Abstract
In the multistep process of leukocyte extravasation, the mechanisms by which leukocytes establish the initial contact with the endothelium are unclear. In parallel, there is a controversy regarding the role for L-selectin in leukocyte recruitment. Here, using intravital microscopy in the mouse, we investigated leukocyte capture from the free flow directly to the endothelium (primary capture), and capture mediated through interactions with rolling leukocytes (secondary capture) in venules, in cytokine-stimulated arterial vessels, and on atherosclerotic lesions in the aorta. Capture was more prominent in arterial vessels compared with venules. In venules, the incidence of capture increased with increasing vessel diameter and wall shear rate. Secondary capture required a minimum rolling leukocyte flux and contributed by ∼20–50% of total capture in all studied vessel types. In arteries, secondary capture induced formation of clusters and strings of rolling leukocytes. Function inhibition of L-selectin blocked secondary capture and thereby decreased the flux of rolling leukocytes in arterial vessels and in large (>45 μm in diameter), but not small (<45 μm), venules. These findings demonstrate the importance of leukocyte capture from the free flow in vivo. The different impact of blockage of secondary capture in venules of distinct diameter range, rolling flux, and wall shear rate provides explanations for the controversy regarding the role of L-selectin in various situations of leukocyte recruitment. What is more, secondary capture occurs on atherosclerotic lesions, a fact that provides the first evidence for roles of L-selectin in leukocyte accumulation in atherogenesis.
Keywords: venule, artery, cell adhesion molecules, rolling string, mice
Introduction
Extravasation of leukocytes requires sequential events of leukocyte–endothelial interactions. It includes leukocyte rolling along, firm adhesion to and transmigration through the vascular endothelium, all steps that are mediated by cell adhesion molecules (CAMs) on leukocytes and endothelial cells 1 2. In this process, the efficiency of leukocyte extravasation is dependent on each link in this chain of events. Consequently, inhibition of any of the steps in this inverted cascade will have powerful effects on the outcome of leukocyte recruitment 3 4 5 6.
Rolling of leukocytes along venular endothelium is mediated primarily by the selectin family of CAMs. Endothelial P- and E-selectin have distinct but overlapping functions in this process 5. P-selectin is a principal receptor for leukocyte rolling and can be upregulated both from preformed Weibel-Palade bodies in endothelial cells as well as upon stimulation from certain mediators such as TNFα or IL-1β 7. E-selectin, on the other hand, mediates slow rolling and transition to firm adhesion and is expressed from de novo synthesis only 8 9 10. Cytokine-induced expression of P- and E-selectin has also been demonstrated in arteries, vessels that rarely express CAMs capable of mediating leukocyte rolling 11 12 13. Both endothelial selectins bind to the P-selectin glycoprotein ligand 1 (PSGL-1) which is constitutively expressed on many leukocyte subclasses 14. Other selectin ligands on leukocytes remain to be clearly defined 14 15 16.
L-selectin is constitutively expressed on many leukocytes and binds to ligands expressed on the endothelium in lymphoid tissues and at sites of inflammation 17 18. In lymphoid tissues, these ligands have been well characterized both with regard to their structure as well as their functional significance as endothelial homing receptors for lymphocytes. In contrast, the identity of the endothelial ligand(s) for L-selectin in inflammation is unclear. However, a recent study could show that the functional epitope on inflamed endothelium mediating interactions with L-selectin is identical with the HECA-452 binding cutaneous lymphocyte antigen (CLA) 19. In addition to the unclear identity of endothelial ligand(s) on inflamed endothelium, the functional roles for L-selectin–dependent leukocyte–endothelial interactions in inflammation are controversial. In some studies, function inhibition of L-selectin in trauma-induced leukocyte rolling has resulted in marked decreases in the flux of rolling leukocytes 1 20 21. In contrast, other studies have found no decrease in leukocyte rolling after blockage of L-selectin function 5 16. This apparent inconsistency may be partly explained by the fact that trauma-induced leukocyte rolling in venules becomes increasingly dependent on L-selectin at later time points after surgical preparation of the tissue exposed for intravital microscopy 22, suggesting that differences in experimental setup could account for part of the contrariety between published sets of data. However, the controversy as to the role of L-selectin in leukocyte recruitment still remains.
Apart from the roles for L-selectin in mediating leukocyte rolling along the endothelium, L-selectin has also been implicated in other events potentially occurring in the process of leukocyte recruitment. To initiate rolling, leukocytes need to establish contact with the endothelium. However, the mechanisms by which leukocytes achieve this contact in vivo are unclear. Hypothetically, rolling can be initiated by transition from nonspecific leukocyte–endothelial contact to adhesion receptor–dependent rolling interactions when leukocytes enter venules from the capillaries. Alternatively, leukocyte rolling may commence upon leukocyte capture from the free flow. In flow chambers in vitro, capture of leukocytes from the free flow is a prerequisite for leukocyte rolling along the adhesive surface and in this situation, capture provides a rate-limiting step in leukocyte accumulation. Capture in vitro has been shown to occur through two distinct mechanisms (see Fig. 1 A). First, leukocytes can attach directly to the endothelium and subsequently initiate rolling interactions, a phenomenon called primary capture. Second, a freely flowing leukocyte can transiently interact with a rolling leukocyte, subsequently attach to the endothelium, and initiate rolling interactions in contact with, or immediately downstream of, the previously rolling cell. This is known as secondary capture 23 24. Secondary capture in vitro is mediated by L-selectin that interacts mainly with PSGL-1, which presents L-selectin binding HECA-452 defined epitopes on leukocytes 15 25 26 27 28 29. However, the physiological roles for capture, primary or secondary, have been questioned. In the only study addressed to investigate these events in vivo, capture was found to be of minimal importance in leukocyte–endothelial interactions 30. Moreover, a recent study could demonstrate that in flow chambers in vitro, secondary capture is markedly decreased when whole blood rather than suspensions of leukocytes free from erythrocytes are used 31. Consequently, primary and secondary capture have been regarded mainly as in vitro phenomena with only minimal impact on leukocyte recruitment in vivo. Instead, it has been postulated that leukocytes interacting with the endothelium in inflammation initiate rolling interactions immediately when entering venules from the capillaries and hence, when they are already in contact with the vessel wall.
Figure 1.
The concept of capture and the influences of vessel type, pretreatment, vessel diameter, and WSR on capture in vivo. (A) Capture in vitro occurs through either of two distinct mechanisms. Leukocytes may attach directly to the endothelium and subsequently initiate rolling interactions, an event called primary capture. Alternatively, a freely flowing leukocyte can transiently interact with a previously rolling leukocyte, and subsequently initiate a rolling interaction with the endothelium immediately downstream of the previously rolling cell. This event is known as secondary capture. (B) RLF, capture, and detachment in arterioles and venules of the mouse cremaster muscle microcirculation after cytokine stimulation of the tissue, and in response to preparation trauma only. Capture represents the number of leukocytes that initiated rolling within the central part of the field of vision without previously having been in contact with the vessel wall. (C) Capture of leukocytes in various situations in the microcirculation plotted against leukocyte detachment from the endothelium (r = 0.843, P < 0.001). Data for venules and arterioles are indicated by dots or circles, respectively. (D) Capture of leukocytes from the free flow was plotted against vessel diameter and WSR. In arterioles, capture of leukocytes to the endothelium occurs in all vessels where leukocyte rolling is observed. In venules, capture is low at low WSR and in vessels of small diameters whereas in larger venules and at higher WSR, capture is prominent.
In this study, we investigated the roles for capture of leukocytes from the free flow under physiological conditions. Our findings reveal that primary capture as well as L-selectin–dependent secondary capture are important mechanisms by which leukocytes initiate interactions with the endothelium. Moreover, blockage of secondary capture has the potential to decrease leukocyte rolling in many inflammatory situations, a fact that provides increased understanding of the roles for L-selectin in leukocyte recruitment in vivo.
Materials and Methods
Animals
Male wild-type (WT) C57BL/6 mice were obtained from B&K. L-selectin–deficient mice (L−/−) mice and appropriate controls (B6/129F2) were obtained from The Jackson Laboratory. Atherosclerotic ApoE deficient (ApoE0) and ApoE/LDL receptor double deficient (ApoE0LDLR0) mice backcrossed into C57BL/6 mice for at least 10 generations were obtained from M&B. Atherosclerosis prone mice were from 8 wk of age fed western diet (Analyzen) based on cornstarch, sucrose, glucose, cocoa butter, cellulose, minerals, and a vitamin mix. The diet contained 0.15% cholesterol and 21% (wt/wt) total fat. All other animals were fed normal chow. Water was provided ad libitum. Intravital microscopy experiments on atherosclerotic mice were performed on the aorta at an age of 5–10 mo. All experiments were approved by the regional ethical committee for animal experimentation.
Cytokine Stimulation
Cremaster Muscle.
Cytokines were given through intrascrotal injections (a combination of 0.05 μg human TNFα (hTNFα) and 0.0125 μg human IL-1β (hIL-1β) in 0.3 ml in PBS given 3–4 h before intravital microscopy).
Femoral Artery.
hTNFα (0.05 μg) and hIL-1β (0.0125 μg) were injected medially on the hindpaw.
Experimental Procedure
In experiments on the aorta, mice were anesthetized by spontaneous inhalation of 2% isoflurane (Forene®; Abbott) in 40% O2. In all other experiments, mice were anesthetized by an intraperitoneal injection of 0.15–0.20 ml of a mixture of ketamine (Ketalar®; Parke-Davis; 25 mg/ml) and xylazine (Narcoxyl vet.®; Veterinaria AG; 5 mg/ml). Catheters were placed in the left carotid artery and in the left jugular vein. Blood pressure was monitored and ranged between 60 and 100 mmHg. Temperature was kept at 37°C with a heating pad and an infrared heat lamp. The exposed tissue was superfused with a thermostated (37°C) bicarbonate-buffered saline solution equilibrated with 5% CO2 in nitrogen to maintain physiological pH. Blood samples (10 μl) were taken through the carotid catheter and later analyzed for systemic leukocyte count (white blood count [WBC]) in a Bürker chamber.
Surgical Procedures
Aorta.
The aorta was prepared as described previously 13. In brief, the abdomen was opened through a midline incision and the intestines were retracted. The aorta was carefully exposed and separated from the vena cava for a distance of 2–3 mm immediately inferior of the renal arteries. The mouse was placed under the microscope and an ultrasonic flowprobe connected to a flowmeter (Transonic T-106 flowmeter, 0.7v probe) was placed around the artery. Direct intravital microscopic observations were performed on the abdominal aorta at least 4–5 mm downstream of the flowprobe.
Femoral Artery.
The skin was opened parallel to the femoral vessels. The skin was retracted exposing the vessels for direct microscopic observations.
Cremaster Muscle.
The cremaster muscle was prepared as described previously 22. In brief, an incision of the skin and fascia ventrally on the right scrotum was made and the tissue was retracted to expose the cremaster muscle. The muscle was incised and spread on a transparent pedestal to allow transillumination. The testis was then pinned to the side.
Intravital Microscopy.
Microscopic observations were made using an intravital microscope (Leitz Biomed or Leitz Orthoplan) with a water immersion objective (Leitz SW25 [observation of leukocytes] or Nikon WI10 [flow measurements]). In experiments on large vessels and in all flow measurements, epi-illumination fluorescence microscopy (Leitz Ploem-o-pac, [filter block M2 or I2] illuminated by a cooled infrared filtered lamp [Osram HBO 200W/4]) was used. In the microcirculation, observation of leukocyte–endothelial interactions was performed using either fluorescent or transmitted light. Labeling of circulating leukocytes was made by an intravenous injection of Rhodamine 6G (0.3 mg/ml, 0.67 mg/kg). Images were televised and recorded on videotape using Panasonic WV-1900 or WV-1550 video cameras.
Analysis of In Vivo Experiments
Vessel diameter (D) was measured from the microscopic image and radius (r) was calculated as D/2. In the microcirculation, flow (q) and wall shear rate (WSR [γw]) were calculated from r and by measuring the velocity of intravenously injected fluorescent beads (2.0 μm in diameter). The fastest beads were approximated to represent axial flow velocity (vax). vax was divided by the empiric factor 2 to achieve mean flow velocity (vm) 32. The factor 2 was controlled by investigating the relation between maximum velocity and mean velocity of fluorescent beads (data not shown). Flow could then be calculated from vm and r according to q = vmπr 2. WSR was subsequently calculated using the formula γw= 2.12 × 4q/πr 3 according to Tangelder et al. 33. Rolling leukocyte flux (RLF) was determined as the number of leukocytes passing a reference line perpendicular to blood flow. RLF fraction (RLFF) was calculated as RLF divided by the total leukocyte flux (TLF) in the observed vessel estimated from flow and WBC. Rolling flux/mm was determined as RLF per mm observed vessel circumference calculated from πD. In large arteries, RLF was determined as the number of leukocytes rolling across a 0.15-mm long reference line perpendicular to blood flow and rolling flux/mm was calculated as RLF/0.15. Leukocytes were regarded as captured if they initiated endothelial contact within the field of vision and had not previously been in contact with the vessel wall. Empirically, it was found that almost all secondary capture in vivo occurred either in contact with, or 0–30 μm downstream of previously rolling leukocytes. Therefore, captured leukocytes were regarded as potentially secondary if they attached in contact with or 0–30 μm downstream of a previously rolling cell. All other captured leukocytes were regarded as primary. To adjust the number of potentially secondary captured leukocytes for primary capture occurring downstream of rolling cells, the number of potentially secondary leukocytes was subtracted by the number of cells that were captured 0–30 μm upstream of rolling leukocytes. The outcome was determined as secondary capture. Capture/mm2 was determined as the number of captured leukocytes divided by luminal vessel area calculated from πDl where l represents the length of the observed vessel segment. In large vessels, capture was examined in a 0.15 × 0.15 mm square area, and capture/mm2 was calculated accordingly. Capture efficiency was calculated as the ratio between the number of captured cells and TLF. Capture efficiency/mm2 was calculated as capture efficiency divided by luminal vessel area calculated from πDl. In cremaster muscle vessels >70 μm in diameter, leukocytes could be observed only on the side of the vessel facing the objective. In such vessels, parameters of leukocyte capture and rolling were adjusted by multiplying these parameters with a factor 2. Leukocytes were regarded as detached if they released from rolling to the free flow without reattaching within the field of vision.
Antibodies and Reagents
mAb MEL-14 against mouse L-selectin (50–70 μg per mouse) and mAb RB40.34 against mouse P-selectin (30 μg per mouse) were obtained from BD PharMingen. Systemic treatment with MEL-14 decreased WBC as described previously 34 and data were carefully adjusted accordingly. Recombinant hIL-1β and hTNFα were obtained from R&D Systems. Rhodamine 6G came from Sigma-Aldrich. FITC-labeled microspheres were obtained from Molecular Probes Inc.
Statistical Analysis
The data represent mean ± SEM of measurements obtained in the indicated number of experiments. Statistical comparison between WT and L−/− mice were performed using the Student's t test or the Mann-Whitney rank sum test, whereas comparison before and after antibody blockage of CAMs were performed using paired t test or Wilcoxon signed rank test for paired samples. Nonparametric tests were used in the cases where samples were not normally distributed. In all comparisons on normally distributed samples, nonparametric tests rendered values of significance similar to what was found using t tests. In experiments investigating the relation between two parameters, linear regression was used. Multiple linear regression was used to investigate how one parameter was dependent on two independent parameters. Statistical significance was set at P < 0.05. In the figures, *, **, and *** denote difference from control value by significance of P < 0.05, P < 0.01, and P < 0.001, respectively.
Results
For specification of all parameters, see Materials and Methods. WBC, vessel diameters, and WSR in different experimental settings are shown in Table . In arterioles treated with a combination of 0.05 μg hTNFα and 0.0125 μg hIL-1β, leukocyte rolling was observed in vessels >25 μm in diameter. In venules, leukocyte rolling was observed in all types of vessels regardless of size or treatment. In trauma-induced leukocyte rolling in venules, RLFF was 9.1 ± 1.3% and 13 ± 2.7% in C57BL/6 and B6/129F2 mice, respectively (P = 0.846). In venules treated with cytokines, RLFF in C57BL/6 was 4.9 ± 1.6% whereas in B6/129F2 mice it was 6.6 ± 1.3% (P = 0.085). In addition, RLFF in arterioles was also similar in the two control mouse strains (6.0 ± 0.64% and 7.7 ± 1.5% for C57BL6 and B6/129F2, respectively; P = 0.332). Thus, for RLFF as well as for all other parameters of leukocyte–endothelial interactions (not shown), no significant differences between C57BL/6 and B6/129F2 control mice were found and therefore, data are pooled as WT mice. In trauma-induced rolling in venules, RLFF was inversely correlated to diameter and flow velocity (r = 0.347, P < 0.001; and r = 0.179, P < 0.05, respectively), as described previously 22. In arterioles, vessel diameter did not influence RLFF significantly (r = 0.072, P = 0.518) whereas RLFF was highly dependent on flow velocity (r = 0.484, P < 0.001). In all experiments in venules, RLFF was lower compared with what has been observed in most previous studies. This may be due to the fact that the average diameter of the vessels observed in this study is ∼10–25 μm larger compared with those studied in most previous reports 5 16 22. Thus, the low RLFFs observed in this study are consistent with a trend of decreasing RLFF in venules of increasing diameters observed in previous studies.
Table 1.
Systemic Leukocyte Counts, Vessel Diameters, and WSR in Different Experimental Situations
| Vessel type | Animal | Number of animals/vessels | WBC | Mono | Poly | Vessel diameter | WSR |
|---|---|---|---|---|---|---|---|
| 106 cells/ml | % | % | μm | s−1 | |||
| Arterioles | |||||||
| hIL1β + hTNFα | WT | 20/83 | 5.1 ± 0.53 | 54 ± 3.0 | 46 ± 3.0 | 45 ± 0.95 | 705 ± 39 |
| R: 29–71 | R: 165–1,924 | ||||||
| L−/− | 7/34 | 3.7 ± 0.62 | 40 ± 5.2 | 60 ± 5.2 | 45 ± 2.2 | 882 ± 81 | |
| R: 22–74 | R: 245–2,124 | ||||||
| Venules | |||||||
| Trauma-induced rolling | WT | 23/157 | 9.2 ± 0.54 | 80 ± 1.6 | 20 ± 1.6 | 48 ± 1.4 | 493 ± 18.2 |
| R: 16–106 | R: 68–1,317 | ||||||
| L−/− | 7/51 | 7.2 ± 2.1 | 78 ± 3.6 | 22 ± 3.6 | 49 ± 3.1 | 567 ± 32 | |
| R: 21–111 | R: 141–1,902 | ||||||
| hIL1β + hTNFα | WT | 7/22 | 5.3 ± 1.5 | 43 ± 6.2 | 57 ± 6.2 | 61 ± 5.4 | 211 ± 21 |
| R: 29–138 | 60–444 | ||||||
| Femoral artery | |||||||
| hIL1β + hTNFα | WT | 5/25 | 5.1 ± 0.94 | 51 ± 8.4 | 49 ± 8.4 | 212 ± 3.7 | − |
| R: 175–333 | |||||||
| L−/− | 6/19 | 5.0 ± 1.0 | 47 ± 12 | 53 ± 12 | 179 ± 5.4 | − | |
| R: 119–270 | |||||||
| Aorta athero | |||||||
| ApoE0, ApoE0/LDLR0 | 7/17 | 6.2 ± 1.1 | 64 ± 2.4 | 36 ± 2.4 | − | − |
All parameters are expressed as mean ± SEM in the indicated number of experiments. R indicates range of the indicated parameter.
Capture of Leukocytes Occurs in Various Types of Vessels In Vivo and Correlates with the Rate of Leukocyte Detachment from the Endothelium.
In all types of microvessels studied, leukocyte capture to, and detachment from, the endothelium was observed. Capture was more prominent in arterioles as compared with venules (Fig. 1 B). Regardless of inductive stimulus or vessel type, a close relation between capture and detachment was found (r = 0.843, P < 0.001; Fig. 1 C) indicating that in the situations where capture is distinct, there is a prominent turnover of leukocytes attaching to and detaching from the endothelium. In situations of low detachment (i.e., stable rolling), the contribution of capture to leukocyte–endothelial interactions is less substantial.
Capture of Leukocytes in Venules Is Strongly Dependent on Vessel Diameter and WSR.
In arterioles, capture was prominent in all arterioles where leukocyte rolling was observed regardless of vessel diameter or WSR indicating that capture is a prerequisite for leukocyte rolling in arterial vessels (Fig. 1 D, left). In contrast, capture in venules was rare at small diameters and at low WSR whereas in larger venules and at higher WSR, capture was significant and could reach levels equal to what was seen in arterioles (multiple regression: r = 0.488, P < 0.001; Fig. 1 D, right). This indicates that leukocyte rolling in small venules is not initiated by capture of leukocytes from the free flow but rather through direct transition from mechanical leukocyte–endothelial contact in narrow capillaries where leukocytes are caught against the vessel wall, to CAM-dependent rolling interactions when leukocytes enter into venular vessels. At higher WSR or at larger vessel diameters than depicted in Fig. 1 D, capture was found in all types of vessels where leukocyte rolling was observed.
Primary and Secondary Capture Contribute to Capture of Leukocytes In Vivo.
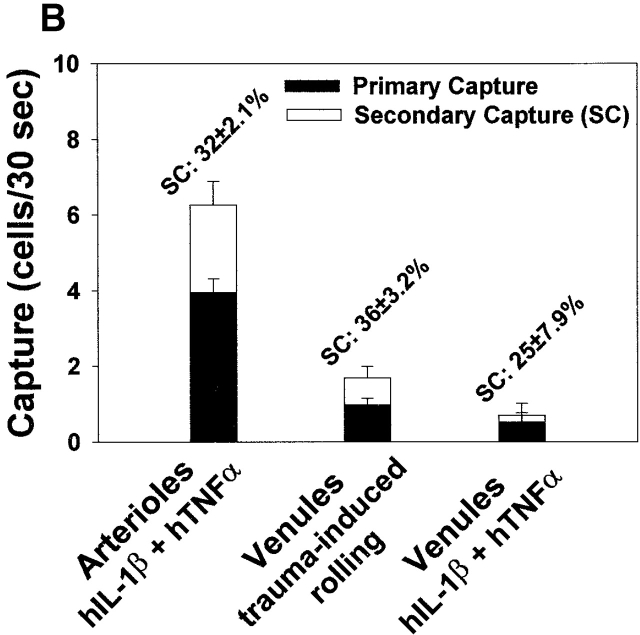
As capture proved to be a significant feature of leukocyte–endothelial interactions in various situations, we investigated the respective contribution of primary and secondary capture to total capture of leukocytes in the microcirculation in vivo. Interestingly, secondary capture was regularly observed in all situations where primary capture was evident. Sequential images of primary and secondary capture in an arteriole treated with hIL-1β and hTNFα are shown in Fig. 2 A. Secondary capture occurred through interactions between rolling and freely flowing leukocytes whereas secondary capture mediated by firmly adherent cells was hardly observed. Primary and secondary capture increased with increasing flux of rolling leukocytes (not shown). In addition, secondary capture seemed to require a minimum rolling flux to become prominent. This is likely because rolling leukocytes will present the required adhesive surface towards freely flowing cells for secondary capture to occur, and increased rolling flux will thus increase secondary capture.
Figure 2.
Primary and secondary capture in the microcirculation. (A) Intravital microscopy images of leukocyte rolling, primary capture, and secondary capture in a cytokine-treated mouse cremaster muscle arteriole. Vessel diameter is indicated with D. Rolling leukocytes are indicated with white circles and arrows. P and S indicate primary and secondary capture, respectively. Large white arrow represents direction of flow. Bar, 50 μm. Images were taken at times 0, 0.72, 0.96, and 1.56 s. (B) The respective contribution of primary and secondary capture in cytokine-induced leukocyte rolling in arterioles and venules, and in trauma-induced rolling in venules. Mean ± SEM of the percentage of total capture that was made up of secondary capture is indicated.
Secondary Capture Is L-Selectin Dependent and Increases Cytokine-induced Leukocyte Rolling in Arterioles and Trauma-induced Rolling in Venules In Vivo.
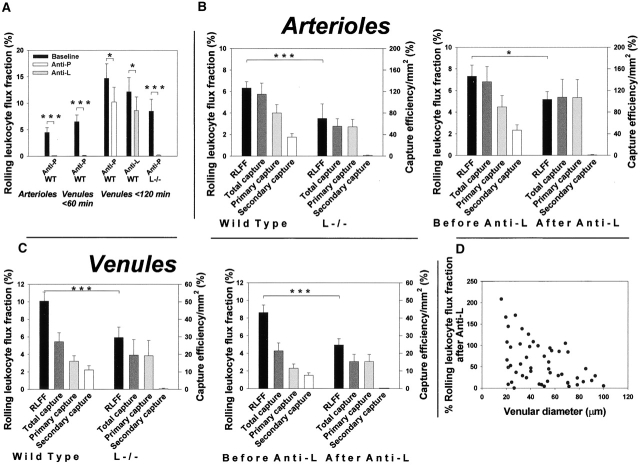
Because secondary capture in vitro has been shown to be mediated by L-selectin interacting with PSGL-1 15 25 26 27 28 29 we sought to analyze whether this holds true also in the in vivo situation. In the mouse cremaster muscle, trauma-induced rolling in venules at early time points after tissue exteriorization (<60 min) as well as cytokine-induced rolling in arterioles have been shown to be critically dependent on endothelial P-selectin 12 22. In this study, these data were confirmed (Fig. 3 A). As previous data combined do not support a role for interactions between L-selectin and P-selectin 14 23 35 36, a possible contribution of L-selectin to secondary capture, as well as a contribution of secondary capture to leukocyte rolling, could then be unmasked through inhibition of L-selectin function, without interfering with direct interactions between leukocytes and endothelial cells.
Figure 3.
Secondary capture is L-selectin dependent and contributes to cytokine-induced leukocyte rolling in arterioles and trauma-induced rolling in venules in vivo. (A) RLFFs in the microcirculation after antibody blockage of P- or L-selectin. RLFF was determined as rolling flux divided by the total number of leukocytes traveling in the vessel estimated from flow and WBC. (B and C) Impact of inhibition of L-selectin on RLFF and capture efficiencies in cytokine-treated arterioles and in trauma-induced rolling in venules at early time-points. Results were obtained in arterioles and venules of WT and L−/− mice (left panels), and in WT mice before and after antibody blockage of L-selectin (right panels). Figures are based on data presented in Table . Capture efficiency/mm2 represents the ratio between leukocytes that were captured within the field of vision and the total number of leukocytes traveling in the vessel adjusted for differences in luminal vessel area. (D) The percentage of RLFF after function inhibition of L-selectin in venules compared with RLFF before antibody treatment plotted against vessel diameter. Antibody blockage of L-selectin function (which abolished secondary capture) decreased RLFF in large venules (>45 μm in diameter) whereas in venules of diameters less than 45 μm, RLFF remained unchanged.
Having determined that direct interactions between leukocytes and endothelial cells in cytokine-treated arterioles as well as in venules at early time points after tissue exteriorization are P-selectin dependent (and L-selectin independent), we analyzed interactions in such vessels in situations of inhibited L-selectin function. Data are shown in Table and in Fig. 3B–D. In arterioles, RLFF was lower in L−/− mice compared with WT (P < 0.001; Fig. 3 B, left). In addition, secondary capture was virtually absent in L−/− mice (P < 0.001) resulting in lower total capture (P < 0.001) and lower total capture efficiency/mm2 (P < 0.01) than what was seen in WT mice. Similar findings were made in WT mice treated with a function-blocking antibody against L-selectin (MEL-14; Fig. 3 B, right). Absence of secondary capture was also found in trauma-induced rolling in venules of L−/− mice and in WT mice treated with MEL-14 (Fig. 3 C). However, decreased RLFF was evident only in venules larger than ∼45 μm (P < 0.001) whereas no significant reduction in RLFF was detected in smaller venules (L−/−; P = 0.246, and MEL-14; P = 0.219; Table ). The persistence of RLFF in small venules is in accordance with the limited capture seen in these vessels (Fig. 1 D), making absence of secondary capture less significant in this situation. The distinct impact of antibody blockage of L-selectin in various-sized venules is clearly illustrated in Fig. 3 D. Importantly, in all experiments involving L-selectin inhibition, the efficiency of primary capture was not altered compared with the intact situation. Hence, despite the fact that the number of leukocytes that initiated contact with the endothelium directly was similar in the compared situations, and that endothelial cells thus seemed equally adhesive for leukocytes, rolling flux was decreased when secondary capture was blocked. Taken together, the data strongly indicate that secondary capture increases the ability for leukocytes to initiate interactions with the endothelium in cytokine-induced rolling in arterioles as well as in trauma-induced rolling in venules in vivo, and that recruitment of leukocytes to the rolling cell population is promoted by this phenomenon. In addition, these data also suggest that L-selectin does not participate in primary capture under these conditions.
Table 2.
Leukocyte Capture and Rolling in Various Situations in the Microcirculation
| Vessel type/ treatment | Animal(N/n) | Rolling flux | RLFF | Total capture | TCE/mm2 | Primary capture | PCE/mm2 | Secondary capture | SCE/mm2 |
|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | ||||||
| Arterioles | |||||||||
| WT (20/83) | 23 ± 1.8 | 6.3 ± 0.59 | 6.3 ± 0.63 | 115 ± 20 | 4.0 ± 0.36 | 80 ± 16 | 2.3 ± 0.31 | 35 ± 6.6 | |
| hIL1β + hTNFα | L−/− (7/34) | 7.7 ± 1.5 | 3.5 ± 1.3 | 3.4 ± 0.54 | 55 ± 14 | 3.3 ± 0.50 | 54 ± 14 | 0.13 ± 0.048 | 1.1 ± 0.45 |
| P < 0.001 | P < 0.001 | P = 0.001 | P = 0.005 | P = 0.118 | P = 0.090 | P < 0.001 | P < 0.001 | ||
| WT anti-L: | Before (5/34) | 24 ± 2.6 | 7.3 ± 1.0 | 5.3 ± 0.59 | 136 ± 28 | 3.2 ± 0.34 | 90 ± 21 | 2.2 ± 0.35 | 47 ± 10 |
| After (5/34) | 8.3 ± 0.90 | 5.2 ± 0.72 | 1.9 ± 0.30 | 108 ± 33 | 1.9 ± 0.30 | 107 ± 33 | 0.017 ± 0.017 | 0.70 ± 0.70 | |
| P < 0.001 | P < 0.034 | P < 0.001 | P = 0.505 | P < 0.001 | P = 0.258 | P < 0.001 | P < 0.001 | ||
| Venules | |||||||||
| Trauma | WT (23/157) | 38 ± 2.5 | 10 ± 1.1 | 1.7 ± 0.25 | 27 ± 5.1 | 0.98 ± 0.14 | 16 ± 3.1 | 0.71 ± 0.11 | 11 ± 2.3 |
| All ∅ | L−/− (7/51) | 20 ± 2.0 | 5.9 ± 1.2 | 0.85 ± 0.28 | 20 ± 8.8 | 0.83 ± 0.27 | 19 ± 8.7 | 0.020 ± 0.014 | 0.37 ± 0.34 |
| P < 0.001 | P < 0.001 | P = 0.100 | P = 0.074 | P = 0.487 | P = 0.303 | P < 0.001 | P < 0.001 | ||
| WT anti-L: | Before (7/52) | 43 ± 3.8 | 8.6 ± 0.86 | 1.9 ± 0.32 | 19 ± 3.6 | 1.1 ± 0.20 | 12 ± 2.4 | 0.81 ± 0.14 | 7.5 ± 1.3 |
| After (7/52) | 12 ± 1.5 | 5.0 ± 0.72 | 0 .45 ± 0.10 | 16 ± 4.2 | 0.45 ± 0.099 | 16 ± 4.2 | 0.0052 ± 0.0052 | 0.011 ± 0.011 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P = 0.097 | P < 0.001 | P = 0.915 | P < 0.001 | P < 0.001 | ||
| Trauma | WT (23/71) | 30 ± 3.2 | 14 ± 2.0 | 1.1 ± 0.33 | 41 ± 9.9 | 0.61 ± 0.16 | 24 ± 6.1 | 0.50 ± 0.17 | 17 ± 4.5 |
| ∅ < 45 μm | L−/− (7/24) | 17 ± 3.0 | 10 ± 2.2 | 0.43 ± 0.18 | 36 ± 18 | 0.41 ± 0.16 | 35 ± 18 | 0.020 ± 0.020 | 0.73 ± 0.73 |
| P = 0.033 | P = 0.246 | P = 0.379 | P = 0.294 | P = 0.580 | P = 0.564 | P = 0.021 | P = 0.023 | ||
| WT anti-L: | Before (7/25) | 25 ± 3.9 | 9.1 ± 1.4 | 0.60 ± 0.20 | 17 ± 6.3 | 0.37 ± 0.14 | 11 ± 4.4 | 0.23 ± 0.071 | 6 ± 2.1 |
| After (7/25) | 7.2 ± 1.4 | 6.9 ± 1.2 | 0.19 ± 0.075 | 18 ± 7.4 | 0.19 ± 0.075 | 18 ± 7.4 | 0 | 0 | |
| P < 0.001 | P = 0.219 | P = 0.021 | P = 0.946 | P =0.147 | P = 0.622 | P = 0.004 | P = 0.004 | ||
| Trauma | WT (23/86) | 45 ± 3.6 | 6.4 ± 0.64 | 2.2 ± 0.36 | 15 ± 3.3 | 1.3 ± 0.23 | 8.6 ± 1.7 | 0.88 ± 0.15 | 6.3 ± 1.7 |
| ∅ > 45 μm | L−/− (7/27) | 23 ± 2.4 | 2.0 ± 0.27 | 1.2 ± 0.51 | 4.5 ± 1.6 | 1.2 ± 0.49 | 4.4 ± 1.5 | 0.019 ± 0.019 | 0.052 ± 0.052 |
| P = 0.009 | P < 0.001 | P = 0.194 | P = 0.057 | P = 0.758 | P = 0.275 | P < 0.001 | P < 0.001 | ||
| WT anti-L: | Before (7/27) | 62 ± 4.1 | 7.7 ± 0.89 | 3.3 ± 0.49 | 22 ± 3.9 | 1.9 ± 0.32 | 12 ± 2.5 | 1.4 ± 0.21 | 9.3 ± 1.7 |
| After (7/27) | 16 ± 2.3 | 3.3 ± 0.68 | 0.68 ± 0.17 | 13 ± 3.6 | 0.067 ± 0.16 | 13 ± 3.6 | 0.010 ± 0.010 | 0.022 ± 0.022 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P = 0.042 | P = 0.002 | P = 0.516 | P < 0.001 | P < 0.001 | ||
| hIL1β + hTNFα | WT (7/22) | 12 ± 3.3 | 5.6 ± 1.0 | 0.74 ± 0.34 | 5.2 ± 2.0 | 0.48 ± 0.25 | 3.5 ± 1.5 | 0.26 ± 0.14 | 1.7 ± 0.98 |
All parameters are expressed as mean ± SEM in the indicated number of animals and vessels (N/n). Values of significance (P) are indicated below compared groups in the respective columns. Total capture efficiency (TCE)/mm2, primary capture efficiency (PCE)/mm2 and secondary capture efficiency (SCE)/mm2 represent total-, primary, and secondary capture efficiency adjusted for differences in the observed vessel area, respectively. Anti-L indicates treatment with the L-selectin blocking antibody MEL-14. ∅ indicates vessel diameter.
Primary and Secondary Capture Are Prominent Features of Leukocyte–Endothelial Interactions in Large Arteries and in Atherosclerosis In Vivo.
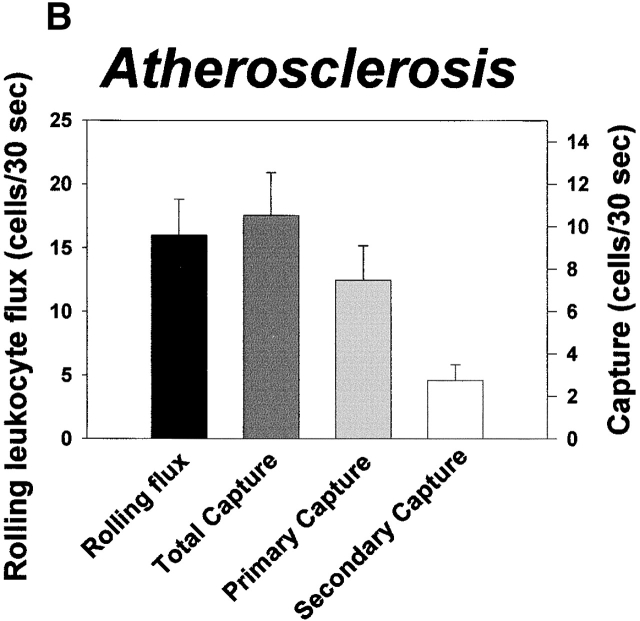
To address the impact of secondary capture on leukocyte recruitment in large vessels, we studied capture and rolling of leukocytes in the femoral artery of cytokine-stimulated WT and L−/− mice, and in the aorta of atherosclerotic ApoE0 and ApoE0LDLR0 mice. In these situations, interactions between leukocytes and endothelium are critically dependent on P-selectin 13 37. Nonetheless, rolling flux in the femoral artery in WT mice was higher compared with L−/− mice (19 ± 2.4 cells/30 s vs. 11 ± 2.5 cells/30 s, P < 0.01; Fig. 4 A). This correlated to complete absence of secondary capture in knockout animals (P < 0.001) whereas primary capture was similar in both strains (3.3 ± 0.48 and 3.6 ± 0.54 cells/30 s in WT and L−/− mice, respectively; P = 0.648). Moreover, capture was prominent in leukocyte–endothelial interactions on atherosclerotic lesions in the mouse aorta (Fig. 4 B). On average, primary capture and secondary capture over a period of 30 s were 7.5 ± 1.6 and 2.7 ± 0.75 cells, respectively. However, similar to what was observed in microvessels, secondary capture was prominent only when rolling flux exceeded a minimum threshold value (∼60–70 cells/mm × 30 s; Fig. 4 C), indicating that secondary capture in atherosclerosis may be significant at sites of high endothelial CAM expression and hence, at high inflammatory activity.
Figure 4.
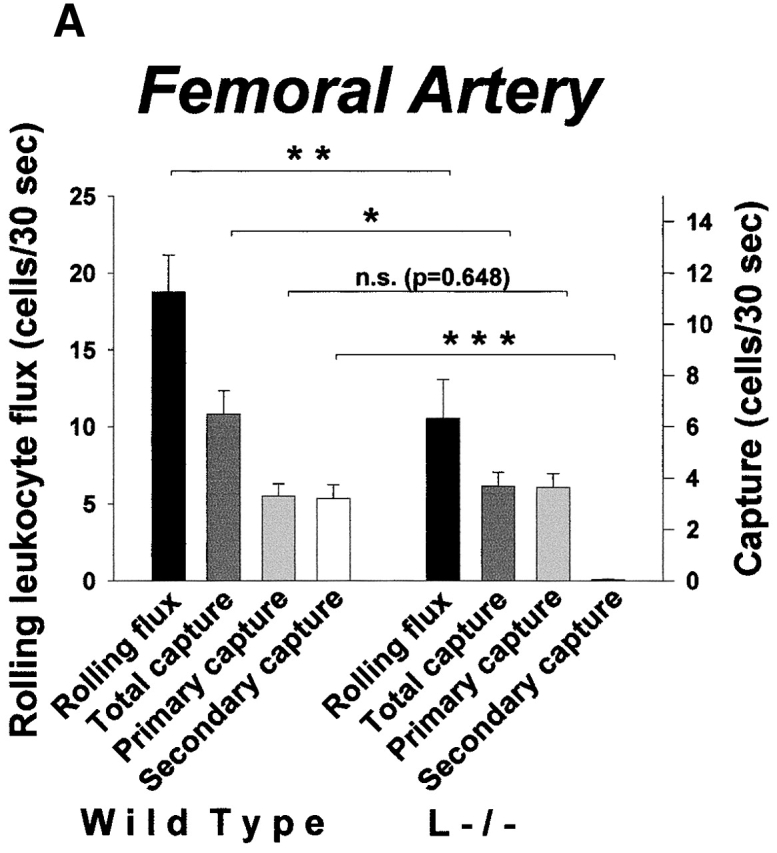
Secondary capture increases leukocyte recruitment in large arteries and in atherosclerosis in vivo. (A) Characteristics of capture and rolling in the cytokine-treated mouse femoral artery. Data for rolling flux and capture are indicated. Leukocyte–endothelial interactions were abolished by treatment with a function-blocking antibody against P-selectin (not shown). (B) Leukocyte capture and rolling on atherosclerotic lesions in the aorta of ApoE0 and ApoE0/LDLR0 mice. Data for capture and rolling flux are shown. (C) Secondary capture on atherosclerotic lesions in the mouse aorta plotted against RLF. RLF/mm represents the flux of rolling leukocytes adjusted for differences in the width of the vessel section observed. Secondary capture/mm2 represents the number of leukocytes that initiated contact with atherosclerotic endothelium through secondary capture adjusted for differences in luminal vessel area.
Secondary Capture Gives Rise to Rolling Clusters and Rolling Strings of Leukocytes in Arterioles and Arteries.
In flow chambers in vitro, secondary capture induces formation of strings of leukocytes rolling along the adhesive surface. Therefore, we investigated the patterns of leukocyte rolling that were generated in WT and L−/− mice. No rolling strings were observed in microvessels. However, we found that leukocytes rolling in arterioles, but not in venules, of WT mice typically formed clusters of three or more leukocytes with variable time delay in between (Fig. 5 A1). Rolling clusters were by direct observation of their formation found to be induced by secondary capture. Consequently, rolling clusters were not evident in L−/− mice. In large arteries, rolling clusters were prominent both in the cytokine-treated femoral artery as well as in the aorta of atherosclerotic mice (Fig. 5A Fig. 2a6). Moreover, in large arteries, strings of rolling leukocytes were sometimes observed. Formation of a rolling string downstream to an atherosclerotic plaque is illustrated in Fig. 5 B.
Figure 5.

Secondary capture in arterial vessels induces formation of rolling clusters and rolling strings of leukocytes. (A) Demonstration of rolling clusters in arterial vessels. In A1, the number of leukocytes passing a reference line during 20 consecutive 3-s periods in a WT (top) and an L−/− (bottom) arteriole are shown. The respective parameters of capture and rolling in the depicted experiments were: Rolling flux, 95 (WT) and 75 (L−/−); primary capture, 16 (WT) and 22 (L−/−); and secondary capture, 20 (WT) and 0 (L−/−). In A2–A6, the buildup of a rolling cluster in a cytokine-stimulated femoral artery is demonstrated. D indicates vessel diameter. P and S indicate primary and secondary capture, respectively. Large arrow indicates direction of flow. Bar, 100 μm. Images were taken at times 0, 0.64, 1.24, 1.52, and 2.52 s. (B) Sequential video frames showing the formation of a rolling string on an atherosclerotic lesion in the right iliac artery of an ApoE0/LDLR0 mouse in vivo. The orientation of the microscopic images is shown in B1. In B2–B6, the lesion is visible in the bottom left as a bright area. Large arrow indicates direction of flow. Bar, 100 μm. Images were taken at times 0, 0.54, 2.36, 3.20, and 3.96 s.
Discussion
In the multistep process of leukocyte recruitment, the mechanisms involved in the initial contact between leukocytes and endothelium are unclear. Previous studies in vitro and in vivo are contradictory inasmuch as capture of leukocytes from the free flow, primary and secondary, has been shown to be of importance in in vitro models whereas other data question a role for capture in physiological systems. Moreover, the roles for L-selectin in leukocyte recruitment remain controversial.
In this study, we hypothesized that primary and secondary capture might be of importance in leukocyte recruitment in vivo. Interestingly, capture was found to exist in all vessel types studied. The significance of capture is greater in all arterial vessels compared with venules, inasmuch as the ratio between capture and rolling flux was up to 10-fold higher in arterial vessels. This corresponds to a high turnover of leukocytes attaching to and detaching from the endothelium in arterioles, and demonstrates that leukocyte rolling in these vessels is not as stable as rolling in venules, including those venules that have received no proinflammatory treatment before observation. As WSR in venules and arterioles are not dramatically different, the differences in stability of leukocyte rolling in microvessels are likely due to differences in CAM expression between these vessel types 11.
Capture in arterioles was observed in all vessels where leukocyte rolling was detected, regardless of vessel diameter or WSR. This strongly indicates that leukocyte rolling in arterial vessels is dependent on initial capture as no capillaries, where leukocytes can initiate interactions with the endothelium without being captured from the free flow, are located upstream. In contrast, leukocyte rolling in venules is not always dependent on capture from the free flow. Instead, capture is rare in small venules (<45 μm in diameter) and at low WSR indicating that leukocyte rolling in these vessels is initiated mainly when leukocytes enter venules from the capillaries and hence, when they are already in contact with the vessel wall. However, capture is clearly significant in venules of larger diameters. These data provide likely explanations why capture has previously not been recognized in vivo, as a prior study, where no role for capture in vivo was detected, investigated this phenomenon in the formation of stable clusters of leukocytes in cytokine-treated venules with mean diameters of 36 μm 30. In this study, we found that interactions between leukocytes and endothelial cells in such a situation are stable and that capture is limited. However, in other situations of leukocyte–endothelial interactions, capture is clearly of importance.
Capture in the microcirculation occurs through both primary and secondary capture. Primary capture is regularly more prominent than secondary capture and the contribution of secondary capture to total capture is dependent on rolling flux inasmuch as secondary capture becomes more significant above a minimum flux of rolling leukocytes. In L−/− mice as well as in WT mice treated with a function-blocking antibody against L-selectin, secondary capture is virtually absent. This strongly indicates that the leukocyte–leukocyte interactions observed in this study are L-selectin dependent and that these interactions are identical to secondary capture previously observed in vitro. Furthermore, the presence of secondary capture increases RLF. Blockage of secondary capture through function inhibition of L-selectin decreases leukocyte rolling even in situations where direct interactions between leukocytes and endothelial cells are independent of this CAM. This is apparent in all arterial vessels and in venules of diameters larger than ∼45 μm, although this mechanism likely influences leukocyte rolling also in somewhat smaller venules (35–40 μm), especially at high WSR (Fig. 1 D). These findings could, together with previous observations regarding the sequential contribution of P- and L-selectin in the time frame of trauma-induced leukocyte rolling 22, explain the somewhat contradictory data concerning the role for L-selectin in leukocyte–endothelial interactions. Evidently, depending on the size of venules subjected to study of leukocyte–endothelial interactions, opposite conclusions may be reached as to the impact of blockage of L-selectin. Indeed, on evaluation of previous studies in models where direct interactions between leukocytes and endothelial cells were likely L-selectin independent, blockage of L-selectin function was found to have no impact on leukocyte rolling in reports where venules of small diameters were subjected to study 5 16 38. In contrast, in the case of clear-cut effects of function inhibition of L-selectin reported in the literature, larger venules have been observed 22 39. Increased importance of L-selectin with increasing venular diameters has been suggested previously, however, without taking into account the possibility of secondary capture 40. Furthermore, because leukocyte rolling is a prerequisite for secondary capture, low flux of rolling leukocytes may in some studies also have limited the impact of L-selectin blockage 41. This may also hold true for leukocyte rolling in arterioles 12. Hence, our findings demonstrating a role for secondary capture in the microcirculation do not contradict previous data but rather explain the apparent inconsistency in the literature regarding the role for L-selectin in leukocyte recruitment, and extends L-selectin–dependent adhesion to involve the two separate mechanisms of leukocyte–endothelial and leukocyte–leukocyte interactions. Consequently, in many situations of leukocyte recruitment, L-selectin–dependent secondary capture will be of importance and should be taken into consideration.
Interestingly, the impact of secondary capture may also hold true for venules exposed to inflammatory stimuli. In this study, we demonstrate that primary and secondary capture occur in venules after treatment with inflammatory mediators. However, the contribution of secondary capture to leukocyte rolling was not specifically addressed due to the difficulty in investigating the impact of blockage of secondary capture through function inhibition of L-selectin when L-selectin–dependent leukocyte–endothelial interactions occur in parallel to leukocyte–leukocyte interactions. Nonetheless, previous studies have shown that function inhibition of L-selectin in the inflamed microcirculation can decrease venular flux of rolling leukocytes by as much as 40–80% 17 39 42 43, despite the fact that in mice deficient in both P- and E-selectin, L-selectin–dependent leukocyte rolling in cytokine-stimulated venules may be as low as 1% of the rolling flux seen in WT mice 17. However, about half of the neutrophils in E- and P-selectin double knockout mice have very low surface expression of L-selectin, a fact that may contribute to the low L-selectin–dependent rolling seen in these mice 44. Nonetheless, between reports, there may be a discrepancy between the impact of inhibiting L-selectin function and the potential for L-selectin in mediating direct interactions between leukocytes and endothelial cells. This discrepancy may at least in part be due to secondary capture.
The presence of secondary capture in mediator-induced inflammation also introduces conceivable explanations for the discrepancy in several experimental setups between the impact of function inhibition of L-selectin on leukocyte rolling on one hand and leukocyte extravasation on the other, i.e., despite that L-selectin blockage may decrease leukocyte rolling as well as it may decrease extravascular migration of leukocytes 38, leukocyte recruitment to the extravascular space may remain largely unaffected 39 45 46. Plausibly, because RLFF is higher in small venules compared with large venules (this study, reference 22), extravasation is likely to take place mainly in the former, where secondary capture is of minor importance. In contrast, when studying leukocyte rolling after similar stimuli, large venules may have been investigated, and in these vessels function inhibition of L-selectin, and thus blockage of secondary capture, may decrease rolling flux. Furthermore, differences in local blood flow and WSR caused by treatment with different inflammatory stimuli may also influence the role of secondary capture and hence, the role of L-selectin. Taken together, this study indicates that variables distinct from the induction of endothelial L-selectin ligands may influence the outcome of L-selectin–mediated leukocyte rolling and recruitment.
In large arteries, we clearly show the impact of primary and secondary capture in leukocyte–endothelial interactions. In the femoral artery, secondary capture contributes to recruitment of leukocytes to the rolling cell pool. Correspondingly, secondary capture of leukocytes occurs on atherosclerotic lesions. Similar to what is found in other vessels, secondary capture in atherosclerosis is more significant at high flux of rolling leukocytes. Thus, the contribution of secondary capture to leukocyte recruitment in atherosclerosis is more prominent in areas where interactions between leukocytes and endothelium are abundant and hence, at sites of high inflammatory activity.
In flow chambers in vitro, secondary capture induces formation of so called rolling strings of leukocytes 15 24 27. However, in this in vivo study, rolling strings were not observed in vessels in the microcirculation. The discrepancy between in vivo and in vitro appearance of capture may be due to differences in the geometry of flow chambers and that of microvessels. On the other hand, in large arteries, which have a geometrical structure that more closely resembles the geometry of in vitro flow chambers, rapidly moving strings of leukocytes were sometimes observed. Moreover, in all arterial vessels, secondary capture induces formation of clusters of leukocytes rolling along the endothelium. Rolling clusters were directly observed to be dependent on secondary capture and were clearly attenuated in L−/− mice, further indicating the importance of secondary capture in their formation.
In summary, we have defined roles for primary and secondary capture in a number of situations of leukocyte recruitment in vivo. Capture of both types are more prominent in vessels of the arterial tree compared with venules, and in arterial vessels secondary capture induces formation of rolling clusters and rolling strings of leukocytes. In venules, capture of leukocytes from the free flow is low in small venules and at low WSR, whereas in larger venules and at higher WSR, capture is significant. This indicates that leukocytes rolling in small venules initiate rolling interactions immediately when entering venules from the capillaries. Blockage of secondary capture through inhibition of L-selectin decreases the flux of rolling leukocytes in arterial vessels and in large, but not small, venules. The distinct impact of blockage of L-selectin in venules of different diameter range provides a likely explanation for discrepancies in the literature as to the contribution of L-selectin in leukocyte recruitment. What is more, secondary capture occurs on atherosclerotic lesions and supports recruitment of leukocytes to the arterial wall. We thereby define a previously not recognized, L-selectin–dependent, cell adhesion pathway in leukocyte recruitment in atherosclerosis.
Acknowledgments
The authors thank the Haephtes Society.
This work was supported by the Wallenberg Foundation, the Swedish Medical Research Council (4764, 14X-4342), the Swedish Heart and Lung Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research (A2001068), IngaBritt and Arne Lundbergs Foundation, and Karolinska Institutet.
Footnotes
Abbreviations used in this paper: CAM, cell adhesion molecule; PSGL-1, P-selectin glycoprotein ligand 1; RLF, rolling leukocyte flux; RLFF, rolling leukocyte flux fraction; WBC, white blood count; WSR, wall shear rate; WT, wild-type.
References
- von Andrian U.H., Chambers J.D., McEvoy L.M., Bargatze R.F., Arfors K.E., Butcher E.C. Two-step model of leukocyte-endothelial cell interaction in inflammationdistinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc. Natl. Acad. Sci. USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Lindbom L., Xie X., Raud J., Hedqvist P. Chemoattractant-induced firm adhesion of leukocytes to vascular endothelium in vivo is critically dependent on initial leukocyte rolling. Acta Physiol. Scand. 1992;146:415–421. doi: 10.1111/j.1748-1716.1992.tb09442.x. [DOI] [PubMed] [Google Scholar]
- Arfors K.E., Lundberg C., Lindbom L., Lundberg K., Beatty P.G., Harlan J.M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. [PubMed] [Google Scholar]
- Jung U., Ley K. Mice lacking two or all three selectins demonstrate overlapping and distinct functions for each selectin. J. Immunol. 1999;162:6755–6762. [PubMed] [Google Scholar]
- Werr J., Johansson J., Eriksson E.E., Hedqvist P., Ruoslahti E., Lindbom L. Integrin alpha(2)beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- Carlos T.M., Harlan J.M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Kunkel E.J., Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- Jung U., Norman K.E., Scharffetter-Kochanek K., Beaudet A.L., Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M.P., Stengelin S., Gimbrone M.A., Jr., Seed B. Endothelial leukocyte adhesion molecule 1an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Jung U., Ley K. Regulation of E-selectin, P-selectin, and intercellular adhesion molecule 1 expression in mouse cremaster muscle vasculature. Microcirculation. 1997;4:311–319. doi: 10.3109/10739689709146794. [DOI] [PubMed] [Google Scholar]
- Kunkel E.J., Jung U., Ley K. TNF-alpha induces selectin-mediated leukocyte rolling in mouse cremaster muscle arterioles. Am. J. Physiol. 1997;272:H1391–H1400. doi: 10.1152/ajpheart.1997.272.3.H1391. [DOI] [PubMed] [Google Scholar]
- Eriksson E.E., Werr J., Guo Y.C., Thoren P., Lindbom L. Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte-endothelium interactions in the mouse aorta. Circ. Res. 2000;86:526–533. doi: 10.1161/01.res.86.5.526. [DOI] [PubMed] [Google Scholar]
- Yang J., Hirata T., Croce K., Merrill-Skoloff G., Tchernychev B., Williams E., Flaumenhaft R., Furie B.C., Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin–mediated but not E-selectin–mediated neutrophil rolling and migration. J. Exp. Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.C., Snapp K., Kansas G.S., Camphausen R., Ding H., Luscinskas F.W. Important contributions of P-selectin glycoprotein ligand-1-mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin, and TNF-alpha-activated endothelium under flow in vitro. J. Immunol. 1998;161:2501–2508. [PubMed] [Google Scholar]
- Weninger W., Ulfman L.H., Cheng G., Souchkova N., Quackenbush E.J., Lowe J.B., von Andrian U.H. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- Jung U., Ramos C.L., Bullard D.C., Ley K. Gene-targeted mice reveal importance of L-selectin-dependent rolling for neutrophil adhesion. Am. J. Physiol. 1998;274:H1785–H1791. doi: 10.1152/ajpheart.1998.274.5.H1785. [DOI] [PubMed] [Google Scholar]
- Butcher E.C., Williams M., Youngman K., Rott L., Briskin M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Tu L., Delahunty M.D., Ding H., Luscinskas F.W., Tedder T.F. The cutaneous lymphocyte antigen is an essential component of the L-selectin ligand induced on human vascular endothelial cells. J. Exp. Med. 1999;189:241–252. doi: 10.1084/jem.189.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M.S., Lasky L.A., Rosen S.D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- Arbones M.L., Ord D.C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D.J., Tedder T.F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Ley K., Bullard D.C., Arbones M.L., Bosse R., Vestweber D., Tedder T.F., Beaudet A.L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargatze R.F., Kurk S., Butcher E.C., Jutila M.A. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J. Exp. Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R., Fuhlbrigge R.C., Finger E.B., Springer T.A. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J. Cell Biol. 1996;135:849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Cordey A.S., Monai N., Giuffre L., Schapira M. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J. Cell Biol. 1996;135:523–531. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L., Murphy P.G., Li X., Tedder T.F. L-selectin ligands expressed by human leukocytes are HECA-452 antibody-defined carbohydrate epitopes preferentially displayed by P-selectin glycoprotein ligand-1. J. Immunol. 1999;163:5070–5078. [PubMed] [Google Scholar]
- Walcheck B., Moore K.L., McEver R.P., Kishimoto T.K. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J. Clin. Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L., Chen A., Delahunty M.D., Moore K.L., Watson S.R., McEver R.P., Tedder T.F. L-selectin binds to P-selectin glycoprotein ligand-1 on leukocytesinteractions between the lectin, epidermal growth factor, and consensus repeat domains of the selectins determine ligand binding specificity. J. Immunol. 1996;157:3995–4004. [PubMed] [Google Scholar]
- Ramos C.L., Smith M.J., Snapp K.R., Kansas G.S., Stickney G.W., Ley K., Lawrence M.B. Functional characterization of L-selectin ligands on human neutrophils and leukemia cell linesevidence for mucinlike ligand activity distinct from P-selectin glycoprotein ligand-1. Blood. 1998;91:1067–1075. [PubMed] [Google Scholar]
- Kunkel E.J., Chomas J.E., Ley K. Role of primary and secondary capture for leukocyte accumulation in vivo. Circ. Res. 1998;82:30–38. doi: 10.1161/01.res.82.1.30. [DOI] [PubMed] [Google Scholar]
- Mitchell D.J., Li P., Reinhardt P.H., Kubes P. Importance of L-selectin-dependent leukocyte-leukocyte interactions in human whole blood. Blood. 2000;95:2954–2959. [PubMed] [Google Scholar]
- Ley K., Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ. Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- Tangelder G.J., Slaaf D.W., Arts T., Reneman R.S. Wall shear rate in arterioles in vivoleast estimates from platelet velocity profiles. Am. J. Physiol. 1988;254:H1059–H1064. doi: 10.1152/ajpheart.1988.254.6.H1059. [DOI] [PubMed] [Google Scholar]
- Lepault F., Gagnerault M.C., Faveeuw C., Boitard C. Recirculation, phenotype and functions of lymphocytes in mice treated with monoclonal antibody MEL-14. Eur. J. Immunol. 1994;24:3106–3112. doi: 10.1002/eji.1830241229. [DOI] [PubMed] [Google Scholar]
- Picker L.J., Warnock R.A., Burns A.R., Doerschuk C.M., Berg E.L., Butcher E.C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Zollner O., Lenter M.C., Blanks J.E., Borges E., Steegmaier M., Zerwes H.G., Vestweber D. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J. Cell Biol. 1997;136:707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson E.E., Xie X., Werr J., Thoren P., Lindbom L. Direct viewing of atherosclerosis in vivo; plaque invasion by leukocytes is initiated by the endothelial selectins. FASEB J. 2001;15:1149–1157. doi: 10.1096/fj.00-0537com. [DOI] [PubMed] [Google Scholar]
- Hickey M.J., Forster M., Mitchell D., Kaur J., DeCaigny C., Kubes P. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J. Immunol. 2000;165:7164–7170. doi: 10.4049/jimmunol.165.12.7164. [DOI] [PubMed] [Google Scholar]
- Kanwar S., Steeber D.A., Tedder T.F., Hickey M.J., Kubes P. Overlapping roles for L-selectin and P-selectin in antigen-induced immune responses in the microvasculature. J. Immunol. 1999;162:2709–2716. [PubMed] [Google Scholar]
- Stein J.V., Cheng G., Stockton B.M., Fors B.P., Butcher E.C., von Andrian U.H. L-selectin–mediated leukocyte adhesion in vivomicrovillous distribution determines tethering efficiency, but not rolling velocity. J. Exp. Med. 1999;189:37–50. doi: 10.1084/jem.189.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte D., Schmid P., Jager U., Botzlar A., Roesken F., Hecht R., Uhl E., Messmer K., Vestweber D. Leukocyte rolling in venules of striated muscle and skin is mediated by P-selectin, not by L-selectin. Am. J. Physiol. 1994;267:H1637–H1642. doi: 10.1152/ajpheart.1994.267.4.H1637. [DOI] [PubMed] [Google Scholar]
- Davenpeck K.L., Steeber D.A., Tedder T.F., Bochner B.S. P- and L-selectin mediate distinct but overlapping functions in endotoxin-induced leukocyte-endothelial interactions in the rat mesenteric microcirculation. J. Immunol. 1997;159:1977–1986. [PubMed] [Google Scholar]
- Steeber D.A., Campbell M.A., Basit A., Ley K., Tedder T.F. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc. Natl. Acad. Sci. USA. 1998;95:7562–7567. doi: 10.1073/pnas.95.13.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette P.S., Mayadas T.N., Rayburn H., Hynes R.O., Wagner D.D. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Sharar S.R., Chapman N.N., Flaherty L.C., Harlan J.M., Tedder T.F., Winn R.K. L-selectin (CD62L) blockade does not impair peritoneal neutrophil emigration or subcutaneous host defense to bacteria in rabbits. J. Immunol. 1996;157:2555–2563. [PubMed] [Google Scholar]
- Catalina M.D., Estess P., Siegelman M.H. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammationparticipation of E- and P- but not L-selectin. Blood. 1999;93:580–595. [PubMed] [Google Scholar]