Abstract
Although CD4+ T cells have been shown to mediate protective cellular immunity against respiratory virus infections, the underlying mechanisms are poorly understood. For example, although phenotypically distinct populations of memory CD4+ T cells have been identified in different secondary lymphoid tissues, it is not known which subpopulations mediate protective cellular immunity. In this report, we demonstrate that virus-specific CD4+ T cells persist in the lung tissues and airways for several months after Sendai virus infection of C57BL/6 mice. A large proportion of these cells possess a highly activated phenotype (CD44hi, CD62Llo, CD43hi, and CD25hi) and express immediate effector function as indicated by the production of interferon γ after a 5-h restimulation in vitro. Furthermore, intratracheal adoptive transfer of lung memory cells into β2m-deficient mice demonstrated that lung-resident virus-specific CD4+ T cells mediated a substantial degree of protection against secondary virus infection. Taken together, these data demonstrate that activated memory CD4+ T cells persisting at mucosal sites play a critical role in mediating protective cellular immunity.
Keywords: immunologic memory, immunity, mucosal, paramyxovirus, CD4+ T lymphocytes
Introduction
CD4+ T lymphocytes play a major role in the immune responses to respiratory virus infections, such as those mediated by influenza and parainfluenza viruses, through the regulation of antibody production 1. In addition, it has also been established that CD4+ T lymphocytes, in association with CD8+ T cells, are involved in the cellular immune response to this class of infection, although the mechanisms are unclear 1 2 3 4. In general, primed memory CD4+ T cells accumulate more rapidly in the lung than naive T cells and mediate accelerated virus clearance in immune-competent mice 5. This protection is mediated at least in part by IFN-γ production and does not depend on antibody (references 4 and 6; unpublished observations). Although it is clear that CD4+ T cells play a role in protection, the recall of memory CD4+ T lymphocytes has generally been difficult to study, as the memory cells are present in the spleen at very low frequencies relative to CD8+ T cells. For example, CD4+ frequencies established to influenza and Sendai virus (a murine parainfluenza-1 virus) are typically in the range of 0.1% of CD4+ T cells in the spleen, whereas memory CD8+ T cells tend to be maintained at around 1–10% of CD8+ T cells 7 8 9. These memory CD4+ T cells persist at stable frequencies in the spleen and lymph nodes many months after the initial infection 7. However, the relative contribution of memory CD4+ T cells from different anatomical sites to protective cellular immune responses is unknown.
We have recently shown that memory CD8+ T cells persist in the lung tissue and airways for over 1 yr after recovery from a respiratory virus infection and that the numbers of cells present in the lungs correlates with the degree of protective immunity 10 11. Lung memory CD8+ T cells express a highly activated phenotype and can be induced to proliferate and express various effector functions. Thus, we speculated that memory CD4+ T cell would also persist in the lung after resolution of the initial infection and that these cells would correlate with protective immunity. In this report, we show that functional memory CD4+ T cells persist at very high frequencies in the lungs after resolution of a Sendai virus infection. In addition, by intratracheal transfer of cells we show that these lung memory CD4+ T cells provide a substantial degree of protection against a secondary infection.
Materials and Methods
Viruses, Animals, and Infections.
The Enders strain of Sendai virus and influenza virus A/HK-x31 (x31, H3N2) were grown, stored, and titrated as described previously 12 13. Female C57BL/6, B6.SJL-Ptprca Pep3b/BoyJ (Ly5.1+), and C57BL/6J-B2mtm1Unc (β2m-deficient) mice were purchased from the Animal Breeding Facility at the Trudeau Institute, The Jackson Laboratory, or Taconic Farms. Mice (6–12 wk) were anesthetized by intraperitoneal injection with 2,2,2 tribromoethanol and infected intranasally with 500 50% egg infectious doses (EID50) of Sendai virus or 300 EID50 of x31 influenza virus.
Peptides.
Sendai virus peptides (hemagglutinin-neuraminidase [HN]421–436 and nucleoprotein [NP]324–332), and influenza virus (hemagglutinin [HA]192–207 and NP366–374) peptides were purchased from New England Peptide Inc. Peptide purity was evaluated using reverse-phase HPLC analysis.
Tissue Preparation.
Single cell suspensions were prepared from spleens and mediastinal lymph nodes (MLNs) by passage through cell strainers. Spleen cells were depleted of erythrocytes by treatment with buffered ammonium chloride solution. Bronchoalveolar lavage (BAL) cells were collected by lavage of the lungs 3–4 times with 1 ml of HBSS. Cells were prepared from lung tissue by passing lavaged lungs through cell strainers. The cells were subsequently resuspended in 80% isotonic percoll and layered with 40% isotonic percoll. After centrifugation at 400 g for 25 min, the cells at the 80%/40% interface were collected, washed, and counted.
Enzyme-linked Immunospot Assay.
The relative frequencies and numbers of IFN-γ–secreting cells derived from spleen, lung, MLN, and BAL tissues were determined after stimulation with Sen-HN421–436, Flu-HA192–207, Sen-NP324–332, or Flu-NP366–374 peptides in a standard enzyme-linked immunospot (ELISPOT) assay system. In brief, 96-well Multiscreen HA nitrocellulose plates (Millipore) were coated overnight at 4°C with 100 μl per well of rat anti–mouse IFN-γ (clone R4-6A2; BD PharMingen), at a concentration of 10 μg/ml. The plates were then washed and blocked before the addition of titered numbers of responding cells, irradiated (3,000 rad) syngeneic normal spleen cells, peptide, and 10 U/ml rhIL-2. Plates were then incubated 24–48 h at 37°C and developed overnight with a biotinylated detection antibody, rat anti–mouse IFN-γ (clone XMG1.2; BD PharMingen), followed by streptavidin–horseradish peroxidase (Sav-HRP; BD PharMingen) for 2 h at room temperature. Visible spots of IFN-γ–secreting cells were then enumerated using an Olympus SZH stereo zoom microscope system. Absolute numbers of antigen-specific CD4+ T cells were calculated based on the number of live cells recovered per mouse tissue. The frequencies of antigen-specific T cells were determined by correcting the data for the number of CD4+ T cells put into the assay based on flow cytometric data. The number of spots in wells containing irrelevant peptides was below detection (<1 in 106).
Intracellular IFN-γ Staining.
Cells isolated from the spleen, MLNs, lung tissue, and BAL (after plastic adherence) were mixed with naive spleen cells from Ly5.1+ H-2b mice. The cells were then cultured at 37°C for 5 h in the presence of 10 μg of the indicated peptide in 250 μl complete tumor medium (CTM) containing 10 μg/ml brefeldin A (BFA). After culture, the cells were blocked with monoclonal antibodies to FcRIII/II receptor and stained with anti-CD4–FITC, anti-Ly5.2 biotin, and streptavidin Cychrome (BD PharMingen) in PBS/BFA. The cells were then fixed in 1% formaldehyde, permeabilized with PBS containing 0.5% saponin, and stained with anti–IFN-γ PE or isotype control IgG1 PE monoclonal antibodies. The data were acquired using a FACScan™ flow cytometer and analyzed with CELLQuest™ software (Becton Dickinson).
Intratracheal Cell Transfer.
BAL cells were collected from C57BL/6 mice at >35 d after Sendai virus or influenza virus infection. The donor cells washed and resuspended in PBS at a concentration of 107 cells/ml. Naive C57BL/6 or β2m-deficient recipient mice were anesthetized and 100 μl (0.5–1 × 106 cells) of the cell suspension or PBS were instilled into the lungs (via the trachea) using a 1-ml syringe fitted with a blunted 20-G needle. Cells isolated from the BAL of mice that have recovered from a Sendai virus infection typically contain <0.5% B cells or plasma cells, as determined by flow cytometric analysis using antibodies specific for B220, CD19, and CD138.
Results and Discussion
Activated CD4+ T Cells Persist in the Lungs After Resolution of a Primary Sendai Virus Infection.
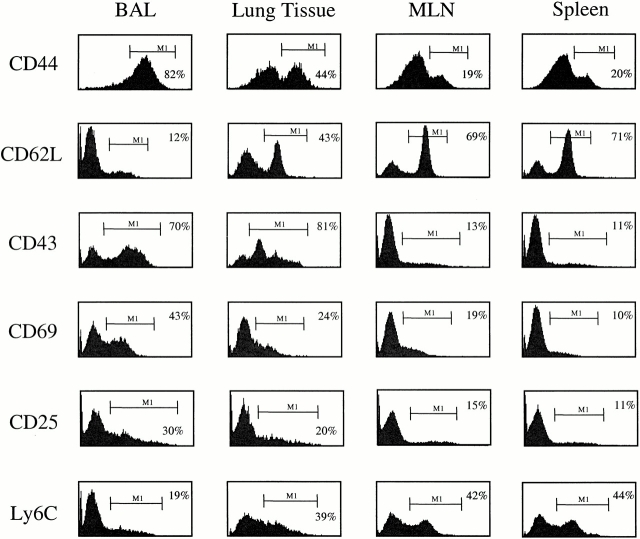
To determine whether CD4+ T cells persisted in the lungs after recovery from a Sendai virus infection, we isolated cells from both the lung tissue and airways at day 41 after Sendai virus infection. Substantial numbers of CD4+ T cells were detected in both the lung tissue and airways, ranging from 30,000 to 100,000 cells per mouse lung (data not shown). Phenotypic analysis indicated that the CD4+ T cell populations in the spleen, MLNs, lung airways, and lung tissue displayed remarkably different phenotypes. As shown in Fig. 1, the vast majority of CD4+ T cells isolated from the BAL displayed high levels of CD44, low levels of CD62L, and low levels of Ly6C expression, indicating that they are predominantly “memory” T cells. In contrast, the levels of CD44 and CD62L expression in the lung tissue indicate that both naive and “memory” CD4+ T cells are present at this site. As expected, the CD44/CD62L profile from the spleen and MLNs show a large number of naive T cells (CD44loCD62Lhi) and a much smaller number of antigen-experienced CD4+ T cells (CD44hiCD62Llo).
Figure 1.
CD4+ T cells in the lung airways (BAL) and lung tissues express an activated phenotype. Cells were isolated from the spleen, MLNs, lung tissue, and lung airways (BAL) of mice that had recovered from a Sendai virus infection (day 41 after infection). After isolation, the cells were stained with anti-CD4–PE and FITC-conjugated CD44, CD62L, CD43, CD69, and Ly6C antibodies. Cell surface expression of CD25 was determined using anti-CD25–PE and anti-CD4–FITC antibodies. The histograms show the expression of the indicated markers among live CD4+ lymphocytes. The bars and numbers in each panel show the percentage of CD4+ cells expres-sing the indicated marker. Data are representative of four independent experiments.
Analysis of the CD4+ T cells in the BAL showed that a large proportion of the cells were of a highly activated phenotype, characterized by the expression of acute activation markers, CD43 and CD69. In contrast, much lower frequencies of CD4+ T cells isolated from the lung tissue, MLNs, and spleen expressed CD43 and CD69. This difference in phenotype persisted even when CD4+CD44hi “memory” T cells were analyzed separately. Thus, as shown in Table , a large proportion of cells isolated from the BAL fluid possess an activate phenotype as indicated by CD43 and CD69 expression. In contrast, decreasing levels of activation marker expression were observed on CD4+CD44hi “memory” T cells in the lung tissue, spleen, and MLNs. This pattern of activation marker expression is remarkable given that many weeks have passed since resolution of the primary infection and is very similar to the description of CD8+ memory T cells that persist in the lungs after recovery from Sendai or influenza virus infections 11. Although the activated phenotype of the cells in the lungs suggests that a pool of viral antigen may persist, we and others have been unable to demonstrate persistent viral antigen by PCR or by using a sensitive virus-specific hybridoma assay (data not shown; reference 14).
Table 1.
The Proportion of CD4+CD44+ Cells Expressing the Indicated Markers After Sendai Virus Infection
| CD4+CD44+ | ||||||
|---|---|---|---|---|---|---|
| Percent CD44+ among CD4+ | CD62L+ | CD43+ | CD69+ | CD25+ | Ly6C+ | |
| BAL (n = 5) | 82 | 6 | 72 | 40 | 23 | 13 |
| Lung tissue (n = 3) | 36 | 15 | 63 | 17 | 9 | 20 |
| MLNs (n = 3) | 25 | 19 | 27 | 33 | 28 | 13 |
| Spleen (n = 3) | 26 | 28 | 38 | 25 | 15 | 25 |
Mice were intranasally infected with 500 EID50 Sendai virus and tissues were analyzed on day 34 after infection.
Virus-specific Effector CD4+ T Cells Persist in the Lungs After Sendai Virus Infection.
To determine whether any of the CD4+ T cells that persisted in the lung were virus specific, we performed ELISPOT assays using cells isolated from the BAL, lung tissue, spleen, and MLNs of Sendai memory mice (day 41 after infection). The antigen used for these studies was a peptide representing an immunodominant I-Ab–restricted epitope derived from the HN protein. Previous studies had shown that this epitope drives about 15% of the CD4+ T cell response to Sendai virus infection in the lung (reference 15; and data not shown). As shown in Table , a substantial population of HN421–436/Ab-specific CD4+ T cells, representing >13% of the total CD4+ T cell pool), is present in the BAL after resolution of the primary infection. Interestingly, the frequency of HN421–436/Ab-specific CD4+ T cells in the lungs is much higher than the frequencies of HN421–436/Ab-specific cells in the spleen and MLNs. For example the frequency of antigen-specific CD4+ T cells in the BAL is >200-fold higher than in the spleen (Table ). When absolute numbers of cells were compared, the population of virus-specific CD4+ T cells in the lungs (BAL and tissue) was more than twice the number isolated from the MLNs and ∼20% of the number in the spleen. Phenotypic and ELISPOT analyses of cells isolated from the lungs of mice 90 d after Sendai virus infection showed similar results, indicating that antigen-specific CD4+ T cells persist in the lungs for >11 wk after infection.
Table 2.
ELISPOT Analysis of Virus-specific T Cells in the BAL, Lung Tissue, MLNs, and Spleens of C57BL/6 Mice After Resolution of a Primary Sendai Virus Infection
| Sendai HN421–436 | Sendai NP324–332 | |||
|---|---|---|---|---|
| Sen-HN421–436 specific among CD4+ | Total no. of Sen-HN421–436–specific cells per mouse | Sen-NP324–332 specific among CD8+ | Total no. of Sen-NP324–332–specific cells per mouse | |
| % | % | |||
| BAL (n = 9) | 13.2 | 1,964 | 59.5 | 11,032 |
| Lung tissue (n = 8) | 2.1 | 1,679 | 13.9 | 7,400 |
| MLNs (n = 8) | 0.11 | 1,512 | 0.37 | 4,992 |
| Spleen (n = 3) | 0.06 | 18,320 | 0.44 | 88,578 |
Mice were intranasally infected with 500 EID50 Sendai virus and tissues were analyzed on day 41 after infection. Data are representative of two independent experiments.
As a control for these studies, we also determined the frequencies of CD8+ T cells in the lungs specific for the immunodominant NP324–332/Kb epitope by ELISPOT analysis 16. As expected, NP324–332/Kb-specific CD8+ T cells are present at very high frequencies and absolute numbers in the lung airways and lung tissue at day 41 after Sendai virus infection (Table ), consistent with previous reports 11. As an additional control, we also included an influenza peptide representing a dominant I-Ab epitope (HA421–436/Ab). This peptide was always negative (i.e., frequencies of <1:1 × 106) in these ELISPOT assays (data not shown). Taken together, these data show that high frequencies of antigen-specific memory CD4+ T cells persist in the lungs after recovery from a Sendai virus infection and that these cells can be induced to secrete IFN-γ in a 48-h restimulation (ELISPOT) assay.
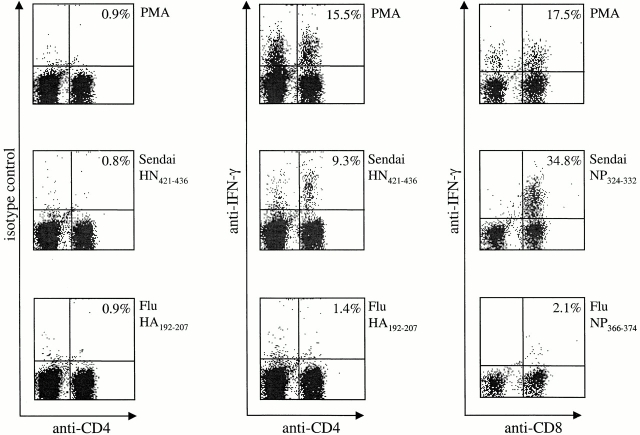
Having established that activated virus-specific CD8+ and CD4+ T cells persist in the lungs, we next investigated the ability of both populations to produce IFN-γ directly ex vivo using an intracellular cytokine assay. Cells were isolated from the lung airways of Sendai memory mice (40 d after infection) by lavage and restimulated for 5 h in vitro in the presence of BFA, peptide, IL-2, and Ly5.1+ spleen cells. Ly5.1+ spleen cells were used as APCs to allow us to specifically examine IFN-γ production in the BAL cell population (Ly5.2+). As shown in Fig. 2, stimulation with PMA and ionomycin induced IFN-γ production in ∼15% of both CD4+ and CD8+ T cells. Furthermore, significant populations of CD4+ and CD8+ BAL cells also produced IFN-γ after stimulation with the relevant Sendai virus peptides. In contrast, memory cells cultured with irrelevant control peptides corresponding to the CD4 and CD8 immunodominant epitopes of influenza virus produced only background levels of IFN-γ (Fig. 2). Staining with an isotype control antibody also confirmed the specificity of the assay. In additional experiments, BAL cells were labeled with carboxyfluorescein diacetate-succinimidyl ester (CFSE) and restimulated in vitro. After a 4-d incubation, BAL-derived CD4+ T cells exhibited marked proliferation (unpublished observations). Taken together, these data confirm that functional virus-specific T cells persist in the lungs and demonstrate that these cells can rapidly produce high levels of IFN-γ in response to relevant antigen 17.
Figure 2.
BAL memory cells produce IFN-γ directly ex vivo. Cells were isolated from the lung airways (BAL) of mice that had recovered from Sendai virus infection (day 34). The cells were restimulated with PMA/ionomycin or Sen-HN421–436, Flu-HA192–207, Sen-NP324–332, or Flu-NP366–374 peptides in the presence of BFA, IL-2, and naive Ly5.1+ spleen cells as APCs. After 5 h in culture, all cells were stained with anti-Ly5.2 biotin followed by streptavidin-allophycocyanin and the indicated antibodies. The data are presented as live Ly5.2+ lymphocytes (BAL). The numbers indicate the percentages of IFN-γ–secreting cells among the total Ly5.2+CD8+ or Ly5.2+CD4+ cell population. Data are representative of three independent experiments.
CD4+ Cells in the Lung Airways Can Mediate Protective Immunity.
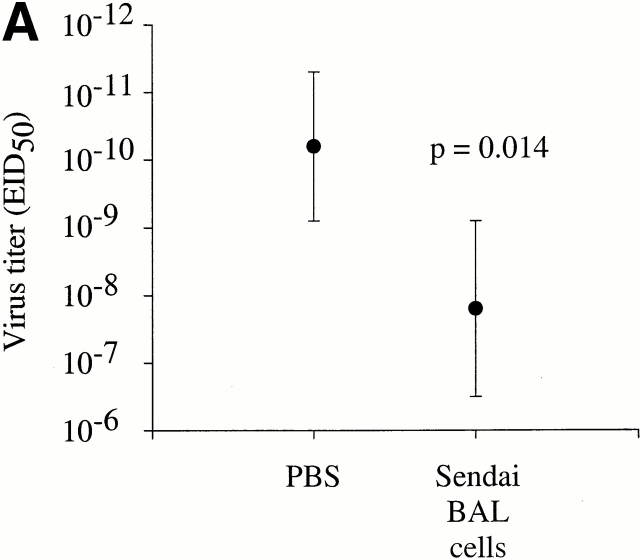
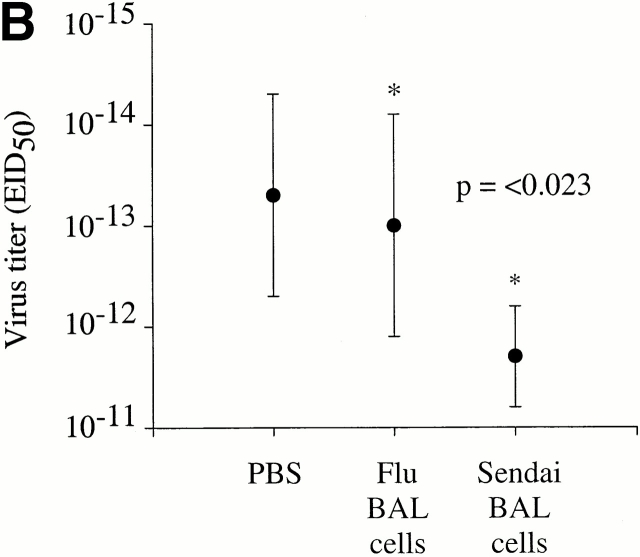
Given the ability of antigen-specific T cells in the lungs to rapidly produce IFN-γ directly ex vivo, we next asked whether these cells would mediate protection against subsequent Sendai virus infection. Thus, total BAL cells were isolated from the lungs of mice that had recovered from a Sendai virus infection (day 35 after infection) and intratracheally transferred directly into the lungs of naive C57BL/6 mice. This procedure instills cells directly into the lung airways and it has been shown that these cells are able persist in the airways for several weeks after transfer (unpublished observations). 4 h after transfer, the mice were infected with Sendai virus and viral titers in the lung were then assessed 4 d later. As shown in Fig. 3 A, mice that received the memory BAL cells had a significantly reduced viral load in the lung compared with PBS control mice (an average reduction of 250-fold, P = 0.014). Although the intratracheal transfer of memory BAL cells had a significant impact on lung virus titers, it was not clear exactly which cells in the BAL mediated this effect, or whether CD4+ T cells were involved. Thus, to specifically investigate the role of virus-specific CD4+ T cells in the lung airways, we intratracheally transferred Sendai memory BAL cells (day 40 after infection) into naive β2m-deficient mice. These mice express undetectable levels of H-2Kb resulting in a significant impairment of the CD8+ T cell response to the immunodominant NP324–332/Kb-epitope 16 18. It should be noted that β2m-deficient mice do express low levels of functional H-2Db, but that this restricting element is not involved in the effector CD8+ T cell response to Sendai virus in H-2b mice 16. Two sets of control β2m-deficient mice received either PBS or BAL cells isolated from influenza memory mice (day 41 after infection). As shown in Fig. 3 B, the β2m-deficient mice that received Sendai memory BAL cells were significantly more efficient at controlling the virus load than the PBS control group (P < 0.009) or the influenza control group (P < 0.023). These data cannot be readily explained by the transfer of memory B or antibody-producing plasma cells, as these cells typically represent <0.5% of the cells in the BAL. Similarly, the fact that the BAL cells were washed before transfer rules out the transfer of passive antibody. Thus, the data suggest that the presence of virus-specific CD4+ T cells in the lungs has a significant impact on viral load during the initial stages of infection.
Figure 3.
BAL memory cells confer protection against Sendai virus infection. (A) 10 naive C57BL/6 mice were intranasally infected with 500 EID50 Sendai virus. 4 h before infection, the mice received either memory BAL cells (5 × 105 cells in 100 μl, n = 5), or control PBS (100 μl, n = 5) intratracheally. The BAL cells in this experiment were derived from C57BL/6 mice that had recovered from infection with 500 EID50 Sendai virus (35 d after infection). (B) 16 naive β2m-deficient mice were intranasally infected with 500 EID50 Sendai virus. 4 h before infection, each mouse received either Sendai memory BAL cells (106 cells in 100 μl, n = 6), influenza memory BAL cells (106 cells in 100 μl, n = 6), or control PBS (100 μl, n = 4) intratracheally. In this experiment, Sendai memory BAL cells were obtained from C57BL/6 mice at day 40 after infection, whereas influenza memory BAL cells were obtained from C57BL/6 mice at day 41 after infection. In both A and B, virus titers in the lung were determined 4 d after transfer by titrating lung homogenates in embryonated chicken eggs, followed by hemagglutination assays. Where indicated, the difference in viral titers among each group was determined to be statistically significant as based on a standard t test. The data are expressed as log10EID50 and are representative of three independent experiments.
The mechanism by which CD4+ T cells mediate protection against respiratory virus infections is unclear. However, there is substantial evidence that IFN-γ production is a key player in this protection. In this regard, the presence of both CD4+ and CD8+ T cells in the lungs capable of rapidly producing IFN-γ is consistent with the idea that this cytokine plays a critical role. The absolute numbers of antigen-specific CD4+ T cells in the lung is relatively small (∼20% of the number in the spleen; Table ). But these cells are present in a highly activated state and are able to respond immediately at the site of infection when the viral load is still very low. Thus, a relatively small number of cells may be sufficient to have a significant impact on the early stages of the infection, especially if the cells proliferate and mediate sustained cytokine production. In addition, these cells may function to rapidly recruit other inflammatory cells to the site of infection through the production of chemokines. We have also shown that the development of an effective CD4+ T cell response depends on the presence of CD8+ T cells, suggesting a regulatory role for CD8+ T cells (unpublished observations). It should be noted that both CD4+ and CD8+ T cells were physically present in the intratracheal transfer experiments that demonstrated protective immunity, but that the CD8+ T cell function was significantly impaired due to a deficient H-2Kb expression on the host respiratory epithelium.
It is currently unclear how memory cells persist in the lung tissues and airways. Based on observations with the CD8+ memory T cells, we have speculated that memory CD4+ and CD8+ cells in the spleen are slowly dividing and that some of the activated cells enter the circulation 10 11. Given the activated phenotype of these cells, they are likely to accumulate in mucosal tissues, including the lung and lung airways. For example, there is substantial evidence that activated T cells accumulate in the lung and extravasate across epithelium into the airways 19. The absolute number of cells in the lung would depend on the relative numbers and replication rates of memory cells in secondary lymphoid organs that seed the circulation, and the length of time that the cells persist at mucosal sites. In support of this general idea, we have shown that vaccination protocols that do not involve infection of the lung nevertheless induce substantial populations of memory CD4+ and CD8+ T cells in the lungs (data not shown).
Taken together, the data demonstrate that memory CD4+ T cells with an activated phenotype and capable of immediate effector function persist at very high frequencies in the lungs after resolution of a respiratory virus infection, and that these cells are capable of mediating protective cellular immunity. These findings have significant implications for our understanding of cellular immunity to infection and vaccine development.
Acknowledgments
We would like to thank Simon Monard for assistance with the flow cytometry, Jean Brennan for help with the intratracheal transfers, and Dr. Marcy Blackman for critically reviewing the manuscript.
This work was supported by funds from the Trudeau Institute and National Institutes of Health grants F32 AI10590 (R.J. Hogan), R01 AI37597 (D.L. Woodland), and R01 HL63925 (D.L. Woodland).
References
- Doherty P.C., Topham D.J., Tripp R.A., Cardin R.D., Brooks J.W., Stevenson P.G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Kast W.M., Bronkhorst A.M., de Waal L.P., Melief C.J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J. Exp. Med. 1986;164:723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.B., Braciale V.L., Braciale T.J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.B., Braciale T.J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte–deficient mice. J. Exp. Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Marshall D., Coleclough C., Woodland D.L. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J. Immunol. 2000;164:3274–3282. doi: 10.4049/jimmunol.164.6.3274. [DOI] [PubMed] [Google Scholar]
- Bot A., Bot S., Bona C.A. Protective role of gamma interferon during the recall response to influenza virus. J. Virol. 1998;72:6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham D.J., Doherty P.C. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J. Immunol. 1998;161:4530–4535. [PubMed] [Google Scholar]
- Usherwood E.J., Hogan R.J., Crowther G., Surman S.L., Hogg T.L., Altman J.D., Woodland D.L. Functionally heterogeneous CD8+ T-cell memory is induced by Sendai virus infection of mice. J. Virol. 1999;73:7278–7286. doi: 10.1128/jvi.73.9.7278-7286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K.J., Belz G.T., Altman J.D., Ahmed R., Woodland D.L., Doherty P.C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Woodland D.L., Hogan R.J., Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol. Res. 2001;In press doi: 10.1385/IR:24:1:53. [DOI] [PubMed] [Google Scholar]
- Hogan R.J., Usherwood E.J., Zhong W., Roberts A.D., Dutton R.W., Harmsen A.G., Woodland D.L. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Hou S., Doherty P.C., Zijlstra M., Jaenisch R., Katz J.M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J. Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- Daly K., Nguyen P., Woodland D.L., Blackman M.A. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J. Virol. 1995;69:7416–7422. doi: 10.1128/jvi.69.12.7416-7422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberger M.C., Wang M.L., Allan W., Webster R.G., Doherty P.C. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J. Gen. Virol. 1991;72:1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- Cole G.A., Katz J.M., Hogg T.L., Ryan K.W., Portner A., Woodland D.L. Analysis of the primary T-cell response to Sendai virus infection in C57BL/6 miceCD4+ T-cell recognition is directed predominantly to the hemagglutinin-neuraminidase glycoprotein. J. Virol. 1994;68:6863–6870. doi: 10.1128/jvi.68.11.6863-6870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G.A., Hogg T.L., Woodland D.L. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 micea single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int. Immunol. 1994;6:1767–1775. doi: 10.1093/intimm/6.11.1767. [DOI] [PubMed] [Google Scholar]
- Meyer K.C., Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999;54:697–700. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D.H. MHC class I-deficient mice. Adv. Immunol. 1994;55:381–421. doi: 10.1016/s0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- Hamann A., Klugewitz K., Austrup F., Jablonski-Westrich D. Activation induces rapid and profound alterations in the trafficking of T cells. Eur. J. Immunol. 2000;30:3207–3218. doi: 10.1002/1521-4141(200011)30:11<3207::AID-IMMU3207>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]