Abstract
Signal transducer and activator of transcription (Stat)4 and Stat6 are transcription factors that provide type 1 and type 2 response, respectively. Here, we explored the role of Stat4 and Stat6 in innate immunity during septic peritonitis. Stat4−/− and Stat6−/− mice were resistant to the lethality compared with wild-type (WT) mice. At the mechanistic level, bacterial levels in Stat6−/− mice were much lower than in WT mice, which was associated with increased peritoneal levels of interleukin (IL)-12, tumor necrosis factor (TNF)-α, macrophage-derived chemokine (MDC), and C10, known to enhance bacterial clearance. In Stat4−/− mice, hepatic inflammation and injury during sepsis were significantly ameliorated without affecting local responses. This event was associated with increased hepatic levels of IL-10 and IL-13, while decreasing those of macrophage inflammatory protein (MIP)-2 and KC. Sepsis-induced renal injury was also abrogated in Stat4−/− mice, which was accompanied by decreased renal levels of MIP-2 and KC without altering IL-10 and IL-13 levels. Thus, Stat6−/− and Stat4−/− mice appeared to be resistant to septic peritonitis by enhancing local bacterial clearance and modulating systemic organ damage, respectively, via balancing cytokine responses. These results clearly highlight an important role of local type 1 and systemic type 2 cytokine response in protective immunity during sepsis, which can be regulated by Stat proteins.
Keywords: sepsis, innate immunity, cytokines, bacteria, multiple organ failure
Introduction
Sepsis is a severe infection in the body and bloodstream that commonly originates in the lung, urinary tract, and abdomen 1 2. Although the overall mortality rate of sepsis is ∼25–35%, patients with septic peritonitis have a higher mortality rate, reaching 60–80% 3. Septic peritonitis is characterized by a massive leukocyte infiltration into the peritoneum, where these cells are the first line of defense for clearing microorganisms 4 5. However, once the host fails to restrict microbes to a local area, microbes may invade into the bloodstream and their products trigger an overwhelming systemic response via the overzealous production of inflammatory mediators including cytokines, leading to shock or multiple organ failure (MOF) 6.
The immune response is generally divided into two types, termed type 1 and type 2 responses, based on the distinct cytokine secretion patterns 7. Although the type 1 cytokine response is essentially important in host defense to bacterial infection 8, the type 2 cytokine response also appears to play a protective role when microbes have disseminated into the body. In a murine model of septic peritonitis, we have shown that IL-12 (type 1 promoting cytokine) detected in the peritoneum is beneficial to clear bacteria from the peritoneum 9, while IL-13 (a type 2 cytokine) detected specifically in organs, functions as an immunomodulator of systemic organ failure 10. Thus, mice with septic peritonitis exhibit a mixed type 1 and type 2 cytokine response, leading to our hypothesis that a balanced type 1/type 2 cytokine response may be critical in host defense during sepsis.
Signal transducer and activator of transcription (Stat) proteins are transcription factors that provide a direct link between the cytokine receptors and cytokine-induced gene transcription 11. Stat4 and Stat6 are members of this family that are essential in mediating responses to IL-12 and IL-13, respectively 11 12. In the present study, we employed mice deficient in Stat4 or Stat6 gene (Stat4−/− or Stat6−/−), and further examined the contribution and regulation of type 1 and type 2 cytokine response during sepsis induced by cecal ligation and puncture (CLP), which possesses several hallmarks of clinical sepsis with peritonitis associated with postsurgical or accidental trauma 13. Since IL-12 and IL-13 are important in host defense during CLP-induced sepsis by enhancing bacterial clearance and modulating organ damage, respectively 9 10, we hypothesized that both Stat4−/− and Stat6−/− mice would be susceptible to CLP because of an impaired bacterial clearance and severe organ damage, respectively. Alternatively, a possible reduction in organ damage in Stat4−/− mice and an increase in bacterial clearance in Stat6−/− mice may result in an improved survival of these mice after CLP. In this study, we have shown that both Stat4−/− and Stat6−/− mice are resistant to the lethality initiated by CLP, by balancing the local (peritoneal) and systemic (organ-associated) cytokine responses. The results clearly highlight an important protective role of the local type 1 cytokine and the systemic type 2 cytokine response in innate immunity during sepsis, which is regulated by Stat proteins.
Materials and Methods
Mice.
Stat4−/− and Stat6−/− mice were generated as described previously 14 15, backcrossed 10 generations to BALB/c mice, and maintained in the Indiana University Laboratory Animal Resource Center, and then transferred to the animal care facility unit at the University of Michigan. Female Stat4−/− and Stat6−/− mice (6–8 wk of age) were used in this study. Age- and sex-matched BALB/c mice were purchased from The Jackson Laboratory. All mice were housed for at least 7 d before manipulations under specific pathogen-free conditions. The Animal Use Committee at the University of Michigan approved all studies.
CLP.
The mice underwent CLP surgery as described previously 13. In brief, the mice were anesthetized with intraperitoneal injection of sodium pentobarbital (Veterinary Laboratories, Inc.), followed by ketamine HCL (Vetamine; Mallinckrodt Veterinary, Inc.). Under sterile conditions, the cecum was exposed through a 1-cm incision of the lower left abdomen, ligated with a 3-0 silk suture below the ileocecal valve, and punctured through once with a 21-gauge needle. The cecum was replaced in the peritoneum and the abdomen was closed with surgical staples. The mice were injected with 1 ml of saline subcutaneously for fluid resuscitation, and placed on a heating pad until they recovered from anesthesia.
Experimental Protocol.
In the first set of experiments, survival rates of Stat4−/−, Stat6−/−, and wild-type (WT) mice were monitored for 14 d after CLP. In the next set of experiments, the CLP mice were anesthetized, bled, and killed at appropriate intervals after CLP. The peritoneal cavities were washed with 2 ml of sterile saline, and the lavage fluids were harvested under sterile conditions. After taking a 10-μl aliquot of lavage fluids for assessment of bacteria CFU, the fluids were centrifuged at 6,000 g for 1 min at 4°C and cell-free peritoneal fluids were stored at −20°C. Cell pellets were resuspended in saline and the cell numbers were counted in a hemocytometer. Differential cell analysis was made after Diff-Quik staining of the smear slides (Dade AG). The liver, lung, and kidney were excised, weighed, snap frozen in liquid nitrogen, and stored at −20°C for subsequent analyses. A part of tissues was fixed in 4% paraformaldehyde, embedded in paraffin, and the tissue sections were stained with hematoxylin and eosin.
In other experiments, passive neutralization of TNF-α or IL-12 was carried out by intraperitoneal injection of 0.5 ml of neutralizing anti–murine TNF-α antiserum or anti–murine IL-12 antiserum, 2 h before CLP. The antibodies did not cross-react with several other murine cytokines. As a control, 0.5 ml of preimmune serum was used. The endotoxin content in these sera was below detection level (<0.05 EU/ml, Pyrogent; BioWhittaker).
Determination of CFU.
10 μl of peritoneal lavage fluids and peripheral blood from each mouse was placed on ice and serially diluted with sterile saline. 10 μl of each dilution was aseptically plated on tryptose soy agar (TSA) blood agar plates (Difco Laboratories) and incubated overnight at 37°C, after which the number of colonies was counted. Data were expressed as CFU/10 μl.
Clinical Chemistry.
Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), and creatinine were measured by Clinical Pathology at the University of Michigan Medical School using standardized techniques.
Measurement of Cytokines and Myeloperoxidase.
The protein concentrations of murine TNF-α, IFN-γ, IL-4, IL-10, IL-12, IL-13, macrophage inflammatory protein (MIP)-2, KC, macrophage-derived chemokine (MDC), and C10 were measured by specific ELISAs, as described previously 10 16 17. The ELISAs employed in this study did not cross-react with other murine cytokines, and consistently detected murine cytokine concentrations >25 pg/ml. Myeloperoxidase (MPO) in tissue extracts was measured by ELISA kit (Calbiochem-Novabiochem) according to the manufacturer's instruction. The lower detection limit was 1.6 ng/ml.
Preparation of Organ Extracts.
0.1 g of excised organs was placed in 1 ml of PBS containing complete protease inhibitor (Roche Diagnostics), and homogenized with a Tissue Tearor (model 985-370; Biospec Products, Inc.). The homogenates subsequently received freeze–thaw extraction once for ELISA and twice for MPO assay. The homogenates were centrifuged at 6,000 g for 20 min at 4°C, and the cleared supernatants were used for measurement of cytokines or MPO. Protein concentration in the supernatants was measured by protein dye binding assay (protein assay; Bio-Rad Laboratories). The levels of cytokines and MPO in organ extracts were expressed as nanogram per milligram of protein.
Measurement of Lysozomal Enzyme Release and Superoxide Production.
The lysozomal enzyme release from cells was determined by β-glucuronidase activity in the culture supernatants, according to the methods described previously 18. Peritoneal macrophages were harvested from untreated mice. Peripheral neutrophils (>94%) were isolated from heparinized mice blood by dextran sedimentation, followed by Ficoll gradient centrifugation and hypotonic lysis of erythrocytes. Cells (106 ) were suspended in antibiotics-free RPMI 1640 containing 5% FCS, cultured in 24-well dishes with Escherichia coli, LPS (100 μg/ml; Sigma-Aldrich), or 106 CFU of E. coli recovered from the peritoneum after CLP, incubated overnight at 37°C, and the cleared supernatants were assayed.
The superoxide production from cells was measured using the reduction of ferricytochrome c 19. In brief, cells (106) were cultured in Phenol red–free RPMI 1640 containing cytochrome c (1.3 mg/ml; Sigma-Aldrich), stimulated with LPS or E. coli for 30 min, and the supernatants were measured spectrophotometrically at 550 nm as a function of ferricytochrome c reduction.
Statistics.
Statistical significance was evaluated by analysis of variance. In case of survival curve and CFU count, the data were analyzed by the log-rank test and Mann-Whitney test, respectively. A P < 0.05 value was regarded as statistically significant. All data were expressed as mean ± SEM.
Results
Stat4−/− and Stat6−/− Mice Were Resistant to CLP-induced Lethality.
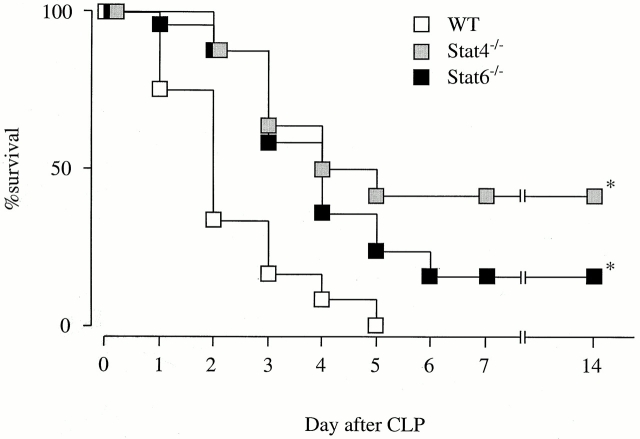
To evaluate the contribution of Stat4 and Stat6 in sepsis induced by CLP, initial studies were carried out to determine the survival rates in Stat4−/−, Stat6−/−, and WT mice after CLP. As shown in Fig. 1, survival rates in Stat4−/− and Stat6−/− mice were significantly higher than in WT mice. On day 2, the survival rate in Stat4−/− and Stat6−/− mice was 87.5% (21/24 mice) and 88.0% (22/25 mice), respectively, but 33.3% (8/24 mice) in WT mice. All WT mice died by day 5 while 41.7% (10/24 mice) and 24% (6/25 mice) of Stat4−/− and Stat6−/− mice, respectively, were alive on the same day (Fig. 1). There were no statistical differences in the survival curves between Stat4−/− and Stat6−/− mice. These data clearly indicate that both Stat4−/− and Stat6−/− mice are resistant to the lethality induced by CLP.
Figure 1.
Stat4−/− and Stat6−/− are resistant to CLP-induced lethality. The survival rates in Stat4−/− (24 mice), Stat6−/− (25 mice), and WT mice (24 mice) were monitored for 14 d after CLP. Two different experiments were carried out, and the data were pooled. The mortality rates were very similar in individual experiments. There is a statistically significant difference between WT and Stat4−/− or Stat6−/− mice (log-rank test). *P < 0.0001 compared with WT.
Bacterial Clearance and Neutrophil Influx Were Augmented in Stat6− / − Mice, but Not in Stat4− / − Mice.
In an attempt to identify the mechanism whereby Stat4−/− and Stat6−/− mice were resistant to CLP, we first examined the bacterial load in the peritoneal fluids and blood of mice after CLP. No bacteria were recovered from the peritoneum and blood of untreated WT, Stat4−/−, and Stat6−/− mice. The peritoneal fluids at 24 h after CLP in WT mice contained a significant number of bacteria in the peritoneum (Fig. 2). At this time point, the bacterial load in the peritoneum in Stat4−/− mice was similar to WT mice. All WT and Stat4−/− mice contained bacteria in the peritoneum. In Stat6−/− mice, however, only 1 out of 11 mice contained bacteria in the peritoneum. Consequently, the mean peritoneal CFU in Stat6−/− mice was 10-fold lower than in WT mice (Fig. 2). Likewise, the bacterial load recovered from peripheral blood in Stat6−/− mice was lower than that in WT or Stat4−/− mice, although it was not statistically significant (Fig. 2). Thus, Stat6−/− mice exhibited the capacity to localize the infection within the peritoneal cavity.
Figure 2.
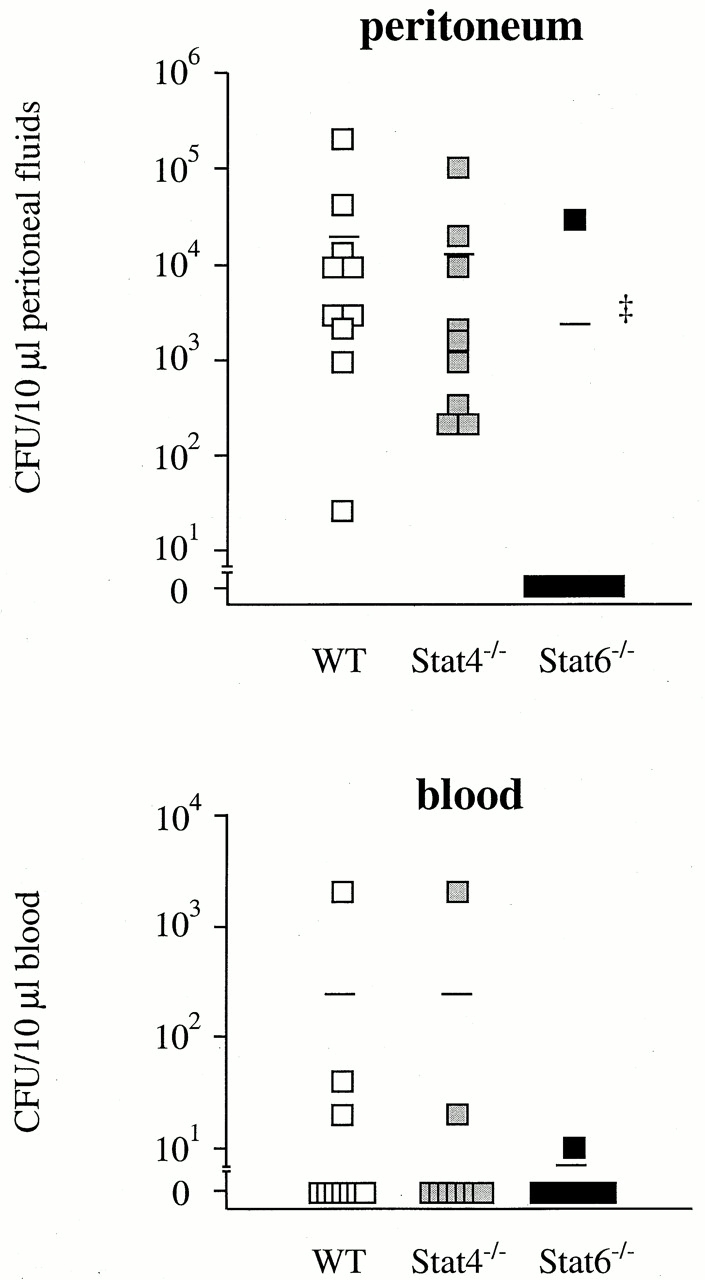
Stat6−/− mice, but not Stat4−/− mice, clear bacteria more effectively than WT mice. At 24 h after CLP (WT, 10 mice; Stat4−/−, 9 mice; Stat6−/−, 11 mice), mice were killed, and the peritoneal fluids and sera were harvested. 10 μl of peritoneal fluids and blood was serially diluted and plated on TSA blood agar plates. Line represents median CFU count. ‡ P < 0.001 compared with WT mice.
CLP induces a rapid leukocyte infiltration in the peritoneum directed at eliminating bacteria from the peritoneum 13 20. Experiments were next conducted to assess the numbers of leukocyte populations in the peritoneum after CLP. The number of resident leukocytes in the peritoneum (>98% macrophages) was similar between WT, Stat4−/−, and Stat6−/− mice (Fig. 3). There were no differences in the numbers of infiltrating neutrophils and macrophages between WT and Stat4−/− mice. However, the number of neutrophils at 6 h after CLP in Stat6−/− mice was significantly increased compared with WT mice (Fig. 3). The leukocyte activation was also assessed in vitro, by measuring β-glucuronidase release (a lysozomal enzyme) and superoxide anion (O2 −) generation (an NADPH-dependent oxidase enzyme) from neutrophils and macrophages after the stimulation with LPS or E. coli. Consequently, there were no differences in the levels of these enzymes between WT and Stat4−/− or Stat6−/− mice, suggesting that the activation of leukocytes by LPS or E. coli in itself is independent of Stat4 and Stat6. Thus, Stat6−/− mice, but not Stat4−/− mice, cleared bacteria more effectively than WT mice, an event that was accompanied by an increase in the number of neutrophils in the peritoneum of Stat6−/− mice, but was not directly associated with the function of innate immune cells.
Figure 3.
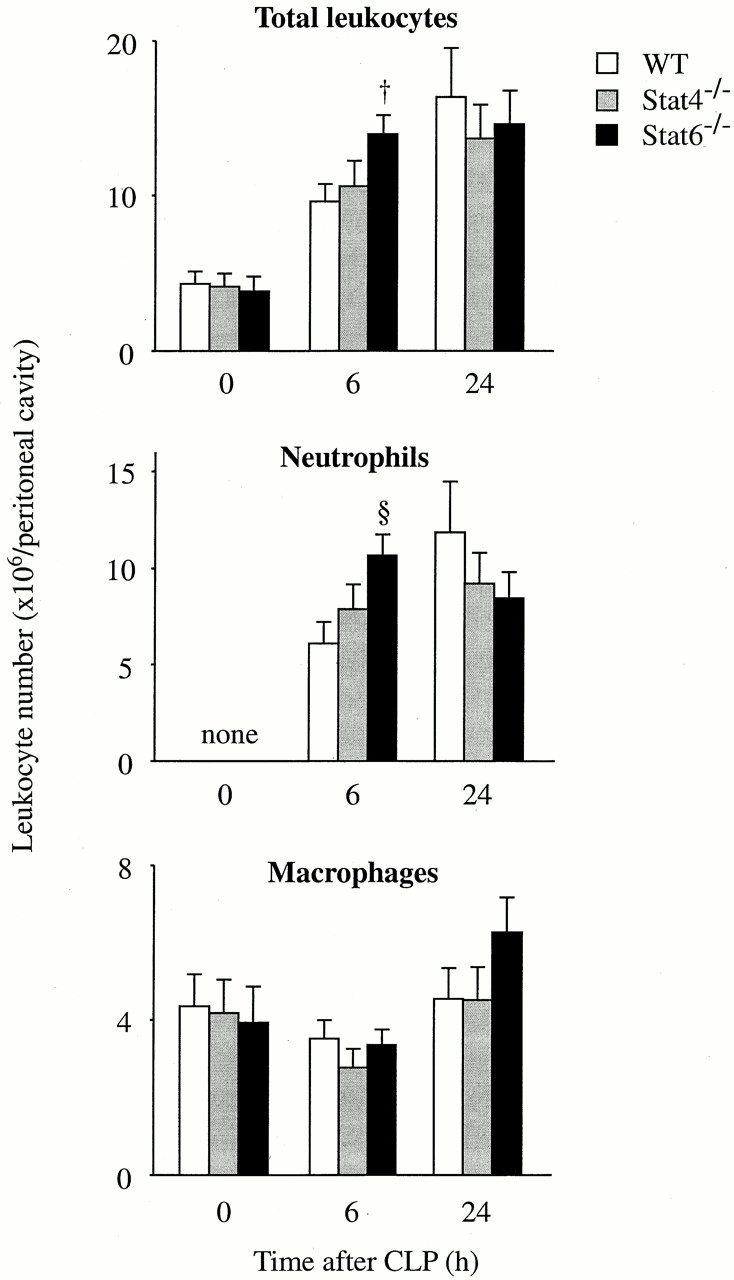
The leukocyte infiltration in the peritoneum after CLP. At 0, 6, and 24 h after CLP, mice were killed and the infiltrating leukocytes in the peritoneal cavity were harvested. The numbers of total leukocytes, neutrophils, and macrophages were then counted. Each point represents the mean ± SEM of 7–11 estimations from separate mice. † P < 0.05, § P < 0.01 compared with WT mice.
Peritoneal Levels of IL-12, TNF-α, MDC, and C10 Were Augmented in Stat6−/− Mice.
To examine the mechanism underlying the increased bacterial clearance and neutrophil infiltration in Stat6−/− mice, peritoneal levels of cytokines in Stat6−/− mice were then measured and compared with those in WT mice. Interestingly, the level of IL-12 in Stat6−/− mice at 6 h after CLP was significantly higher than in WT mice (Fig. 4), although IFN-γ level was not augmented in Stat6−/− mice (data not shown). The levels of TNF-α were increased in Stat6−/− mice compared with WT mice (Fig. 4). When TNF-α, but not IL-12, was neutralized with its antibodies, the neutrophil influx at 6 h after CLP in Stat6−/− mice was significantly reduced compared with control antibodies (Fig. 5 A), suggesting that augmented TNF-α in Stat6−/− mice was responsible for the increased number of neutrophils in Stat6−/− mice. The bacterial load in the peritoneum of Stat6−/− mice was increased by either anti–TNF-α or anti–IL-12 treatment compared with control (Fig. 5 B), indicating that elevated levels of IL-12 and TNF-α contribute to the improved bacterial clearance in Stat6−/− mice. MDC levels at 6 h after CLP were dramatically increased while decreasing at 24 h, suggesting that the production kinetics of MDC was altered in Stat6−/− mice. In addition, C10 levels after CLP were increased in Stat6−/− mice compared with WT mice (Fig. 4). CC chemokines MDC and C10 are shown to enhance bacterial killing activity of macrophages in this model of sepsis 16 17. On the other hand, CLP failed to induce IL-4 and IL-13 in the peritoneum at any time checked in both WT and Stat6−/− mice. IL-10 was increased in the peritoneum after CLP, but there was no statistical significance between WT and Stat6−/− mice (data not shown). Thus, Stat6−/− mice showed an altered cytokine profile in the peritoneum in favor of bacterial clearance.
Figure 4.
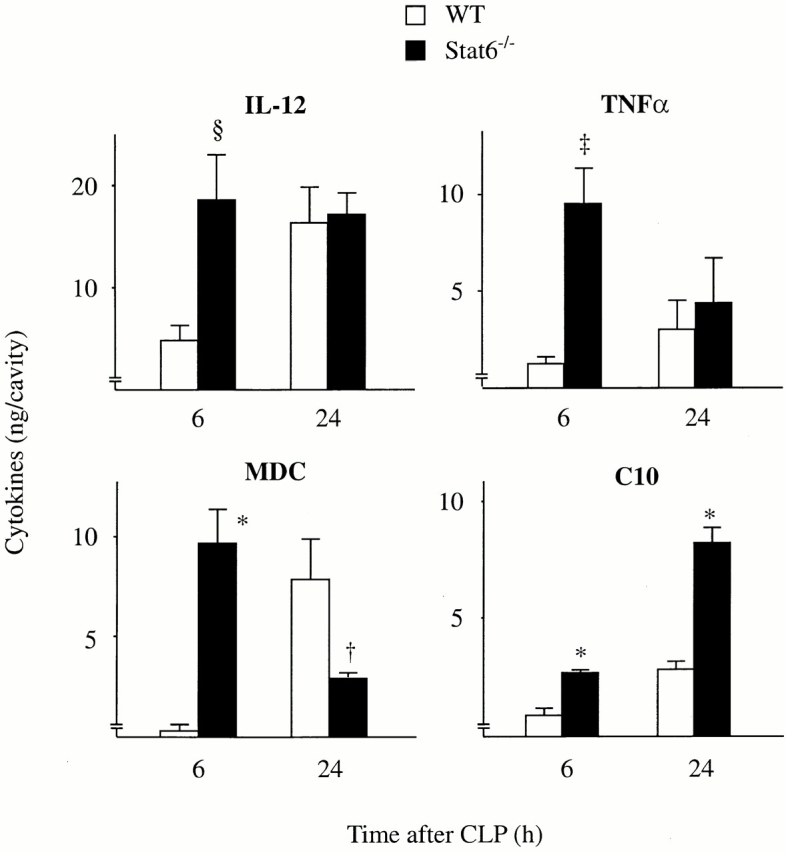
Stat6−/− mice augment the production of IL-12 and TNF-α, MDC, and C10 in the peritoneum after CLP. At 6 and 24 h after CLP, mice were killed, bled, and the peritoneal fluids were harvested. The protein concentrations of IL-12, TNF-α, MDC, and C10 were measured by ELISA. The data represent the mean ± SEM of 8–11 estimations from separate mice. † P < 0.05, § P < 0.01, ‡ P < 0.001, *P < 0.0001 compared with WT mice. Cytokines harvested from normal mice were below detection level.
Figure 5.
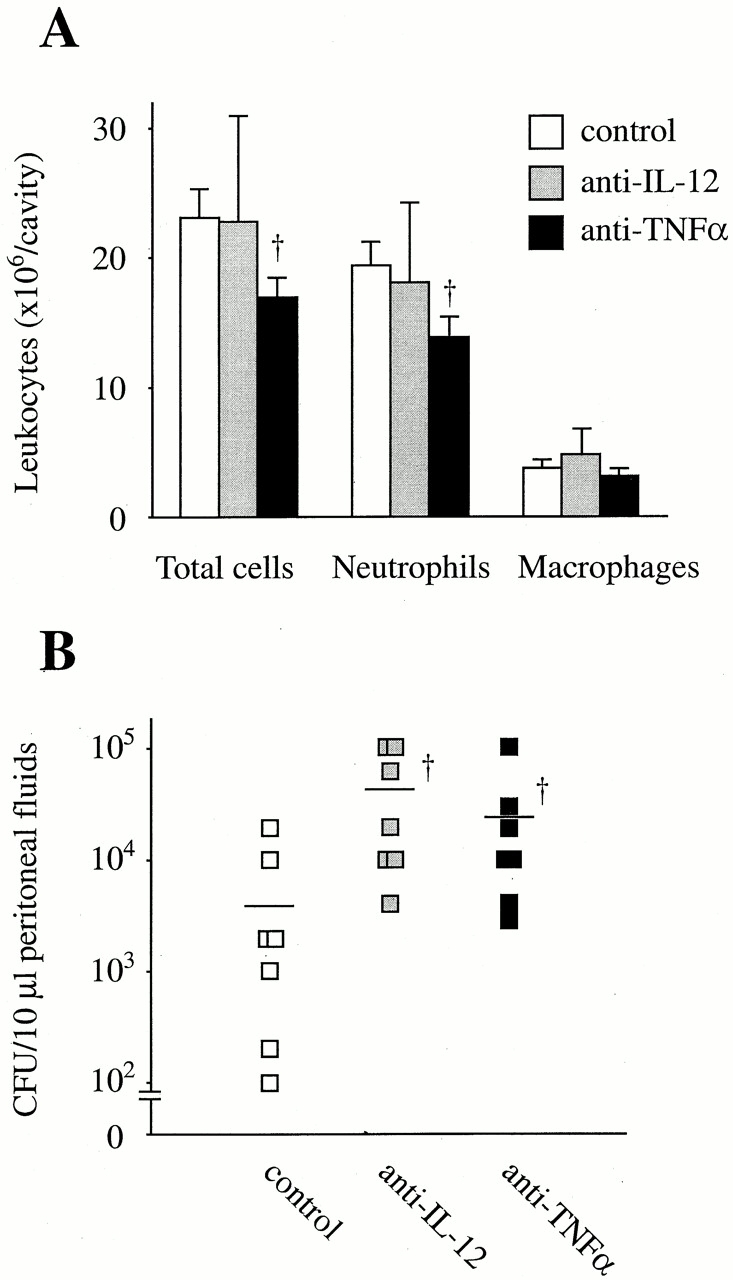
Participation of elevated levels of TNF-α and IL-12 in the neutrophil infiltration and bacterial clearance in Stat6−/− mice. Anti–TNF-α, anti–IL-12, or control antibodies were intraperitoneally injected, 2 h before CLP. (A) At 6 h after CLP, mice were killed and the infiltrating leukocytes in the peritoneal cavity were counted. (B) 10 μl of peritoneal fluids harvested at 24 h after CLP was serially diluted and plated on TSA blood agar plates. Line represents median CFU count. Each point represents the mean ± SEM of seven estimations from separate mice. † P < 0.05 compared with control.
CLP-initiated Organ Injury Was Ameliorated in Stat4− / − Mice.
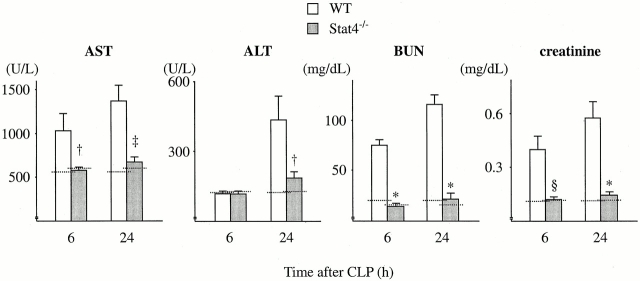
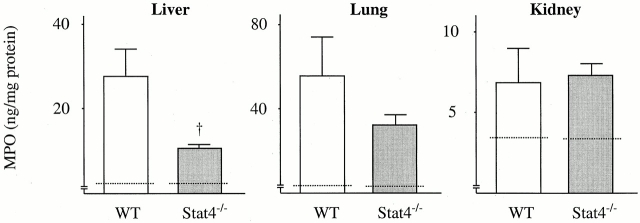
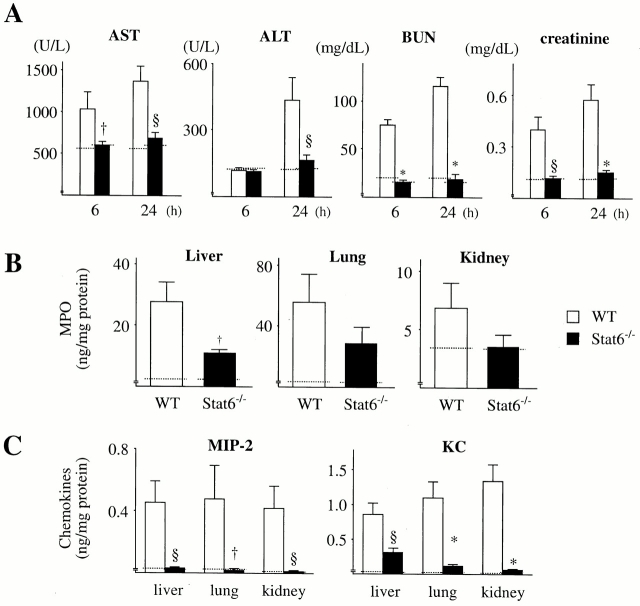
Sepsis frequently causes MOF, a condition that leads to death 1 2. To understand the mechanism whereby Stat4−/− mice were resistant to CLP, experiments were conducted to assess the organ damage induced by CLP. The data in Fig. 6 show that CLP resulted in increases in the serum level of AST, ALT, BUN, and creatinine in WT mice at 6 and 24 h after CLP, indicating that CLP caused liver and kidney damage in WT mice. However, the levels of AST, ALT, BUN, and creatinine in Stat4−/− mice after CLP were remarkably reduced compared with WT mice, and the levels were comparable to those in untreated mice (Fig. 6), suggesting that organ injury was evaded in Stat4−/− mice. Histologically, CLP induced a significant number of infiltrating neutrophils in the liver in WT mice, which was dramatically reduced in Stat4−/− mice (Fig. 7). Correspondingly, MPO level in the liver, an indirect means of determining the recruitment of neutrophils, was significantly decreased in Stat4−/− mice compared with WT mice (Fig. 8). CLP also caused an elevated level of MPO in the lung, which was decreased in Stat4−/− mice although it was not statistically significant. MPO level in the kidney was slightly increased in WT mice after CLP, which was not altered in Stat4−/− mice (Fig. 8). CLP did not cause any histological changes in the kidney of WT and Stat4−/− mice (data not shown). Thus, organ damage was ameliorated in Stat4−/− mice without affecting the bacterial load in the peritoneum, which was partly associated with decreased organ inflammation.
Figure 6.
Stat4−/− mice ameliorate organ injury induced by CLP. At 6 and 24 h after CLP, mice were killed, bled, and the sera were harvested. The amounts of AST, ALT, BUN, and creatinine in the sera were measured. The data represent the mean ± SEM of 8–11 estimations from separate mice. † P < 0.05, § P < 0.01, ‡ P < 0.001, *P < 0.0001 compared with WT mice. Dotted line, mean data obtained from untreated mice (six mice each).
Figure 7.
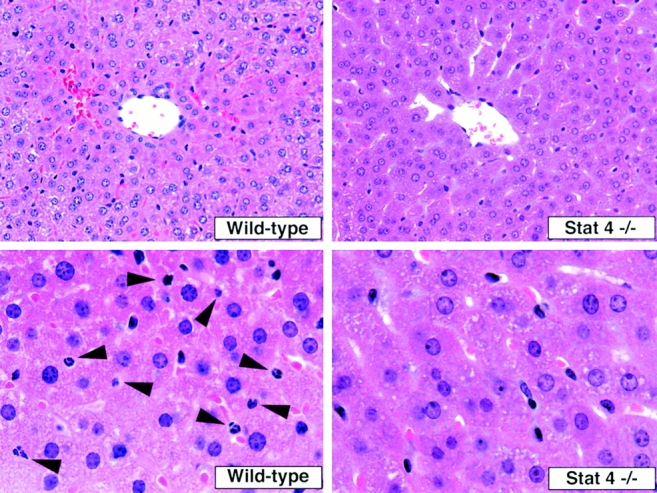
Histological alteration in the liver after CLP. The photograph are representative of findings in the liver harvested at 24 h after CLP from WT and Stat4−/− mice. There are few neutrophils in the liver of Stat4−/− mice. Arrows indicate the infiltrating neutrophils in the liver of WT mice. Original magnifications: top, ×400; bottom, ×1,000.
Figure 8.
MPO level in organs in Stat4−/− mice after CLP. At 24 h after CLP, mice were killed, and the liver, lung, and kidney were resected. The organs were extracted and the amounts of MPO were measured. The data represent the mean ± SEM (WT, eight mice; Stat4−/−, eight mice). Dotted line, mean data obtained from untreated tissues (six mice each). † P < 0.05 compared with WT mice.
Organ-associated Cytokine Profile Was Altered in Stat4− / − Mice.
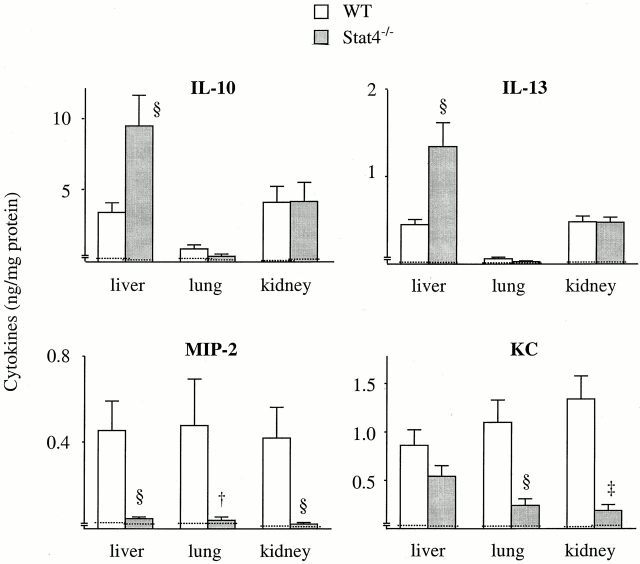
We next measured organ-associated cytokine levels in Stat4−/− mice. The level of IFN-γ in the liver, lung, and kidney in Stat4−/− mice was comparable to WT mice at any time checked. There was no difference in the level of IL-4 between WT and Stat4−/− mice (data not shown). In contrast, the levels of type 2 cytokine IL-10 and IL-13 in Stat4−/− mice were significantly augmented in the liver compared with WT mice (Fig. 9). Conversely, MIP-2 level in the liver of Stat4−/− mice was much lower than that in WT mice. KC level in the liver was also decreased in Stat4−/− mice, although it was not statistically significant (Fig. 9). Similarly, the levels of MIP-2 and KC in the kidney and lung of Stat4−/− mice were significantly decreased compared with WT mice, although there were no differences in the level of IL-10 and IL-13 (Fig. 9). Thus, cytokine profile in organs during sepsis was altered in Stat4−/− mice.
Figure 9.
Cytokine levels in organs of Stat4−/− mice after CLP. At 24 h after CLP, mice were killed, bled, and the liver, lung, and kidney were harvested. The protein concentrations of IL-10, IL-13, MIP-2, and KC were measured by ELISA. The data represent the mean ± SEM (WT, 10 mice; Stat4−/−, 9 mice). † P < 0.05, § P < 0.01, ‡ P < 0.001 compared with WT mice. Dotted line, mean data obtained from untreated mice (six mice each).
Systemic Inflammatory Response in Stat6−/− Mice.
Systemic inflammatory response in Stat6−/− mice was also investigated. Serum levels of AST, ALT, BUN, and creatinine in Stat6−/− mice were not significantly increased at 6 and 24 h after CLP compared with untreated Stat6−/− mice (Fig. 10 A). MPO level in the liver, lung, and kidney at 24 h after CLP was decreased in Stat6−/− mice compared with WT mice, although the level in the lung and kidney was not statistically significant (Fig. 10 B). MIP-2 and KC levels in Stat6−/− organs were significantly decreased compared with WT mice, and comparable to untreated mice (Fig. 10 C). These data suggest that more effective bacterial clearance in the peritoneum may result in a reduction in organ inflammation and damage.
Figure 10.
Systemic inflammatory response in Stat6−/− mice. (A) At 6 and 24 h after CLP, mice were killed, bled, and the sera were harvested. The amounts of AST, ALT, BUN, and creatinine in the sera were measured. (B and C) The liver, lung, and kidney were resected at 24 h after CLP, extracted, and the levels of MPO (B) and MIP-2 and KC (C) were measured. The data represent the mean ± SEM (WT, 10 mice; Stat6−/−, 11 mice). † P < 0.05, § P < 0.01, *P < 0.0001 compared with WT mice. Dotted line, mean data obtained from untreated mice (six mice each).
Discussion
Recent studies using Stat4−/− mice have demonstrated that the type 1 cytokine response mediated by Stat4 is critical for the protective immunity against Leishmania major and Trypanosoma cruzi 21 22. Others have shown using Stat6−/− mice that type 2 cytokine response mediated by Stat6 is necessary for the development of airway hyperresponsiveness in allergen-induced airway inflammation 23 24 and Schistosoma mansoni OVA-induced granuloma 25. Thus, Stat4−/− and Stat6−/− mice appear to be an excellent experimental system for analyzing the importance of type 1 and type 2 cytokine responses in vivo. We have recently shown that IL-12 (type 1 promoting cytokine) and IL-13 (a type 2 cytokine), both of which are endogenously produced during sepsis initiated by CLP, play beneficial roles in CLP-induced sepsis by enhancing local bacteria clearance and modulating systemic organ injury, respectively 9 10. Since Stat4 and Stat6 are essential transcription factors that respond to IL-12 and IL-13, respectively, we had initially hypothesized that Stat4−/− and Stat6−/− mice would be susceptible to septic peritonitis induced by CLP. In contrast to the hypothesis, the data clearly demonstrated that Stat4−/− and Stat6−/− mice were resistant to the lethality induced by CLP. In an attempt to understand these findings, we found that bacterial clearance was enhanced in Stat6−/− mice, whereas systemic organ damage initiated by CLP was ameliorated in Stat4−/− mice without affecting bacterial load in the peritoneum and peripheral blood.
Leukocytes play a crucial role in bacterial clearance 5. To understand the enhanced bacterial clearance in Stat6−/− mice, lysozomal enzyme release and superoxide production from leukocytes were examined in vitro, which showed that the activation of leukocytes in itself was similar between Stat6−/− and WT mice, suggesting a possibility that Stat6−/− mice cleared bacteria more effectively than WT mice based on the situation of the cells. When cytokines were measured in the peritoneum after CLP, levels of IL-12 and TNF-α were significantly increased in Stat6−/− mice compared with WT mice. IL-12 and TNF-α have been shown to enhance bacterial clearance in several bacterial infection models including septic peritonitis 9 26. In this study, treatment of Stat6−/− mice with anti–IL-12 or anti–TNF-α antibodies increased the bacterial load in the peritoneum, indicating that elevated levels of these cytokines contribute to the improved bacterial clearance in Stat6−/− mice. TNF-α also contributed to the increase in the number of infiltrating neutrophils, the cells that play a crucial role in bacterial clearance 5. In addition, MDC and C10 were elevated in Stat6−/− mice compared with WT mice. These CC chemokines are generally regarded to be associated with the type 2 response, as these chemokines can be induced by IL-4 and IL-13 27 28 and both are involved in the development of type 2–associated chronic inflammation 29 30. However, we have recently demonstrated that both MDC and C10 exhibit a strong type 1 response in innate immunity during septic peritonitis, as these chemokines are capable of inducing macrophage activation and protect mice from CLP-induced lethality by enhancing bacterial clearance of macrophages 16 17. Expression of MDC in macrophages is enhanced by TNF-α and IL-1β 31. IL-1β induces C10 production from peritoneal macrophages and C10 stimulates TNF-α production from macrophages in the presence of IL-1β 17. Thus, MDC and C10 appear to be unique chemokines that play a divergent role depending on the immune situation and contributing cell types. It should be noted that IL-4 and IL-13 were not detected in the peritoneum after CLP and T cells were minor cell populations in the infiltrating leukocytes in the peritoneum. Another type 2 cytokine, IL-10, was detected in the peritoneum, but the level in WT mice was similar to that in Stat6−/− mice. These findings suggest that Stat6−/− mice were resistant to septic peritonitis by exerting an improved bacterial clearance via an altered cytokine profile in the peritoneum in favor of bacterial clearance. However, unlike in vivo data, the levels of IL-12, TNF-α, MDC, and C10 were not augmented when innate immune cells from in Stat6−/− mice were stimulated with LPS or E. coli (data not shown). Further study will be necessary to understand the mechanism underlying the regulation of cytokine production.
Sepsis frequently causes MOF, a fatal condition to the host 1 2. The liver is a major target organ, and the injury generally arises from excessive hepatic inflammation 32 33, which was significantly modulated in Stat4−/− mice compared with WT mice. Interestingly, hepatic levels of IL-10 and IL-13 in Stat4−/− mice were significantly higher than those in WT mice. IL-10 and IL-13 are type 2 cytokines that have antiinflammatory properties both in vitro and in vivo 34 and have been shown to play a protective role in this model of sepsis, by downregulating inflammatory cytokines 10 35. In Stat4−/− mice, hepatic levels of MIP-2 and KC, CXC chemokines known to attract and activate neutrophils, were markedly lower than those in WT mice. These chemokines were also decreased in Stat4−/− lung, and there was a trend toward a decrease in the level of MPO in Stat4−/− lung. The renal system is also a target in sepsis, and the injury is likely to be caused by renal hypoperfusion, not by inflammation 36. This was partly supported by our present data, as renal function was significantly impaired in CLP mice without being accompanied by a significant increase in the kidney level of MPO. Interestingly, CLP-induced kidney injury was not observed in Stat4−/− mice, and this was associated with a dramatic decrease in the kidney level of MIP-2 and KC in Stat4−/− mice while levels of IL-4, IL-10, and IL-13 were comparable to those in WT mice. Thus, Stat4−/− kidney also exhibited an altered cytokine balance in favor of antiinflammatory cytokine. Although the precise role of these chemokines in the kidney is unclear, reduced production of MIP-2 and KC in Stat4−/− mice may be responsible for the improved kidney function.
Previous investigations have shown that T cells from Stat4−/− mice impair type 1 response and exhibit enhanced type 2 response 15 37, whereas T cells from Stat6−/− mice lack type 2 response, intensifying type 1 response 14 38 39. A recent in vivo study has demonstrated that Stat6−/− mice are resistant to infection against Leishmania mexicana via developing type 1 cytokine response 40. Thus, Stat6−/− mice exhibited an enhanced type 1 response while Stat4−/− mice showed the inverse phenotype. Our results are consistent with these findings, as Stat6−/− and Stat4−/− mice were resistant to CLP-induced sepsis by enhancing local type 1 cytokine response and organ-associated type 2 cytokine response, respectively. Studies have suggested that a shift from a type 1 to a type 2 cytokine response contributes to the late immunosuppression seen during sepsis 41 42. However, in addition to an importance of the local type 1 cytokine response, the results in this and our previous study 10 suggest the importance of organ-associated type 2 cytokine response in host defense during sepsis. It is possible that prevention of mortality in Stat4−/− and Stat6−/− mice after CLP is because of interference with signal transduction of other cytokines that are not measured or discussed in this study. However, it appears that the balance of type 1 and type 2 cytokine responses influences the outcome of sepsis and septic shock.
A very interesting observation in this study is that the type 1/type 2 cytokine balance can be controlled for the convenience of the host defense if one can regulate the activation of Stat4 and Stat6 proteins at the right time in the right place. The management of sepsis is largely supportive and based on prevention and vigorous resuscitation including early nutritional support and adequate oxygenation. Sepsis remains a serious disorder and the mortality attributable to sepsis and sepsis-mediated MOF has not significantly improved over the past three decades 1 2. Development of new drugs that specifically regulate Stat4 and Stat6 activity may pave the way for novel therapeutic interventions in sepsis. Innate immune response is tightly linked to acquired immune response 43. Augmenting the innate responses by Stat proteins has the potential to deliver immediate effects, as well as immunoregulatory functions promoting the most beneficial downstream adaptive responses, for defense against particular pathogens.
Acknowledgments
We thank Ms. Pamela M. Lincoln and Ms. Holly L. Evanoff for their excellent technical assistance, and Ms. Robin G. Kunkel for her excellent artistic work.
This work was supported in part by National Institutes of Health grants P50HL60289 and HL31237.
Footnotes
Abbreviations used in this paper: AST, aspartate transaminase; ALT, alanine transaminase; BUN, blood urea nitrogen; CLP, cecal ligation and puncture; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory protein; MPO, myeloperoxidase; MOF, multiple organ failure; Stat, signal transducer and activator of transcription; WT, wild-type.
References
- Astiz M.E., Rackow E.C. Septic shock. Lancet. 1998;351:1501–1505. doi: 10.1016/S0140-6736(98)01134-9. [DOI] [PubMed] [Google Scholar]
- Bone R.C., Balk R.A., Cerra F.B., Dellinger R.P., Fein A.M., Knaus W.A., Schein R.M., Sibbald W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Holzheimer R.G., Muhrer K.H., L'Allemand N., Schmidt T., Henneking K. Intraabdominal infectionsclassification, mortality, scoring and pathophysiology. Infection. 1991;19:447–452. doi: 10.1007/BF01726463. [DOI] [PubMed] [Google Scholar]
- Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Sawyer D.W., Donowitz G.R., Mandell G.L. Polymorphonuclear neutrophilsan effective antimicrobial force. Rev. Infect. Dis. 1989;11:S1532–S1544. doi: 10.1093/clinids/11.supplement_7.s1532. [DOI] [PubMed] [Google Scholar]
- Hack C.E., Aarden L.A., Thijs L.G. Role of cytokines in sepsis. Adv. Immunol. 1997;66:101–195. doi: 10.1016/s0065-2776(08)60597-0. [DOI] [PubMed] [Google Scholar]
- Constant S.L., Bottomly K. Induction of Th1 and Th2 CD4+ T cell responsesthe alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Infante-Duarte C., Kamradt T. Th1/Th2 balance in infection. Springer Semin. Immunopathol. 1999;21:317–338. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- Steinhauser M.L., Hogaboam C.M., Lukacs N.W., Strieter R.M., Kunkel S.L. Multiple roles for IL-12 in a model of acute septic peritonitis. J. Immunol. 1999;162:5437–5443. [PubMed] [Google Scholar]
- Matsukawa A., Hogaboam C.M., Lukacs N.W., Lincoln P.M., Evanoff H.L., Strieter R.M., Kunkel S.L. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J. Immunol. 2000;164:2738–2744. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Wurster A.L., Tanaka T., Grusby M.J. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- Fink M.P., Heard S.O. Laboratory models of sepsis and septic shock. J. Surg. Res. 1990;49:186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- Kaplan M.H., Schindler U., Smiley S.T., Grusby M.J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Kaplan M.H., Sun Y.L., Hoey T., Grusby M.J. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Matsukawa A., Hogaboam C.M., Lukacs N.W., Lincoln P.M., Evanoff H.L., Kunkel S.L. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J. Immunol. 2000;164:5362–5368. doi: 10.4049/jimmunol.164.10.5362. [DOI] [PubMed] [Google Scholar]
- Steinhauser M.L., Hogaboam C.M., Matsukawa A., Lukacs N.W., Strieter R.M., Kunkel S.L. Chemokine C10 promotes disease resolution and survival in an experimental model of bacterial sepsis. Infect. Immun. 2000;68:6108–6114. doi: 10.1128/iai.68.11.6108-6114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J.I., Fletcher M.P., Seligmann B.E., Hoffstein S., Cehrs K., Mounessa N. Human neutrophil-specific granule deficiencya model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982;59:1317–1329. [PubMed] [Google Scholar]
- Markert M., Andrews P.C., Babior B.M. Measurement of O2 − production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- Matsukawa A., Hogaboam C.M., Lukacs N.W., Lincoln P.M., Strieter R.M., Kunkel S.L. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitiscross-talk between MCP-1 and leukotriene B4. J. Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- Stamm L.M., Satoskar A.A., Ghosh S.K., David J.R., Satoskar A.R. STAT-4 mediated IL-12 signaling pathway is critical for the development of protective immunity in cutaneous leishmaniasis. Eur. J. Immunol. 1999;29:2524–2529. doi: 10.1002/(SICI)1521-4141(199908)29:08<2524::AID-IMMU2524>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tarleton R.L., Grusby M.J., Zhang L. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi . J. Immunol. 2000;165:1520–1525. doi: 10.4049/jimmunol.165.3.1520. [DOI] [PubMed] [Google Scholar]
- Akimoto T., Numata F., Tamura M., Takata Y., Higashida N., Takashi T., Takeda K., Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J. Exp. Med. 1998;187:1537–1542. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D., Schofield B., Wills-Karp M., Grusby M.J. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J. Exp. Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.H., Whitfield J.R., Boros D.L., Grusby M.J. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- Malaviya R., Ikeda T., Ross E., Abraham S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNFα. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Andrew D.P., Chang M.S., McNinch J., Wathen S.T., Rihanek M., Tseng J., Spellberg J.P., Elias C.G., III. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J. Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- Orlofsky A., Wu Y., Prystowsky M.B. Divergent regulation of the murine CC chemokine C10 by Th1 and Th2 cytokines. Cytokine. 2000;12:220–228. doi: 10.1006/cyto.1999.0535. [DOI] [PubMed] [Google Scholar]
- Gonzalo J.A., Pan Y., Lloyd C.M., Jia G.Q., Yu G., Dussault B., Powers C.A., Proudfoot A.E., Coyle A.J., Gearing D., Gutierrez-Ramos J.C. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- Hogaboam C.M., Gallinat C.S., Taub D.D., Strieter R.M., Kunkel S.L., Lukacs N.W. Immunomodulatory role of C10 chemokine in a murine model of allergic bronchopulmonary aspergillosis. J. Immunol. 1999;162:6071–6079. [PubMed] [Google Scholar]
- Rodenburg R.J., Brinkhuis R.F., Peek R., Westphal J.R., Van Den Hoogen F.H., van Venrooij W.J., van de Putte L.B. Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interleukin-1β, tumor necrosis factor α, and lipopolysaccharide. J. Leukoc. Biol. 1998;63:606–611. doi: 10.1002/jlb.63.5.606. [DOI] [PubMed] [Google Scholar]
- Smets D., Spapen H., Diltoer M., Nguyen D.N., Hubloue I., Huyghens L. Liver perfusion and hepatocellular inflammatory response in sepsis. Acta Clin. Belg. 1999;54:201–206. [PubMed] [Google Scholar]
- Wang P., Chaudry I.H. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am. J. Physiol. 1996;270:R927–R938. doi: 10.1152/ajpregu.1996.270.5.R927. [DOI] [PubMed] [Google Scholar]
- Opal S.M., DePalo V.A. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Marchant A., Buurman W.A., Berman L., Keogh C.V., Lazarus D.D., Nguyen L., Goldman M., Moldawer L.L., Lowry S.F. Endogenous IL-10 protects mice from death during septic peritonitis. J. Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- Groeneveld A.B. Pathogenesis of acute renal failure during sepsis. Nephrol. Dial. Transplant. 1994;9:47–51. [PubMed] [Google Scholar]
- Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T., Sangster M.Y., Vignali D.A., Doherty P.C., Grosveld G.C., Ihle J.N. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Shimoda K., van Deursen J., Sangster M.Y., Sarawar S.R., Carson R.T., Tripp R.A., Chu C., Quelle F.W., Nosaka T., Vignali D.A. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Stamm L.M., Raisanen-Sokolowski A., Okano M., Russell M.E., David J.R., Satoskar A.R. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J. Immunol. 1998;161:6180–6188. [PubMed] [Google Scholar]
- Volk H.D., Reinke P., Docke W.D. Clinical aspectsfrom systemic inflammation to ‘immunoparalysis’. Chem. Immunol. 2000;74:162–177. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- Ayala A., Chaudry I.H. Immune dysfunction in murine polymicrobial sepsismediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6:S27–S38. [PubMed] [Google Scholar]
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]