Abstract
Behavioral sex pheromone responsiveness in the male moth Agrotis ipsilon was previously shown to be controlled by juvenile hormone (JH). However, this morphogenetic hormone did not change the sensitivity of antennae to sex pheromones. To analyze the possible involvement of JH in the central integration of the female-produced sex pheromone, we investigated the pheromone response of olfactory antennal lobe (AL) interneurons in male A. ipsilon as a function of age and JH status by using intracellular recordings. When the antennae were stimulated with the sex pheromone blend, the sensitivity of olfactory AL neurons increased with age, as does the JH-dependent behavioral and physiological development of A. ipsilon males. Furthermore, males surgically deprived of JH showed a significant decrease in the sensitivity of the AL neurons. JH injection in operated or in young males restored or induced, respectively, a high sensitivity of the AL neurons. JH seems likely to be involved in the plasticity of the adult insect brain by modulating the central nervous processing of olfactory information, thus allowing mate recognition and reproduction at the optimal time.
In adult insects, juvenile hormone (JH) has been shown to play a role in many physiological processes, but its effects on the nervous system have been little studied (1). JH was shown to be involved in central nervous regulation of phonotaxis (2) and in neurogenesis in potential oviposition steering brain areas in the house cricket (3). JH also plays a role in the honeybee related to behavioral maturation and brain structure (see ref. 4).
In the male moth, Agrotis ipsilon, JH was shown to be involved in behavioral and physiological processes linked with reproduction. Males of this species do not respond to the sex pheromone when they are newly emerged (5). Their behavioral responsiveness toward the female sex pheromone increases with age coinciding with an increasing JH biosynthesis activity (5, 6). Moreover, the presence of the corpora allata, the retrocerebral gland producing JH, is required for the male to respond to the sex pheromone (5). Allatectomy suppressed male responsiveness, and JH was able to restore the sexual behavior of the operated males (7). Electrophysiological studies revealed that the antennal sensory system of newly emerged and allatectomized males was as sensitive as in old intact males, thus suggesting that the target of JH to influence the pheromone responsiveness of the male moth is the central olfactory system (5).
In moths, olfactory information is detected by antennal receptor neurons and integrated by interneurons in the antennal lobe (AL), which is part of the deutocerebrum. In males, the central nervous processing of female-produced sex pheromone occurs in male-specific glomeruli, called the macroglomerular complex within the AL (8). In this study, we examined the response characteristics of pheromone-responding AL interneurons in A. ipsilon males of different ages and hormonal status, by using intracellular recordings while stimulating the antennae with various concentrations of a synthetic sex pheromone blend. The results indicate that the sensitivity of AL interneurons to the pheromone in the male moth, A. ipsilon, is controlled by JH.
MATERIALS AND METHODS
Preparation.
The colony of A. ipsilon originated from moths caught in southern France. Adults from field catches are introduced into the colony each spring. Larvae of the black cutworm were reared on an artificial diet (9) and maintained in individual plastic cups until pupation under a 16:8 hour light/dark photoperiod and at 21 ± 1°C. Pupae containers were observed each day for newly emerged adults. Adults were held in plastic boxes and had access to 20% sucrose solution. For electrophysiological experiments, a male moth was mounted head-up in a plastic pipette (Finnpipette; Labsystems, Helsinki, Finland) tip. The cuticle, tracheal sacs, and muscles were carefully removed to expose the brain. The AL was manually desheathed to facilitate microelectrode penetration. A flow system perfused the head cavity with saline (10).
Intracellular Recordings and Stimulation.
Intracellular recordings were performed according to standard methods (10). Pheromone-sensitive AL interneurons were penetrated in the male-specific macroglomerular complex (11). A glass microelectrode filled with 2 M KCl was used as the recording electrode. The antennae of the moths were stimulated with either a synthetic three-component pheromone blend or clean air as a control. To separate plant-specific neurons from our study, a common green leaf volatile, (E)-2-hexenal, also was used as a stimulus. The pheromone blend was a mixture of (Z)-7-dodecenyl acetate (Z7-12:OAc), (Z)-9-tetradecenyl acetate (Z9-14:OAc), and (Z)-11-hexadecenyl acetate (Z11-16:OAc) at a ratio of 4:1:4, as used in field trapping experiments (12). The pheromone blend was applied at amounts between 1 pg and 100 ng on a filter paper in a Pasteur pipette. A 500-msec pulse of air was sent through the pipette containing either clean air or an odorant by means of a stimulatory device (Syntech, Hilversum, The Netherlands) (13). Medium-dose odor stimuli were always given first, otherwise stimuli were chosen at random, interspersed by at least 10 sec. The physiological data were stored on a Vetter video recorder and visualized on a Tektronix digital oscilloscope.
Data Analysis.
Spikes were counted manually from the storage oscilloscope. Net-spikes were calculated from the number of spikes during a 600-msec period after the stimulus minus the number of spikes counted during the preceding 600 msec. The net number of spikes produced in response to the blank stimulus was subtracted from the net number of spikes produced in response to an odor stimulus to quantify the response to a specific stimulus (14). A neuron was classified to respond to a stimulus when the odor response exceeded the blank response by at least 10%. For statistical treatment, three groups of neurons were created: one with high threshold (neurons with thresholds of 100 ng and 10 ng), one with medium threshold (1 ng and 0.1 ng), and another with low threshold (0.01 ng and 1 pg). Nonresponding neurons and neurons responding exclusively to the plant odor were not taken into account for the statistical analysis. A G test was performed comparing the three groups of neurons pairwise for the different ages and treatments.
Surgical and Injection Treatments.
Surgical removal of the corpora allata was performed on day 3 of adult life (15). Three-day-old allatectomized males were injected with 2 μl of olive oil or with 10 μg of JH-III dissolved in 2 μl of olive oil (7). Newly emerged males were injected with 10 μg of JH dissolved in 2 μl of olive oil. Intracellular recordings were in all cases performed 2 days after the treatment.
RESULTS
Response Characteristics of AL Neurons.
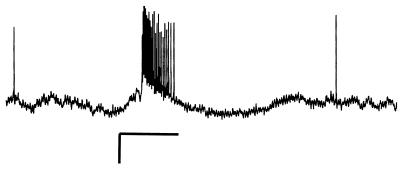
Nine of 441 studied neurons responded only to the plant odor E-2-hexenal and were excluded from data analysis. Responses of spontaneously active AL interneurons to sex pheromone stimuli consisted of an excitatory period followed by an inhibitory period (Fig. 1). The vast majority of all AL neurons responded to the respective lowest above-threshold stimulus with a spike frequency between 70 and 90 Hz, independent of the age or treatment of the males and independent of the threshold. The spike frequency increased in a dose-dependent manner with increasing stimulus amounts (the number of spikes increased between 15 and 30 Hz from threshold to the next concentration). The lowest stimulus amount tested (1 pg) never elicited a spike frequency higher than 80 Hz, which gives a hint that a 1 pg of stimulus is close to the absolute detection threshold of AL neurons in A. ipsilon. The responses of AL neurons to the pheromone blend did not differ between males of different age or treatment apart from the response threshold. Constant penetration area for the electrode and very uniform spontaneous activity and response patterns in our recordings make it likely that we mainly recorded from MGC projection neurons. Exemplary intracellular fills of electrophysiologically characterized neurons also revealed MGC projection neurons (data not shown).
Figure 1.
Typical response of an AL interneuron to the female sex pheromone blend in A. ipsilon. Horizontal scale bar marks the stimulation (500 ms), vertical scale bar = 10 mV.
Effects of Age on the Sensitivity of AL Neurons.
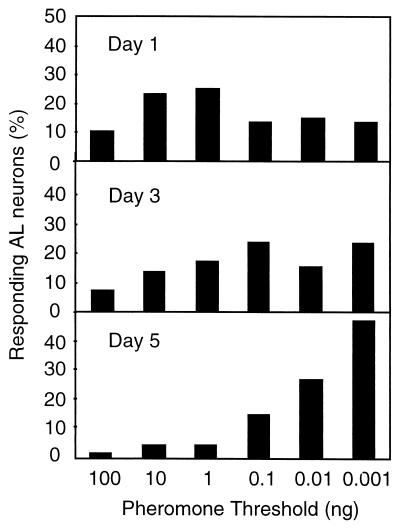
Eighty-eight AL interneurons in 14 1-day-old males, 63 neurons in 12 3-day-old males, and 69 neurons in 18 5-day-old males were tested, of which 28, 4, and 2 neurons, respectively, did not respond to any stimuli. The percentage of highly sensitive neurons in the AL increased with age, whereas the proportion of neurons with a high threshold decreased clearly with age (Fig. 2). The sensitivity increased significantly between AL interneurons of 1-day-old and 5-day-old males (G = 31.05, P ≤ 0.001, df = 2) and between AL interneurons of 3-day-old and 5-day-old males (G = 17.22, P ≤ 0.001, df = 2), but not between 1-day-old and 3-day-old males (G = 2.93, df = 2). From these results we conclude that the sensitivity of the AL interneurons involved in central nervous processing of the sex pheromone in A. ipsilon males is age-dependent.
Figure 2.
Age-dependent sensitivity of AL interneurons in A. ipsilon males to the sex pheromone blend. The proportion of responding neurons with a certain threshold in males of different ages is represented in the diagrams. Day 1, 1-day-old males, n = 60 neurons. Day 3, 3-day-old males, n = 59 neurons. Day 5, 5-day-old males, n = 67 neurons.
Effect of JH Treatments on the Sensitivity of AL Neurons.
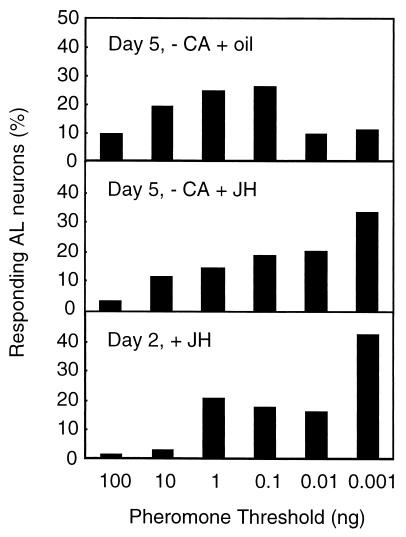
The involvement of JH in AL interneuron sensitivity was first tested in JH-deprived 5-day-old males. Eighty AL interneurons in 15 allatectomized males were characterized physiologically, of which 7 did not respond to any of the stimuli. The responding neurons showed a very low sensitivity to the female sex pheromone blend, not significantly different from that of 1-day-old males (G = 2.17, df = 2) (Fig. 3). To check whether it was possible to restore high sensitivity, we injected JH into 20 3-day-old allatectomized males and performed intracellular recordings of 72 AL interneurons 2 days later. Of these neurons, 2 did not respond to any stimuli. JH injection induced a high sensitivity, although it did not reach the level of 5-day-old control males completely (Fig. 3). The sensitivity of AL interneurons in allatectomized males injected with JH was significantly higher than that of allatectomized males (lacking JH) (G = 16.83, P ≤ 0.001, df = 2). Both experiments show that JH is involved in the regulation of AL interneuron sensitivity.
Figure 3.
Effect of JH on the sensitivity of AL interneurons in A. ipsilon males to the sex pheromone blend. The proportion of responding neurons with a certain threshold in treated males is represented in the diagrams. Males were tested 2 days after the treatment. Day 5, −CA + oil, 3-day-old allatectomized males injected with olive oil, n = 73 neurons. Day 5, −CA + JH, 3-day-old allatectomized males injected with JH, n = 70 neurons. Day 2, + JH, 0-day-old control males injected with JH, n = 69 neurons.
To investigate whether the hormone acts on the olfactory system in nonmature males, we injected JH into 14 newly emerged males and tested the response characteristics of 69 AL interneurons 2 days later. There were no nonresponding neurons. A high sensitivity in response to the synthetic pheromone blend was observed after injection of JH (Fig. 3). The sensitivity of AL interneurons in JH-treated young males was statistically different from that of 1-day-old and 3-day-old males (G = 23.17, P ≤ 0.001, df = 2 and G = 9.72, P ≤ 0.01, df = 2, respectively), and was not statistically different from that of 5-day-old males (G = 5.64, df = 2) (see Fig. 2). These last results show that the presence of JH is necessary for AL neurons to be sensitive and that JH modulates activity in the AL rapidly also in nonmature males.
DISCUSSION
In the present study, we show the occurrence of plasticity of central olfactory neuron responses related to the age and to the JH level in a male moth. Whereas it was shown in different insect species that the sensitivity of antennal responses to odors increases with age (16, 17, 18), the antennal system of male A. ipsilon is fully functional at emergence and does not change sensitivity with age (5). The age-dependent sensitivity changes of AL neurons in A. ipsilon coincide with that of behavioral pheromone responsiveness and with that of the JH biosynthetic activity. The low sensitivity of AL neurons in JH-deprived mature males, found in the present study, is in good correlation with the inhibition of pheromone responsiveness of allatectomized males as demonstrated in wind tunnel experiments (5). Our results indicate that JH controls behavioral responsiveness to female-produced sex pheromone by acting on the sensitivity of central olfactory neurons. Of the 441 tested neurons in control and treated males, 43 did not respond to any of the stimuli. Although we cannot be sure of the reason for the neurons not responding, most of the nonresponding neurons were found in young immature control males and in mature allatectomized males, in correlation with a low JH level. Considering the uniform design for all experiments, we conclude that nonresponding AL neurons in males with low JH level exhibit an extremely high threshold.
Although corpora allata of male moths produce only JH acids (JHA) because they lack the JHA methyltransferase, which is the necessary enzyme allowing the last step of the JH biosynthesis (6), injection of JH instead of JHA into allatectomized males restored the high behavioral and neuronal sensitivity observed in mature males. JH injected in vivo can readily be converted into JHA, but we have never been able to show the JHA methyltransferase activity in any tissue of the insect (L. Duportets, unpublished results). It is therefore unclear whether it is JH or JHA that controls the sensitivity of the AL neurons.
The induction of high sensitivity of AL neurons in young immature males through JH injection shows that the central olfactory system is most likely fully developed at the tested age and that the presence of JH is necessary as a “switch” to reach high sensitivity. The sensitivity changes of pheromone-responding AL interneurons with the hormonal status indicate that hormone-mediated neuronal plasticity occurs in the adult moth brain. Age-dependent plasticity in adult brain structure was previously shown in social insects (19, 20), and an influence of JH on neurogenesis in the brain was found in crickets (3). JH also was shown in crickets to act on the sensitivity of auditory interneurons controlling phonotaxis (21). In mammals, gonadal steroids have been shown to control the behavioral responses to pheromones by actions on the central nervous system (22). Several studies have localized receptors for gonadal steroids within neurons (23). It is suggested that steroids could influence the behavior by regulating the synaptic transmission between peripheral neurons and more central areas. In insects, nothing is known about the exact structure of JH receptors in the central nervous system, although JH binding proteins have been found in the brain of Diploptera punctata (24). The question of whether JH acts directly on central neurons in A. ipsilon or indirectly via other neuroactive substances, remains open. In A. ipsilon, JH could act directly or indirectly on the AL neurons. Serotonin can be seen as a potential mediator of JH action, as it has been shown to modulate the growth and the response of olfactory neurons in the moth Manduca sexta (25–27).
Three criteria have been defined so far to be essential for sex pheromone processing in the male moth brain: quality, quantity, and intermittency of the stimulus (28). We could now add a fourth criterion, the physiological state of the male, represented by its hormonal level. By regulating the sensitivity of the olfactory center, JH participates in processing of the sex pheromone, thus allowing the optimal timing for pheromone integration and reproduction events. Both the development of reproductive glands (6) and the sensitivity of the olfactory system to sex pheromone seem to be controlled by the JH level in the male moth, synchronizing these vital processes that are needed for a successful mating. Further investigations will be needed to show whether JH acts directly or indirectly on central olfactory neurons and whether JH controls pheromone integration in other insects with a long adult life.
Acknowledgments
We thank L. Peypelut for his help in the statistical analysis, M. Nilsson and J. Zhang for technical help, F. Couillaud, B. S. Hansson, J. G. Hildebrand, and A. Strambi for critical reading of an earlier version of the manuscript, and the anonymous reviewers for their helpful comments. This work, dedicated to the memory of Lionel Peypelut, was supported by French and Swedish Research Councils [Institut National de la Recherche Agronomique, Swedish Council for Forestry and Agricultural Research (SJFR), and Swedish Natural Science Research Council (NFR)].
ABBREVIATIONS
- AL
antennal lobe
- JH
juvenile hormone
- JHA
juvenile hormone acid
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Wyatt G R, Davey K G. Adv Insect Physiol. 1996;26:1–157. [Google Scholar]
- 2.Walikonis R, Schoun D, Zacharias D, Henley J, Coburn P, Stout J. J Comp Physiol A. 1991;169:751–764. doi: 10.1007/BF00194903. [DOI] [PubMed] [Google Scholar]
- 3.Cayre M, Strambi C, Strambi A. Nature (London) 1994;368:5759. [Google Scholar]
- 4.Fahrbach S E, Robinson G E. Dev Neurosci. 1996;18:102–114. doi: 10.1159/000111474. [DOI] [PubMed] [Google Scholar]
- 5.Gadenne C, Renou M, Sreng L. Experientia. 1993;49:721–724. [Google Scholar]
- 6.Duportets L, Dufour M C, Couillaud F, Gadenne C. J Exp Biol. 1998;201:2425–2432. doi: 10.1242/jeb.201.16.2425. [DOI] [PubMed] [Google Scholar]
- 7.Duportets L, Dufour M C, Bécard J M, Gadenne C, Couillaud F. Arch Insect Biochem Physiol. 1996;32:601–611. [Google Scholar]
- 8.Hansson B S. Experientia. 1995;51:1003–1027. [Google Scholar]
- 9.Poitout S, Buès R. Ann Zool Ecol Anim. 1974;2:79–91. [Google Scholar]
- 10.Christensen T A, Hildebrand J G. J Comp Physiol A. 1987;160:553–569. doi: 10.1007/BF00611929. [DOI] [PubMed] [Google Scholar]
- 11.Gemeno C, Anton S, Zhu J W, Haynes K F. Int J Insect Morphol Embryol. 1998;27:185–191. [Google Scholar]
- 12.Causse R, Buès R, Barthès J, Toubon J F. In: Médiateurs Chimiques: Comportement et Systématique des Lépidoptères. Application en Agronomie. Descoins C, Frérot B, editors. Paris: Institut National de la Recherche Agronomique; 1988. pp. 75–82. [Google Scholar]
- 13.Hansson B S, Anton S, Christensen T A. J Comp Physiol A. 1994;175:547–562. [Google Scholar]
- 14.Anton S, Löfstedt C, Hansson B S. J Exp Biol. 1997;200:1073–1087. doi: 10.1242/jeb.200.7.1073. [DOI] [PubMed] [Google Scholar]
- 15.Gadenne C. J Insect Physiol. 1993;39:25–29. [Google Scholar]
- 16.Seabrook W D, Hirai K, Shorey H H, Gaston L K. J Chem Ecol. 1979;5:587–594. [Google Scholar]
- 17.Masson C, Arnold G. J Insect Physiol. 1984;1:7–14. [Google Scholar]
- 18.Crnjar R, Yin C M, Stoffolano J G, Barbarossa I T, Liscia A, Angioy A M. J Insect Physiol. 1990;36:917–921. [Google Scholar]
- 19.Withers G S, Fahrbach S E, Robinson G E. J Neurobiol. 1995;26:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]
- 20.Gronenberg W, Heeren S, Hölldobler B. J Exp Biol. 1996;199:2011–2019. doi: 10.1242/jeb.199.9.2011. [DOI] [PubMed] [Google Scholar]
- 21.Stout J, Atkins J G, Zacharias D. J Comp Physiol A. 1991;169:765–772. doi: 10.1007/BF00194904. [DOI] [PubMed] [Google Scholar]
- 22.Swann J M. Brain Res. 1997;750:189–194. doi: 10.1016/s0006-8993(96)01348-0. [DOI] [PubMed] [Google Scholar]
- 23.Wood R I, Brabec R K, Swann J M, Newman S E. Brain Res. 1992;596:89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- 24.King L E, Zhang J, Tobe S S. Gen Comp Endocrinol. 1994;94:11–22. doi: 10.1006/gcen.1994.1055. [DOI] [PubMed] [Google Scholar]
- 25.Mercer A R, Kloppenburg P, Hildebrand J G. J Comp Physiol A. 1995;178:21–31. doi: 10.1007/BF00189587. [DOI] [PubMed] [Google Scholar]
- 26.Kloppenburg P, Hildebrand J G. J Exp Biol. 1995;198:603–611. doi: 10.1242/jeb.198.3.603. [DOI] [PubMed] [Google Scholar]
- 27.Mercer A R, Kirchhof B S, Hildebrand J G. J Neurobiol. 1996;29:49–64. doi: 10.1002/(SICI)1097-4695(199601)29:1<49::AID-NEU4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrand J G. Proc Natl Acad Sci USA. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]