Abstract
Cellular differentiation is a complex process involving integrated signals for lineage specification, proliferation, endowment of functional capacity, and survival or cell death. During embryogenesis, spatially discrete environments regulating these processes are established during the growth of tissue mass, a process that also results in temporal separation of developmental events. In tissues that undergo steady-state postnatal differentiation, another means for inducing spatial and temporal separation of developmental cues must be established. Here we show that in the postnatal thymus, this is achieved by inducing blood-borne precursors to enter the organ in a narrow region of the perimedullary cortex, followed by outward migration across the cortex before accumulation in the subcapsular zone. Notably, blood precursors do not transmigrate the cortex in an undifferentiated state, but rather undergo progressive developmental changes during this process, such that defined precursor stages appear in distinct cortical regions. Identification of these cortical regions, together with existing knowledge regarding the genetic potential of the corresponding lymphoid precursors, sets operational boundaries for stromal environments that are likely to induce these differentiative events. We conclude that active cell migration between morphologically similar but functionally distinct stromal regions is an integral component regulating differentiation and homeostasis in the steady-state thymus.
Keywords: thymus, stromal cells, cell differentiation, cell movement, histology
Introduction
T cell development in the postnatal thymus occurs in three relatively distinct phases consisting of lymphopoiesis, TCR-mediated selection, and functional maturation. The lymphopoietic phase of intrathymic development occurs over a 2-wk interval (for a review, see reference 1), and includes four phenotypically and genetically distinct stages 2 3 4 5 6 7 8. The first of these, defined as CD4−8−25−44hi (lineage double negative stage 1, [DN1]), contains cells with multilineage potential, including T and B lymphocytes as well as dendritic and NK cells, but not myeloid cells 9 10 11. Cells at the next stage of development (DN2) are CD4−8−25+44hi; such cells have apparently lost the potential to give rise to B and NK cells, but can still give rise to both α/β and γ/δ T lineages and dendritic cells 9 10 11 12. The next stage of development (DN3) is identified as being CD4−8−25+44lo; these cells have absolutely committed to the T lineage, but can still give rise to both α/β and γ/δ T cells 12. The final major lymphopoietic stage, marked by loss of CD25 expression, includes cells that are no longer CD4−8− by biochemical or genetic traits 7 8 13 14, but in fact represent the early CD4+8+ (predouble positive [preDP]) stage of development. In addition to such lineage commitment events, the lymphopoietic phase of T cell development includes substantial cell proliferation; each precursor entering the postnatal thymus gives rise to approximately one million progeny, representing a 4,000-fold expansion during the DN stages, and an additional 250-fold expansion during the early DP stage (for a review, see reference 1).
In contrast to the relatively well-described sequence of changes that occurs among thymocytes during differentiation, data regarding the signals that induce this process is sparse. To date, only four receptors expressed by lymphopoietic precursors have been shown to play essential and nonredundant roles in this process, including c-kit 15, IL-7R 16, Notch 17, and the preTCR 18 19 20. It is worth noting that even in these cases, the precise nature of the signals that are transduced (i.e., proliferation, differentiation, or survival) remain controversial. In any case, it is clear that this limited array is inadequate to account for the numerous biochemical and genetic changes that occur during thymic lymphopoiesis. To address the question of what other signals may be involved, we began with the assumption that differentiation and proliferation are not intrinsic to the precursor cell, but rather are induced by signals emanating from the external environment. To determine the nature of such signals, we therefore sought to determine the precise histologic location of each stage of lymphopoietic development. Early DN precursors are generally believed to reside in the subcapsular zone (SCZ)of the thymus based on analysis of proliferation in situ 21. However, this is at odds with anatomic and functional studies 22 23 24 25 26 27 28 29 showing that blood-borne precursors arrive either within the medulla, or near the junction of cortical and medullary regions (CMJ). In this study, we resolve this discrepancy by showing that blood precursors arrive in a narrow region of the perimedullary cortex, followed by outward migration across the cortex to the SCZ. Further, we find that relative location within the cortex correlates with specific developmental events, such that each lymphopoietic stage is in a relatively distinct anatomic subregion. In addition to revealing a previously unreported pattern of precursor migration within the thymus, these findings operationally define stratified stromal regions that correspond to distinct developmental processes in lymphoid precursors. Such findings should facilitate identification of the microenvironmental signals that induce and control lymphopoiesis in the steady-state thymus.
Materials and Methods
Cells, Tissues, and Antibodies.
Mice were 4–6-wk-old C57BL/6 males or females bred under SPF conditions at Memorial Sloan-Kettering Cancer Center. Recombination activating gene (RAG)-2/B6.PL/Thy-1a bone marrow chimeras were prepared as described previously 19. In brief, sublethally irradiated Ly5.1+ wild-type mice were transplanted with a 4:1 mixture of RAG-deficient (Ly5.2+) and syngeneic marrow, followed by a 6–8-wk recovery period. Intact thymuses removed after euthanasia were embedded in OCT mounting medium (Fisher Scientific) and stored at −70°C until use. Antibodies specific for mouse CD3 (clone KT3), CD4 (clone GK-1.5), CD8 (clone 53-6.7), CD25 (clone PC-61), CD117 (clone ACK-2), CD45R (clone RA3-3A1/6.1), Mac-1 (clone M1-70), Thy-1.2 (clone 30H12), and a marker of erythroid cells (clone TER-119) were produced by the Monoclonal Antibody Core Facility at Memorial Sloan-Kettering Cancer Center. RB6-8C5 (anti–GR-1) was used as a tissue culture supernatant. FITC-conjugated anti-BrdU antibodies were purchased from BD Biosciences.
Immunohistochemistry.
Frozen sections of 4–5-μm thickness were dried overnight at 4°C in a desiccated container, followed by fixation in acetone at 4°C for 30 min. Endogenous peroxidase activity was quenched by incubating slides for 30 min in a solution of 1 mM NaN3, 10 mM glucose, and 1 U/ml of glucose oxidase (Calbiochem). All antibody incubations (biotin conjugates) were performed for 30 min at room temperature. Single-color histochemical detection was performed using an avidin-peroxidase conjugate system (VectaStain Elite; Vector Laboratories), and antibody–enzyme complex was visualized with 3′3′-diaminobenzidene (DAB; Sigma-Aldrich) and 0.1% H2O2. For two-color detection, sections were thoroughly rinsed, treated a second time with glucose oxidase, stained again with the appropriate antibodies, and visualized using nickel-conjugated DAB (Vector Laboratories). Sections were then counterstained using Harris' modified hematoxylin (Fisher Scientific) or methyl green (Vector Laboratories), dehydrated through graded alcohol, cleared in Hemo-De (Fisher Scientific), and cover slipped using permount (Fisher Scientific). For detection of BrdU incorporation, animals were injected intraperitoneally with 1 mg of BrdU 4 h before killing. Tissue sections were prepared as described above but were fixed using 4% formaldehyde. After washing in PBS, endogenous peroxidase activity was quenched as described previously. Tissues were then treated with 1 N HCl in normal saline for 10 min at room temperature, and stained using the Histo-Mouse SP kit (Zymed Laboratories), following the manufacturer's instructions. DAB detection and counterstaining were performed as described previously.
Immunofluorescent Microscopy.
CD25/BrdU two-color immunofluorescence was performed exactly as described 21. For all other immunofluorescent stains, slides were first dried and fixed as described previously. Antibodies and secondary reagents were diluted in PBS containing 5% FBS. Incubations with primary and secondary reagents were for 30 min at room temperature. After staining, samples were washed with PBS and fixed again for 10 min in 70% EtOH at −20°C, followed with washing in PBS. Slides were mounted using Prolong anti-fade media (Molecular Probes); in some cases, mounting medium contained DAPI (1 μg/ml) as a nuclear counterstain. Fluorescent images were acquired using a Zeiss LSM510 confocal microscope.
CFSE Labeling and Marrow Transplantation.
C57BL/6 bone marrow was harvested from the femurs and tibias of adult mice and depleted of mature cells of all lineages using a cocktail of antibodies (anti-CD3, -CD4, -CD8, -B220, –GR-1, –Mac-1, and –TER-119), followed by stringent depletion with antiimmunoglobulin-coated magnetic beads. Fluorescent labeling of lineage depleted bone marrow cells was accomplished using the Vybrant CFDA-SE Cell Tracer kit (C-1157; Molecular Probes). In brief, lineage-depleted cells were washed once in PBS and resuspended in 1 μM CFDA SE/PBS for 15 min at 37°C. After labeling, cells were washed twice in PBS, and one mouse equivalent of lineage-negative cells was injected intravenously into each normal, nonablated recipient. After 24–48 h, vascular tissues were identified by intravenous administration of biotinylated lectin (see below), followed by removal of the thymus, cryopreservation, and sectioning as described previously.
Identification of Vascular Endothelial Cells.
In some experiments, vascular endothelial cells were identified by injecting donor mice with 50–100 μg of biotinylated Lycopersico esculentum lectin (Vector Laboratories) immediately before killing. Biotinylated lectin was visualized as described in the sections on immunohistochemistry or immunofluorescence, as appropriate, using Texas Red– (see Fig. 2) or peroxidase– (see Fig. 3) streptavidin conjugates.
Figure 2.
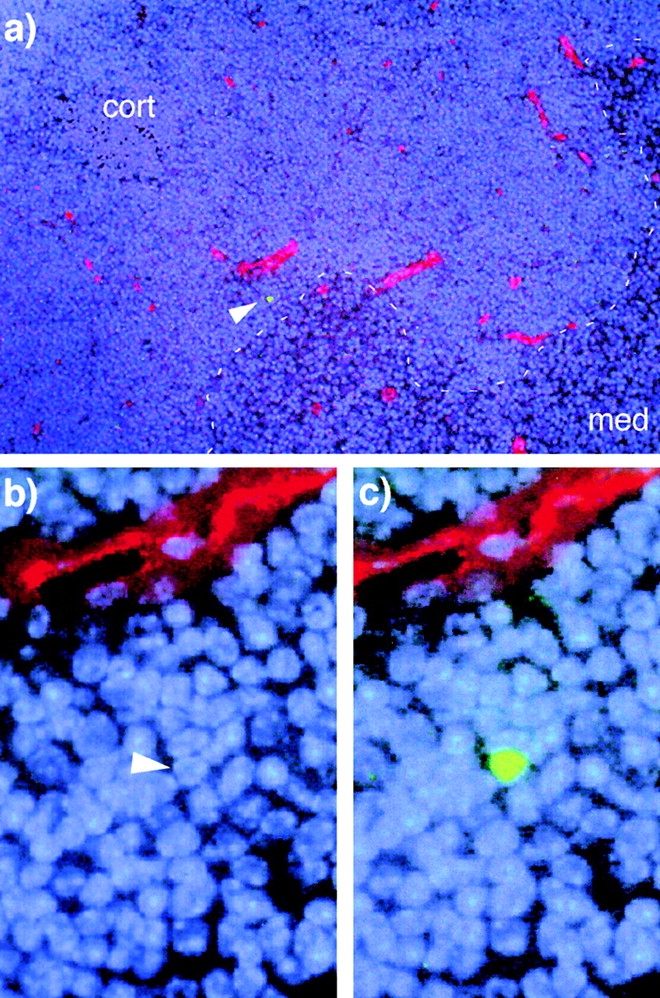
Marrow-derived precursors extravasate deep in the cortex adjacent to the medulla. CFSE-labeled, lineage-depleted bone marrow cells were administered intravenously to nonablated recipients. 1–2 d later, thymuses were recovered, cryogenically sectioned, and examined for the presence of CFSE+ cells. In all cases where CFSE+ cells (green) were found, they were in the deep cortex, near the CMJ (dashed line, a) and in close proximity to large blood vessels (red) that appear to be postcapillary venules. The outer capsule of the section shown in a is just outside of the field of view, to the left and above the image. The nuclear counterstain is DAPI (blue), blood vessels are revealed using Texas Red, and the single CFSE+ cell is indicated by an arrowhead. b and c show magnified views of this region; in b, green fluorescence is not shown so that the nucleus of the CFSE+ cell can be visualized.
Figure 3.
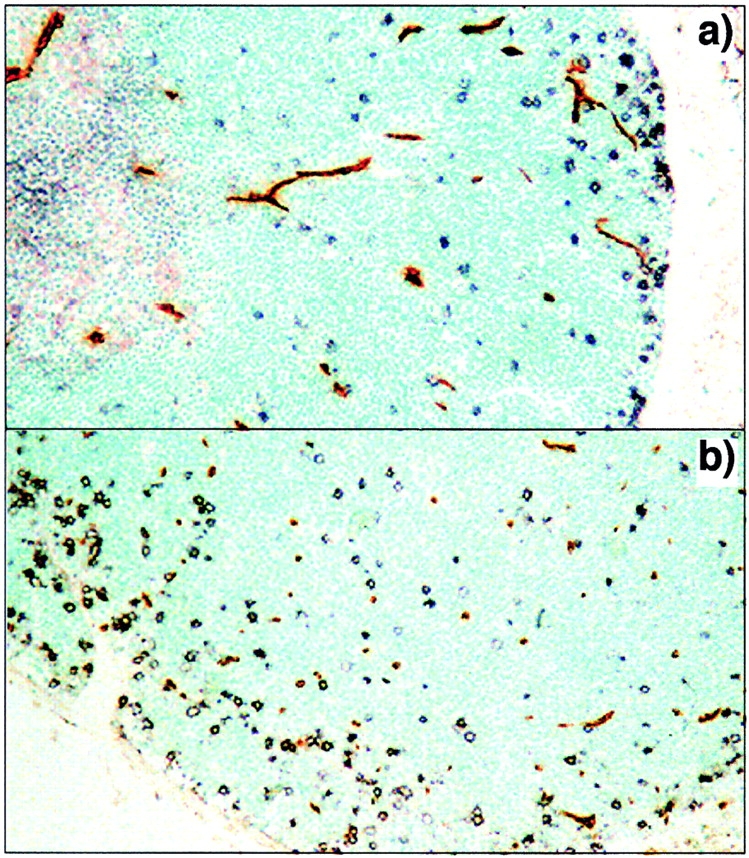
Parenchymal entry correlates with the site of extravasation in the thymic cortex. The relationship between early precursors and vascular/perivascular tissues was examined using CD25 staining (black) as a marker of DN precursors and tomato lectin (brown) as a marker of vascular endothelium. The transverse section shown in a includes cortical and medullary regions and is parallel to cortical capillary loops. In b, the plane of sectioning is through the deep cortex perpendicular to the orientation of capillary loops. No correlation between CD25+ precursors and vascular tissue is seen in either orientation, indicating that the perivascular sheaths are not conduits for the transcortical migration of early thymocytes and that parenchymal entry occurs deep within the tissue.
Results
Stratified Distribution of Lymphopoietic Progenitors in the Thymic Cortex.
To determine the subanatomic location of staged T cell precursors, microscopic analysis was performed on transverse sections of thymus from 4–6-wk-old mice. Since many nonlymphoid cells also express CD44, CD117 staining was used to identify DN1 and DN2 precursors, as this receptor is expressed in much the same way as CD44 6. The data in Fig. 1 a–d show that the earliest developmental stages, marked by expression of CD117, are abundant at the CMJ, and are scattered at lower density throughout the cortex, but are rare in the SCZ. In contrast, later DN stages, marked by CD25 expression, are virtually absent from the CMJ, but increase in frequency moving outward through the cortex, and are most concentrated in the SCZ. Since the morphology of some CD117+ cells near the CMJ was indistinct, and since some mature thymocytes can express CD25, the overall distribution of DN cells was further confirmed by marking RAG-2–deficient cells (Thy-1.2+) in chimeric thymuses from wild-type (Thy-1.1+)/RAG-mutant bone marrow chimeras 19. The distribution of RAG-deficient DN cells in chimeric thymuses (Fig. 1e–f) is essentially equivalent to the sum of CD117 and CD25 distribution in normal thymus; cells are completely absent from the medulla but span the boundaries of the cortex in a gradient that peaks in the SCZ.
Figure 1.
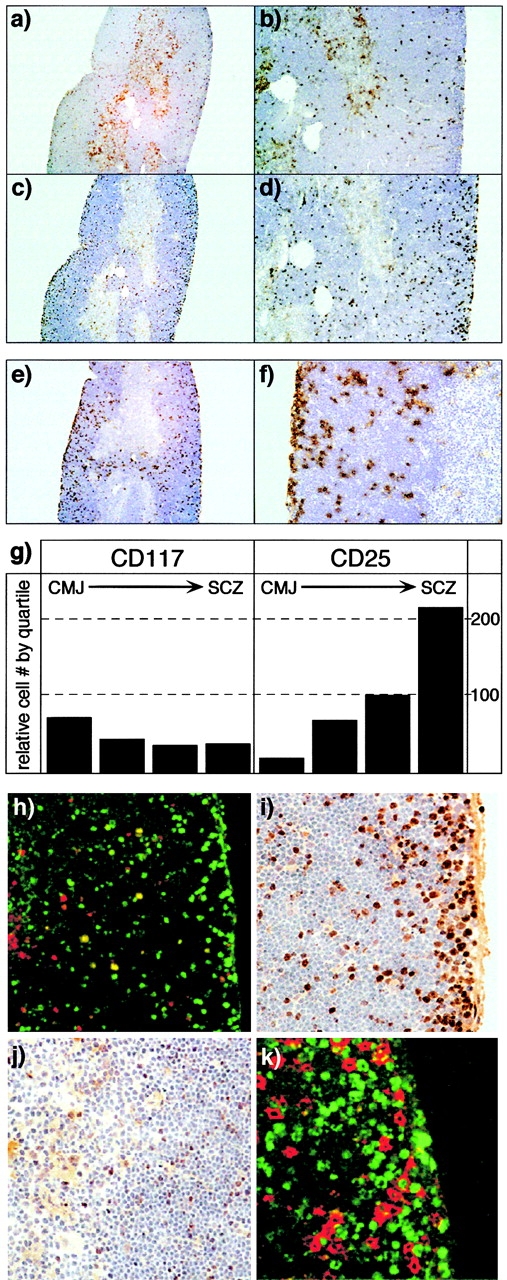
Anatomic localization of early precursors in the adult thymus. Low magnification (a and c, original magnifications: 40×) and high magnification (b and d, original magnifications: 100×) views of serial sections of adult thymus, stained with anti-CD117 (brown, a and b) or anti-CD25 (c and d) antibodies. The counterstain (blue) is hematoxylin. CD117+ cells are most frequent near the CMJ and are also scattered at lower density throughout the cortex, but are relatively infrequent in the SCZ. In contrast, CD25+ cells are rare in the vicinity of the CMJ, but increase in frequency with proximity to the SCZ. The total anatomic range for CD4−8− precursors is seen in e (original magnification: 40×) and f (original magnification: 200×), using a congenic marker for RAG-2–deficient cells (Thy-1.2) in thymuses from RAG-2/wild-type (Thy-1.1+) bone marrow chimeras. The relative distribution of CD117+ or CD25+ cells in the cortex, divided into quartiles, is illustrated in g; data are derived from counting multiple CD25/117 serial sections. h shows two-color immunofluorescent staining of CD117 (red) and CD25 (green) in a single cortical field (original magnification: 200×; left, CMJ). DN2 cells (yellow, CD117+CD25+) are generally found in the midcortical region. i and j illustrate the location of proliferating cells (brown, anti-BrdU) in the SCZ/outer cortex or inner cortex/CMJ, respectively (original magnification: 400×) after a single 4-h pulse of BrdU. As reported previously, the vast majority of cell proliferation is found to occur in the SCZ, although infrequent BrdU+ cells are found throughout the cortex. However, two-color staining (k) with CD25 (red) and BrdU (green) reveals that most of the proliferating cells in the SCZ are not DN cells (CD25+), and therefore represent amplification of the early DP compartment (preDP).
Quantitation of lymphoid cells from such numerous serial sections (Fig. 1 g) reveals that DN1 cells (CD117+25−) are mainly limited to the very inner regions of the cortex, since this is the only site where CD117+ cells outnumber CD25+ cells. Likewise, the predominance of CD25+ cells over CD117+ cells in the outer third of the cortex (Fig. 1 g) indicates that this is the primary location for most DN3 cells (CD25+117lo). To further define the regions for cell differentiation, two-color immunofluourescent analysis of CD117 and CD25 was performed (Fig. 1 h). Consistent with the predictions made above, DN2 cells (CD117+25+) begin to appear in the second quartile of the cortex, and generally span at least 50% of the cortical width. Together, the data in Fig. 1 a–h suggest that DN2 cells are highly motile and are largely responsible for the migration that occurs from the inner to the outer cortex.
DN3 cells represent the most numerically dominant phase of early development (for a review, see reference 1). Consequently, the localization of this population to the outer cortex/SCZ is consistent with the widely accepted view that DN cells (identified as proliferating precursors; reference 21) are concentrated in this region. However, the data in Fig. 1 show that earlier stages of DN development (i.e., DN1 and DN2) are in fact much deeper in the cortex, consistent with the hypothesis that blood-borne cells enter deep within the organ. Our group and others have shown that both of these deep cortical populations are proliferating; in fact, DN2 cells are the second-most rapidly proliferating stage of intrathymic development 30 31. In vivo DNA synthetic labeling confirms the presence of a small number of proliferating cells scattered throughout the deep cortex (Fig. 1i and Fig. j), consistent with the notion that DN proliferation may not be limited to the SCZ. To determine the identity of proliferating cells in the SCZ, we performed two-color immunofluorescent analysis (Fig. 1 k), using antibodies recognizing CD25 (to mark DN precursors) and BrdU (to mark DNA synthesis). Although a few BrdU+CD25+ cells are seen in the SCZ, most proliferating cells in this region are not true DN cells (i.e., CD25+), but rather represent the proliferative burst that accompanies transition to the DP stage 30 31 32. The localization of most DN cells to the deeper cortex, together with the knowledge that these deep cortical DN do proliferate, indicates that migration of precursor cells to the SCZ is not obligated by proliferation, but rather because the signals that induce differentiation to the DP stage are restricted to this region.
Bone Marrow Precursors Extravasate within the Cortex in a Narrow Region Adjacent to the Medulla.
The data shown in Fig. 1, together with the findings of others 26 27 28, support the view that blood-borne bone marrow–derived precursors first appear in the thymus deep within the organ. However, the region for cell entry into the thymus has remained fairly vague, due to the relatively broad distribution of postcapillary venules and complications related to the effects of surgery, radiation, recirculating mature cells, and other similar matters. Precise localization of this event is necessary not only to corroborate the location of DN1 precursors in the perimedullary cortex, but also to facilitate identification of the signals leading to extravasation of precursors from the blood, since these are likely to be found in the corresponding regions. To precisely define the location of this event, lineage-depleted bone marrow cells were labeled with the fluorescent dye CFSE and were transferred intravenously into nonablated normal recipients, followed by removal of the recipient thymus 24–48 h later. In this system, labeled T cell precursors contained within the lineage-negative population would be expected to compete with endogenous (unlabeled) precursors for access to an essentially unmodified thymus. This system thus differs from archetypal studies of precursor entry into the postnatal thymus which, due to the consequences of radiation or other ablative regimens, apparently require a lag period between transplantation and thymic homing. A total of six mice were treated in this fashion. Numerous frozen sections of thymus were examined until a total of nine labeled cells were found; at this point, screening was stopped, because all were found within a narrow region of the perimedullary cortex, in the vicinity of blood vessels exhibiting the morphology of postcapillary venules (Fig. 2, a–c). Subsequent experiments using this nonablated transplant system have shown that cells entering the thymus in this manner progress through the known stages of T cell development with normal kinetics (supplemental data not shown), thus validating the histologic findings. Together with the intrathymic localization studies described above, these experiments identify a narrow region of the perimedullary cortex as being critical for signaling the extravasation of precursors from the blood, consistent with the localization of DN1 cells to this region.
Extravasation from the Bloodstream and Thymic Parenchymal Entry Are Spatially Coincident Events.
Extravasation from the bloodstream and entry into the thymic parenchyma are physically and functionally separable. Relatively large perivascular spaces are found between the basement membrane of thymic blood vessels and the surrounding perivascular epithelium, and these frequently contain large numbers of lymphocytes 24. Consequently, it is possible that while extravasation (i.e., transendothelial migration) occurs in the perimedullary cortex, formal entry into the parenchyma (i.e., transepithelial migration) could occur at a distal site, even as far away as the SCZ, if cortical transmigration were to occur within perivascular sheaths. In this case, the signals required for extravasation and parenchymal entry would be expected to be spatially separate. To further define where newly arrived precursors enter the thymic parenchyma, colocalization studies were performed using a marker for DN thymocytes (CD25) together with a second marker to reveal the location of blood vessels (intravenous tomato lectin; Materials and Methods). Fig. 3 a shows a deep transverse section of thymus stained in this manner; no correlation is seen between the location of DN cells and vascular tissues, either in the perimedullary regions or elsewhere in the cortex. To eliminate the possibility that interactions between DN cells and blood vessels were above or below the plane shown in Fig. 3 a, other sections were examined in which the plane was perpendicular to that of cortical blood vessels, with identical results (Fig. 3 b). Together, these data show that newly arrived precursors dissociate from vascular regions very soon after leaving the bloodstream, and must directly navigate the cortical parenchyma to reach the SCZ.
Discussion
The experiments described in this study provide new insights into two aspects of thymus biology. The first is a precise description of lymphoid precursor cell migration into and within the steady-state thymus. Although anatomic assessment of vascular tissue distribution in the thymus 23 24 25 and studies using irradiation bone marrow chimeras 26 27 28 long ago implicated deep-tissue regions in the entry of cells into the thymus, the precise sites of such entry have never been defined, especially in a nonablated, steady-state system. Precise identification of the sites for precursor entry is critical, since the cryptic signals that induce multipotent precursors to leave the blood and enter the thymus are likely to be found in these regions. Further, an unresolved discrepancy has endured between the ostensible location of blood precursor entry (deep in the tissue) and the apparent tissue location of DN cells (in the SCZ). In this study, we reconcile these by showing that in the postnatal thymus, blood precursor cells enter the thymic parenchyma in a narrow region of the perimedullary cortex, followed by outward migration across the cortex and accumulation in the SCZ. However, blood precursors do not transmigrate the cortex in an undifferentiated state. Rather, movement between different regions of the cortex correlates with defined stages of early lymphoid development. For instance, the earliest DN1 precursors predominate in a region roughly corresponding to the inner 15–20% of the cortex (Fig. 1, a, b, and g), an area that also includes the specific regions for precursor entry from the blood (Fig. 2 and Fig. 3). In contrast, DN2 cells are virtually absent from this region (Fig. 1 h), but are found throughout the mid to outer cortex (Fig. 1g and Fig. h). DN3 cells begin to predominate in the outer third of the cortex and continue to accumulate in number in the SCZ (Fig. 1c, Fig. d, and Fig. g). Thus, although the SCZ does in fact represent the principal location for DN cells, this is only because the numerically dominant DN3 stage is found here. However, most proliferating cells in the SCZ are not DN precursors at all (Fig. 1 k), but rather represent cells in the early DP (preDP) stage. Further, the most actively proliferating DN population, DN2 30 31, is not found in the SCZ, but rather is localized to midcortical regions (Fig. 1 h). Consequently, our findings show that the SCZ is not an exclusive location for lymphopoietic proliferation in the steady-state thymus, but rather provides an environment for the final stages of proliferation that occur very late in DN development and after transition to the DP stage 30 31.
The nonhomogenous distribution of DN stages in the thymic cortex reveals another novel aspect of thymus biology, namely, boundaries of the stromal environments that may induce and/or support each progressive stage of development. Although it is recognized that a variety of stable stromal elements (epithelial cells, fibroblasts, vascular tissues, etc.) and their products are differentially localized in the thymic cortex (for examples, see references 33 34 35 36 37 38 39), the implications of this for lymphoid development has not been obvious. Our data show that distinct stages of DN development are differentially located in the cortex in a manner that corresponds to distinct stromal layers. Identification of the anatomic range for each stage of lymphoid development (Fig. 1 and Fig. 2), together with knowledge of their genetic potential, provides a functional map for these stromal environments and the signals that they impose (illustrated in Fig. 4). For instance, localization of DN1 cells to the inner 15–20% of the cortex identifies this as the first thymic stromal region encountered by T cell progenitors (region 1). The main function of this region appears to be proliferative expansion of cells arriving from the blood, since no apparent differences in lineage potential are seen between DN1 cells and their blood-borne precursors 9 10 40 41. Consequently, production of cytokines and growth factors by stromal cells in this region is strongly implicated. Region 1 also includes specialized sites for precursor cell entry from the blood (Fig. 2 Fig. 3 Fig. 4). It is not clear whether stromally derived signals mediating extravasation and parenchymal entry are common to all of region 1, with cell entry being limited to perimedullary areas by the structure of postcapillary venules, or whether the stromal elements immediately surrounding the sites of extravasation are biochemically distinct. Experiments to distinguish these possibilities, by analysis of gene expression profiles in microdissected tissue regions, are currently underway.
Figure 4.
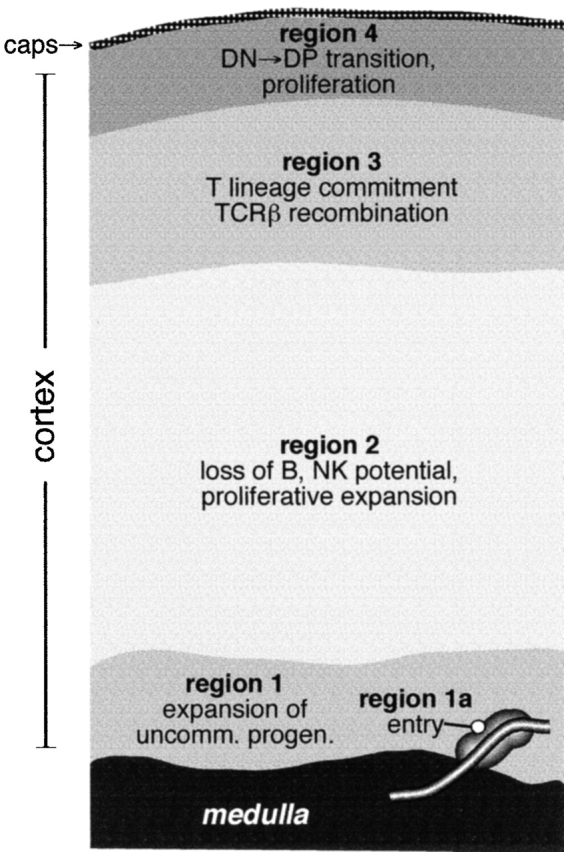
Functionally distinct zones for lymphopoietic precursor differentiation in the postnatal thymus. A map of cortical regions, as defined by the approximate location of each lymphopoietic stage, is shown together with some of the developmental events that occur during migration between them.
The next stromal region (region 2) is defined by onset of the appearance of DN2 cells, ∼20–25% of the distance into the cortex. Given the variety of biochemical changes that occur upon differentiation to the DN2 stage, including loss of B and NK lineage potential 9 11 42, upregulation of mitotic activity 30 31, and initiation of TCR-γ/δ gene rearrangements 43, among others, the signals elucidated by stromal cells in this region are likely to be quite complex. Consequently, characterization of the genes expressed by stromal cells in this region is likely to provide important clues about the factors regulating lymphopoietic development in the thymus. The third functional region of the cortex, revealed by transition to the DN3 stage of development, appears to begin ∼2/3 of the way across the cortex. Again, precise developmental events correlate with migration into this region, including decreased mitotic activity 30 31 and the induction of rearrangements at the TCR-β locus 6 44. Given that D-Jβ and V-DJβ rearrangements are temporally separate events during development 30 44 45 46, it is interesting to note that DN3 cells span two functional regions in the cortex, the fourth region being defined by the induction of rapid cell proliferation and differentiation into the DP stage (Fig. 1). Since these latter functions quickly follow V-DJβ recombination, our data indicate the possibility that signals delivered by entry into regions 3 and 4 may be responsible for sequentially regulating accessibility of D-J and V regions of the TCR-β locus, respectively, to the recombinase. Further, consistent with previously published findings 19 21, our data show that differentiation into the DP stage only occurs after migration into the SCZ (region 4). Thus, like stromal region 2, entry into stromal region 4 (i.e., the SCZ) appears to mitigate a number of cellular responses, including activation of the preTCR checkpoint, differentiation to the DP stage, induction of the mitogenesis that is coincident with these events, and accessibility of the TCR-α locus to recombination. Identification of the specific cellular and/or humoral signals expressed in this region will thus be crucial in elucidating the enigmatic events that govern this important developmental transition.
Successful encounters with distinct stromal environments for differentiation and mitogenesis, as described previously, obviously require migration of lymphopoietic precursors between these regions. This, in turn, reveals the presence of another category of stratified stromal signals, namely, those inducing cell migration from the deep cortex to the SCZ. The data in Fig. 1 e and f show that DN precursors move exclusively in the direction of the capsule after entry into the thymus, never in the opposite direction. Such polarized migration (chemotaxis) implicates the presence of chemoattractive stimuli emanating from stable stromal elements in the outer cortex, capsule, and/or SCZ, as well as receptors for these chemoattractants on deep cortical DN precursors. In addition, cells that are actively transmigrating the cortex (mainly DN2 cells) must express receptors that allow them to adhere to a stable matrix and move in the direction of chemoattractive signals. Recent work from our laboratory indicates that DN2 migration is mediated via adhesion of α4-containing integrins (α4β1 and α4β7) to a matrix composed of VCAM-1+ cortical epithelial cells (unpublished data). Further, we find that the chemokine receptors CCR7 and CCR8 are differentially expressed on deep cortical precursors (i.e., DN1/2 cells), implicating expression of these chemokines by stromal elements in the outer cortex/SCZ in the outward migration of early precursors (unpublished data). Although we have not addressed the movement of later stages of T cell development in this manuscript, it is worth pointing out that a similar migration through the cortex occurs among DP cells, albeit in the opposite direction, during intrathymic development 21. Ultimately, cells that undergo successful TCR–MHC interaction migrate into the medulla, a process that is critical for functional maturation 47 48. Thus, polarized migration of developing lymphoid cells between distinct stromal environments appears to be a universal requirement during postnatal T cell development. In this regard, it is interesting to note that precursor development in the fetal thymus does not require a similar process, since compartmentalization of the fetal organ occurs simultaneously with, rather than before, lymphoid differentiation. Further, the fetal organ is not vascularized at the time of hematopoietic seeding 49 50, a process that is integrally linked to the compartmentalization process 51. Consequently, although many parallels can be drawn between fetal and postnatal development, the arrival of precursors through deep vascular tissues, followed by outward migration through discrete stromal zones, indicate the presence of numerous regulatory parameters in the adult that do not function in the embryo. We conclude that spatial separation of differentiative and proliferative compartments in the fully formed organ represents a critical regulatory element in the control of T cell homeostasis during postnatal life.
Acknowledgments
The authors wish to express their gratitude to Drs. Kathryn Anderson (Memorial Sloan-Kettering Cancer Center), Richard Boyd (Monash Medical School, Melbourne, Australia), Andrew Farr (University of Washington, Seattle, WA), Paul Kincade (Oklahoma Medical Research Foundation, Oklahoma City, OK), Michelle Letarte (Hospital for Sick Children, Toronto, Canada), Dennis Osmond (McGill University, Montreal, Canada), and Juan Carlos Zuniga-Pflucker (University of Toronto, Toronto, Canada) for their invaluable advice and discussions.
This work was supported by Public Health Service grants AI33940 (to H.T. Petrie) and P30-CA08748 (to Memorial Sloan-Kettering Cancer Center), and by funds from the DeWitt Wallace Foundation.
Footnotes
Abbreviations used in this paper: CMJ, cortical and medullary junction; DN, double negative; DP, double positive; SCZ, subcapsular zone.
References
- Shortman K., Egerton M., Spangrude G.J., Scollay R. The generation and fate of thymocytes. Semin. Immunol. 1990;2:3–12. [PubMed] [Google Scholar]
- Pearse M., Wu L., Egerton M., Wilson A., Shortman K., Scollay R. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc. Natl. Acad. Sci. USA. 1989;86:1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R.P., Husmann L.A., Bevan M.J., Crispe I.N. Transient expression of IL-2 receptor precedes the differentiation of immature thymocytes. Nature. 1987;329:157–159. doi: 10.1038/329157a0. [DOI] [PubMed] [Google Scholar]
- Lesley J., Trotter J., Schulte R., Hyman R. Phenotypic analysis of the early events during repopulation of the thymus by the bone marrow prothymocyte. Cell. Immunol. 1990;128:63–78. doi: 10.1016/0008-8749(90)90007-e. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J.W., Nabholz M., MacDonald H.R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Kennedy J., Mombaerts P., Tonegawa S., Zlotnik A. Onset of TCR-β gene rearrangement and role of TCR-β expression during CD3−CD4−CD8− thymocyte differentiation. J. Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- Nikolic-Zugic J., Moore M.W., Bevan M.J. Characterization of the subset of immature thymocytes which can undergo rapid in vitro differentiation. Eur. J. Immunol. 1989;19:649–653. doi: 10.1002/eji.1830190412. [DOI] [PubMed] [Google Scholar]
- Petrie H.T., Hugo P., Scollay R., Shortman K. Lineage relationships and developmental kinetics of immature thymocytesCD3, CD4, and CD8 acquisition in vivo and in vitro. J. Exp. Med. 1990;172:1583–1588. doi: 10.1084/jem.172.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardavin C., Wu L., Li C.L., Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–773. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- Wu L., Antica M., Johnson G.R., Scollay R., Shortman K. Developmental potential of the earliest precursor cells from the adult mouse thymus. J. Exp. Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Li C.L., Shortman K. Thymic dendritic cell precursorsrelationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J. Exp. Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie H.T., Scollay R., Shortman K. Commitment to the T cell receptor-α/β or -γ/δ lineages can occur just prior to the onset of CD4 and CD8 expression among immature thymocytes. Eur. J. Immunol. 1992;22:2185–2188. doi: 10.1002/eji.1830220836. [DOI] [PubMed] [Google Scholar]
- Dudley E.C., Petrie H.T., Shah L.M., Owen M.J., Hayday A.C. T cell receptor β chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Akashi K., Kondo M., Weissman I.L. Two distinct pathways of positive selection for thymocytes. Proc. Natl. Acad. Sci. USA. 1998;95:2486–2491. doi: 10.1073/pnas.95.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald H.R., Ogawa M., Haller C., Waskow C., DiSanto J.P. Pro-thymocyte expansion by c-kit and the common cytokine receptor γ chain is essential for repertoire formation. Immunity. 1997;6:265–272. doi: 10.1016/s1074-7613(00)80329-5. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F., Wilson A., Stark G., Bauer M., van Meerwijk J., MacDonald H.R., Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Voll R.E., Jimi E., Phillips R.J., Barber D.F., Rincon M., Hayday A.C., Flavell R.A., Ghosh S. NF-κB activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13:677–689. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Petrie H.T., Tourigny M., Burtrum D.B., Livak F. Precursor thymocyte proliferation and differentiation are controlled by signals unrelated to the pre-TCR. J. Immunol. 2000;165:3094–3098. doi: 10.4049/jimmunol.165.6.3094. [DOI] [PubMed] [Google Scholar]
- Fehling H.J., Krotkova A., Saint-Ruf C., von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of α/β but not γ/δ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- Penit C. Localization and phenotype of cycling and post-cycling murine thymocytes studied by simultaneous detection of bromodeoxyuridine and surface antigens. J. Histochem. Cytochem. 1988;36:473–478. doi: 10.1177/36.5.2895787. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Schreyer M. Immunohistochemical localization of host and donor-derived cells in the regenerating thymus of radiation bone marrow chimeras. Thymus. 1984;6:15–26. [PubMed] [Google Scholar]
- Raviola E., Karnovsky M.J. Evidence for a blood-thymus barrier using electron-opaque tracers. J. Exp. Med. 1972;136:466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiki T. A scanning electron-microscopic study of the rat thymus with special reference to cell types and migration of lymphocytes into the general circulation. Cell Tissue Res. 1986;244:285–298. doi: 10.1007/BF00219204. [DOI] [PubMed] [Google Scholar]
- Kato S., Schoefl G.I. Microvasculature of normal and involuted mouse thymus. Light- and electron-microscopic study. Acta. Anat. 1989;135:1–11. doi: 10.1159/000146715. [DOI] [PubMed] [Google Scholar]
- Brahim F., Osmond D.G. Migration of bone marrow lymphocytes demonstrated by selective bone marrow labeling with thymidine-H3. Anat. Rec. 1970;168:139–159. doi: 10.1002/ar.1091680202. [DOI] [PubMed] [Google Scholar]
- Brumby M., Metcalf D. Migration of cells to the thymus demonstrated by parabiosis. Proc. Soc. Exp. Biol. Med. 1967;124:99–103. doi: 10.3181/00379727-124-31675. [DOI] [PubMed] [Google Scholar]
- Kyewski B.A. Seeding of thymic microenvironments defined by distinct thymocyte-stromal cell interactions is developmentally controlled. J. Exp. Med. 1987;166:520–538. doi: 10.1084/jem.166.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezine S., Weissman I.L., Rouse R.V. Bone marrow cells give rise to distinct cell clones within the thymus. Nature. 1984;309:629–631. doi: 10.1038/309629a0. [DOI] [PubMed] [Google Scholar]
- Tourigny M.R., Mazel S., Burtrum D.B., Petrie H.T. T cell receptor (TCR)-β gene recombinationdissociation from cell cycle regulation and developmental progression during T cell ontogeny. J. Exp. Med. 1997;185:1549–1556. doi: 10.1084/jem.185.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C., Lucas B., Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J. Immunol. 1995;154:5103–5113. [PubMed] [Google Scholar]
- Hoffman E.S., Passoni L., Crompton T., Leu T.M., Schatz D.G., Koff A., Owen M.J., Hayday A.C. Productive T-cell receptor β-chain gene rearrangementcoincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- Felli M.P., Maroder M., Mitsiadis T.A., Campese A.F., Bellavia D., Vacca A., Mann R.S., Frati L., Lendahl U., Gulino A., Screpanti I. Expression pattern of notch1, 2 and 3 and Jagged1 and 2 in lymphoid and stromal thymus componentsdistinct ligand-receptor interactions in intrathymic T cell development. Int. Immunol. 1999;11:1017–1025. doi: 10.1093/intimm/11.7.1017. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Sharrow S.O., Farr A.G., Singer A., Udey M.C. Expression of the homotypic adhesion molecule E-cadherin by immature murine thymocytes and thymic epithelial cells. J. Immunol. 1994;152:5653–5659. [PubMed] [Google Scholar]
- Takahama Y., Letterio J.J., Suzuki H., Farr A.G., Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor β. J. Exp. Med. 1994;179:1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug D.B., Carter C., Crouch E., Roop D., Conti C.J., Richie E.R. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc. Natl. Acad. Sci. USA. 1998;95:11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander G.A., Wang B., Nichogiannopoulou A., Platenburg P.P., van Ewijk W., Burakoff S.J., Gutierrez-Ramos J.C., Terhorst C. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373:350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- van Ewijk W. Cell surface topography of thymic microenvironments. Lab. Invest. 1988;59:579–590. [PubMed] [Google Scholar]
- Wallin J., Eibel H., Neubuser A., Wilting J., Koseki H., Balling R. Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development. 1996;122:23–30. doi: 10.1242/dev.122.1.23. [DOI] [PubMed] [Google Scholar]
- Kondo M., Weissman I.L., Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Spangrude G.J., Muller-Sieburg C.E., Heimfeld S., Weissman I.L. Two rare populations of mouse Thy-1lo bone marrow cells repopulate the thymus. J. Exp. Med. 1988;167:1671–1683. doi: 10.1084/jem.167.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie A.M., Carlyle J.R., Schmitt T.M., Ljutic B., Cho S.K., Fong Q., Zuniga-Pflucker J.C. Clonal characterization of a bipotent T cell and NK cell progenitor in the mouse fetal thymus. J. Immunol. 2000;164:1730–1733. doi: 10.4049/jimmunol.164.4.1730. [DOI] [PubMed] [Google Scholar]
- Livak F., Tourigny M., Schatz D.G., Petrie H.T. Characterization of TCR gene rearrangements during adult murine T cell development. J. Immunol. 1999;162:2575–2580. [PubMed] [Google Scholar]
- Petrie H.T., Livak F., Burtrum D., Mazel S. T cell receptor gene recombination patterns and mechanismscell death, rescue, and T cell production. J. Exp. Med. 1995;182:121–127. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Yague J., Palmer E., Kappler J., Marrack P. Rearrangement of T-cell receptor β-chain genes during T-cell development. Proc. Natl. Acad. Sci. USA. 1985;82:2925–2929. doi: 10.1073/pnas.82.9.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F.W., Yancopoulos G.D., Blackwell T.K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall R., Nikolic-Zugic J. The majority of postselection CD4+ single-positive thymocytes requires the thymus to produce long-lived, functional T cells. J. Exp. Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie H.T., Strasser A., Harris A.W., Hugo P., Shortman K. CD4+8− and CD4−8+ mature thymocytes require different post-selection processing for final development. J. Immunol. 1993;151:1273–1279. [PubMed] [Google Scholar]
- Suniara R.K., Jenkinson E.J., Owen J.J. Studies on the phenotype of migrant thymic stem cells. Eur. J. Immunol. 1999;29:75–80. doi: 10.1002/(SICI)1521-4141(199901)29:01<75::AID-IMMU75>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M., Jotereau F.V. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J. Exp. Med. 1975;142:17–40. doi: 10.1084/jem.142.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M., Anderson S.K., Farr A.G. Thymic vasculatureorganizer of the medullary epithelial compartment? Int. Immunol. 2000;12:1105–1110. doi: 10.1093/intimm/12.7.1105. [DOI] [PubMed] [Google Scholar]


