells did not alter their constitutive expression of CTLA-4, and they continued to express this inhibitory molecule at significantly higher levels than their CD25−CD4+ counterparts (Fig. 3 C).
In Vitro–expanded CD25+CD4+ Tr Cells Remain Anergic and Retain Their Suppressive Capacity.
In vitro–expanded CD25+CD4+ Tr cells failed to proliferate in response to anti-CD3 mAbs alone, and proliferation could only be completely restored by the addition of exogenous IL-2 (Fig. 4 A). Moreover, cultured CD25+CD4+ Tr cells failed to proliferate in response to alloantigens and retained their ability to suppress the proliferation of autologous CD25− CD4+ T cells (at a 1:1 ratio, an average of 64 ± 3.8%, n = 3, suppression was observed; Fig. 4 B). These data indicate that CD25+CD4+ Tr cells expanded in vitro maintain their regulatory functions and behave similarly to freshly isolated CD25+CD4+ Tr cells. Since in these experiments the CD25−CD4+ responder T cells had been activated previously and expanded in vitro, these data demonstrate that CD25+CD4+ Tr cells suppress not only the response of freshly isolated naive CD25−CD4+ T cells, but also of previously activated memory CD25−CD4+ T cells (Fig. 4 B). Finally, similar to observations with freshly isolated CD25+CD4+ Tr cells, the suppression mediated by in vitro–expanded CD25+CD4+ Tr cells was not reversed by the addition of neutralizing anti–IL-10R, anti–TGF-β, or anti–CTLA-4 mAbs (Fig. 4 C).
Figure 4.
Cultured CD25+CD4+ Tr cells retain their suppressive capacity. CD25+ and CD25−CD4+ T cells were purified and activated with anti-CD3 mAbs, allogeneic feeder cell mixture, and exogenous IL-2. After 14 d of culture, T cells were tested for their ability to proliferate in response to 10 μg/ml anti-CD3 mAbs in the absence or presence of 1 μg/ml soluble anti-CD28 mAbs and/or 100 U/ml IL-2 (A). Cultured CD25−CD4+ T cells (50,000 cells per well) were tested for their ability to proliferate in response to alloantigens in the absence or presence of increasing numbers of in vitro–cultured, autologous CD25+CD4+ Tr cells (B). Cultured CD25−CD4+ T cells were activated by allogeneic APCs (from a donor different from that used for expansion) with or without CD25+CD4+ Tr cells (1:1 ratio) in the presence of the indicated mAbs (10 μg/ml). Numbers represent the percent reduction in proliferation compared with culture in the absence of CD25+CD4+ Tr cells (C). For all cultures, after 48 h, [3H]thymidine was added for an additional 16 h. Results are representative of three independent experiments.
In this study we show that human CD4+ T cells that express CD25 in vivo are a unique subset of Tr cells. Human CD25+CD4+ Tr cells are anergic, fail to produce IL-2, constitutively express CTLA-4, and suppress the proliferation of naive CD4+ T cells as described for murine CD25+CD4+ Tr cells 2 3. In addition, after polyclonal TCR-mediated activation, human CD25+CD4+ Tr cells strongly upregulate CTLA-4, display reduced expression of CD40L, and produce cytokines. CD25 and CTLA-4 remain constitutively expressed on in vitro–expanded human CD25+CD4+ Tr cells, while after activation, upregulation of CD40L is still defective. More interestingly, in vitro–expanded CD25+CD4+ Tr cells retain their potent suppressive activity, even towards previously activated memory T cells. The observation that functional Tr cells can be expanded in vitro and can regulate responses of memory T cells is of great clinical relevance for the use of CD25+CD4+ Tr cells as a cellular therapy in T cell–mediated diseases where there are preactivated T cells.
The role of immunoregulatory cytokines in the suppression mediated by CD25+CD4+ Tr cells remains an open question. Alloantigen-activated CD25+CD4+ Tr cells did not proliferate but produced IL-10, IFN-γ, and TGF-β, and indeed possessed a profile of cytokine production which is comparable to Tr1 cells (i.e., IL-10+IFN-γ+TGF-β+IL-4−IL-2−/low) 6. However, we observed only a slight reversal of suppression in the presence of both neutralizing anti–IL-10R and anti–TGF-β mAbs, which is consistent with previous observations that production of IL-10 and TGF-β is dispensable for the regulatory function of CD25+CD4+ Tr cells 15 16. On the other hand, in a murine model of experimentally induced colitis, both IL-10 and TGF-β were found to be required for suppression mediated by CD25+CD4+ Tr cells 5 10. The basis for this discrepancy in the involvement of IL-10 and TGF-β is unclear. CD25+CD4+ Tr cells have the capacity to produce IL-10 and TGF-β, but production of these cytokines may depend on their maturation state and the environmental context in which they are activated.
Previous reports demonstrated that direct cell–cell contact is required for murine CD25+CD4+ Tr cells to exert their suppressive effects 15 16. Despite constitutive and persistent expression of CTLA-4, anti–CTLA-4 mAbs failed to abrogate the suppressive activity of human CD25+CD4+ Tr cells. These data are in agreement with a study indicating that signals via CTLA-4 were dispensable for suppression by mouse CD25+CD4+ Tr cells in vitro 15. However, more recent reports show that expression of CTLA-4 is essential for suppression mediated by these cells 10 18. It is possible that suppression of proliferation operates via mechanisms which differ depending on the stimuli and microenvironment, or that human and mouse CD25+CD4+ Tr cells act through different mechanisms. Finally, suppression is not simply due to consumption of IL-2 as murine CD25+CD4+ Tr cells suppressed IL-2 production at the transcriptional level 15. In addition, human CD4+ T cells which expressed CD25 after activation in vitro did not suppress responses of CD25−CD4+ T cells, indicating that high expression of CD25 does not simply result in sequestration of IL-2.
Human CD25+CD4+ Tr cells expand in vitro and maintain their unique cell surface marker profile and suppressive functions. To our knowledge, these data represent the first report of in vitro–expansion of human CD25+CD4+ T suppressor cell lines. The ability to isolate and expand human CD25+CD4+ Tr cells will allow the further biological and biochemical characterization of this unique T cell subset and expedite progress towards clinical use of Tr cells as a cellular therapy for T cell–mediated diseases.
Acknowledgments
We thank Paul Orban for helpful discussions and critical reading of the manuscript.
This work was partially supported by a grant from the Italian Telethon Foundation. M.K. Levings is a recipient of a postdoctoral fellowship from the Canadian Institutes for Health Research.
References
- Roncarolo M.G., Levings M.K. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr. Opin. Immunol. 2000;12:676–683. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cellskey controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Shevach E.M. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Cottrez F., Hurst S.D., Coffman R.L., Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J. Immunol. 2000;165:4848–4853. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Powrie F., Carlino J., Leach M.W., Mauze S., Coffman R.L. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CD45RBlowCD4+ T cells. J. Exp. Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V.K., Inobe J., Hafler D.A., Weiner H.L. Regulatory T cell clones induced by oral tolerancesuppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri-Payer E., Amar A.Z., Thornton A.M., Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Asano M., Toda M., Sakaguchi N., Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. Thymus and autoimmunityproduction of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cellsinduction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbom L., Hall H., Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bacchetta R., Bigler M., Touraine J.L., Parkman R., Tovo P.A., Abrams J., de Waal Malefyt R., de Vries J.E., Roncarolo M.G. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J. Exp. Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Griend R.J., Bolhuis R.L. Rapid expansion of allospecific cytotoxic T cell clones using nonspecific feeder cell lines without further addition of exogenous IL-2. Transplantation. 1984;38:401–406. doi: 10.1097/00007890-198410000-00017. [DOI] [PubMed] [Google Scholar]
- Kitani A., Chua K., Nakamura K., Strober W. Activated self-MHC-reactive T cells have the cytokine phenotype of Th3/T regulatory cell 1 T cells. J. Immunol. 2000;165:691–702. doi: 10.4049/jimmunol.165.2.691. [DOI] [PubMed] [Google Scholar]
- Kuniyasu K., Takahashi T., Itoh M., Shimizu J., Toda G., Sakaguchi S. Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell population. Int. Immunol. 2000;12:1145–1155. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]