Abstract
Mucosal organs such as the intestine are supported by a rich and complex underlying vasculature. For this reason, the intestine, and particularly barrier-protective epithelial cells, are susceptible to damage related to diminished blood flow and concomitant tissue hypoxia. We sought to identify compensatory mechanisms that protect epithelial barrier during episodes of intestinal hypoxia. Initial studies examining T84 colonic epithelial cells revealed that barrier function is uniquely resistant to changes elicited by hypoxia. A search for intestinal-specific, barrier-protective factors revealed that the human intestinal trefoil factor (ITF) gene promoter bears a previously unappreciated binding site for hypoxia-inducible factor (HIF)-1. Hypoxia resulted in parallel induction of ITF mRNA and protein. Electrophoretic mobility shift assay analysis using ITF-specific, HIF-1 consensus motifs resulted in a hypoxia-inducible DNA binding activity, and loading cells with antisense oligonucleotides directed against the α chain of HIF-1 resulted in a loss of ITF hypoxia inducibility. Moreover, addition of anti-ITF antibody resulted in a loss of barrier function in epithelial cells exposed to hypoxia, and the addition of recombinant human ITF to vascular endothelial cells partially protected endothelial cells from hypoxia-elicited barrier disruption. Extensions of these studies in vivo revealed prominent hypoxia-elicited increases in intestinal permeability in ITF null mice. HIF-1–dependent induction of ITF may provide an adaptive link for maintenance of barrier function during hypoxia.
Keywords: epithelium, gastrointestinal disease, transcription factor, intestinal permeability, endothelium
Introduction
Epithelial cells are anatomically positioned to provide a barrier to antigenic flux and luminal bacteria 1. Mucosal organs, such as the intestine, rely on an extensive underlying vasculature, and therefore are primary sites for conditions of diminished blood flow and resultant tissue hypoxia 2. Under such conditions, maintenance of barrier function depends on structures within the intercellular tight junction at the boundary of the apical and basolateral membrane domain. At present, it is not known if protective mechanisms exist to support functional epithelial barrier properties during episodes of diminished blood flow and resultant hypoxia.
Both adaptive (e.g., erythropoietin [EPO], glycolytic enzymes) and proinflammatory (e.g., TNF-α, IL-8) responses have been attributed to conditions of diminished tissue oxygen delivery (hypoxia), and it is now clear that responses to hypoxia include transcriptionally regulated gene expression 2 3. One such transcriptional pathway relies on the activation of hypoxia-inducible factor (HIF)-1, a member of the rapidly growing per-ARNT-sim family of basic helix-loop-helix (bHLH) transcription factors 4 5 6. Functional HIF-1 exists as an αβ heterodimer, the activation of which is dependent on stabilization of an O2-dependent degradation pathway 7 . Binding of HIF-1 to DNA consensus domains (5′-RCGTG-3′) results in the transcriptional induction of HIF-1–bearing gene promoters 3. HIF-1 is widely expressed and recent studies indicate that consensus HIF-1–binding sequences exist in a number of genes 3. Here we demonstrate that hypoxia induces the epithelial-specific, barrier-protective protein intestinal trefoil factor (ITF) through an HIF-1–dependent mechanism.
Materials and Methods
Growth and Maintenance of Cell Lines.
T84 cells and a subclone of Caco-2 B Be cells with high epithelial resistance were grown as monolayers on polycarbonate permeable supports as described previously 8 9. The KB cell line is a human oral epithelial cell line derived from native human mucosa 10. Human microvascular endothelial cells (MVECs) are primary culture endothelial cells (Cascade Biologics, Inc.) derived from the human dermis and were cultured as described previously 11. Madin-Darby canine kidney (MDCK) cells were cultured and passaged as described previously 12. Confluent cells were exposed to hypoxia in a humidified hypoxic cell chamber (Coy Laboratory Products, Inc.) as described previously 13. Standard hypoxic conditions (based on previous work; references 13 14 15) were pO2 = 20 Torr, pCO2 = 35 Torr, with the balance made up of nitrogen and water vapor. Normoxic controls were cells exposed to the same experimental protocols under conditions of atmospheric oxygen concentrations (pO2 = 147 Torr and pCO2 = 35 Torr within a tissue culture incubator). Paracellular permeability was assessed as described previously 11 using FITC-labeled dextran (40 kD).
Transcriptional Analysis.
The transcriptional profile of epithelial cells exposed to ambient hypoxia was assessed in RNA derived from control or hypoxic epithelia (T84 cells at 6- or 18-h hypoxia) using quantitative gene chip expression arrays (Affymetrix, Inc.; reference 16). Reverse transcription (RT)-PCR analysis of mRNA levels was performed using DNase-treated total RNA as described previously 14 using primers specific for human ITF 17 resulting in a 108-bp fragment or β-actin 14 resulting in a 661-bp fragment. In subsets of experiments, the transcription inhibitor actinomycin D (10 ng/ml final concentration) was used to determine the prevalence of newly synthesized RNA in this response.
Western Blot Analysis.
After experimental treatment of epithelial cells, cell culture supernatants (for examination of soluble ITF) were prepared as described previously 11. Nuclear extracts (for analysis of HIF-1α) were isolated from confluent monolayers of epithelia on 100-mm petri dishes as described before 18. Samples (25 μg/lane) were resolved by reducing SDS-PAGE, transferred to nitrocellulose, and blocked overnight in blocking buffer (250 mM NaCl, 0.02% Tween 20, 5% goat serum, 3% BSA). For Western blotting, affinity-purified anti–HIF-1α (generated by immunizing rabbits with an antigenic peptide encoded in the HIF-1α protein structure encoding amino acids 527–541 [MVNEFKLELVEKLFA] of HIF-1α) or anti-ITF 19 was added for 3 h, blots were washed, and species-matched peroxidase-conjugated secondary Ab is added, as described previously 14. Labeled bands from washed blots were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Electrophoretic Mobility Shift Assay.
Nuclear extracts of cells exposed to indicated experimental conditions were obtained as described elsewhere. Synthetic oligonucleotides were synthesized (Genosys Biotechnologies, Inc.) and used as probes in electrophoretic mobility shift assays (EMSAs). The hypoxic response element (HRE) motif lies at −129 to −122 relative to the transcription start site in the ITF promoter 17. Oligonucleotide probes for EMSA were digoxigenin-labeled, incubated with nuclear lysates, and resolved on a 6% nondenaturing polyacrylamide gel as described previously (Boehringer; reference 14). DNA–protein complexes were transblotted to nylon membrane, probed with antidigoxigenin-peroxidase, and developed by enhanced chemiluminescence. Controls consisted of free probe alone, excess unlabeled probe, or a species-matched irrelevant Ab (anti-cAMP response element–binding protein [CREB]; Transduction Labs, Inc.).
HIF-1α Antisense Oligonucleotide Treatment.
HIF-1α depletion in epithelial cells was accomplished by using antisense oligonucleotide loading as described previously 20 using phosphorothioate derivatives of antisense (GCC GGC GCC CTC CAT) or control sense (ATG GAG GGC GCC GGC) oligonucleotides. T84 epithelial cells were washed in serum free media, then in media containing 20 μg/ml effectene transfection reagent (QIAGEN) with 2 μg/ml HIF-1α antisense or sense oligonucleotide. Cells were incubated for 4 h at 37°C and then replaced with serum containing growth media. Treated cells were exposed to hypoxia or normoxia for indicated periods of time. As indicated, ITF mRNA was quantified by RT-PCR as described above (see Transcriptional Analysis).
HIF-1 Transfection.
Stable cell lines overexpressing HIF-1α (plasmid provided by H.F. Bunn, Harvard Medical School; reference 7) were generated in the high resistance Caco-2 B Be cell line as described previously 8. Primary screening for HIF-1 expression was by RT-PCR analysis. Clones with the highest expression underwent secondary screens based on electrophysiologic characteristics (high resistance monolayers) and Western blot analysis of HIF-1α.
Intestinal Permeability In Vivo.
The generation of ITF null mice has been described previously 21. Intestinal permeability was examined in 6–10-wk-old wild-type Bl6129 (Taconic, Inc.) or ITF null mice using an FITC-labeled dextran method as described previously 22. Mice were gavaged with 60 mg/100 g body weight of FITC-dextran (4,000 kD at 80 mg/ml; Sigma-Aldrich) and exposed to ambient hypoxia (8% O2, 92% N2) or ambient room air for various times (n = 4–6 per condition). In some experiments, mice were administered either recombinant human ITF (30 mg/kg, 75% gastric lavage, 25% enema) or PBS 1 h before hypoxia. Cardiac puncture was performed and serum analysis of FITC concentration performed. In subsets of experiments, tissues were collected from wild-type and ITF null mice after hypoxia and fixed in 10% buffered formalin. 5-μm sections were cut, stained with hematoxylin and eosin, and histologically characterized. Colonic tissue was harvested and homogenized and RNA extracted with Trizol as described above. Another section of colonic mucosal scrapings was harvested and processed for Western blot analysis as described above. This protocol was in accordance with National Institutes of Health guidelines for use of live animals and was approved by the Institutional Animal Care and Use Committee at Brigham and Women's Hospital.
Statistical Analyses.
ITF bioactivity and paracellular permeability data were compared by one- or two-factor analysis of variance or by Student's t test where appropriate. Values are expressed as the mean and SEM from at least three separate experiments.
Results
Colonic Epithelia Resist Hypoxia-elicited Barrier Changes.
Previous reports indicate that colonic epithelial barrier function is maintained during even severe conditions of hypoxia 13 14. In contrast, other cell types (e.g., vascular endothelia) appear to markedly lose barrier qualities during ambient hypoxia 23. As shown in Table , comparison of six barrier cell types revealed that colonic epithelial cells (T84 and Caco-2 cells), as opposed to other epithelia (KB, A549, and MDCK) or MVECs, appear to be uniquely resistant to increased permeability induced by hypoxia, suggesting that intestinal epithelial cells possess mechanisms that sustain barrier function under such conditions.
Table 1.
Intestinal Epithelia Resist Hypoxia-elicited Disruption
| Paracellular flux | ||||
|---|---|---|---|---|
| Cell type | Normoxia | Hypoxia | Fold increase | P value |
| pM/cm2/h | pM/cm2/h | |||
| Epithelia (T84, colon) | 5 ± 0.4 | 5 ± 0.3 | 0.0 | NS |
| Epithelia (Caco-2, colon) | 8 ± 1.6 | 9 ± 0.8 | 0.1 | NS |
| Epithelia (KB, oral) | 97 ± 8.1 | 430 ± 12.6 | 4.4 | <0.001 |
| Epithelia (MDCK, kidney) | 63 ± 10.4 | 245 ± 12.2 | 3.8 | <0.001 |
| Epithelia (A549, lung) | 197 ± 19.5 | 482 ± 52.5 | 2.4 | <0.007 |
| Epithelia (MVEC, dermis) | 127 ± 5.1 | 395 ± 14.6 | 3.1 | <0.001 |
Monolayers of indicated cell type were grown to confluence on semipermeable supports. Monolayers were exposed to normoxia (pO2 = 147 Torr) or hypoxia (pO2 = Torr) for 24 h (48 for A549 cells) at 37°C. Paracellular permeability of fluoresceinated dextran (40 kD, 70 kD for A549 cells) was assessed over a 60-min period and flux was calculated from a standard curve. Results represent mean and SEM from at least three separate experiments.
Hypoxia Induces ITF Production.
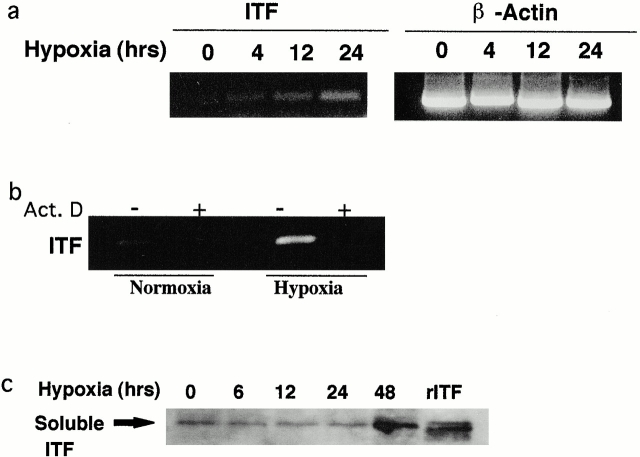
Transcriptional profiling has been used to identify potential factors contributing to maintenance of barrier during hypoxia 24 25. Microarray analysis 26 was adopted to broadly screen hypoxia-regulated genes in RNA derived from intestinal epithelia (T84 cells), and identified a time-dependent induction of the intestinal-specific, barrier-protective ITF gene (2.8- and 4.2-fold increase over control normoxia at 6- and 18-h hypoxia, respectively). Subsequently, RT-PCR analysis was employed to corroborate results of microarray screening (Fig. 1 a). This analysis of Caco-2 cells demonstrated a time-dependent induction of ITF mRNA expression, and was evident by 4-h exposure to hypoxia. Similar results were seen in T84 cells (data not shown). Epithelial exposure to the RNA synthesis inhibitor actinomycin D blocked this hypoxia-elicited ITF induction (Fig. 1 b), indicating that this response reflects new transcriptional activity. To confirm these mRNA observations at the protein level, Western blot analysis of soluble supernatants confirmed an increase of secreted ITF protein by 48 h (Fig. 1 b).
Figure 1.
ITF induction of hypoxia. Confluent Caco-2 epithelial monolayers were exposed to indicated periods of ambient hypoxia (pO2 = 20 Torr) or normoxia (pO2 = 147 Torr). In a, total RNA was isolated and examined for ITF transcript by RT-PCR. β-Actin transcript was used as an internal control. In b, Caco-2 cells were pretreated with vehicle or actinomycin D (Act. D) before 24 h of hypoxia or normoxia. Total RNA was isolated and examined for ITF mRNA. In c, soluble supernatants derived from monolayers exposed to indicated periods of hypoxia were examined for ITF content by SDS-PAGE and Western blot analysis.
ITF Production Is HIF-1 Dependent.
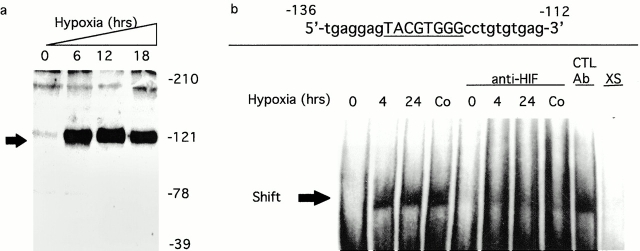
Prompted by these results, a search of the cloned ITF gene identified a previously unappreciated HIF-1α binding site at positions −129 to −122 relative to the transcription start site 17 (DNA consensus motif 5′-TACGTGGG-3′; reference 6). Consequently, we assessed the induction of HIF-1α and ITF by hypoxia. First, we determined whether conditions of hypoxia induce HIF-1α in intestinal epithelia (Fig. 2 a). Western blot analysis of nuclear lysates derived from hypoxic intestinal epithelia Caco-2 cells demonstrated abundant HIF-1α in nuclei. These data are consistent with previous studies suggesting that HIF-1α is expressed in most cell types during periods of hypoxia 3. Next, we examined the binding of HIF-1α to the HRE consensus in the ITF promoter by EMSA (Fig. 2 b). As can be seen, nuclear extracts derived from hypoxic, but not normoxic, Caco-2 cells bind to the HIF-1α consensus of the ITF promoter. Pretreatment of epithelia with 100 μM CoCl2 for 4 h, conditions that promote nuclear accumulation of HIF-1α 5, resulted in a similar band shift. Addition of anti–HIF-1α mAb, but not isotype-matched mAb, resulted in a diminution of shifted signal, but no obvious supershift consistent with Ab interference with DNA–protein interaction. Incubation with 100-fold excess unlabeled primer resulted in complete loss of signal. Collectively, these results indicate that hypoxia elicits nuclear accumulation of HIF-1α, which binds to the consensus HRE located within the ITF promoter.
Figure 2.
Parallel induction of HIF-1 and ITF by hypoxia. Confluent Caco-2 epithelial monolayers were exposed to indicated periods of ambient hypoxia (pO2 = 20 Torr) or normoxia (pO2 = 147 Torr). In a, nuclear lysates derived from monolayers exposed to normoxia or hypoxia were examined for HIF-1α content by SDS-PAGE Western blot analysis. In b, interactions between HIF-1α and the HRE of the ITF promoter gene were examined by EMSA using synthetic oligonucleotides and nuclear lysates derived from cells exposed to normoxia, 4- or 24-h hypoxia, or CoCl2 (100 μM for 4 h). Specificity was determined with the use of anti–HIF-1α Ab, anti-CREB Ab (control), or excess (XS) unlabeled probed. Note diminution of signal in the presence of anti–HIF-1α, but not by anti-CREB Ab.
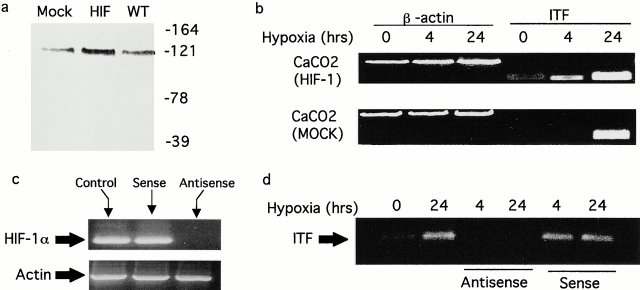
As proof of principle for this concept, intestinal epithelial cell lines overexpressing HIF-1α were generated by stable transfection (Fig. 3 a). As shown in Fig. 3 b, overexpression of HIF-1α resulted in increased basal expression of ITF and enhanced hypoxia-elicited induction of ITF. Next, antisense oligonucleotides directed against HIF-1α were used to block HIF-1α expression (Fig. 3 c) and the influence of HIF-1α depletion on ITF mRNA induction by hypoxia was assessed (Fig. 3 d). As can be seen, the directed loss of HIF-1α resulted in a nearly complete loss of ITF mRNA expression at 4- and 24-h periods of hypoxia. Control oligonucleotides generated to the sense strand of HIF-1α did not influence ITF hypoxia inducibility. Taken together, these results indicate that hypoxia induces a time-dependent HIF-1α nuclear accumulation and parallel ITF induction.
Figure 3.
Induction of ITF is HIF-1α dependent. (a) Analysis of HIF-1α in transfected Caco-2 cells. Shown are mock transfected (Mock), cells overexpressing HIF-1α (HIF), and wild-type (WT) Caco-2 cells exposed to hypoxia and examined for HIF-1α expression by Western blot analysis. In b, ITF expression was analyzed by RT-PCR in HIF-1α transfectants and mock transfectants after exposure to indicated periods of hypoxia. In c, cells were loaded with antisense or sense oligonucleotides directed against HIF-1α as indicated and examined for HIF-1α mRNA. In d, Caco-2 cells pretreated with sense or antisense oligonucleotides as indicated were exposed to hypoxia or normoxia, and ITF mRNA expression was analyzed by RT-PCR.
ITF Promotes Barrier Function In Vitro and In Vivo.
Given the findings described above, studies were undertaken to determine whether ITF played a functional role in maintenance of the epithelial integrity during hypoxia. For this purpose, Caco-2 monolayers were exposed to hypoxia in the presence or absence of anti-ITF (Fig. 4 a). Increasing concentrations of anti-ITF sera (titer ∼1:100,000), but not isotype-matched antisera, resulted in a dose-dependent increase in epithelial permeability in response to hypoxia (no Ab versus anti–human [h] ITF at 1:100 dilution; P < 0.001). These results demonstrate that ITF participates in the maintenance of barrier function during hypoxia. Further evidence in support of ITF as a barrier-protective agent were provided by addition of recombinant hITF to hypoxia-sensitive cells. Monolayers of endothelial cells (which respond to hypoxia with increase paracellular permeability, Table ) were used to define this principle. As shown in Fig. 4 b, addition of hITF to confluent endothelial cells during conditions of hypoxia resulted in a concentration-dependent protection against barrier disruption (no hITF versus 1 mg/ml hITF; P < 0.01). These data provide direct evidence for a key role of ITF protein in barrier protection and suggest that ITF's functional action may be independent of the cell type, as illustrated here by the vascular endothelia.
Figure 4.
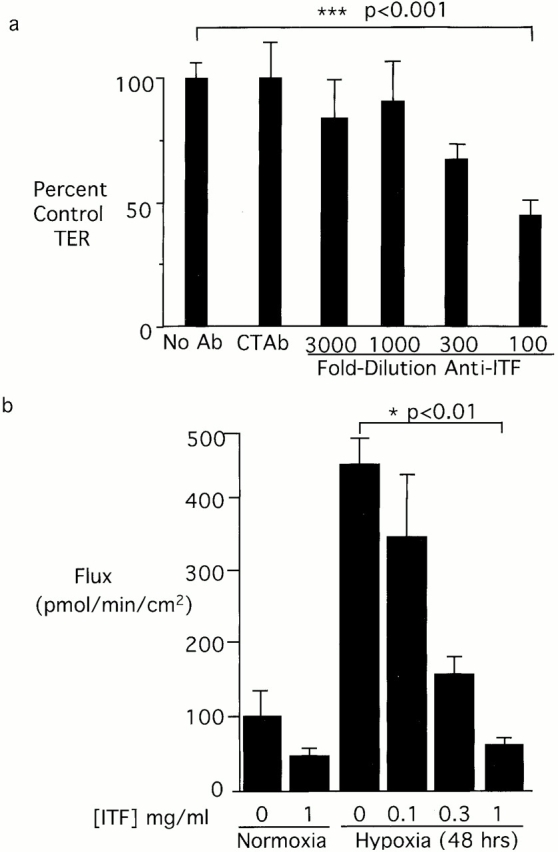
Functional role of ITF peptide on barrier function. In a, Caco-2 cells were grown to electrical confluence on polycarbonate supports. Anti-ITF immune serum at indicated concentrations or control anti-CREB (1:100) was added to the apical well of the support. Cells were exposed to indicated periods of ambient hypoxia (pO2 = 20 Torr) or normoxia. Transepithelial electrical resistance was measured at 48 h and indicated as data normalized to untreated control monolayers. 1:100 anti-ITF versus no Ab; ***P < 0.001. (b) The influence of recombinant ITF protein on permeability changes elicited in human MVEC exposure to hypoxia. Endothelia were grown to confluence on polycarbonate supports. Recombinant human ITF at indicated concentrations were applied to the apical well, and cells were exposed to indicated periods of ambient hypoxia (pO2 = 20 Torr) or normoxia. Flux of 40-kD FITC-labeled dextran was used to assess endothelial paracellular permeability. 1 mg/ml hITF versus no recombinant protein; *P < 0.001.
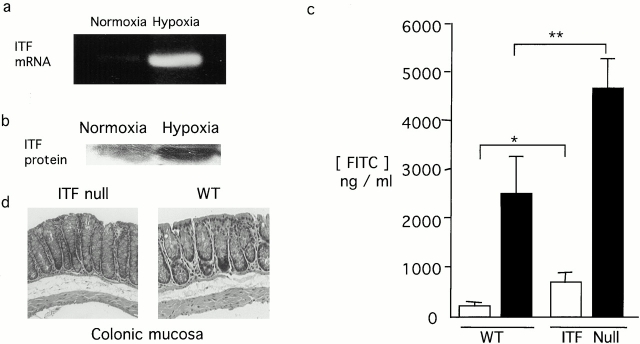
To define the role of hypoxia-elicited ITF on mucosal barrier in vivo, intestinal permeability was examined in wild-type and ITF null mice 21 during 4-h exposure to ambient hypoxia (8% O2). Under such conditions, increases in both ITF mRNA (Fig. 5 a) and protein (Fig. 5 b) were induced in tissues derived from wild-type animals subjected to hypoxia. As shown in Fig. 5 c, upon exposure to hypoxia, ITF null mice exhibited a significantly greater increase in intestinal permeability to FITC-dextran (4 kD) when compared with wild-type mice (ITF knockout versus wild-type exposed to hypoxia; P < 0.01). Interestingly, no histologically evident lesions were observed in the colonic mucosa with 4-h exposure to hypoxia (representative section from wild-type and ITF null animals are shown in Fig. 5 d). Longer periods of time in hypoxia did not significantly increase intestinal permeability beyond the 4-h time point. For example, intestinal permeability of wild-type animals subjected to hypoxia for 18 h (3.3 ± 1.2-fold increase in permeability, n = 4 animals) were not different than animals exposed to 4-h hypoxia (4.4 ± 1.3-fold increase, n = 19 animals). Moreover, these longer time periods resulted in an unexpected death in ITF null (four out of six animals tested) but not wild-type animals (zero out of six animals tested, data not shown). Interestingly, increased intestinal permeability was also evident in ITF null mice not exposed to hypoxia as compared with wild-type (P < 0.05 for ITF null compared with wild-type exposed to normoxia), consistent with the presumption that ITF may contribute to the maintenance of intestinal barrier under physiological conditions.
Figure 5.
Role of ITF in vivo. ITF null or wild-type Bl6129F1 mice were gavaged with 60 mg/100 g body weight of FITC-dextran and exposed to 4-h ambient hypoxia (8% O2) or ambient room air. Mice were killed, colonic tissue harvested for RNA (a), Western blot analysis (b), histologic analysis original magnification: ×10 (d), and serum FITC-dextran was quantified as a measure of intestinal permeability (c). *P < 0.05; **P < 0.01.
To further define these principles, wild-type and ITF null mice were administered recombinant ITF protein (30 mg/kg in PBS with 75% intragastric and 25% rectal infusion) before hypoxia. These studies revealed that administration of ITF to normoxic ITF null animals resulted in a significant decrease in permeability (550 ± 40 vs. 320 ± 90 ng/ml in the absence and presence of ITF administration, respectively; n = 4 mice per condition, P < 0.04). ITF addback to ITF null mice in hypoxia resulted in a trend toward resolution of increased permeability (1,310 ± 310 and 970 ± 110 ng/ml in the absence and presence of ITF administration; n = 6 mice per condition), however, these results were not statistically significant (P > 0.05). Similar to these results, ITF administration to wild-type mice in normoxia and hypoxia trended toward resolution of increased permeability but were not statistically significant (data not shown).
Discussion
These studies provide the first molecular link between hypoxia and intestinal epithelial barrier protection. Here, we identify a previously unappreciated HIF-1α regulated pathway for induction of ITF. ITF is a small, protease-resistant peptide that is abundant throughout the mammalian intestinal tract 27 28. Increased ITF expression has been observed in proximity to sites of injury in the gastrointestinal tract, including peptic ulcers and active inflammatory bowel disease 27 28 29. ITF can promote epithelial barrier function, protecting against injury, and facilitating repair after damage occurs irrespective of the nature of the initial wound. However, specific transcriptional pathways for ITF induction are not well understood 30.
The hypoxia-dependent production of ITF represents a novel, innate protective mechanism that may guard the immunologic components of the lamina propria from exposure to pathogenic luminal bacteria, antigens, and toxins during episodes of diminished oxygen delivery. Accumulating evidence suggests a variety of physiological roles for ITF 28, however, molecular mechanisms regulating ITF expression have not been studied in detail. Rather, the signaling pathways activated by the addition of exogenous ITF have been more closely examined and include the inactivation of extracellular signal regulatory kinase, regulated expression of α- and β-catenin, and transactivation of the epidermal growth factor receptor 31 32 33. Recent work has delineated that ITF can elicit distinct signaling pathways for epithelial restitution and inhibition of apoptosis, the latter of which involves activation of mitogen-activated protein kinase pathways independent of epidermal growth factor receptor phosphorylation 33. Important for the present studies, these same extracellular signal regulatory kinase pathways were recently demonstrated to critically function in the development of intestinal epithelial barrier function through regulated expression of claudin-2 and organization of membrane proteins within the tight junction 34. Thus, it is likely that ITF-specific, extracellular signaling pathways regulate epithelial barrier under these defined conditions.
At the tissue and cellular level, an array of genes pivotal to survival in low oxygen states are activated 4 35 36 37. Original studies of hypoxia-induced EPO production concentrated on the regulation of gene transcription. Sequences from the EPO promoter were isolated and identified as a heterodimer with independently regulated subunits termed HIF-1, a member of the rapidly growing per-ARNT-sim family of basic helix-loop-helix transcription factors 5 6. HIF-1 exists as an αβ heterodimer, the activation of which is dependent on stabilization of an O2-dependent degradation domain of the α subunit by the ubiquitin–proteasome pathway 7. Although not certain, HIF-1 appears to reside in the cytoplasm of normoxic cells, and like a number of other transcription factors (e.g., nuclear factor κB, β-catenin), HIF-1 translocates to the nucleus to form a functional complex 38 39. Binding of HIF-1 to consensus domains in the EPO enhancer results in the transcriptional induction of HIF-1–bearing gene promoters 4. Subsequently, it was determined that HIF-1 is widely expressed and that consensus HIF-1–binding sequences exist in a number of genes other than EPO and are termed HRE 4. Of note on this accord, we did not precisely map the ITF HRE. Attempts to define this site in exact terms were hampered by the complexity of the flanking region around the HIF-1 consensus sequence (data not shown). For example, the immediate region of the HIF-1 consensus site also contains consensus binding sites for the transcription factors myeloid zinc finger-1 (MZF1, consensus 5′-AGGGGGA-3′), GATA-1 (consensus 5′-GGGGATTCTG-3′), GATA-2 (consensus 5′-CAGGATAAGG-3′), Sp1 (consensus 5′-GTGGCAGGGT-3′), MyoD (consensus 3′-GCTGTCCACCGG-5′), and upstream stimulatory factor (USF, consensus 5′-CCACCTGT-3′). Important in this regard, at least three of these transcription factors (GATA-1, GATA-2, and MyoD) have been recently implicated in either induction or repression of genes in hypoxia 40 41 42 43. Therefore, it is possible that regulation of the ITF gene at this site could be an interplay of positive and negative signaling pathways, and given this complexity, more work will be necessary to define the exact details of this HRE.
Our results suggest that ITF may represent a potential novel therapeutic approach for a variety of diseases associated with mucosal hypoxia and altered epithelial barrier function, including inflammatory bowel disease, necrotizing enterocolitis, and ischemic colitis 44. In addition, these results suggest that a receptor/binding site for ITF may be present at sites other than the gastrointestinal tract, such as the endothelium. Thus, the protective effect of ITF on the hypoxia-induced decline in endothelial permeability suggests that ITF may have even broader therapeutic relevance in conditions of vascular leakiness such as septic shock. Although it is unknown whether ITF would function alone in these responses, it is possible, as previously proposed, that ITF acts in concert with and complementary to other protective growth factors and cytokines resident at sites of tissue damage 29. Efforts to better understand ITF signaling pathways and to identify other hypoxia-elicited protective elements could provide future focus for the development of novel treatments.
Acknowledgments
We thank Eric Black, Kristin Synnestvedt, and Robyn Carey for their superb technical assistance. The authors also thank I.K. Ezedi and I. Rosenberg for production of recombinant ITF used in these studies.
This work was supported by National Institutes of Health grants DK02564 (to G.T. Furuta), DK02682 (to C.T. Taylor), DK02503 (to J. Turner), DK43351 and DK46776 (to D.K. Podolsky), and DK50189 and HL60569 (to S.P. Colgan).
Footnotes
Abbreviations used in this paper: EMSA, electrophoretic mobility shift assay; EPO, erythropoietin; HIF, hypoxia-inducible factor; HRE, hypoxic response element; ITF, intestinal trefoil factor; MDCK, Madin-Darby canine kidney; MVEC, microvascular endothelial cell; RT, reverse transcription.
References
- Madara J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Taylor C.T., Colgan S.P. Therapeutic targets for hypoxia-elicited pathways. Pharmacol. Res. 1999;16:1498–1505. doi: 10.1023/a:1011936016833. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Perspectives on oxygen sensing. Cell. 1999;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-inducible factor 1master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular oxygen tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.E., Gu J., Schau M., Bunn H.F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R., Lencer W.I., Carlson S., Madara J.L. Carboxy-terminal vesicular stomatitis virus G protein-tagged intestinal Na+-dependent glucose cotransporter (SGLT1)maintenance of surface expression and global transport function with selective perturbation of transport kinetics and polarized expression. J. Biol. Chem. 1996;271:7738–7744. doi: 10.1074/jbc.271.13.7738. [DOI] [PubMed] [Google Scholar]
- Madara J.L., Stafford J., Dharmsathaphorn K., Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92:1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- Eagle H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc. Soc. Exp. Biol. Med. 1955;94:362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- Lennon P.F., Taylor C.T., Stahl G.L., Colgan S.P. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J. Exp. Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambuy Y., Rodriguez-Boulan E. Isolation and characterization of the apical surface of polarized Madin-Darby canine kidney epithelial cells. Proc. Natl. Acad. Sci. USA. 1988;85:1529–1533. doi: 10.1073/pnas.85.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan S.P., Dzus A.L., Parkos C.A. Epithelial exposure to hypoxia modulates neutrophil transepithelial migration. J. Exp. Med. 1996;184:1003–1015. doi: 10.1084/jem.184.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.T., Fueki N., Agah A., Hershberg R.M., Colgan S.P. Critical role of cAMP response element binding protein expression in hypoxia-elicited induction of epithelial TNFα. J. Biol. Chem. 1999;274:19447–19450. doi: 10.1074/jbc.274.27.19447. [DOI] [PubMed] [Google Scholar]
- Zünd G., Nelson D.P., Neufeld E.J., Dzus A.L., Bischoff J., Mayer J.E., Colgan S.P. Hypoxia enhances stimulus-dependent induction of E-selectin on aortic endothelial cells. Proc. Natl. Acad. Sci. USA. 1996;93:7075–7080. doi: 10.1073/pnas.93.14.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart D.J., Dong H., Byrne M.C., Follettie M.T., Gallo M.V., Chee M.S., Mittmann M., Wang C., Kobayashi M., Horton H., Brown E.L. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Seib T., Dooley S., Welter C. Characterization of the genomic structure and the promoter region of the human intestinal trefoil factor. Biochem. Biophys. Res. Commun. 1995;214:195–199. doi: 10.1006/bbrc.1995.2274. [DOI] [PubMed] [Google Scholar]
- Zünd G., Uezono S., Stahl G.L., Dzus A.L., McGowan F.X., Hickey P.R., Colgan S.P. Hypoxia enhances endotoxin-stimulated induction of functional intercellular adhesion molecule-1 (ICAM-1) Am. J. Physiol. 1997;273:C1571–C1580. doi: 10.1152/ajpcell.1997.273.5.C1571. [DOI] [PubMed] [Google Scholar]
- Ogata H., Podolsky D.K. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am. J. Physiol. 1997;273:G348–G354. doi: 10.1152/ajpgi.1997.273.2.G348. [DOI] [PubMed] [Google Scholar]
- Caniggia I., Mostachfi H., Winter J., Gassmann M., Lye S.J., Kuliszewski M., Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ(3) J. Clin. Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo H., Wu D.C., Podolsky D.K., Fishman M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- Napolitano L.M., Koruda M.J., Meyer A.A., Baker C.C. The impact of femur fracture with associated soft tissue injury on immune function and intestinal permeability. Shock. 1996;5:202–207. doi: 10.1097/00024382-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R., Ogawa S., Cozzolino F., Torcia G., Braunstein N., Butura C., Brett J., Leiberman H.B., Furie M.B., Joseph-Silverstien J., Stern D. Macrovascular and microvascular endothelium during long-term hypoxiaalterations in cell growth, monolayer permeability, and cell surface coagulant properties. J. Cell. Physiol. 1991;146:8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- Furuta G.T., Dzus A.L., Taylor C.T., Colgan S.P. Parallel induction of epithelial surface-associated chemokine and proteoglycan by cellular hypoxiaimplications for neutrophil activation. J. Leukoc. Biol. 2000;68:251–259. [PubMed] [Google Scholar]
- Taylor C.T., Furuta G.T., Synnestvedt K., Colgan S.P. Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc. Natl. Acad. Sci. USA. 2000;97:12091–12096. doi: 10.1073/pnas.220211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweiger G. Knowledge discovery in gene-expression-microarray datamining the information output of the genome. Trends Biotechnol. 1999;17:429–436. doi: 10.1016/s0167-7799(99)01359-1. [DOI] [PubMed] [Google Scholar]
- Thim L. Trefoil peptidesfrom structure to function. Cell. Mol. Life Sci. 1997;53:888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A.S. X. Trefoil peptide and EGF receptor/ligand transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G501–G506. doi: 10.1152/ajpgi.2000.278.4.G501. [DOI] [PubMed] [Google Scholar]
- Beck P.L., Podolsky D.K. Growth factors in inflammatory bowel disease. Inflamm. Bowel Dis. 1999;5:44–60. doi: 10.1097/00054725-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Ogata H., Inoue N., Podolsky D.K. Identification of a goblet cell-specific enhancer element in the rat intestinal trefoil factor gene promoter bound by a goblet cell nuclear protein. J. Biol. Chem. 1998;273:3060–3067. doi: 10.1074/jbc.273.5.3060. [DOI] [PubMed] [Google Scholar]
- Efstathiou J.A., Noda M., Rowan A., Dixon C., Chinery R., Jawhari A., Hattori T., Wright N.A., Bodmer W.F., Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc. Natl. Acad. Sci. USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Mullen C., Podolsky D.K. Intestinal trefoil factor induces inactivation of extracellular signal-regulated protein kinase in intestinal epithelial cells. Proc. Natl. Acad. Sci. USA. 1998;95:178–182. doi: 10.1073/pnas.95.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K., Taupin D.R., Itoh H., Podolsky D.K. Distinct pathways of cell migration and antiapoptotic response to epithelial injurystructure-function analysis of human intestinal trefoil factor. Mol. Cell. Biol. 2000;20:4680–4690. doi: 10.1128/mcb.20.13.4680-4690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugasa T., Sakaguchi T., Gu X., Reinecker H.C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- Bunn H.F., Poyton R.O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Wenger R.H. HIF-1, a mediator of the molecular response to hypoxia. News Physiol. Sci. 1997;12:214–218. [Google Scholar]
- Ratcliffe P.J., O'Rourke J.F., Maxwell P.H., Pugh C.W. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J. Exp. Biol. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- Presta L.G., Chen H., O'Conner S.J., Chisholm V., Meng Y.G., Krummen L., Winkler M., Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- Kallio P.J., Wilson W.J., O'Brien S., Makino Y., Poellinger L. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J. Biol. Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- Minet E., Mottet D., Michel G., Roland I., Raes M., Remacle J., Michiels C. Hypoxia-induced activation of HIF-1role of HIF-1α-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- De Maria R., Zeuner A., Eramo A., Domenichelli C., Bonci D., Grignani F., Srinivasula S.M., Alnemri E.S., Testa U., Peschle C. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Kramer M.F., Gunaratne P., Ferreira G.C. Transcriptional regulation of the murine erythroid-specific 5-aminolevulinate synthase gene. Gene. 2000;247:153–166. doi: 10.1016/s0378-1119(00)00103-7. [DOI] [PubMed] [Google Scholar]
- Tarumoto T., Imagawa S., Ohmine K., Nagai T., Higuchi M., Imai N., Suzuki N., Yamamoto M., Ozawa K. N(G)-monomethyl-l-arginine inhibits erythropoietin gene expression by stimulating GATA-2. Blood. 2000;96:1716–1722. [PubMed] [Google Scholar]
- Unno N., Fink M.P. Intestinal epithelial hyperpermeability. Mechanisms and relevance to disease. Gastroenterol. Clin. North Am. 1998;27:289–307. doi: 10.1016/s0889-8553(05)70004-2. [DOI] [PubMed] [Google Scholar]