Abstract
Latent membrane protein 1 (LMP1) plays a critical role in B cell transformation by Epstein-Barr virus (EBV) and appears to mimic a constitutively active CD40 receptor. Intracellular tumor necrosis factor (TNF) receptor–associated factor (TRAF) adapter proteins, shown to contribute to signaling by both CD40 and LMP1, were recruited by both molecules to lipid-enriched membrane rafts. However, we found that TRAFs 2 and 3 were subsequently degraded after CD40- but not LMP1-induced signaling. This degradation was proteasome-dependent and required direct TRAF binding by CD40. Using a model system designed to directly compare the signaling potency of the cytoplasmic domains of LMP1 and CD40 in B lymphocytes, we found that LMP1 more potently activates c-Jun kinase and nuclear factor κB and induces higher levels of several B cell effector functions than does CD40. This suggests that LMP1 utilizes a modified CD40 signaling pathway. Failure to regulate TRAFs may contribute to the enhanced capacity of LMP1 to activate B cells as well as promote B cell transformation.
Keywords: HHV4, CD40, signal transduction, B lymphocyte, lymphoma
Introduction
EBV is a B lymphotropic human herpes virus that infects a majority of the world's population. EBV has been strongly associated with the development of several human lympho-proliferative conditions including posttransplant lymphoproliferative disease, AIDS-related lymphoma, Burkitt's lymph-oma, and Hodgkin's disease 1. Of the nine EBV genes expressed as proteins in EBV-transformed cell lines, latent membrane protein 1 (LMP1) is the best characterized, and is the only EBV-encoded gene product capable of transforming cells in vitro 2. Additionally, LMP1 is essential for transformation of EBV-infected B lymphocytes 3. Together these data suggest a primary role for LMP1 in the development of EBV-lymphoproliferative disorders.
Structurally, LMP1 is composed of a short NH2-terminal cytoplasmic region, six transmembrane domains, and a signaling COOH-terminal cytoplasmic tail. The NH2 terminus and transmembrane domains appear responsible for promoting constitutive activation by ligand-independent oligomerization of LMP1, but are not required for signaling per se 4 5. The signaling COOH-terminal cytoplasmic tail of LMP1 appears to mimic many aspects of signaling by CD40, an activating receptor normally expressed on B lymphocytes 6 7 8. After ligation by its ligand CD154, expressed on activated T cells, CD40 activates B cells by a signaling pathway that begins with the association of adapter proteins known as TNF receptor–associated factors (TRAFs; reference 9). After binding CD40, TRAFs are thought to interact with as yet uncharacterized signaling proteins that link CD40 to nuclear factor (NF)-κB and c-Jun kinase (JNK) activation. Similar to CD40, LMP1 also binds TRAFs which are then thought to interact with kinases such as NF-κB–inducing kinase (NIK; reference 10) that ultimately promote activation of NF-κB.
TRAFs are a class of adapter proteins initially characterized by their ability to interact with the TNF receptor (for a review, see reference 11). CD40 is reported to directly bind TRAFs 2, 3, 5, and 6 (and can associate with TRAF1 via its heterodimerization with TRAF2), whereas LMP1 is reported to bind TRAFs 1, 2, 3, and 5 12 13 14 15 16. Different TRAF proteins appear to play distinct roles in signaling through CD40, LMP1, and other TNF receptor family members. TRAF2 appears to be primarily required for JNK activation and also contributes to NF-κB activation by both molecules 12 17 18. TRAF2 also plays a role in CD40-mediated surface molecule upregulation and IgM secretion in B lymphocytes 19 20. In contrast, TRAF3 may antagonize TRAF2's effects in promoting NF-κB activation and CD40-mediated IgM secretion 18 20. The TRAF binding domains of LMP1 have been shown to be essential to its ability to transform B cells 21.
Despite similarities in CD40 and LMP1 signaling, differences have been noted in addition to differences in TRAF utilization. When CD40-deficient mice were manipulated to transgenically express the LMP1 gene under control of the Ig promoter, they showed a B cell activation phenotype intermediate between that of wild-type (WT) and CD40−/− mice 8. This suggests that although LMP1 mimics some aspects of CD40 signaling, differences do exist. Additionally, we reported previously that CD40 and LMP1 can cooperate in signaling, also indicating that CD40 and LMP1 signaling pathways contain distinct features 7.
Together, these data suggested the hypothesis that CD40 and LMP1 utilize distinct signaling mechanisms to affect B cell activation. As stated above, both molecules initiate signaling at least in part via association with TRAF adapter proteins. Additionally, it is known that LMP1 recruits TRAF3 to lipid-rich membrane microdomains, or rafts 22, and we have shown that upon CD40 ligation, CD40 itself relocates to membrane rafts, as well as recruiting TRAFs 2 and 3 to these complexes 23. Engagement of several members of the TNFR family of molecules, CD30 24 and TNFR2 25, results in degradation of TRAF2, and we have recently reported that CD40 ligation also stimulates TRAF2 loss 23. Remarkably, LMP1 signaling failed to induce TRAF degradation. As TRAF degradation may be an important mechanism for regulating and limiting signal transduction, we were thus curious to determine whether the signaling potency of CD40 and LMP1 differs. Experiments using WT LMP1 molecules and chimeric LMP1 molecules with CD40 cytoplasmic domains indicated that the LMP1 cytoplasmic domain delivers stronger activation signals to the B cell. However, because the transmembrane domains of LMP1 aggregate and initiate signaling constitutively as the molecule is expressed, it was difficult to directly compare signaling between subclones expressing the WT or hybrid molecules. To examine this question more carefully, we needed an experimental system that would allow direct comparison of signaling effectiveness.
Previous work using a chimeric molecule with the external domain of the class I molecule HLA-A2 demonstrated that the COOH-terminal cytoplasmic tail of LMP1 is sufficient to initiate LMP1 signaling in B lymphocyte cell lines 7, confirming earlier findings 26. When stimulated by anti-A2 mAb the chimeric molecule faithfully replicated every aspect of LMP1 signaling. However, use of this molecule did not permit a direct comparison of signaling potencies of CD40 and LMP1 as different Abs (anti-CD40 versus anti-A2) were required to initiate signaling, and might differ in their affinities and/or cross-linking ability. To permit a direct comparison of the activity of the CD40 and LMP1 cytoplasmic tails in stimulating signaling, we constructed a recombinant human CD40-LMP1 chimera. Stable transfectants of B cell lines expressing similar levels of either WT human (h)CD40 or the hCD40-LMP1 chimera were produced. This chimera allowed us to use the same type and level of stimulus to initiate signaling by molecules containing either cytoplasmic domain. We determined that both recruitment of TRAFs and their degradation is dependent on whether the cytoplasmic domain of the molecule is from hCD40 or LMP1, verifying that it is this domain of each molecule that determines its signaling behavior. CD40-mediated TRAF degradation was found to be proteasome dependent, require direct TRAF binding, and dominate over the inability of LMP1 to induce degradation.
Using this model we also directly compared signaling effectiveness of the two cytoplasmic domains, and found that the cytoplasmic domain of LMP1 more potently activates B lymphocytes in both early and late measures of signaling, including activation of JNK and NF-κB, upregulation of surface molecules, and secretion of IL-6 and IgM. We propose that differential TRAF regulation contributes to the observed differences in signaling potencies of the cytoplasmic domains of CD40 and LMP1, and may contribute to the transforming activity of LMP1.
Materials and Methods
Cell Lines.
The mouse B cell lines CH12.LX 27 and M12.4.1 28 were maintained in RPMI 1640 with 10% FCS, 10 μM 2-mercaptoethanol, and antibiotics. M12.4.1 cells were chosen for analysis of surface molecule upregulation, because CH12.LX cells have a higher background level of the surface molecules studied, and thus are not optimal in this assay. CH12.LX cells were chosen for analysis of IgM and IL-6 secretion, as M12.4.1 cells produce little to no IL-6 or Ig after any stimulus. Both cell lines activate both NF-κB and JNK after CD40 and LMP1 signaling 7 29, so both were tested in these assays. Transfected B cell lines were maintained in culture medium supplemented with 400 μg/ml G418 sulfate (Life Technologies). Human CD154-expressing Chinese hamster ovary (CHO) cells were provided by Dr. A. Black (IDEC Pharmaceuticals Corporation, San Diego, CA). Mouse CD154-expressing CHO cells prepared in our laboratory have been described previously 30. CHO cells were maintained in DMEM with 10% FCS, nonessential amino acids, and antibiotics.
Abs.
The mAbs 16\10A1 (FITC-labeled anti–B7-1, Armenian hamster IgG), G235-2356 (FITC-hamster IgG isotype control, hamster IgG), HM40-3 (anti–mouse CD40, hamster IgM), G235-1 (isotype control, hamster IgM), and C4 (anti–mouse actin, mouse IgG1) were purchased from BD PharMingen. Mouse IgG1 isotype control mAb (MOPC-21) was purchased from Sigma-Aldrich. Rabbit anti-TRAF1 (N-19), rabbit anti-TRAF2 (C-20), rabbit anti-TRAF3 (H-122), and rabbit anti-IκBα (FL) Abs were purchased from Santa Cruz Biotechnology, Inc. Sheep polyclonal anti–glutathione S-transferase (GST)–hCD40 (external domain) Ab was prepared for us by Elmira Biologicals (Iowa City, IA). Goat anti–rabbit horseradish peroxidase (HRP) and goat anti–mouse HRP Abs were purchased from Bio-Rad Laboratories. Donkey anti–sheep HRP Ab was purchased from Jackson ImmunoResearch Laboratories. The following Abs were produced in our laboratory by hybridomas purchased from American Type Culture Collection or were gifts of the indicated individuals: anti-hCD40 (G28-5, mIgG1; American Type Culture Collection), anti-LMP1 (S-12, mIgG2a) from Dr. F. Wang (Harvard Medical School, Boston, MA), anti–mouse CD40 (1C10, rat IgG2a) from Dr. F. Lund (Trudeau Institute, Saranac Lake, NY), anti–mouse CD23 (clone B3B4, rat IgG2a), and anti–mouse IgE (EM95.3, rat IgG2a isotype control) from Dr. T. Waldschmidt (University of Iowa, Iowa City, IA), anti–mouse intercellular adhesion molecule (ICAM)-1 (YN1/1.7.4; American Type Culture Collection), anti–mouse lymphocyte function–associated antigen (LFA)-1 (M17/4.4.11.9, rat IgG2a; American Type Culture Collection), anti–mouse IL-6 hybridomas 20F3.11 (rat IgG; American Type Culture Collection) and 32C11.4 (rat IgG1; American Type Culture Collection).
Chemicals.
Recombinant mouse IL-6 was purchased from BD PharMingen. Octylglucoside and 0-phenylenediamide dihydrochloride tablets were purchased from Amersham Pharmacia Biotech. MG132 proteasome inhibitor was purchased from Calbiochem. Isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Amresco.
DNA Constructs.
The generation of the WT-hCD40 expression plasmid and hCD40 structural mutants has been described previously 19. PCR SOEing 31 was used to generate a direct fusion between the first 215 amino acids of CD40 (extracellular and transmembrane domains) and the terminal 200 amino acids of LMP1 (cytoplasmic COOH terminus). The same technique was used to generate a fusion between the membrane spanning external domains of LMP1 and the cytoplasmic domain of hCD40. DNA sequencing of the chimeric molecules confirmed that the constructs were free of undesired mutations. Constructs expressed inducibly contained LacR binding sites upstream of the cDNA insertion site in the plasmid, and were transfected into subclones of M12.4.1 and CH12.LX already stably constitutively expressing LacR. Expression is induced by incubation in the presence of IPTG. This expression system has been described previously 7.
Transfections.
Stable transfections of mouse B cell lines with DNA constructs were conducted as described previously 32. G418 resistant subclones were analyzed either for surface expression of hCD40 or hCD40-LMP1 using a FACScan™ flow cytometer (Becton Dickinson), or by Western blotting for WT-LMP1 and LMP1-hCD40, and expression-matched clones isolated. B cell transfectants expressing mutant hCD40 molecules have been described previously 19. All experiments presented were performed with two to three individual stably transfected, expression-matched subclones of each type analyzed.
TRAF Degradation Assays.
TRAF degradation after induced expression of WT-LMP1 or LMP1-hCD40 was analyzed after incubation of B cell transfectants with 100 μM IPTG for varying time periods. TRAF degradation in transfectants expressing WT-hCD40 or hCD40-LMP1 was determined by stimulating B cells (107) for 10 min, 30 min, 2 h, or 6 h with 1 μg/ml of either anti-hCD40 (G28-5) or isotype control (MOPC-21) mAbs. Degradation induced via stimulation through endogenous mCD40 was as above, using anti-mCD40 mAb (1C10) or its isotype control (EM-95). After IPTG incubation or mAb stimulation, cells were lysed in 1% SDS, 2% β-ME, and 62.5 mM Tris, pH 6.8. Total cell lysates were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes. To determine dependence of degradation on cellular proteasome activity, expression-matched WT-hCD40– or hCD40-LMP1–expressing M12.4.1 cells (107) were preincubated for 2 h in the presence of either 50 μM MG132 33 or DMSO vehicle at a concentration of 106 cells/ml. Cells were then stimulated with either 1 μg/ml anti-hCD40 (G28-5), mouse IgG1 isotype control (MOPC-21), anti-mCD40 (1C10), or rat IgG2a isotype control (EM95.3) mAbs for 2 h. Cells in IκBα control experiments were stimulated for 20 min as maximal depletion was previously demonstrated at this time point 29. TRAF degradation by signaling via CD40 mutants was determined by stimulating WT-hCD40, hCD40Δ22, or hCD40-T234A–expressing M12.4.1 cells for 10 min, 20 min, or 2 h with 1 μg/ml anti-hCD40 (G28-5) or isotype control (MOPC-21) mAbs.
TRAF Recruitment to Receptors in Detergent-insoluble Fractions.
To determine TRAF movement into detergent-insoluble fractions, WT-hCD40– or hCD40-LMP1– expressing M12.4.1 cells (107) were stimulated for 10 min in 1 ml with either 1 μg anti-hCD40 (G28-5) or mouse IgG1 isotype control (MOPC-21) mAbs. Cells were lysed in Brij lysis buffer (1% Brij 58, 150 mM NaCl, and 20 mM Tris, pH 7.5) containing protease and phosphatase inhibitors for 30 min on ice. Detergent-soluble fractions were separated by centrifugation and the detergent-insoluble pellet was isolated. The pellet was resuspended and sonicated in buffer containing SDS to dissolve membrane rafts (0.5% SDS, 1% β-ME, 1% Brij 58, 150 mM NaCl, and 20 mM Tris, pH 7.5). Detergent-soluble and -insoluble fractions for each condition were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes. Density gradient separation of raft fractions used ultracentrifugation (200,000 g for 4 h at 4°C) of cells lysed as above, then loaded onto a step gradient of 25, 21.5, 18, 15, and 8% Nycodenz (Amersham Pharmacia Biotech), as described previously 23. 11 fractions collected after centrifugation were subjected to SDS-PAGE and Western blotting, as above. A chemiluminescent substrate (Pierce Chemical Co.) was used to detect HRP-labeled Abs on Western blots for this and all additional Western blots.
Electrophoretic Mobility Shift Assays and JNK Activation.
To measure nuclear translocation of NF-κB and Sp1, WT-hCD40– or hCD40-LMP1–expressing M12.4.1 or CH12.LX cells (107) were stimulated for 30, 60, and 120 min with either 1 μg/ml anti-hCD40 (G28-5), mouse IgG1 isotype control (MOPC-21), anti-mCD40 (1C10), or rat IgG2a isotype control (EM95.3) mAbs at a concentration of 106 cells/ml. Nuclear extracts were prepared and electrophoretic mobility shift assays (EMSAs) performed as described previously 34. The probe for NF-κB was described previously 34; the Sp1 probe was the gift of Dr. W. Maury (University of Iowa). Samples were separated on a 5% native polyacrylamide gel at a constant current of 20 mA and x-ray film exposed to dried gels overnight at −70°C.
To measure JNK activation, WT-hCD40– or hCD40-LMP1–expressing M12.4.1 or CH12.LX cells (2 × 106/ml) were stimulated for 15 min (M12.4.1 transfectants) or 30 min (CH12.LX transfectants) with either 1 μg/ml anti-hCD40 (G28-5), mouse IgG1 isotype control (MOPC-21), anti-mCD40 (HM40-3), or hamster IgM isotype control (G235-1) mAbs. Cells were also stimulated with 0.6 M sorbitol (an osmotic stress) as a positive control. The times chosen represent the point of maximal JNK activation for each of these cell lines, determined in previous studies. Cell lysates were prepared and JNK activity measured as described previously 29. Reactions were separated by SDS-PAGE, and phosphorylated c-Jun was visualized by autoradiography of dried gels.
Assays for B Cell Effector Functions.
To evaluate the upregulation of surface proteins in B cells expressing inducible WT-LMP1 or LMP1-hCD40 molecules, stable transfectants of M12.4.1 cells were incubated with 100 μM IPTG. To analyze ligation-induced surface molecule upregulation, 5 × 105 WT-hCD40– or hCD40-LMP1–expressing M12.4.1 cells were stimulated with either 100 ng/ml of anti-hCD40 (G28-5), mouse IgG1 isotype control (MOPC-21), anti-mCD40 (1C10), or rat IgG2a isotype control (EM95.3) in 24-well plates. After 48–72 h, cells were washed and stained with FITC-labeled Abs to surface proteins or with isotype controls. Cells were then evaluated for surface protein expression using a FACScan™ flow cytometer (Becton Dickinson).
IL-6 secretion was evaluated as described previously 7 30. Membrane-bound CD154 is required to induce B cell IL-6 secretion 30. In brief, 105 WT-hCD40– or hCD40-LMP1–expressing CH12.LX cells were stimulated with either untransfected CHO cells, CHO cells expressing mouse CD154, or CHO cells expressing human CD154 at a 1:10 ratio (CHO cells/B cells) in a 96-well microtitration plate for 48 h. IL-6 present in the supernatant was detected by ELISA 7 30.
CH12.LX and its transfected subclones express surface IgM specific for phosphatidylcholine, an Ag found on the surface of SRBCs 35. Enumeration of SRBC-specific IgM-secreting cells was by direct plaque assay, as described previously 36. In brief, 1.5 × 103 cells were stimulated in flat-bottomed 96-well plates in a total volume of 200 μl for 72 h. Ab-secreting cells are measured as cells capable of forming lytic plaques on a lawn of SRBCs in the presence of complement, and are quantitated as plaque-forming cells (PFCs) per 106 viable cells recovered from replicate cultures.
Results
To study potential differences in signaling by the cytoplasmic domains of CD40 and LMP1, stimulated by normal self-aggregation of the LMP1 membrane-spanning domains, we constructed an inducible recombinant LMP1-hCD40 gene encoding the NH2-terminal and transmembrane domains of LMP1 fused to the cytoplasmic CD40 domain. To study signaling events in a manner permitting greater control over signal initiation and more precise matching in protein expression, we also constructed a hCD40-LMP1 gene encoding extracellular and transmembrane CD40 domains fused to the COOH-terminal cytoplasmic LMP1 domain. Two mouse B cell lines (M12.4.1 and CH12.LX) were stably transfected with either of these constructs and signaling activity compared between WT-LMP1 and the LMP1-hCD40 chimera and the wild-type hCD40 and hCD40-LMP1 chimera, respectively. The cytoplasmic domains of mouse and hCD40 are similar in structure and indistinguishable in function 19, and LMP1 functions in a similar manner in both mouse and human B cells 7.
Induced TRAF Degradation by CD40 but Not LMP1.
We have reported previously that after CD40 stimulation, the amounts of TRAF2 decrease in total cell lysates 23, indicating that TRAFs may be degraded after initiation of CD40 signaling. TRAF degradation may ultimately attenuate a signal by CD40 and serve to regulate signaling by the CD40 receptor. LMP1 also utilizes TRAF2 and TRAF3 in its signaling pathway, so we wished to determine if this degradation was also a feature of LMP1 signaling. Induced expression of chimeric LMP1-hCD40 led to TRAF2 and TRAF3 degradation as early as 6 h after IPTG induction of receptor expression. In contrast, induced expression of WT-LMP1 stimulated no demonstrable TRAF2 or TRAF3 degradation for up to 24 h after IPTG induction, although stimulation of these transfectants through endogenous mouse CD40 (samples at right side of panels) led to degradation of both TRAFs (Fig. 1A and Fig. B). Induced expression of LMP1 in this system has been previously shown beginning at 6 h 7. Numbers below each lane indicate densitometry values for the bands shown.
Figure 1.
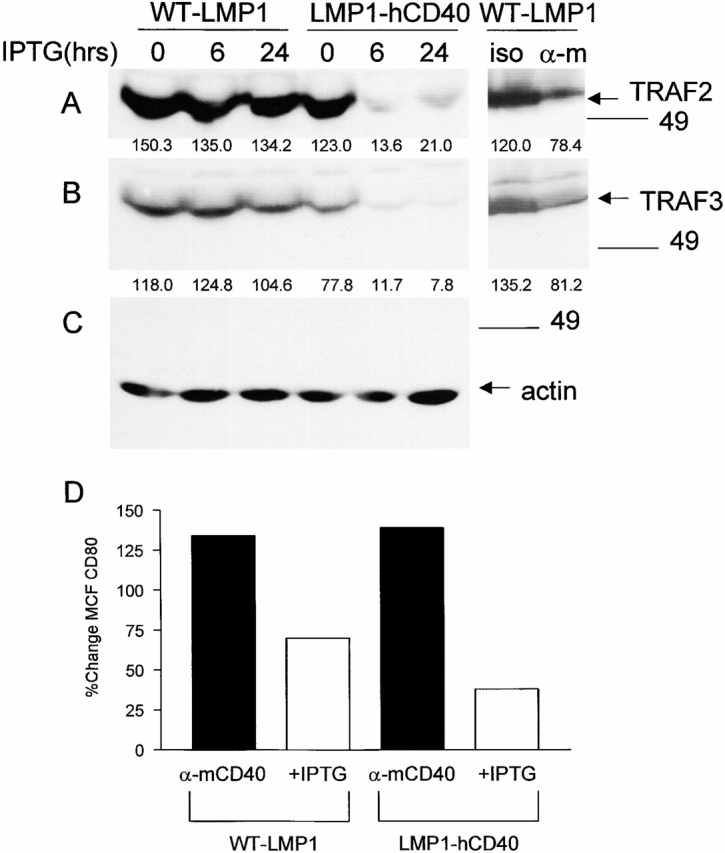
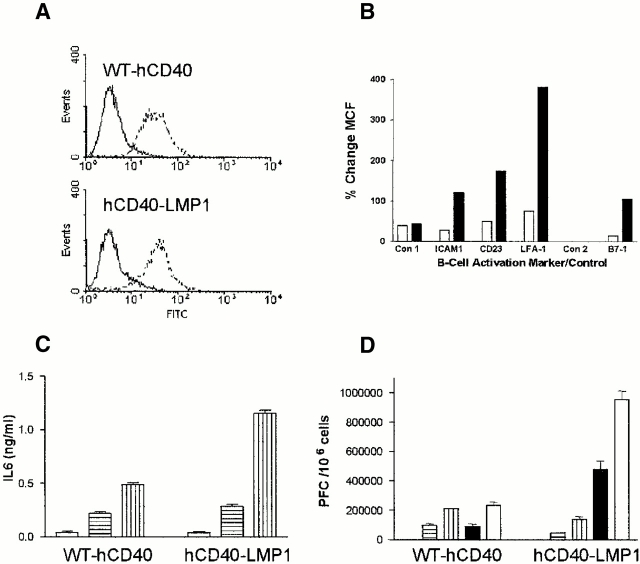
TRAF degradation after induced expression of WT-LMP1 or LMP1-hCD40. (A) M12.4.1 B cells stably transfected with inducible WT-LMP1 or LMP1-hCD40 were induced with IPTG for the indicated number of hours, or stimulated with anti-mCD40 mAb or its isotype control (iso; rightmost two lanes) for 2 h as described in Materials and Methods. Total cell lysates were prepared, separated, and transferred to nitrocellulose membranes as described in Materials and Methods. Western blotting was performed to detect TRAF2 (A), TRAF3 (B), or actin as a loading control (C). Numbers below each lane indicate densitometry values for the bands present in the lane. (D) Stable transfectants as in A were treated with anti-mCD40 mAb, isotype control mAb, medium alone, or IPTG for 72 h, before immunostaining for CD80. Staining and analysis by immunofluorescence flow cytometry were performed as described in Materials and Methods. Data are expressed as the percentage of change in MCF of cells treated with anti-mCD40 mAb/isotype control mAb (black bars) or treated with IPTG/B cell medium (white bars). Data are representative of three independent experiments performed with two sets of stably transfected subclones.
To permit a more direct comparison of the kinetics of TRAF degradation stimulated via the CY domains of CD40 and LMP1, we examined degradation after engagement of the reverse chimeric molecules, WT-hCD40 or chimeric hCD40-LMP1. Stable transfectants tested were expression matched for these two molecules, as shown in Fig. 6 A. TRAF2 and TRAF3 degradation is evident as early as 10 min after ligation of WT-hCD40, but it is strikingly absent after engagement of the hCD40-LMP1 chimera for up to 6 h (Fig. 2A and Fig. B). Addition of the 26S proteasome inhibitor, MG132, led to a partial block in TRAF2 and TRAF3 degradation induced by CD40 but had little effect on TRAF2 and TRAF3 levels in hCD40-LMP1–stimulated cells (Fig. 2D and Fig. E). Simultaneously stimulating B cells through both chimeric hCD40-LMP1 and the endogenous mouse CD40 led to an overall decrease in TRAF2 and TRAF3 levels (Fig. 2D and Fig. E), indicating that CD40-induced degradation predominates over LMP1's inability to induce degradation.
Figure 6.
B cell effector functions stimulated by WT-hCD40 vs. hCD40-LMP1. (A) Expression of transfected WT-hCD40 and hCD40-LMP1 in B cell lines. M12.4.1 transfectants were analyzed by immunofluorescence flow cytometry as described in Materials and Methods, using anti-hCD40 (dotted lines) or isotype control (solid lines) mAbs. All transfectants used in this study, including those in CH12.LX cells, were expression matched in this manner. (B) Summary data of increased surface molecule expression after ligation of WT-hCD40 (white bars) or hCD40-LMP1 (black bars). Transfected M12.4.1 B cells were stimulated with anti-hCD40 or isotype control mAbs for 48 h as described in Materials and Methods. Cells were then analyzed by immunofluorescence flow cytometry as above, using FITC-labeled mAbs to ICAM-1, CD23, LFA-1, or B7-1. Two isotype control mAbs were used for staining; Con 1 is the isotype control for mAbs specific for ICAM-1, CD23, and LFA-1; Con 2 is the control for B7-1 staining. Data are presented as percentage of change in MCF, calculated as MCF of cells stimulated with anti-hCD40/MCF of cells stimulated with control mAbs. Data are representative of three independent experiments, using two sets of stably transfected subclones of B cells. (C) IL-6 secretion induced via WT-hCD40 or hCD40-LMP1. Expression-matched CH12.LX transfect-ants were stimulated with either untransfected CHO cells (white bars), CHO transfectants expressing mouse CD154 (horizontally striped bars), or CHO transfectants expressing human CD154 (vertically striped bars). Conditions of stimulation and details of the IL-6 ELISA assay are described in Materials and Methods; values presented are the mean ± SE of triplicate samples. Data are representative of three independent experiments each for two sets of transfected B cell subclones. (D) IgM secretion induced via WT-hCD40 or hCD40-LMP1. Expression-matched CH12.LX transfectants were stimulated with Ag (SRBC) alone (checked bars; not visible due to low values on this scale), anti-mCD40 (horizontally striped bars), anti-mCD40 plus Ag (vertically striped bars), anti-hCD40 (black bars), or anti-hCD40 plus Ag (white bars). The SRBC-specific plaque-forming cell (PFC) assay was performed as described in Materials and Methods. Values represent mean ± SE of replicate samples, and are representative of two independent experiments each for two sets of transfected B cell subclones.
Figure 2.
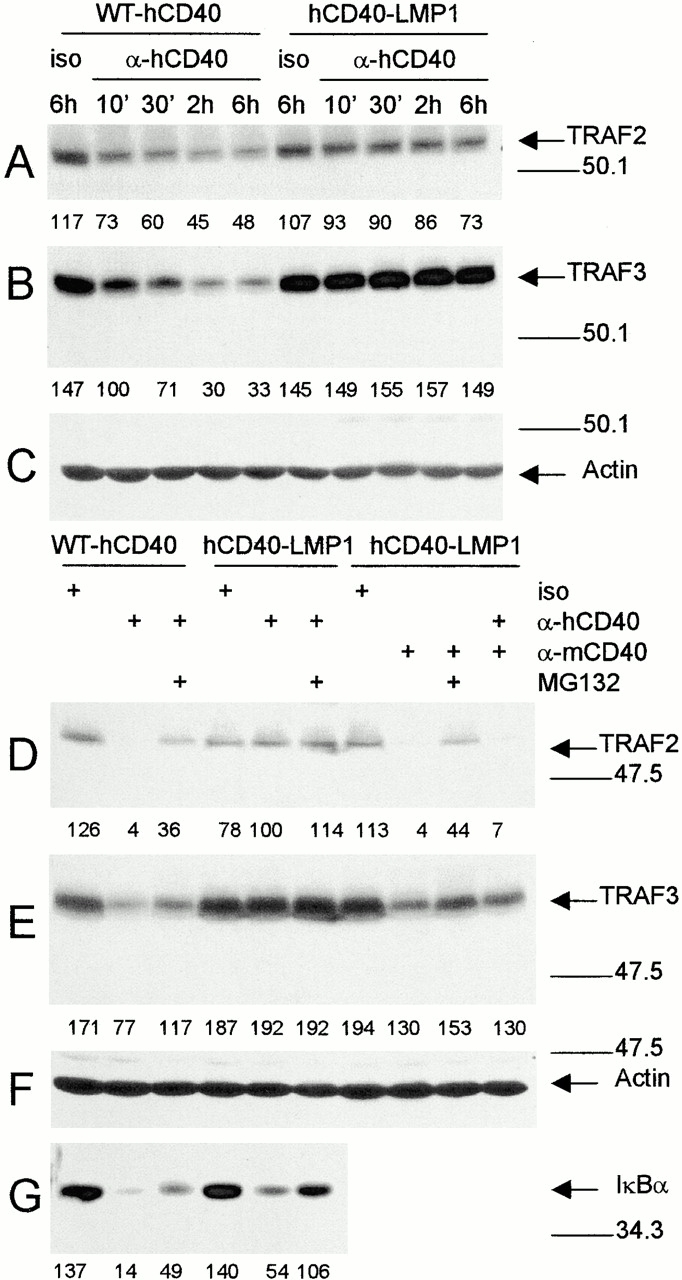
TRAF degradation after ligation of WT-hCD40 or hCD40-LMP1. (A–C) Expression-matched M12.4.1 subclones stably transfected with WT-hCD40 or hCD40-LMP1 were stimulated for the indicated number of minutes or hours with anti-hCD40 or isotype control mAbs (iso), as described in Materials and Methods. Total cell lysates were prepared and analyzed as in the legend to Fig. 1, using blotting Abs specific for (A) TRAF2, (B) TRAF3, or (C) actin. (D–G) Stable transfectants of M12.4.1 cells expressing matched amounts of the molecules indicated at the top of the lanes were stimulated for 2 h (D–F) or 20 min (G) with either anti-hCD40, anti-mCD40, or isotype control mAbs appropriate for each, as indicated to the left at the top of the figure. Where indicated, stimulation was performed in the presence of the proteasome inhibitor MG132. Total cell lysates were prepared and analyzed as above. Blotting Abs were specific for (D) TRAF2, (E) TRAF3, (F) actin, and (G) IκBα. Densitometric values are indicated below each band. Data presented are representative of three independent experiments performed with two sets of stably transfected subclones.
TRAF Degradation Requires Binding to CD40.
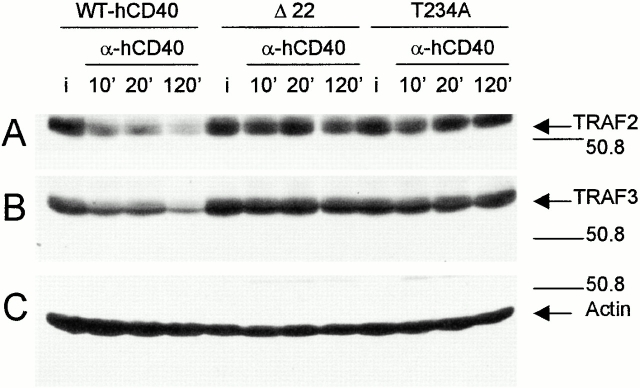
Several reports have indicated that some CD40-mediated activation events are independent of TRAF2 and TRAF 3 binding 17 19 20 30 34 37. To test whether CD40-induced TRAF2 and 3 degradation could be occurring independent of CD40 binding (as an indirect effect of CD40 signaling), two mutants of CD40 were examined, a 22-amino acid deletion of the cytoplasmic tail of hCD40 (hCD40Δ22) and a point mutant in the PXQXT motif of cytoplasmic hCD40 (hCD40T234A). These mutants are defective in binding TRAFs 2 and 3 20. To examine TRAF degradation, WT-hCD40–, hCD40Δ22–, or hCD40T234A–expressing M12.4.1 cells, matched for hCD40 surface expression 19, were stimulated with anti-hCD40 and total lysates prepared. Stimulation of WT-hCD40 led to marked decreases in TRAF2 and TRAF3, but this decrease was noticeably absent after ligation of the hCD40Δ22 and hCD40T234A mutants (Fig. 3A and Fig. B). Analysis of LMP1 cytoplasmic domain mutants, including a 53-amino acid COOH-terminal truncation mutant, a deletion mutant of the proximal COOH-terminal TRAF binding motif, and a point mutant in the distal COOH-terminal TRAF binding motif did not identify mutants with an enhanced ability to degrade TRAFs (data not shown).
Figure 3.
TRAF degradation requires direct binding to hCD40. Expression-matched M12.4.1 cell lines stably expressing WT-hCD40, hCD40Δ22, or hCD40T234A were stimulated with either anti-hCD40 or isotype control mAbs (i) as described in Materials and Methods, for the number of minutes indicated above the lanes. Total cell lysates were prepared and separated as in previous figures, then analyzed by Western blotting for (A) TRAF2, (B) TRAF3, or (C) actin. All cell lines used showed similar TRAF degradation after ligation of their endogenously expressed mCD40 molecules (not shown). Data are representative of three experiments each performed with two sets of stably transfected subclones.
Recruitment of TRAFs into Membrane Rafts.
TRAFs are adapter proteins that play a critical role in signaling by both CD40 and LMP1, and different TRAFs may play different roles in distinct signaling pathways 12 20. We wished to determine if differential TRAF utilization could explain differences in TRAF degradation mediated via CD40 versus LMP1. LMP1 recruits TRAF3 to detergent-insoluble membrane microdomains (membrane rafts; reference 22), and we have shown previously that TRAF2 and TRAF3 are recruited with CD40 to membrane rafts 23. Membrane rafts are cholesterol- and sphingolipid-enriched microdomains within the plasma membrane that may play a role in concentrating receptors with signaling molecules (for reviews, see references 38 and 39).
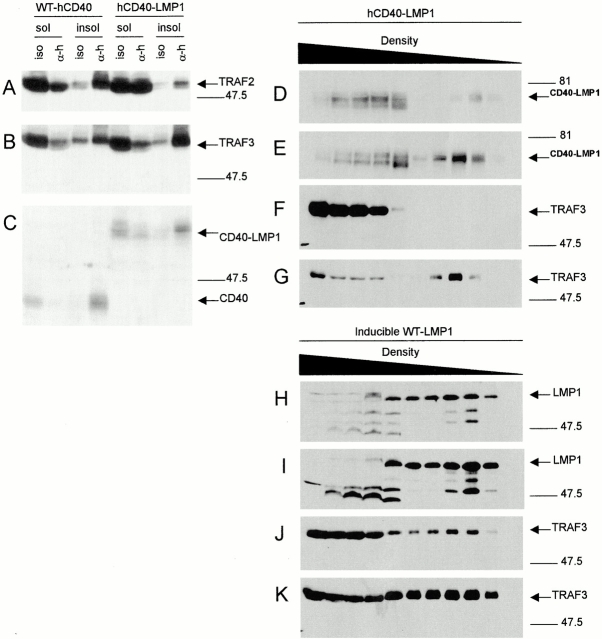
To examine CD40 or LMP1-induced recruitment of TRAFs to rafts, the detergent insoluble fraction of cell lysates was examined after stimulation through WT-hCD40 or hCD40-LMP1. Stimulated cells were lysed in 1% Brij-58 and the insoluble pellet resuspended in 0.5% SDS and sonicated, to obtain detergent soluble and insoluble fractions. Ligation of both WT-hCD40 and hCD40-LMP1 initiated recruitment of TRAFs 2 and 3 to detergent-insoluble fractions (Fig. 4A and Fig. B), although there were reproducible differences in the relative amount of TRAF recruitment. Specifically, WT-hCD40 stimulation appeared more effective in inducing TRAF2 recruitment, whereas hCD40-LMP1 stimulation appeared more effective in inducing TRAF3 recruitment (Fig. 4A and Fig. B). These differences were not due to clonal variation as endogenous mCD40 stimulation in the hCD40-LMP1 transfected cell line induced recruitment of TRAFs 2 and 3 as effectively as did WT-hCD40 in cells expressing this molecule (data not shown). Thus, it is possible that relative differences in the amounts of TRAF recruitment contribute to differences seen in TRAF degradation. However, as hCD40-LMP1 recruits more TRAF3 than does WT-hCD40, but does not cause its degradation, it seems unlikely that TRAF recruitment differences are important to differences in degradation. Both WT-hCD40 and hCD40-LMP1 receptors themselves were recruited into detergent-insoluble fractions after their stimulation, indicating that differences in membrane raft localization of receptors are not responsible for the observed differences in TRAF recruitment (Fig. 4 C).
Figure 4.
(A–C) TRAF recruitment into detergent-insoluble fractions by WT-hCD40 or hCD40-LMP1. Expression-matched M12.4.1 subclones stably transfected with WT-hCD40 or hCD40-LMP1 were stimulated for 10 min with either anti-hCD40 (α-h) or isotype control mAbs (iso), as described in Materials and Methods. Detergent-soluble (sol) and -insoluble (insol) fractions of total cell lysates were prepared as described in Materials and Methods, separated by SDS-PAGE, and analyzed by Western blotting for recruitment of (A) TRAF2 or (B) TRAF3. C was blotted with anti-hCD40 to show the presence of WT and chimeric receptor molecules in the lysate fractions. Data are representative of three independent experiments. (D–K) TRAF recruitment into membrane rafts by hCD40-LMP1 or inducible WT-LMP1. M12.4.1 subclones stably transfected with hCD40-LMP1 (D–G) were stimulated for 10 min with either anti-hCD40 (E and G) or isotype control mAb (D and F) as described in Materials and Methods. M12.4.1 subclones stably transfected with inducible WT-LMP1 (H–K) were uninduced (H and J) or induced with IPTG for 18 h (I and K) as described in Materials and Methods. Density gradient ultracentrifugation was performed as described in Materials and Methods. Cell lysate fractions were separated by SDS-PAGE and analyzed by Western blotting for LMP1 (D, E, H, and I) or TRAF3 (F, G, J, and K). Buoyant density of the fractions collected decreases from left to right as indicated above the blots. Data are representative of three independent experiments performed with two sets of transfected subclones.
Our previous studies have shown that Brij-58–insoluble fractions of cell lysates correspond to high-buoyancy membrane raft fractions isolated by density gradient centrifugation 23. To verify this in this study, lysates from unstimulated (Fig. 4D and Fig. F) or stimulated (Fig. 4E and Fig. G) B cells expressing hCD40-LMP1 were separated by density gradient centrifugation, and fractions analyzed for CD40-LMP1 and TRAF3. Fig. 4H–K, shows the same analysis performed on cells able to inducibly express WT-LMP1, either in the absence (Fig. 4H and Fig. J) or presence (Fig. 4I and Fig. K) of IPTG. Results corroborate those seen in Fig. 4A–C; stimulation of hCD40-LMP1 or induced expression of WT-LMP1 induced their enhanced migration to membrane raft fractions, together with recruitment of TRAF3.
Activation of NF-κB and JNK.
Several signaling events have been suggested to play important roles in CD40- and LMP1-mediated B cell activation, including NF-κB and JNK activation 17 29 40 41. We wished to determine if differences in TRAF degradation between CD40 and LMP1 correlated with the capacity of these receptors to activate these signaling pathways. To examine JNK activation, WT-hCD40 or hCD40-LMP1 transfectants were stimulated with either anti-hCD40 or anti-mCD40 as an internal control. Sorbitol stimulation (an osmotic stress) was used as a positive control. After stimulation, an in vitro kinase reaction was performed using a GST–c-Jun fusion protein substrate as described previously 29. It can be seen in Fig. 5 A that compared with stimulation with isotype control mAb, stimulation through hCD40-LMP1 resulted in two- to threefold greater c-jun phosphorylation than stimulation through WT-hCD40.
Figure 5.
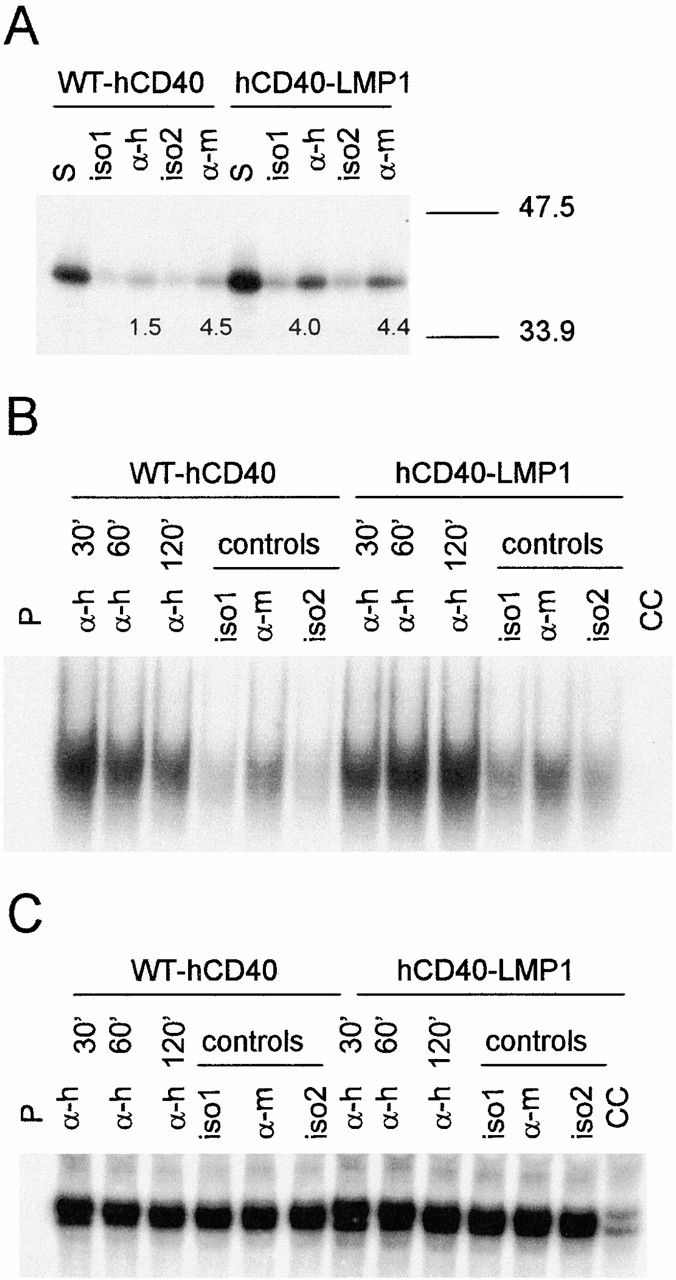
Early B cell activation events triggered via WT-hCD40 vs. hCD40-LMP1. (A) JNK activation. Expression-matched M12.4.1 subclones stably transfected with WT-hCD40 or hCD40-LMP1 were stimulated for 15 min with the positive control reagent sorbitol (S), anti-hCD40 (α-h), anti-mCD40 (α-m), or isotype control mAbs for each (iso1 and iso2, respectively). Stimulation conditions and in vitro kinase assays for the detection of JNK activation were performed as described in Materials and Methods. Numbers below lanes labeled α-h are densitometry values of phosphorylated c-jun stimulated by anti-hCD40 mAb minus values of samples stimulated with iso1. Numbers below lanes labeled α-m are densitometry values of phosphorylated c-jun stimulated by anti-mCD40 mAb minus values of samples stimulated with iso2. Data represent results of three independent experiments; similar results were obtained in CH12.LX transfectants (not shown). (B) Nuclear translocation of NF-κB. Expression-matched CH12.LX subclones stably transfected with WT-hCD40 or hCD40-LMP1 were stimulated for the indicated number of minutes with 1 μg/ml anti-hCD40 mAb. Control stimuli (described in A) were given for 120 min. Cells were lysed and EMSA performed as described in Materials and Methods. The lane labeled P contained radioactive probe alone; the lane labeled CC contained a 10-fold excess of unlabeled probe as a cold competitor. (C) Nuclear Sp1. EMSA was performed on lysates as in B, using an Sp1-specific probe and cold competitor. As in B, the lane labeled P indicates probe alone, and CC contained a 10-fold excess of cold competing probe.
To examine NF-κB activation, WT-hCD40 or hCD40-LMP1 transfectants were stimulated with either anti-hCD40 or anti-mCD40 as an internal control for various times. After stimulation, EMSAs for NF-κB or Sp1 (as a control) were performed. At early time points, WT-hCD40 stimulation induced higher levels of NF-κB nuclear translocation than hCD40-LMP1 (Fig. 5 B), although NF-κB activation stimulated by WT-hCD40 returned to baseline values relatively rapidly. In contrast, translocated NF-κB continued to increase after hCD40-LMP1 stimulation (Fig. 5 B), demonstrating that the cytoplasmic domain of LMP1 induced a more sustained signal. This higher level of NF-κB activation was confirmed by reporter gene analysis (not shown). No change in the amount of nuclear Sp1 was seen after either CD40 or LMP1 stimulation (Fig. 5 C).
Activation of B Cell Effector Functions.
Induction of an activated B cell phenotype, and B cell effector functions, has been demonstrated after stimulation of CD40 or expression of LMP1 4 7 19. We wished to determine if differences in CD40- and LMP1-mediated TRAF degradation and early signaling events had functional implications for the capacity of these receptors to activate B cells. B cell activation was determined by surface molecule upregulation, IL-6 secretion, and IgM secretion. Several surface molecules are upregulated upon B cell activation, including adhesion receptors such as ICAM-1, LFA-1, and CD23, as well as costimulatory molecules such as B7. Signaling via both CD40 and LMP1 upregulates these molecules 7 19, although a direct comparison between the capacity of the two receptors to do so has not been possible previously. M12.4.1 cells stably expressing matched levels of either WT-hCD40 or hCD40-LMP1 (Fig. 6 A) were stimulated for 48 h with either anti-hCD40 or anti-mCD40 as an internal control, and examined for surface expression of ICAM-1, LFA-1, CD23, and B7-1. Anti-hCD40 stimulation of hCD40-LMP1 transfectants resulted in greater cell surface expression of all receptors examined, compared with cells transfected with WT-hCD40 (Fig. 6 B). Differences in upregulation of surface molecule expression between cell lines were not seen after stimulation with anti-mCD40 mAb, indicating these transfectants were equally responsive. Mean channel fluorescence (MCF) values for stimulation via mCD40 in the two stable transfectants were: for hCD40-expressing cells, isotype control staining, 3.73; ICAM-1, 20.15; CD23, 5.71; LFA-1; 5.58, anti-TNP control Ab, 4.47; B7-1, 5.42; hCD40-LMP1–expressing cells, isotype control staining, 3.32; ICAM-1, 19.64; CD23, 5.34; LFA-1, 6.50; anti-TNP control Ab, 4.86; and B7-1, 5.00. To ensure that differences in effector function were seen whether or not the LMP1 membrane-spanning domains are present, the two chimeras described for Fig. 1 were inducibly expressed and tested for their ability to upregulate B7-1. Although it is not possible to precisely match these cell lines for expression of the chimeric molecules, induction of WT-LMP1 expression led to approximately twofold higher levels of B7-1 expression compared with induction of the LMP1-hCD40 chimera (Fig. 1 D), showing that stimulation via self-aggregation of LMP1 leads to the same result as engagement of the external domain of CD40.
We have demonstrated previously that, unlike most CD40-induced B cell functions, production of IL-6 requires stimulation with a membrane-bound form of CD154 30. To evaluate CD40- and LMP1-stimulated IL-6 secretion, CH12.LX cells expressing equal levels of either WT-hCD40 or hCD40-LMP1 were stimulated for 48 h with CHO cells expressing hCD154 (CHO-hCD154). Untransfected CHO cells were a negative control and CHO cells expressing mouse CD154 were an internal positive control. Stimulation of CH12.LX cells expressing hCD40-LMP1 resulted in approximately twofold greater IL-6 production than cells expressing WT-hCD40 (Fig. 6 C). No differences were seen between WT-hCD40– and hCD40-LMP1–expressing cells after stimulation with CHO cells expressing mCD154 (Fig. 6 C, middle bars).
Both CD40 engagement and LMP1 expression induce B cell IgM secretion 7 42, and CH12.LX cells provide an excellent model for studying induced IgM production, as they inducibly secrete IgM specific for a known antigen, phosphatidylcholine 35. CH12.LX cells expressing either WT-hCD40 or hCD40-LMP1 were stimulated with antigen (SRBCs as a source of membrane phosphatidylcholine), with or without anti-hCD40 or anti-mCD40 as an internal control. Stimulation of CH12.LX cells through hCD40-LMP1 resulted in approximately four- to fivefold greater secretion of IgM than cells stimulated through WT-hCD40 (Fig. 6 D). Secreted IgM levels were somewhat higher with anti-mCD40 stimulation in WT-hCD40–expressing cells than in hCD40-LMP1–expressing cells, suggesting differences may be even greater. As described previously 7, both CD40 and LMP1 stimulation synergize with stimulation by antigen through the B cell receptor (Fig. 6 D, white bars).
Discussion
Both CD40 and LMP1 stimulate B cell activation and NF-κB/JNK activation 7 29 41 43. Previous studies have shown that the cytoplasmic domain of LMP1 is necessary and sufficient to deliver these activation signals 7 26 41 44, and have suggested that the LMP1 cytoplasmic domain can mimic that of CD40 5 45, the major difference between the two being that LMP1 signals constitutively via self-aggregation, whereas CD40 requires engagement by CD154. However, several reports have also presented evidence that important differences exist between the signaling pathways used by CD40 and LMP1. LMP1 expression in the Jurkat T cell line stimulates detectable CD54 upregulation, whereas CD40 does not 44, and transgenic B cell expression of LMP1 in CD40−/− mice does not restore the normal development of B cell memory 8. Additionally, earlier studies from our laboratory showed that CD40 and LMP1 signals can cooperate in B cell activation, further supporting the concept that the two receptors use overlapping but distinct mechanisms of signaling 7.
A very early event associated with signaling by both LMP1 and CD40 is the recruitment of the cytoplasmic adapter proteins TRAFs 1, 2, and 3 to cholesterol-rich, detergent-insoluble membrane microdomains, or rafts 22 23. We have recently shown that degradation of TRAF 2 rapidly follows CD40-mediated raft recruitment 23, a finding we have confirmed in normal splenic B cells (unpublished observations). Although TRAF1 is also recruited to rafts by both CD40 and LMP1, signaling through neither molecule stimulates any detectable degradation of TRAF1 (data not shown). To determine whether LMP1 also stimulates degradation of TRAFs 2 and 3, we used an inducibly expressed LMP1 molecule that we previously found stimulates NF-κB activation and B cell effector functions 7. Unlike CD40, self-aggregation of WT-LMP1 stimulated no detectable TRAF degradation. This was not due to a deficiency in the strength of the self-aggregation stimulus compared with normal ligand-induced aggregation of CD40, as a chimeric molecule with the membrane-spanning domains of LMP1 and the cytoplasmic domain of CD40 induced TRAF degradation as effectively as did WT CD40 (Fig. 1 versus Fig. 2).
The failure of LMP1 to mimic CD40 in stimulating the degradation of TRAFs 2 and 3 led us to ask whether this difference is reflected in altered signal strength or duration in downstream effects on B cell activation. Although both CD40 engagement and induced LMP1 expression have been shown to stimulate increased expression of CD80 7, it is not possible to directly compare this effect between WT CD40 and LMP1, because the modes of initiation of the stimulus are quite different. However, when signaling through the cytoplasmic domains of WT-LMP1 and LMP1-hCD40 molecules was initiated for both molecules by self-aggregation of the LMP1 membrane-spanning domains, induced WT-LMP1 expression stimulated greater upregulation of CD80 than did induced expression of the LMP1-hCD40 molecule (Fig. 1 B). This finding suggested that the failure of LMP1 signaling to induce TRAF degradation may contribute to enhanced B cell activation signals delivered via LMP1. However, direct comparison of signaling, particularly early signaling events, is difficult to make between molecules with LMP1 external domains. These molecules begin to signal as their B cell expression is induced, so initiation of signaling cannot be carefully regulated or synchronized between cell lines. Additionally, as their relative expression must be determined by Western blotting, a technique that is only semiquantitative, it is difficult to ensure that two different transfected cell lines are expressing the same amounts of different LMP1 molecules.
As mentioned above, we and others have shown previously that the cytoplasmic domain of LMP1 is necessary and sufficient for B cell activation signals, and Fig. 1 shows that TRAF degradation behavior also maps to the cytoplasmic domain of CD40 versus LMP1. We thus produced a chimeric molecule consisting of the external and transmembrane domains of human CD40 and the cytoplasmic domain of LMP1. This molecule allows quantitation of cell surface expression in transfectants by flow cytometry, and, like CD40, does not signal unless and until it is engaged by CD154 or anti-CD40 mAb. B cell transfectants were generated that were expression matched for either WT-hCD40 or the hCD40-LMP1 chimera (Fig. 6 A). Ligation of these two receptors led to the same outcome as self-aggregation of LMP1 versus LMP1-hCD40; namely, WT-hCD40 induced TRAF 2 and 3 degradation, whereas hCD40-LMP1 did not (Fig. 2). These data validated the use of the hCD40-LMP1 molecule to directly compare signaling by the cytoplasmic domains of CD40 and LMP1. The ability of an inhibitor of the cellular 26S proteasome to diminish the CD40-induced TRAF degradation suggests that the process of ubiquitination may be involved in the degradation, a possibility we are currently investigating.
The inability of the LMP1 molecule to degrade TRAFs 2 and 3 cannot be solely accounted for by strength of TRAF binding. Fig. 4 demonstrates that properties of TRAF binding in B cells confirm previous in vitro binding assays 16. That is, although CD40 appears to bind TRAF2 more effectively, LMP1 shows stronger binding than CD40 to TRAF3. However, CD40 signaling stimulates degradation of both TRAFs, whereas LMP1 signaling stimulates degradation of neither. This suggests that there may be a specific alteration induced in TRAFs 2 and 3 by association with CD40, but not LMP1. This idea is supported by two pieces of evidence. First, although we and others have shown that certain CD40-mediated activation signals are independent of the binding of TRAFs 2 and 3 17 19 20 30 34 46, data presented in Fig. 3 show that hCD40 mutants that cannot bind TRAFs 2 or 3 cannot stimulate TRAF degradation. Second, Fig. 2 shows that when B cells receive signals from both CD40 and LMP1 cytoplasmic domains, TRAFs 2 and 3 are degraded. Thus, LMP1 signaling does not block CD40-mediated TRAF degradation, although in these transfectants the hCD40-LMP1 molecule is expressed at higher levels than the endogenous mCD40 molecule (not shown). This finding argues against the hypothesis that additional intracellular molecules that bind to LMP1 but not CD40, such as TNFR-associated death domain (TRADD), block TRAF degradation. Additionally, LMP1 mutant molecules lacking the TRADD binding site do not gain the ability to degrade TRAFs (unpublished observations). However, intracellular molecules that associate with CD40 but not LMP1 may participate in the differential ability to degrade TRAFs 2 and 3. For example, CD40 but not LMP1 binds TRAF6, and CD40 utilizes TRAF6 in several of its signaling functions 15 47 48. We are currently examining the potential role of TRAF6 and other intracellular molecules in mediating degradation of TRAFs 2 and 3 by CD40 signals.
The model system described in this report permitted a direct comparison of the effects of signaling via CD40 and LMP1 on early B cell activation and later B cell effector functions, and allowed us to determine whether the differential ability to degrade TRAFs 2 and 3 was reflected by differences in downstream signaling through CD40 and LMP1. Analysis in both M12.4.1 and CH12.LX transfectants indicated that the cytoplasmic domain of LMP1 was a more potent stimulus for JNK activation, a very early event after initiation of signaling through each of these molecules. Additionally, NF-κB activation was considerably sustained in cells signaled via the LMP1 cytoplasmic domain compared with those signaled via WT-hCD40 (Fig. 5). Both JNK and NF-κB activation have been shown to play important roles in signaling by both CD40 (for a review, see reference 49) and LMP1 18 41 50. To determine whether enhancement in these early signals correlated with enhanced B cell effector functions, we compared surface molecule upregulation, IL-6 and IgM secretion induced by ligation of either WT-hCD40 or hCD40-LMP1, and found that for all three effector functions, the LMP1 cytoplasmic domain provided a markedly greater stimulus than that of CD40 (Fig. 6).
This study identifies a novel and important mechanism by which TRAF signaling and thereby the signaling of receptors that utilize TRAFs may be regulated. The potential importance of these results is underscored by the fact that LMP1 is a transforming protein 2. Previous reports have concluded that the transforming effect of LMP1 on B cells is mostly due to its constitutive CD40 mimicry (see above). The data presented here suggest that the capacity of LMP1 to transform cells may also be due to the maintenance of an amplified and sustained LMP1 signal, permitted by avoiding TRAF degradation. As the TRAF binding region of LMP1 appears to play a necessary and sufficient role in cellular transformation by LMP1 51 52, stimulation through CD40 may be able to ultimately attenuate LMP1 signaling by degrading the TRAFs necessary for its transforming effect. This hypothesis is supported by experimental data indicating CD40 signals protect EBV-infected human peripheral B cells from undergoing transformation in vitro and in vivo 53.
In summary, we have identified a novel mechanism for regulating CD40 signaling. This mode of regulation involves rapid proteasome-dependent degradation of TRAF adapter molecules after initiation of signaling through CD40. Strikingly, this mode of regulation is absent after initiation of signaling through LMP1, possibly contributing to the enhanced signaling potency of LMP1 and its ability to transform B cells.
Acknowledgments
This work was supported by National Institutes of Health grants AI28847, CA66570, and VA Merit Review 383 to G.A. Bishop. Core support was provided by National Institutes of Health grant DK25295 to The University of Iowa Diabetes and Endocrinology Research Center. K.D. Brown received support from a predoctoral fellowship from the American Heart Association. B.S. Hostager received support from a postdoctoral fellowship from the Arthritis Foundation.
Footnotes
Abbreviations used in this paper: CHO, Chinese hamster ovary; EMSA, electrophoretic mobility shift assay; HRP, horseradish peroxidase; ICAM; intercellular adhesion molecule; JNK, c-Jun kinase; LFA, lymphocyte function–associated antigen; LMP, latent membrane protein; MCF, mean channel fluorescence; NF, nuclear factor; TRAF, TNF receptor–associated factor; WT, wild-type.
References
- Lyons S.F., Liebowitz D.N. The roles of human viruses in the pathogenesis of lymphoma. Semin. Oncol. 1998;25:461–475. [PubMed] [Google Scholar]
- Wang D., Liebowitz D.N., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Kaye K.M., Izumi K.M., Kieff E. EBV LMP1 is essential for B-lymphocyte growth transformationEBV strategy in normal and neoplastic B cells. Proc. Natl. Acad. Sci. USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. EBV LMP alters the human B-lymphocyte phenotypedeletion of the amino terminus abolishes activity. J. Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou E., Miller W.E., Raab-Traub N., Kieff E., Mosialos G. A fusion of the EBV LMP1 transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of EGF-R expression, NF-κB, and stress-activated protein kinase. J. Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- Kilger E., Kieser A., Baumann M., Hammerschmidt W. EBV-mediated B cell proliferation is dependent upon LMP1, which simulates an activated CD40 receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch L.K., Bishop G.A. The EBV transforming protein, LMP1, mimics and cooperates with CD40 signaling in B lymphocytes. J. Immunol. 1999;162:2555–2561. [PubMed] [Google Scholar]
- Uchida J., Yasui T., Takaoka-Shichijo Y., Muraoka M., Kulwichit W., Raab-Traub N., Kikutani H. Mimicry of CD40 signals by EBV LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- Kuhné M.R., Robbins M., Hambor J.E., Mackey M.F., Kosaka Y., Nishimura T., Gigley J.P., Noelle R.J., Calderhead D.M. Assembly and regulation of the CD40 receptor complex in human B cells. J. Exp. Med. 1997;186:337–342. doi: 10.1084/jem.186.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla B.S., Hung S.C., Davidson D.M., Hatzivassiliou E., Malinn N.L., Wallach D., Gilmore T.D., Kieff E., Mosialos G. EBV-transforming protein LMP1 activates transcription factor NF-κB through a pathway that includes NIK and the IκB kinases IKKα and IKKβ. Proc. Natl. Acad. Sci. USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch R.H., Gedrich R.W., Thompson C.B. TRAFs - a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- Rothe M., Sarma V., Dixit V.M., Goeddel D.V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Cheng G., Cleary A.M., Ye Z., Hong D.I., Lederman S., Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- Ishida T., Tojo T., Aoki T., Kobayashi N., Ohishi T., Watanabe T., Yamamoto T., Inoue J.-I. TRAF5, a novel TNF-R-associated factor family protein, mediates CD40 signaling. Proc. Natl. Acad. Sci. USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Mizushima S., Azuma S., Kobayashi N., Tojo T., Suzuki K., Aizawa S., Watanabe T., Mosialos G., Kieff E., Yamamoto T., Inoue J. Identification of TRAF6, a novel TRAF protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J. Biol. Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Hammerschmidt W., Sugden B. Characterization of LMP1 association with TRAF1, 2 and 3. J. Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Reichlin A., Santana A., Sokol K.A., Nussenzweig M.C., Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:701–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- Devergne O., Hatzivassiliou E., Izumi K.M., Kaye K.M., Kleignen M.F., Kieff E., Mosialos G. Association of TRAF1, TRAF2 and TRAF3 with an EBV LMP1 domain important for B-lymphocyte transformationRole in NF-κB activation. Mol. Cell. Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostager B.S., Hsing Y., Harms D.E., Bishop G.A. Different CD40-mediated signaling events require distinct CD40 structural features. J. Immunol. 1996;157:1047–1053. [PubMed] [Google Scholar]
- Hostager B.S., Bishop G.A. Cutting edgecontrasting roles of TRAF2 and TRAF3 in CD40-mediated B lymphocyte activation. J. Immunol. 1999;162:6307–6311. [PubMed] [Google Scholar]
- Devernge O., Hatzivassiliou E., Izumi K.M., Kaye K.M., Kleijnen M.F., Kieff E., Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an EBV LMP1 domain important for B-lymphocyte transformationrole in NF-κB activation. Mol. Cell. Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila-Osorio H., Clausse B., Mishal Z., Wiels J., Tursz T., Busson P. Evidence of LMP1-TRAF3 interactions in glycosphingolipid-rich complexes of lymphoblastoid and nasopharyngeal carcinoma cells. Int. J. Cancer. 1999;81:645–649. doi: 10.1002/(sici)1097-0215(19990517)81:4<645::aid-ijc22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hostager B.S., Catlett I.M., Bishop G.A. Recruitment of CD40, TRAF2 and TRAF3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- Duckett C.S., Thompson C.B. CD30-dependent degradation of TRAF2implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F.K., Lenardo M.J. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals to T lymphocytes. Eur. J. Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Peng-Pilon M., Ruuth K., Lundgren E., Brodin P. The cytoplasmic C-terminal domain but not the N-terminal domain of LMP1 of EBV is essential for B cell activation. J. Gen. Virol. 1995;76:767–777. doi: 10.1099/0022-1317-76-4-767. [DOI] [PubMed] [Google Scholar]
- Bishop G.A., Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc. Natl. Acad. Sci. USA. 1986;83:7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T., Kim K.J., Leiserson W.M., Asofsky R. Establishment of B cell hybridomas with B cell surface antigens. J. Immunol. 1982;129:1403–1411. [PubMed] [Google Scholar]
- Hsing Y., Bishop G.A. Requirement for NF-κB activation by a distinct subset of CD40-mediated effector functions in B lymphocytes. J. Immunol. 1999;162:2804–2811. [PubMed] [Google Scholar]
- Baccam M., Bishop G.A. Membrane-bound CD154, but not anti-CD40 mAbs, induces NF-κB independent B cell IL-6 production. Eur. J. Immunol. 1999;29:3855–3866. doi: 10.1002/(SICI)1521-4141(199912)29:12<3855::AID-IMMU3855>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ho S.N., Hunt H.D., Horton R.M., Pullen J.K., Pease L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Bishop G.A., Frelinger J.A. Haplotype-specific differences in signaling by transfected class II molecules to a Ly-1+ B-cell clone. Proc. Natl. Acad. Sci. USA. 1989;86:5933–5937. doi: 10.1073/pnas.86.15.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H., Kumar S., Howley P.M. Regulation of the Src family tyrosine kinase Blk through E6AP-mediated ubiquitination. Proc. Natl. Acad. Sci. USA. 1999;96:9557–9562. doi: 10.1073/pnas.96.17.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing Y., Hostager B.S., Bishop G.A. Characterization of CD40 signaling determinants regulating NF-κB activation in lymphocytes. J. Immunol. 1997;159:4898–4906. [PubMed] [Google Scholar]
- Mercolino T.J., Arnold L.W., Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J. Exp. Med. 1986;163:155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.A. Requirements of class II-mediated B cell differentiation for class II crosslinking and cAMP. J. Immunol. 1991;147:1107–1114. [PubMed] [Google Scholar]
- Yeh W., Shahinian A., Speiser D., Kraunus J., Billia F., Wakeham A., de la Pampa J.L., Ferrick D., Hum B., Iscove N. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Horejsí V., Drbal K., Cebecauer M., Cerny J., Brdicka T., Angelisová P., Stockinger H. GPI-microdomainsa role in signalling via immunoreceptors. Immunol. Today. 1999;20:356–361. doi: 10.1016/s0167-5699(99)01489-9. [DOI] [PubMed] [Google Scholar]
- Kaye K.M., Devergne O., Harada J.N., Izumi K.M., Yalamanchili R., Kieff E., Mosialos G. TRAF2 is a mediator of NF-κB activation by LMP1, the EBV transforming protein. Proc. Natl. Acad. Sci. USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos A.G., Blake S.M., Floettmann J.E., Rowe M., Young L.S. EBV-encoded LMP1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.A., Warren W.D., Berton M.T. Signaling via MHC class II molecules and antigen receptors enhances the B cell response to gp39/CD40 ligand. Eur. J. Immunol. 1995;25:1230–1238. doi: 10.1002/eji.1830250515. [DOI] [PubMed] [Google Scholar]
- Huen D.S., Henderson S.A., Croom-Carter D., Rowe M. The EBV LMP1 mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;4:549–560. [PubMed] [Google Scholar]
- Floettmann J.E., Eliopoulos A.G., Jones M., Young L.S., Rowe M. EBV LMP1 signalling is distinct from CD40 and involves physical cooperation of its two C-terminus functional regions. Oncogene. 1998;17:2383–2392. doi: 10.1038/sj.onc.1202144. [DOI] [PubMed] [Google Scholar]
- Poe J.C., Wagner D.H., Miller R.W., Stout R.D., Suttles J. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1β synthesis and rescue from apoptosis. J. Immunol. 1997;159:846–852. [PubMed] [Google Scholar]
- Xu Y., Cheng G., Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- Lomaga M.A., Yeh W.C., Sarosi I., Duncan G.S., Furlonger C., Ho A., Morony S., Capparelli C., Van G., Kaufman S. TRAF6 deficiency results in osteopetrosis and defective IL-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1021. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalukar S.V., Hostager B.S., Bishop G.A. Characterization of the roles of TRAF6 in CD40-mediated B lymphocyte effector functions. J. Immunol. 2000;164:623–630. doi: 10.4049/jimmunol.164.2.623. [DOI] [PubMed] [Google Scholar]
- Bishop G.A., Hostager B.S. Molecular mechanisms of CD40 signaling. Arch. Immunol. Ther. Exp. 2001;49:129–137. [PubMed] [Google Scholar]
- Laherty C., Hu H., Opipari A., Wang F., Dixit V. The EBV LMP1 gene product induces A20 zinc finger protein expression by activating NF-κB. J. Biol. Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- Kaye K.M., Izumi K.M., Mosialos G., Kieff E. The EBV LMP1 cytoplasmic C-terminus is essential for B lymphocyte transformation; fibroblast cocultivation complements a critical function within the terminal 155 residues. J. Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K.M., Kaye K.M., Kieff E.D. The EBV LMP1 amino acid sequence that engages TRAFs is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi S., Taub D.D., Asai O., Hirano A., Ruscetti F.W., Longo D.L., Murphy W.J. Effects of CD40 stimulation in the prevention of human EBV-lymphomagenesis. Leuk. Lymphoma. 1997;24:187–199. doi: 10.3109/10428199709039007. [DOI] [PubMed] [Google Scholar]