Abstract
Type-B T cells raised against the immunodominant peptide in hen egg lysozyme (HEL48–62) do not respond to whole lysozyme, and this has been thought to indicate that peptide can bind to l-Ak in different conformations. Here we demonstrate that such T cells recognize a deamidated form of the HEL peptide and not the native peptide. The sequence of the HEL epitope facilitates rapid and spontaneous deamidation when present as a free peptide or within a flexible domain. However, this deamidated epitope is not created within intact lysozyme, most likely because it resides in a highly structured part of the protein. These findings argue against the existence of multiple conformations of the same peptide–MHC complex and have important implications for the design of peptide-based vaccines. Furthermore, as the type-B T cells are known to selectively evade induction of tolerance when HEL is expressed as a transgene, these results suggest that recognition of posttranslationally modified self-antigen may play a role in autoimmunity.
Keywords: posttranslational/chemical modification, autoimmunity, peptide, deamidation, T cell
Introduction
MHC class I and class II molecules have evolved to bind short peptides and present them at the cell surface to circulating T lymphocytes. Recognition of these peptides by the TCR can be exquisitely specific, such that minor changes in the peptide–MHC complex structure can radically affect T cell activation. Much of our understanding of the interactions between peptide, MHC, and TCR comes from the study of the immunodominant epitope of hen egg lysozyme restricted by I-Ak (HEL48–62). Interestingly, the majority of peptide-specific T cells isolated from mice that have been immunized with this peptide fragment do not recognize APC incubated with whole lysozyme (type-B T cells) 1 2. This is in contrast to the HEL48–62-specific T cells isolated from mice immunized with HEL, all of which respond to both peptide and whole antigen (type-A T cells) 1 2 3. The type-B T cells also fail to respond to APCs engineered to express HEL in the vesicular compartment or to APCs that express the HEL48–62 peptide covalently linked to I-Ak. However, as the type-B cells recognize APCs incubated with trypsin-digested HEL 2, it is clear that these cells do not simply recognize impurities present within the synthetic peptide immunogen 4. Detailed analysis of the HEL48–62 peptide binding to I-Ak demonstrated a critical requirement for Asp-52 to bind in the P1 pocket of this class II molecule 5 6, ruling out the possibility that the type-A and type-B T cells recognize the HEL peptide bound in different registers to I-Ak. Instead, Unanue and colleagues 1 7 have proposed that the HEL peptide could bind to I-Ak in at least two stable conformations. According to this hypothesis, the type-A conformation should be generated by the endogenous processing of HEL to peptide and subsequent intracellular loading on to I-Ak, while peptides binding directly to I-Ak at the cell surface were proposed to generate both type-B and type-A conformers.
An alternative explanation could be that the two populations of T cells recognize structurally distinct peptides that result from a spontaneous modification. Indeed, it can be predicted from the primary sequence of the HEL48–62 epitope that the asparagine at residue 59 should spontaneously deamidate 8. This is particularly interesting because crystallographic studies of the I-Ak–HEL48–62 complex have demonstrated that this residue, positioned at P8, is solvent exposed and available for direct contact with the TCR 9. This type of asparagine deamidation is the most common nonenzymatic protein modification occurring under physiological conditions 10 11. The reaction proceeds via a succinimide intermediate to form isoaspartate and aspartate, as well as small amounts of d-aspartate and d-isoaspartate (for review see reference 12). The deamidation of asparagine is strongly promoted when the C-flanking residue is a glycine, serine, or histidine, and in the HEL48–62 epitope the asparagine residue is proceeded by a serine. The spontaneous deamidation of asparagine is also influenced by pH, temperature, and the position of the residue within a given protein 12. In fact, constraints imposed when an asparagine is situated within a highly structured region of a protein can override the sequence-specific influence of the C-flanking residue and dramatically reduce the rate of deamidation 10 13. Interestingly, biochemical studies have found no evidence that this residue deamidates within intact lysozyme, although evidence for deamidation was found at other residues 10 14 15. The Asn-59 is situated within a β-stranded secondary structure close to the active site of lysozyme, and it may well be protected from nonenzymatic deamidation unless it is present as a synthetic or tryptic peptide fragment. We therefore hypothesized that the type-B T cell hybridomas may respond to a deamidated peptide that can be formed when the epitope is present as a free peptide, but not when present as a whole antigen.
Materials and Methods
Antigens.
Synthetic peptides were prepared by multiple solid-phase peptide synthesis on a robotic system (Syro MultiSynTech) using Fmoc/OtBu chemistry and 2-chlorotrityl resin (Senn Chemicals AG). For coupling, 10 equivalents (eq) of diisopropylcarbodiimide, 5 eq of 1-hydroxybenzotriazole, and 5 eq of protected amino acids were used. Peptides were cleaved off the resin, and side chains were deprotected with trifluoroacetic acid/triisopropylsilane/water (94:5:1) 16. Peptides were precipitated by adding ice cold diethylether, washed three times with diethylether, and lyophilized from tert-butyl-alcohol/water (4:1). Identity of the peptides was confirmed by electrospray mass spectrometry, and purity was analyzed by RP-HPLC. Peptides with purities <90% were purified by preparative RP-HLPC. The following peptides were synthesized: p48–61, DGSTDYGILQINSR; p48–61/D59, DGSTDYGILQIDSR; P48–61/isoD59, DGSTDYGILQI-isoD-SR; p48–62, DGSTDYGILQINSRW; p48–62/D59, DGSTDYGILQIDSRW; and p48–62/T62, DGSTDYGILQINSRT. Peptides were weighed, re-suspended in water, aliquoted, dried under vacuum, and stored at −80°C until use.
HEL that was crystallized and dialysed three times was purchased from Sigma-Aldrich (cat no. L-6876) and unless otherwise stated was freshly weighed and resuspended in PBS before use.
The genes coding for the recombinant αIgD–IgG3HEL46–61 and αIgD–IgG3HEL46–61/59D antibodies (unpublished data), which contain residues 46–61 from HEL inserted into the L3 loop in the CH1 domain, were introduced into an expression vector using a strategy similar to that described previously 17. After transfection of NSO cells, single G418-resistant colonies that expressed the antibody were isolated and grown at high density in a miniPERM bioreactor (Heraeus Instruments). Antibody was used either as supernatant directly frozen after 17 h culture or as purified protein. Antibody was purified at room temperature on a protein L (Actigen) column, eluted with 0.1 M Gly–HCL, pH 2.7, neutralized, and dialyzed for several days against PBS and then RPMI growth medium before freezing at −20°C.
High Resolution Mass Spectrometry and Micro-HPLC.
Chemical deamidation within asparagine side chains was demonstrated by high resolution ESI-FTICR-MS (electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry) measurements (4.7 Tesla APEXTMII-ESI/MALDI-FTICR Mass Spectrometer, passively shielded; Bruker Daltonik). On a UNIX-based Silicon Graphics O2 Workstation, the mass spectrometry software XMASS version 5.0.7 (Bruker Daltonik) was used for mass calculation, data acquisition, and processing. ESI was performed in the positive mode with a grounded capillary sprayer needle mounted 60° off-axis. For coupling with micro-HPLC, the spray was supported with nitrogen nebulizing gas at ambient pressure and 50 psi. For working with direct infusion, no nebulizer gas was used. The ESI source (Analytica of Branford) was connected with the diode array detector of the HPLC system by using a fused silica capillary (365 μm OD, 75 μm ID) without splitting the solvent stream. For liquid chromatography FTICR-MS coupling, an HP series 1100 HPLC (Hewlett Packard) and an RP-C18 column (GROM-Sil 120 ODS-4 HE; 1 × 60 mm) was used. The samples were eluted with 0.1% formic acid in water (a) and 0.1% formic acid in acetonitrile (b) using a linear gradient (0–30% b over 40 min), a flow rate of 50 μl/min, and monitoring at 214 nm. FTICR-MS detection took place by measuring sequenced experiments, which included four averaged scans with a total scan time of ∼3 s.
T Cell Hybridoma IL-2 Secretion Assays.
The T cell hybridoma 3A9 was generated by Allen et al. using HEL as the immunogen 18. The ALV-48 hybridoma was generated after immunization with a peptide covering HEL48–61, and the DAV-21 hybridoma was generated using a peptide covering HEL48–62 1. APCs were the B cell lymphoma C3.F6 that has been engineered to express I-Ak 19 or freshly isolated spleen cells from CBA (Bomholtgaard) or B10.BR mice (Harlan UK Ltd.). All cell lines and assays were cultured in DMEM supplemented with 5% inactivated newborn calf serum, penicillin/streptomycin, and 0.01 M 2-ME. Secretion of IL-2 was used as a marker for T cell activation using the following protocol: peptides were added in duplicate at the final concentration indicated to 96-well U-bottomed plates containing 5 × 104 APCs/well in a total volume of 100 μl. T cell hybridomas were then added either directly or after incubation at 37°C for 1 h, followed by three washes in 200 μl of DMEM plus 2% newborn calf serum. T cells were added at 5 × 104 cells per well to give a total volume of 200 μl. Supernatants were collected after 16 h and quantified for IL-2 by time resolved fluorometry using DELFIA® reagents (Wallac) and rat anti–murine IL-2 antibodies (JES6–1A12 and biotinylated JES6–5H4; PharMingen). All T cell assays were performed at least three times, with results from a single representative experiment presented.
Results
Type-B Hybridomas Preferentially Respond to Peptides Containing a Modified Residue 59.
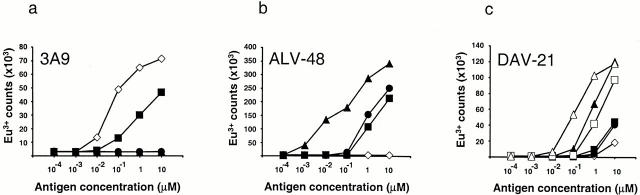
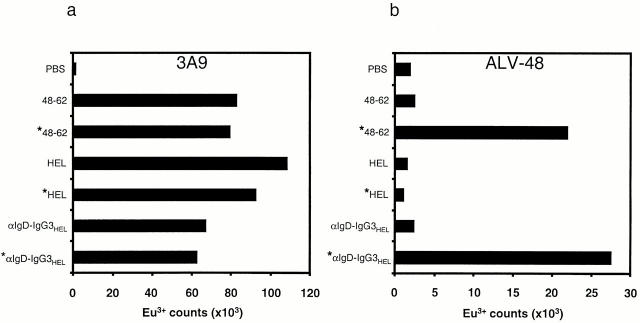
To investigate whether type-B T cells preferentially recognize modified peptides, we synthesized the HEL peptide with asparagine (p48–61 and p48–62), aspartate (p48–61/D59 and p48–62/D59), or isoaspartate (p48–61/isoD59) at position 59 and compared their ability to stimulate a type-A T cell hybridoma (3A9) and two type-B T cell hybridomas (ALV-48 and DAV-21) (Fig. 1, a–c). As expected, the type-A hybridoma responded well to a B lymphoma cell line expressing I-Ak (C3.F6) when either whole HEL or the native peptide was added to the assay. No detectable response to synthetic peptides containing aspartate or isoaspartate at residue 59 was made by 3A9.
Figure 1.
Type-B cells preferentially respond to deamidated peptide. T cell responses of (a) a type-A hybridoma (3A9) and (b and c) two type-B hybridomas (ALV-48 and DAV-21) were measured using IL-2 secretion detected by the europium-based DELFIA® assay. HEL (⋄), p48–61 (▪), p48–61/D59 (▴), and p48–61/isoD59 (•) were added to C3.F6 B cell lymphoma cells at the concentrations indicated and remained in the assay for the duration of the experiment. DAV-21 was also tested for recognition of p48–62 (□) and p48–62/59D (▵). CBA spleen cells were also used as APCs in other experiments and gave comparable results.
The ALV-48 hybridomas gave a typical type-B profile by responding to native peptide but not whole HEL. However, in contrast to the lack of recognition of the modified peptides by the type-A hybridoma, ALV-48 was between two and three logs more sensitive to the 48–61/59D peptide than the native peptide. ALV-48 also gave a response to the peptide containing an isoaspartate at position 59 that was comparable to the response to the native peptide. Although initial experiments testing the DAV-21 hybridoma clearly showed preferential recognition of the 48–61/59D peptide over the native peptide, the responses to both peptides were weak, and the superior recognition of the deamidated peptide was not as pronounced as for the ALV-48 T cell. As this hybridoma was raised against the HEL48–62 peptide rather than the HEL48–61 peptide used to generate ALV-48 1, we suspected that this hybridoma required the additional tryptophan residue at the COOH terminus for optimal recognition. Indeed, this was found to be the case (Fig. 1 c). Moreover, the pattern of recognition of the modified peptides was now similar to the response of ALV-48, indicating that the DAV-21 also preferentially recognized peptides with aspartate at residue 59 (Fig. 1 c). We found no evidence for the recognition of peptides containing isoaspartate at residue 59 by DAV-21.
The HEL48–61 Peptide Undergoes Rapid and Spontaneous Deamidation.
The type-B T cell response to both the native and aspartate-containing peptides could be due to truly cross-reactive TCRs, or, alternatively, to deamidation of the native peptide during the course of the assay. As there is a charge difference between asparagine and aspartate, and as residue 59 is known to be a major TCR contact residue within this epitope, we thought it unlikely that the T cells were cross-reactive. We therefore analyzed the native peptide for spontaneous deamidation by incubating the peptide at 37°C in PBS, pH 7.5, and analyzing it by micro-HPLC-FTICR mass spectrometry. The native and deamidated peptide differs by only a single dalton, which can lead to the monoisotopic signal from the deamidated peptide being overlooked due to the first isotopic signal of the native species. While the very high mass resolution provided by FTICR-MS can discriminate between these signals, we chose to couple FTICR-MS to micro-HPLC so that very small amounts of deamidated peptide could be detected within an excess of native peptide.
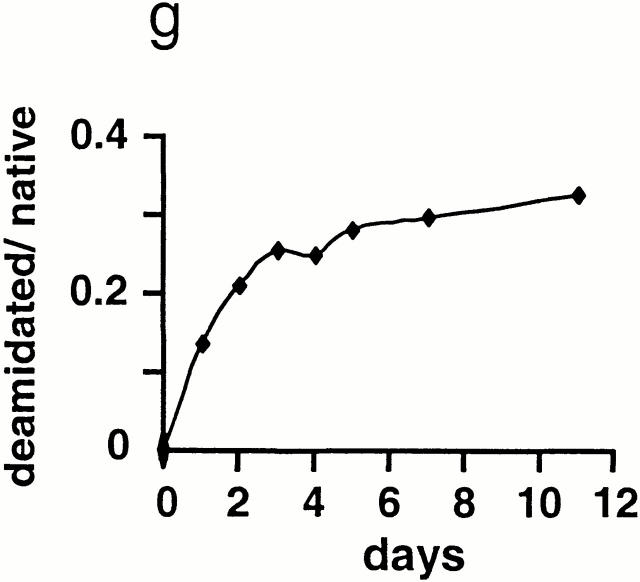
Conditions that allowed separation of native and deamidated peptides by micro-HPLC were first established using p48–61 and p48–61/59D (Fig. 2, a and b). Prior to incubation, no deamidated peptide was detectable by HPLC or MS analysis in p48–61 (Fig. 2, a and d). However, after incubating p48–61 for 5 d, a major fraction of the peptide had become deamidated (Fig. 2c and Fig. f). Kinetic measurements of deamidation over 12 d were done by HPLC and showed that the conversion proceeds via first order kinetics (Fig. 2 g). The deamidation occurs rapidly; >10% of the peptide becomes deamidated after just 24 h of incubation at 37°C.
Figure 2.
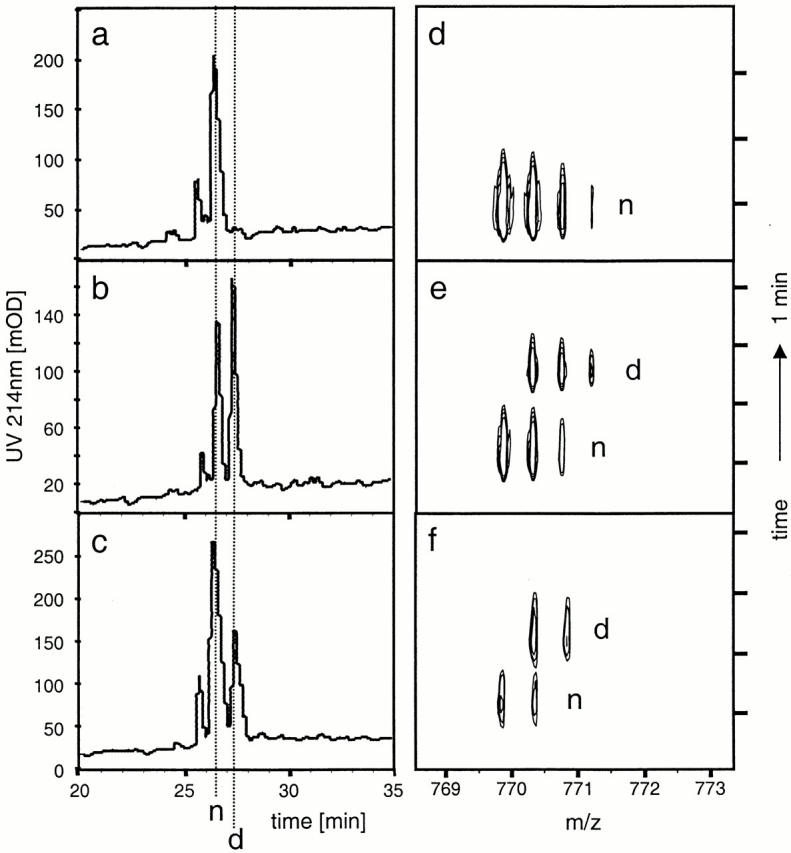
Rapid and spontaneous deamidation of p48–61. To determine possible deamidation within asparagine residues under physiological conditions, p48–61 and p48–61/D59 peptides were incubated for different time periods at 37°C in PBS, pH 7.5, and then analyzed by micro-HPLC-FT-ICR-MS. HPLC profiles are shown on the left with the corresponding MS contour plot on the right for freshly resuspended HEL48–61 (a and d), freshly resuspended p48–61 and p48–61/D59 in equimolar mix (b and e), and p48–61 after 5-d incubation (c and f ). The elution times for p48–61 and p48–61/D59 were identified using synthetic peptides and are indicated with dashed lines marked “n” and “d,” respectively. The signals on the contour plots are also marked with “n” and “d” to indicate the HPLC peak from which they were derived. Panel g shows the kinetics of the deamidation by plotting the percentage of deamidated peptide over nondeamidated peptide calculated by integrating the area under the respective peaks in the HPLC trace.
Type-B Cells Do Not Recognize APCs Pulsed with Native HEL48–62 Peptide.
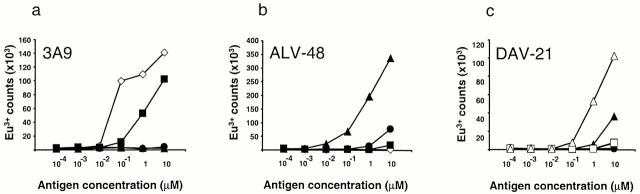
The finding that p48–61 rapidly converted to a deamidated form at 37°C led us to speculate that type-B cells could be responding to deamidated peptide created during the assay rather than to native peptide. To investigate this, we performed experiments where APCs were pulsed with antigen for 1 h and then washed to remove excess peptide that either had not been internalized by the APC or bound to I-Ak at the cell surface. Using this approach, the response pattern of 3A9 was identical to that made when antigen was left in the assay (i.e., strong responses to HEL and the native peptide but no response to the modified peptides; Fig. 3 a). In contrast, the type-B hybridoma reactivity to the native peptide that was left in the assay was lost when APCs were pulsed, while the strong responses to the peptides containing aspartate at residue 59 were maintained (Fig. 3b and Fig. c). These results indicate not only that the type-B cells do not recognize the native peptide but also that, once bound to I-Ak or internalized, p48–62 is inhibited from further spontaneous deamidation.
Figure 3.
Type-B T cell hybridomas do not respond to APCs pulsed with HEL48–61 peptide. T cell responses of (a) a type-A hybridoma (3A9) and (b and c) two type-B hybridomas (ALV-48 and DAV-21) were measured as in Fig. 1. The assays were performed in a manner identical to that of Fig. 1, except CBA splenocytes were used as APCs and antigen was washed from the APCs after a 1-h pulse and before the addition of T cells. Symbols identical to those in Fig. 1 are used.
Type-B Cells Recognize Endogenously Processed HEL Peptide.
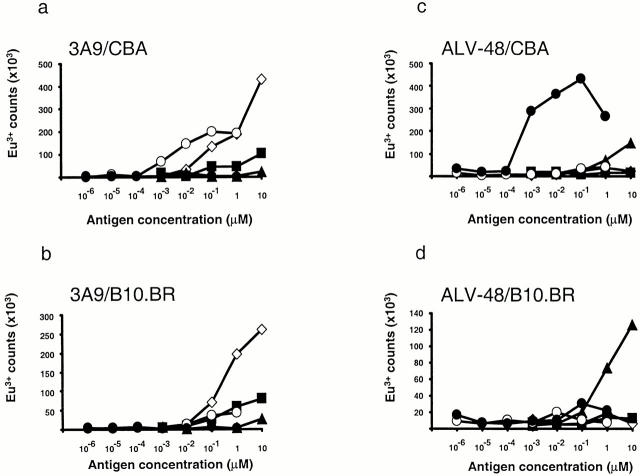
The lack of recognition by type-B T cells of APCs fed whole HEL antigen, APCs engineered to express full length HEL, or APCs that express HEL48–62 covalently linked to I-Ak was thought to indicate that type-B cells could not recognize endogenously processed peptide 2. To investigate this further, we exploited a novel antigen delivery system using a recombinant antibody specific for IgD that has been engineered to express the HEL46–61 or the HEL46–61/59D sequence in a loop inserted into the CH1 domain (unpublished data). This type of antibody expressing another T cell epitope (91–101λ2315) has been shown to be internalized by splenic B cells and subsequently processed and efficiently presented to T cells 17. Recognition by 3A9 of fresh splenocytes from CBA mice that have been pulsed with the purified αIgD–IgG3HEL46–61 antibody confirmed the efficiency (an enhancement of between 1,000 and 10,000 times, compared with the synthetic peptide) of this delivery strategy (Fig. 4 a). Notably, when splenocytes from B10.BR mice were used as APCs, the response to αIgD–IgG3HEL46–61 was almost abolished, while the response to the HEL48–61 peptide was unaltered. B10.BR and CBA mice share identical H-2 haplotypes but differ in their IgD allotype, with the ‘a’ allotype on the CBA splenocytes being recognized by the IgDa-specific antibody, while the ‘b’ allotype on the B10.BR B cells is not. The requirement for IgDa expression on the splenocytes strongly suggests that intact antibody has to be internalized by receptor-mediated endocytosis and that recognition is not caused by the presentation of peptide that could result from extracellular degradation of the αIgD–IgG3HEL46–61 antibody. Consistent with this, CBA and B10.BR splenocytes presented nontargeted (anti-NIP) recombinant antibodies containing the HEL epitope equally poorly (data not shown). Splenocytes pulsed with either the HEL48–61/59D peptide or αIgD–IgG3HEL46–61/59D antibody failed to stimulate this type-A hybridoma as would be expected.
Figure 4.
Type-B hybridomas recognize endogenously processed peptide. IL-2 secretion by (a and b) 3A9 and (c and d) ALV-48 in response to splenocytes pulsed for 1 h with whole lysozyme (⋄), p48–61 (▪), p48–61/D59 (▴), recombinant αIgD–IgG3HEL46–61 (○), or recombinant αIgD–IgG3HEL46–61/59D (•) were detected by a europium-based DELFIA® assay.
ALV-48 exhibited an extremely vigorous response to the αIgD–IgG3HEL46–61/59D antibody when pulsed onto APCs expressing the IgDa allotype (Fig. 4 c), but only a weak response was observed when the same antibody was pulsed onto APCs expressing the IgDb allotype (Fig. 4 d). These data clearly demonstrate that type-B T cells can readily respond to intracellularly processed antigen and are not limited to recognizing peptides loaded onto MHC molecules at the cell surface. DAV-21 did not respond to αIgD–IgG3HEL antibody, which was to be expected given the dependence of this hybridoma on the COOH-terminal tryptophan in p48–62. The antibody contains the shorter HEL46–61 sequence, which in the immunoglobulin is followed by a threonine. DAV-21 responds only weakly to the synthetic peptide containing a threonine at the residue equivalent to 62 (data not shown), a finding that supports this explanation.
Interestingly, ALV-48 also gave a weak but significant response to αIgD–IgG3HEL46–61 when presented by CBA splenocytes. This is likely due to the presence of a minor fraction of the antibody that has become deamidated during production and purification. The HEL sequences replace a four–amino acid loop connecting β-strands. This loop is exposed and surface accessible in the IgG molecule 20, and the amino acid sequence in the loop varies among IgGs 21. Furthermore, no interactions have been reported to exist between loop amino acids and their supporting framework 20, which suggests to us that the loop may well be flexible and probably not subject to strong secondary structure that could inhibit its deamidation.
T Cell Recognition of HEL48–61 Is Strongly Influenced by Antigen Structure.
The response of ALV-48 to αIgD–IgG3HEL46–61 indicates that deamidation of the HEL epitope is possible when it is within the structural context of the antibody, but not within intact HEL. To further probe what influence structure may have on deamidation of this peptide, we compared the stimulation of 3A9 and ALV-48 by HEL48–61 peptide, fresh αIgD–IgG3HEL supernatant, and whole HEL, with or without preincubating these antigens in PBS at 37°C for 16 h (Fig. 5, a and b). After a 1-h pulse onto splenocytes, all three antigens strongly stimulated 3A9 regardless of whether they had been preincubated or not (Fig. 5 a). Results were different with the ALV-48 hybridoma: free peptide and αIgD–IgG3HEL were only able to stimulate ALV-48 if they had undergone a period of preincubation at 37°C. HEL was not stimulatory under any circumstances (Fig. 5 b). These results illustrate that the structural context of epitope within an antigen can have a radical effect on its recognition by T cells. In addition, they provide further evidence that the response toward the native peptide by the type-B hybridomas is due to the spontaneous deamidation during the course of the assay.
Figure 5.
T cell recognition of antigens preincubated at 37°C. HEL, p48–61, and fresh αIgD–IgG3HEL46–61 antibody supernatant was tested for T cell hybridoma recognition by (a) 3A9 or (b) ALV-48, either freshly prepared or after incubation at 37°C for 16 h (marked with *). Both the fresh antigen and the preincubated antigen were pulsed onto CBA splenocytes for 1 h before washing and the addition of T cells. HEL and p48–61 were tested at 10 μM, whereas the concentration of αIgD–IgG3HEL was 1 μM. IL-2 released into the assay was then detected using the europium DELFIA® assay.
Discussion
We have shown that T cells raised against an immunodominant peptide from HEL can be divided into those T cells that recognize the native peptide and whole lysozyme antigen (type-A) and those that recognize the deamidated peptide (type-B). Biochemical studies aimed at determining which residues are prone to deamidation within intact proteins are difficult and can be misleading, as residues may deamidate during the identification procedure rather than in the intact protein. Nevertheless, studies of intact HEL have found no evidence that the asparagine at position 59 is deamidated, although deamidation of other asparagine residues has been identified 10 14 15. The Asn-59 is positioned close to the active site of lysozyme, and the highly ordered structure within this region is likely to be responsible for this lack of deamidation and consequent failure of the type-B cells to recognize whole antigen. The T cell hybridomas used in this study are the archetypal type-B T cells that display no significant responses to HEL, and as demonstrated here, they possess little or no cross-reactivity between the asparagine- and aspartate-containing peptides. Clearly, other T cells that demonstrate more cross-reactivity would be expected to display a less pronounced type-B phenotype. Notably, Mamula et al. have generated specific T cell responses to murine cytochrome C and snRNP d-isoaspartate-containing peptides by vaccination with synthetic modified peptides 22. As we observed that the ALV-48 hybridomas cross-reacted with the isoaspartate-containing peptide, this indicates that this peptide can bind to I-Ak and could conceivably be recognized by other type-B hybridomas.
Studies of type-B T cell recognition of the HEL48–62 epitope have provided some persuasive evidence supporting the idea that a peptide can bind to MHC molecules in more than one distinct and stable conformation. In an elegant experiment, Viner et al. 1 purified I-Ak from APCs fed HEL that by definition were not recognized by type-B hybridomas. When peptides were eluted from these I-Ak molecules and then added back to APCs, the eluted peptides were found to stimulate the type-B T cells. The authors reasoned that this was due to the binding of the peptide to I-Ak at the cell surface rather than through an endogenous route 2. However, this can now be explained by the spontaneous deamidation of the eluted peptide after its release from the binding cleft of I-Ak, where deamidation is inhibited. Thus, our data strongly argues against the existence of different stable peptide–MHC conformations and suggests that when T cell recognition of peptide and whole antigen is discordant, then this phenomenon more likely could be explained by a posttranslational modification of peptide or antigen.
Expression of HEL as a transgenic self-protein in mice has been shown to result in a profound T cell tolerance to a subsequent challenge with this antigen 23. Notably, Peterson et al. have demonstrated that type-B T cells specific for the HEL48–62 peptide can escape this negative selection 2. Based on our results, the failure in negative selection and peripheral tolerance can be explained by a lack of presentation of deamidated peptide, both in the thymus and in the periphery. Interestingly, it could be envisaged that during periods of tissue injury or stress, or after abnormal antigen expression or clearance, antigen may be released in a manner that would allow deamidation to occur. Thus, the creation of deamidated peptides may be concomitant with a local milieu that is likely to contain activated APCs, a situation likely to initiate and propagate a T cell response to the modified peptide and possibly lead to autoimmunity.
The generation of T cell responses to posttranslationally modified peptides is of particular relevance when designing peptide- and recombinance-based vaccines, where a response to unmodified antigen is likely to be desirable. Our data illustrates that a potential danger of using such artificial antigens is that asparagine(s) that are stable in the native antigen could be converted into a labile one, and hence a T cell response could be generated to a modified epitope that did not cross-react with the unmodified sequence. Moreover, responses to the synthetic or recombinant peptides in vitro could be erroneously interpreted as an ability of the induced T cells to recognize unmodified antigen. This argument might be of special relevance to cancer vaccines 24 and warrants caution in interpretation of results obtained with synthetic tumor-specific peptides or recombinant antigens. Our study provides a conceptual framework for understanding how and why type-B T cells can be generated and will allow identification of peptides that are prone to these types of modification.
We have demonstrated that posttranslational modification plays a major role in the recognition of one of the most intensively studied T cell epitopes in immunology, and this finding underscores the difficulty of characterizing T cells specific for posttranslationally modified epitopes. The frequency and biological importance of posttranslationally modified T cell epitopes in immunology may well be underappreciated.
Acknowledgments
We thank John Haurum, Øyvind Molberg, and Erik Thorsby for helpful discussions and critical reading of the manuscript and Peter O. Hofgaard for excellent technical help.
This work is funded by research grants from the Research Council of Norway, The Norwegian National Cancer Society, and the European Commission (BMH4-CT98-3087) and by the Deutsche Forschungsgemeinschaft DFG (SFB 510, project D4).
Footnotes
Abbreviations used in this paper: ESI, electrospray ionization; FTICR-MS, Fourier transform ion cyclotron resonance mass spectrometry; HEL, hen egg lysozyme.
References
- Viner N.J., Nelson C.A., Deck B., Unanue E.R. Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J. Immunol. 1996;156:2365–2368. [PubMed] [Google Scholar]
- Peterson D.A., DiPaolo R.J., Kanagawa O., Unanue E.R. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- Viner N.J., Nelson C.A., Unanue E.R. Identification of a major I-Ek-restricted determinant of hen egg lysozymelimitations of lymph node proliferation studies in defining immunodominance and crypticity. Proc. Natl. Acad. Sci. USA. 1995;92:2214–2218. doi: 10.1073/pnas.92.6.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H., Batocchi A.P., Hawke S., Nicolle M., Jacobson L., Vincent A., Newsom-Davis J., Willcox N. Peptide-selected T cell lines from myasthenia gravis patients and controls recognized epitopes that are not processed from whole acetylcholine receptor. J. Immunol. 1995;155:3683–3692. [PubMed] [Google Scholar]
- Nelson C.A., Viner N.J., Unanue E.R. Appreciating the complexity of MHC class II peptide bindinglysozyme peptide and I-Ak . Immunol. Rev. 1996;151:81–105. doi: 10.1111/j.1600-065x.1996.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Latek R.R., Petzold S.J., Unanue E.R. Hindering auxiliary anchors are potent modulators of peptide binding and selection by I-Ak class II molecules. Proc. Natl. Acad. Sci. USA. 2000;97:11460–11465. doi: 10.1073/pnas.210384197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latek R.R., Unanue E.R. Mechanisms and consequences of peptide selection by the I-Ak class II molecule. Immunol. Rev. 1999;172:209–228. doi: 10.1111/j.1600-065x.1999.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Robinson A.B., Tedro S. Sequence dependent deamidation rates for model peptides of hen egg-white lysozyme. Int. J. Pept. Protein Res. 1973;5:275–278. doi: 10.1111/j.1399-3011.1973.tb03461.x. [DOI] [PubMed] [Google Scholar]
- Fremont D.H., Monnaie D., Nelson C.A., Hendrickson W.A., Unanue E.R. Crystal structure of I-Ak in complex with a dominant epitope of lysozyme. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- Wright H.T. Sequence and structure determinants of the nonenzymatic deamidation of asparagine and glutamine residues in proteins. Protein Eng. 1991;4:283–294. doi: 10.1093/protein/4.3.283. [DOI] [PubMed] [Google Scholar]
- Sarioglu H., Lottspeich F., Walk T., Jung G., Eckerskorn C. Deamidation as a widespread phenomenon in two-dimensional polyacrylamide gel electrophoresis of human blood plasma proteins. Electrophoresis. 2000;21:2209–2218. doi: 10.1002/1522-2683(20000601)21:11<2209::AID-ELPS2209>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Aswad D.W., Paranandi M.V., Schurter B.T. Isoaspartate in peptides and proteinsformation, significance, and analysis. J. Pharm. Biomed. Anal. 2000;21:1129–1136. doi: 10.1016/s0731-7085(99)00230-7. [DOI] [PubMed] [Google Scholar]
- Kossiakoff A.A. Tertiary structure is a principal determinant to protein deamidation. Science. 1988;240:191–194. doi: 10.1126/science.3353715. [DOI] [PubMed] [Google Scholar]
- Mine S., Ueda T., Hashimoto Y., Tanaka Y., Imoto T. High-level expression of uniformly 15N-labeled hen lysozyme in Pichia pastoris and identification of the site in hen lysozyme where phosphate ion binds using NMR measurements. FEBS Lett. 1999;448:33–37. doi: 10.1016/s0014-5793(99)00332-4. [DOI] [PubMed] [Google Scholar]
- Tomizawa H., Yamada H., Hashimoto Y., Imoto T. Stabilization of lysozyme against irreversible inactivation by alterations of the Asp-Gly sequences. Protein Eng. 1995;8:1023–1028. doi: 10.1093/protein/8.10.1023. [DOI] [PubMed] [Google Scholar]
- Jung G., Beck-Sickinger A.G. Multiple peptide synthesis methods and their applications. Angew. Chem. Int. Ed. Engl. 1992;31:367–383. [Google Scholar]
- Lunde E., Munthe L.A., Vabo A., Sandlie I., Bogen B. Antibodies engineered with IgD specificity efficiently deliver integrated T-cell epitopes for antigen presentation by B cells. Nat. Biotechnol. 1999;17:670–675. doi: 10.1038/10883. [DOI] [PubMed] [Google Scholar]
- Allen P.M., Babbitt B.P., Unanue E.R. T-cell recognition of lysozymethe biochemical basis of presentation. Immunol. Rev. 1987;98:171–187. doi: 10.1111/j.1600-065x.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Wade W.F., Chen Z.Z., Maki R., McKercher S., Palmer E., Cambier J.C., Freed J.H. Altered I-A protein-mediated transmembrane signaling in B cells that express truncated I-Ak protein. Proc. Natl. Acad. Sci. USA. 1989;86:6297–6301. doi: 10.1073/pnas.86.16.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L.J., Larson S.B., Hasel K.W., McPherson A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry. 1997;36:1581–1597. doi: 10.1021/bi962514+. [DOI] [PubMed] [Google Scholar]
- Eidem J.K., Rasmussen I.B., Lunde E., Gregers T.F., Rees A.R., Bogen B., Sandlie I. Recombinant antibodies as carrier proteins for sub-unit vaccinesinfluence of mode of fusion on protein production and T-cell activation. J. Immunol. Methods. 2000;245:119–131. doi: 10.1016/s0022-1759(00)00274-x. [DOI] [PubMed] [Google Scholar]
- Mamula M.J., Gee R.J., Elliott J.I., Sette A., Southwood S., Jones P.J., Blier P.R. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J. Biol. Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- Adelstein S., Pritchard-Briscoe H., Anderson T.A., Crosbie J., Gammon G., Loblay R.H., Basten A., Goodnow C.C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Tanzarella S., Fleischhauer K., van Endert P., Bordignon C., Traversari C. Characterization of antigenic peptide epitopes by reverse immunologyinduction of cytotoxic T lymphocytes specific for exogenous peptide only. Int. J. Cancer. 1997;72:912–915. doi: 10.1002/(sici)1097-0215(19970904)72:5<912::aid-ijc32>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]