It is well established that cancer is a progressive disease, occurring in a series of well-defined steps, typically arising as a consequence of activating mutations (oncogenes) or deactivating mutations (tumor suppressor genes) in proliferating cells. From studies exploiting cultured tumor cells, two-stage carcinogenesis protocols in mice, and transgenic models of tumorigenesis, it is now evident that a single mutagenic event does not result in formation of a malignant tumor 1. Additional genetic and epigenetic events are necessary for progression to the tumor state. Initiated cells therefore require alterations rendering them self-sufficient for growth, insensitive to growth-inhibitory signals, resistant to programs of terminal differentiation, senescence, or apoptosis, as well as endowing them with unlimited self-renewal capacity, the ability to orchestrate and direct sustained angiogenesis, and the ability to invade and thrive in ectopic tissue environments 1. In this issue, Lin et al. 2 report that CSF-1 expression is a critical factor in a transgenic mouse model of mammary cancer development. This study provides compelling data that one subset of inflammatory cells, macrophages, and the dynamic microenvironment in which they live facilitate malignant outgrowth and eventual metastatic spread of evolving neoplastic cells.
CSF-1, a dimeric polypeptide growth factor, acts through a cell surface tyrosine kinase receptor encoded by the cfms protooncogene 3. This growth factor–receptor complex is an important regulator of proliferation, differentiation, and survival of macrophages and their bone marrow progenitors 3. Interestingly, elevated expression of CSF-1 and cfms has long been associated with poor prognosis in several types of human epithelial cancer, e.g., breast, uterine, and ovarian 4. Elevated expression of CSF-1 also correlates with intense leukocyte infiltration during development and progression of human breast and ovarian cancer 5 6; however, the functional significance of increased CSF-1 and infiltrating leukocytes in neoplastic tissue has remained unclear. The study by Lin et al. provides a connection between autocrine CSF-1 expression, macrophage infiltration, and development of late-stage mammary carcinoma and its pulmonary metastases 2. The broader implications of this study are that inflammatory cells potentiate neoplastic progression via paracrine factors that are as important to tumor evolution as oncogenes and tumor suppressor genes (Fig. 1).
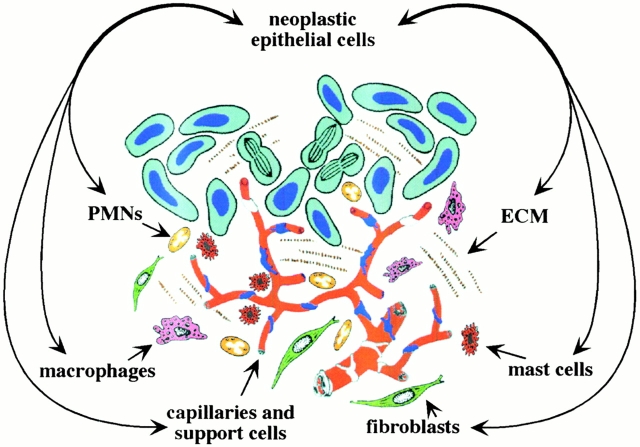
Figure 1.
Tumors consist of neoplastic epithelial cells and their microenvironment. ECM in and around the forming tumor is populated with capillaries and their support cells (pericytes, smooth muscle cells, and fibroblasts), and inflammatory cells (macrophages, PMNs, and mast cells).
In this study, the authors have crossed transgenic mice susceptible to development of mammary cancer (PyMT mice) with mice containing a recessive null mutation in the CSF-1 gene (Csf1op). Whereas absence of CSF-1 during early neoplastic development is without apparent consequence, development of late-stage invasive carcinoma and its metastatic pulmonary derivatives are significantly attenuated. The key difference between PyMT mice and PyMT/Csf1op/Csf1opmice is not in the proliferative capacity of neoplastic epithelial cells, but failure to recruit mature macrophages into neoplastic tissue in the absence of CSF-1. Macrophage recruitment is restored by restoring CSF-1 expression specifically to mammary epithelium in CSF-1–null/PyMT mice, as was characteristic primary and metastatic tumor development. A similar study shows that subcutaneous growth of Lewis lung cancer is impaired in Csf1op/Csf1opmice 7. However, in the latter example, tumors display a decreased mitotic index and pronounced necrosis, apparently resulting from diminished angiogenesis and impaired tumor stroma formation. These defects can be corrected by treatment of tumor-bearing mice with recombinant CSF-1 7. Together, these genetic experiments provide a causal link between CSF-1–dependent infiltrating macrophages and the malignant potential of epithelial cells.
Although the association of various inflammatory cell types with cancer is not new, with Westphal reporting dense areas of mast cells at the periphery of tumor as early as 1891 8, until recently, inflammatory cells have been largely ignored as “promoting” forces in tumor development (Fig. 2). Several independent studies using human clinical samples show that epithelial cell turnover is affected by inflammation 9 10. More significantly, proliferation in the setting of chronic inflammation predisposes humans to carcinoma in the breast, liver, large bowel, urinary bladder, prostate, gastric mucosa, ovary, and skin 5 9 10 11 12 13. Perhaps the best evidence for the importance of inflammation during neoplastic progression comes from study of cancer risk among long-term users of aspirin and nonsteroidal antiinflammatory drugs (NSAIDs). A large body of data indicates that use of these drugs reduces colon cancer risk by 40–50%, and may be preventative for lung, esophagus, and stomach cancer 14 15. The mechanism(s) underlying the chemopreventive effects of NSAIDs has to do with their ability to inhibit cyclooxygenases (COX-1 and COX-2). COX-2 converts arachidonic acid to prostaglandins, which in turn induces inflammatory reactions in damaged tissues 16.
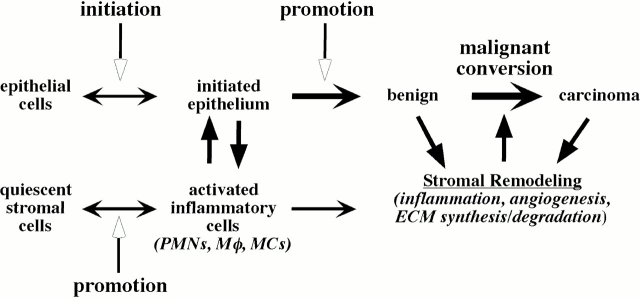
Figure 2.
Epithelial-stromal cross-talk during neoplastic progression. Epithelial neoplasia is initiated with mutational events; however, these cells remain dormant until wounding or tumor promoters activate the quiescent stromal cells. These promotion events recruit and activate inflammatory cells such as PMNs, macrophages (Mφ), or mast cells (MCs). In turn, these activated inflammatory cells stimulate growth and progression of the epithelial cells to form a benign tumor. These cells further activate additional inflammation and the ensuing angiogenesis, ECM remodeling. This altered microenvironment further destabilizes the epithelial cells to undergo malignant conversion to full carcinomas and facilitate metastasis.
What do infiltrating inflammatory cells provide to evolving neoplasms? The inflammatory component of a developing neoplasm includes a diverse leukocyte population, e.g., macrophages, neutrophils, eosinophils, and mast cells, all of which are variably loaded with an assorted array of cytokines, cytotoxic mediators including reactive oxygen species, serine-, cysteine-, and metallo-proteases, membrane-perforating agents, and soluble mediators of cell killing, such as TNF-α, ILs, and IFNs 9 17 18. Neutrophils (PMNs) are the most abundant circulating blood leukocytes. They provide the first line of defense against infection and release soluble chemotactic factors and proteases that alter the microenvironment and guide the recruitment of both nonspecific and specific immune effector cells 17. Mast cells play an important role in acute inflammation due to their release of stored and newly synthesized inflammatory mediators after activation. Mast cells release diverse factors known to enhance angiogenic phenotypes, including heparin, heparanase, histamine, metallo- and serine proteinases, and various polypeptide growth factors, including basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF; reference 19). Thus, mast cells provide direct mitogens for fibroblasts, endothelial, and epithelial cells, as well as diverse enzymatic activities involved in (extracellular matrix [ECM]) remodeling. Eosinophils, which have many bioactive molecules in their granules, are generally regarded as cells recruited to tissue as a host defense against parasites or during allergic responses 20. Macrophages produce several potent angiogenic cytokines, growth factors, and proteases. Macrophage infiltration is closely associated with the depth of invasion of primary melanoma due, in part, to macrophage-regulated tumor-associated angiogenesis 21. Macrophages express many bioactive molecules, including proteases, arachidonate metabolites, TGF-β, TNF-α, and IL-1α 20. In response, melanocytes express IL-8 and VEGF, thereby inducing angiogenesis through paracrine control 22.
But macrophages are not unique among inflammatory cells in potentiation of neoplastic processes. PMNs, mast cells, and activated T lymphocytes can also contribute to malignancies by releasing proteases, angiogenic factors, and chemokines 9 17 19 23 24. mast cells and neutrophils also potentiate the actions of oncogenes encoded by human papillomavirus type 16 (HPV16) in transgenic mice predisposed to squamous carcinoma development 19 23. These data support a model in which mast cells, macrophages, and PMNs amplify neoplastic cell proliferation and angiogenesis largely by release of matrix metalloproteinase-9 (MMP-9). HPV16 mice carrying a homozygous null mutation in the MMP-9 gene have decreased epithelial proliferation, delayed angiogenesis, reduced tumor incidence, and altered differentiation characteristics of rarer emergent tumors 23. As infiltration of neoplastic tissues by mast cells and PMNs is not impaired in HPV16/MMP-9–deficient mice (unpublished observations), the data suggest that MMP-9 is a key factor released by these leukocytes mediating potentiation of tumorigenic progression.
How do inflammatory cells get co-opted into the neoplastic process? A plausible hypothesis is that many malignancies arise from areas of infection and inflammation, simply as part of the normal host response. Indeed, there is a growing body of evidence that many malignancies, e.g., gastric, cervical, and colon, are initiated by infections 9 25. In fact, upwards of 15% of malignancies worldwide can be attributed to infections, a global total of 1.2 million cases per year 9. The three main mechanisms by which infections can cause cancer involve initiation as well as promotion (for a review, see reference 9). Persistent infections within the host induce chronic inflammation. Leukocytes and other phagocytic cells induce DNA damage in proliferating cells through their generation of reactive oxygen and nitrogen species that are produced normally by these cells in order to fight infection 26. Unfortunately, these react to form peroxynitrite, a mutagenic agent 26. Hence, repeated tissue damage and regeneration of tissue in the presence of highly reactive nitrogen and oxygen species, released from inflammatory cells, interacts with DNA in proliferating epithelium resulting in permanent genomic alterations, e.g., point mutations, deletions, or rearrangements. The gram-negative bacterium Helicobacter pylori is established as a definite carcinogen for the development of gastric cancer, the second most common type of cancer globally 9 25. Infection by H. pylori has a very high cancer-associated risk of 75% 25. DNA damage resulting from chronic inflammation is believed to be the mechanism. Exacerbating DNA damage induced by inflammatory cells is expression of macrophage migration inhibitory factor (MIF) by macrophages and T lymphocytes. MIF is a potent cytokine that overcomes p53 function by suppressing its transcriptional activity 27. Chronic bypass of p53 regulatory functions in infiltrated tissues can enhance proliferation and extend life span, while also creating an environment with a deficient response to DNA damage, amplifying accumulation of potential oncogenic mutations.
Infectious agents may also directly transform cells by inserting active oncogenes into the host genome, e.g., DNA tumor viruses and human immunodeficiency. It is clear that many types of infectious agents, particularly viruses, are present in mammals. However, virus-associated malignancies are relatively rare in infected individuals. This likely reflects the necessity of cofactors necessary for promotion and the fact that a neoplasia can only develop if viral infection has affected that tissues pluripotent stem cells, as differentiated cells are not easily immortalized. Although this does occur, stem cells are typically low in abundance and located in regions of tissues protected from agents that would otherwise harm them 28. In Rous sarcoma virus infections, inflammation is essential for tumor development, and this requirement is mediated by factors such as TGF-β and other cytokines produced by the inflammatory cells 29. Another intriguing possibility arises from the observation that leukocyte adhesion molecules are required for effective tumor progression 30. Just as microorganisms hijack macrophages for growth and spread, perhaps the formation of tumor-platelet-leukocyte emboli allows tumor cells to piggyback on macrophages and other leukocytes to their interactions with the endothelium of distant organs.
Are tumor-associated macrophages and other inflammatory cells targets for cancer therapy? The incredible efficacy of NSAIDs in chemoprevention argues for antiinflammatory therapy at the earliest stages of neoplastic progression. Although the results with CSF-1 suggest that macrophages contribute to metastasis 2, inflammatory and other immune cells undoubtedly also take part in antitumor surveillance. In the absence of certain cells or functions, it is possible that some tumors will progress more rapidly 19 20 24.
Acknowledgments
Supported by grants from the National Cancer Institute (CA72006), the American Cancer Society (AC-04-02), the V Foundation for Cancer Research, and the Edward Mallinckrodt, Jr. Foundation for Medical Research.
References
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Lin E.Y., Nguyen A.V., Russell R.G., Pollard J.W. CSF-1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 2001;193:727–739. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley B.M., Guillert L.T., Tushinski R.J., Bartelmez S.H. CSF-1 a mononuclear phagocyte lineage-specific hemopoietic growth factor. J. Cell. Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Kacinski B.M. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann. Med. 1995;27:79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- Scholl S.M., Pallud C., Beuvon F., Hacene K., Stanley E.R., Rohrschneider L.R., Tang R., Pouillart P., Lidereau R. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J. Natl. Cancer Inst. 1994;86:120–126. doi: 10.1093/jnci/86.2.120. [DOI] [PubMed] [Google Scholar]
- Tang R.P, Kackinski B., Valisire P., Beuvon F., Sastre X., Benoit P., dela Rochefordiere A., Mosseri V., Pouillart P., Scholl S. Oncogene amplification correlates with dense lymphocyte infiltration in human breast cancersa role for hematopoietic growth factor release by tumor cells? J. Cell. Biochem. 1990;44:189–198. doi: 10.1002/jcb.240440307. [DOI] [PubMed] [Google Scholar]
- Nowicki A., Szenajch J., Ostrowska G., Wojtwicz A., Wojtowicz K., Kruszewski A.A., Maruszynski M., Aukerman S.L., Wiktor-Jedrezjczak W. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouseevidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int. J. Cancer. 1996;65:112–119. doi: 10.1002/(SICI)1097-0215(19960103)65:1<112::AID-IJC19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Westphal E. Uber mastzellen. In: Ehrlich P., editor. Farbenanalytische Utersuchuugen. Zur Histologie und Klinik des PlutesGesammelte Mitt(h)eilungen. Vol. 1. Hirschwald Press; Berlin, Germany: 1891. p. 17. [Google Scholar]
- Kuper H., Adami H.O., Trichopoulos D. Infections as a major preventable cause of human cancer. J. Int. Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- DeMarzo A.M., Marchi V.L., Epstein J.I., Neson W.G. Proliferative inflammatory atrophy of the prostateImplications for prostatic carcinogenesis. Am. J. Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst P., Gold B.D. The disease spectrum of Helicobacter pyloriThe immunopathogenesis of gastroduodenal ulcer and gastric cancer. Ann. Rev. Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- Ness R.B., Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J. Natl. Cancer Inst. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- Brocker E.B., Zwaldo G., Holzmann B., Macher E., Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int. J. Cancer. 1988;41:562–567. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- Baron J.A., Sandler R.S. Nonsteroidal anti-inflammatory drugs and cancer prevention. Ann. Rev. Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez L.A., Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- Williams C.S., Mann M., DuBois R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- DiCarlo E., Fomi G., Lollini P.L., Colombo M.P., Modesti A., Musiani P. The intriguing tole of polymorphonuclear neutrophils in anticancer reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- Wahl L.M., Kleinman H.K. Tumor-associated macrophages as targets for cancer therapy. J. Natl. Cancer Inst. 1998;90:1583–1584. doi: 10.1093/jnci/90.21.1583. [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Raymond W.W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G., Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M.E. Eosiniphilia. N. Engl. J. Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- Ono M., Torisu H., Fukushi J., Nisjie A., Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother. Pharmacol. 1999;43:69–71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- Torisu H., Ono M., Kiryu H., Furue M., Ohmoto Y., Nakayama J., Nishioka Y., Sone S., Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanomapossible involvement of TNF-a and IL-1a. Int. J. Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- Coussens L.M., Tinkle C.L., Hanahan D., Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Brekken R., McMahon G., Vu T.H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M.J., Chyou P.H., Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:561–565. [PubMed] [Google Scholar]
- Maeda H., Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry. 1998;63:854–865. [PubMed] [Google Scholar]
- Hudson J.D., Shoaibi M.A., Maestro R., Carnero A., Hannon G.J., Beach D.H. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen U.B., Lowell S., Watt F.W. The spatial relationship between stem cells and their progeny in the basal layer of human epidermisa new view based on whole mount labeling and lineage analysis. Development. 1999;126:2409–2418. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- Martins-Green M.N., Boudreau N., Bissell M.J. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–4341. [PubMed] [Google Scholar]
- Kim Y.J., Borsig L., Varki N.M., Varki A. P-selectin deficiency attenuates tumor growth and metastasis and metastasis. Proc. Natl. Acad. Sci. USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]