Abstract
In human breast carcinomas, overexpression of the macrophage colony–stimulating factor (CSF-1) and its receptor (CSF-1R) correlates with poor prognosis. To establish if there is a causal relationship between CSF-1 and breast cancer progression, we crossed a transgenic mouse susceptible to mammary cancer with mice containing a recessive null mutation in the CSF-1 gene (Csf1op) and followed tumor progression in wild-type and null mutant mice. The absence of CSF-1 affects neither the incidence nor the growth of the primary tumors but delayed their development to invasive, metastatic carcinomas. Transgenic expression of CSF-1 in the mammary epithelium of both Csf1op/Csf1op and wild-type tumor-prone mice led to an acceleration to the late stages of carcinoma and to a significant increase in pulmonary metastasis. This was associated with an enhanced infiltration of macrophages into the primary tumor. These studies demonstrate that the growth of mammary tumors and the development to malignancy are separate processes and that CSF-1 selectively promotes the latter process. CSF-1 may promote metastatic potential by regulating the infiltration and function of tumor-associated macrophages as, at the tumor site, CSF-1R expression was restricted to macrophages. Our data suggest that agents directed at CSF-1/CSF-1R activity could have important therapeutic effects.
Keywords: mouse, proliferation, macrophages, metastasis, breast cancer
Introduction
Breast cancer represents the highest incidence and second leading cause of cancer death in American women 1. Nonetheless, little is known about the molecular and cellular factors that contribute to the progression and especially to the metastasis of this cancer. Normal and malignant mammary epithelial and their surrounding stromal cells produce and respond to various growth factors such as TGF α and βs 2, fibroblast growth factors (FGFs 3) and insulin-like growth factors 4. A major effort has been made to determine the role of such growth factors both in the normal development and progression to malignancy of mammary epithelial cells. However, our knowledge of the factors involved in breast cancer progression, especially in the interactions between the mammary epithelial, stromal, and adipose cells, is still limited.
CSF-1 (or macrophage-CSF) is a dimeric polypeptide growth factor that acts through the cell surface receptor (CSF-1R) encoded by the cfms protooncogene 5. CSF-1 was originally identified as a regulator of the proliferation, differentiation, and survival of macrophages and their bone marrow progenitors 5. However, in addition to its normal role in mononuclear phagocyte biology, elevated expression of CSF-1 and cfms has been found in breast, uterine, and ovarian tumor cells, and the extent of expression in these tumors correlates with high grade and poor prognosis 6 7. In breast carcinomas, CSF-1 expression is prevalent in invasive tumor cells as opposed to the intraductal (preinvasive) cancer 8. The coexpression of CSF-1 and CSF-1R in these cells suggests that CSF-1 might be involved in tumor invasion through its direct influence on CSF-1R–bearing tumor cells. However, a strong correlation of CSF-1 expression with dense leukocytic infiltration that contains a large percentage of CSF-1R–bearing cells has also been found in human breast carcinomas 8 9, suggesting that tumor-produced CSF-1 might also be a paracrine modifier of host immune function. Solid tumors are infiltrated by a heterogeneous population of leukocytes of which macrophages represent a major component 10. The biological role and possible clinical significance of these macrophages are still unknown and remained controversial. Studies have shown that macrophages can serve as both positive and negative mediators of tumor growth 11. Macrophages are known to mediate direct antitumor cytotoxicity and the presentation of tumor-associated antigens 12 13. On the other hand, macrophages have also been found to promote tumor angiogenesis 14 and to secrete a wide range of growth factors which may promote tumor growth 15. However, as most of these data are derived from studies of cultured tumor cells or from clinical observations, the functions for macrophages in the tumor microenvironment have still not been determined.
Given the strong association of CSF-1 and CSF-1R expression with tumor progression and macrophage infiltration at the tumor site, it is essential to determine the role of CSF-1 in these processes, especially in the development of metastatic disease. To address this question, we have established a mouse mammary cancer model in the absence of CSF-1 by breeding the CSF-1 null recessive mutation, Csf1op(16), onto a mammary cancer–susceptible strain of mice (polyoma middle T antigen [PyMT] mice), in which the expression of the transgene (Tg), PyMT, was directed to the mammary epithelium by the mouse mammary tumor virus (MMTV) LTR 17. Previous studies have shown that in the CSF-1 null mutant (Csf1op/Csf1op) mice, the development of the myeloid lineage was severely affected with a relative absence of mature macrophages found in many tissues, including the mammary gland 16 18. In this study, we demonstrated that in the absence of CSF-1 the formation and the growth of the primary mammary tumors in PyMT mice was not affected, but the recruitment of macrophages and both the progression to malignancy and the metastasis of these tumors to the lung were significantly delayed. Furthermore, restoration of expression of CSF-1 specifically in the mammary epithelium of CSF-1 null mutant mice and overexpression of CSF-1 in wild-type mice using transgenic techniques accelerated both tumor progression and metastasis.
Materials and Methods
Mice.
All procedures involving mice were conducted in accordance with National Institutes of Health regulations concerning the use and care of experimental animals. The study of mice was approved by the Albert Einstein College of Medicine Animal Use Committee. Detailed descriptions of the origin, care, and identification of Csf1op/Csf1op mice and their heterozygote controls have been given previously 16. +/Csf1op mice have normal serum concentrations of CSF-1, normal tissue populations of macrophages, and are in all aspect tested equivalent to wild-type (+/+) mice 16. Transgenic mice expressing the PyMT oncogene under the control of MMTV LTR were provided by Dr. W.J. Muller (McMaster University, Ontario, Canada). Male Csf1op/Csf1op PyMT mice on a C3H/B6 × FVB-C3H/B6 background were randomly bred with C3H/B6 +/Csf1op females lacking the PyMT Tg to obtain female mice heterozygous for PyMT on either Csf1op/Csf1op or +/Csf1op background. All of the mice analyzed in this study were heterozygous for the PyMT Tg.
The preparation and genotyping of CSF-1–transgenic mice (MMTV/tetracycline activator [Ta]+ and Tg[CSF-1]+) will be described in detail separately (unpublished observations). In brief, the CSF-1 cDNA 19 was ligated downstream of a minimally active CMV promoter controlled by seven tetracycline operators in plasmid 609, provided by Dr. L. Hennighausen (Laboratory of Genetics and Physiology, National Institutes of Health, Bethesda, MD), and transgenic mice were made by routine methods. Trangenic mice carrying this construct (Tg[CSF-1]+) were bred with transgenic mice that expressing the Ta under the control of the MMTV LTR (MMTV/Ta+; reference 20). Male PyMT Csf1op/Csf1op MMTV/Ta+ mice on a C3H/B6 × FVB-C3H/B6 background were crossed with MMTV/Ta+Tg (CSF-1)+ females in a FVB-C3H/B6 background lacking the PyMT Tg. The expression of the CSF-1 Tg in the mammary gland was determined by a reverse transcription (RT)-PCR assay that specifically detects transgenic CSF-1 mRNA. Total mammary gland RNA was prepared following the guanidium-cesium chloride method 21. 2 μg of total RNA was used for RT following the protocol for SuperScript II RNase H-Reverse Transcriptase (GIBCO BRL). Primers used in the PCR reaction were located at the 3′ end of the CSF-1 cDNA (5′-GGGTGCCTGGTTACATCGGAGCAGGGG-3′) and in plasmid 609 (5′-GGGTCCCCAAACTCACCCTG-3′). The level of CSF-1 protein in various tissues was detected by a radioimmunoassay specific for CSF-1 as described previously 22.
Whole Mount Analysis of Mammary Tumor Growth.
Right abdominal mammary glands were used for whole mount preparation as described 18. Whole mounts were photographed with a ruler, scanned, and the size of the primary tumor on the Adobe Photoshop image measured using the NIH Image 1.61 program.
5-Bromo-2-deoxy-uridine Incorporation.
Female PyMT mice were injected intraperitoneally with 100 μg/g body wt of 5-bromo-2-deoxy-uridine (BrdU; GIBCO BRL) 2 h before killing. Left abdominal mammary glands were fixed in formalin, processed for histology, and BrdU-positive cells detected using a BrdU Staining Kit (Oncogene Research Products). The entire primary tumors was scanned into Adobe Photoshop 5 and the area of BrdU-positive cells, as a fraction of the total scanned area of the tumor, determined using NIH Image 1.61 with a computer program written to determine the density of staining.
Lung Metastasis.
Pulmonary metastasis of mammary tumors in PyMT transgenic mice was determined using Northern analysis for the expression of the Tg, PyMT, in the lung. The lungs were removed before removing mammary glands to avoid cross-contamination and RNA was prepared following standard procedures 21. 10 μg of RNA was separated by formaldehyde-agarose gel electrophoresis and blotted onto a nylon filter 21. The expression of PyMT mRNA was detected using [α-32P]dCTP-labeled cDNA probes for PyMT, provided by Dr. W.J. Muller 17, and expression normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Ambion, Austin, TX). After densitometry, the relative level of PyMT mRNA in the lungs was determined between samples by normalizing to the level to a control sample from the mammary gland of a tumor-bearing +/Csf1op PyMT mouse at 18 wk of age. To identify metastatic mammary foci, lungs from mammary tumor–bearing mice were fixed in 10% buffer neutralized formalin, sectioned, and stained with hematoxylin and eosin.
Expression of PyMT in Mammary Epithelium.
Northern blots containing total mammary gland RNA were hybridized with [α-32P]dCTP-labeled cDNA probes for both PyMT and keratin 18 following standard procedures 21. The hybridization signal of PyMT in each mammary gland was normalized to the keratin 18 signal from the same gland.
Histological Analysis and Classification of Mammary Tumors.
The left abdominal mammary glands were formalin fixed, sectioned, and stained with hematoxylin and eosin. Primary mammary tumors were classified into four categories by our histopathologist (R.G. Russell), who was unaware of the genotype of the mice (blinded). These four stages represented the morphologic events of tumor progression from early hyperplasia to advanced adenocarcinoma and conformed to the recommendations for classification of mouse mammary tumor pathology 23. (a) Alveolar hyperplasia: the preneoplastic lesion. The terminal ductal lobular unit comprised of multiple alveoli (acini) branching at the end of the terminal ducts that were lined by cuboidal cells with hyperchromatic nuclei and a reduced cytoplasmic to nuclear ratio. Some acini exhibited orderly, multiple layers of epithelial cells that partially or completely filled the lumen. At this stage, the basement membrane of the secretory unit was intact and the outline of the alveoli distinct. (b) Adenoma: the advance stage of the premalignant tumor. This stage contained well-differentiated, multilobulated, and coalescing solid acinar arrangements that were locally expansile. They were composed of solid sheets of cells, usually uniform, with basophilic round to oval nuclei. The outline of the tumor was well defined within the affected lobule(s). (c) Early adenocarcinoma: early malignancy. Tumors were enlarged, locally expansive, but still well defined. They were composed of locally coalescing solid acini and lobules with cells having pleomorphic nuclei and evidence of the loss of an intact basal lamina. There was a mild increase in connective tissue stroma, particularly among the periductal and ductal hyperplasias. Adjacent lobules in the same mammary gland also showed varying degrees of hyperplasia and adenoma. (d) Late adenocarcinoma: advanced malignancy. Tumors had extensive neoplastic nodules composed of multilobulation of solid neoplastic epithelial cells, sheets of neoplastic cells, poorly differentiated acinar, and sometimes cribiform arrangements. Elevated mitotic indexes and prominent rates of apoptosis were observed in some tumors. Nuclear atypia and multiple local areas of basement membrane loss were found. Some tumors showed cystic alveolar spaces containing protein secretion and lined by irregular multilayered and irregular papillary pleomorphic epithelium.
Immunohistochemistry.
To identify macrophages at the tumor site, fomalin-fixed mammary gland sections were immunostained using the pan-macrophage anti-F4/80 rat monoclonal antibody (CALTAG Laboratories) and specific reactivity was detected using a peroxidase-based detection kit (Vector Laboratories) as described 18.
In Situ Hybridization.
Frozen mammary tissues were sectioned and briefly fixed in 4% paraformaldehyde/PBS, embedded, and sectioned. To prepare the cfms probes, plasmid p755 24 was linearized by digestion with either SalI or NdeI. Digoxigenin (DIG)-labeled sense or antisense RNA probe was synthesized from the Sp6 or T7 promoter, respectively, in a reaction containing 1 μg of DNA template, using a DIG RNA labeling kit (Boehringer Mannheim, Leverkusen, FRG). The hybridization signal was detected using a DIG Nucleic Acid Detection Kit (Boehringer). Placental sections (day 14 gestation) were used as a positive control 25.
Results
Absence of CSF-1 Does Not Effect Growth of the Primary Mammary Tumor.
To determine the effect of the absence of CSF-1 on mammary tumor development and progression, PyMT transgenic mice (PyMT mice), heterozygous and homozygous for the Csf1op mutation (+/Csf1op and Csf1op/Csf1op), together with nontransgenic control mice were examined. Mammary tumors were found in all of PyMT mice as early as 4 wk of age regardless of their background, consistent with the previous report for wild-type PyMT mice on an FVB background 17. No mammary tumors were found in any of the nontransgenic mice, indicating that the development of the tumor was due to the expression of the PyMT Tg.
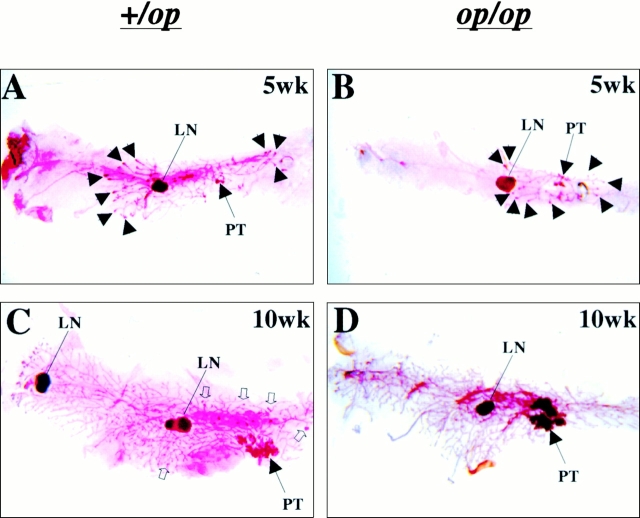
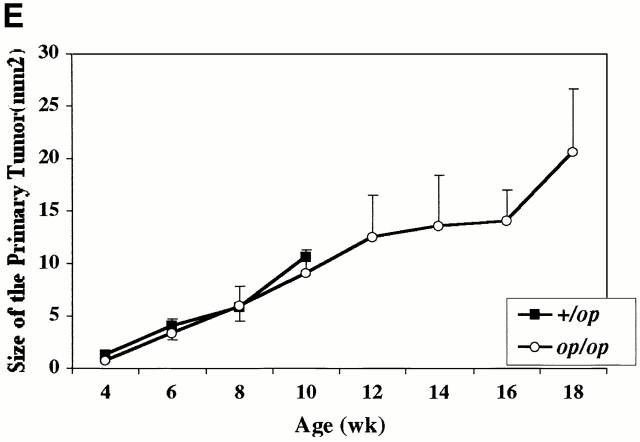
The development of tumors in the mammary glands of both +/Csf1op and Csf1op/Csf1op PyMT mice has an identical pattern. At first, a single focus always developed on the ducts emanating from the nipple (primary tumor [PT]; Fig. 1A and Fig. B). Subsequently, other tumors arose in the ducts distant to the nipple (Fig. 1 C, open arrowheads). Although the development of multiple foci on the distal ducts was reduced in the Csf1op/Csf1op mammary glands (Fig. 1 D), the rate of growth of the primary tumors was comparable to that of +/Csf1op PyMT mice (Fig. 1C Fig. e). In both genotypes, the size of the primary tumor increased linearly from 4 to 10 wk without significant differences in the growth rate between tumors in Csf1op/Csf1opand +/Csf1op PyMT mice (Fig. 1 E). At 12 wk of age in +/Csf1op mice, it became impossible to differentiate the primary tumor from the multiple secondary tumors that had arisen, and data could not be collected beyond 10 wk. However, in Csf1op/Csf1op mice the area of the primary tumor could be measured up to 18 wk and the tumor continued to grow linearly until this point (Fig. 1 E).
Figure 1.
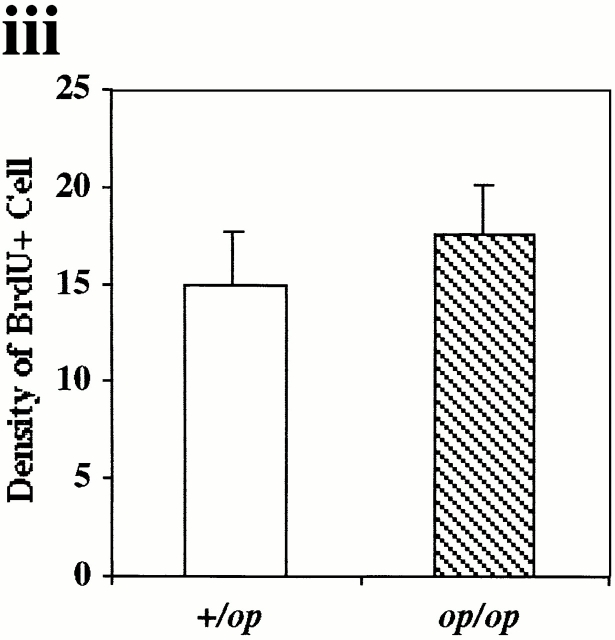
Growth of mammary tumors was not inhibited in Csf1op/Csf1op PyMT mice. (A–D) Representative mammary whole mounts of +/Csf1op(A and C) and Csf1op/Csf1op PyMT mice (B and D) at ages indicated (original magnification: ×2.5). PT, the primary tumor in the nipple area; LN, the subiliac lymph node. Black arrowheads point to terminal-end buds and open arrowheads point to the tumor foci on ducts distal to the nipple. (E) Growth of primary mammary tumors in +/Csf1op(▪; n = 30) and Csf1op/Csf1op (○; n = 53) PyMT mice was measured in mammary whole mounts at the age indicated. Data are presented as the mean ± SE of at least four mice per group. No significant difference was found between the two groups at 4, 6, 8, and 10 wk (unpaired Student's t test). (F) Cell proliferation in primary tumors was determined by BrdU incorporation. Typical distribution of BrdU-positive cells (brown spots) in mammary tumor of +/Csf1op (i) and Csf1op/Csf1op (ii) PyMT mice at 8 wk of age (original magnification: ×100); (iii) comparison of densities of BrdU positive cells in primary tumors of +/Csf1op and Csf1op/Csf1op PyMT mice at 8 wk of age. No significant difference was found (unpaired Student's t test, n = 8). In this and other figures, +/op and op/op indicate +/Csf1op and Csf1op/Csf1op, respectively.
To further determine whether the lack of CSF-1 in Csf1op/Csf1opPyMT mice inhibited the growth of the tumor cells, the proliferative rate of the primary tumors was measured by BrdU incorporation (Fig. 1 F). No significant difference in the distribution and the density of BrdU-positive cells was found in the primary tumors of +/Csf1op and Csf1op/Csf1op PyMT mice (Fig. 1 F, i, ii, and iii). These results further demonstrated that the growth of the primary tumor was not inhibited in the absence of CSF-1.
Absence of CSF-1 Resulted in Delayed Lung Metastasis.
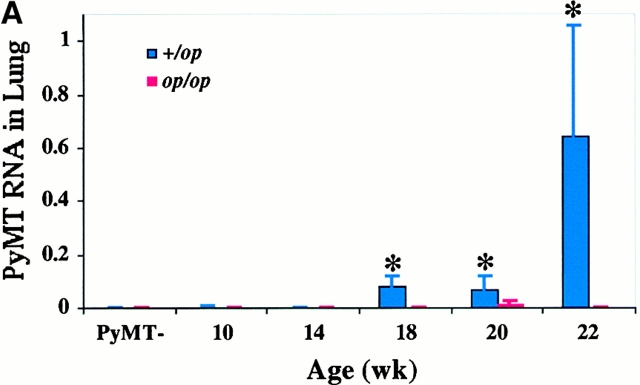
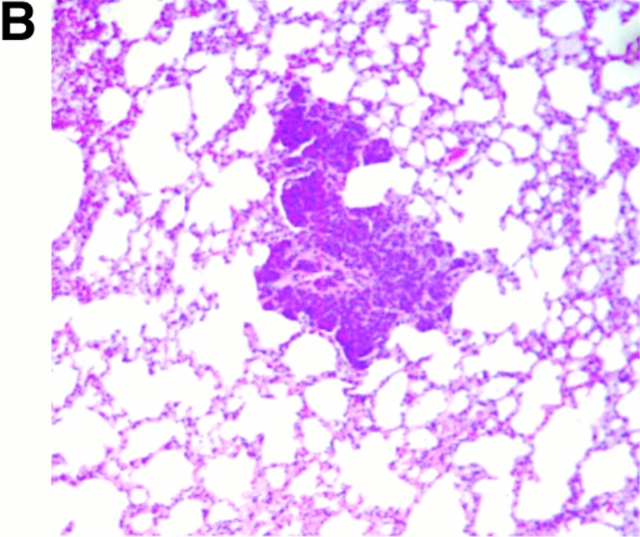
We next determined whether pulmonary metastasis of the mammary tumors in Csf1op/Csf1opPyMT mice was comparable to the +/Csf1op littermates. Previous studies have shown that the expression of PyMT mRNA acts as a reliable biochemical marker for these metastases 17. The expression of PyMT mRNA in lungs of Csf1op/Csf1opand +/Csf1op PyMT mice from 10 to 22 wk was examined by Northern analysis corrected for RNA loading by expression of the housekeeping GAPDH gene. In 10–14-wk-old +/Csf1op mice a very low level of PyMT mRNA was detected in the lungs. By 18 wk of age this expression increased ∼20-fold (P = 0.048) and increased a further 8-fold by 22 wk of age (Fig. 2 A). The metastasis of the mammary tumor in +/Csf1op PyMT transgenic mice was confirmed by histological analysis that showed small colonies of mammary tumor cells growing in the lung (Fig. 2 B). In contrast, only baseline levels of PyMT mRNA were found in lungs of Csf1op/Csf1op mice examined over the same period with the exception of one mouse at 20 wk that showed significant expression of PyMT mRNA (Fig. 2 A). A significant difference in PyMT mRNA level in the lung was found between +/Cf1op and Csf1op/Csf1op mice at 18 to 22 wk (P < 0.0001; Fig. 2 A). No metastatic lesions were found in Csf1op/Csf1opPyMT lungs after histologic examination of a limited number of samples. These results show that by these measurements the metastasis of the mammary tumors to the lung in Csf1op/Csf1op PyMT mice was delayed compared with +/Csf1op littermates.
Figure 2.
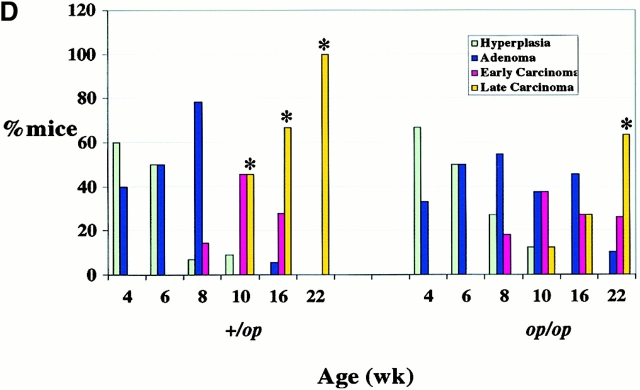
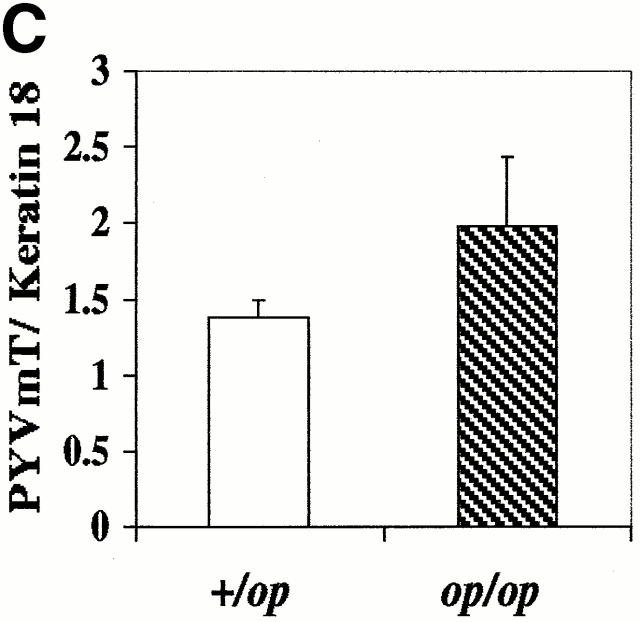
Histopathological progression and metastasis of mammary tumors in Csf1op/Csf1op PyMT mice was delayed. (A) Pulmonary metastasis of mammary tumors in +/Csf1op and Csf1op/Csf1op PyMT mice was compared by Northern analysis of PyMT mRNA expression in lungs. Data are presented as the mean ± SE of at least three mice for each time point. Asterisk indicate the significant difference of +/Csf1op PyMT mice between 10 to 14 and 18 to 22 wk of age (Mann-Whitney test, P = 0.0016, n = 21), and the significant difference between +/Csf1op and Csf1op/Csf1op PyMT mice from 18 to 22 wk of age (Mann-Whitney test, P < 0.0001, n = 28). (B) Hematoxylin and eosin–stained lung section from a +/Csf1op PyMT mouse at 18 wk of age (original magnification: ×100). (C) PyMT mRNA levels in mammary gland epithelium of +/Csf1op and Csf1op/Csf1op PyMT mice from 6 to 12 wk of age were compared using Northern analysis. Mean ± SE of eight mice/group. No significant difference was found between the two groups (unpaired Student's t test). (D) Histopathological progression of primary tumors to late carcinoma in Csf1op/Csf1op PyMT mice was delayed compared with +/Csf1op littermates. Data are presented as the percentile distribution of primary tumors of +/Csf1op (n = 68) and Csf1op/Csf1op PyMT mice (n = 70) in four histopathological stages at the ages indicated. Asterisks indicate a significant difference in the frequency of late carcinoma stage tumors in mice older than 8 wk of age compared with mice of the same genotype at 8 wk of age (Fisher's exact test, P = 0.0087, 0.0004, and 0.0006 for +/Csf1op and P = 0.42, 0.077, and 0.0006 for Csf1op/Csf1op PyMT mice at 10, 16, and 22 wk of age, respectively).
One trivial explanation for the lower rate of tumor progression and metastasis in Csf1op/Csf1opmice is that the Tg, PyMT, is not expressed at the same level in Csf1op/Csf1op and +/Csf1op PyMT mammary epithelium. Therefore, Northern analysis was performed to compare the level of PyMT mRNA in the mammary glands of these mice at various ages. To correct for the variable epithelial cell concentrations in the +/Csf1op and Csf1op/Csf1opmammary glands, PyMT mRNA level in each mammary gland was normalized to the expression of the mRNA for keratin 18, an intermediate filament expressed in simple epithelia and their tumor cells 26. No significant difference was found in the PyMT mRNA levels in +/Csf1op and Csf1op/Csf1op mammary glands (Fig. 2 C), suggesting that mammary epithelial and tumor cells in these mice express similar levels of PyMT.
Histopathological Progression of the Mammary Tumor in Csf1op/Csf1op Mice Is Delayed.
To investigate the basis for the delayed metastasis in Csf1op/Csf1op mice, the histology of the primary mammary tumors in PyMT mice was evaluated in a blinded fashion. The development of the tumor was classified into four histopathological stages, as described in Materials and Methods, from the nonmalignant alveolar hyperplasia and adenoma to the malignant early and late adenocarcinoma. A similar distribution of histopathological stages was found in +/Csf1op and Csf1op/Csf1op primary tumors from 4 to 8 wk of age (Fig. 2 D). Over this age range, the majority of mice in either background developed nonmalignant hyperplasia and adenomas. However, a dramatic progression of the tumor to malignant stages, especially to the most advanced invasive late carcinoma stage, occurred in +/Csf1op mice beginning at 10 wk of age. 50% of +/Csf1op PyMT mice examined at 10 wk had primary tumors that had developed to the most malignant late carcinoma stage, whereas none of the 2 wk younger +/Csf1op PyMT mice had progressed to this stage (P = 0.0087; Fig. 2 D, indicated by *). On the other hand, only 13% of Csf1op/Csf1op PyMT mice examined at 10 wk of age had developed to the late carcinoma stage, which was not a significant increase compared with the Csf1op/Csf1op PyMT mice examined at 8 wk of age (P = 0.42; Fig. 2 D). In fact, compared with Csf1op/Csf1op PyMT mice at 8 wk, a significant increase of primary tumor to the late carcinoma stage did not occur in Csf1op/Csf1op PyMT mice until these mice reached 22 wk of age (Fig. 2 D, indicated by *). These data indicate that the tumor progression to this most aggressive carcinoma stage in Csf1op/Csf1op PyMT mice was >10 wk delayed compared with +/Csf1op littermates (Fig. 2 D).
Paucity of Tumor-associated Macrophages Is Correlated with Delayed Tumor Progression.
CSF-1 is a macrophage growth factor, suggesting that the recruitment of macrophages to the tumor might be a factor that promotes the aggressive development of the +/Csf1op tumors. Consequently, we examined the histology of the mammary tumors and the surrounding stroma from both +/Csf1op and Csf1op/Csf1op PyMT mice at the period of the transition to carcinoma (∼10 wk of age). Before the transition at 7 to 8 wk of age, a dramatic increase of leukocytic infiltration was found around the primary mammary tumors in +/Csf1op mammary glands (Fig. 3 A, indicated by arrows). No such increase was observed at the tumor site in Csf1op/Csf1op mammary glands (Fig. 3 B), though the primary tumors in both Csf1op/Csf1op and +/Csf1op PyMT mice had progressed to similar nonmalignant stages (adenoma). Immunohistochemical analysis, using a monoclonal antibody against the macrophage lineage–specific marker, F4/80, showed that a large percentage of infiltrated leukocytes in the +/Csf1op mammary gland were F4/80 positive (Fig. 3 C) and that few such cells were found around Csf1op/Csf1op primary tumors (Fig. 3 D). In concert with the histopathological development of the primary tumors to the carcinoma stage, the infiltration of leukocytes became more intense and focal infiltration sites were often seen in the mammary glands of +/Csf1op PyMT mice. Densely infiltrated cells with the morphology of granulocytes, mast cells, and monocyte-like cells were found in these sites and the tumor acini adjacent to them often displayed a disrupted boundary (Fig. 3 E, arrows). This suggests that the basement membrane of the acini at the infiltration site had lost its integrity, potentially allowing tumor cells to migrate into the adjacent connective tissue. These leukocytic infiltration sites were detected in +/Csf1op PyMT mice as early as 9 wk of age but were absent in Csf1op/Csf1op mammary glands at the same age (Fig. 3 F). Furthermore, an intensive infiltration of F4/80+ cells was found in the vicinity of +/Csf1op tumor that had developed to the carcinoma stage (Fig. 3G and Fig. I), whereas the density of F4/80+ cells was still reduced in Csf1op/Csf1op tumors, even in those that had developed to the same histological stage (Fig. 3H and Fig. J).
Figure 3.
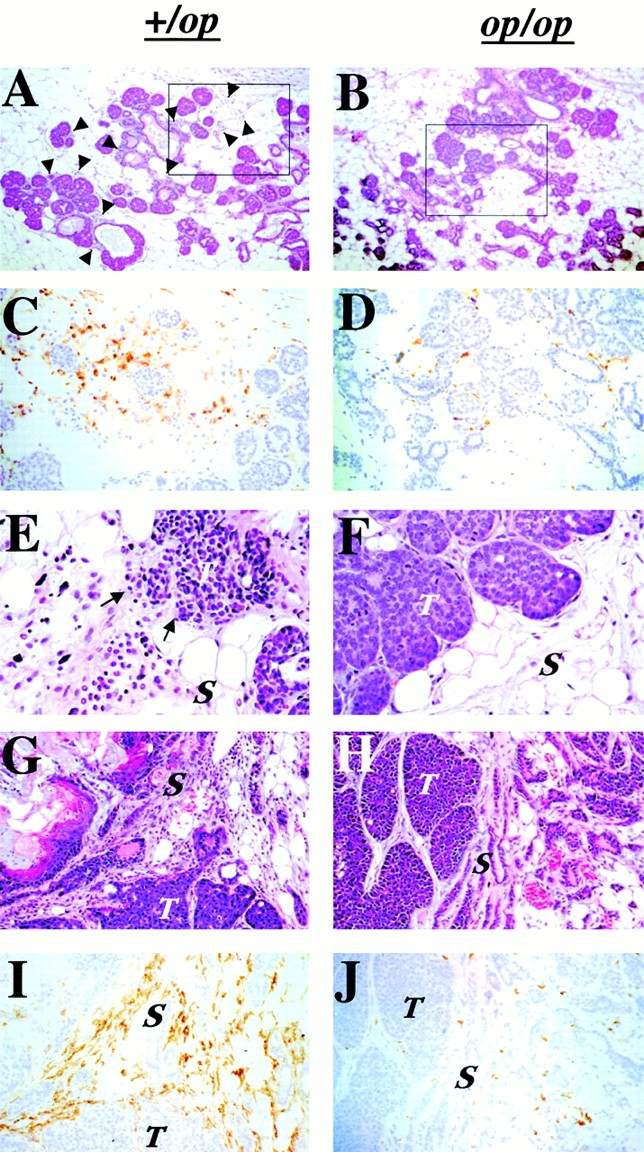
Infiltration of leukocytes and F4/80+ cells at the tumor site was reduced in Csf1op/Csf1op PyMT mice. (A–D) Primary tumors in PyMT mice at 7 wk of age. Hematoxylin and eosin–stained +/Csf1op (A) and Csf1op/Csf1op (B) tumors (original magnification: ×100). Arrowheads indicate infiltrated leukocytes. The insets in A and B are shown in C and D as adjacent sections immunostained with anti-F4/80 monoclonal antibody (original magnification: ×250). (E and F) Hematoxylin and eosin–stained primary tumors from +/Csf1op (E) and Csf1op/Csf1op (F) PyMT mice at 9 wk. Arrows indicate the site at which the tumor acini adjacent to zones of leukocyte infiltration display disrupted boundaries (original magnification: ×400). (G–J) Primary mammary tumors at late adenocarcinoma stage. (G and H) Hematoxylin and eosin–stained mammary primary tumors; (I and J) adjacent sections immunostained with anti-F4/80 monoclonal antibody from +/Csf1op and Csf1op/ Csf1op PyMT mice at 19 and 20 wk of age, respectively (original magnification: ×250). T, tumor; S, stroma.
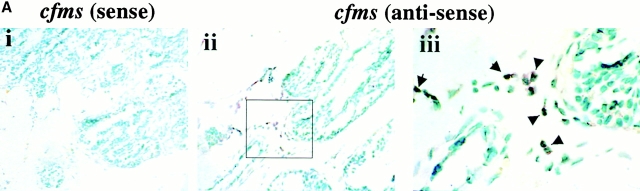
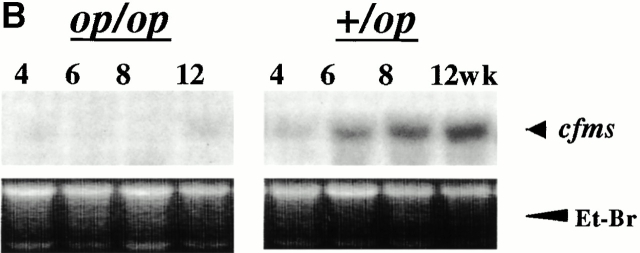
These results are consistent with the hypothesis that CSF-1 acts through macrophages in its promotion of tumor progression. However, in human mammary tumor, CSF-1 receptor expression has been detected in tumor cells as well as infiltrated macrophages 8. To determine if macrophages are the only target cell at the tumor site in mice, we performed in situ hybridization for CSF-1R expression. CSF-1R–positive cells were found to be in the infiltrated cells surrounding the tumor in +/Csf1op mammary glands but the tumor cells were consistently negative (Fig. 4 A, ii). The positively stained cells were mononuclear, dendritic cells with elongated cell bodies, and were in the same location as F4/80+ cells consistent with their identification as macrophages (Fig. 4 A, iii). Very few positive cells were found when the same cfms antisense probe was hybridized to tumor-bearing Csf1op/Csf1op mammary glands (data not shown). The sense cfms probe on adjacent +/Csf1op mammary gland sections was consistently negative (Fig. 4 A, i). To obtain a quantitative comparison of macrophage infiltration in the mammary tumors of Csf1op/Csf1op and +/Csf1opPyMT mice, the expression of cfms was determined by Northern analysis. Expression of cfms mRNA was detected in +/Csf1op mammary glands and an approximately threefold increase of the mRNA was observed at 6 wk compared with 4 wk of age (Fig. 4 B). The cfms mRNA in Csf1op/Csf1op mammary gland was barely detectable until 12 wk, at which age the level of cfms mRNA was less than the level found in +/Csf1op mammary glands at 4 wk of age (Fig. 4 B). As all mononuclear phagocytes express the CSF-1R, this data is consistent with both the F4/80+ immunohistochemistry and the sole localization of the CSF-1R in macrophages.
Figure 4.
Expression of CSF-1R was found in macrophage-like cells. (A) CSF-1R expressing cells at the tumor site were identified by in situ hybridization for cfms. Primary mammary tumor from a +/Csf1op PyMT mouse at 14 wk of age was hybridized with sense (i) and antisense (ii) cfms probes, respectively (original magnification: ×250). The inset in panel (ii) is shown in panel (iii; original magnification: ×1,000). Arrows indicate positively stained cells. (B) Northern analysis of cfms mRNA in mammary glands of +/Csf1op and Csf1op/Csf1op PyMT mice at the ages indicated. Et-Br, the same gel stained with ethidium bromide.
Transgenic Expression of CSF-1 in Csf1op/Csf1op Mammary Epithelium Accelerates Tumor Progression.
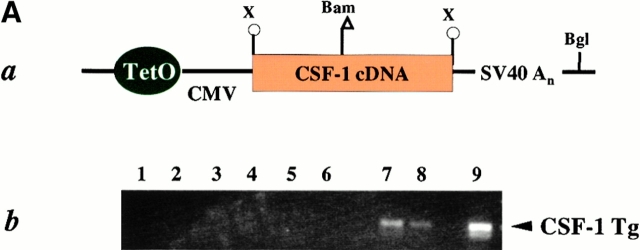
To determine whether the progression and metastasis of mammary tumors in PyMT mice can be affected by CSF-1 produced locally in the mammary gland, transgenic mice expressing CSF-1 specifically in the mammary epithelium were prepared. The transgenic construct consisted of the full-length CSF-1 cDNA which encodes predominately the secretory form of CSF-1 19. The CSF-1 cDNA was ligated downstream of a minimally active CMV promoter controlled by tetracycline operators (Fig. 5 A, a), and the transgenic mice carrying this construct (Tg[CSF-1]+)were bred with mice that expressed the transgenic Ta under the control of MMTV LTR (MMTV/Ta+; reference 20). The progeny having both these Tgs, MMTV/Ta+ and Tg(CSF-1)+, express CSF-1 specifically in mammary and salivary glands (unpublished observations). The expression of the Tg in the mammary glands of CSF-1 transgenic mice (MMTV/Ta+Tg[CSF-1]+) was detected by RT-PCR (Fig. 5 A, b; lanes 7 and 8) but was not found in the mice only carrying the tetracycline transactivator (MMTV/Ta+; Fig. 5 A, b; lanes 3–6). Using a radioimmunoassay specific for CSF-1 22, the concentration of CSF-1 protein in the mammary gland lysates of CSF-1–transgenic Csf1op/Csf1opPyMT mice was comparable to nontransgenic +/Csf1op PyMT controls (data not shown). At 8 wk of age, ∼43 pg/mg of CSF-1 protein was found in the mammary gland of CSF-1–transgenic Csf1op/Csf1opPyMT mice, a level that was similar to +/Csf1op PyMT controls at 18 wk (31 pg/mg). More than a twofold increase of CSF-1 protein concentration was found in the mammary gland of transgenic Csf1op/Csf1op PyMT mice examined at 18 wk of age. Unlike nontransgenic +/Csf1op PyMT control mice, no CSF-1 protein was detected in the serum of CSF-1–transgenic Csf1op/Csf1opPyMT mice and the extended estrous cycle found in Csf1op/Csf1op mice 27 was not corrected in these CSF-1–transgenic mice. This indicates the expression of CSF-1 is restricted to mammary and does not result in a general correction of phenotype (unpublished observations).
Figure 5.
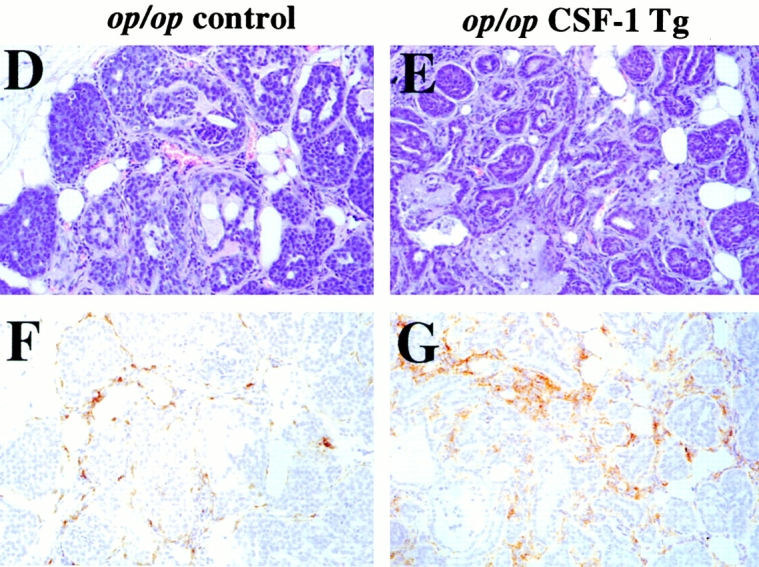
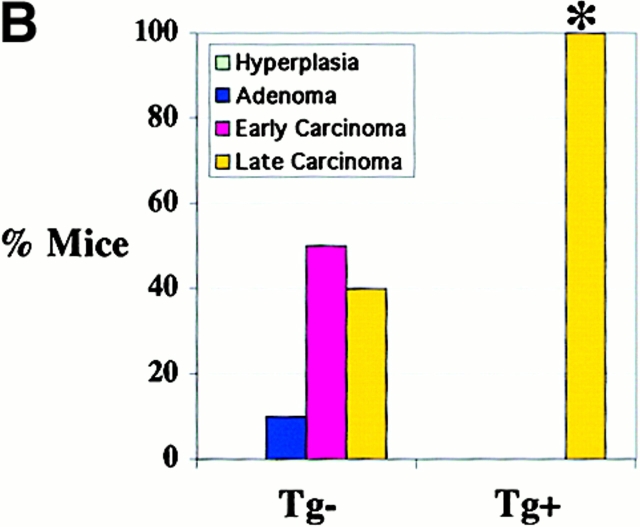
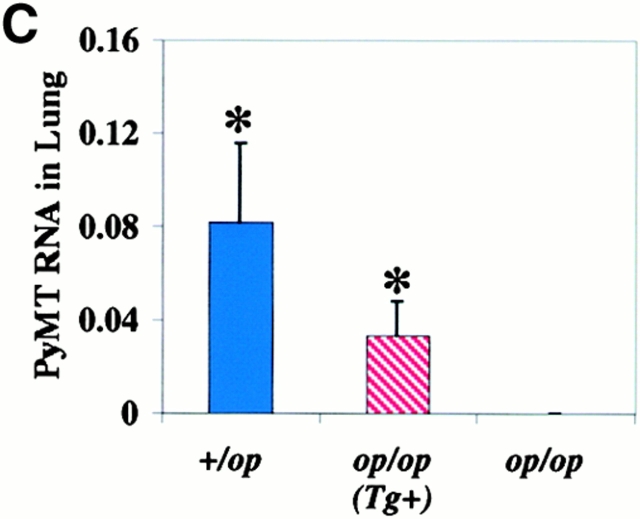
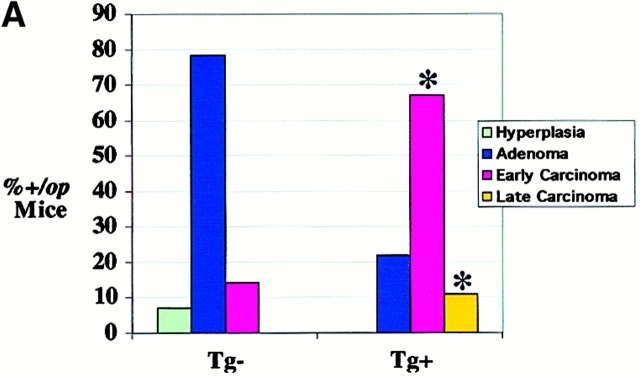
Progression of mammary tumor to malignancy was accelerated in CSF-1–transgenic Csf1op/Csf1op PyMT mice. (A, a) Schematic diagram of CSF-1 transgenic construct. CSF-1 cDNA was ligated downstream of a CMV promoter controlled by tetracycline operators, TetO. SV40An, polyadenylation site from SV40 small t antigen; X, restriction site for XbaI, Bam, restriction site for BamHI; Bgl, restriction site for BglII. (A, b) RT-PCR analysis of transgenic CSF-1 expression in mammary glands. RNA samples were analyzed from four nontransgenic control (lanes 3–6), two CSF-1–transgenic (lanes 7 and 8), and the same CSF-1–transgenic PyMT mice without reverse transcriptase (lanes 1 and 2). Lane 9, genomic DNA from a CSF-1–transgenic mouse. CSF-1 Tg, positive 300-bp band. (B) Histopathological distribution of primary mammary tumors in CSF-1–transgenic and control Csf1op/Csf1opPyMT mice at 18 wk of age. Data are presented as the percentile distribution of four histopathological stages of primary mammary tumors in CSF-1–transgenic (Tg+, n = 6) and control (Tg−, n = 10) Csf1op/Csf1opPyMT mice. Asterisk indicates a significant difference in the frequency of late carcinoma stage tumors between CSF-1–transgenic and control Csf1op/Csf1opPyMT mice (Fisher's exact test, P = 0.039). (C) Pulmonary metastasis of mammary tumors in CSF-1–transgenic Csf1op/Csf1op and control PyMT mice at 18 wk of age were compared by Northern analysis of PyMT mRNA expression in lung. Data are presented as the mean ± SE of at least five mice/point. Asterisks indicate significant differences for both CSF-1–transgenic Csf1op/Csf1op(Tg+, n = 6) and control +/Csf1op PyMT mice (n = 5) when compared with control Csf1op/Csf1op PyMT mice (n = 6) (Mann-Whitney test, P = 0.03 and 0.008, respectively). No significant difference was found between CSF-1–transgenic Csf1op/Csf1opand control +/Csf1op PyMT mice at the same age (Mann-Whitney test, P = 0.33). (D–G) Histology of mammary tumors from CSF-1–transgenic (E and G) and control (D and F) Csf1op/Csf1op PyMT mice at 18 wk of age. Stained with hematoxylin and eosin (D and E) and with anti-F4/80 antibody (F and G; original magnification: ×250).
To determine whether restoration of CSF-1 expression in Csf1op/Csf1op mammary glands could accelerate tumor progression, MMTV/Ta+Tg(CSF-1)+ mice were bred onto Csf1op/Csf1op PyMT mice. Enhanced tumor progression was found in these CSF-1–transgenic Csf1op/Csf1opPyMT mice. Primary mammary tumors in all of the six CSF-1–transgenic Csf1op/Csf1opPyMT mice examined at ∼18 wk had developed to late carcinoma, whereas only 40% (4 out of 10) control Csf1op/Csf1op PyMT mice examined at this age had tumors of this stage (P = 0.039; Fig. 5 B). This histopathological distribution in CSF-1–transgenic mice was identical to that found in the +/Csf1op PyMT controls mice at a comparable age. Pulmonary metastasis had occurred in CSF-1–transgenic Csf1op/Csf1op PyMT mice by 18 wk of age in a fashion similar to +/Csf1op control PyMT mice. The mean level of PyMT mRNA in lungs of 18-wk-old CSF-1–transgenic Csf1op/Csf1op PyMT mice was >100-fold higher than the Csf1op/Csf1op PyMT controls (P = 0.03) and not significantly different from the level in +/Csf1op lungs at the same age (P = 0.33; Fig. 5 C). A marked increase of F4/80+ cells was found in the mammary tumors of these CSF-1–transgenic Csf1op/Csf1opmice (Fig. 5E and Fig. G) compared with the control Csf1op/Csf1opmice that had developed to the same carcinoma stage (Fig. 5D and Fig. F). The distribution of F4/80+ cells in CSF-1–transgenic Csf1op/Csf1op PyMT mice had a different appearance from the infiltration pattern found in +/Csf1op PyMT control mice. In the CSF-1–transgenic Csf1op/Csf1op carcinomas, patches of intensive infiltration were adjacent to areas lacking infiltrated cells whereas in +/Csf1op control carcinomas, F4/80+ cells were often evenly distributed throughout the tumor. These findings are consistent with reports that the expression of the MMTV promoter is mosaic in mammary epithelium 20.
Transgenic Expression of CSF-1 in +/Csf1op Mammary Gland Accelerates Tumor Progression.
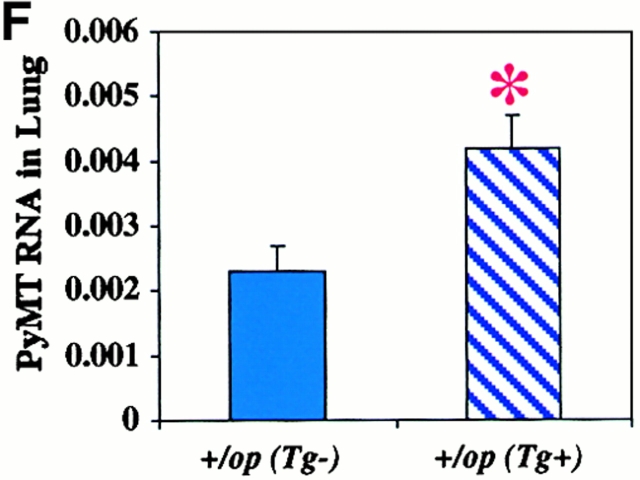
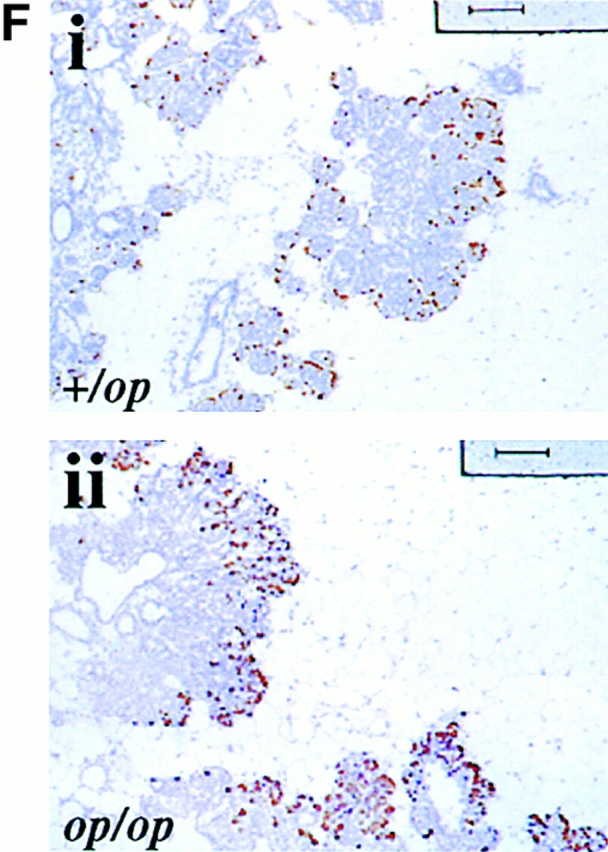
We next asked whether local overexpression of CSF-1 could drive tumor progression in +/Csf1op PyMT mice that are phenotypically normal with respect to the serum CSF-1 level and hematopoietic and mammary gland development 16. We found that both the local progression of the tumor in the mammary gland and pulmonary metastases in these mice were greatly accelerated. At 8 wk of age, a significant advance in tumor histology was found in the CSF-1–transgenic +/Csf1op PyMT mice (Fig. 6 A). Seven out of nine primary tumors of the CSF-1–transgenic +/Csf1op PyMT mice examined at 8 wk were at the carcinoma stage, compared with only 2 out of 14 +/Csf1op control PyMT mice at this age (P = 0.0066; Fig. 6 A). The density of infiltrating leukocytes and F4/80+ cells in the mammary glands of CSF-1–transgenic +/Csf1op PyMT mice was also greater than in control +/Csf1op PyMT mice, and this infiltration occurred much earlier in the transgenic mice compared with the nontransgenic controls. A high density of F4/80+ cells was found in the vicinity of mammary tumors in the CSF-1–transgenic +/Csf1op PyMT mice as early as 5 wk of age (Fig. 6C and Fig. E), whereas very few infiltrates were seen in the control +/Csf1op PyMT mice at this age (Fig. 6B and Fig. D). Compared with 14-wk-old control +/Csf1op PyMT mice a significantly higher level of PyMT mRNA was found in lungs of CSF-1–transgenic +/Csf1op PyMT mice at 13 wk of age (Fig. 6 F; P = 0.029). As only a basal level of PyMT mRNA was found in the lungs of control +/Csf1op PyMT mice until 18 wk of age (Fig. 2 A), this result indicates that the lung metastasis of the mammary tumor in CSF-1–transgenic +/Csf1op PyMT mice occurred several weeks earlier than in the nontransgenic +/Csf1op controls. Thus, local overexpression of CSF-1 not only resulted in an enhancement of tumor progression in Csf1op/Csf1op PyMT mice but also accelerated this process in wild-type PyMT mice expressing normal level of systemic CSF-1.
Figure 6.
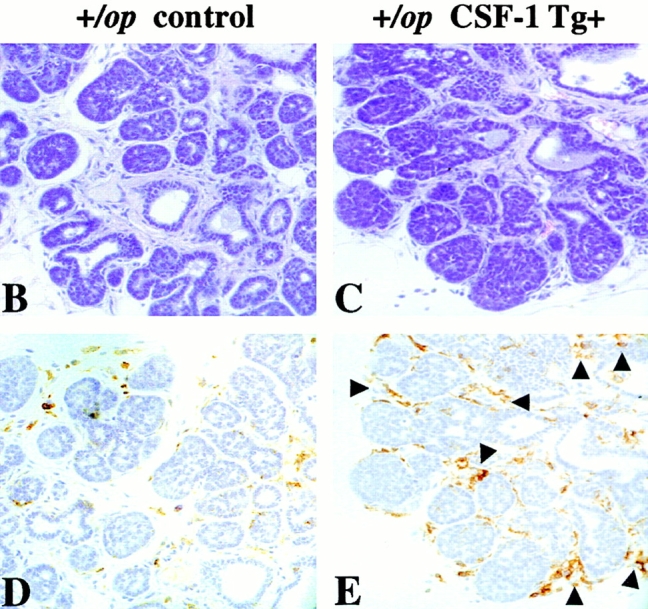
Tumor progression was accelerated in CSF-1–transgenic +/Csf1op PyMT mice. (A) Histopathological distribution of primary mammary tumors in CSF-1–transgenic and control +/Csf1op PyMT mice at 8 wk of age. Data are presented as the percentile distribution of histopathological stages of primary mammary tumors in CSF-1–transgenic (Tg+, n = 9) and control +/Csf1op PyMT mice (Tg−, n = 14). Asterisks indicate a significant difference in the frequency of carcinoma stage tumor between CSF-1–transgenic and control +/Csf1op PyMT mice (Fisher's exact test, P = 0.007). (B–E) Tumor histology and the distribution of F4/80+ cells in tumors of control (B and D) and CSF-1–transgenic (C and E) +/Csf1op PyMT mice at 5 wk of age. (B and C) Hematoxylin and eosin staining; (D and E) the adjacent sections stained with anti-F4/80 monoclonal antibody. Arrows indicate areas with high density of F4/80 cells. (F) Pulmonary metastasis of mammary tumors in CSF-1–transgenic and control +/Csf1op PyMT mice at 13 and 14 wk of age were compared by Northern analysis of PyMT mRNA expression in lung. Data are presented as the mean ± SE of at least three mice/point. Asterisks indicate a significant difference between CSF-1–transgenic +/Csf1op PyMT mice at 13 wk (Tg+) and control +/Csf1op PyMT mice at 14 wk of age (Tg−) (Mann-Whitney test, P = 0.029).
Discussion
The transition from the normal to a malignant state is a multistep process involving genetic and epigenetic changes. Alterations in cellular regulatory circuits have been identified during this progression 28. Many tumor cells produce growth factors and/or their receptors, such as platelet-derived and vascular endothelial growth factor and TGF-α produced by glioblastomas and sarcomas 29 30, fibroblast growth factor 2 by melanomas 31, and epidermal growth factor (EGF) receptor or HER-2/neu overexpressed in breast carcinomas 32. Signaling pathways that are involved in the transduction of mitogenic stimuli, such as the mitogen-activated protein (MAP) kinase and the phosphatidylinositol (PI)-3 kinase pathways, are often constitutively activated in tumor cells 33 34, while upregulated expression of antiapoptotic genes also occurs frequently 35. All of these alterations enable tumor cells to elude the commitment to terminal differentiation and quiescence that normally regulate tissue homeostasis. However, little is known about how these cells exhibiting uncontrolled proliferation or increased life span become malignant and able to metastasize to a distant organ, a feature that differentiates them from benign tumor cells. Many genes specifically expressed in malignant tumors have been identified. However, only a very few of these have been shown to influence the acquisition of invasiveness and metastatic potential 36. In this paper we demonstrate that CSF-1 is one such regulatory factor that has no effect on mammary tumor initiation and growth but promotes tumor progression to malignancy.
Clinically, overexpression of CSF-1 and CSF-1R has been found in a large percentage of breast cancers where expression is correlated with poor prognosis 6. In these tumors, CSF-1R was expressed in both tumor cells and infiltrating macrophages 8. A marked leukocytic infiltration in these tumors also correlated with poor prognosis with the majority of these cells being macrophages 8 37. CSF-1 is the major growth factor for the mononuclear phagocytic lineage and is a chemoattractant for these cells 5 38. Consequently we proposed that CSF-1 might not only act as an autocrine factor for tumor cells but also to recruit macrophages to the tumor site where they promote the progression of the tumor 39.
To test whether CSF-1 has a causal role in mammary tumorigenesis, we established a mouse model whereby a tumor-prone mouse strain was made homozygous or heterozygous for a recessive null mutation in the CSF-1 gene, and the effect of the absence of CSF-1 on tumor progression analyzed. This model more likely mimics the situation in humans, as the tumor develops within the natural mammary environment and all of the factors involved in tumor progression and metastasis are present in the environment. In contrast, previous studies have mainly focused on direct effects of CSF-1 on cultured tumor cells. In these studies, or in tumor cells transplanted into immuocompromised mice models, both positive and negative effects of CSF-1 have been reported 40 41 42 43. However, our data clearly indicates that the absence of CSF-1, although not affecting the incidence or growth of the primary tumor, dramatically reduced its progression to malignancy as assessed by tumor morphology and lung metastasis. A caveat to this interpretation is that the necessary breeding results in a mixing of genetic backgrounds that could potentially expose a modifier gene that might affect the rate of tumor progression. However, our closed colony was randomly bred to ensure that independently segregating genes would be randomly assorted to each genotype. Thus, although modifier genes might increase the data variance, they would not affect the final conclusion, as this breeding strategy ensures that such genes would not be enriched in a particular genotype. Furthermore, restoration of CSF-1 specifically in the mammary gland but not elsewhere except the salivary gland, accelerated mammary tumor progression and increased metastasis. This treatment did not restore serum CSF-1 concentrations in the null mutant mice, or rescue other phenotypes including the extended estrous cycles, osteopetrosis, nor tissue populations of macrophages other than in the mammary gland (unpublished observations). Furthermore, overexpression of CSF-1 in +/Csf1op mice also accelerated mammary gland progression to the metastatic state despite the normal CSF-1 serum and tissue concentrations in these mice. These data argues strongly that the effects of CSF-1 on tumor progression are not secondary to some other phenotype in the mutant mice but are directly due to a requirement in the tumor. They also argue strongly that CSF-1 will have a causal role in tumor progression in humans and that this explains the correlation between CSF-1 overexpression and poor prognosis.
In +/Csf1op PyMT mice, a transition point of the primary mammary tumor to malignancy was identified at 10 wk of age when there was a significant increase in the number of mice that developed late carcinomas, followed by pulmonary metastases at 18 wk of age. This transition to carcinoma was preceded by recruitment of abundant macrophages to the tumor. In contrast, in CSF-1 null mutant PyMT mice, macrophage infiltration at the tumor site was not observed and both malignant transition and pulmonary metastasis were markedly delayed. In addition, increased macrophage density at the tumor site was associated with accelerated tumor progression and metastasis in both Csf1op/Csf1opand +/Csf1op PyMT mice expressing the CSF-1 Tg in the mammary gland. In this mouse model, in contrast to human tumors, CSF-1R expression could only be detected in macrophages and not in the tumor cells. Although it cannot be completely ruled out that tumor cells could also express CSF-1R mRNA at a level below detection in our experiments, our data strongly argues that macrophages are the only target for CSF-1 action. In humans, CSF-1 is also expressed in the tumor cells. However, in mice, analysis of CSF-1 transcripts in the mammary gland and their tumors showed a low level of expression that was not enriched as the tumors grew (data not shown). This suggests that the lack of macrophages in the tumors of Csf1op/Csf1opmice is due primarily to the systemic loss of CSF-1 leading to few circulating monocytes, and that in mice there are chemoattractants other than CSF-1 which recruit macrophages to the tumor site. Nevertheless, as CSF-1 is found in serum and mammary tissue under normal circumstance, these macrophages will be exposed to CSF-1.
Overall, our data suggests a role for infiltrated macrophages in the progression to malignancy. The biological basis of this is unknown. However, we observed high densities of macrophages at sites where the basement membrane of the acini had lost its integrity. Macrophages are potent producers of proteases and, at least, plasminogen activator is CSF-1–regulated in these cells 44. Macrophages also produce many angiogenic factors, including thymidine phosphorylase and vascular endothelial–derived growth factor (VEGF), suggesting that these could also promote this essential process for metastasis 45. Macrophages are also major producers of growth factors 46 such as epidermal growth factor that could act as a paracrine factor on tumor cells promoting their migration and invasion through the acinar basement membrane and into blood vessels 15. However, given the extraordinary range of product that macrophages can synthesize, it seems likely that macrophages play multifunctional roles in the progression of mammary tumors.
The dramatic reduction in tumor progression and metastasis in the absence of CSF-1 represents the first occasion in which histological progression and metastasis have been coordinately modulated in the PyMT breast cancer model. Pulmonary metastasis was found to be reduced in Mgat5-deficient PyMT mice. However, the effect on metastasis could be attributed to an inhibitory effect of Mgat5 deficiency on the growth of the primary tumor 47. Modified tumor organization and increased infiltration of monocytes/macrophages at the tumor site were observed in tenascin-C–deficient PyMT mice; however, neither metastasis nor primary tumor progression were altered in the mice 48. Suppression of pulmonary metastasis was observed in plasminogen-deficient PyMT transgenic mice in the absence of effect on the growth of primary mammary tumor 49. However, no histological difference was detected between tumors in plasminogen-deficient and wild-type mice: all tumors presented as well differentiated adenocarcinomas 49. The age range (10–20 wk) of mice in the latter study was similar to that in the current report, suggesting that the histological defect observed in Csf1op/Csf1op PyMT mice is a specific feature of CSF-1 deficiency and may reflect CSF-1–dependent steps that are antecedent to those affected by plasminogen.
In conclusion, this study has identified CSF-1 as an important regulatory factor in mammary tumor progression to metastasis, possibly acting through macrophages. These data in mice have provided a causal explanation for the correlation between poor prognosis in breast cancer patients with CSF-1 overexpression and leukocytic invasion into the tumor. Consequently, therapeutics targeted at CSF-1 or the CSF-1R could potentially benefit patients.
Acknowledgments
We thank Dr. William J. Muller for generously providing the MMTV-PyMT mice, PyMT cDNA, and for helpful discussions. We thank Drs. John Condeelis, Thomas Graf, Amos Orlofsky, and Jeffrey E. Segall for critical comments upon the manuscript, Dr. Valerie Gouon-Evans for helpful discussion, L. Zhu, J. Lee, and J. Lauridsen for technical support, M. Cammer for designing computer programs for image analysis, and Drs. X.P. Yang and Y. Gu for helping in statistical analysis.
This work was supported by grants from US Army DOD no. 17-97-1-7153 and the Albert Einstein Comprehensive Cancer Center P30-CA13330. J.W. Pollard is the Sheldon and Betty E. Feinberg Senior Faculty Scholar in Cancer Research. E.Y. Lin and A.V. Nguyen were recipients of National Research Service Awards 5-T32-AG00194 and AI-CA09060, respectively.
Footnotes
Abbreviations used in this paper: BrdU, 5-bromo-2-deoxy-uridine; DIG, digoxigenin; MMTV, mouse mammary tumor virus; PyMT, polyoma middle T antigen; RT, reverse transcription; Ta, tetracycline activator; Tg, transgene.
References
- Greenlee R.T., Murray T., Bolden S., Wingo P.A. Cancer statistics, 2000. CA Cancer J. Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- Xie B., Tsao S.W., Wong Y.C. Sex hormone-induced mammary carcinogenesis in female Noble ratsexpression of TGF-beta1 and its receptors, TGF-alpha, and EGF-R in mammary carcinogenesis. Breast Cancer Res. Treat. 1999;58:227–239. doi: 10.1023/a:1006349532643. [DOI] [PubMed] [Google Scholar]
- Dickson C., Creer A., Fantl V. Mammary gland oncogenes as indicators of pathways important in mammary gland development. Oncogene. 2000;19:1097–1101. doi: 10.1038/sj.onc.1203267. [DOI] [PubMed] [Google Scholar]
- Hovey R.C., Davey H.W., Mackenszie D.D.S., McFadden T.B. Ontogeny and epithelial-stromal interactions regulate IGF expression in the ovine mammary gland. Mol. Cell. Endocrinol. 1998;136:139–144. doi: 10.1016/s0303-7207(97)00223-2. [DOI] [PubMed] [Google Scholar]
- Stanley E.R., Guilbert L.T., Tushinski R.J., Bartelmez S.H. CSF-1 A mononuclear phagocyte lineage-specific hemopoietic growth factor. J. Cell. Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Kacinski B.M. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann. Med. 1995;27:79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- Smith H.O., Anderson P.S., Kuo D.Y.K., Goldberg G.L., DeVictoria C.L., Boocock C.A., Jones J.G., Runowicz C.D., Stanley E.R., Pollard J.W. The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin. Cancer Res. 1995;1:313–325. [PubMed] [Google Scholar]
- Scholl S.M., Pallud C., Beuvon F., Hacene K., Stanley E.R., Rohrschneider L.R., Tang R., Pouillart P., Lidereau R. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J. Natl. Cancer Inst. 1994;86:120–126. doi: 10.1093/jnci/86.2.120. [DOI] [PubMed] [Google Scholar]
- Tang R.P., Kackinski B., Validire P., Beuvon F., Sastre X., Benoit P., dela Rochefordiere A., Mosseri V., Pouillart P., Scholl S. Oncogene amplification correlates with dense lymphocyte infiltration in human breast cancersa role for hematopoietic growth factor release by tumor cells? J. Cell. Biochem. 1990;44:189–198. doi: 10.1002/jcb.240440307. [DOI] [PubMed] [Google Scholar]
- O'Sullivan C., Lewis C.E. Tumour-associated leucocytesfriends or foes in breast carcinoma. J. Pathol. 1994;172:229–235. doi: 10.1002/path.1711720302. [DOI] [PubMed] [Google Scholar]
- Elgert K.D., Alleva D.G., Mullins D.W. Tumor-induced immune dysfunctionthe macrophage connection. J. Leukoc. Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- Herberman R.B., Holden H.T., Djeu J.Y., Jerrells T.R., Varesio L., Tagliabue A., White S.L., Oehler J.R., Dean J.H. Macrophages as regulators of immune responses against tumors. Adv. Exp. Med. Biol. 1979;121:361–379. doi: 10.1007/978-1-4684-8914-9_35. [DOI] [PubMed] [Google Scholar]
- Bonta I.L., Ben-Efraim S. Involvement of inflammatory mediators in macrophage antitumor activity. J. Leukoc. Biol. 1993;54:613–626. doi: 10.1002/jlb.54.6.613. [DOI] [PubMed] [Google Scholar]
- Kerbel R.S. Tumor angiogenesispast, present and the near future. Carcinogen. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- Chan A.Y., Raft S., Bailly M., Wyckoff J.B., Segall J.E., Condeelis J.S. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J. Cell Sci. 1998;111:199–211. doi: 10.1242/jcs.111.2.199. [DOI] [PubMed] [Google Scholar]
- Pollard J.W., Stanley E.R. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic (op) Adv. Dev. Biochem. 1996;4:153–193. [Google Scholar]
- Guy C.T., Cardiff R.D., Muller W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogenea transgenic mouse model for metastatic disease. Mol. Cell. Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V., Rothenberg M.E., Pollard J.W. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- Ladner M.B., Martin G.A., Noble J.A., Wittman V.P., Warren M.K., McGrogan M., Stanley E.R. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc. Natl. Acad. Sci. USA. 1988;85:6706–6710. doi: 10.1073/pnas.85.18.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Wall R.J., Tillmann U., Li M., Furth P.A. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J. Cell. Biochem. 1995;59:463–472. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Bartocci A., Pollard J.W., Stanley E.R. Regulation of colony-stimulating factor 1 during pregnancy. J. Exp. Med. 1986;164:956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R.D., Anver M.R., Gusterson B.A., Hennighausen L., Jensen R.A., Merino M.J., Rehm S., Russo J., Tavassoli F.A., Wakefield L.M., Ward J.M., Green J.E. The mammary pathology of genetically engineered micethe consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- Rothwell V.M., Rohrschneider L.R. Murine c-fms cDNAcloning, sequence analysis and retroviral expression. Oncogene Res. 1987;1:311–324. [PubMed] [Google Scholar]
- Arceci R.J., Shanahan F., Stanley E.R., Pollard J.W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc. Natl. Acad. Sci. USA. 1989;86:8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R.G., Baribault H., Caulin C. Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev. 1996;15:445–471. doi: 10.1007/BF00054012. [DOI] [PubMed] [Google Scholar]
- Cohen P.E., Zhu L., Pollard J.W. The absence of CSF-1 in osteopetrotic (csfmop/csfmop) mice disrupts estrous cycles and ovulation. Biol. Reprod. 1997;56:110–118. doi: 10.1095/biolreprod56.1.110. [DOI] [PubMed] [Google Scholar]
- Compagni A., Christofori G. Recent advances in research on multistage tumorigenesis. Br. J. Cancer. 2000;83:1–5. doi: 10.1054/bjoc.2000.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M.T., Hart C.E., Commers P.A., Whitlock J.A., Martincic D., Maciunas R.J., Moots P.L., Shehab T.M. Transforming growth factor beta as a potential tumor progression factor among hyperdiploid glioblastoma culturesevidence for the role of platelet-derived growth factor. J. Neurooncol. 1997;31:233–254. doi: 10.1023/a:1005767616500. [DOI] [PubMed] [Google Scholar]
- Gerharz C.D., Ramp U., Reinecke P., Schardt C., Friebe U., Dejosez M., Nitsch T., Gabbert H.E. Analysis of growth factor-dependent signalling in human epithelioid sarcoma cell lines. clues To the role of autocrine, juxtacrine and paracrine interactions in epithelioid sarcoma. Eur. J. Cancer. 2000;36:1171–1179. doi: 10.1016/s0959-8049(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Becker D., Meier C.B., Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:3685–3691. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard S., Tagliabue E., Campiglio M., Pupa S.M. Role of HER2 gene overexpression in breast carcinoma. J. Cell. Physiol. 2000;182:150–162. doi: 10.1002/(SICI)1097-4652(200002)182:2<150::AID-JCP3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wells A. Tumor invasionrole of growth factor-induced cell motility. Adv. Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]
- Glading A., Chang P., Lauffenburger D.A., Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Escaping cell deathsurvival proteins in cancer. Exp. Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Yokota J. Tumor progression and metastasis. Carcinogen. 2000;21:497–503. doi: 10.1093/carcin/21.3.497. [DOI] [PubMed] [Google Scholar]
- Lewis C.E., Leek R., Harris A., McGee J.O. Cytokine regulation of angiogenesis in breast cancerthe role of tumor-associated macrophages. J. Leuk. Biol. 1995;57:747–751. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- Pollard J.W. Role of colony-stimulating factor-1 in reproduction and development. Mol. Reprod. Dev. 1997;46:54–61. doi: 10.1002/(SICI)1098-2795(199701)46:1<54::AID-MRD9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Scholl S.M., Crocker P., Tang R., Pouillart P., Pollard J.W. Is colony stimulating factor-1 a key mediator in breast cancer invasion and metastasis? Mol. Carcinog. 1993;7:207–211. doi: 10.1002/mc.2940070402. [DOI] [PubMed] [Google Scholar]
- Nowicki A., Szenajch J., Ostrowska G., Wojtowicz A., Wojtowicz K., Kruszewski A.A., Maruszynski M., Aukerman S.L., Wiktor-Jedrzejczak W. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouseevidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int. J. Cancer. 1996;65:112–119. doi: 10.1002/(SICI)1097-0215(19960103)65:1<112::AID-IJC19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Yano S., Nishioka Y., Nokihara H., Sone S. Macrophage colony-stimulating factor gene transduction into human lung cancer cells differentially regulates metastasis formations in various organ microenvironments of natural killer cell-depleted SCID mice. Cancer Res. 1997;57:784–790. [PubMed] [Google Scholar]
- Sapi E., Flick M.B., Rodov S., Gilmore-Hebert M., Kelley M., Rockwell S., Kacinski B.M. Independent regulation of invasion and anchorage-independent growth by different autophosphorylation sites of the macrophage colony-stimulating factor 1 receptor. Cancer Res. 1996;56:5704–5712. [PubMed] [Google Scholar]
- Morita T., Ikeda K., Douzono M., Yamada M., Kimura F., Kawakami K., Sasaki K., Motoyoshi K., Takahara J., Irino S. Tumor vaccination with macrophage colony-stimulating factor-producing Lewis lung carcinoma in mice. Blood. 1996;88:955–961. [PubMed] [Google Scholar]
- Hamilton J.A., Stanley E.R., Burgess A.W., Shadduck R.K. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J. Cell. Physiol. 1980;103:435–445. doi: 10.1002/jcp.1041030309. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C., Steinbrink K., Goebeler M., Bhardwaj R., Sorg C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Leek R.D., Harris A.L., Lewis C.E. Cytokine networks in solid human tumorsregulation of angiogenesis. J. Leukoc. Biol. 1994;56:423–435. doi: 10.1002/jlb.56.4.423. [DOI] [PubMed] [Google Scholar]
- Granovsky M., Fata J., Pawling J., Muller W.J., Khokha R., Dennis J.W. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- Talts J.F., Wirl G., Dictor M., Muller W.J., Fassler R. Tenascin-C modulates tumor stroma and monocyte/macrophage recruitment but not tumor growth or metastasis in a mouse strain with spontaneous mammary cancer. J. Cell Sci. 1999;112:1855–1864. doi: 10.1242/jcs.112.12.1855. [DOI] [PubMed] [Google Scholar]
- Bugge T.H., Lund L.R., Kombrinck K.K., Nielsen B.S., Holmback K., Drew A.F., Flick M.J., Witte D.P., Dano K., Degen J.L. Reduced metastasis of Polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene. 1998;16:3097–3104. doi: 10.1038/sj.onc.1201869. [DOI] [PubMed] [Google Scholar]