In this issue, Matsumoto et al. 1 report that histamine exocytosed from brain mast cells (MCs) after activation by antigenic cross-linking of the high affinity IgE receptor (FcεRI) acts via Hl receptors at the hypothalamus to elicit corticotropin-releasing factor (CRF). These findings reveal a homeostatic response to a pathobiologic event in the dog. Conversely, CRF can also be proinflammatory by mediating activation of tissue MCs 2. This action is implicated in the acute immobilization stress response of the rat, in which degranulation of brain MCs is associated with a histamine H2 receptor–mediated increase in permeability of the blood–brain barrier 3. These models remind us that the inflammatory response is homeostatic in principle and pathobiologic only when the same pathways lead to an outcome that is more detrimental than beneficial to the host. Thus, the elegant demonstration that experimental allergic encephalomyelitis in the mouse can be MC dependent 4 invites readers to think of the contribution of the MC to dysfunction of the blood–brain barrier rather than its homeostatic hypothalamic-pituitary-adrenal signal function. As the documented role of the MC expands beyond recognition of its contribution to adverse local and systemic allergic responses, it is pertinent to review its development, remarkable proinflammatory armamentarium, and participation in an evolving number of models of pathobiologic processes.
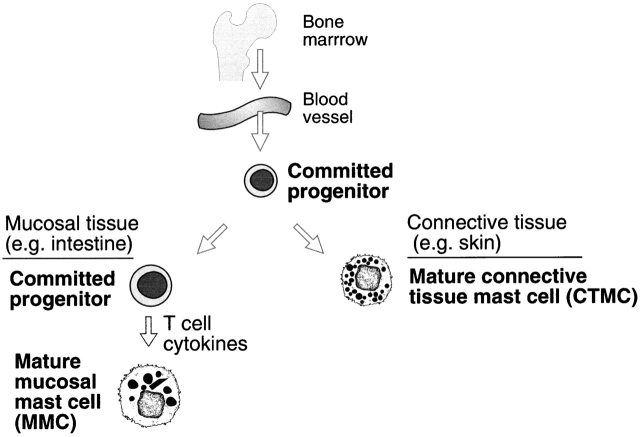
Although MCs are derived, like other leukocytes, from hematopoietic stem cells 5, they do not mature before exiting the bone marrow and circulate as committed progenitors. These progenitors complete their maturation with concomitant phenotypic diversity after moving into diverse peripheral tissues (Fig. 1). Their presence in these peripheral tissues depends on the action of their cell surface tyrosine kinase, c-kit, and its ligand, stem cell factor (SCF). The circulating progenitors have been isolated from human blood as c-kit +CD34+CD13+FcεRI− cells. This population contains both committed progenitors and cells that are bipotent, that is, able to differentiate into either MCs or monocytes 6. Progenitors also have been identified in the blood of 15.5-d-old fetal mice, where they represent an usually high proportion (∼2.5%) of the mononuclear cells at this point in development 7. These cells are poorly granulated, express high levels of c-kit, low levels of Thy-1, and no FcεRI and show no capacity to differentiate into any other cell type. Thus, these cells are committed MC progenitors. In the adult mouse, the mucosa of the intestine contains the largest peripheral pool of these committed progenitors 8. However, in the absence of inflammation, these cells do not develop into mature MCs. The rejection of many intestinal parasites requires the intestinal hyperplasia of a differentiated MC population 9 and therefore, the large reservoir of undifferentiated but committed progenitors provides homeostasis in an environment in which intestinal parasitism is a constant threat.
Figure 1.
MC development and diversity. MC lineage progenitors arise in the bone marrow, circulate through the vasculature, and move into tissues to complete their development. In skin and connective tissues of mice, mature MCs present different protease phenotypes within their secretory granules in different tissues. In mucosal tissues of mice, MCs remain as committed progenitors until acted on by T cell–derived cytokines.
The progenitors represent a single lineage that gives rise to distinct phenotypes after moving into different tissues and under different situations within a tissue 10 11 12. In the initial identification of MCs based on fixation properties and histochemical stains, two prominent phenotypes were recognized in rodents that reflect the biochemical properties of these cells in connective tissues or intestinal mucosa 13 and gave rise to the trivial nomenclature of connective tissue MCs (CTMCs) and mucosal MCs (MMCs). We now recognize phenotypic differences in the protease expression profile even within these anatomically defined cell populations of the mouse 10 11 12. Their histochemical differences are associated with heparin glycosaminoglycan-rich proteoglycans for CTMCs compared with mono- and disulfated chondroitin sulfate glycosaminoglycans linked to the same peptide core in MMCs 14 15. Human MCs do not provide these histochemical distinctions, and human MCs obtained from dispersed lung exhibit both heparin and chondroitin sulfate proteoglycans 16. An important distinction for the MMCs of both mouse and human is their T cell dependence for these cells are lacking in athymic mice and in humans with acquired immunodeficiency disease 17 18. In both settings, CTMCs are present in the submucosa, contrasting their constitutive appearance with the reactive character of MMCs. These two phenotypes of MCs also have other biochemical differences, with the MMC being low in histamine and high in activation-elicited cysteinyl leukotriene (cys-LT) production and the CTMC having a high histamine content and generating the prostanoid, prostaglandin (PG)D2, in marked preference to cys-LT 19. These distinctions may be pertinent to the understanding of animal models, but the available data for T cell–determined phenotypic MC changes are best defined for the intestine and are available only by implication for other mucosal surfaces.
In the mouse, 12 different proteases are stored along with the amines, histamine and serotonin, in the secretory granules as a complex with different proteoglycans that share the same peptide core (for a review, see reference 20). Distinct functions have been recognized for some of the proteases. Mouse MC protease (mMCP)-5, a chymase (chymotryptic-like secretory granule protease), has been deleted directly by targeted disruption of the gene and indirectly by targeted disruption of the N-sulfotransferase-2 gene needed for the production of heparin glycosaminoglycan 21 22 23. In both cases, there was coincident loss of mMC-carboxypeptidase A expression. In both instances, the cutaneous MCs representing the CTMC phenotype were abnormally small with poor granule morphology, whereas the Trichinella spiralis–elicited MMCs were robust and of normal phenotype. These findings emphasize that the tissue dictates the phenotypic diversity for the single MC lineage. There were also severe developmental abnormalities in several organs such as the eyes. As a developmental abnormality of the eye, for example, is not present in the W/Wv strain, either the short-lived progenitors in the bone marrow of W/Wv mice are sufficient for normal development or the phenotype results from an imbalance of proteases created by the disruption. Recombinant mouse tryptase mMCP-6, recombinant human βI tryptase, and a tryptase preparation isolated from human MCs all cause neutrophil accumulation in vivo, whereas recombinant mMCP-7 causes eosinophil accumulation in vivo and human MC chymase induces the accumulation of neutrophils and other leukocytes in vivo 24 25 26 27. Furthermore, the directed migration of neutrophils into the lung by recombinant human βI tryptase protects the W/Wv mouse against pulmonary infection by Klebsiella pneumoniae 25. The mouse tryptase mMCP-7 cleaves fibrinogen in vivo and blocks its function; this action could limit the functions of fibrinogen in MC-mediated reactions 28. The mouse jejunal MMC–specific chymase mMCP-1 is important in the rejection of T. spiralis, as mice rendered deficient in mMCP-1 by targeted disruption of the gene have an impaired ability to expel this nematode 29. Other studies with gene-disrupted mice have confirmed the association of the T cell cytokines with the appearance of the MMCs and rejection of various helminths 30 31. In a model of neoplasia, the CTMC proteases mMCP-4 (chymase family) and mMCP-6 were implicated in the MC-mediated upregulation of angiogenesis 32. Other possible functions not yet proven in vivo include cleavage of angiotensin I to angiotensin II, stimulation of mucus secretion, activation of metalloproteases, and activation of protease-activated receptors 20.
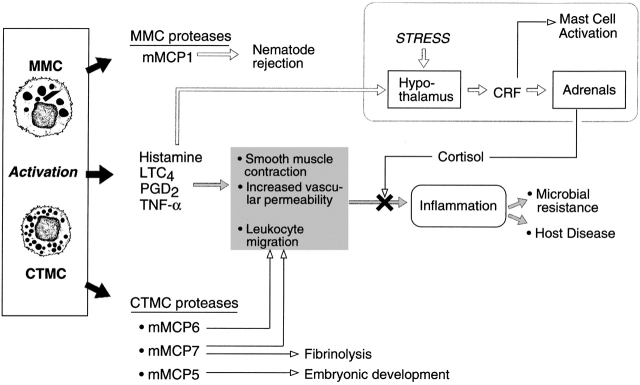
Whereas the secretory granule amines, proteases, and proteoglycans are stored for immediate release by exocytosis, the perinuclear membrane and endoplasmic reticulum respond to the same activation signal with the release of arachidonic acid for processing into eicosanoids. For the MC, PGD2 is the dominant prostanoid product, and the cys-LT, LTC4, dominates over the dihydroxy leukotriene, LTB4. In mice with a targeted disruption of the gene for LTC4 synthase (LTC4S), which provides LTC4, the parent of all receptor-active cys-LTs, the augmented vascular permeability causing edema in innate and adaptive immune inflammation is attenuated 33. Both zymosan-elicited, monocyte/macrophage-mediated intraperitoneal plasma influx and IgE/antigen-initiated MC-dependent ear edema were reduced by one half. The latter is noteworthy because cutaneous MCs, exemplifying the CTMC phenotype, were projected from studies with dispersed tissue MCs to be prostanoid-producing rather than cys-LT–producing phenotypes 19. Disruption of the classical PGD2 receptor, DP, confirmed the action of PGD2 on the microvasculature and airway smooth muscle in an aerosol antigen challenge of a sensitized mouse 34. The subsequent recognition by in vitro assays that PGD2 is also a ligand for a chemokine-like receptor (termed CRTH2) on human T cells, basophils, and eosinophils 35 provides a candidate feedback pathway for the Th2/MC duo in allergic inflammation.
A direct role of MC-derived cytokines in vivo has been demonstrated only for TNF-α. Mice that either have a targeted disruption of the TNF-α gene or are MC deficient due to a functional inactivation of their c-kit (W/Wv) are highly susceptible to death after cecal ligation and puncture, compared with their normal littermates 36 37. W/Wv mice can be protected by reconstitution of their peritoneal MC population through the adoptive transfer of immature MCs derived in vitro from the bone marrow of their normal littermates, in combination with the cytoprotective and mitogenic MC effects of administered recombinant stem cell factor. The serosal cavity MCs implicated in this innate host resistance are of the CTMC phenotype. In contrast, the MMCs developed in a T cell–dependent manner from intestinal progenitors are effective in the expulsion of adult T. spiralis in mice with a disruption of their TNF-α receptor gene 30. Cutaneous MC-derived TNF-α also induces endothelial leukocyte adhesion molecule-1 in humans 38, and its role in the mouse cecal ligation and puncture model is attributed to neutrophil recruitment 36 37. In this context, a proinflammatory function is clearly homeostatic.
The observation that ischemia-reperfusion injury of a hind limb of a mouse can be associated with remote pulmonary injury implies a mediator signal. That the lung is spared the injury, neutrophil extravasation, and edema by the lack of MCs in the W/Wv mouse 39 or by deficiency of the fifth complement component in an otherwise normal mouse 40 links two proinflammatory pathways, complement and MCs, but does not define their order. Of equal note are the findings that the W/Wv mouse is protected not only against the remote site injury, but also partially against the permeability enhancement, PMN extravasation, and myofibril disruption of the targeted hind limb 41.
In contrast to the in vivo proinflammatory actions of MCs that form the basis of our limited knowledge of their “diverse roles” (Fig. 2), Matsumoto et al. 1 suggest that MCs in the central nervous system may participate in the counter-regulation of an immune inflammatory response through interactions with the hypothalamic-pituitary-adrenal axis. In this study, the dogs were passively sensitized by the administration of IgE either intracerebroventricularly or intravenously and were challenged with specific antigen, either intracerebroventricularly or intravenously, resulting in cortisol release from the adrenal glands. The effect could be mimicked by intracerebroventricular injection of the MC secretagogue compound 48/80 and was blocked by corticotropin-releasing hormone (CRH) antibodies or histamine H1-blockers. Because glucocorticoids can be used to downmodulate immune reactions, Matsumoto et al. suggest that this pathway is an immunomodulator in which the MCs act as the switch, detecting high levels of systemic antigen and activating the hypothalamic-pituitary-adrenal axis to prevent anaphylaxis.
Figure 2.
MC function. Mature cells release preformed mediators, proteases, and vasoactive amines; vasoactive de novo–derived arachidonic acid metabolites, LTC4 and PGD2; and activation-induced gene products, e.g., TNF-α. These mediators have pleiotropic and even redundant effects on various tissues such as smooth muscle, leukocytes, and hypothalamic neurons (indicated by box in top right). Of note, the interaction between neurons and MCs can result in activation of MCs via CRF with resultant inflammation and disruption of the blood–brain barrier or release of adrenal-derived glucocorticoids with the potential downmodulation of an inflammatory response. This scheme is based primarily on studies done in rats and mice with the antiinflammatory production of cortisol derived from the dog.
The armamentarium and distribution of tissue MCs foretells a role for these cells in host integrity and disease that has not yet been realized. The reasons relate to their biology. Mature MCs do not circulate like cells of most hematopoietic lineages and do not dominate a single organ like parenchymal cells. The tissue-based progenitors cannot yet be recognized except by limiting dilution of dispersed tissue cells with culture-driven lineage identification. Their diverse differentiated tissue phenotypes are defined reasonably well for the secretory granule compartment for the mouse, but only minimally for humans and rats. Furthermore, the functions of these proteases, which dominate the total protein of this cell, are largely unknown, especially in vivo. The ability to appreciate their capacity for gene induction or eicosanoid generating preference in situ is limited by issues of identification with product quantitation. Even a presumptive straightforward systemic anaphylactic response is severely species related, based in part on the anatomic location of the targeted MCs. Humans experience laryngeal edema or acute emphysema with hypoxia or encounter a direct cardiovascular demise without antecedent hypoxia 42. Only the guinea pig resembles the human with fatal acute emphysema and no other species is known to have laryngeal edema. The dog experiences a hypovolemic death due to pooling in the liver because of a MC distribution that attenuates outflow. Nonetheless, progress in understanding the biology of the MC can be derived from models that use the MC-deficient W/Wv strain, mice with targeted disruptions of MC–selective genes (e.g., mMCP-1−/−, mMCP-5−/−), and mice with targeted disruption of genes expressed nonexclusively in MC (e.g., LTC4S−/−). After calibration of MC-selective responses such as IgE-mediated anaphylaxis for CTMCs and helminth infection for MMCs, more complex models can be approached. As the developmental and functional biology of the MC continues to unfold, the role of this cell in surrogate models of human diseases and its direct assessment in clinical settings will address the mysteries of its nature.
Acknowledgments
Supported by grants HL36110-16, AI31599-10, AI07306, and HL63284-02 from the National Institutes of Health.
References
- Matsumoto I., Inoue Y., Shimada T., Aikama T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J. Exp. Med. 2001;194:71–78. doi: 10.1084/jem.194.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L.K., Boucher W., Pang X., Letourneau R., Seretakis D., Green M., Theoharides T.C. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J. Pharmacol. Exp. Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- Esposito P., Gheorghe D., Kandere K., Pang X., Connolly R., Jacobson S., Theoharides T.C. Acute stress increases permeability of the blood¯brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Secor V.H., Secor W.E., Gutekunst C.-A., Brown M.A. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- Kirshenbaum A.S., Goff J.P., Semere T., Foster B., Scott L.M., Metcalfe D.D. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- Rodewald H.R., Dessing M., Dvorak A.M., Galli S.J. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Dy M., Luffau G., Vassalli P. Gut mucosal mast cells. Origin, traffic, and differentiation. J. Exp. Med. 1984;160:12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J.F., Jr., Schopf L., Morris S.C., Orekhova T., Madden K.B., Betts C.J., Gamble H.R., Byrd C., Donaldson D., Else K., Finkelman F.D. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J. Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- Gurish M.F., Pear W.S., Stevens R.L., Scott M.L., Sokol K., Ghildyal N., Webster M.J., Hu X., Austen K.F., Baltimore D., Friend D.S. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity. 1995;3:1–20. doi: 10.1016/1074-7613(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Friend D.S., Ghildyal N., Austen K.F., Gurish M.F., Matsumoto R., Stevens R.L. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J. Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R.L., Friend D.S., McNeil H.P., Schiller V., Ghildyal N., Austen K.F. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc. Nat. Acad. Sci. USA. 1994;91:128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback L. Mast cells in rat gastrointestinal mucosa. II. Dye-binding and metachromatic properties. Acta. Pathol. Microbiol. Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Stevens R.L., Lee T.D., Seldin D.C., Austen K.F., Befus A.D., Bienenstock J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J. Immunol. 1986;137:291–295. [PubMed] [Google Scholar]
- Tantravahi R.V., Stevens R.L., Austen K.F., Weis J.H. A single gene in mast cells encodes the core peptides of heparin and chondroitin sulfate proteoglycans. Proc. Natl. Acad. Sci. USA. 1986;83:9207–9210. doi: 10.1073/pnas.83.23.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R.L., Fox C.C., Lichtenstein L.M., Austen K.F. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Nat. Acad. Sci. USA. 1988;85:2284–2287. doi: 10.1073/pnas.85.7.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E.J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264:258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Irani A.M., Craig S.S., DeBlois G., Elson C.O., Schechter N.M., Schwartz L.B. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J. Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- Heavey D.J., Ernst P.B., Stevens R.L., Befus A.D., Bienenstock J., Austen K.F. Generation of leukotriene C4, leukotriene B4, and prostaglandin D2 by immunologically activated rat intestinal mucosa mast cells. J. Immunol. 1988;140:1953–1957. [PubMed] [Google Scholar]
- Huang C., Sali A., Stevens R.L. Regulation and function of mast cell proteases in inflammation. J. Clin. Immunol. 1998;18:169–183. doi: 10.1023/a:1020574820797. [DOI] [PubMed] [Google Scholar]
- Li L., Humphries D.E., Krilis S.A., Stevens R.L. The role of mast cell-derived, preformed granule mediators in embryonic development FASEB J 14 2000. A1128(Abstr.) [Google Scholar]
- Stevens R.L., Qui D., McNeil H.P., Friend D.S., Hunt J.E., Austen K.F., Zhang J. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene cannot store carboxypeptidase A in their granules FASEB J. 10 1996. 1307(Abstr.) [Google Scholar]
- Humphries D.E., Wong G.W., Friend D.S., Gurish M.F., Qiu W.T., Huang C., Sharpe A.H., Stevens R.L. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- Huang C., Friend D.S., Qiu W.T., Wong G.W., Morales G., Hunt J., Stevens R.L. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 1998;160:1910–1919. [PubMed] [Google Scholar]
- Huang C., De Sanctis G.T., O'Brien P.J., Mizerd J.P., Friend D.S., Drazen J.M., Brass L.F., Stevens R.L. Human mast cell tryptase betaIevaluation of its substrate specificity and demonstration of its importance in bacterial infections of the lung. J. Biol. Chem. 2001;In press doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- He S., Walls A.F. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br. J. Pharmacol. 1998;125:1491–1500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Peng Q., Walls A.F. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptaseselective enhancement of eosinophil recruitment by histamine. J. Immunol. 1997;159:6216–6225. [PubMed] [Google Scholar]
- Huang C., Wong G.W., Ghildyal N., Gurish M.F., Sali A., Matsumoto R., Stevens R.L. The tryptase, mouse mast cell protease 7, degrades fibrinogen in V3-mastocytosis mice. J. Biol. Chem. 1997;272:31885–31893. doi: 10.1074/jbc.272.50.31885. [DOI] [PubMed] [Google Scholar]
- Knight P.A., Wright S.H., Lawrence C.E., Paterson Y.Y., Miller H.R. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell–specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C.E., Paterson J.C., Higgins L.M., MacDonald T.T., Kennedy M.W., Garside P. IL-4-regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 1998;28:2672–2684. doi: 10.1002/(SICI)1521-4141(199809)28:09<2672::AID-IMMU2672>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lantz C.S., Boesiger J., Song C.H., Mach N., Kobayashi T., Mulligan R.C., Nawa Y., Dranoff G., Galli S.J. Role for interleukin-3 in mast cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Raymond W.W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G.H., Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y., Maekawa A., Penrose J.F., Austen K.F., Lam B.K. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;In press doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- Matsuoka T., Hirata M., Tanaka H., Takahashi Y., Murata T., Kabashima K., Sugimoto Y., Kobayashi T., Ushikubi F., Aze Y. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001;193:255–262. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M., Echtenacher B., Hültner L., Kollias G., Männel D.N., Langley K.E., Galli S.J. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Ikeda T., Ross E., Abraham S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Walsh L.J., Trinchieri G., Waldorf H.A., Whitaker D., Murphy G.F. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman G., Welbourn R., Klausner J.M., Kobzik L., Valeri C.R., Shepro D., Hechtman H.B. Mast cells and leukotrienes mediate neutrophil sequestration and lung edema after remote ischemia in rodents. Surgery. 1992;112:578–586. [PubMed] [Google Scholar]
- Kyriakides C., Austen W.G., Jr., Wang Y., Favuzza J., Moore F.D., Jr., Hechtman H.B. Neutrophil mediated remote organ injury after lower torso ischemia and reperfusion is selectin and complement dependent. J. Trauma. 2000;48:32–38. doi: 10.1097/00005373-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Mukundan C., Gurish M.F., Austen K.F., Hechtman H.B., Friend D.S. Mast cell mediation of muscle and pulmonary injury following hind limb ischemia-reperfusion. J. Histochem. Cytochem. 2001;In press doi: 10.1177/002215540104900813. [DOI] [PubMed] [Google Scholar]
- James L.P., Jr., Austen K.F. Fatal systemic anaphylaxis in man. N. Engl. J. Med. 1964;270:597–603. doi: 10.1056/NEJM196403192701202. [DOI] [PubMed] [Google Scholar]