Abstract
T cell receptor (TCR)-interacting molecule (TRIM) is a recently identified transmembrane adaptor protein, which is exclusively expressed in T cells. Here we demonstrate that in mature T cells, TRIM preferentially interacts with the TCR via the TCR-ζ chains and to a lesser extent via the CD3-ε/γ heterodimer. Transient or stable overexpression of TRIM in Jurkat T cells results in enhancement of TCR expression on the cell surface and elevated induction of Ca2+ mobilization after T cell activation. TRIM-mediated upregulation of TCR expression results from inhibition of spontaneous TCR internalization and stabilization of TCR complexes on the cell surface. Collectively, our data identify TRIM as a novel integral component of the TCR complex and suggest that one function of TRIM might be to modulate the strength of signals transduced through the TCR through regulation of TCR expression on the cell surface.
Keywords: T lymphocytes, signal transduction, T cell receptor complex, ζ chains, transmembrane adaptor proteins
Introduction
Hematopoietic antigen receptors such as the TCR, the B cell receptor, or Fc receptors are generally composed of at least two different subunits, one representing the ligand binding site, the other being responsible for signal transduction after receptor engagement. The molecular structure of these two subunits reflects their different tasks for eliciting cellular responses. Thus, the ligand binding site normally comprises a long extracellular domain (serving as recognition element), an α-helical transmembrane domain that carries at least one charged amino acid, and a very short cytoplasmic segment devoid of any signaling motifs. In contrast, the signal-transducing component is composed of a very short extracellular domain (lacking an external ligand), a transmembrane domain that also contains a charged amino acid (to allow stable interaction with the ligand binding subunit), and a comparably long cytoplasmic tail carrying particular amino acid motifs, which are necessary for signal transduction. These tyrosine-based signaling motifs have been termed immunoreceptor tyrosine-based activation motifs (ITAMs) and comprise the sequence YxxL(x)7–8YxxL (x = nonconserved amino acid). After binding of an external ligand (e.g., antigen), ITAMs become phosphorylated by protein tyrosine kinases of the src family such as Lck, Fyn, and Lyn, and this initial phosphorylation event then allows propagation of the signal from the cell surface into the cytoplasmic compartment 1.
In the case of the TCR, the ligand binding site is constructed from the polymorphic α- and β chains, which form a disulfide-linked heterodimer that interacts with peptide/MHC complexes presented on the surface of antigen presenting cells 2 3 4. Signal transduction after binding of antigen/MHC to the TCR is then mediated via the TCR-associated components of the CD3 complex (CD3 γ, -δ, and -ε) and the ζ chains 5 6 7 8 9. However, besides serving as signal transducing elements for the TCR, expression of the CD3 molecules and ζ chains is required for proper assembly of the TCR complex and its transportation to and expression on the cell surface. Loss of each of the individual members of the CD3 complex or the ζ chains results in the synthesis of unstable TCRs which cannot be transported to the cell surface and become either degraded in lysosomes (in the case of hexameric αβγεδε-TCRs lacking ζ) or in the endoplasmic reticulum (in the case of TCRs lacking any component of the TCR/CD3 complex besides ζ; references 10 11 12 13 14).
Although it now seems clear that all components necessary for efficient assembly, transportation, and cell surface expression of the TCR have been identified 15, it is not yet fully understood how the expression levels of the TCR on the cell surface are regulated. Based on analysis of ζ-deficient murine T cell hybridomas, it was proposed that the availability of ζ might be the limiting factor that determines how many TCRs are expressed on the cell surface 11. Thus, although all components of the TCR are synthesized in large amounts, relatively few ζ molecules are synthesized by T cells (∼10% compared with other TCR/CD3 chains). As only TCR/CD3 complexes that are associated with ζ can be transported to the plasma membrane whereas those devoid of ζ are degraded in lysosomes, the amount of available ζ regulates cell surface expression of the TCR.
However, the ζ chain only loosely associates with the TCR. Indeed, a recent report demonstrated that in Jurkat T cells, ζ is transported to the Golgi apparatus independently from other TCR chains 16. Moreover, it had been suggested that in human peripheral blood T cells and in the Jurkat T cell line, ζ chains cycle independently from the other components of the TCR 17. Thus, the majority of the ζ chains that are expressed on the cell surface are rapidly internalized, degraded in lysosomes, and replaced by newly synthesized ζ, whereas the turnover of the remaining TCR/CD3 complex seems to be much slower. The significance of the rapid turnover of TCR-ζ and its partial independence from the other components of the TCR is unclear at present. Neither is it understood to what extent the turnover of ζ can influence the expression or function of the TCR/CD3 complex (for a review about the fate of the TCR, see reference 18)
Recently we reported the identification and molecular cloning of a novel transmembrane adaptor protein termed TCR-interacting molecule (TRIM) (reference 19). Based on the findings that TRIM coprecipitates with the TCR in resting T lymphocytes and various T cell lines as well as our observation that TRIM expression is strongly downregulated after modulation of the TCR from the cell surface, we had proposed that TRIM represents a so far uncharacterized integral component of the TCR/CD3/ζ complex. However, the function of TRIM is not yet clear. In addition, it is not known how the interaction between TRIM and the TCR is mediated and whether TRIM plays a role in the assembly and/or the cell surface expression of the TCR/CD3/ζ complex. Here we have addressed these questions by studying the association between TRIM and the TCR in peripheral blood T lymphocytes and in Jurkat T cells.
Materials and Methods
Cells.
Jurkat T cells and COS cells were maintained in RPMI 1640 (GIBCO BRL) supplemented with 10% FCS (GIBCO BRL), 1% penicillin-streptomycin (GIBCO BRL), and 2% glutamine (GIBCO BRL) at 37°C and 100% humidity. The Jurkat variants 18B3 (lacking TCR-α), JBN (lacking TCR-β), and JGN (lacking CD3γ; provided by Dr. C. Geisler, Institute of Medical Microbiology and Immunology, University of Copenhagen, Copenhagen, Denmark) have been described previously 16. Peripheral blood T lymphocytes were prepared by E-rosetting from freshly drawn venous blood of healthy donors as described elsewhere 20.
cDNA Constructs and Molecular Cloning of the Mouse TRIM cDNA.
The cDNA constructs encoding wild-type TRIM, TRIM/linker for activation of T cells (LAT) chimeras, and TRIM mutants that were used in this study are all based on the TRIM cDNA constructs that we have described previously 19. They were generated by in vitro mutagenesis using the QuickChange™ site directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. All constructs were verified by DNA sequencing. A mouse TRIM cDNA clone (sequence data are available from GenBank/EMBL/DDBJ under accession no. AJ29769) was isolated by screening a mouse spleen cDNA library at low stringency with a human TRIM probe according to standard procedures.
Transfection Experiments.
Jurkat cells were transfected by electroporation and stable clones selected in hygromycin-containing medium as described previously 21. For transfection of COS cells, 2 × 105 exponentially growing cells were incubated overnight in a six-well plate (Nunc). Adherent cells were washed twice with 2 ml of PBS and incubated for 4 h in 1 ml of transfection solution (2 μg DNA, 10 mg/ml DEAE-dextran, 5.6 mg/ml chloroquine in RPMI 1640 supplemented with 2% FCS). Subsequently, the solution was removed and 1 ml of a 10% DMSO (Merck) in RPMI was added and allowed to incubate for 2 min. Then cells were washed once with PBS and incubated for 36 h in 2–3 ml of culture medium. Transfected cells were washed in PBS and further processed for immunoprecipitation or confocal laserscan microscopy.
Abs.
The following monoclonal and polyclonal Abs were used for immunoprecipitation, confocal laser scan microscopy, Western blotting, and indirect immunofluorescence: HLA-I (W6/32, for FACS® analysis and cap formation), anti-ζ mAb, clone 6B10.2 (Santa Cruz Biotechnology, Inc.; confocal laser scan analysis and immunoprecipitation), anti-ζ (H146 from hamster; a gift of Dr. K. Campell, Fox Chase Cancer Center, Philadelphia, PA; for immunoprecipitation), anti-ζ clone 1C10 (Western blotting; donated by Dr. M. Peter, University of Chicago, Chicago, IL), CD3ε mAb OKT3 (for cocapping and FACS® analysis), CD3ε clone SP34 (from M. Peter), CD3ε clone 2Ad2a2 (IgM; donated by Dr. E. Reinherz, Dana Farber Cancer Center, Boston, MA; for modulation experiments), PE-labeled CD3ε clone UCHT-1 (BD PharMingen), and anti-TCR mAb BMA-031 (a gift from Dr. Kurrle, Hoechst AG, Frankfurt, Germany; for FACS® analysis). To raise mouse mAbs to the intracellular domain of TRIM, F1(BALB/c × B10.A) hybrid mice were immunized with a TRIM cytoplasmic fragment corresponding to amino acid residues 29–186 of human TRIM. For bacterial expression, a cDNA fragment encoding this intracellular part of TRIM was cloned into the BamHI site of the pET-15b expression vector (Novagen), generating a construct with NH2-terminal His tag. The protein was isolated from bacterial lysates by affinity chromatography and used for intrasplenic immunization followed by standard procedures to produce Ab-secreting hybridomas. Two anti-TRIM mAbs were used in this study, namely TRIM-4 and TRIM-7. The protein A–purified Abs were covalently linked to CNBr-activated sepharose beads at a concentration of 6 mg/ml. Polyclonal antisera directed at mouse CD3γ, -δ, and -ε used for Western blotting have been described previously 22 and were donated by Dr. E. Palmer (Basel Institute for Immunology, Basel, Switzerland). Affinity-purified polyclonal anti-TRIM, anti-Src kinase–associated phosphoprotein homologue (SKAP-HOM), and anti-lymphocyte phosphatase-associated protein (LPAP) antisera were prepared as described elsewhere 19 23 24 and used at 5–10 μg/ml for confocal laser scan analysis. Anti-TCR mAb C305 (IgM) was a gift of Dr. A. Weiss (University of California at San Francisco, San Francisco, CA) and used as culture supernatant. For the capping experiment shown in Fig. 1 B, the following Abs (all IgM) were used: CD4 (MEM-16), CD5 (MEM-128), and CD28 (248.23.2; reference 25).
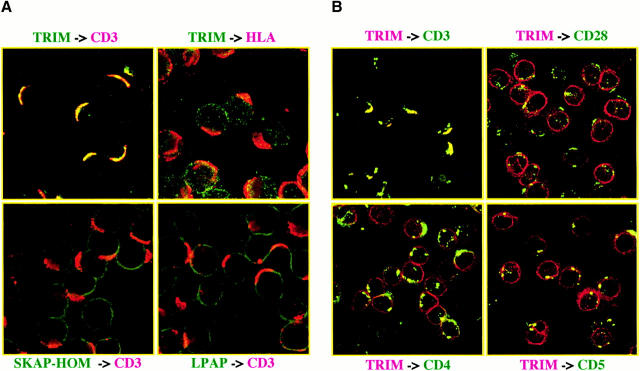
Figure 1.
Interaction between TRIM and the TCR in peripheral blood T cells. (A) Top: T cells were incubated with CD3ε (OKT3) or anti-HLA I (W6/32) mAbs followed by induction of cap formation using Texas red–labeled donkey anti–mouse Ab. Cells were fixed and the subcellular localization of TRIM determined using polyclonal TRIM Ab and Cy2-labeled donkey anti–rabbit antiserum. Bottom: subcellular localization of SKAP-HOM (left) and LPAP (right) in TCR-capped peripheral blood T cells. (B) T cells were incubated with mAbs directed at CD3, CD4, CD5, and CD28 (all of the IgM isotype) to induce cap formation. Cells were fixed and the distribution of TRIM determined using biotinylated TRIM mAb (TRIM-4) followed by streptavidin Texas red. In parallel, cap formation was monitored by incubating the IgM-treated cells with a FITC-labeled goat anti–mouse IgM antiserum.
Cocapping Experiments.
Resting peripheral blood T cells were washed twice with RPMI (without FCS) and adjusted to 7 × 106/ml in RPMI 1640. 10 μl of this suspension was pipetted onto each reaction field of an adhesion slide (Bio-Rad Laboratories) and allowed to adhere for 10 min at room temperature. Adherent cells were washed twice with RPMI and the slide was blocked for 15 min with complete culture medium. Cap formation was subsequently induced by incubating the cells for 15 min with 15 μl mAb solution (concentration 10 μg/ml in complete culture medium), followed by two washes with RPMI 1640 (50 μl per field) and cross-linking of the primary Abs with 15 μl of Texas red–labeled donkey anti–mouse antiserum (Dianova; diluted 1:50 vol/vol in culture medium) for 35 min at room temperature in the dark. Capped cells were washed twice with 50 μl PBS supplemented with 0.2% NaN3 and then fixed for 3 min with methanol (stored at −20°C). The methanol was removed and the cells were fixed for an additional 5 s with ice-cold acetone. Fixed cells were washed twice with PBS and blocked again for 10 min with PBS/1% BSA at room temperature. Subsequently, affinity-purified polyclonal Abs directed at TRIM, SKAP-HOM, and LPAP were added (15 μl per field, concentration 10 μg/ml) and incubated for 30 min at room temperature in the dark. The Ab-labeled cells were then washed once with PBS/BSA (40 μl per field) and three times with PBS. Then, 15 μl of the secondary Cy2-labeled donkey anti–rabbit Ab (Dianova) was added (concentration 1:125 vol/vol in PBS/1% BSA) and incubated for 30 min s at room temperature in the dark. Cells were washed once with PBS/BSA, three times with PBS, and finally for at least 5 min with distilled water. The slides were then covered with a cover slip, which was fixed with Elvanol (Calbiochem) for at least 16 h.
For colocalization studies in transfected Jurkat T cells or COS cells, cells were subjected to adhesion slides and fixed with methanol and acetone as described above. Fixed cells were then incubated with 10 μl CD3ζ mAb 6B10.2 (10 μg/ml in PBS/1% BSA) and incubated for 30 min at room temperature. The stained cells were then washed with PBS/1% BSA for 3 min and three more times with plain PBS. Then, 10 μl of Cy2-labeled donkey anti–mouse antiserum were added (Dianova; diluted 1:150 vol/vol in PBS/1% BSA) and incubated for 30 min at room temperature. For detection of FLAG-tagged molecules, cells were incubated with 10 μl of biotinylated anti-FLAG Ab (M2; 10 μg/ml in PBS/BSA; Sigma-Aldrich) for 30 min at room temperature, washed as above, and incubated again for 30 min at room temperature with 10 μl of streptavidin–Texas red solution (1:100 vol/vol in PBS/1% BSA). Cells were then washed again and the slide preparation finished as described above. For detection of TRIM, essentially the same steps were conducted with the exception that Texas red–labeled donkey anti–rabbit Ab (Dianova; diluted 1:125 vol/vol in PBS/1% BSA) was used as second step reagent. Slides were viewed on a Laserscan microscope (Ernst Leitz GmbH) using 570 nm (red emission) and 508 nm (green emission) filters, respectively. In general, the magnification was ×40.
Analysis of Spontaneous TCR Internalization.
Jurkat T cells were either transfected with empty vector or with a cDNA construct encoding wild-type TRIM. 40 h later, 107 cells were incubated on ice with PE-labeled mAb UCHT-1 for 30 min in precooled HBSS (GIBCO BRL) supplemented with 2% FCS. Unbound Abs were removed by washing and the externally labeled cells were then resuspended in 4 ml prewarmed medium (37°C) for the indicated periods of time. The reaction was terminated by adding 4 ml of ice-cold HBSS/2% FCS/0.1% NaN3 and the cells were pelleted by centrifugation. After removing the supernatant, cells were resuspended in 100 μl of ice-cold C305 (anti-TCR, IgM) hybridoma culture supernatant and incubated on ice for 30 min. After washing, cells were incubated on ice with a 1:40 vol/vol (in HBSS/2% FCS/0.1% NaN3) dilution of a FITC-labeled polyclonal goat anti–mouse IgM serum for 20 min, washed, and further analyzed by flow cytometry and confocal laser scanning microscopy.
Intracellular Ca2+ Mobilization.
Ca2+ flux in response to TCR ligation was investigated using variable wavelength Ca2+ indicators according to standard protocols. Jurkat T cell clones were loaded with 4 μM fura-2 AM (Molecular Probes Inc.) for 30 min at 37°C. Dye-loaded cells were washed and incubated on ice with C305 anti-TCR IgM mAb for 30 min before centrifugation and resuspending in warm serum-free medium. Fluorescence intensity was measured on a PerkinElmer luminescence spectrometer LS50 with excitation at 340 and 380 nm and emission fixed at 510 nm. Cells were maintained at 37°C with continuous gentle stirring throughout the analysis. Maximum fluorescence (F max) was determined by lysing the cells with 0.1% Triton X-100 and minimum fluorescence (F min) by the addition of 40 mM Tris, 4 mM EGTA. [Ca2+]i was calculated using FL WinLab software according to the Grynkiewitz equation:
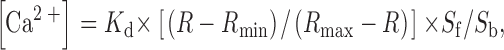 |
where K d = 135 nM for fura-2 AM and Ca2+ and where R is F at 340 nm/F at 380 nm, and S f (or S b) is F min (or F max) at 380 nm.
Cell Surface Biotinylation and Other Experimental Procedures.
For biotinylation of cell surface molecules, Jurkat T cells were washed twice with ice cold PBS, adjusted to 10 × 106 cells/ml, and labeled with 100 μl biotin (10 mg/ml in PBS; Pierce Chemical Co.) for 20 min at 4°C. The reaction was stopped by adding an equal volume of ice cold PBS supplemented with 10 mM glycine for 5 min, and the externally labeled cells were then washed twice with PBS to remove unbound biotin. 35 × 106 cells were then lysed in 2 ml of lysis buffer supplemented with 1% digitonin and postnuclear supernatants were subjected to immunoprecipitation using 75 μl of packed CNBr-activated Sepharose beads coupled with protein A–purified CD3ε mAb OKT-3 (6 mg mAb/ml of packed beads). After washing, the beads pellet was resuspended in 75 μl of lysis buffer supplemented with 1% Triton X-100 and 0.5% SDS and incubated for 5 min at 95°C. The SDS was quenched by adding 1.25 ml of lysis buffer, the beads were spun down, and the supernatant carefully removed and subjected to reprecipitation using either polyclonal rabbit antisera directed at CD3γ, -δ, and -ε or a mAb directed at ζ (H146). After 14% SDS-PAGE, samples were blotted onto nitrocellulose which was then revealed by peroxidase-labeled streptavidin followed by enhanced chemoluminescence (ECL) detection.
Immunoprecipitation, Western blotting, and FACS® analysis were essentially performed as described elsewhere 19 20 26.
Results
TRIM Is a Novel Integral Component of the TCR/CD3/ζ Complex.
Recently, we reported the molecular cloning of a novel 29/30-kD disulfide-linked transmembrane adaptor protein called TRIM 19. We had chosen the name TRIM because several lines of evidence had suggested that this novel polypeptide preferentially interacts with the TCR/CD3/ζ complex. For example, in transformed human T cell lines such as HPB-ALL, TRIM coprecipitates with CD3ε but not with CD4, CD8, CD28, or CD45 when using the detergent digitonin. In addition, in nontransformed peripheral blood T lymphocytes and the cutaneous T cell lymphoma PBLCPCS, the expression levels of TRIM in total cellular lysates are strongly downregulated after ligand-induced modulation of the TCR, whereas the expression levels of, e.g., CD8 and HLA class I remain unchanged 19.
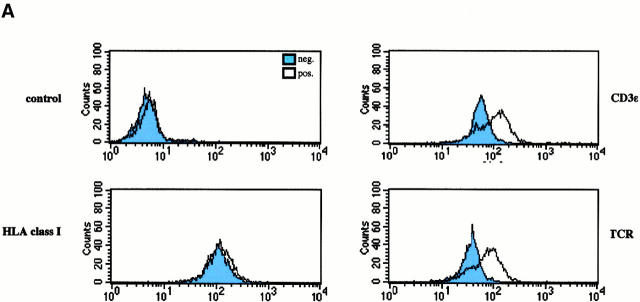
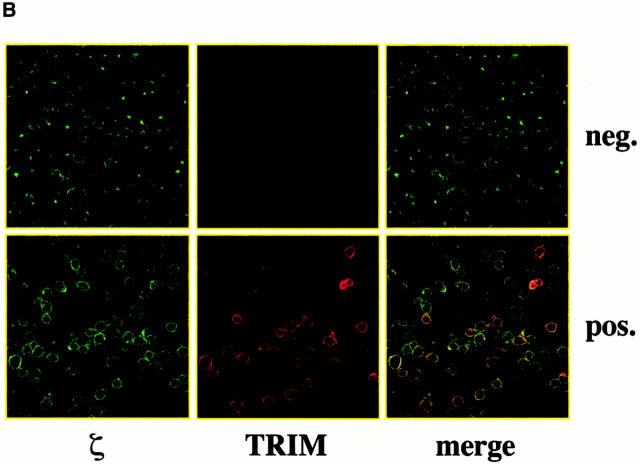
To provide additional evidence for an association between TRIM and the TCR in vivo in intact and nontransformed cells, human peripheral blood T lymphocytes were incubated with mAbs directed at the extracellular domains of CD3ε, or as a control HLA class I followed by induction of cap formation using goat anti–mouse polyclonal antiserum. Subsequently, the subcellular localization of TRIM was determined by confocal laser scan microscopy. The top left panel of Fig. 1 A indicates that under these experimental conditions, TRIM cocaps with the TCR. In contrast, under the same experimental conditions, TRIM does not cocap with HLA class I molecules (top right panel of Fig. 1 A). Similarly, TRIM does not cocap with the CD4, CD5, and CD28 coreceptors (Fig. 1 B). Finally, as shown in the bottom panels of Fig. 1 A, cocapping with the TCR is a particular property of TRIM, as other cytoplasmic and/or transmembrane adaptor proteins such as SKAP-HOM or LPAP do not colocalize with capped CD3ε under the same experimental conditions. Thus, these data further confirm that TRIM represents a novel component of the TCR/CD3/ζ complex.
Regulation of TCR Expression by TRIM.
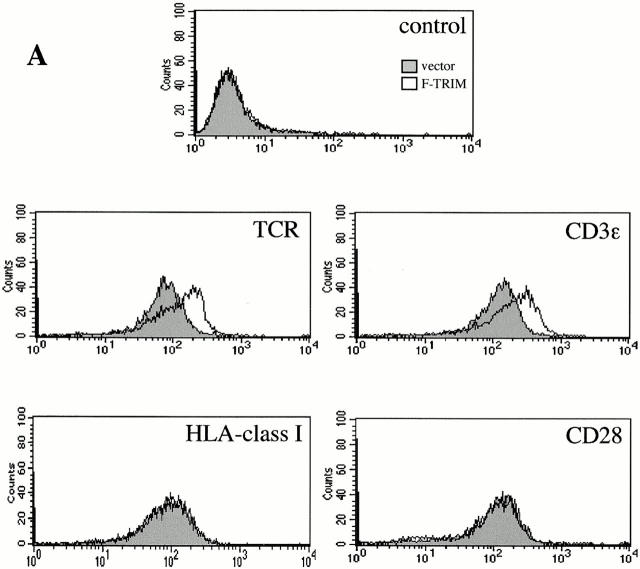
We next assessed whether the association between TRIM and the TCR might be of functional relevance. To this end we transiently overexpressed F-TRIM in Jurkat cells and analyzed the expression levels of the TCR-α/β heterodimer and of CD3ε by indirect immunofluorescence using the mAbs BMA031 (TCR-α/β) and OKT-3 (detecting CD3δ/ε and CD3 γ/ε heterodimers), respectively. As demonstrated in Fig. 2 A, overexpression of TRIM resulted in a considerable increase in TCR-α/β and CD3ε expression on the cell surface, whereas the expression levels of CD28 and HLA class I remained unchanged. Analysis of the mean channels of fluorescence of TCR expression indicated that TRIM transfectants expressed approximately twice as many TCRs on the cell surface as vector transfectants. In contrast, overexpression of the transmembrane adaptor proteins LAT or SHP2-interacting transmembrane adaptor protein (SIT) did not influence the expression levels of the TCR under the same experimental conditions (not shown here but see Fig. 3 C and 6 C). This suggests that upregulation of TCR expression is a particular property of TRIM and that it is not simply induced by transfecting the cells. The data depicted in Fig. 2 B further indicate that overexpression of TRIM does not substantially alter the subunit composition of the TCR/CD3/ζ complexes that are expressed on the cell surface.
Figure 2.
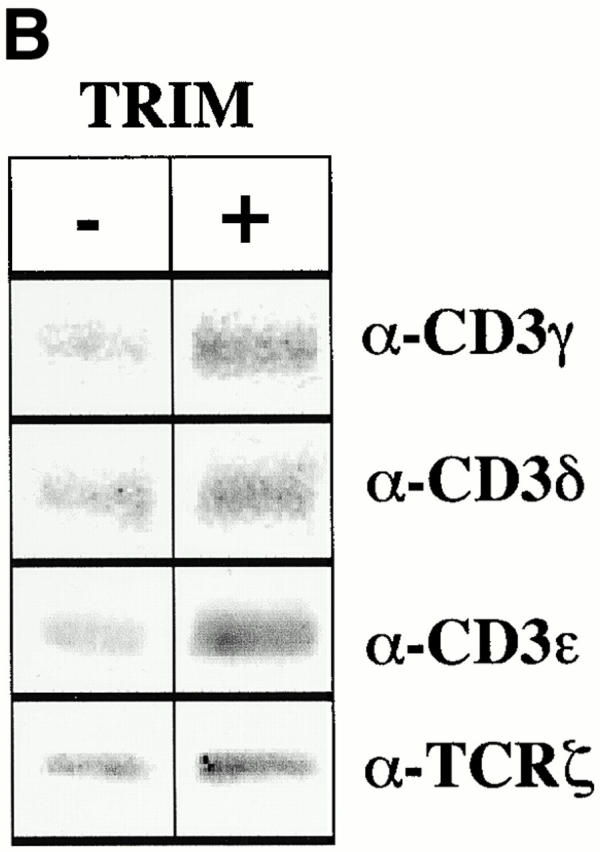
Overexpression of TRIM in Jurkat cells results in upregulation of TCR expression. (A) Jurkat T cells were transiently transfected with empty vector or with a cDNA construct encoding FLAG-tagged wild-type TRIM. 16 h later, expression of the TCR-α/β, CD3ε, CD28, or HLA class I on the cell surface was determined by flow cytometry. (B) Jurkat T cells were transfected with empty vector or with a cDNA coding for F-TRIM. After 40 h, cell surface molecules expressed by vector transfectants and TRIM transfectants were biotinylated. Cells were then lysed in digitonin-containing buffer and subjected to CD3ε immunoprecipitation. After washing, the precipitated molecules immunoprecipitates were boiled in SDS-containing buffer and the released material subjected to reprecipitation using either polyclonal Abs directed at CD3γ, CD3δ, or CD3ε, or a mAb directed at ζ. The secondary immunoprecipitates were separated on 14% reducing SDS-PAGE, blotted onto nitrocellulose, which was then probed with peroxidase-labeled streptavidin followed by ECL detection.
Figure 3.
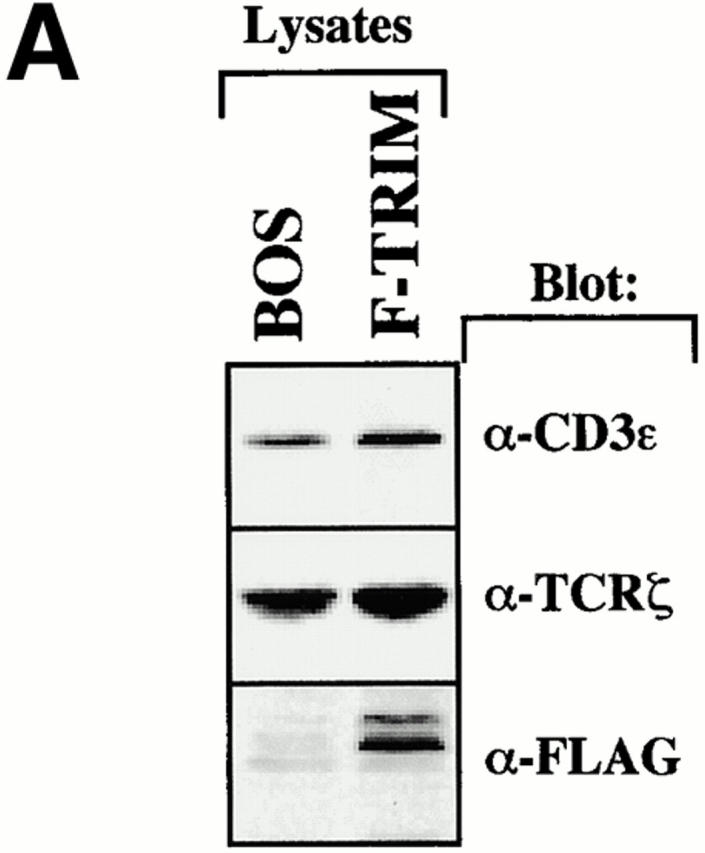
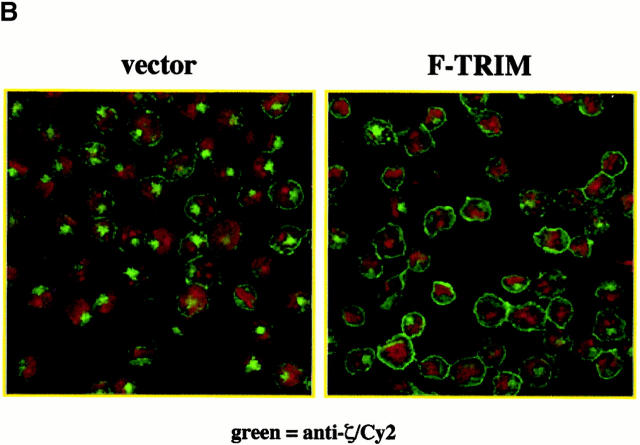
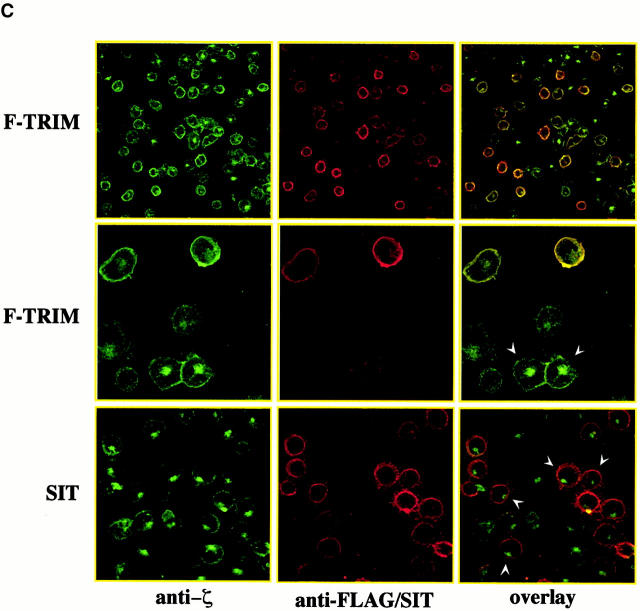
Overexpression of TRIM in Jurkat T cells alters the subcellular localization of ζ. (A) Lysates of Jurkat T cells transfected with empty vector or a cDNA coding for F-TRIM were analyzed for the expression of CD3ε (using mouse mAb SP34), TCR-ζ (using mouse mAb 1C10), and F-TRIM (anti-FLAG) by Western blotting. (B) Jurkat T cells were transfected with empty vector alone (left) or with a cDNA construct encoding F-TRIM (right). 40 h later, the subcellular localization of ζ (using mAb 6B10.2) was determined by confocal laser scan microscopy. Nuclei were stained with propidium iodine. (C) Cells were transfected as in B and the subcellular localization of ζ (mAb 6B10.2, green fluor-escence, Cy2) or F-TRIM determined by confocal laserscan analysis. F-TRIM was detected using a biotinylated anti-FLAG Ab. Bottom: specificity control showing that overexpression of the transmembrane adapter protein SIT does not alter the subcellular localization of ζ. Note that additional data indicating that overexpression of SIT also does not influence the expression levels of the TCR on the cell surface are shown in Fig. 6.
TRIM Inhibits Spontaneous Internalization of TCR/CD3/ζ Complexes.
Despite the fact that overexpression of TRIM leads to enhanced expression of all TCR subunits on the cell surface, the overall amounts of CD3ε and TCR-ζ in total lysates remain rather unchanged (Fig. 3 A). This suggests that the functional effect of TRIM cannot be simply explained by an increase in protein synthesis of particular TCR components. Therefore, we argued that TRIM might enhance TCR expression on the cell surface by either facilitating transportation of preassembled TCR complexes to the cell surface or by preventing spontaneous TCR internalization. To obtain first insights on whether one of these mechanisms could play a role in TRIM-mediated upregulation of TCR expression, we compared the subcellular localization of ζ in Jurkat T cells transfected with either empty vector or with a cDNA encoding F-TRIM using confocal laser scan microscopy.
The left panel of Fig. 3 B depicts that in vector-transfected cells, similar fractions of endogenous ζ localize at the plasma membrane and in an intracellular compartment. This pattern of ζ-distribution within the cell seems to be typical for Jurkat T cells and resting peripheral blood T cells 16. In marked contrast, in Jurkat cells transfected with the plasmid encoding F-TRIM, most ζ molecules localize at the plasma membrane (Fig. 3 B, right panel) and only some cells show the typical intracellular localization of ζ that is visible in vector transfectants. To analyze whether the latter cells are those which had not been transfected, we performed double immunofluorescence using anti-ζ (green fluorescence, Cy2) and anti-FLAG (red fluorescence, Texas red) mAbs, respectively. The top and middle panels of Fig. 3 C show that endogenous ζ is targeted to the cell membrane only in cells expressing F-TRIM, whereas in cells which do not express F-TRIM or which express only very low amounts of the molecule, a fraction of ζ is still present in the cytoplasmic compartment. Thus, overexpression of TRIM in Jurkat T cells increases the amounts of ζ at the plasma membrane and concomitantly decreases its intracellular pool. This is a specific property of TRIM, as overexpression of the transmembrane adaptor protein SIT does not alter the subcellular localization of ζ (Fig. 3 C, bottom panel). Collectively, these data indicate that one of the above discussed mechanisms likely is responsible for TRIM-mediated upregulation of TCR expression.
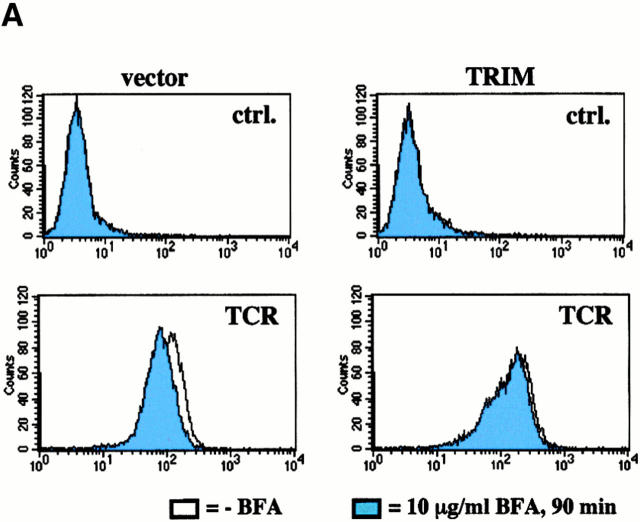
To distinguish between the two possibilities, we next incubated vector transfectants and TRIM transfectants with brefeldin A (BFA) and monitored BFA-mediated downregulation of TCR expression by flow cytometry. BFA inhibits exocytosis of proteins while leaving endocytic processes largely unaffected 27. Thus, we argued, that if TRIM upregulates transportation of preassembled TCR complexes to the cell surface, BFA treatment should lead to an accelerated loss of cell surface–expressed TCR complexes in TRIM transfectants compared with vector transfectants. In contrast, if TRIM would inhibit spontaneous endocytosis of TCR complexes, one would expect that TRIM transfectants are less sensitive to BFA treatment than vector transfectants.
As reported previously 28, BFA treatment of vector-transfected Jurkat T cells induced only an ∼20% downregulation of TCR expression (Fig. 4 A). This contrasts the situation in T cell hybridomas in which BFA treatment has been reported most recently to induce a much stronger downregulation of TCR expression 29. Nevertheless, in Jurkat T cells overexpressing TRIM, BFA had almost completely lost its capability to induce downregulation of TCR expression. This suggests that the higher levels of TCR expression in Jurkat T cells overexpressing TRIM likely result from TRIM-mediated inhibition of spontaneous TCR internalization rather than from facilitating transport of newly synthesized receptors to the cell surface.
Figure 4.
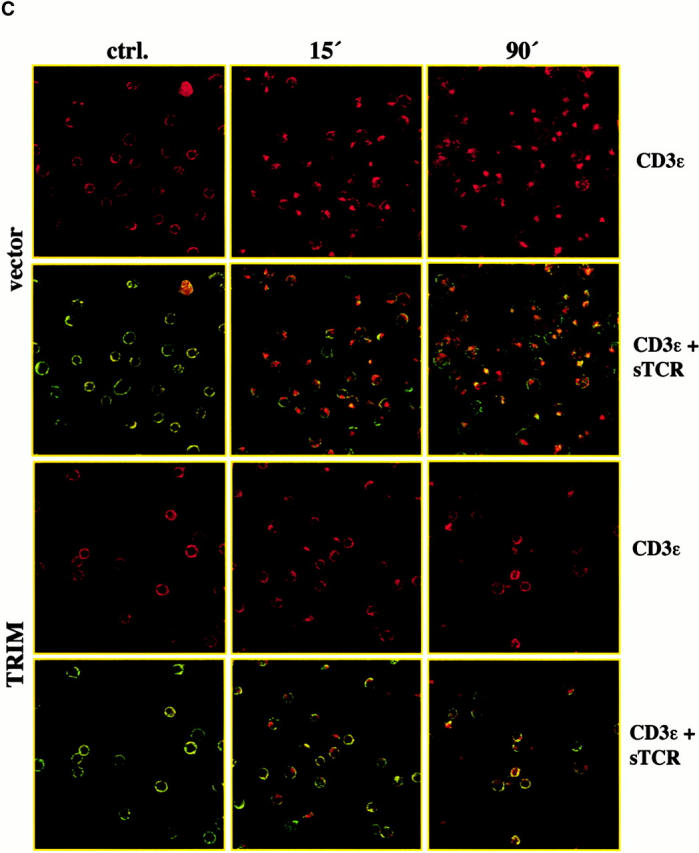
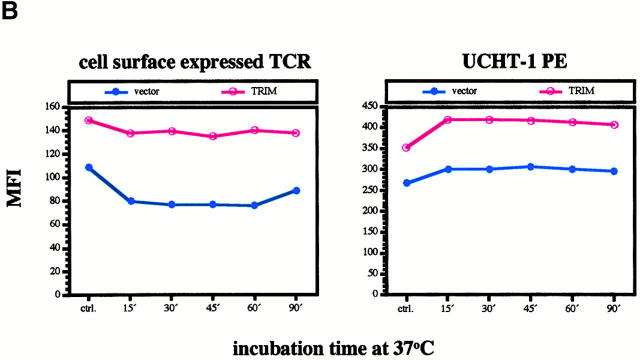
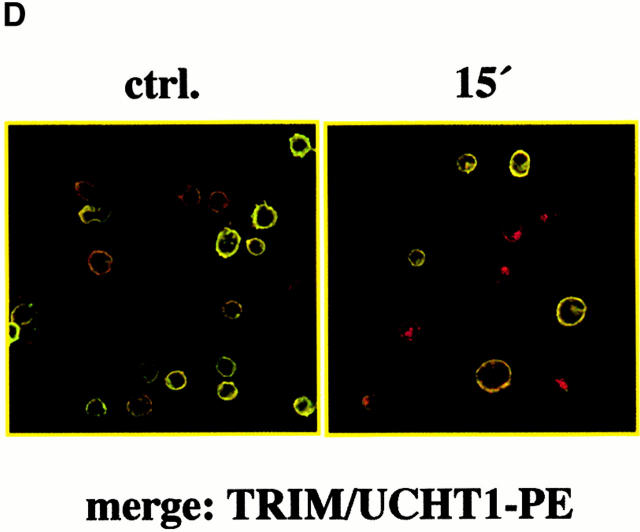
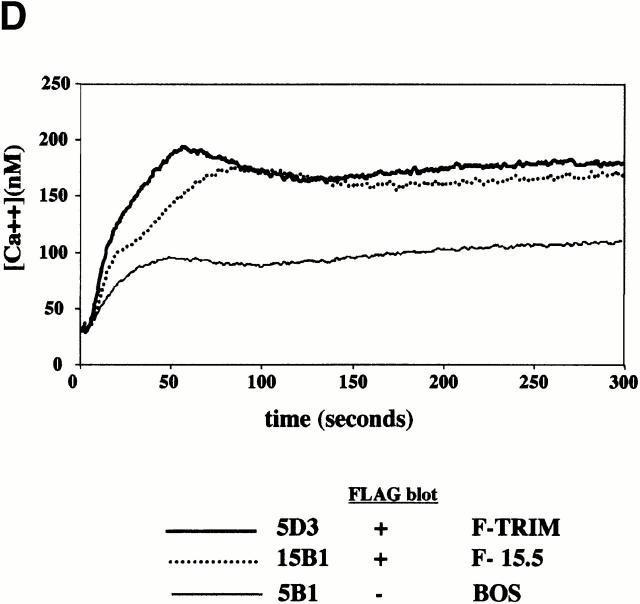
Inhibition of spontaneous TCR internalization by TRIM. (A) Vector transfectants or TRIM transfectants were treated for 90 min with BFA at 37°C. Subsequently, the expression of the TCR-α/β heterodimer was determined by indirect immunofluorescence. ctrl., control. (B and C) Vector transfectants or TRIM transfectants were externally labeled on ice with PE-labeled CD3ε mAb UCHT-1. After washing the temperature was shifted to 37°C for the indicated periods of time. After incubation at 37°C, the temperature was again lowered to 4°C and cells were externally labeled with the anti-TCR mAb C305 (IgM) followed by FITC-labeled goat anti–mouse IgM serum. Cells were then analyzed by flow cytometry (B) or by confocal laserscanning analysis (C). sTCR, surface TCR. (D) TCR internalization is only downregulated in Jurkat T cells overexpressing TRIM. Jurkat T cells transfected with a plasmid encoding TRIM were labeled on ice with UCHT1-PE as described above. After washing, cells were either incubated on ice (left) or incubated for 15 min at 37°C (right). Cells were then permeabilized and stained with biotinylated TRIM-4 mAb followed by detection using streptavidin Cy2.
To substantiate this assumption we transfected Jurkat T cells with empty vector or with a cDNA encoding TRIM. 40 h later, cells were externally labeled with a PE-labeled CD3ε mAb (UCHT1) on ice. After washing, cells where shifted to 37°C for increasing periods of time and then externally labeled with an IgM Ab directed at the anti–TCR-α/β heterodimer (C305). These cells were then subjected to FACS® analysis as well as analysis by confocal laserscanning microscopy.
As shown in Fig. 4 B, in both vector and TRIM transfectants the PE signal does not substantially decline even after a 90-min incubation at 37°C. An extended kinetics even revealed that the PE signal remains stable for up to 18 h (not shown). These findings rule out the possibility that the CD3ε molecules become degraded intracellularly (e.g., in lysosomes) after shifting the temperature to 37°C and thus confirm previous data showing that the TCR/CD3 complexes are long lived 29. However, when the same cells are analyzed by confocal laserscanning microscopy a rapid segregation of the PE- and the FITC signals can be observed. Thus, already after 15 min of incubation at 37°C a substantial portion of the PE signal accumulates in intracellular compartment which has recently been shown to represent early endosomes 29. In contrast, the FITC signal remains on the cell surface. Identical data were obtained with nontransfected Jurkat T cells (not shown). Collectively, these data corroborate previous data showing that TCR/CD3 complexes are rapidly internalized and subjected to the recycling pathway without being degraded 29 30 31. The difference between the rather moderate reduction of TCR expression after BFA treatment shown in Fig. 4 A versus the apparently rapid and strong intracellular accumulation of CD3ε shown in Fig. 4 B could indicate that binding of the PE-labeled mAb to CD3ε accelerates spontaneous endocytosis of the TCR.
However, when the same experiment was applied to Jurkat T cells overexpressing TRIM, we observed that the PE-labeled CD3ε molecules were internalized at a much slower rate than in the vector-transfected cells. Indeed, confocal laserscanning microscopy revealed that in cells overexpressing TRIM, a fraction of the PE-labeled CD3ε molecules were still present on the cell surface and colocalized with the TCR-α/β heterodimers even after 90 min of incubation at 37°C (Fig. 4c and Fig. d). Further analysis of the transfectants using TRIM mAb revealed that only in cells overexpressing TRIM was internalization of the PE-labeled CD3ε molecules abolished (Fig. 4 D). From these data, we conclude that overexpression of TRIM primarily upregulates TCR expression on the cell surface by inhibiting its spontaneous internalization.
TRIM Preferentially Associates with the TCR-ζ Chain.
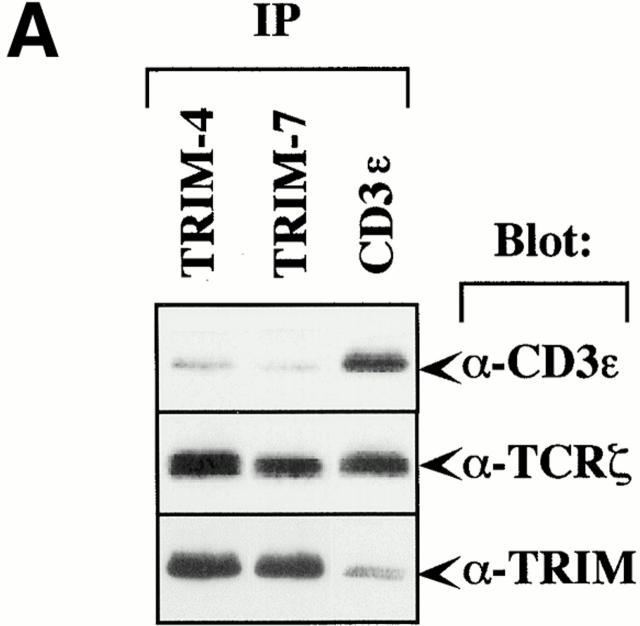
We next wished to determine how TRIM interacts with the TCR/CD3/ζ complex. To assess this question, we analyzed TRIM and CD3ε immunoprecipitates prepared from digitonin lysates of Jurkat T cells for the relative amounts of CD3ε and TCR-ζ, respectively, by Western blotting. Fig. 5 A depicts that identical amounts of CD3ε and TCR-ζ are detectable in CD3ε immunoprecipitates. In contrast, under the same experimental conditions, ∼10 times more ζ than CD3ε coprecipitate with TRIM (as judged from densitometric analysis of the shown blot). Similar results as shown here for Jurkat T cells are obtained when the immunoprecipitates are prepared from resting peripheral blood T cells (not shown). This suggests that TRIM interacts with the TCR preferentially via the TCR-ζ chain.
Figure 5.
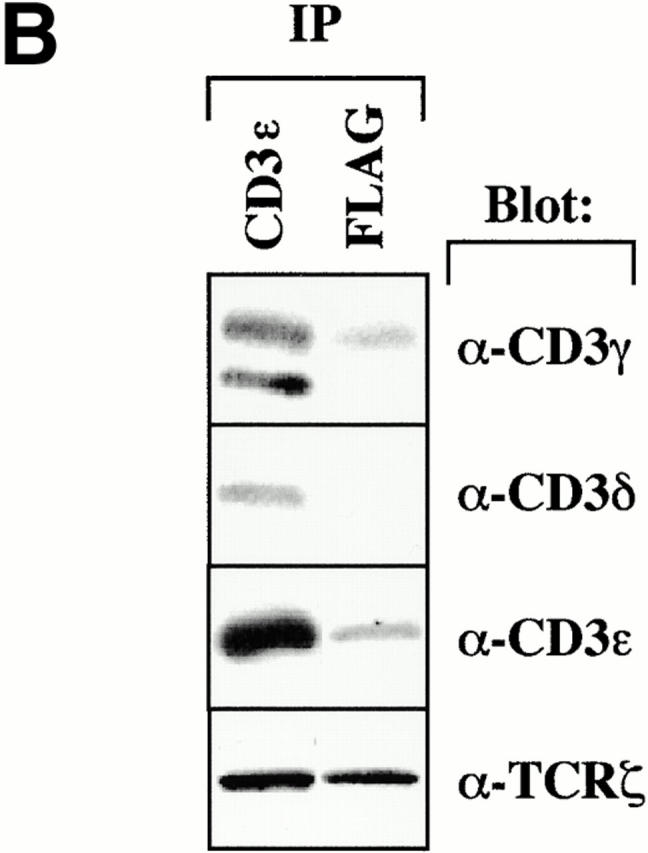
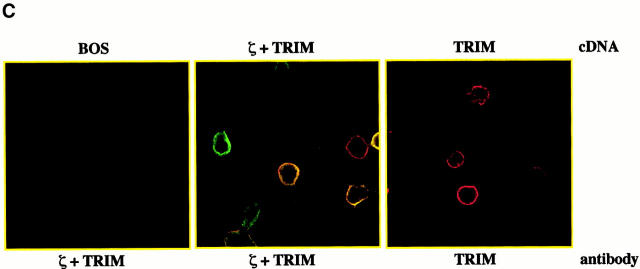
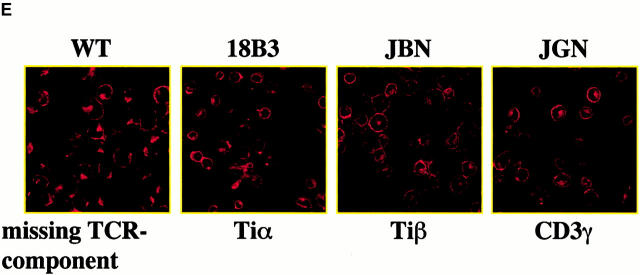
TRIM preferentially associates with the TCR-ζ chain. (A) 3 × 107 Jurkat T cells were lysed in digitonin-containing buffer and subjected to TRIM or CD3ε (OKT-3) immunoprecipitation. Two different monoclonal TRIM Abs (TRIM-4 and TRIM-7) were used for immunoprecipitation. Precipitates were analyzed by sequential anti-CD3ε (a polyclonal rabbit antiserum), anti-ζ (hamster mAb H146), and anti-TRIM (a polyclonal rabbit antiserum) Western blotting. (B) Jurkat cells were transiently transfected with empty vector or with a cDNA construct encoding FLAG-tagged wild-type TRIM. 40 h after transfection, cells were lysed in digitonin-containing buffer and subjected to anti-CD3ε or anti-FLAG immunoprecipitation followed by sequential anti-CD3γ, anti-CD3δ, anti-CD3ε, or TCR-ζ (hamster mAb H146) Western blotting. (C) COS cells were transiently transfected with a cDNA coding for human TCR-ζ together with a construct encoding FLAG-tagged wild-type TRIM (F-TRIM). After 40 h of incubation, double immunofluorescence was applied on the transfected cells using anti-ζ (mAb 6B10.2, green fluorescence, Cy2) and anti-TRIM (the affinity-purified polyclonal antiserum, red fluorescence, Texas red) Abs. (D) A portion of the transfectants was lysed in digitonin-containing buffer and subjected to ζ immunoprecipitation (using mAb 6B10.2) followed by anti-TRIM immunoblotting (using the polyclonal antiserum). (E) Wild-type (WT) Jurkat T cells, as well as the Jurkat variants 18B3 (lacking expression of TCR-α), JBN (lacking expression of TCR-β), and JGN (lacking expression of CD3γ) were assessed for the subcellular localization of TRIM (using mAb TRIM-4) by confocal laserscan microscopy.
To further corroborate this hypothesis, we also analyzed CD3ε or anti-FLAG immunoprecipitates prepared from vector- or F-TRIM–transfected Jurkat T cells by Western blotting for coprecipitation of the individual components of the CD3 complex and the ζ chains. Fig. 5 B demonstrates that, even after overexpression, large amounts of endogenous ζ coprecipitate with TRIM, whereas only low amounts of CD3ε are detectable in the TRIM immunoprecipitates. Interestingly, the TRIM-associated fraction of CD3ε exclusively consisted of CD3γ/ε heterodimers. Although the CD3δ Ab that was used in these experiments had a somewhat weaker reactivity compared with the CD3γ Ab, we did not observe coprecipitation of CD3δ with TRIM in several experiments and even after prolonged overexposure of the autoradiograph. Thus, also when overexpressed in Jurkat T cells, TRIM preferentially interacts with the TCR-ζ chain and to a much lesser extent with the CD3γ/ε heterodimer.
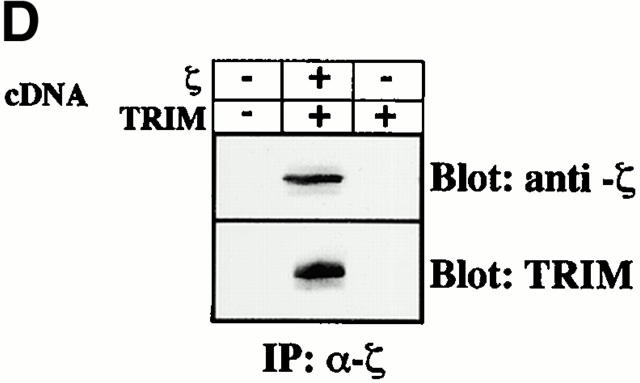
We next determined whether TRIM associates with ζ also in the absence of other TCR components. Fig. 5c and Fig. d, demonstrate that when coexpressed in COS7 cells, TRIM and ζ coprecipitate with each other and colocalize in the transfectants. This suggests that TRIM and ζ can interact with each other in the absence of other components of the TCR. Importantly, the right panel of Fig. 5 C also demonstrates that TRIM does not need additional components of the TCR (including ζ) to be expressed on the cell surface. This was confirmed using a series of well-characterized Jurkat mutant clones, each of which lack one component of the TCR/CD3 complex and thus does not express TCR complexes on the cell surface (Fig. 5 E). In all three Jurkat mutants tested, TRIM showed the same subcellular localization as in wild-type Jurkat T cells, namely a preferential localization at the plasma membrane and an intracellular compartment.
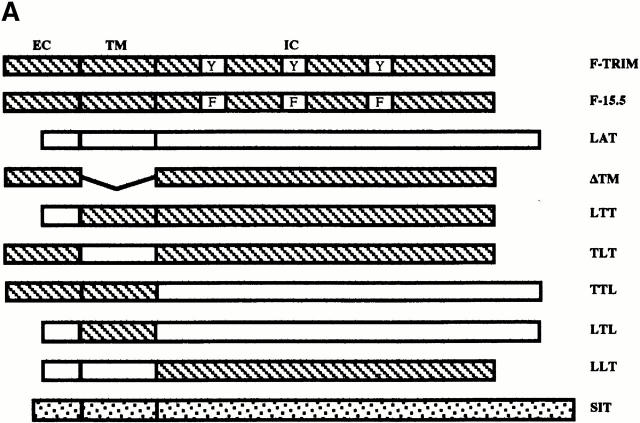
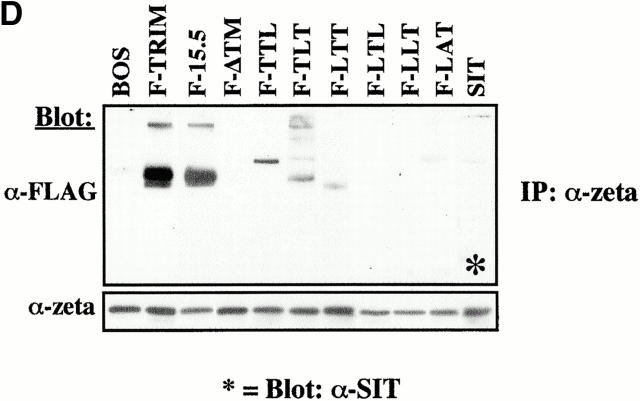
The Molecular Basis for the Association between TRIM and ζ.
To determine the region(s) of TRIM that is/are required for its association with ζ and for upregulation of TCR expression on the cell surface, we overexpressed chimeric FLAG-tagged TRIM molecules (in which individual parts of TRIM were replaced by the corresponding regions of LAT; Fig. 6 A) in Jurkat cells and assessed the expression levels of the TCR on the cell surface (Fig. 6 C) and the association between TRIM and endogenous ζ by anti-FLAG Western blot analysis of ζ immunoprecipitates (Fig. 6 D). These experiments revealed that, as already shown above, F-TRIM strongly associates with ζ and facilitates upregulation of TCR expression. Importantly, almost identical results are obtained when a TRIM mutant, in which the three cytoplasmic tyrosine residues representing the major sites of phosphorylation are mutated to phenylalanine (F-15.5; Fig. 6a Fig. b Fig. c), is overexpressed in Jurkat T cells. This indicates that all three tyrosine residues of TRIM are dispensable for the association between TRIM and ζ and also for TRIM-mediated upregulation of TCR expression.
Figure 6.
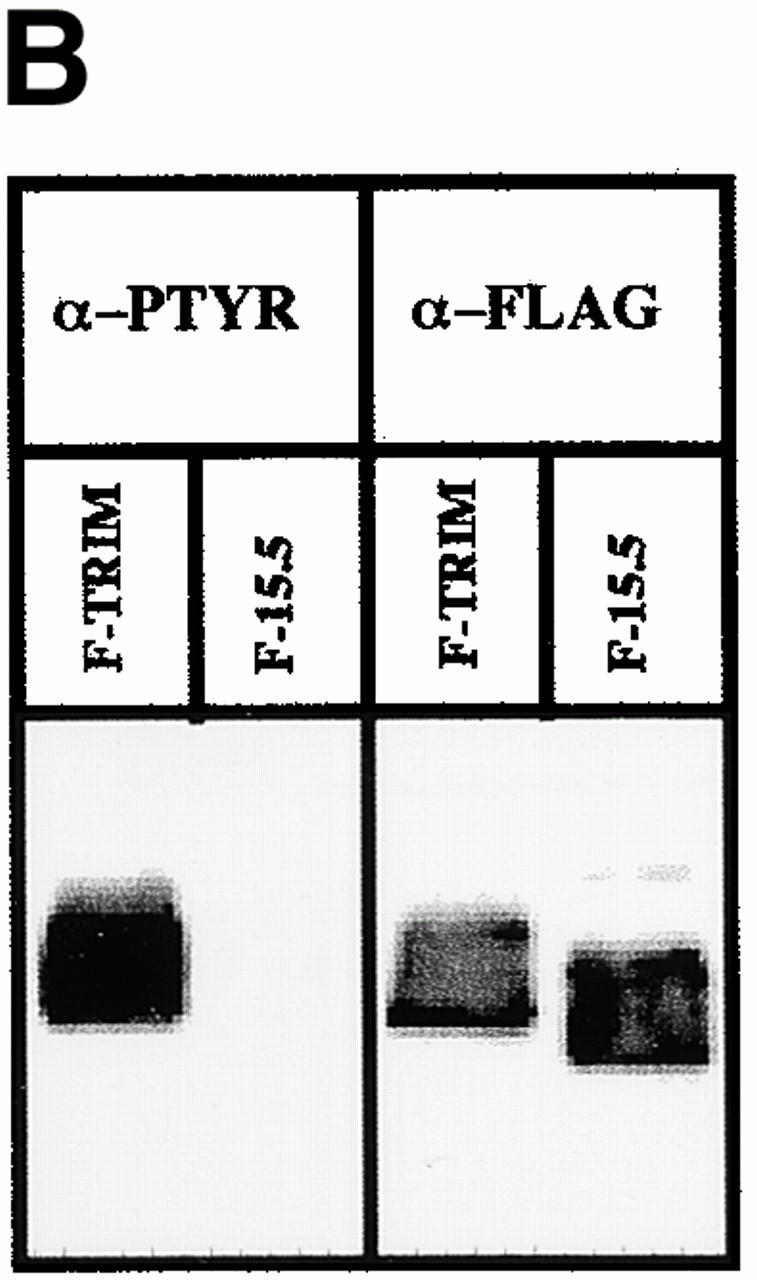
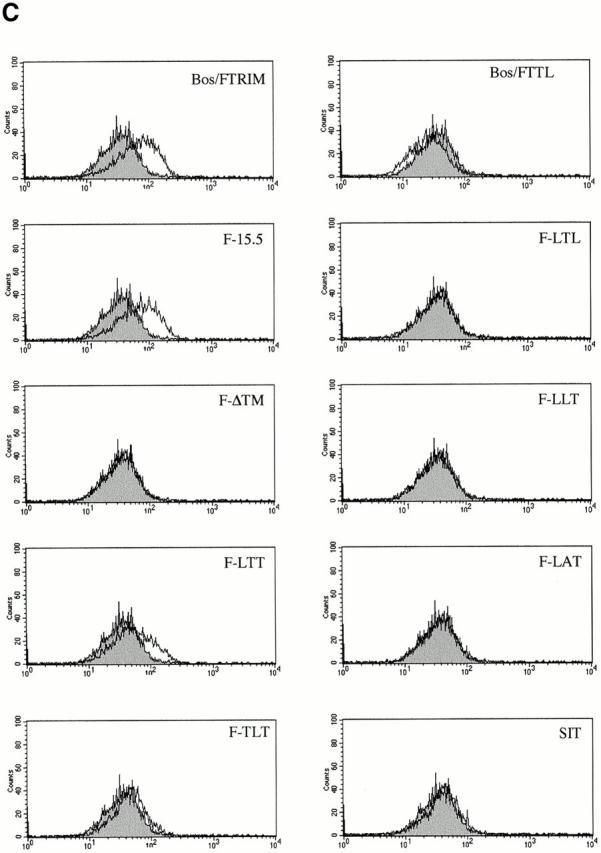
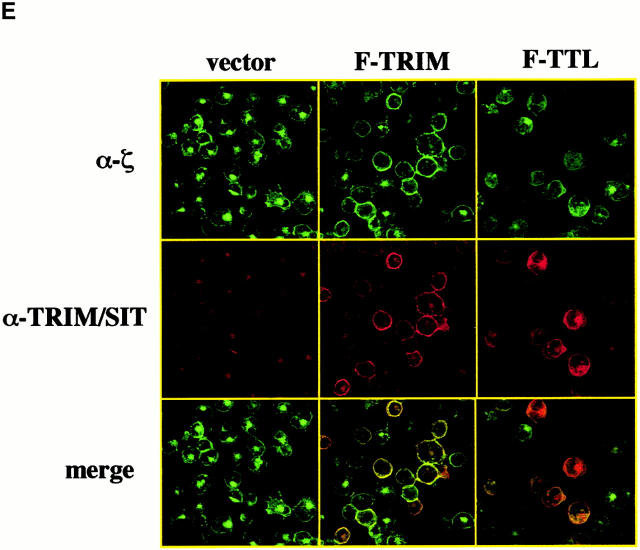
The molecular basis for association between TRIM and TCR-ζ. (A) Scheme depicting the various TRIM/LAT chimeras that were analyzed in this figure. The chimeras are composed of parts TRIM (labeled with T) or LAT (labeled with L). The three-letter code stands for the extracellular (EC), transmembrane (TM), and intracellular (IC) domains, respectively. (B) Jurkat T cells were transfected with cDNAs encoding F-TRIM or the F.15.5 mutant in which the potential tyrosine based signaling motifs (Y63GNL, Y79EQM, and Y110ASL) were mutated to phenylalanine (F). 40 h after transfection, the cells were briefly treated with pervanadate (2′). Then, anti-FLAG immunoprecipitates were prepared which were first analyzed by anti-PTYR immunoblotting. Blots were then stripped and reprobed with anti-FLAG Ab. Note that mutation of the three tyrosines slightly alters the molecular weight of the F-15.5 mutant. (C) cDNA constructs encoding the various TRIM/LAT chimeras shown in A were transfected into Jurkat T cells by electroporation. 40 h later, the expression of CD3ε on the cell surface was determined by flow cytometry. (D) In parallel, cells were lysed in digitonin-containing buffer, and subjected to ζ (mAb 6B10.2) immunoprecipitation and nonreducing SDS-PAGE followed by anti-FLAG immunoblotting. Note that the differences in molecular weight of the TRIM/LAT chimeras results from the different length of the TRIM and LAT extracellular and cytoplasmic domains, respectively. (E) Subcellular localization of ζ and TRIM/ζ colocalization in Jurkat T cells transfected with cDNA encoding F-TRIM or the F-TTL chimera. Note that the TTL chimera is not properly transported to the cell membrane but colocalizes with ζ inside the cell.
However, despite comparable levels of expression (not shown), none of the TRIM/LAT chimeras investigated is capable of mediating upregulation of TCR expression in a similar fashion as wild-type TRIM or the F-15.5 mutant. In fact, when only a single domain of TRIM is replaced by the corresponding portion of LAT (LTT-, TLT-, and TTL chimeras), the ability of TRIM to upregulate TCR expression and to associate with ζ is already largely impaired. Of the three chimeras tested, the TTL chimera even downregulates TCR expression to some extent (Fig. 6 C), although the coprecipitation experiments indicate that this chimera weakly interacts with ζ (Fig. 6 D). Analysis of the subcellular localization of the TTL chimera revealed that it cannot be properly transported to the plasma membrane and is to a large extent retained in the cytoplasm (Fig. 6 E). Apparently, its weak association with ζ is sufficient to prevent some ζ chains to be transported to the plasma membrane, thus resulting in downregulation of TCR expression. It is also interesting to note that in several experiments we observed that the LTT chimera associates with ζ as a dimeric molecule (Fig. 6 D), although this chimera does not possess the cysteine residue (C7) that normally confers TRIM dimerization (thus, LTT would be expected to run only as a monomeric molecule of ∼30 kD even under nonreducing conditions). For reasons that will be discussed below, we think that the ζ association of the LTT chimera as a dimer is due to a particular property of the TRIM transmembrane domain.
The interaction with ζ is completely lost when TRIM chimeras are expressed in which two domains of TRIM are replaced by LAT (LTL- and LLT chimeras). Consequently, these chimeras do not influence the expression levels of the TCR on the cell surface at all. Similarly, the transmembrane adaptor proteins SIT and LAT do not associate with ζ and thus do not alter TCR expression.
Interestingly, we found that a TRIM mutant in which the transmembrane domain of TRIM was deleted (F-ΔTM) completely fails to associate with ζ and to alter TCR expression (Fig. 6c and Fig. d). This finding somehow contradicted the results that were obtained with the TLT-, the LTT-, and the TTL chimeras, namely that the presence of two domains of TRIM allows a weak interaction with ζ. However, confocal laserscanning analysis revealed that the ΔTM mutant diffusely localizes within the cell, including the nuclear compartment (data not shown). This particular subcellular localization might explain its inability to associate with ζ. Nevertheless, from the data shown in Fig. 6 we draw the following conclusions: first, upregulation of TCR expression is a particular property of TRIM and cannot be mediated by other transmembrane adaptor proteins such as SIT or LAT. Second, in order to associate with ζ and to facilitate TCR expression on the cell surface, all three domains of TRIM are required.
TCR Expression in Jurkat Cells Stably Overexpressing TRIM.
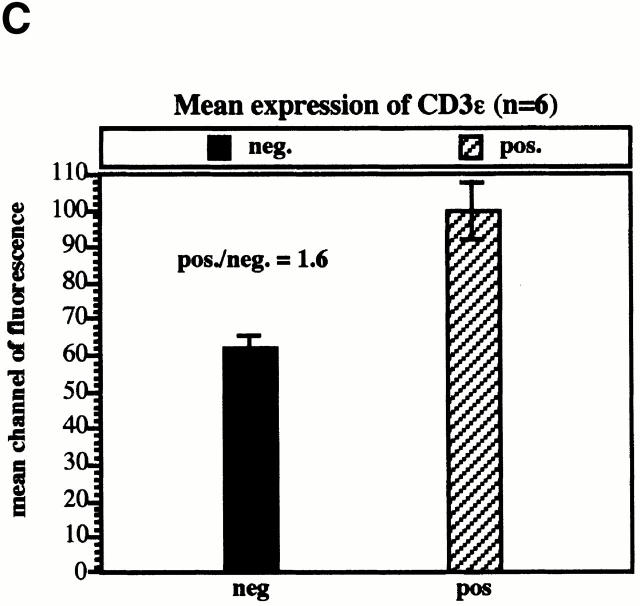
The experiments described above were performed using transiently transfected Jurkat T cells. To exclude the possibility that upregulation of TCR expression after overexpression of F-TRIM could only be observed in transiently transfected Jurkat T cells, we established a series of transfectants stably overexpressing F-TRIM. Fig. 7 A demonstrates that a representative Jurkat clone overexpressing F-TRIM also expresses higher amounts of TCR and CD3ε on the cell surface than a vector transfectant. Confocal laser scan analysis further revealed that also in this cell line the majority of endogenous ζ is localized at the plasma membrane (Fig. 7 B). Identical results were obtained for six additional clones of each group. On average, TRIM transfectants expressed 1.6 times more TCRs on the cell surface compared with the vector transfectants (Fig. 7 C). However, it is important to note that after prolonged periods of cell culture, the transfectants downregulated TCR expression to a certain extent. The molecular basis of this counterregulation is not known at present.
Figure 7.
Characterization of Jurkat clones stably overexpressing TRIM. (A) and (B) CD3ε and TCR-α/β expression (A) and subcellular localization of ζ (B) in a representative stably transfected Jurkat T clone (pos.; clone 2D1) compared with a transfectant not expressing TRIM (neg.; clone 2C3). (C) Mean channels of CD3ζ expression in six vector transfectants and in six TRIM transfectants as determined by flow cytometry. (D) Enhanced TCR-mediated Ca2+ flux in Jurkat T cells stably expressing F-TRIM and the F-15.5 mutant.
Enhanced TCR-mediated Ca2+ Flux in Jurkat T Cells Stably Expressing F-TRIM.
Next, it was important to determine whether overexpression of TRIM and the resulting upregulation of TCR expression would alter the signaling capacity of the TCR. To assess this, we compared TCR-mediated Ca2+ fluxes in Jurkat clones stably overexpressing F-TRIM with those obtained after TCR triggering of vector transfectants. Fig. 7 D indicates that TCR triggering of the stably transfected clones resulted in an approximately twofold higher level of Ca2+ compared with the vector transfectants. However, the amplification of TCR-mediated Ca2+ fluxes mediated by overexpression of TRIM was not influenced by Tyr to Phe substitutions, as almost identical Ca2+ responses were observed in the F-TRIM transfected cells compared with cells overexpressing the F-15.5 mutant. These data also suggest that the capacity of TRIM to enhance TCR-mediated signal transduction does not depend on the expression of the tyrosine-based signaling motifs in its cytoplasmic domain.
Discussion
In this report we provide further evidence that the recently cloned transmembrane adaptor protein TRIM represents a novel integral component of the TCR/CD3/ζ complex 19. Thus, besides coprecipitating with the TCR, our results indicate that TRIM but not other signaling molecules such as LPAP and SKAP-HOM specifically cocaps with the TCR (Fig. 1). Biochemical analysis of TRIM immunoprecipitates prepared from Jurkat T cells further indicated that the major component of the TCR complex that is present in TRIM immunoprecipitates is the TCR-ζ chain (Fig. 5 A). From these data, as well as from the observation that the preferential association between TRIM and ζ was also observed after transient overexpression of TRIM (Fig. 5 B), we conclude that in mature T cells, TRIM preferentially interacts with the TCR via TCR-ζ. The association between TRIM and ζ is most likely direct and does not depend on the expression of other components of the CD3 complex, as it also occurs in COS cells (Fig. 5c and Fig. d). However, we certainly cannot exclude the possibility that COS cells express an unknown molecule that facilitates the association between TRIM and ζ. In addition, it is unknown at present whether TRIM associates with the ζ chain already in the cytosol or only after the complete TCR/CD3/ζ complex has reached the cell surface. Clearly, additional studies are required to elucidate this question. However, confocal laserscanning analysis of COS7 overexpressing TRIM or of Jurkat variants lacking cell surface expression of the TCR (Fig. 5c and Fig. e) clearly indicated that TRIM does not need additional components of the TCR/CD3/ζ complex to be transported to the cell surface.
The most striking finding of our work is that transient and stable overexpression of TRIM in Jurkat T cells induces an approximately twofold higher expression of the TCR/CD3 complex compared with wild-type Jurkat T cells (Fig. 2, Fig. 6, and Fig. 7) and that these cells responded towards TCR triggering with elevated levels of Ca2+ mobilization. Comparative confocal laser scanning analysis of wild-type Jurkat T cells and TRIM transfectants indicated an altered subcellular localization of the TCR-ζ chain in Jurkat cells overexpressing TRIM (Fig. 3b and Fig. c, and Fig. 7 D). Thus, in accordance with a recent publication 16, we observed that in wild-type Jurkat T cells, only half of endogenous ζ localizes at the plasma membrane, whereas the other half is retained in cytoplasm. In marked contrast, upon overexpression of TRIM, the majority of endogenous ζ localizes at the plasma membrane (Fig. 3b and Fig. c). Upregulation of TCR expression by TRIM and enhanced presence of ζ at the plasma membrane requires a physical association between TRIM and ζ. This conclusion is drawn from experiments which demonstrated that overexpression of TRIM chimeras that do not associate with ζ are also unable to alter TCR expression (Fig. 6). Surprisingly, we observed that the association between TRIM and ζ apparently involves all three domains of TRIM. Thus, replacement of any portion of TRIM by the corresponding part of the transmembrane adaptor protein LAT produced a TRIM chimera, which had largely lost its ability to associate with ζ (and to upregulate TCR expression).
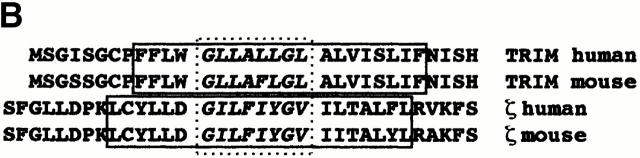
With regard to the extracellular and the transmembrane domains of TRIM, this finding is not surprising. Bolliger et al. have recently identified a dimerization motif (Fig. 8) within the transmembrane domain of ζ, which has first been described in glycophorin A, a cell surface receptor expressed on erythrocytes 32. This dimerization motif allows formation of SDS-stable protein dimers in the absence of disulfide bonds linking the two polypeptide chains. The integrity of the dimerization motif in the transmembrane domain of ζ (the amino acid sequence is: GLxxxxGVxxT) seems to be necessary to facilitate reexpression of the TCR/CD3 complex in a ζ-deficient T cell hybridoma 32. Importantly, a similar dimerization motif as in ζ is also present in the transmembrane domain of TRIM (GLxxxxGLxxV). The assumption that this dimerization motif might be of functional relevance is supported by our repeated observation that treatment of TRIM with reducing agents alone (e.g., 2-ME) is not sufficient to generate its monomeric form even in SDS-containing buffers (unpublished data). Moreover, a chimeric TRIM molecule in which the extracellular domain of TRIM (which normally carries the cysteine residue (C7) that mediates TRIM dimerization via the S-S bridge) was replaced by the corresponding part of the transmembrane adaptor protein LAT still can associate with ζ as an SDS-stable dimer (Fig. 6 D).
Figure 8.
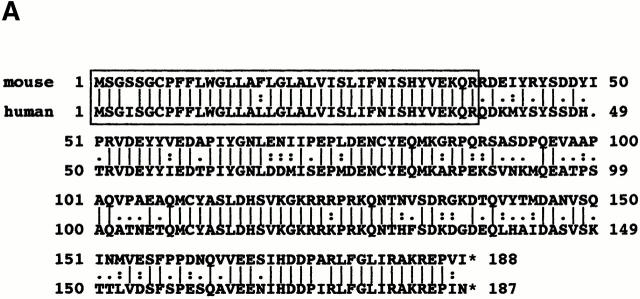
(A) Comparison between human and mouse TRIM indica-ting a strong conservation of the extracellular domain, the transmembrane region, and the first 10 cytoplasmic amino acids (boxed). (B) Similarity between the putative dimerization motifs in the transmembrane domains of ζ, and TRIM. The transmembrane domains are indicated by solid lines, and the putative dimerization motif by a dotted box. The GenBank/EMBL/DDBJ accession no. for mouse TRIM is AJ29769.
These data could indicate that TRIM can only associate with ζ as a dimeric molecule, and that TRIM dimerization is mediated in two different ways, namely (a) the dimerization motif within the transmembrane domain and (b) the extracellular domain of TRIM including C7. With regard to the latter point, it is important to note that preliminary data obtained in our laboratory indicate that mutation of C7 generates a monomeric TRIM molecule, which is no longer able to associate with ζ and consequently does not alter the expression level of the TCR. Moreover, it is important to note that recent data also suggest that the expression and function of the TCR is not only dependent on the integrity of the transmembrane domain of ζ but also on its extracellular domain 33 34. Whether or not the association between TRIM and ζ contributes to this finding is unclear at present but represents an intriguing possibility.
In contrast to a requirement of both the extracellular and transmembrane domains of TRIM to allow a stable interaction between TRIM and ζ, we were surprised to observe that this interaction apparently also involves the cytoplasmic domain of TRIM (Fig. 6c and Fig. d). However, a comparison of the mouse and human TRIM amino acid sequences indicates that the extracellular domain, the transmembrane domain, and the first 11 cytoplasmic amino acids directly downstream of the transmembrane domain are highly conserved between the two species. In marked contrast, the remaining parts of TRIM show a much lesser conservation (95 vs. 62%, respectively; Fig. 8). This could indicate that the membrane proximal cytoplasmic part of TRIM is also involved in its association with ζ. However, our attempts to clarify this question directly were so far unsuccessful because truncated TRIM molecules in which the majority of the cytoplasmic domain was deleted could not be expressed stably in Jurkat T cells.
Analysis of the fate of externally labeled TCR components by confocal laserscanning analysis revealed that TRIM upregulates TCR expression on the cell surface by inhibiting spontaneous TCR internalization (Fig. 4). The precise mechanism underlying this phenomenon is not yet clear. However, it has been proposed that spontaneous internalization of the TCR/CD3/ζ complex in nonactivated T cells is mainly regulated by a motif within the cytoplasmic domain of CD3γ that becomes phosphorylated by protein kinase C 30 31 35. Moreover, it has been reported that CD16/CD3γ chimeras capable of associating with TCR-ζ can be stably expressed on the cell surface and have a low rate of spontaneous internalization whereas CD4/CD3γ chimeras, which cannot interact with ζ are rapidly internalized resulting in low levels of expression on the cell surface 36. These findings collectively indicate that, via its association with the TCR, the ζ chain is capable of influencing the function of the internalization motif within CD3γ and that ζ is involved in regulating cycling of the TCR.
Therefore, one possibility to explain upregulation of TCR expression after overexpression of TRIM would be that TRIM inhibits TCR internalization by enhancing the amounts of plasma membrane–associated ζ, which then alters the function of the CD3γ internalization motif. Whether this is due to masking the phosphorylation site within the motif, to displacement of the serine kinase phosphorylating CD3γ, or to recruitment of a serine phosphatase that dephosphorylates the motif, requires further analysis. However, our data suggest that, by altering the expression levels of TRIM, T cells could regulate the levels of cell surface–expressed TCR molecules and, via this mechanism, T cell responsiveness towards externally applied stimuli.
Previously we have reported that after phosphorylation by src kinases, TRIM recruits the regulatory p85 subunit of PI3 kinase via a phosphorylated YxxM motif 19. In addition, most recent experiments performed in our laboratory revealed that, in addition to PI3 kinase, tyrosine-phosphorylated TRIM associates with at least two unknown polypeptides of 43 and 50 kD (unpublished data). It is tempting to speculate that the complex between TRIM, PI3 kinase, and the two unknown polypeptides serves a signaling function during T cell activation. However, so far we have not been able to dissect which signaling pathway is initiated/altered by TRIM. Thus, the data shown in this report demonstrated that a TRIM mutant in which all tyrosine residues are mutated to phenylalanine (F-15.5) has exactly the same properties as wild-type TRIM (at least with regard to regulation of TCR expression [Fig. 6], inhibition of spontaneous TCR internalization [not shown], and TCR-mediated induction of Ca2+ fluxes [Fig. 7]). Moreover, analysis of Jurkat T cells that stably overexpress the F-15.5 mutant revealed that these cells are indistinguishable from Jurkat T cells overexpressing wild-type TRIM. This applies, for example, for TCR-mediated induction of mRNA expression coding for several cytokines or upregulation of CD69 expression after TCR triggering. Thus, additional studies are required to elucidate the signaling function of the cytoplasmic domain of TRIM.
Acknowledgments
We would like to thank Drs. K. Campbell, C. Geisler, R. Kurrle, E. Palmer, M. Peter, E. Reinherz, and A. Weiss for kindly providing our laboratory with reagents.
This work was supported by the Deutsche Forschungsgemeinschaft grants SCHR 533/4-4, an internal research grant of the Medical Faculty of the University of Heidelberg (both to B. Schraven), and by the Wellcome Trust, UK (to P. Isomaki and A.P. Cope). J. Dietrich is a recipient of a postdoctoral fellowship from the Carlsberg foundation.
Footnotes
Abbreviations used in this paper: BFA, brefeldin A; LAT, linker for activation of T cells; SIT, SHP2-interacting transmembrane adaptor protein; SKAP-HOM, Src kinase–associated phosphoprotein homologue; TRIM, TCR-interacting molecule.
References
- Weiss A., Littman D.R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Meuer S.C., Acuto O., Hussey R.E., Hodgdon J.C., Fitzgerald K.A., Schlossman S.F., Reinherz E.L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983;303:808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S.C., Cooper D.A., Hodgdon J.C., Hussey R.E., Fitzgerald K.A., Schlossmann S.F., Reinherz E.L. Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes. Science. 1983;222:1239–1242. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987;238:1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Wegener A.-M.K., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992;68:83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Irving B.A., Weiss A. The cytoplasmic domain of the T cell receptor ζ-chain is sufficient to couple to receptor-associated signal transduction pathway. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Irving B.A., Chan A.C., Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J. Exp. Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashima M., Irving B.A., Van Oers N.S.C., Chan A.C., Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R.D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 ε. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Minami Y., Weissman A.M., Samelson L.E., Klausner R.D. Building a multichain receptorsynthesis, degradation and assembly of the T-cell antigen receptor. Proc. Natl. Acad. Sci. USA. 1987;84:2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J.J., Bonifacino J.S., Lippincott-Schwartz J., Weissman A.M., Saito T., Klausner R.D., Ashwell J.D. Failure to synthesize the T cell CD3-ζ chainstructure and function of a partial T cell receptor complex. Cell. 1988;52:85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Chen C., Bonifacino J.S., Yuan L.C., Klausner R.D. Selective degradation of T cell antigen receptor chains retained in a pre-golgi compartment. J. Cell Biol. 1988;107:2149–2161. doi: 10.1083/jcb.107.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Suzuki C.K., Lippincott-Schwartz J., Weissman A.M., Klausner R.D. Pre-golgi degradation of newly synthesized T-cell antigen receptor chainsintrinsic sensitivity and the role of subunit assembly. J. Cell Biol. 1989;109:73–83. doi: 10.1083/jcb.109.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Carson G.R., Concino M., Ahmed A., Terhorst C. The γ and ε subunits of the CD3 complex inhibit pre-golgi degradation of newly synthesized T cell antigen receptors. J. Cell Biol. 1990;110:973–986. doi: 10.1083/jcb.110.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppa J.B., Ploegh H. In vitro translation and assembly of a complete T cell receptor-CD3 complex. J. Exp. Med. 1997;186:393–403. doi: 10.1084/jem.186.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Kastrup J., Laurirtsen J.P.H., Menné C., von Bülow F., Geisler C. TCRζ is transported to and retained in the Golgi apparatus independently of other TCR chainsimplications for TCR assembly. Eur. J. Immunol. 1999;29:1719–1728. doi: 10.1002/(SICI)1521-4141(199905)29:05<1719::AID-IMMU1719>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ono S., Ohno H., Saito T. Rapid turnover of the CD3 zeta chain independent of the TCR-CD3 complex in normal T cells. Immunity. 1995;2:639–644. doi: 10.1016/1074-7613(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Alcover A., Alarcón B. Internalization and intracellular fate of TCR-CD3 complexes. Crit. Rev. Immunol. 2000;20:325–346. [PubMed] [Google Scholar]
- Bruyns E., Marie-Cardine A., Kirchgessner H., Sagolla K., Shevchenko A., Mann M., Autschbach F., Bensussan A., Meuer S., Schraven B. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-ζ complex, recruits intracellular signaling proteins to the plasma membrane. J. Exp. Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraven B., Roux M., Hutmacher B., Meuer S.C. Triggering of the alternative pathway of human T cell activation involves members of the T200 family of glycoproteins. Eur. J. Immunol. 1989;19:397–403. doi: 10.1002/eji.1830190226. [DOI] [PubMed] [Google Scholar]
- Bruyns E., Kirchgessner H., Meuer S., Schraven B. Biochemical analysis of the CD45-p56lck complex in Jurkat T cells lacking expression of lymphocyte phosphatase associated phosphoprotein. Int. Immunol. 1998;10:185–194. doi: 10.1093/intimm/10.2.185. [DOI] [PubMed] [Google Scholar]
- Bäckström B.T., Rubin B., Peter A., Tiefenthaler G., Palmer E. T cell receptor α-chain is required for protein kinase C-mediated down-regulation, but not for signaling. Eur. J. Immunol. 1997;27:1433–1441. doi: 10.1002/eji.1830270621. [DOI] [PubMed] [Google Scholar]
- Schraven B., Schoenhaut D., Bruyns E., Koretzky G., Eckerskorn C., Wallich R., Kirchgessner H., Sakorafas P., Labkovsky B., Ratnofsky S., Meuer S. LPAP, a novel 32-kDa phosphoprotein that interacts with CD45 in human lymphocytes. J. Biol. Chem. 1994;269:29102–29111. [PubMed] [Google Scholar]
- Marie-Cardine A., Verhagen A.M., Eckerskorn C., Schraven B. SKAP-HOM, a novel adaptor protein homologous to the Fyn-associated protein SKAP55. FEBS Lett. 1998;435:55–60. doi: 10.1016/s0014-5793(98)01040-0. [DOI] [PubMed] [Google Scholar]
- Liang Q., Schürmann G., Betzler M., Meuer S.C. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology. 1991;101:1529–1536. doi: 10.1016/0016-5085(91)90388-2. [DOI] [PubMed] [Google Scholar]
- Schraven B., Wild M., Kirchgessner H., Siebert B., Wallich R., Henning S., Samstag Y., Meuer S.C. Alterations of CD2 association with T cell receptor signaling molecules in “CD2 unresponsive” human T lymphocytes. Eur. J. Immunol. 1993;23:119–123. doi: 10.1002/eji.1830230119. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R.D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lauritsen J.P., Christensen M.D., Dietrich J., Kastrup J., Odum N., Geisler C. Two distinct pathways exist for down-regulation of the TCR. J. Immunol. 1998;161:260–267. [PubMed] [Google Scholar]
- Liu H., Rhodes M., Wiest D., Vignali D. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- Niedergang F., San José E., Rubin B., Alarcón B., Dautry-Varsat A., Alcover A. Differential cytosolic tail dependence and intracellular fate of T-cell receptors internalized upon activation with superantigens and phorbol ester. Res. Immunol. 1997;148:225–239. doi: 10.1016/s0923-2494(97)80865-6. [DOI] [PubMed] [Google Scholar]
- Dietrich J., Bäckström T., Lauritsen P.H., Kastrup J., Christensen M.D., von Bülow F., Palmer E., Geisler C. The phosphorylation state of CD3γ influences T-cell responsiveness and controls T-cell receptor cycling. J. Biol. Chem. 1998;273:24232–24238. doi: 10.1074/jbc.273.37.24232. [DOI] [PubMed] [Google Scholar]
- Bolliger L., Johansson B. Identification and functional characterization of the ζ-chain dimerization motif for TCR surface expression. J. Immunol. 1999;163:3867–3876. [PubMed] [Google Scholar]
- Bolliger L., Johannson B., Palmer E. The short extracellular domain of the T cell receptor zeta chain is involved in assembly and signal transduction. Mol. Immunol. 1998;34:819–827. doi: 10.1016/s0161-5890(97)00122-3. [DOI] [PubMed] [Google Scholar]
- Johannson B., Palmer E., Bolliger L. The extracellular domain of the zeta-chain is essential for TCR function. J. Immunol. 1999;162:878–885. [PubMed] [Google Scholar]
- Dietrich J., Hou X., Wegener A.-M., Geisler C. CD3γ contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Geisler C. T-cell receptor ζ allows stable expression of receptors containing the CD3γ leucine-based receptor-sorting motif. J. Biol. Chem. 1998;273:26281–26284. doi: 10.1074/jbc.273.41.26281. [DOI] [PubMed] [Google Scholar]